Abstract
In diet-induced obesity, adipose tissue (AT) is in a chronic state of inflammation predisposing the development of metabolic syndrome. Cocoa (Theobroma cacao) is a polyphenol-rich food with putative anti-inflammatory activities. Here, we examined the impact and underlying mechanisms of action of cocoa on AT inflammation in high fat-fed mice. In the present study, male C57BL/6J mice were fed a high fat diet (HF), a HF diet with 8% (w/w) unsweetened cocoa powder (HFC), or a low-fat diet (LF) for 18 wk. Cocoa supplementation decreased AT mRNA levels of tumor necrosis factor-α, interleukin-6, inducible nitric oxide synthase, and EGF-like module-containing mucin-like hormone receptor-like 1 by 40 – 60% compared to HF group, and this was accompanied by decreased nuclear protein levels of nuclear factor-κB. Cocoa treatment reduced the levels of arachidonic acid in the AT by 33% compared to HF controls. Moreover, cocoa treatment also reduced protein levels of the eicosanoid-generating enzymes, adipose-specific phospholipase A2 and cycloxygenase-2 by 53% and 55%, respectively, compared to HF-fed mice. Finally, cocoa treatment ameliorated metabolic endotoxemia (40% reduction in plasma endotoxin) and improved gut barrier function (as measured by increased plasma levels of glucagon-like peptide-2). In conclusion, the present study has shown for the first time that long-term cocoa supplementation can reduce AT inflammation in part by modulating eicosanoid metabolism and metabolic endotoxemia.
Keywords: cocoa, Theobroma cacao, polyphenol, obesity, inflammation, adipose tissue
1. Introduction
Obesity and related health problems (e.g. type 2 diabetes, hypertension, and atherosclerosis) are the most prevalent nutrition-related issues in the United States [1], affecting over 50% of the adult population [2]. The cluster of obesity-related metabolic diseases is known as the metabolic syndrome. One emerging feature of the metabolic syndrome is its linkage with chronic inflammation in adipose tissue (AT) that becomes systemic [1,3]. This chronic systemic inflammation is driven by the infiltration of macrophages into AT, which, together with adipocytes, perpetuate a cycle of macrophage recruitment and secretion of free fatty acids and deleterious cytokines/chemokines that predispose that drive the development of metabolic syndrome [4].
During the progression of obesity, the adipocytes undergoes hyperplasia and hypertrophy: these enlarged adipocyte begin recruiting macrophages [5,6]. Adipose tissue macrophages (ATMs) secrete pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukins (e.g. IL-6), and recruitment of additional macrophages by secreting chemokines including monocyte chemotactic protein-1 (MCP-1) [5,7]. These newly released inflammatory cytokines can interact with their receptors at the surface of nearby adipocytes to signal a further activation of nuclear factor-κB (NF-κB), the key transcription factor that drives the inflammatory responses of the innate immune system[8]. Pro-inflammatory genes such as inducible nitric oxide synthase (Nos2) and cyclooxygenase-2 (Cox2) are activated by NF-κB, and contribute to the progression of systemic inflammation [3].
Inflammatory lipid mediators play a role in the development of obesity-induced AT inflammation [4,9]. Eicosanoids, a large family of compounds generated from arachidonic acid (AA), represent one of the most potent classes of endogenous inflammatory mediators. In AT, upon activation of adipose-specific phospholipase A2 (AdPLA), AA is released from membrane phospholipids and becomes available as a substrate for the intracellular biosynthesis of eicosanoids through two major enzymatic routes: the cyclooxygenase (COX) and lipoxygenase (LOX) pathways[9]. Studies have shown that mice that are deficient in key eicosanoid-generating enzymes including COX-2, 5-LOX and 12/15-LOX exhibit decreases in adipocyte differentiation, macrophage infiltration and are protected from high-fat (HF) diet-induced elevation of inflammatory cytokines [10–12]. Thus, the metabolism of eicosanoids represents a novel target for the prevention or treatment of obesity-associated inflammation.
An increasing number of studies have suggested that metabolic endotoxemia, characterized by an excess of circulating bacterial wall lipopolysaccharide (LPS), is also associated with obesity and systemic inflammation [13,14]. Studies have shown that consumption of a HF diet can alter the composition of gut microbiota, increase the incorporation of LPS into chylomicrons as well as affect the intestinal permeability, which allow excess endotoxin to enter systemic circulation [15,16]. Metabolic endotoxemia is believed to trigger the AT inflammation via CD14 and toll-like receptor 4 (TLR4) signaling [14,17].
One potential strategy to reduce obesity-related inflammation is consumption of polyphenol-rich foods, which have been reported to have anti-inflammatory properties in a number of model systems [1]. Cocoa (Theobroma cacao) and cocoa-based products are among the richest food sources of polyphenols. Cocoa polyphenols are primarily composed of monomeric (epicatechin and catechin) and oligomeric (proanthocyanidins, PACs) flavan-3-ols or flavanols. PACs with a degree of polymerization (DP) up to ten (i.e. decamer) have been identified in cocoa [18]. In addition to polyphenols, cocoa also contains a significant amount of dietary fibers (approximately 40%) as well as theobromine (2–3%) and a small amount of caffeine is also present (0.2%) [19]. Over the last decade, a growing number of studies have reported the health benefits of cocoa and cocoa flavan-3-ols, particularly reduced risk of cardiometabolic diseases by promoting nitric oxide bioavailability, as well as, through antioxidant, anti-inflammatory, and anti-platelet activities [20–24]. The impact of cocoa on eicosanoid metabolism and metabolic endotoxemia in vivo remains an understudied area.
