Abstract
Post-traumatic osteoarthritis (PTOA) is characterized by progressive cartilage degeneration in injured joints. Since fibronectin fragments (Fn-fs) degrade cartilage mainly through up-regulating matrix metalloproteinases (MMPs) and pro-inflammatory cytokines, we hypothesized that Fn-fs play a key role in PTOA by promoting chondrolysis in and around injured cartilage. To test this hypothesis, we profiled the catabolic events focusing on fibronectin fragmentation and proteinase expression in bovine osteochondral explants following a single blunt impact on cartilage with a drop tower device which created partial-thickness tissue damage. Injured and control explants were cultured for up to 14 days. The presence of Fn-fs, MMPs (-1, -3, -13), ADAMTS-5 in culture media and in cartilage was determined with immunoblotting. The daily proteoglycan (PG) depletion of cartilage matrix was assessed with DMMB assay. The effect of explant-conditioned media on chondrocytes was also examined with immunoblotting. Impacted cartilage released significantly higher amount of native Fn, three chondrolytic Fn-fs and PG than non-impacted controls did. Those increases coincided with up-regulation of MMP-3 both in conditioned media and in impacted cartilage. These findings support our hypothesis that PTOA may be propelled by Fn-fs which act as catabolic mediators through up-regulating cartilage-damaging proteinases.
Keywords: cartilage, fibronectin fragments, impact, matrix metalloproteinase-3, proteoglycan
INTRODUCTION
Ligamentous and meniscal injuries and intra-articular fractures raise the risk for premature osteoarthritis (OA) by 20% to 50%. This secondary form of OA, referred to as post-traumatic OA (PTOA), accounts for more than 10% of all cases1,2. How a focal cartilage damage initiated by joint trauma sustains and propagates till the complete loss of the tissue remains as a central enigma. Searching for the mediators of trauma-induced cartilage destruction has become one of the focuses of PTOA study. Fibronectin (Fn), a matrix glycoprotein, emerges as a potential candidate since its content rises up to 20-fold in osteoarthritic cartilage3,4.
Native Fn as a structural protein does not damage cartilage. However, since it contains several proteinase sensitive flexible regions, the elevated Fn in diseased joints may lead to increased levels of Fn fragments (Fn-fs). In 1989, Griffiths et al. found abundant Fn fragments (Fn-fs) ranging from 30 kDa to 200 kDa in synovial fluids from patients diagnosed with OA or from patients with joint trauma. The pattern of Fn fragmentation included three bands between 90 kDa and 100 kDa, two around 60 kDa and one band at 50 kDa5. In 1992, Homandberg et al. reported that at least 50% of the Fn in all OA synovial fluids was fragmented. The Fn-f concentrations were above 1.0 μM6. Later, the same group demonstrated that three Fn-fs, the N-terminal 29-kDa, the gelatin-binding 50-kDa, the cell-binding 110–140 kDa, exert robust chondrolytic activity, depleting cartilage proteoglycan (PG) by 9-, 6-, and 2-fold respectively over untreated controls. In fact, native Fn was inactive7. Moreover, a single intra-articular injection of 3.0 μM of the 29-kDa or the 50-kDa Fn-fs, into a rabbit knee joint caused nearly complete PG depletion in just two days8.
This remarkable effect of Fn-fs on PG depletion has been linked to the action of matrix metalloproteinases-3 (MMP-3). For instance, in just 24 hrs the 29-kDa Fn-f caused 25-fold greater MMP-3 release from cartilage over un-treated controls. After 3 days of treatment, this Fn-f induced MMP-3 release was 33 fold higher than controls. When the cultures were treated with specific antibodies to MMP-3, the PG depletion rate by the 29-kDa Fn-f was inhibited by 76%9. In primary chondrocyte cultures, the protein expression of MMP-3 was markedly increased by all three Fn-fs by 24 hrs. Furthermore, the 29-kDa and 140-kDa Fn-fs induced detectable MMP-3 expression at only 8 hrs of incubation with the former showing much greater effect10.
Besides up-regulating MMP-3, Fn-fs exert their chondrolytic action through catabolic cy-tokines including TNF-α and IL-1β which were markedly induced in human cartilage cultures by Fn-fs with doses ranging from 0.1–1.0 μM. The cytokine release peaked at Day 2 for TNF-α and at Day 3 for IL-1β. Furthermore, when neutralizing antibodies to those catabolic cytokines were added, the suppressed PG synthesis by the Fn-f was reversed: MMP-3 release was reduced by 63% with TNF-α antibody and by 42% with IL-1β antibody, respectively11.
