Abstract
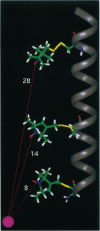
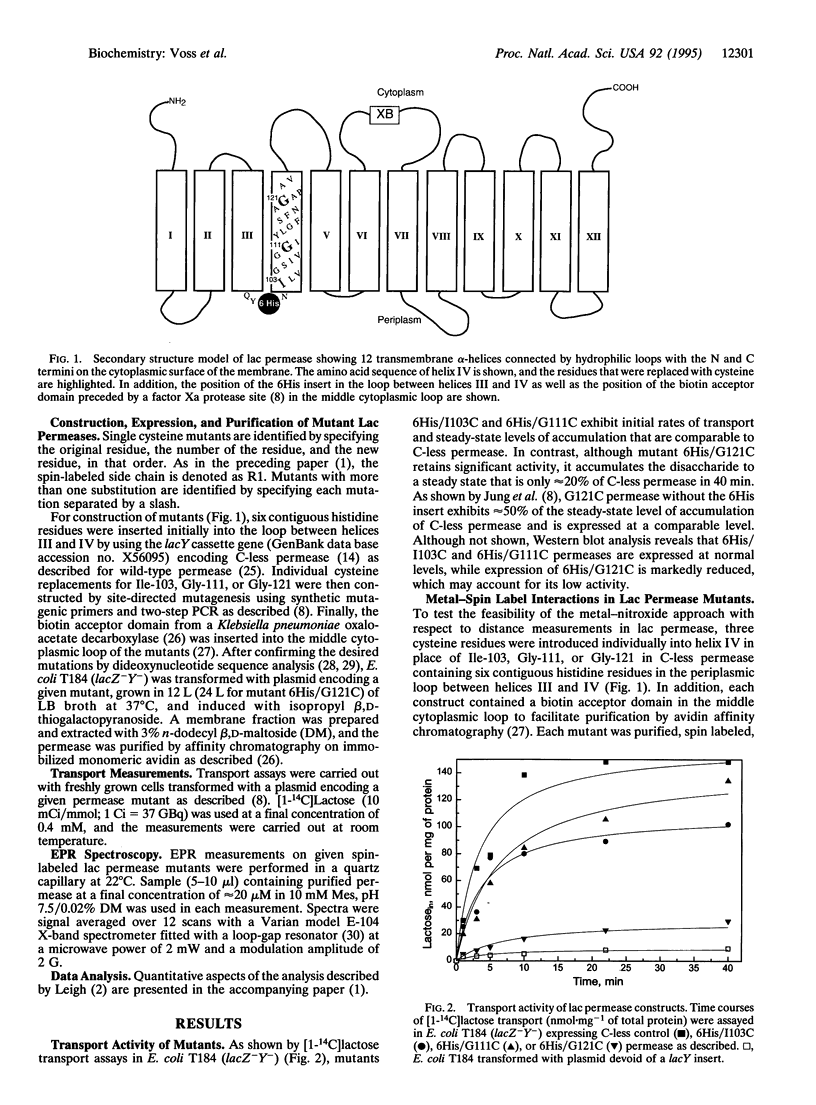
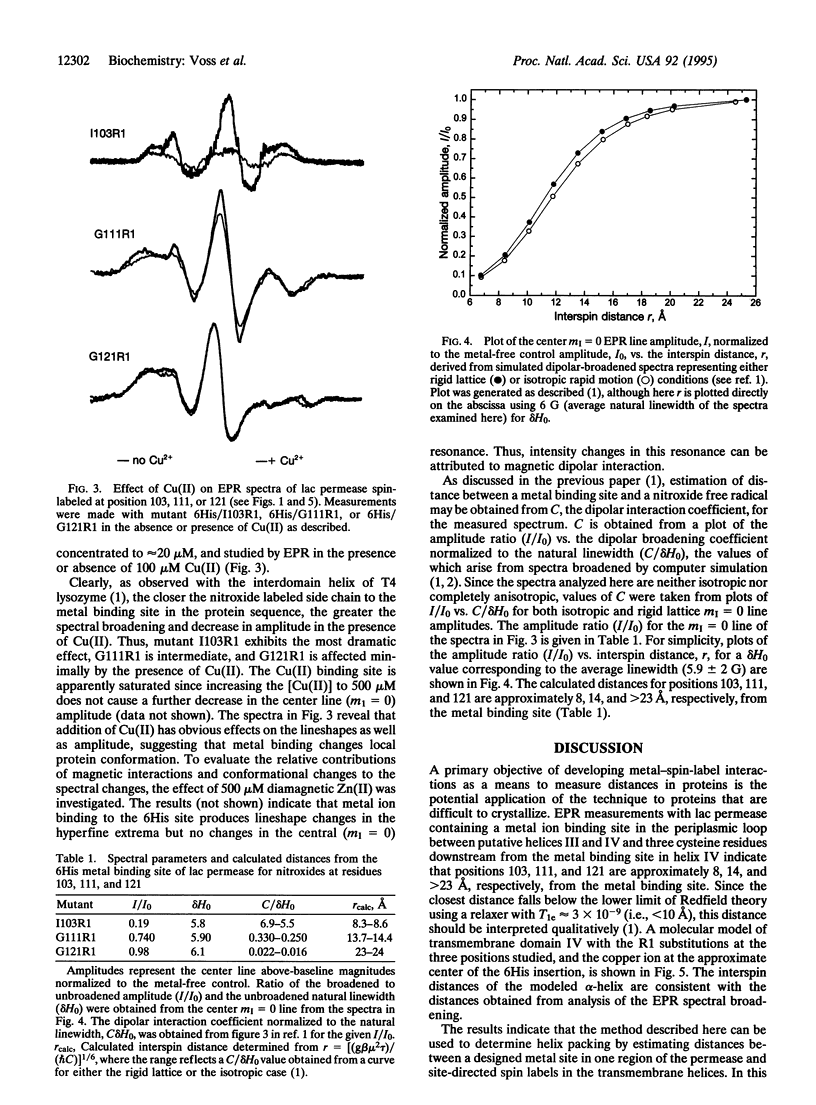
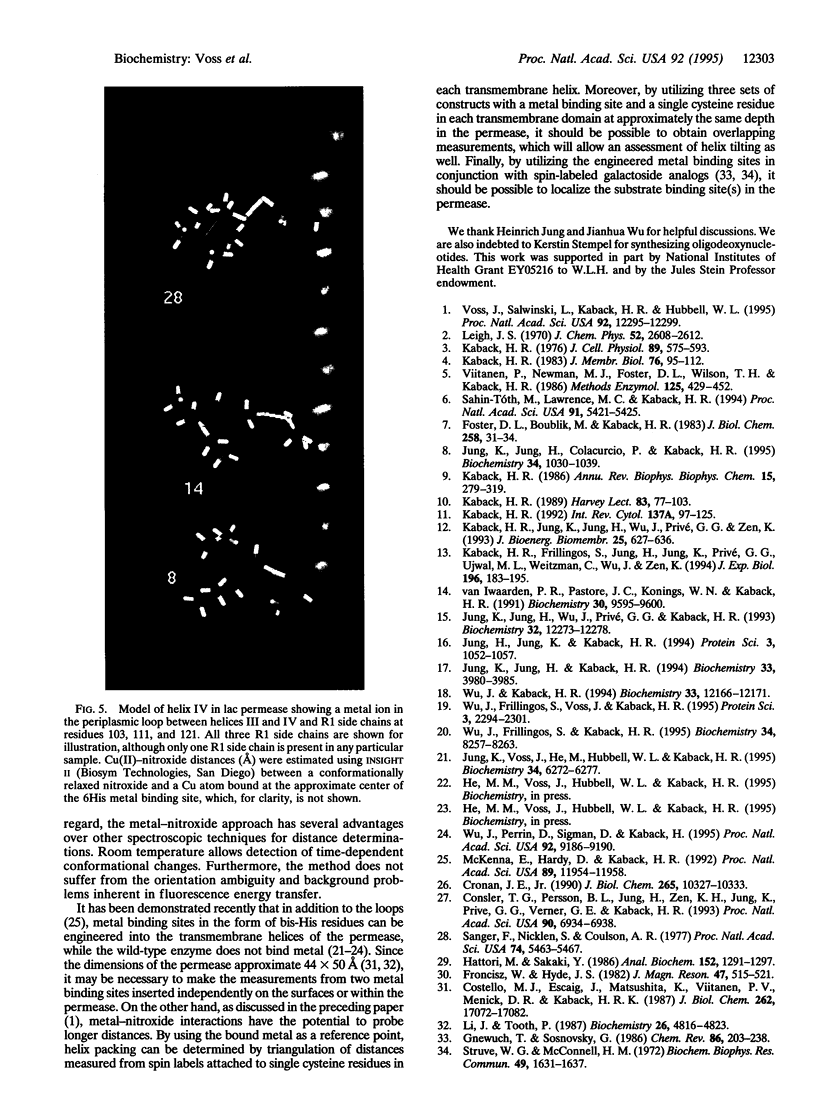
As shown in the accompanying paper, the magnetic dipolar interaction between site-directed metal-nitroxide pairs can be exploited to measure distances in T4 lysozyme, a protein of known structure. To evaluate this potentially powerful method for general use, particularly with membrane proteins that are difficult to crystallize, both a paramagnetic metal ion binding site and a nitroxide side chain were introduced at selected positions in the lactose permease of Escherichia coli, a paradigm for polytopic membrane proteins. Thus, three individual cysteine residues were introduced into putative helix IV of a lactose permease mutant devoid of native cysteine residues containing a high-affinity divalent metal ion binding site in the form of six contiguous histidine residues in the periplasmic loop between helices III and IV. In addition, the construct contained a biotin acceptor domain in the middle cytoplasmic loop to facilitate purification. After purification and spin labeling, electron paramagnetic resonance spectra were obtained with the purified proteins in the absence and presence of Cu(II). The results demonstrate that positions 103, 111, and 121 are 8, 14, and > 23 A from the metal binding site. These data are consistent with an alpha-helical conformation of transmembrane domain IV of the permease. Application of the technique to determine helix packing in lactose permease is discussed.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Consler T. G., Persson B. L., Jung H., Zen K. H., Jung K., Privé G. G., Verner G. E., Kaback H. R. Properties and purification of an active biotinylated lactose permease from Escherichia coli. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6934–6938. doi: 10.1073/pnas.90.15.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello M. J., Escaig J., Matsushita K., Viitanen P. V., Menick D. R., Kaback H. R. Purified lac permease and cytochrome o oxidase are functional as monomers. J Biol Chem. 1987 Dec 15;262(35):17072–17082. [PubMed] [Google Scholar]
- Cronan J. E., Jr Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem. 1990 Jun 25;265(18):10327–10333. [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Jung H., Jung K., Kaback H. R. A conformational change in the lactose permease of Escherichia coli is induced by ligand binding or membrane potential. Protein Sci. 1994 Jul;3(7):1052–1057. doi: 10.1002/pro.5560030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Jung H., Colacurcio P., Kaback H. R. Role of glycine residues in the structure and function of lactose permease, an Escherichia coli membrane transport protein. Biochemistry. 1995 Jan 24;34(3):1030–1039. doi: 10.1021/bi00003a038. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Kaback H. R. Dynamics of lactose permease of Escherichia coli determined by site-directed fluorescence labeling. Biochemistry. 1994 Apr 5;33(13):3980–3985. doi: 10.1021/bi00179a026. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Wu J., Privé G. G., Kaback H. R. Use of site-directed fluorescence labeling to study proximity relationships in the lactose permease of Escherichia coli. Biochemistry. 1993 Nov 23;32(46):12273–12278. doi: 10.1021/bi00097a001. [DOI] [PubMed] [Google Scholar]
- Jung K., Voss J., He M., Hubbell W. L., Kaback H. R. Engineering a metal binding site within a polytopic membrane protein, the lactose permease of Escherichia coli. Biochemistry. 1995 May 16;34(19):6272–6277. doi: 10.1021/bi00019a003. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Active transport in Escherichia coli: passage to permease. Annu Rev Biophys Biophys Chem. 1986;15:279–319. doi: 10.1146/annurev.bb.15.060186.001431. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Frillingos S., Jung H., Jung K., Privé G. G., Ujwal M. L., Weitzman C., Wu J., Zen K. The lactose permease meets Frankenstein. J Exp Biol. 1994 Nov;196:183–195. doi: 10.1242/jeb.196.1.183. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. In and out and up and down with lac permease. Int Rev Cytol. 1992;137:97–125. doi: 10.1016/s0074-7696(08)62674-1. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Jung K., Jung H., Wu J., Privé G. G., Zen K. What's new with lactose permease. J Bioenerg Biomembr. 1993 Dec;25(6):627–636. doi: 10.1007/BF00770250. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology and energetics of membrane transport. J Cell Physiol. 1976 Dec;89(4):575–593. doi: 10.1002/jcp.1040890414. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology of active transport: from membrane to molecule to mechanism. Harvey Lect. 1987;83:77–105. [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- Li J., Tooth P. Size and shape of the Escherichia coli lactose permease measured in filamentous arrays. Biochemistry. 1987 Jul 28;26(15):4816–4823. doi: 10.1021/bi00389a032. [DOI] [PubMed] [Google Scholar]
- McKenna E., Hardy D., Kaback H. R. Insertional mutagenesis of hydrophilic domains in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11954–11958. doi: 10.1073/pnas.89.24.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Tóth M., Lawrence M. C., Kaback H. R. Properties of permease dimer, a fusion protein containing two lactose permease molecules from Escherichia coli. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5421–5425. doi: 10.1073/pnas.91.