A previous study from our laboratory has demonstrated that dietary cocoa supplementation (8% w/w) for 10 weeks can significantly ameliorate the body weight gain, nonalcoholic fatty liver disease and systemic inflammation in HF-fed obese mice, principally through modulation of cytokine secretion and inhibition of dietary fat absorption [26]. In addition, cocoa extracts can dose-dependently inhibit activity of digestive enzymes including secreted phospholipase A2 (PLA2, IC50 < 20 μg/mL) in vitro [27]. In the present study, we investigated the preventative effects of a long-term dietary cocoa powder supplementation on AT inflammation though the regulation of pro-inflammatory gene expression, eicosanoid metabolism and metabolic endotoxemia in HF-fed C57BL/6J mice.
2. Materials and Methods
2.1 Diets and chemicals
The composition of the low-fat (LF, 10% kcal from fat), high-fat (HF, 60% kcal from fat) and HF diet supplemented with 80 mg/g unsweetened cocoa powder (HFC) diet was described previously [26]. The unsweetened cocoa powder used in this study was provided by Blommer Chocolate Co. (Chicago, IL, USA). The composition of the cocoa powder was previously reported [26]. All other chemicals were of the highest grade commercially-available.
2.2 Animals and treatment
The animal experiment was conducted in accordance with a protocol (IACUC# 37115) approved by the Institutional Animal Care and Use committee at the Pennsylvania State University (University Park, PA, USA). Male C57BL/6J mice (4 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained on 12 h light/dark with access to food and water ad libitum. After a two-week acclimatization period, mice were randomized to LF diet (n=23), HF diet (n=21) or HFC diet (n=24) treatments. Body weight and food intake were recorded weekly. At the end of week 18, mice were food-deprived for 7 h (7 am – 2 pm), anesthetized, and sacrificed by exsanguination via cardiac puncture. Hearts, livers, spleens, kidneys and visceral fat depots (epididymal, retroperitoneal, and mesenteric) were harvested, rinsed and weighed. Plasma samples were isolated by centrifugation at 3200 g for 15 min. All samples were snap-frozen and stored at −80°C until further analysis.
2.3 Glycemic markers
Fasting blood glucose, plasma insulin levels were assessed as previously described [26]. Briefly, fasting blood glucose were measured using a hand-held Contour glucose monitor (Bayer Healthcare, Tarrytown, NY, USA) after 7 h of fasting. Fasting plasma insulin was determined at the end of the experiment using an ELISA kit (Crystal Chem, Downers Grove, IL, USA) according to the manufacturer’s protocol. Homeostasis model assessment of insulin resistance (HOMA-IR) was estimated based on the final blood glucose and insulin values [28].
2.4 Fasting plasma triglycerides and free fatty acids
Fasting plasma concentration of triglycerides was measured by a commercial L-type triglyceride kit (Wako Diagnostics, Richmond, VA, USA). Non-esterified free fatty acids levels were quantitatively determined by an enzymatic colorimetric method (λmax = 546 nm, Zen-bio, Research Triangle, NC, USA).
2.5 Gene expression analysis in stromal vascular fraction (SVF) of AT
Isolation of SVF of epididymal AT and gene expression analysis by quantitative reverse transcriptase polymerase chain reaction (qPCR) were conducted as previously described [26]. In brief, epididymal AT were minced and filtered through a cell strainer and lysed, and the SVF was collected after centrifugation. Total RNA of SVF was extracted and genomic DNA contamination was removed using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Gene expression of Tnfa, Il6, Nos2, and Emr1 was analyzed by reverse-transcriptase real-time PCR using commercial TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA), normalized to Gapdh as an endogenous control. Information of the Taqman® probes used in this study was reported previously [26]. Gene expression was analyzed according to the delta delta Ct (ΔΔCT) method normalized to Gapdh, and expression levels were calculated as 2−ΔΔCT.
2.6 Adipocyte cell size image analysis
Samples from epididymal AT were fixed in paraformaldehyde, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin. The sections were viewed at 100X magnification and images were obtained with a DV-130 digital camera (Hawking Technology, Irvine, CA, USA). A measure scale was added to each image using LissView program (Hawking Technology, Irvine, CA, USA). The images were pre-edited using Picasa 3 (Google Inc., Mountain View, CA, USA). The images were conditioned by changing “Invert Color”, “Fill Light”, “Highlights” and “Shadows”. Minor adjustments were also made by “Sharpen” to make image crisper and less fuzzy. After pre-editing the images, Adobe PhotoShop CS 8.0 (Adobe systems, San Joes, CA, USA) was used to optimize the images for adipocyte measurement. The following commands were used for the conversion: “Bilevel Thresholding”, “Erode”, “Dilate” and “Watershed Segmentation”. The binary black and white images were compared with the original images to ensure an accurate conversion and minor adjustments were made using following commands: “Fill Holes” and “Paintbrush”. The total number and cell diameter of adipocytes were calculated with the command “Measure All” after measure scale calibration. Results were directly loaded into Excel (Microsoft Inc., Redmond, WA, USA) for analysis.
