Abstract
MLL-rearranged leukemias exemplify malignancies with perturbations of the epigenetic landscape. Specific chromatin modifications that aid in the perpetuation of MLL-fusion gene driven oncogenic programs are being defined, presenting novel avenues for therapeutic intervention. Proof-of-concept studies have recently been reported, using small-molecule inhibitors targeting the histone methyltransferase DOT1L or the acetyl-histone binding protein BRD4 showing potent activity against MLL-rearranged leukemias in pre-clinical models. It is apparent that intensive efforts will be made toward the further development of small-molecule inhibitors targeting these, and other chromatin-associated protein targets. These studies may lead to the advent of a new generation of much-needed therapeutic modalities in leukemia and other cancers.
Keywords: chromatin modifications, leukemia, epigenetics, targeted therapy, MLL
Aberrant chromatin modifications in hematologic malignancies
The last two decades have brought profound advances in our molecular understanding of leukemogenesis, and numerous opportunities have arisen to design and develop therapeutic agents that selectively target leukemic cells. Despite this progress, most patients with leukemia continue to be treated with broadly cytotoxic therapeutic regimens such as intensive combination chemotherapy with or without hematopoietic stem cell transplantation. Such regimens have a number of potentially debilitating side effects. Moreover, a large number of patients with leukemia eventually succumb to the disease necessitating the development of less toxic, more efficacious therapies.
There is growing recognition that mutations that lead to deregulated chromatin structure are important in leukemia. Chromatin is controlled in a dynamic fashion by the recruitment of chromatin-associated proteins that fall into three general categories: 1) enzymes that add chemical moieties to histone or DNA substrates such as histone acetyl- or methyl- transferases or DNA methyltransferases (so-called epigenetic “writers”); 2) enzymes that actively remove these covalent modifications such as histone deacetylases (HDACs) and demethylases (termed epigenetic “erasers”); and 3) proteins containing chromatin-binding modules that bind to specific modifications and positively or negatively influence transcription (termed epigenetic “readers”) (1, 2). The concerted action of these broadly classified proteins effectuates a sophisticated pattern of chromatin modifications frequently referred to as the “histone code”. Structural perturbation of the “histone code” can have catastrophic consequences on coordinated transcriptional pathways, one potential outcome of which is cellular transformation. In leukemia, a number of chromatin “writer”, “eraser” and “reader” proteins are found disrupted through diverse genetic alterations strongly pointing to the role of abnormal chromatin-dependent growth pathways in this disease (see Box 1).
Box1: Somatic mutations of chromatin regulators in hematological malignancies.
A large number of chromatin regulating proteins have been found to be mutated in cancer, many of them in hematological malignancies. There are a number of examples of chromatin “writers” found to be mutated in leukemia including several histone methyl- and acetyl-transferases. A classic example is the large trithorax group (trxG) histone methyltransferase MLL, which is involved in recurring chromosomal translocations in leukemia and is the focus of this review. Other notable examples of histone methyltransferases mutated in leukemia include the polycomb group histone 3 lysine 27 (H3K27) methyltransferase EZH2 (82) and proteins of the nuclear receptor-binding SET domain (NSD) family of methyltransferases (NSD1 and NSD3) (83, 84). Histone acetyl-transferases such as CBP/p300, MOZ and MORF are also involved in leukemia-specific translocation events, (85, 86), with some of these enzymes also found to be fused to MLL in rare but recurrent cases of leukemia. In addition, there is evidence of genetic lesions affecting the structure of chromatin “eraser” proteins such as the H3K27me2/3 demethylase UTX (87–89) (90), the ARID domain containing protein JARID1A (91) and the jumonji domain containing protein JHDM1b (92). Lastly, recent studies have indicated that chromatin “reader” modules may also be co-opted for leukemogenesis: e.g. -gain (9) or loss (68) of function of chromatin “reading” PHD domains in leukemia or the involvement of chromatin recognizing bromodomains in leukemogenesis(79, 93).
The FDA-approval of DNA methyltransferase inhibitors (azacytidine and decitabine) for the treatment of myelodysplastic syndrome (3, 4), and the more recent approval of HDAC inhibitors (vorinostat and romidepsin) for use in refractory, cutaneous T cell lymphoma (5) have validated epigenetic enzymes as attractive targets for pharmaceutical drug development. A number of inhibitors of histone acetyltransferases (HATs), histone methyltransfereases (HMTs) and histone demethylases (HDMs) are in various stages of preclinical development (as reviewed in (6)), but the function of these enzymes in normal and disease processes have only recently begun to be explored. Some progress has been made in leukemias bearing MLL-rearrangements and already, pharmacological inhibition of specific chromatin processes has been reported to show efficacy and specificity against MLL-rearranged leukemia cells in model systems (7–10). The focus of the review is to highlight the role of chromatin dysregulation in leukemia and to discuss recent advances towards the development of therapeutic agents targeting these epigenetic aberrations.
MLL-rearranged leukemias as archetypal malignancies with dysregulated chromatin
Genomic rearrangements of the human chromosomal band 11q23 involving MLL are frequent events in pediatric leukemia, appearing in more than 70% of infant ALL and approximately 10% of AML cases (11). Translocations involving MLL are also recurrently observed in adult and therapy-related leukemias, and as with infant leukemias, such rearrangements are associated with a poor prognosis when compared to MLL-germline leukemias (12). In contrast to the high overall success rate in treating childhood ALL where 5-year survival rates have reached ~ 80–90 %, the genetically-defined subset of MLL-rearranged ALL continues to predict poor survival rates of around 50 %. At the molecular level, MLL-rearranged leukemias display characteristic gene expression profiles that distinguish them from other leukemia subtypes. A characteristic hallmark is high expression of the posterior homeobox-A (HOXA) gene cluster (13, 14).
