Abstract
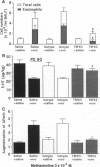
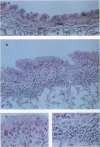
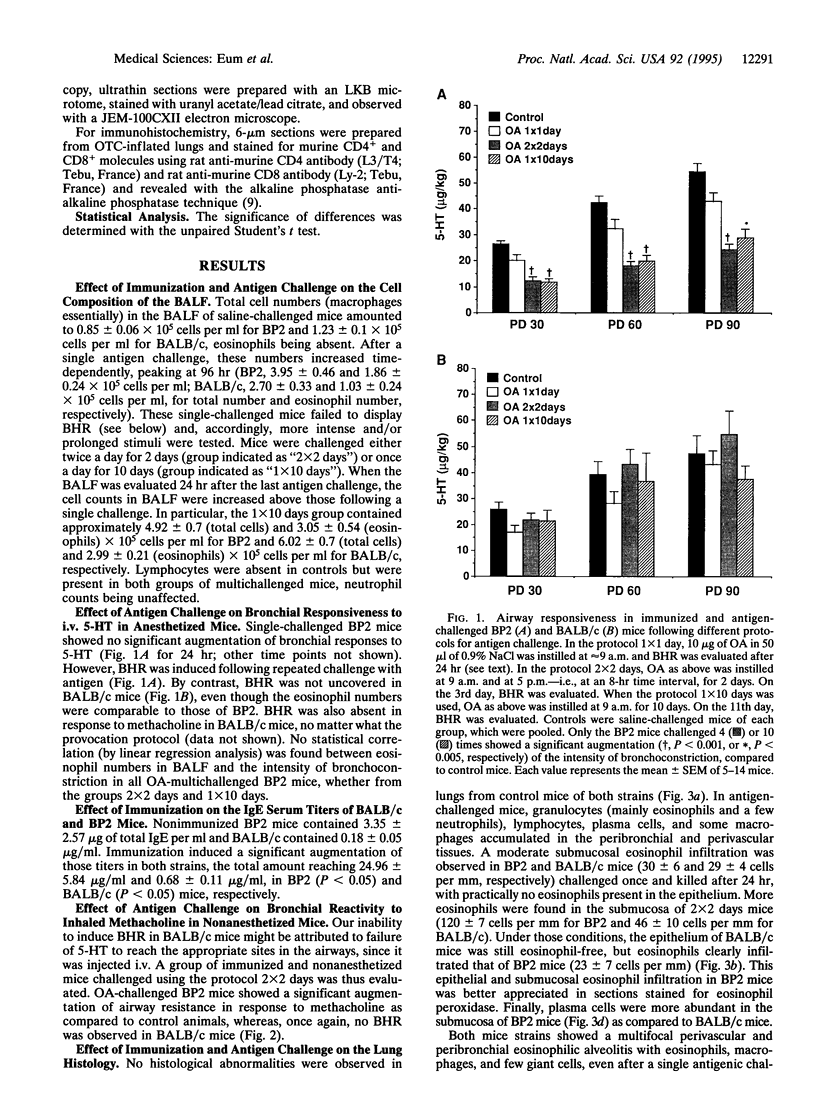
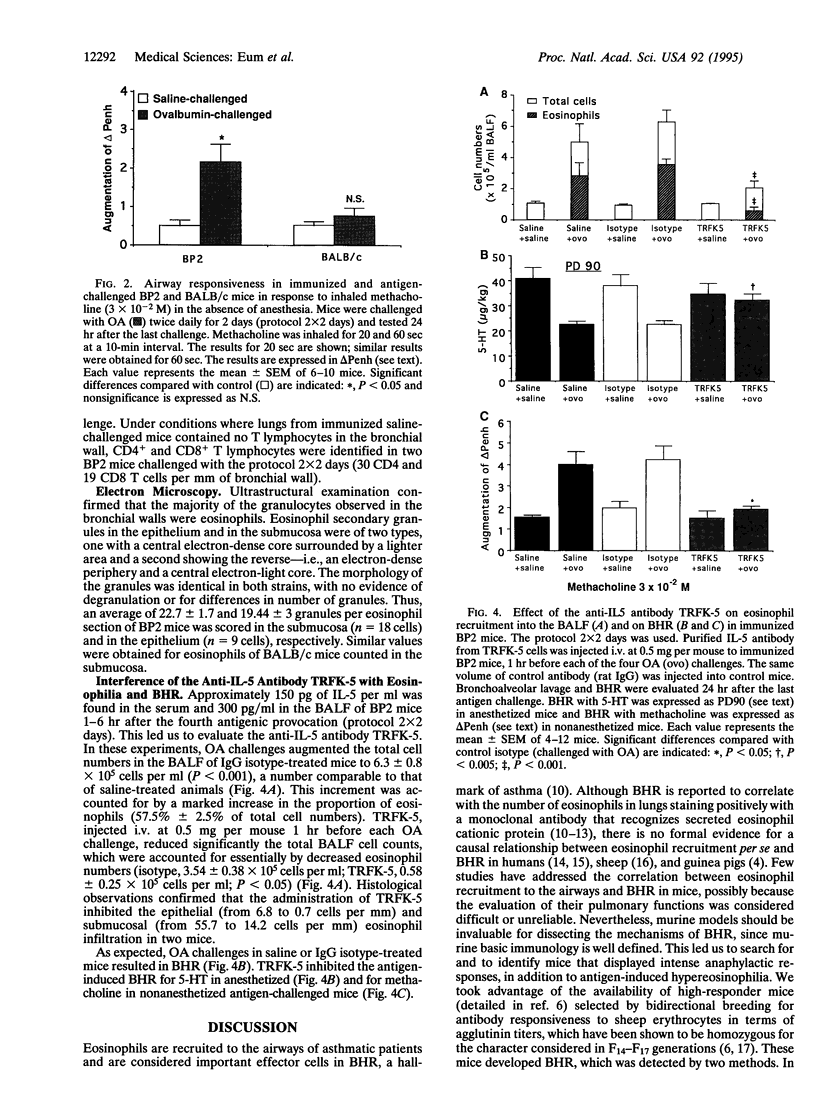
A murine model for antigen-induced bronchial hyperreactivity (BHR) and airway eosinophilia, two hallmarks of asthma, was developed using ovalbumin-immunized mice, which produce large amounts of IgE (named BP2, "Bons Producteurs 2," for High Line of Selection 2). A single intranasal ovalbumin challenge failed to modify the bronchial responses, despite the intense eosinophil recruitment into the bronchoalveolar lavage fluid and airways. When mice were challenged twice a day for 2 days or once a day for 10 days, BHR in response to i.v. 5-hydroxytryptamine or to inhaled methacholine was induced in BP2 mice but not in BALB/c mice. Histological examination showed that eosinophils reached the respiratory epithelium after multiple ovalbumin challenges in BP2 mice but remained in the bronchial submucosa in BALB/c mice. Total IgE titers in serum were augmented significantly with immunization in both strains, but much more so in BP2 mice. Interleukin 5 (IL-5) titers in serum and bronchoalveolar lavage fluid of BP2 mice were augmented by the antigenic provocation, and a specific anti-IL5 neutralizing antibody suppressed altogether airway eosinophilia and BHR, indicating a participation of IL-5 in its development. Our results indicate that the recruitment of eosinophils to the airways alone does not induce BHR in mice and that the selective effect on BP2 mice is related to their increased IgE titers associated with antigen-driven eosinophil migration to the epithelium, following formation and secretion of IL-5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham W. M., Sielczak M. W., Ahmed A., Cortes A., Lauredo I. T., Kim J., Pepinsky B., Benjamin C. D., Leone D. R., Lobb R. R. Alpha 4-integrins mediate antigen-induced late bronchial responses and prolonged airway hyperresponsiveness in sheep. J Clin Invest. 1994 Feb;93(2):776–787. doi: 10.1172/JCI117032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzawi M., Bradley B., Jeffery P. K., Frew A. J., Wardlaw A. J., Knowles G., Assoufi B., Collins J. V., Durham S., Kay A. B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Biozzi G., Mouton D., Sant'Anna O. A., Passos H. C., Gennari M., Reis M. H., Ferreira V. C., Heumann A. M., Bouthillier Y., Ibanez O. M. Genetics of immunoresponsiveness to natural antigens in the mouse. Curr Top Microbiol Immunol. 