2.7 Arachidonic acid quantification by gas chromatography
AA levels were determined in retroperitoneal AT. A one-step lipid extraction and methylation procedure was conducted according to Garces and Mancha [29]. Briefly, AT was heated at 80°C for 2 h in a reagent containing methanol, heptane, toluene, 2,2-dimethoxypropane, and H2SO4. Fatty acid methyl esters (FAME) were extracted in heptane and quantified using a GC (Agilent 6890A, Atlanta, GA, USA) equipped with a silica-fused capillary column (SP-2560, Sigma-Aldrich) and a flame ionization detector. Fatty acid peaks are identified using standard mixtures (GLC 68D, 461, and 780; NU-CHEK Prep Inc.) and tissue fatty acid concentration determined using internal standards (C13:0 and C19:0). Data were corrected for recovery and methyl ester mass.
2.8 Western blot
Frozen retroperitoneal AT was homogenized in tissue protein extraction buffer containing a cocktail of protease inhibitors and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). The nuclear fraction of AT was prepared using the Nuclear Extract Kit (Active Motif, Rixensart, Belgium). After centrifugation, the protein concentration in supernatant is determined by Bradford assay (Sigma-Aldrich, St. Louis, MO, USA). Whole cell lysates and nuclear lysates were then combined with an equal volume of laemmli sample buffer (Bio-Rad, Hercules, CA, USA), denatured at 90 °C for 5 min. Proteins (60 μg) were separated by polyacrylamide (15 %) gel electrophoresis, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and probed with primary antibodies (Cell Signaling Technology, Danvers, MA, USA) for AdPLA (1:200 dilution, Cayman Chemical, Ann Arbor, MI, USA), COX-2 (1:200 dilution, Cayman Chemical), 5-LOX (1:1000 dilution, Cayman Chemical, Ann Arbor, MI, USA), NF-κB p65 (1:100 dilution, Biotechnology, Santa Cruz, CA, USA), GAPDH (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA) and Histone H3 (1:500 dilution, Cell Signaling Technology, Danvers, MA, USA) overnight at 4 °C. After incubation with a fluorescently-labeled secondary antibody (LI-COR Biosciences, Lincoln, NE, USA) proteins were imaged with an Odyssey imaging system (LI-COR, Lincoln, NE, USA). Protein loading was normalized to GAPDH or Histone H3. Band density was quantified using Odyssey Application Software version 3.0.
2.9 Metabolic endotoxemia and gut barrier function
Plasma LPS determinations were performed using a Limulus amaebocyte lysate kit (LAL kit, Lonza, Walkersville, MD, USA). Samples were diluted 1:5 to 1:10 with endotoxin-free LAL reagent water and heated at 70°C for 10 min before analysis. Plasma GLP-2 levels were determined by ELISA (MyBioSource, Inc. San Diego, CA, USA) following the manufacturer’s instructions.
2.10 Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Two-way ANOVA with Bonferroni’s post-test was used for body weight, food intake and blood glucose comparisons over the course of the study. One-way ANOVA with Dunnett’s post-test was used for all other data comparisons. Pearson’s correlation coefficient (r) was used as a measure of the strength of the association between two variables. Analyses were performed using GraphPad Prism 5.0 (San Diego, CA, USA). A P < 0.05 was considered statistically significant.
3 Results
3.1 Physiological parameters
A significant increase in body weight of mice fed the HF diet was observed at the end of experiment compared to that of the LF-treated mice (P < 0.001, Table 1). Dietary cocoa supplementation (HFC group) for 18 weeks did not significantly change the final body weight compared to HF-fed control. Food and energy intake was also unaffected by cocoa treatment (Table 1). Spleen and kidney weight were decreased in HFC mice compared to HF-fed mice (P < 0.05, Table 1). Although no significant differences were found between mean fasting blood glucose (data not shown) and final fasting blood glucose (Table 2) in cocoa-supplemented mice and HF-fed controls, fasting plasma insulin levels determined at the completion of the experiment were decreased by 14.8% in HFC group (P < 0.05). In addition, HF-fed obese mice had increased HOMA-IR scores (P < 0.001) compared to LF-fed lean mice (Table 2), and this increase was attenuated in cocoa-supplemented mice (P < 0.05). Cocoa-supplemented mice also displayed lower fasting plasma triglycerides (P < 0.05) and fasting plasma free fatty acids (P < 0.001) than HF group (Table 2).
Table 1.
Physiological parameters of mice fed with LF, HF and HFC diet1
| LF | HF | HFC | |
|---|---|---|---|
| Final body weight (g) | 34.8±0.7*** | 49.5±1.0 | 47.1±0.4 |
| Food Intake (g/week/mouse) | 20.3±0.3 | 21.4±0.5 | 20.6±0.3 |
| Energy Intake (kcal/week/mouse) | 77.2±1.0*** | 111.1±2.3 | 104.9±1.7 |
| Heart (g) | 0.14±0.004** | 0.17±0.005 | 0.16±0.004 |
| Liver (g) | 1.20±0.01*** | 2.14±0.01 | 2.08±0.01 |
| Spleen (g) | 0.07±0.002*** | 0.10±0.003 | 0.09±0.003* |
| Kidney (g) | 0.31±0.01*** | 0.37±0.01 | 0.34±0.01* |
Values are expressed as mean ± SEM with LF, n=23; HF, n=21; and HFC, n=24. Data were compared by one-way ANOVA with Dunnett’s post-test (HF as control).
P<0.05,
P<0.01,
P<0.001.
Table 2.