Later on, studies on Fn-f signaling revealed that α5β1 integrin receptor12,13, receptor associated protein kinases including Pyk2 and Src14, MAP kinases10, and NF-κB14 are all involved in Fn-f pathways leading to up-regulation of ECM degrading proteinases and catabolic cytokines which further induce more proteinases. Those abnormally elevated proteinases accelerate the degradation of cartilage ECM structural proteins including Fn, which leads to even higher levels of Fn-fs. This forms a positive feedback loop which may propel cartilage degradation. Based on this, we hypothesized that blunt trauma on cartilage triggers Fn fragmentation to generate chondrolytic Fn-fs which then mediate the spread of cartilage damage initiated by the mechanical insult through up-regulating ECM damaging matrix metalloproteinases and catabolic cytokines.
METHODS
Acquisition and Preparation of Bovine Osteochondral Explants
Under sterile conditions, forty-seven in total osteochondral explants (2.0–2.5 cm W × 2.0–2.5 cm L × 0.5–1.0 cm H) were sawed from lateral tibial plateau (one explant per joint) of 18–20 month old cows obtained from a local abattoir. The explants were pre-equilibrated for 72 hrs in serum free DMEM/F12 media containing 50 U/mL Penicillin, 50 mg/L Streptomycin, and 2.5 mg/L Amphotericin B inside a humidified 37 °C incubator supplied with 5% CO2 and 5% O2.
Blunt Impact on Cartilage
Explants were subjected to a single blunt impact delivered by a custom-built drop tower15,16. Briefly, an explant with the cartilage surface uppermost was rigidly secured aseptically in a chamber filled with 1 × HBBS or DMEM/F-12 containing antibiotics. Impaction at 7.0 J/cm2 (lower energy) or 14.0 J/cm2 (higher energy) was delivered by dropping a 2.0 kg mass from a height of 7.0 or 14.0 cm to a flat-faced brass rod (ø = 5.0 mm) with rounded edges resting on the cartilage surface of the explant.
Examination of Native Fn and Fn-fs in Culture Media and Cartilage Extracts (N=3)
Culture media were sampled either daily during the 3-day pre-equilibration, or at Day 3, 7, 14 post-impact in some experiments, or daily in the first 3 days post-impact and then every other day between Day 5 and 9. Samples were then concentrated with Amicon® Ultra-15 Centrifugal Filter Devices (MWCO=3.0 kDa) (EMD Millipore, Billerica, MA) prior to being dialyzed against Milli-Q water. Full-thickness cartilage pieces from three areas as illustrated in Supplementary Fig. 8 were harvested at Day 1, 7, and 14 post-impact. After being washed with PBS containing 1.0 mM EDTA and a 1:1K fold dilution of protease inhibitor cocktail set III (Calbiochem/EMD, Gibbstown, NJ), those pieces were then incubated with 4.0 M GuHCl/10 mM EDTA at 4 °C overnight. Prepared medium or cartilage samples were resolved by SDS-PAGE. Fn and Fn-fs were identified with specific antibodies: goat anti-human N-terminal Fn polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), sheep anti-human Fn polyclonal antisera (The Binding Site Limited, Brimingham, U.K.), and mouse anti-human Fn cell-binding domain monoclonal antibody (Chemicon Corporation, Temecula, CA).
Determination of Major Cartilage Degrading Proteinases in Culture Media and/or Cartilage Extracts (N=3 or 5)
After proteins were transferred onto nitrocellulose membranes, MMP-3 was recognized with a specific antibody against the hinge region of the protein (Chemicon Corporation, Temecula, CA; Abcam®, Cambridge, MA; BIOMOL® International, Plymouth Meeting, PA) while other MMPs (-1 and -13) and ADAMTS-5 were probed with specific antibodies from Abcam® (Cambridge, MA).
Measurements of PG Depletion (N=3 or 5)
The contents of sulfated glycosaminoglycan (sGAG) in culture media sampled daily till the 9th day post-impact were measured with DMMB assay as described before 20. The wet weights of cartilage were used for normalization of the assay results.