There are a number of reasons why the MLL-rearranged leukemias lend themselves very well to the study of aberrant epigenetic pathways in leukemogenesis. First, the MLL protein itself, whose gene rearrangements define this unique sub-set of prognostically poor leukemias, is a histone 3 lysine 4 (H3K4) methyltransferase (15) (16). MLL translocations disrupt the H3K4 methyltransferase activity of MLL by truncating the protein prior to the H3K4 catalyzing SET domain. Moreover, MLL directly or indirectly binds to a number of chromatin modifiers including other (TrxG) proteins (17), members of the polycomb group (PcG) of proteins (18), histone acetyl transferases (HATs) (19) (20), histone deacetylases (HDACs) and members of the SWI/SNF complex (15, 18). Also, there is increasing evidence to show that a number of chromatin modifiers are either associated with MLL fusion partners or, in other rarer cases of leukemia, directly participate in gene rearrangements with the MLL gene (21) (22).
More than 60 different partner genes across disparate chromosomal regions have been identified within MLL fusions. The partners of MLL are nuclear, cytoplasmic or membrane associated proteins involved in diverse functional processes ranging from transcriptional elongation to cellular adhesion, endocytosis, cytoskeletal organization, chromatin modifications and signal transduction (as reviewed in (21)). Significant effort has been directed toward defining a unifying mechanism of oncogenesis for MLL fusion proteins since it would facilitate pharmacologic targeting of these shared leukemogenic mechanisms. Some broad patterns have emerged, and the most commonly occurring MLL fusions can be classified into two main subtypes based on the mechanisms of transformation. The most common MLL fusion partners can be categorized as either 1) nuclear proteins that form large biochemical complexes including components of the transcriptional activation/elongation machinery or 2) predominantly cytoplasmic proteins that contribute dimerizing motifs such as coiled-coil domains to truncated MLL, thereby facilitating oncogenic MLL-homodimerization. Additional subtypes relating to the less frequent MLL fusion partners have been discussed in previous reviews (23–25). In the first category, nuclear proteins such as AF4, AF9, ENL, ELL, AF10, AF17 and AF5q31- fusions of which together account for the vast majority of MLL leukemias are all found to directly or indirectly recruit components of the transcriptional machinery (See Table 1) (26–32). A number of complexes linked to transcriptional regulation have been reported, often with overlapping protein components, such as the ENL associated protein (EAP) complex (28), the AF4/ENL/P-TEFb (AEP) complex (31), the super elongation complex (SEC) (30) and the complex comprising the histone 3 lysine 79 (H3K79) methyltransferase DOT1L (DotCom) (29) (33) (See Table 1, Fig. 1 and Box. 2).
Table 1.
The table depicts some of the biochemical complexes associated with MLL fusion partner proteins that have been described in literature. Some of the striking features that are evident from these biochemical studies are :1) a number of the protein components of these complexes are found as MLL-fusion partners in human leukemia 2) all of these complexes contain sub-units of the transcriptional elongation machinery.
| Biochemical complexes associated with MLL fusion proteins | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Components | Other | Complex name | Reference | ||||||
| ENL | AF4 | P-TEFb | DOT1L | AF9 | AF10 | 25 | |||
| ENL | AF4 | P-TEFb | DOT1L | AF5q31 | BCoR, HSP70, HSP70L, RING1, LAF4, BCX8, p52RO, TUB6, PIP5K2C | EAP | 27 | ||
| ENL | AF4 | P-TEFb | DOT1L | EAP Core | 28 | ||||
| ENL | AF4 | P-TEFb | AF5q31 | AEP | 31 | ||||
| ENL | AF4 | P-TEFb | AF9 | AF5q31 | ELL1, ELL2, ELL3 | SEC | 30 | ||
| ENL | DOT1L | AF9 | AF10 | AF17, TRRAP, SKP1, B-Catenin | DotCom | 29 | |||
Abbreviations: AEP: AF4/ENL/P-TEFb complex, SEC: Secondary elongation complex, EAP: elongation associated proteins, DotCom: Dot1l associated protein complex.
Fig.1. Complexes involved in transcriptional activation in the MLL-rearranged leukemias.
Of the several different complexes that are thought to be important for MLL-leukemogenesis, the Dot1l complex (a), the SEC complex (b) and the Brd4 complex (c) are depicted. Overlapping components are highlighted by enclosing with dotted rectangles. The Dot1l complex (DotCom) consists of the histone methyltransferase Dot1l and the MLL fusion partners AF10, AF9, ENL and AF17, along with components of the Wnt signaling pathway. The AEP/EAP and SEC complexes comprise of a number of unique and overlapping components and notably, several proteins in these complexes participate in fusions with MLL. The PAFc complex, which physically interacts with the N-terminal part of wild-type MLL as well as with MLL fusion genes is a multi-protein complex that consists of the core components PAF1, LEO1, CDC73, CTR9 and WDR61 (reviewed in(96)). Recently, BRD4 was shown to co-purify with some components of both the SEC as well as the PAFc complexes; however, it is yet unclear whether BRD4 is part of the MLL-AF9/PAFc/SEC multi-subunit complex or if it associates with PAFc/SEC in other complexes which are independent from the MLL-fusion protein.
Box 2: Multiple protein complexes regulate oncogenic transcription in the MLL leukemias.
MLL fusion partners are found in a number of multi-protein complexes involved in transcriptional activation/elongation. Some of the most well-defined complexes include the polymerase-associated factor complex (PAFc) that associates with the N-terminus of MLL, the EAP, SEC, and/or AEP complexes that contain a number of MLL fusion partners (28, 31),(30) and the Dot1l complex (DotCom) that also includes multiple MLL-fusion partners (29) (33). Recent data suggest that a number of components of the PAFc and SEC complex, which biochemically interact with MLL-fusion proteins, co-purify with the bromodomain protein BRD4, although it is not yet clear whether MLL-fusions also form a part of the BRD4/SEC/PAFc super-complex (See Fig. 2 legends).
Interestingly, a large number of the protein components of these complexes (e.g., AF4, AF5q31, AF9, ENL, ELL, AF10, AF17, etc) directly participate in leukemia-specific fusions with MLL. The importance of these complexes for the development of MLL-rearranged leukemia has been shown in different models (28, 30, 31). The complexes mentioned above have overlapping components (see Table. 1 and Fig. 2) and recent studies have suggested that the activity of these different complexes may be functionally linked (79, 94, 95). The exact nature of the inter-relationships between these complex transcriptional networks remains to be exactly defined; nevertheless, the importance of these complexes in MLL-rearranged leukemias presents a number of therapeutic possibilities to target this disease.