Markers of insulin resistance and dyslipidemia in mice fed with LF, HF and HFC diet1
| LF | HF | HFC | |
|---|---|---|---|
| Final fasted blood glucose (mmol/L) | 7.31±0.27*** | 9.86±0.28 | 9.78±0.21 |
| Fasted plasma triglycerides (mmol/L) | 0.33±0.01*** | 0.45±0.02 | 0.36±0.02** |
| Fasted plasma free fatty acids (mmol/L) | 0.98±0.05 | 0.89±0.04 | 0.67±0.02*** |
| Fasted plasma insulin (pmol/L) | 165.5±15.5*** | 1176.4±64.6 | 1001.9±55.06* |
| HOMA-IR | 7.33±0.84*** | 71.51±4.18 | 60.64±3.26* |
Values are expressed as mean ± SEM with LF, n=23; HF, n=21; and HFC, n=24. Data were compared by one-way ANOVA with Dunnett’s post-test (HF as control).
P<0.05,
P<0.01,
P<0.001.
3.2 Inflammatory gene expression in ATMs
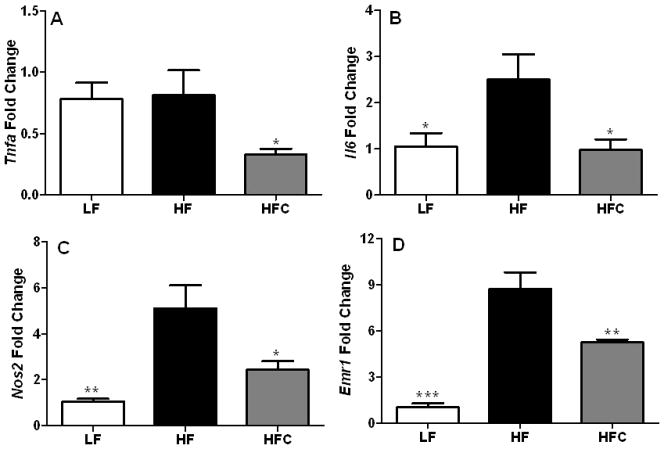
The mRNA levels of Tnfa, Il6, Nos2, and Emr1 in the epididymal AT SVF were determined. Expression of Il6, Nos2, and Emr1 were significantly elevated, whereas Tfna levels were unaffected (P < 0.05) in HF-fed obese mice compared to the LF-fed lean mice (Fig. 1). Supplementation with dietary cocoa remarkably reduced the expression of the four genes by 40 – 61% (P < 0.05) compared to HF-fed mice (Fig. 1).
Figure 1.
The effect of cocoa supplementation on the expression of inflammatory gene expression in the SVF of C57BL/6J mice. (A) Tnfa, (B) Il6, (C) Nos2 and (D) Emr1 expression was determined in adipose tissue macrophages (ATMs) in the SVF of epididymal AT. Expression of pro-inflammatory genes was determined at the end of the experiment using RNA isolated from the epididymal SVF from a set of representative mice from each group. LF, n=10; HF, n=18; and HFC, n=18. Values are expressed as mean ± SEM. Means were compared by one-way ANOVA with Dunnett’s post-test (HF as control). * P<0.05, ** P<0.01, *** P<0.001.
3.3 Adiposity and adipocyte cell size distribution
Visceral adiposity of HF-fed obese mice was significantly elevated compared to LF-fed mice (P < 0.05, Fig. 2A). Cocoa supplementation reduced the mass of retroperitoneal AT, but did not affect the other depots or total visceral adiposity. In spite of the lack of effect on total visceral AT mass, cocoa treatment did influence the adipocyte cell size distribution. Cocoa-treated mice had a significantly larger number of smaller adipocytes (0 – 20 μm, P <0.0001) and a smaller number of larger cells (40 – 60 μm, P <0.01) in epididymal depot (Fig. 2B).
Figure 2.

The effect of cocoa supplementation on (A) visceral adiposity and (B) adipocyte cell size distribution in C57BL/6J mice. Visceral fat depots were weighed at the end of experiment with LF, n=23; HF, n=21; and HFC, n=24. Adipocyte cell size distribution was analyzed from a set of representative histological section samples of epididymal AT with n=8 for each group. (C) Typical photomicrographs are shown with white bars representing 40 μm scale. Values are expressed as mean ± SEM. Means were compared by one-way ANOVA with Dunnett’s post-test (HF as control). * P<0.05, ** P<0.01, *** P<0.001.
3.4 AA levels in AT and its correlation with adiposity
Eicosanoids represent a group of inflammatory lipid mediators derived from AA. Cocoa-supplemented mice had 33% reduction in AA content of AT compared to HF group (P <0.001, Fig. 3A). Moreover, the AA content in AT was positively correlated with mass of the fat depot (Pearson r = 0.57, P = 0.02, Fig. 3B).
Figure 3.
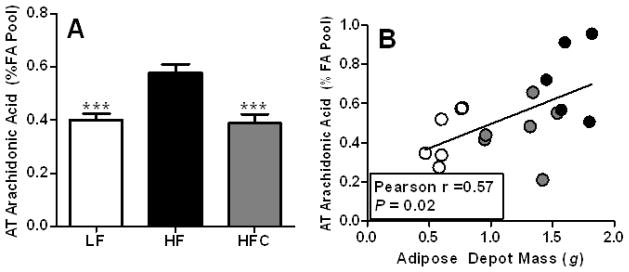
The effect of cocoa supplementation on (A) arachidonic acid levels in AT and (B) its correlation with adiposity in C57BL/6J mice. Arachidonic acid levels were determined in duplicate from a set of representative retroperitoneal AT samples with LF, n=6; HF, n=5; and HFC, n=6. Values are expressed as mean ± SEM. Means were compared by one-way ANOVA with Dunnett’s post-test (HF as control). *** P<0.001. The correlation between arachidonic acid levels and the adiposity was assessed by GraphPad Prism 5.0 (San Diego, CA).