Determination of Effect of Explant-Conditioned Media on Monolayer Chondrocytes (N=3)
Passage 1 bovine chondrocytes from full-thickness tibial plateau cartilage were seeded at 0.3 × 106 cells/cm2 and cultured in DMEM/F12/10% FBS for 3–4 days before serum deprivation. After 24 hrs, cells were subjected to either fresh serum free media or culture media from incubating un-impacted explants or the ones impacted at 7.0 J/cm2 or 14.0 J/cm2 for 1 day. Cells served as positive controls were cultured in media containing 10 ng/mL rhIL-1β or the combination of 10 ng/mL rhIL-1β and 10 nM rhHMGB1 or 100 ng/mL rbTNF-α (R&D Systems®, Minneapolis, MN). After 24 hrs, media were harvested, dialyzed, and concentrated by 30-fold. Equal vol. of denatured and reduced samples was resolved with a 10% SDS acrylamide/bis gel. The expression of MMP-1, 3 and ADAMTS-5 was determined with specific antibodies.
Statistical Analysis
For samples examined on the same gel, protein band intensities were measured with Adobe Photoshop CS3. Means and standard deviations of the treatment group from 3–6 individual experiments were compared to those of un-treated controls with Student t-test. P less than 0.05 is considered statistically significant.
RESULTS
Increased Cumulative Release of Three Fn-fs from the Higher-Energy Impacted Explants
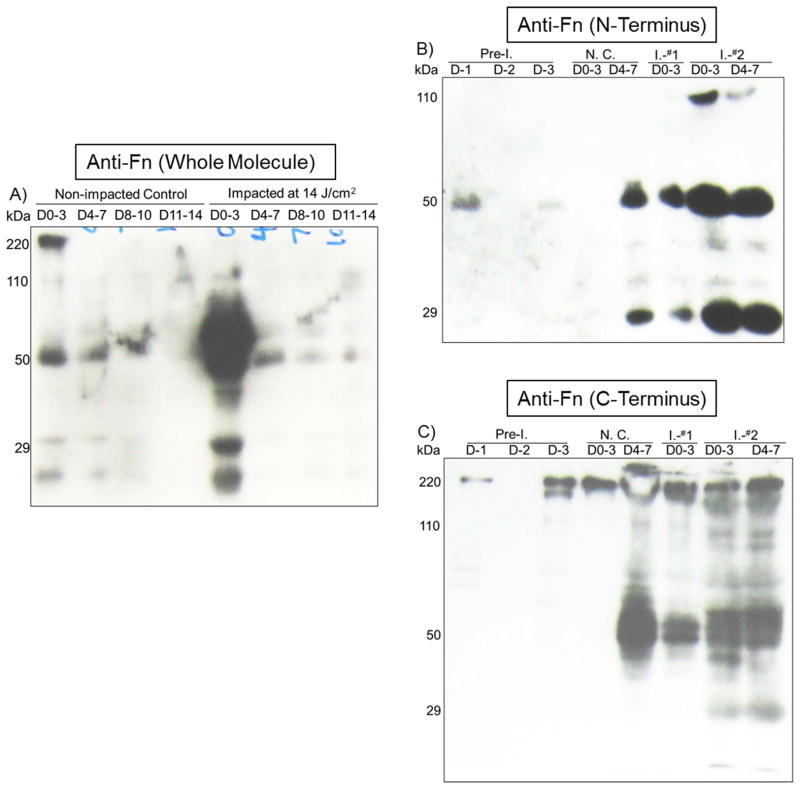
At Day 3, Fn-fs positioned at 50- and 29-kDa, respectively, was greatly elevated in culture media from impacted explants. The 50-kDa Fn-f was still detectable in media collected at Day 14 post-impact while the signal was barely seen in the non-impacted controls (Fig. 1A). These results were confirmed with a polyclonal antibody recognizing the N-terminus of Fn (Fig. 1B). The C-terminal 110–140 kDa Fn-f was also more abundant in culture media from impacted explants (Fig. 1C). Similar results were observed in two repeated experiments (Supplementary Fig. 1)
Figure 1. Cumulative release of Fn and Fn-fs from the higher-energy impacted (I.) explants.
A) Medium samples collected every two days till the 14th day post-impact were probed with an Fn antibody recognizing the whole molecular. B) & C) Medium samples harvested before (Pre-impact; Pre-I.) and a week after the impact were probed with an antibody reacting to the N-terminus of Fn or the C-terminus of the protein, respectively.
Elevated Daily Release of Fn and three Chondrolytic Fn-fs from Impacted Cartilage
Within 9 days, bluntly impacted cartilage released 1.56 ± 0.30 fold of native Fn relative to un-impacted control and this increase was statistically significant (P = 0.005) (Fig. 2A). Moreover, the cell-binding 110–140 kDa Fn-fs, the gelatin-binding 50-kDa Fn-f, and the N-terminus 29-kDa Fn-f were remarkably increased in cultures containing impacted cartilage: 2.69 ± 1.15 fold (P = 0.004), 1.34 ± 0.27 fold (P = 0.015), and 4.98 ± 2.42 fold (P = < 0.001), respectively (Fig. 2A–C).