Prospects for therapeutic targeting in the MLL-rearranged leukemias
The importance of complexes associated with transcriptional elongation for MLL-fusion mediated leukemogenesis is underscored by the fact that recruitment of key components of these complexes is crucial for the oncogenic activation of MLL-fusion target genes and transformation (Fig. 1 and Box. 2). At least some MLL-fusions with predominantly cytoplasmic proteins also seem to recruit these transcriptional elongation complexes, through hitherto unknown mechanisms. Even though the detailed mechanisms by which these complexes are recruited remain to be studied, there is a possibility that cytoplasmic MLL fusion partners may also require the activity of these transcriptional elongation complexes and therefore therapeutic targeting of these complexes may benefit these leukemias as well (34). Recent studies indicate that interactions of MLL fusion proteins with wild-type MLL (35) or with heterologous proteins such as the product of the Men1 gene Menin (36), the polycomb group protein Cbx8 (37) or the polymerase associated factor complex (PAFc) (16) (38) are critical for MLL-fusion mediated leukemogenesis. These interactions may present valid targets for inhibition and indeed, a smallmolecule inhibitor of the Menin-MLL interaction was recently reported to reverse MLL-fusion mediated leukemic transformation(39). The positive transcription elongation factor, P-TEFb has been implicated in the oncogenic activation of MLL-target genes, given its association with the independently identified and possibly related EAP, SEC and AEP complexes (26–28, 30–32). P-TEFb is a heterodimeric complex consisting of Cyclin T proteins and cyclin-dependent kinase 9 (CDK9) that phosphorylates the carboxy-terminal domain of RNA polymerase II. This process is essential for promoting paused RNA Pol II to elongate (reviewed in (40)). Targeting of the P-TEFb core component CDK9 is an interesting therapeutic possibility since flavinoids such as flavopiridol, already in phase two trials for other malignancies, are reported to show activity against this kinase. However, concerns regarding toxicity and the modest efficacy of flavopiridol have been raised (41, 42), indicating that alternate avenues for P-TEFb inhibition might be necessary. A number of other interesting candidates and approaches for therapeutic intervention in the MLL leukemias have been reported (43–47), but as they have been extensively discussed in previous publications (48, 49), this review will focus primarily on pharmacological targeting of chromatin alterations.
H3K79 methylation and the role of the histone methyltransferase DOT1L
The histone methyltransferase Dot1 was discovered in yeast as a non-SET domain containing histone methyltransferase solely responsible for catalyzing the methylation of histone 3 at lysine 79 (H3K79) (50–53). The discovery of orthologous Dot1-like proteins mediating H3K79 methylation in several organisms points to an important, evolutionarily conserved function for this chromatin modification across divergent species. An important potential link between the human Dot1-like counterpart (DOT1L) and hematological malignancy was uncovered in 2005 when it was demonstrated that DOT1L interacts with the protein product of AF10/MLLT10, a gene often fused to MLL or another partner protein CALM in a variety of human hematologic malignancies (54). The Dot1l-Af10 interaction was found to be essential for MLL-AF10 and CALM-AF10 mediated transformation, establishing a direct link between aberrant H3K79 methylation and leukemogenesis (54, 55). The discovery that DOT1L directly or indirectly associates with a number of other MLL fusion partners such as AF4, AF9, ENL, and AF17 (26, 27,56, 57, 58), in addition to its interaction with AF10, suggested that aberrant H3K79 methylation might be a shared mechanism of oncogenic transcriptional activation in MLL leukemias involving these fusion partners. Abnormally high levels of H3K79 methylation on MLL target genes in human and mouse MLL leukemia cells (27, 59, 60) were believed to be a “smoking gun” implicating aberrant DOT1L activity in these leukemias. These findings led to the hypothesis that at least some MLL-fusion oncoproteins may, through DOT1L recruitment, lead to highly elevated H3K79 methylation levels on MLL-target genes, resulting in their sustained expression.
This scenario presents the interesting possibility that inhibiting DOT1L activity may impair leukemic transformation in the MLL-rearranged leukemias. Indeed, it was shown that inducible expression of the MLL-ENL fusion gene activated H3K79 dimethylation (me2) on MLL target genes (27, 61), and the MLL-ENL fusion gene lost its transforming activity upon exclusion of the DOT1L binding motif in the ENL part of the fusion oncoprotein (27). Likewise, H3K79 methylation by DOT1L was also crucial for HOXA gene activation in human MLL-AF4 leukemia cell lines as demonstrated through shRNA-mediated DOT1L knockdown experiments (59). Recently, a number of groups have reported that leukemogenesis driven by the MLL-AF9 fusion gene depends on H3K79 dimethylation, using conditional knockout mice that can be used to genetically inactivate Dot1l enzymatic activity(34, 62–64). Taken together, these studies highlight the importance of aberrant H3K79 methylation for the transforming activity of a number of MLL fusion oncogenes. It should be noted that although H3K79 methylation seems to play a critical role in the oncogenesis of a number of MLL fusion genes, including MLL fusions with predominantly cytoplasmic partner proteins (65), the exact molecular mechanism by which Dot1l is recruited to MLL target genes is not entirely clear. For example, in leukemias bearing MLL-AF4 fusions - which comprise the largest sub-set of MLL-rearranged ALLs - the mechanism by which DOT1L is recruited to MLL target loci is not completely understood (30). These mechanisms are likely to be the focus of research in coming years.
Pharmacologic targeting of DOT1L
Even as the genetic and biochemical studies mentioned above validate DOT1L as a possible therapeutic target in the MLL-rearranged leukemias, translation of these findings into sustainable clinical outcomes mandates the development of targeted small-molecule inhibitors. An important step in this direction was the recent description of a potent small-molecule inhibitor for DOT1L, which selectively inhibits the S-(5′-adenosyl)-l-methionine (SAM) binding activity of this methyltransferase (7) (Fig. 2A). Potent and selective activity of an S-adenosylmethionine structural analogue, EPZ004777, against DOT1L has facilitated the assessment of pharmacologic DOT1L inhibition in MLL- rearranged leukemia cells. A recent study demonstrated that a panel of human MLL-rearranged cell lines bearing MLL-AF4, MLL-AF9 or MLL-ENL translocations are sensitive to EPZ004777 treatment, in contrast to non-MLL rearranged cell lines that do not respond to this small molecule (7). This study demonstrated that pharmacologic DOT1L inhibition results in robust and selective reduction in cellular H3K79 methylation, curtailing the expression of key genes associated with MLL-translocation induced leukemogenesis, thereby selectively inhibiting proliferation of MLL-rearranged leukemia cell lines (7). Apart from reaffirming DOT1L as a bona fide “druggable” target in the MLL leukemias, these results validate small-molecule inhibition of lysine methyltransferases as a potentially important therapeutic modality. Several other potent DOT1L inhibitors have been recently reported (66). While toxicology studies remain to be done, there is a possibility that early stage human clinical trials with Dot1l inhibitors might begin soon (See Box.3).