3.5 Expression of eicosanoid-generating enzymes and NF-κB in AT
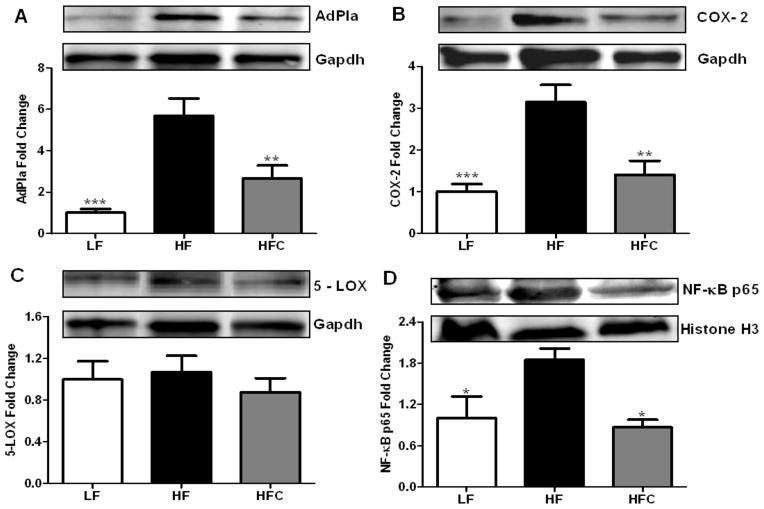
Western blot analysis (Fig. 4A–C) showed that the protein expression of AdPla and COX-2 was reduced by nearly 50% in cocoa treated mice compared to HF fed controls (P <0.01). By contrast, there is no significant effect of cocoa on the expression of 5-LOX among the three groups. Furthermore, the level of NF-κB (p65 subunit) in the nucleus was reduced in cocoa-treated mice compared to HF fed controls (Fig. 4D).
Figure 4.
The effect of cocoa supplementation on the protein expression of (A) AdPLA, (B) COX-2, (C) 5-LOX and (D) nucleus NF-κB p65 in adipose tissue of C57BL/6J mice. Protein expression of eicosanoid-generating enzymes (AdPLA, COX-2 and 5-LOX) was determined in whole cell lysate from a set of representative mouse retroperitoneal AT samples with n=6 for each group. Protein expression of NF-κB p65 was measured in nuclear fraction from a set of representative mouse retroperitoneal AT samples with n=6 for each group. Values are expressed as mean ± SEM. Means were compared by one-way ANOVA with Dunnett’s post-test (HF as control). * P<0.05, ** P<0.01, *** P<0.001.
3.6 Endotoxemia and gut barrier function
HF diet induced a 1.8-fold increase in plasma endotoxin levels (P < 0.001) compared to LF-fed controls (Fig. 5A). Cocoa supplementation ameliorated this elevation resulting in 40.8% lower (P < 0.001) plasma endotoxin levels than HF-fed mice. GLP-2 is a gastrointestinal hormone that has a number of actions in the intestine including stimulation of mucosal growth, improvement of gut barrier function. Compared to LF-fed mice, HF-fed mice had lower levels of plasma GLP-2 (P < 0.01), whereas cocoa treatment prevented this decrease (Fig. 5B). Correlation analysis showed that plasma GLP-2 levels were inversely related to plasma endotoxin levels (Pearson r = −0.52, P = 0.001, Fig. 5C).
Figure 5.
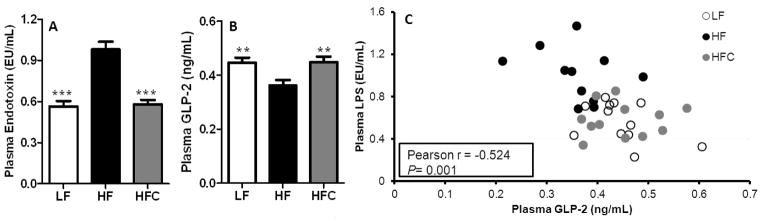
Effect of cocoa supplementation on the plasma endotoxin and GLP-2 levels in C57bl6/J mice. (A) Plasma endotoxin or (B) GLP-2 levels were determined at the end of experiment. Values are expressed as mean ± SEM of N = 23, 21, and 24 for LF, HF, and HFC, respectively. Means were compared by one-way ANOVA with Dunnett’s post-test (HF as control). *** P<0.001. (C) The correlation between plasma endotoxin and GLP-2 levels was determined by examining Pearson’s correlation coefficient. Correlation analysis was performed using GraphPad Prism 5.0 (San Diego, CA).
4. Discussion
AT has an important endocrine function in the regulation of whole-body metabolism. Obesity leads to a chronic inflammation of the AT, which in turn disrupts this endocrine function and results in metabolic derangements, such as type-2 diabetes and cardiovascular diseases [30]. Bioactive food components, such as polyphenols, have been shown to suppress both systemic and AT inflammation and have the potential to attenuate these obesity-associated metabolic disorders [30–32]. In the present study, we provide the first scientific evidence that long-term supplementation with dietary cocoa alleviates AT inflammation in HF-fed C57BL/6J mice. Based on allometric scaling, the dose of cocoa give to the mice is equivalent to approximately 54 g of unsweetened cocoa powder [26].