Figure 2. Time-course release of Fn and three Fn-fs from the higher-energy impacted explants (N = 6).

A) The expression of native Fn and the 110–140 kDa Fn-fs in media culturing impacted (I) or non-impacted control (C) osteochondral explants and the comparison of their band intensities. B & C) The expression and comparison of the release of the 50-kDa and the 29-kDa Fn-fs. Error bars represent standard deviations.
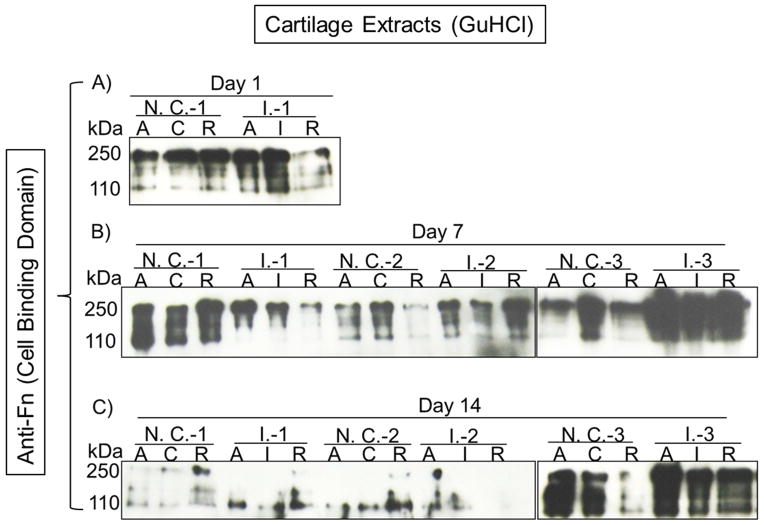
Propagation of Fn Fragmentation Outside of Cartilage Impact Zone
At Day 1, notably increased 110–140 kDa Fn-f was detected inside cartilage impact zone (I.) compared to its counterpart (Center; C) in non-impacted controls (Fig. 3A). Moreover, in two out of three experiments, the shifting of increased Fn fragmentation from the impact zone to the areas either adjacent (Annulus; A) or remote (R) to the zone was observed at Day 7 post-injury (Fig. 3B). This trend was still detectable at Day 14 but only in one of three sets of explants (Fig. 3C).
Figure 3. Expression of Fn and the 110–140 kDa Fn-f in cartilage extracts.
A) One day post-impact, full thickness cartilage pieces removed from the impact zone (I), the adjacent area (A), and the remote area (R) of an osteochondral explant impacted with the higher energy (I.) or from the central (C), the adjacent (A), and the remote (R) areas of a non-impacted control explant (N. C.) were subjected to GuHCl incubation and the extracts were examined with an antibody recognizing the cell binding domain of Fn. B) & C) Cartilage extracts from Day 7 or 14 post-impact were probed (N=3) with the same Fn antibody, respectively.
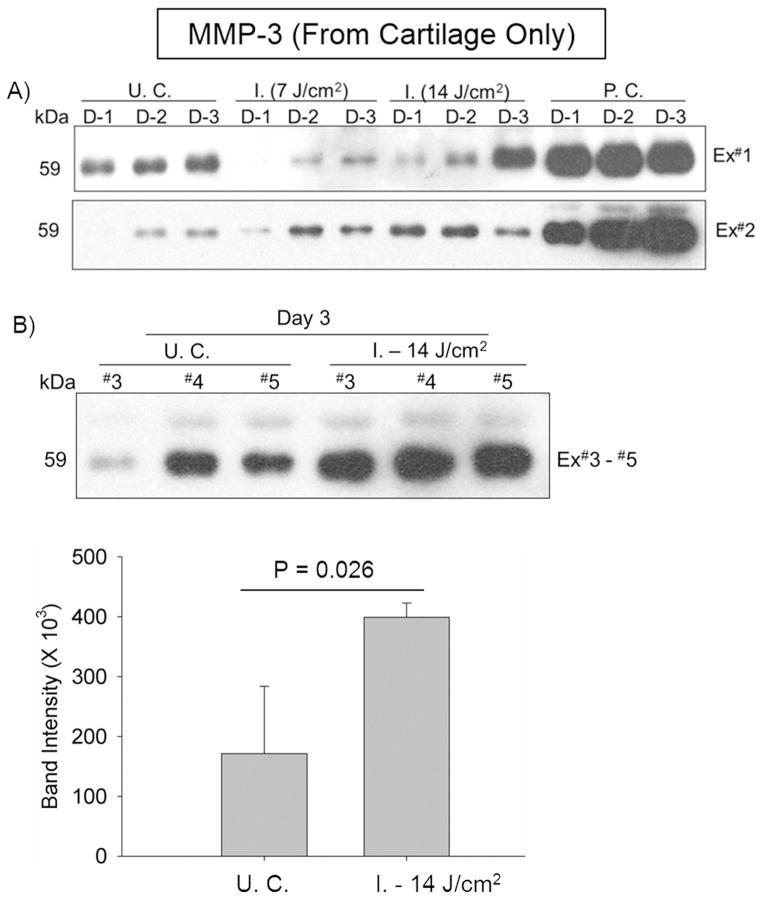
Significantly Increased Release of MMP-3 from Cartilage Only by Higher-Energy Impact
In the first 2 days post-impact, the increase of MMP-3 release from cartilage injured by either energy level was mild. Nonetheless, at Day 3, greatly elevated MMP-3 release was spotted in 50% of cultures impacted with the higher energy (Fig. 4A). This observation was lately confirmed with three more experiments in which MMP-3 release at Day 3 was compared between injured and non-injured cultures side-by-side on the same blot. Statistical analysis indicated that MMP-3 release by the higher-energy impact was 2.33 ± 0.14 fold (P = 0.026) (Fig. 4B).