1979;85:31–98. [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J., Sheller J. R., Nadel J. A. Bronchial hyperreactivity. Am Rev Respir Dis. 1980 Feb;121(2):389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- Brusselle G. G., Kips J. C., Tavernier J. H., van der Heyden J. G., Cuvelier C. A., Pauwels R. A., Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994 Jan;24(1):73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Chand N., Nolan K., Pillar J., Lomask M., Diamantis W., Sofia R. D. Aeroallergen-induced dyspnea in freely moving guinea pigs: quantitative measurement by bias flow ventilated whole body plethysmography. Allergy. 1993 May;48(4):230–235. doi: 10.1111/j.1398-9995.1993.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Chand N., Nolan K., Pillar J., Lomask M., Diamantis W., Sofia R. D. Aeroallergen-induced dyspnea in freely moving guinea pigs: quantitative measurement by bias flow ventilated whole body plethysmography. Allergy. 1993 May;48(4):230–235. doi: 10.1111/j.1398-9995.1993.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Kay A. B. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992 Dec;13(12):501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Garlisi C. G., Falcone A., Kung T. T., Stelts D., Pennline K. J., Beavis A. J., Smith S. R., Egan R. W., Umland S. P. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin Immunol Immunopathol. 1995 Apr;75(1):75–83. doi: 10.1006/clin.1995.1055. [DOI] [PubMed] [Google Scholar]
- Gavett S. H., Chen X., Finkelman F., Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994 Jun;10(6):587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- Gerblich A. A., Salik H., Schuyler M. R. Dynamic T-cell changes in peripheral blood and bronchoalveolar lavage after antigen bronchoprovocation in asthmatics. Am Rev Respir Dis. 1991 Mar;143(3):533–537. doi: 10.1164/ajrccm/143.3.533. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Howarth P. H., Bradding P., Montefort S., Peroni D., Djukanovic R., Carroll M. P., Holgate S. T. Mucosal inflammation and asthma. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 2):S18–S22. doi: 10.1164/ajrccm/150.5_Pt_2.S18. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Ledermann F., Schlienger C., Wagner K., Heusser C. A sensitive and efficient induction system for murine IgE. Single cell analysis at the clonal level. J Immunol Methods. 1991 Aug 9;141(2):263–275. doi: 10.1016/0022-1759(91)90153-7. [DOI] [PubMed] [Google Scholar]