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve W. G., McConnell H. M. A new spin-labelled substrate for -galactosidase and -galactoside permease. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1631–1637. doi: 10.1016/0006-291x(72)90529-3. [DOI] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Voss J., Salwiński L., Kaback H. R., Hubbell W. L. A method for distance determination in proteins using a designed metal ion binding site and site-directed spin labeling: evaluation with T4 lysozyme. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12295–12299. doi: 10.1073/pnas.92.26.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Frillingos S., Kaback H. R. Dynamics of lactose permease of Escherichia coli determined by site-directed chemical labeling and fluorescence spectroscopy. Biochemistry. 1995 Jul 4;34(26):8257–8263. doi: 10.1021/bi00026a007. [DOI] [PubMed] [Google Scholar]
- Wu J., Frillingos S., Voss J., Kaback H. R. Ligand-induced conformational changes in the lactose permease of Escherichia coli: evidence for two binding sites. Protein Sci. 1994 Dec;3(12):2294–2301. doi: 10.1002/pro.5560031214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Kaback H. R. Cysteine 148 in the lactose permease of Escherichia coli is a component of a substrate binding site. 2. Site-directed fluorescence studies. Biochemistry. 1994 Oct 11;33(40):12166–12171. doi: 10.1021/bi00206a020. [DOI] [PubMed] [Google Scholar]
- Wu J., Perrin D. M., Sigman D. S., Kaback H. R. Helix packing of lactose permease in Escherichia coli studied by site-directed chemical cleavage. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9186–9190. doi: 10.1073/pnas.92.20.9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iwaarden P. R., Pastore J. C., Konings W. N., Kaback H. R. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991 Oct 8;30(40):9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]