Figure 4. Time-course release of MMP-3 from injured cartilage only.
A) In the 1st two sets of experiments, medium samples collected daily till the 3rd day of post-impact from cultures either containing cartilage impacted with two levels of energy (7 or 14 J/cm2), respectively, or containing cartilage treated with catabolic cytokines (positive control, P. C.), or containing un-impacted cartilage (U. C.), were examined for MMP-3 expression with immunoblotting. B) In another 3 sets of experiments, the MMP-3 release at Day 3 was compared between the higher energy impacted and un-impacted control cultures. Error bars represent standard deviations.
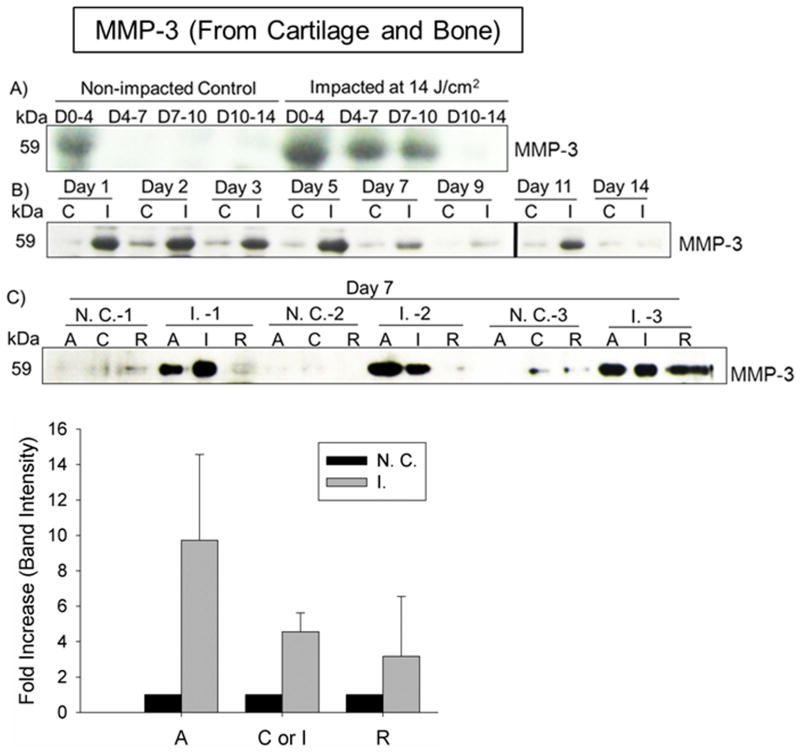
Elevated MMP-3 Release from Impacted Whole Explants and Significant MMP-3 Up-Regulation inside Injured Cartilage
The cumulative release of MMP-3 from impacted explants which were completely submerged in culture media peaked at Day 4 and declined with time to an undetectable level at Day 14 (Fig. 5A). The daily release of MMP-3 from injured explants greatly exceeded that from non-impact controls till Day 5 (Fig. 5B). Moreover, at Day 7, the average MMP-3 level inside cartilage impact zone (I) was 4.56 ± 1.06 fold relative to its counterpart in non-impacted controls (C). More strikingly, the increased of MMP-3 expression was even greater in the area ~1.0 mm adjacent to the impact zone (A), which was 9.72 ± 4.85 fold relative to that in the counterpart of non-impacted cartilage. Strong MMP-3 signal was also detected in an area at least 5.0 mm away from the impact zone (R) in 33% of impacted explants, while MMP-3 expression was barely detectable in its counterpart from control cartilage (Fig. 5 C). However, the expression of other matrix proteinases also critically involved in OA cartilage degradation was only mildly up-regulated by impaction (Supplementary Fig. 2 A–E for ADAMTS-5, Supplementary Fig. 3 A–F for MMP-13), and (Supplementary Fig. 4 A–B for MMP-1).