In this study, although the final body weight of HF-fed mice was unchanged, markers of hyperinsulinemia and hyperlipidemia were significantly improved by cocoa treatment for 18 wks. Dietary supplementation with 8% (w/w) cocoa powder contributes approximately about 50 mg polyphenols/kg body weight. Previous work in this area has provide mixed results. For example, Min et al. found that C57BL/6 mice fed a HF diet with cocoa polyphenol extract (200 mg/kg body weight) for 5 wks exhibited significantly reduced body weight and epididymal fat mass, as well as decreased plasma triglycerides levels, whereas blood glucose was unaffected [33]. In another study, Tomaru et al. found that diabetic obese mice fed a standard diet with 1% cocoa liquor PAC extract for 3 weeks demonstrated lower blood glucose levels, but no significant changes in body weight were observed [34].
Chronic inflammation in obese AT is characterized by increased macrophage infiltration and abnormal cytokine production. We found that mRNA expression of pro-inflammatory cytokines (Tnfα and Il6), Nos2 and macrophage surface marker (Emr1) was elevated in HF-fed obese mice in ATMs derived from epididymal fat. However, cocoa supplementation reduced the expression of these genes by 40 – 61% compared to HF controls. Interestingly, the decreased expression of Emr1 (also known as F4/80 in mouse) indicates that cocoa treatment decreased macrophage infiltration into AT. Studies have also shown that Emr1 was significantly and positively correlated with both adipocyte size and body mass [6].
Fat mass expansion in obesity occurs via adipocyte hyperplasia (increased adipocyte number) and/or adipocyte hypertrophy (increased size of adipocytes) [30]. The latter is often associated with AT dysfunction and inflammation [35]. Although there was no change in the mass of the epididymal fat depot induced by cocoa treatment, the adipocyte cell size distribution was markedly changed with an increase in the number of smaller cells and a decrease in the number of larger cells. Skurk et al. have reported that adipocyte size is an important determinant of the production of cytokines including IL-6, IL-8 and MCP-1 in AT [36].
Eicosanoids are lipid products derived from the metabolism of AA, which play a significant role as mediators of the inflammatory cascade and have important activities for maintaining homeostasis [37]. AA is a ω-6-PUFA and is the primary source of fatty acids that mediate inflammatory responses [9]. Elevated tissue levels of AA have been associated with a number of disease states, including coronary heart disease, breast cancer, obesity and diabetes [38]. A recent study has associated increased AA levels in retroperitoneal AT with a high prevalence of the metabolic syndrome in human subjects [38]. We found that cocoa-supplemented mice had 33% lower levels of AA compared to HF group, furthermore.
In AT, the release of AA can be catalyzed by AdPLA or by other types of PLA2 released from macrophages[9]. AdPLA is highly and exclusively expressed in AT and is associated with adipocyte differentiation and lipolysis [39]. Ablation of AdPLA in mice caused a reduction in triglyceride levels and insulin resistance in response to a HF diet [9,39,40]. In the present study, the protein expression of AdPLA in AT was significantly suppressed by cocoa treatment. Together with reduced AA levels in AT, these results suggest a decreased availability of substrates for the synthesis of eicosanoids. The COX pathway metabolizes AA to form prostanoids, including the prostaglandins (PGs). Ghoshal et al. have reported that COX-2 deficiency attenuated AT differentiation and macrophage-dependent inflammation in mice [10]. The LOX pathway metabolizes AA into many bioactive eicosanoids including leukotrienes (LTs). Recent studies have demonstrated a role of LOX pathway in signaling the AT dysfunction and inflammation [11,12,41]. Here, we found that cocoa supplementation significantly reduced COX-2 protein expression in AT compared HF group, while the expression of 5-LOX was unaffected. To our knowledge, this is the first report of modulatory effects of cocoa on eicosanoid metabolizing enzymes in AT in vivo: previous data was limited to in vitro studies (see [42] for example).
NF-κB is a ubiquitous and well-characterized transcription factor controlling the expression of genes encoding the pro-inflammatory cytokines, chemokines, adhesion molecules, inducible enzymes (NOS2 and COX-2) etc. [43]. In the present study, the protein levels of NF-κB p65 subunit in nucleus of adipocytes were compared, and cocoa supplemented exhibited significant lower levels of nuclear p65 levels than that of HF-fed controls. Together with decreased pro-inflammatory gene expression by cocoa treatment, these results suggest that dietary cocoa may prevent the nuclear translocation and activation of NF-κB pathway which results in down-regulation of inflammatory gene expression. Although, the decrease in nuclear p65 may indicate a decrease in NFκB transcriptional activity, further studies are needed to directly determine if transcriptional activity is reduced. Terra et al. [44] have reported that the administration of a grape-seed PAC extract (30 mg/kg per day) for 14 weeks in HF fed rats significantly decreased Tnfα, Il6 and Emr1 expression in mesenteric AT, and a reduced NF-κB activity in liver is also observed which can be related to low expression rates of hepatic inflammatory markers. Although in vitro studies have shown that cocoa extracts and cocoa polyphenols can inhibit the translocation and activation of NF-κB in several cell models, our study represents the first in vivo demonstration of such activity by cocoa in an obese animal model [45–48].