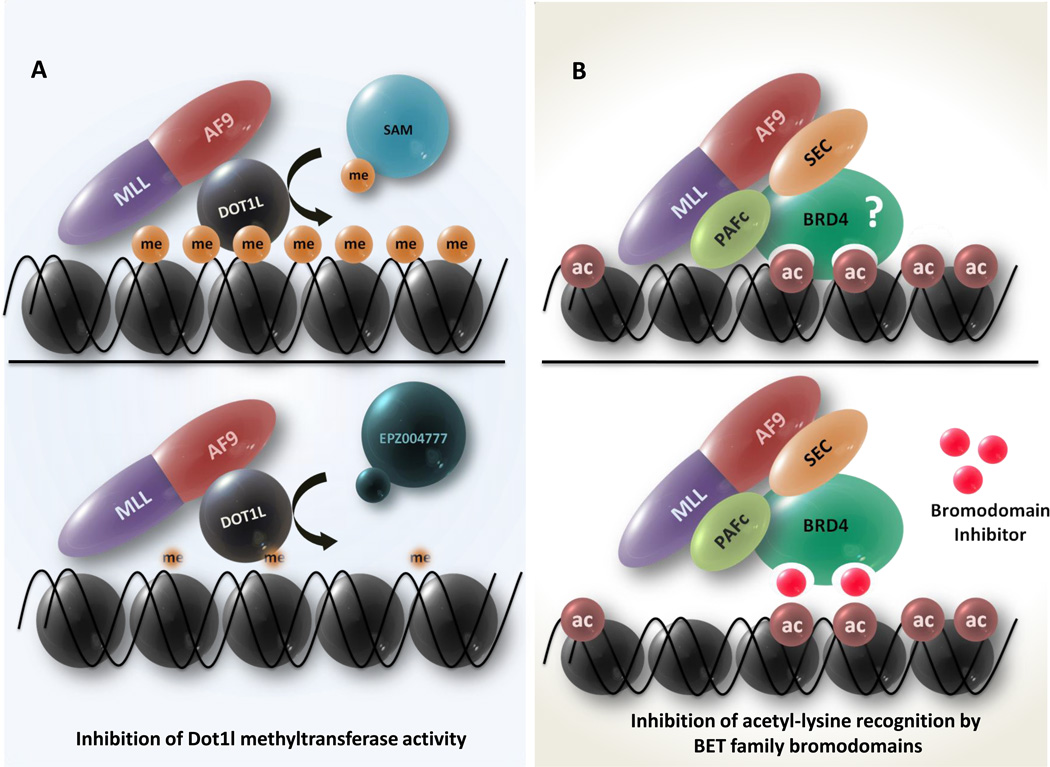
Fig.2. Pharmacological targeting of chromatin regulatory pathways in MLL-rearranged leukemias.
A: Using MLL-AF9 as an example, the cartoon depiction shows the association of the Dot1l histone methyltransferase with the AF9 part of the MLL-AF9 fusion. The Dot1l histone methyltransferase methylates histone 3 at lysine residue 79 (H3K79) using S-(5′-adenosyl)-l-methionine (SAM) as a donor. Current models suggest that aberrant recruitment of this enzyme to MLL-target loci leads to constitutive activation of MLL-target genes. The use of a SAM mimetic, eg., EPZ0004777, (lower panel) that mimics the binding of Dot1l to SAM, can potently inhibit Dot1l activity, serving as a candidate therapeutic agent targeting oncogenic H3K79 hypermethylation.
B: MLL fusions such as MLL-AF9 (used as a typical example) recruit the PAFc complex through the N-terminal portion of MLL. A number of nuclear fusion partners such as AF9, ENL, and AF5q31 are a part of the SEC complex which includes the transcription elongation factor P-TEFb. Both the PAFc and the SEC complexes have been implicated in MLL-fusion mediated leukemia. Recently, it was demonstrated that the acetyl-lysine recognizing bromodomain protein BRD4 co-purifies with a number of components of the PAFc and the SEC complexes. The inhibition of acetyl lysine recognition by BRD4 using a BET family inhibitor (lower panel) was shown to preclude the targeting of the PAFc and SEC complex components to chromatin, leading to down regulation of gene expression. Targeting of acetyl-lysine recognition by BRD4 using small-molecule inhibitors (lower panel) could therefore inhibit MLL-fusion mediated transformation as described in these studies (8, 9). It must be noted however that BRD4 has not yet been identified in MLL-fusion protein complexes. Therefore, it is not clear whether the BRD4/PAFc/SEC complex and the MLL-fusion/PAFc/SEC complexes are overlapping, or control different sub-sets of an oncogenic transcriptional program (See also Box 2).
Box 3: Unique kinetics of cellular response to small molecules that target chromatin regulators.
A number of pharmacological agents targeting oncogenic chromatin processes are likely to be discovered and some of them may enter clinical trials in the near future. These promising new agents may pose novel challenges that merit consideration for effective study design. One potentially important consideration could be the issue of endpoint selection, given that some of these epigenetic therapies may display delayed response kinetics as compared to conventional therapies. An example of this is found with DNA methyltransferase inhibitors where response to these molecules may be delayed by many weeks both in vitro and in vivo. Another example is the profound growth inhibitory effect of the Dot1l inhibitor EPZ0004777 on MLL-fusion transformed cells that manifests only 7–10 days after initiation of drug-exposure (7, 78). The kinetics of these responses are in contrast to standard chemotherapeutic agents which may induce rapid apoptosis or anti-proliferative responses. The importance of the kinetics of response is important both for drug discovery efforts and clinical development. Potentially effective pharmacologic compounds may escape discovery if preclinical assessments or screens fail to take this delayed effect into consideration. Although in vitro studies with leukemia cell lines may not completely recapitulate human patient response, arguably, evidence of biological drug effects such as tumor shrinkage or symptom amelioration may be even more delayed in patients. This possibility will likely need to be factored in to the design of clinical protocols.