- Mouton D., Siqueira M., Sant'Anna O. A., Bouthillier Y., Ibanez O., Ferreira V. C., Mevel J. C., Reis M. H., Piatti R. M., Stiffel C. Genetic regulation of multispecific antibody responsiveness: improvement of "high" and "low" characters. Eur J Immunol. 1988 Jan;18(1):41–49. doi: 10.1002/eji.1830180108. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Tomoe S., Matsumura R., Tomioka H., Takatsu K., Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992 Aug;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- Pretolani M., Ruffié C., Joseph D., Campos M. G., Church M. K., Lefort J., Vargaftig B. B. Role of eosinophil activation in the bronchial reactivity of allergic guinea pigs. Am J Respir Crit Care Med. 1994 May;149(5):1167–1174. doi: 10.1164/ajrccm.149.5.8173756. [DOI] [PubMed] [Google Scholar]
- Pretolani M., Ruffié C., Lapa e Silva J. R., Joseph D., Lobb R. R., Vargaftig B. B. Antibody to very late activation antigen 4 prevents antigen-induced bronchial hyperreactivity and cellular infiltration in the guinea pig airways. J Exp Med. 1994 Sep 1;180(3):795–805. doi: 10.1084/jem.180.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Smith H. R., Henson J. E., Ray B. S., Irvin C. G., Gelfand E. W. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. J Allergy Clin Immunol. 1992 Jun;89(6):1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J., Peterson C., Venge P., Olsson I. Monoclonal antibodies distinguish between storage and secreted forms of eosinophil cationic protein. Nature. 1984 May 10;309(5964):182–184. doi: 10.1038/309182a0. [DOI] [PubMed] [Google Scholar]
- Ten R. M., Pease L. R., McKean D. J., Bell M. P., Gleich G. J. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989 May 1;169(5):1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Ladenius A. R., Savelkoul H. F., Van Ark I., Delsman K. C., Nijkamp F. P. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993 Mar;147(3):548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]