Figure 5. Time-course release of MMP-3 from cartilage and subchondral bone and the zonal MMP-3 expression in impacted cartilage.
A) & B) Cumulative and daily release of MMP-3 from impacted and non-impacted osteochondral explants. C) The side-by-side comparison of zonal expression of MMP-3 in impacted and non-impacted cartilage (N = 3). Error bars represent standard deviations. C = central area of un-impacted cartilage; I = impact site of impacted cartilage; A = adjacent area to impact or central site; R = remote area to impact or central site.
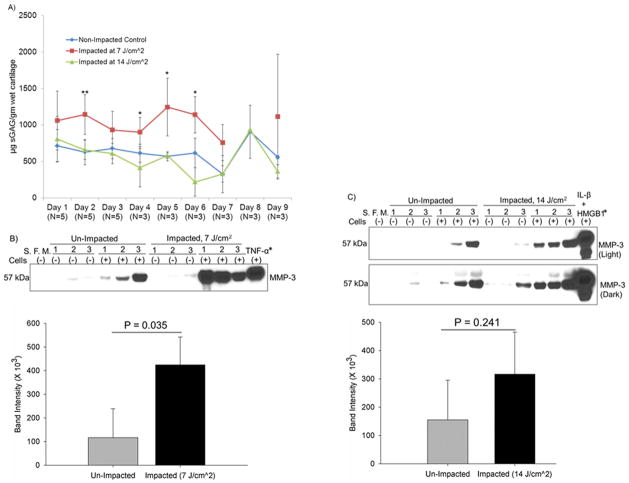
Significant PG Depletion by Lower Energy Impact and Correlation with MMP-3 Up-Regulation
Cartilage PG depletion was significantly increased by the lower energy impact at Day 2 (P = 0.007), 4 (P = 0.026), 5 (P = 0.044), and 6 (P = 0.047) (Fig. 6A). The soluble factors released from the lower-energy-impacted cartilage within 24 hrs markedly up-regulated MMP-3 (P = 0.035). However, the higher energy impact neither increased PG depletion nor significantly up-regulated MMP-3 expression (P = 0.241) (Fig. 6 B&C). Two proteinases also involved in PG degradation, ADAMTS-5 and MMP-1, were not significantly up-regulated (Supplementary Fig. 6 A–C and 7 A–C, respectively).
Figure 6. PG depletion from impacted cartilage and the correlation to MMP-3 expression.
A) The contents of sGAG in medium samples collected daily till the 9th day post-impact were measured with DMMB assay. B) Comparison of MMP-3 up-regulation by soluble factors from the lower-energy-impacted cartilage to non-impacted controls. C) Comparison of MMP-3 up-regulation by soluble factors from the higher-energy-impacted cartilage to non-impacted controls. S. F. M. = serum free media. *The loading vol. was reduced to half of the others due to saturated MMP-3 signal.
DISCUSSION
We have provided novel observations linking trauma-induced cartilage destruction to Fn-fs which may mediate the spread of cartilage degradation in PTOA pathogenesis. Our results clearly showed that a single blunt impact on cartilage triggered remarkably increased expression of native Fn and increased production of three chondrolytic Fn-fs. This elevation occurred as early as 1 day post-impact, peaked around Day 5 and was still detectable at Day 14. An earlier study by Farquhar et al. also reported that native Fn content in cartilage subjected to cyclical injurious impact was increased 109% relative to unloaded controls17. However, they did not look into Fn fragmentation whose products are capable of initiating and amplifying cartilage degradation. We have gathered abundant evidence showing blunt trauma induces increase of Fn-fs. Moreover, within a week of post-impact, we observed a notable shift of the increased Fn-fs from the impact site to the outside.