Histone acetylation in the MLL leukemias
A link to histone acetylation and MLL-rearranged leukemia is evident from the fact that the recruitment of histone deacetylases (HDACs) by the wild type MLL protein, which could be important for silencing of developmentally-regulated genes during differentiation (67), is lost in leukemic fusions of MLL. This loss of recruitment, which occurs due to the disruption of the third PHD domain of MLL, is required for transformation (68). Moreover, in rare but recurrent cases, especially of therapy related of leukemia, MLL is found to be directly involved in fusions to histone acetyl-transferases CBP or p300. It is conceivable that truncated MLL proteins lose repressive HDAC activity and gain aberrant transcriptional activation potential either through recruiting histone modifying enzymes (such as DOT1L) or by directly fusing to them (e.g. MLL fusions to CBP or p300). Intriguingly, inconsistent with the current belief that decreased HDAC recruitment by MLL-fusions could constitutively de-repress MLL-target genes, HDAC inhibitors have shown some activity in MLL leukemias: a number of B-ALL cell lines and patient samples with MLL-AF4 fusions were shown to respond to HDAC inhibition (69). Moreover, complete remission was reported in a patient with an MLL-CBP rearrangement in a phase 1a/2 clinical trial with the investigational HDAC inhibitor, panobinostat (70). It is not known whether the therapeutic response provided by HDAC inhibition in these studies resulted from an increased histone acetylation at MLL-target loci. The exact relationship between histone acetylation and the pathogenesis of MLL rearranged leukemias remains to be elucidated.
Pharmacologic inhibition of acetyl-lysine recognition
A link between the recognition of histone acetylation and MLL-leukemia has been discovered and offers another attractive therapeutic possibility. The recognition of side-chain acetylated lysine residues is mediated by a conserved structural motif called a bromodomain. A large number of chromatin-related proteins harbor bromodomains including members of the bromodomain and extra-terminal domain (BET) family of proteins (including BRD2, BRD3 and BRD4) (71). Various proteins of the BET family such as BRD4 and BRD3 have been implicated in cancer. Most notably, BRD4 is fused to the NUT protein in most cases of NUT midline carcinoma, a rare but particularly aggressive form of squamous cell carcinoma (72). The recent development of direct-acting, competitive inhibitors of BET bromodomains (such as JQ1 and I-BET), has created opportunities for mechanistic studies of chromatin biology in cancer and therapeutic translation in leukemia and other diseases (73) (74).
Recently, two collaborating laboratories identified BRD4 as a unique cancer dependency in MLL-rearranged AML using RNA interference screening (9). Silencing of BRD4 expression resulted in impaired cell cycle progression, growth and leukemogenesis in vivo. Using the first-in-class BET bromodomain inhibitor, JQ1, the collaborating researchers identified potent anti-proliferative activity in a murine model of MLL-AF9 and mutant Nras (NrasG12D). MLL leukemia cells exposed to JQ1 exhibited growth arrest accompanied by monocytic differentiation. Genome-wide transcriptional analysis demonstrated a coordinated down-regulation of the Myc transcriptional program, a feature of BET inhibition which was also identified in studies of multiple myeloma (75). Beyond MLL-dependent leukemia, anti-proliferative effects of the bromodomain inhibitor JQ1 were observed more broadly in a number of genetically-distinct AML cell lines.
The role of BRD4 as a positive regulator of P-TEFb has been well documented (71, 76) ; it has been shown that the BRD4-P-TEFb interaction enhances RNA Pol II dependent transcription, linking the recognition of histone acetyl residues to transcriptional activity. A recent study demonstrated that the BET family of proteins, including BRD4 co-purify with both P-TEFb as well as the polymerase associated factor c (PAFc) complex. (8). The inhibition of acetyl-lysine binding by the BRD4 bromodomains may therefore forestall recruitment of the P-TEFb and possibly PAFc complexes, a step that is crucial in MLL-fusion mediated leukemogenesis (Fig. 2B). Indeed, the authors of this study went on to demonstrate that BRD4 inhibition, using a third reported small-molecule inhibitor of BET family, GSK1210151A (I-BET151) suppressed transforming activity of the MLL-fusions in vitro and in murine xenograft models. Together, these studies validate chromatin “reader” proteins as therapeutic targets in leukemia, and extended the scope of epigenetic anti-cancer therapeutics beyond the much pursued realm of enzyme inhibition.
Despite these promising results, a number of questions remain to be answered. Firstly, most studies demonstrating the role of chromatin modifications in MLL-rearranged leukemias have used murine or human cell line models of the most common MLL fusions (e.g.: MLL-AF9, MLL-ENL, MLL-AF4 etc). It is yet unclear whether leukemic transformation in patients bearing other translocations also involves similar mechanisms. Some less common MLL-rearrangements such as MLL-CBP, MLL-EEN or MLL-tandem duplications may act in ways that are different from the examples discussed in this review. Notably, in the case of the rare MLL-EEN translocation, the EEN part of the fusion recruits the histone methyltransferase PRMT1, leading to increased histone acetylation at MLL target genes (22). This example of another histone methyltransferase directly recruited by an MLL fusion partner - apart from the example of DOT1L described above - demonstrates that MLL-rearranged leukemias may involve other chromatin aberrations. Detailed analysis of molecular mechanisms involving other chromatin modifications in the MLL-rearranged leukemias may reveal additional putative candidates for epigenetic therapies. A recent example is the histone demethylase LSD1 which was shown to play a role in the pathogenesis of MLL–rearranged leukemias through mechanisms that are not yet completely clear (10). Of note, treatment of murine and human leukemia cells bearing MLL-translocations with anti-LSD1 shRNAs or pharmacologic inhibitors of LSD1 led to a significant impairment of in vitro and in vivo leukemic activity. These data demonstrate that in addition to chromatin “writers” such as DOT1L and PRMT1 and “readers” such as BRD4, chromatin “eraser” proteins such as LSD1 could also serve as potential targets of epigenetic therapy in MLL-rearranged leukemias.