A growing number of studies suggests that a HF diet can induce “metabolic endotoxemia” through alteration of gut microbiota and gut barrier functions, which promotes leakage of endotoxin from the gut lumen into the circulation, resulting in inflammatory changes in tissues including AT [14–16]. Because cocoa contains dietary fiber and higher molecular weight polyphenols with low bioavailability, modulation of a target(s) in the gastrointestinal system which exerts an indirect systemic effects represents an attractive explanation for the anti-inflammatory activity of cocoa in HF-fed mice [49,50]. In our study, the HF diet significantly elevated plasma endotoxin (LPS) levels compared to LF-fed controls. By contrast, cocoa supplementation significantly reduced this elevation with levels similar to that of LF group.
GLP-2 is a hormone which can increase intestinal crypt cell proliferation and mucosal thickness resulting in improved gut barrier function [51]. Previously Cani et al. reported that administration of exogenous GLP-2 to ob/ob mice decreased plasma LPS by approximately 50%; plasma inflammatory cytokines were also reduced [52]. Our results demonstrate that cocoa treatment significantly increased plasma GLP-2 levels compared to HF-fed mice. As expected, we observed a strong negative correlation between plasma GLP-2 and endotoxin levels.
It is noteworthy that the decrease in endotoxemia is also associated with reduced plasma triglycerides in cocoa-treated mice. It has been hypothesized that endotoxin is transported from the intestine to target tissues by incorporation into triglyceride-rich lipoproteins, notably chylomicrons synthesized from epithelial intestinal cells in response to a HF diet [14]. Previously we reported that cocoa PACs inhibit the activity of PL in vitro and increased fecal lipid content [26,27]. It is possible that cocoa can also reduce metabolic endotoxemia in part by this alternative mechanism.
One limitation of the present study is that different depots of AT were used to measure adipocyte hypertrophy and gene expression studies (epididymal) and arachidonic acid and protein analysis (retroperitoneal). This was due to the fact that the epididymal depot was exhausted in preparing the SVF faction and histological sections. It is possible that differences in inflammatory response exist between different AT depots. Such questions will need to be investigated in future studies.
In conclusion, the present study has shown for the first time that long-term cocoa supplementation can reduce AT inflammation in HF-fed mice by down-regulating NF-κB target gene expression and modulating eicosanoid metabolism. Although further studies are needed, our results suggest that dietary cocoa powder can exert anti-inflammatory activity in part by modulating gut barrier function and metabolic endotoxemia. These results provide a putative mechanism of action which is congruent with the reported low bioavailability of cocoa polyphenols and high fiber content in cocoa powder.
Acknowledgments
Funding Source: Funded by NIH grant AT004678 and the Silvia and Edith Crespo Faculty Award in Chocolate Research (to JDL)
Abbreviations
- AA
arachidonic acid
- AdPLA
adipose-specific phospholipase A2
- AT
adipose tissue
- ATMs
adipose tissue macrophages
- COX-2
cycloxygenase-2
- DP
degree of polymerization
- FAME
fatty acid methyl ester
- GC
Gas Chromatography
- GLP-2
glucagon-like peptide-2
- HF
high-fat
- HFC
high-fat diet with 8% (w/w) unsweetened cocoa powder
- Nos2
inducible nitric oxide synthase
- LF
low-fat diet
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- LT
leukotriene
- MCP-1
monocyte chemotactic protein-1
- NF-κB
nuclear factor-κB
- PAC
proanthocyanidin
- PG
prostaglandin
- PUFA
polyunsaturated fatty acid
- SVF
stromal vascular fraction
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chuang C-C, McIntosh MK. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr. 2011;31:155–76. doi: 10.1146/annurev-nutr-072610-145149. [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol Res. 2011;64:438–455. doi: 10.1016/j.phrs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 4.González-Périz A, Clària J. Resolution of adipose tissue inflammation. Scientific World Journal. 2010;10:832–56. doi: 10.1100/tsw.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears B, Ricordi C. Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obes. 2011 doi: 10.1155/2011/431985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- 10.Ghoshal S, Trivedi DB, Graf Ga, Loftin CD. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J Biol Chem. 2011;286:889–98. doi: 10.1074/jbc.M110.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mothe-Satney I, Filloux C, Amghar H, Pons C, Bourlier V, Galitzky J, et al. Adipocytes Secrete Leukotrienes: Contribution to Obesity-Associated Inflammation and Insulin Resistance in Mice. Diabetes. 2012;61:2311–9. doi: 10.2337/db11-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, et al. 12/15-Lipoxygenase Is Required for the Early Onset of High Fat Diet-Induced Adipose Tissue Inflammation and Insulin Resistance in Mice. PloS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 15.Chang S, Li L. Metabolic Endotoxemia: A Novel Concept in Chronic Disease Pathology. J Med Sci. 2011;31:191–209. [Google Scholar]
- 16.Moreira APB, Texeira TFS, Ferreira AB, Peluzio MDCG, Alfenas RDCG. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–9. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–58. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 18.Hammerstone JF, Lazarus Sa, Mitchell aE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 1999;47:490–6. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- 19.Katz DL, Doughty K, Alli A. Cocoa and chococlate in human health and disease. Antioxid Redox Signal. 2011;15:2779–811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti R, Flammer AJ, Hollenberg NK, Lüscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–41. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 21.Monahan KD. Effect of cocoa/chocolate ingestion on brachial artery ow-mediated dilation and its relevance to cardiovascular health and disease in humans. Arch Biochem Biophys. 2012;527:90–4. doi: 10.1016/j.abb.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879–86. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 23.Engler MB, Engler MM. The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr Rev. 2006;64:109–18. doi: 10.1111/j.1753-4887.2006.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Lambert JD. Modulation of metabolic syndrome-related inflammation by cocoa. Mol Nutri Food Res. 2013:1–14. doi: 10.1002/mnfr.201200837. [DOI] [PubMed] [Google Scholar]