This trauma induced Fn fragmentation may be caused by up-regulated matrix proteinases since native Fn harbors five domains jointed by four less ordered segments which can be easily hydrolyzed by various proteinases, including MMP-318,19. Three forms of Fn found in plasma, cartilage, and synovial fluids, are all digestible with MMP-3 to similar sized Fn-fs possessing chondrolytic activity20. In our ex vivo cartilage impact model, we did observe greatly up-regulated MMP-3 by blunt impact as early as Day 1 and the increase of MMP-3 was statistically significant at Day 3. More convincingly, at Day 7, we observed even greater MMP-3 up-regulation in the adjacent area to the impact zone, which coincided with the spread of Fn fragmentation. The up-regulation of MMP-3 in the early phase of injury might be the reaction of chondrocytes and osteoblasts21 to the mechanical insult and catabolic factors released from necrotic cells. With the accumulation of Fn-fs, MMP-3 was probably mainly induced by those degradation products of Fn9,22.
Besides MMP-3, another matrix proteinase, ADAM-8, has been demonstrated to cleave Fn into active Fn-fs23. However, we found that the lower energy impact had minimal effect on up-regulating ADAM-8 while the higher energy impact increased its release by less than 2 fold (P=0.194; Supplementary Fig. 5 A–C). This may suggest that MMP-3, not ADAM-8, plays a critical role in Fn fragmentation observed in our ex vivo impact model.
MMP-3 not only degrades Fn but also cleaves the core protein of aggrecan at Asn341 –Phe342 of the G1 domain to generate a new C-terminus (…DIPEN) 24,25. Our data showed that MMP-3 expression was more up-regulated by soluble factors released from the lower-energy-impacted cartilage than from the higher-energy-impacted one. This may partially explain why in our ex vivo impact model the lower energy impact caused more cartilage PG depletion. The critical role of MMP-3 in impact induced PG depletion was strengthened by the observation that the up-regulation of MMP-1 and ADAMTS-5, other two major proteases involved in PG cleavage, was insignificant by either impact energy on cartilage compared to non-impacted controls (Supplementary Fig. 6 &7).
In summary, the results obtained from this ex vivo impact model supported our hypothesis that a blunt impact on cartilage activates Fn fragmentation and MMP-3 which might be a major force driving the generation of Fn-fs in PTOA. Our findings also suggest that Fn-fs and MMP-3 form a positive feed-back loop which may promote sustained cartilage destruction after a single mechanical insult. An early intervention attempting to attenuate this loop may limit even block the propagation of cartilage degeneration inside a traumatized joint. For instance, application of highly selective MMP-3 inhibitors26 to cartilage in the acute phase of joint trauma may alleviate cartilage loss therefore prevent the early onset of OA.
Nonetheless, our ex vivo impact model focuses on the responses to blunt trauma from cartilage and subchondral bone. Since PTOA is an organ disease, other soft tissues inside the joint and immune cells and complements from blood may as well participate in the pathogenesis of cartilage breakdown. Their possible roles may involve the initiation of the catabolic chain reactions to the mechanical insults via up-regulating the expression of catabolic cytokines and MMPs from chondrocytes, synoviocytes, and macrophages, which in turn activates Fn-f pathway to magnify cartilage destruction inside a traumatized joint.
Supplementary Material
Acknowledgments
Supported by US DHHS, National Institutes of Health/NIAMS grant #1 P50 AR055533 and by a Merit Review Award from the Department of Veterans Affairs. We thank Dr. Yubo Gao for statistical assistance and thank John Bierman, Barbara Laughlin, Abigail Lehman, Kee Woong Jang, Theresa Messlein, and Lois Lembke for preparing osteochondral explants, collecting some medium samples, and ordering reagents.
Footnotes
The authors have nothing to disclose.