Concluding remarks
Our understanding of the number and diversity of post-translational covalent modifications of chromatin is rapidly expanding (77). In this scenario, determining specific epigenetic modifications that are the most valuable therapeutic targets for a particular malignancy may prove daunting. In the coming years, intense efforts will have to be made to identify the dependence of individual tumor types on specific chromatin modifications. In the studies on Dot1l and Brd4 discussed in this review, both genetic Dot1l inactivation (78) as well as Brd4 inhibition using GSK1210151A (I-BET151) (79) altered transcription levels of only a select sub-set of genes rather than promoting large scale changes in gene expression, which is interesting given that both strategies target epigenetic modifiers that associate with chromatin on a genome-wide scale. In the case of the Dot1l study, the small sub-set of genes that showed decreased expression following Dot1l deletion was highly enriched for MLL-AF9 target genes, suggesting that MLL-AF9 mediated oncogenesis might be particularly dependent on Dot1l controlled transcriptional regulation. The case of Brd4 inhibition might be more complex, given that it also inhibits expression of the MYC oncogene in at least two hematologic malignancies (75). The possibility that tumor cells may show a greater dependence on a particular epigenetic modification than their normal counterparts is enticing and deserves further attention. Notably, normal hematopoiesis is perturbed following Dot1l deletion (80, 81), but initial studies suggest MLL-fusion driven leukemias display a higher dependence on DOT1L than normal hematopoietic stem cells. Studies will now focus on the relative dependence of normal vs. tumor cells on specific chromatin regulators so that a clinically applicable therapeutic window can be identified. These studies should pave the way for the discovery and application of novel therapies targeting chromatin modifications altered in cancer.
Acknowledgments
We would like to apologize to our colleagues in the field whose work may not have been cited due to space considerations. We would like to acknowledge support from the American Cancer Society, the Leukemia and Lymphoma Society, Gabrielle’s Angel Foundation and the National Cancer Institute (U01CA105423, R01CA140575) to S.A.A. A.J.D. is supported by the NCI Howard Temin K99 Award. S.A.A. is a consultant for Epizyme Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007 Feb 23;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010 Jul;10(7):457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Issa JP, Kantarjian HM, Kirkpatrick P. Azacitidine. Nat Rev Drug Discov. 2005 Apr;4(4):275–276. doi: 10.1038/nrd1698. [DOI] [PubMed] [Google Scholar]
- 4.Gore SD, Jones C, Kirkpatrick P. Decitabine. Nat Rev Drug Discov. 2006 Nov;5(11):891–892. doi: 10.1038/nrd2180. [DOI] [PubMed] [Google Scholar]
- 5.Marks PA. The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin Investig Drugs. 2010 Sep;19(9):1049–1066. doi: 10.1517/13543784.2010.510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boumber Y, Issa JP. Epigenetics in cancer: what's the future? Oncology (Williston Park) 2011 Mar;25(3):220–226. 8. [PubMed] [Google Scholar]
- 7.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011 Jul 12;20(1):53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson M, Prinjha R, Dittmann A, Giotopoulos G, Bantscheff M, Chan W-I, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–562. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011 Oct 27;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y, et al. The Histone Demethylase KDM1A Sustains the Oncogenic Potential of MLL-AF9 Leukemia Stem Cells. Cancer Cell. 2012 Apr 17;21(4):473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Biondi A, Cimino G, Pieters R, Pui CH. Biological and therapeutic aspects of infant leukemia. Blood. 2000 Jul 1;96(1):24–33. [PubMed] [Google Scholar]
- 12.Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009 Sep 17;114(12):2489–2496. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002 Jan;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 14.Ferrando AA, Armstrong SA, Neuberg DS, Sallan SE, Silverman LB, Korsmeyer SJ, et al. Gene expression signatures in MLL-rearranged T-lineage and B-precursor acute leukemias: dominance of HOX dysregulation. Blood. 2003 Jul 1;102(1):262–268. doi: 10.1182/blood-2002-10-3221. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, et al. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002 Nov;10(5):1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 16.Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002 Nov;10(5):1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 17.Miller T, Krogan NJ, Dover J, Erdjument-Bromage H, Tempst P, Johnston M, et al. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):12902–12907. doi: 10.1073/pnas.231473398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia ZB, Anderson M, Diaz MO, Zeleznik-Le NJ. MLL repression domain interacts with histone deacetylases, the polycomb group proteins HPC2 and BMI-1, and the corepressor C-terminal-binding protein. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8342–8347. doi: 10.1073/pnas.1436338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, et al. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005 Jun 17;121(6):873–885. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 20.Ernst P, Wang J, Huang M, Goodman RH, Korsmeyer SJ. MLL and CREB bind cooperatively to the nuclear coactivator CREB-binding protein. Mol Cell Biol. 2001 Apr;21(7):2249–2258. doi: 10.1128/MCB.21.7.2249-2258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, Trka J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009 Aug;23(8):1490–1499. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- 22.Cheung N, Chan LC, Thompson A, Cleary ML, So CW. Protein arginine-methyltransferase-dependent oncogenesis. Nat Cell Biol. 2007 Oct;9(10):1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- 23.Krivtsov A, Armstrong S. MLL translocations, histone modifications and leukaemia stem-cell development. Nature reviews Cancer. 2007;7(11):823–856. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 24.Somervaille TC, Cleary ML. Grist for the MLL: how do MLL oncogenic fusion proteins generate leukemia stem cells? Int J Hematol. 2010 Jun;91(5):735–741. doi: 10.1007/s12185-010-0579-8. [DOI] [PubMed] [Google Scholar]