- 25.Selmi C, Mao TK, Keen CL, Schmitz HH, Gershwin ME. The Anti-inflammatory Properties of Cocoa Flavanols. J Cardiovasc Pharmacol. 2006;47:163–71. doi: 10.1097/00005344-200606001-00010. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Yu S, Lambert JD. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur J Nutr. 2013 doi: 10.1007/s00394-013-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Y, Hurst WJ, Stuart Da, Lambert JD. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem. 2011;59:5305–11. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mlinar B, Marc J, Janez AMP. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Garcés R, Mancha M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem. 1993;211:139–43. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- 30.Siriwardhana N, Kalupahana NS, Cekanova M, Lemieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24:613–23. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 31.González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104S:15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 32.Cherniack EP. Polyphenols: planting the seeds of treatment for the metabolic syndrome. Nutrition. 2011;27:617–23. doi: 10.1016/j.nut.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Min SY, Yang H, Seo SG, Shin SH, Chung M-Y, Kim J, et al. Cocoa polyphenols suppress adipogenesis in vitro and obesity in vivo by targeting insulin receptor. Int J Obes. 2013;37:584–92. doi: 10.1038/ijo.2012.85. [DOI] [PubMed] [Google Scholar]
- 34.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, et al. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–5. doi: 10.1016/j.nut.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Arner E, Westermark PO, Spalding KL, Britton T, Ryde M, Frise J, et al. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes. 2010;59:105–9. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 37.Imig JD. Eicosanoids and renal damage in cardiometablic syndrome. Expert Opin Drug Metab Toxicol. 2008;4:165–74. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams ES, Baylin A, Campos H. Adipose Tissue Arachidonic Acid and the Metabolic Syndrome in Costa Rican Adults. Clin Nutr. 2007;26:474–82. doi: 10.1016/j.clnu.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady Ka, Hellerstein MK, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–68. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf G. Adipose-specific phospholipase as regulator of adiposity. Nutr Rev. 2009;67:551–4. doi: 10.1111/j.1753-4887.2009.00227.x. [DOI] [PubMed] [Google Scholar]
- 41.Horrillo R, González-Périz A, Martínez-Clemente M, López-Parra M, Ferré N, Titos E, et al. 5-Lipoxygenase Activating Protein Signals Adipose Tissue Inflammation and Lipid Dysfunction in Experimental Obesity. J Immunol. 2010;184:3978–87. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W-Y, Liu H-Q, Xie K-Q, Yin L-L, Li Y, Kwik-Uribe CL, et al. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] suppresses the expression of cyclooxygenase-2 in endotoxin-treated monocytic cells. Biochem Biophys Res Commun. 2006;345:508–15. doi: 10.1016/j.bbrc.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 43.Calixto J, Otuki M, Santos A. Anti-inflammatory compounds of plant origin. Part I. Action on arachidonic acid pathway, nitric oxide and nuclear factor kappa B (NF-kappaB) Planta Med. 2003;69:973–83. doi: 10.1055/s-2003-45141. [DOI] [PubMed] [Google Scholar]
- 44.Terra X, Pallarés V, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, et al. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem. 2011;22:380–7. doi: 10.1016/j.jnutbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Zeng H, Locatelli M, Bardelli C, Amoruso A, Coisson JD, Travaglia F, et al. Anti-inflammatory Properties of Clovamide and Theobroma cacao Phenolic Extracts in Human Monocytes: Evaluation of Respiratory Burst, Cytokine Release, NF-KB Activation, and PPARγ Modulation. J Agric Food Chem. 2011;59:5342–50. doi: 10.1021/jf2005386. [DOI] [PubMed] [Google Scholar]
- 46.Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18:167–9. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 47.Recio MC, Giner RM, Cienfuegos-jovellanos E, Laghi S, Ríos L. Inhibition of Ulcerative Colitis in Mice after Oral Administration of a Polyphenol-Enriched Cocoa Extract Is Mediated by the Inhibition of STAT1 and STAT3 Phosphorylation in Colon Cells. J Agric Food Chem. 2011;59:6474–83. doi: 10.1021/jf2008925. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-prieto MA, Bettaieb A, Haj FG, Fraga CG, Oteiza PI. (−)-Epicatechin prevents TNFa-induced activation of signaling cascades involved in inflammation and insulin sensitivity in 3T3-L1 adipocytes. Arch Biochem Biophys. 2012;527:113–8. doi: 10.1016/j.abb.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Déprez S, Brezillon C, Rabot S, Philippe C, Mila I, Lapierre C, et al. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J Nutr. 2000;130:2733–8. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 50.Massot-Cladera M, Pérez-Berezo T, Franch A, Castell M, Pérez-Cano FJ. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch Biochem Biophys. 2012;527:105–12. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Marathe CS, Rayner CK, Jones KL, Horowitz M. Glucagon-like peptides 1 and 2 in health and disease: A review. Peptides. 2013;44:75–86. doi: 10.1016/j.peptides.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard a, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzounis X, Rodriguez-mateos A, Vulevic J, Gibson GR, Kwik-uribe C. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]