References
- 1.Martin JA, Brown T, Heiner A, et al. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41(3–4):479–91. [PubMed] [Google Scholar]
- 2.Buckwalter JA, Brown TD. Joint injury, repair and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;(423):7–16. [PubMed] [Google Scholar]
- 3.Wurster NB, Lust G. Fibronectin in osteoarthritic canine articular cartilage. Biochem Biophys Res Commun. 1982;109(4):1094–101. doi: 10.1016/0006-291x(82)91889-7. [DOI] [PubMed] [Google Scholar]
- 4.Carnemolla B, Cutolo M, Castellani P, et al. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984;27(8):913–21. doi: 10.1002/art.1780270811. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths AM, Herbert KE, Perrett D, et al. Fragmented fibronectin and other synovial fluid proteins in chronic arthritis: their relation to immune complexes. Clin Chim Acta. 1989;184(2):133–46. doi: 10.1016/0009-8981(89)90283-0. [DOI] [PubMed] [Google Scholar]
- 6.Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J Rheumatol. 1992;19(9):1448–52. [PubMed] [Google Scholar]
- 7.Xie D, Homandberg GA. Fibronectin fragments bind to and penetrate cartilage tissue resulting in proteinase expression and cartilage damage. Biochim Biophys Acta 8. 1993;1182(2):189–96. doi: 10.1016/0925-4439(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 8.Homandberg GA, Kang Y, Zhang J, et al. A single injection of fibronectin fragments into rabbit knee joints enhances catabolism in the articular cartilage followed by reparative responses but also induces systemic effects in the non-injected knee joints. Osteoarthritis Cartilage. 2001;9(8):673–83. doi: 10.1053/joca.2001.0419. [DOI] [PubMed] [Google Scholar]
- 9.Xie DL, Hui F, Meyers R, et al. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch Biochem Biophys. 1994;311(2):205–12. doi: 10.1006/abbi.1994.1228. [DOI] [PubMed] [Google Scholar]
- 10.Ding L, Guo D, Homandberg GA. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage. 2008;16(10):1253–62. doi: 10.1016/j.joca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Homandberg GA, Hui F, Wen C, et al. Fibronectin-fragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem J. 1997;321(Pt 3):751–7. doi: 10.1042/bj3210751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homandberg GA, Costa V, Wen C. Fibronectin fragments active in chondrocytic chondrolysis can be chemically cross-linked to the alpha5 integrin receptor subunit. Osteoarthritis Cartilage. 2002;10(12):938–49. doi: 10.1053/joca.2002.0854. [DOI] [PubMed] [Google Scholar]
- 13.Homandberg GA, Costa V, Ummadi V, et al. Antisense oligonucleotides to the integrin receptor subunit alpha(5) decrease fibronectin fragment mediated cartilage chondrolysis. Osteoarthritis Cartilage. 2002;10(5):381–93. doi: 10.1053/joca.2002.0524. [DOI] [PubMed] [Google Scholar]
- 14.Ding L, Guo D, Homandberg GA. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-src, NF-kappaB and protein kinase Cdelta. Osteoarthritis Cartilage. 2009;17(10):1385–92. doi: 10.1016/j.joca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Ding L, Heying E, Nicholson N, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18(11):1509–17. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JA, McCabe D, Walter M, et al. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–7. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farquhar T, Xia Y, Mann K, et al. Swelling and fibronectin accumulation in articular cartilage explants after cyclical impact. J Orthop Res. 1996;14(3):417–23. doi: 10.1002/jor.1100140312. [DOI] [PubMed] [Google Scholar]
- 18.Okada Y, Nagase H, Harris ED., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986;261(30):14245–55. [PubMed] [Google Scholar]
- 19.Murphy G, Cawston TE, Galloway WA, et al. Metalloproteinases from rabbit bone culture medium degrade types IV and V collagens, laminin and fibronectin. Biochem J. 1981;199(3):807–11. doi: 10.1042/bj1990807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6(4):231–44. doi: 10.1053/joca.1998.0116. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki K, Takagi M, Konttinen YT, et al. Upregulation of matrix metalloproteinase (MMP)-1 and its activator MMP-3 of human osteoblast by uniaxial cyclic stimulation. J Biomed Mater Res B Appl Biomater. 2007;80(2):491–8. doi: 10.1002/jbm.b.30622. [DOI] [PubMed] [Google Scholar]
- 22.Homandberg GA, Hui F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys. 1996;334(2):325–31. doi: 10.1006/abbi.1996.0461. [DOI] [PubMed] [Google Scholar]
- 23.Zack MD, Malfait AM, Skepner AP, et al. ADAM-8 isolated from human osteoarthritic chondrocytes cleaves fibronectin at Ala(271) Arthritis Rheum. 2009;60(9):2704–13. doi: 10.1002/art.24753. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CE, Caterson B, Fosang AJ, et al. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995;305(Pt 3):799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homandberg GA, Hui F. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch Biochem Biophys. 1996;334(2):325–31. doi: 10.1006/abbi.1996.0461. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock GA, Dack KN, Dickinson RP, et al. A novel series of highly selective inhibitors of MMP-3. Bioorg Med Chem Lett. 2007;17(24):6750–3. doi: 10.1016/j.bmcl.2007.10.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.