- 25.Slany R. The molecular biology of mixed lineage leukemia. Haematologica. 2009;94(7):984–1077. doi: 10.3324/haematol.2008.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitoun E, Oliver P, Davies K. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Human molecular genetics. 2007;16(1):92–198. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- 27.Mueller D, Bach C, Zeisig D, Garcia-Cuellar M-P, Monroe S, Sreekumar A, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110(13):4445–4499. doi: 10.1182/blood-2007-05-090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller D, García-Cuéllar M-P, Bach C, Buhl S, Maethner E, Slany R. Misguided transcriptional elongation causes mixed lineage leukemia. PLoS biology. 2009;7(11) doi: 10.1371/journal.pbio.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan M, Herz H-M, Takahashi Y-H, Lin C, Lai K, Zhang Y, et al. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom) Genes & development. 2010;24(6):574–663. doi: 10.1101/gad.1898410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C, Smith E, Takahashi H, Lai K, Martin-Brown S, Florens L, et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Molecular cell. 2010;37(3):429–466. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary M. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17(2):198–410. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monroe S, Jo S, Sanders D, Basrur V, Elenitoba-Johnson K, Slany R, et al. MLL-AF9 and MLL-ENL alter the dynamic association of transcriptional regulators with genes critical for leukemia. Experimental hematology. 2011;39(1):77. doi: 10.1016/j.exphem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas D, Milne TA, Basrur V, Kim J, Elenitoba-Johnson KS, Allis CD, et al. Function of leukemogenic mixed lineage leukemia 1 (MLL) fusion proteins through distinct partner protein complexes. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15751–15756. doi: 10.1073/pnas.1111498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang M-J, Wu H, Achille N, Reisenauer M, Chou C-W, Zeleznik-Le N, et al. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer research. 2010;70(24):10234–10276. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiel AT, Blessington P, Zou T, Feather D, Wu X, Yan J, et al. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell. 2010 Feb 17;17(2):148–159. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama A, Somervaille T, Smith K, Rozenblatt-Rosen O, Meyerson M, Cleary M. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207–225. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Jones M, Koseki H, Nakayama M, Muntean A, Maillard I, et al. CBX8, a polycomb group protein, is essential for MLL-AF9-induced leukemogenesis. Cancer Cell. 2011;20(5):563–638. doi: 10.1016/j.ccr.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muntean AG, Tan J, Sitwala K, Huang Y, Bronstein J, Connelly JA, et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 2010 Jun 15;17(6):609–621. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012 Jan 29; doi: 10.1038/nchembio.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brès V, Yoh S, Jones K. The multi-tasking P-TEFb complex. Current opinion in cell biology. 2008;20(3):334–374. doi: 10.1016/j.ceb.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang LM, Ren DM. Flavopiridol, the first cyclin-dependent kinase inhibitor: recent advances in combination chemotherapy. Mini Rev Med Chem. 2010 Oct;10(11):1058–1070. doi: 10.2174/1389557511009011058. [DOI] [PubMed] [Google Scholar]
- 42.Rizzolio F, Tuccinardi T, Caligiuri I, Lucchetti C, Giordano A. CDK inhibitors: from the bench to clinical trials. Curr Drug Targets. 2010 Mar;11(3):279–290. doi: 10.2174/138945010790711978. [DOI] [PubMed] [Google Scholar]
- 43.Nakanishi H, Nakamura T, Canaani E, Croce CM. ALL1 fusion proteins induce deregulation of EphA7 and ERK phosphorylation in human acute leukemias. Proc Natl Acad Sci U S A. 2007 Sep 4;104(36):14442–14447. doi: 10.1073/pnas.0703211104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stubbs MC, Kim YM, Krivtsov AV, Wright RD, Feng Z, Agarwal J, et al. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia. 2008 Jan;22(1):66–77. doi: 10.1038/sj.leu.2404951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008 Oct 30;455(7217):1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010 Mar 26;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeung J, Esposito MT, Gandillet A, Zeisig BB, Griessinger E, Bonnet D, et al. beta-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell. 2010 Dec 14;18(6):606–618. doi: 10.1016/j.ccr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 48.Liedtke M, Cleary ML. Therapeutic targeting of MLL. Blood. 2009 Jun 11;113(24):6061–6068. doi: 10.1182/blood-2008-12-197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012 Feb 28;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Q, Wang H, Ng HH, Erdjument-Bromage H, Tempst P, Struhl K, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol. 2002 Jun 25;12(12):1052–1058. doi: 10.1016/s0960-9822(02)00901-6. [DOI] [PubMed] [Google Scholar]
- 51.Lacoste N, Utley RT, Hunter JM, Poirier GG, Cote J. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J Biol Chem. 2002 Aug 23;277(34):30421–30424. doi: 10.1074/jbc.C200366200. [DOI] [PubMed] [Google Scholar]
- 52.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002 Jun 14;109(6):745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 53.Ng HH, Feng Q, Wang H, Erdjument-Bromage H, Tempst P, Zhang Y, et al. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 2002 Jun 15;16(12):1518–1527. doi: 10.1101/gad.1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005 Apr 22;121(2):167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 55.Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y. Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat Cell Biol. 2006 Sep;8(9):1017–1024. doi: 10.1038/ncb1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeisig D, Bittner C, Zeisig B, García-Cuéllar M-P, Hess J, Slany R. The eleven-nineteen-leukemia protein ENL connects nuclear MLL fusion partners with chromatin. Oncogene. 2005;24(35):5525–5557. doi: 10.1038/sj.onc.1208699. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem. 2006 Jun 30;281(26):18059–18068. doi: 10.1074/jbc.M601903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reisenauer MR, Anderson M, Huang L, Zhang Z, Zhou Q, Kone BC, et al. AF17 competes with AF9 for binding to Dot1a to up-regulate transcription of epithelial Na+ channel alpha. J Biol Chem. 2009 Dec 18;284(51):35659–35669. doi: 10.1074/jbc.M109.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krivtsov AV, Feng Z, Lemieux ME, Faber J, Vempati S, Sinha AU, et al. H3K79 methylation profiles define murine and human MLL-AF4 leukemias. Cancer Cell. 2008 Nov 4;14(5):355–368. doi: 10.1016/j.ccr.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guenther MG, Lawton LN, Rozovskaia T, Frampton GM, Levine SS, Volkert TL, et al. Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev. 2008 Dec 15;22(24):3403–3408. doi: 10.1101/gad.1741408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milne TA, Martin ME, Brock HW, Slany RK, Hess JL. Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Res. 2005 Dec 15;65(24):11367–11374. doi: 10.1158/0008-5472.CAN-05-1041. [DOI] [PubMed] [Google Scholar]
- 62.Bernt K, Zhu N, Sinha A, Vempati S, Faber J, Krivtsov A, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20(1):66–144. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen A, Taranova O, He J, Zhang Y. DOT1L, the H3K79 methyltransferase, is required for MLL-AF9-mediated leukemogenesis. Blood. 2011;117(25):6912–6934. doi: 10.1182/blood-2011-02-334359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jo S, Granowicz E, Maillard I, Thomas D, Hess J. Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood. 2011;117(18):4759–4827. doi: 10.1182/blood-2010-12-327668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang MJ, Wu H, Achille NJ, Reisenauer MR, Chou CW, Zeleznik-Le NJ, et al. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res. 2010 Dec 15;70(24):10234–10242. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Y, Chen P, Diao J, Cheng G, Deng L, Anglin JL, et al. Selective inhibitors of histone methyltransferase DOT1L: design, synthesis, and crystallographic studies. J Am Chem Soc. 2011 Oct 26;133(42):16746–16749. doi: 10.1021/ja206312b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Song J, Milne TA, Wang GG, Li H, Allis CD, et al. Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression. Cell. 2010 Jun 25;141(7):1183–1194. doi: 10.1016/j.cell.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muntean AG, Giannola D, Udager AM, Hess JL. The PHD fingers of MLL block MLL fusion protein-mediated transformation. Blood. 2008 Dec 1;112(12):4690–4693. doi: 10.1182/blood-2008-01-134056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stumpel DJ, Schneider P, Seslija L, Osaki H, Williams O, Pieters R, et al. Connectivity mapping identifies HDAC inhibitors for the treatment of t(4;11)-positive infant acute lymphoblastic leukemia. Leukemia. 2011 Oct 21; doi: 10.1038/leu.2011.278. [DOI] [PubMed] [Google Scholar]
- 70.Burbury KL, Bishton MJ, Johnstone RW, Dickinson MJ, Szer J, Prince HM. MLL-aberrant leukemia: complete cytogenetic remission following treatment with a histone deacetylase inhibitor (HDACi) Ann Hematol. 2011;90(7):847–849. doi: 10.1007/s00277-010-1099-6. Epub 2010 Oct 15. [DOI] [PubMed] [Google Scholar]
- 71.Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005 Aug 19;19(4):535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 72.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008 Apr 3;27(15):2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 73.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010 Dec 23;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010 Dec 23;468(7327):1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011 Sep 16;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005 Aug 19;19(4):523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 77.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011 Sep 16;146(6):1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011 Jul 12;20(1):66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011 Oct 27;478(7370):529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jo SY, Granowicz EM, Maillard I, Thomas D, Hess JL. Requirement for Dot1l in murine postnatal hematopoiesis and leukemogenesis by MLL translocation. Blood. 2011 May 5;117(18):4759–4768. doi: 10.1182/blood-2010-12-327668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen AT, He J, Taranova O, Zhang Y. Essential role of DOT1L in maintaining normal adult hematopoiesis. Cell Res. 2011 Sep;21(9):1370–1373. doi: 10.1038/cr.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011 May 1;17(9):2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. [DOI] [PubMed] [Google Scholar]
- 83.Morishita M, di Luccio E. Cancers and the NSD family of histone lysine methyltransferases. Biochim Biophys Acta. 2011 Dec;1816(2):158–163. doi: 10.1016/j.bbcan.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Lucio-Eterovic AK, Carpenter PB. An open and shut case for the role of NSD proteins as oncogenes. Transcription. 2011 Jul;2(4):158–161. doi: 10.4161/trns.2.4.16217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004 May 24;23(24):4225–4231. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- 86.Yang XJ, Ullah M. MOZ and MORF, two large MYSTic HATs in normal and cancer stem cells. Oncogene. 2007 Aug 13;26(37):5408–5419. doi: 10.1038/sj.onc.1210609. [DOI] [PubMed] [Google Scholar]
- 87.van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat Genet. 2009 May;41(5):521–523. doi: 10.1038/ng.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011 Mar 24;471(7339):467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wartman LD, Larson DE, Xiang Z, Ding L, Chen K, Lin L, et al. Sequencing a mouse acute promyelocytic leukemia genome reveals genetic events relevant for disease progression. J Clin Invest. 2011 Apr;121(4):1445–1455. doi: 10.1172/JCI45284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mar BG, Bullinger L, Basu E, Schlis K, Silverman LB, Dohner K, et al. Sequencing histone-modifying enzymes identifies UTX mutations in acute lymphoblastic leukemia. Leukemia. 2012 Mar 1; doi: 10.1038/leu.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Zutven LJ, Onen E, Velthuizen SC, van Drunen E, von Bergh AR, van den Heuvel-Eibrink MM, et al. Identification of NUP98 abnormalities in acute leukemia: JARID1A (12p13) as a new partner gene. Genes Chromosomes Cancer. 2006 May;45(5):437–446. doi: 10.1002/gcc.20308. [DOI] [PubMed] [Google Scholar]
- 92.He J, Nguyen AT, Zhang Y. KDM2b/JHDM1b, an H3K36me2-specific demethylase, is required for initiation and maintenance of acute myeloid leukemia. Blood. 2011 Apr 7;117(14):3869–3880. doi: 10.1182/blood-2010-10-312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, et al. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature. 2009 Jun 11;459(7248):847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briggs SD, Xiao T, Sun ZW, Caldwell JA, Shabanowitz J, Hunt DF, et al. Gene silencing: trans-histone regulatory pathway in chromatin. Nature. 2002 Aug 1;418(6897):498. doi: 10.1038/nature00970. [DOI] [PubMed] [Google Scholar]
- 95.He N, Chan CK, Sobhian B, Chou S, Xue Y, Liu M, et al. Human Polymerase-Associated Factor complex (PAFc) connects the Super Elongation Complex (SEC) to RNA polymerase II on chromatin. Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):E636–E645. doi: 10.1073/pnas.1107107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tan J, Muntean AG, Hess JL. PAFc, a key player in MLL-rearranged leukemogenesis. Oncotarget. 2010 Oct;1(6):461–465. doi: 10.18632/oncotarget.181. [DOI] [PMC free article] [PubMed] [Google Scholar]