Abstract
Despite significant advances in biology and medicine, the incidence and mortality due to breast cancer world-wide is still unacceptably high. Thus, there is an urgent need to discover new molecular targets. In this paper, we show evidence for a novel target in human breast cancer, the tetraspan protein epithelial membrane protein-2 (EMP2). Using tissue tumor arrays, protein expression of EMP2 was measured and found to be minimal in normal mammary tissue, but it was upregulated in 63% of invasive breast cancer tumors and in 73% of triple negative tumors tested. To test the hypothesis that EMP2 may be a suitable target for therapy, we constructed a fully human IgG1 antibody specific for a conserved domain of human and murine EMP2. Treatment of breast cancer cells with the anti-EMP2 IgG1 significantly inhibited EMP2 mediated signaling, blocked FAK/Src signaling, inhibited invasion, and promoted apoptosis in vitro. In both human xenograft and syngeneic metastatic tumor monotherapy models, anti-EMP2 IgG1 retarded tumor growth without detectable systemic toxicity. This anti-tumor effect was in part attributable to a potent ADCC response as well as direct cytotoxicity induced by the monoclonal antibody. Together, these results identify EMP2 as a novel therapeutic target for invasive breast cancer.
Keywords: Epithelial membrane protein-2, breast cancer, triple negative breast cancer, antibody therapy, recombinant IgG1, tumor xenograft
Introduction
Over the last decade, several advances have been made in the diagnosis and treatment of breast cancer centered on the expression of specific molecular markers. Breast cancer is now divided into three major subtypes: tumors expressing estrogen receptors (ERs) and/or progesterone receptors (PRs) (commonly referred to as hormone receptor–positive [HR-positive]), ERBB2-amplified (also known as human epidermal receptor 2–amplified [HER2-amplified]), and tumors lacking the expression of ERs and PRs and normal or negative HER2 expression termed triple-negative breast cancer (TNBC) (1–4). While this grouping has led to targeted therapies for some subtypes, in particular hormone receptor positive and HER2 positive tumors, breast cancer still remains the most common malignancy and second deadliest among women worldwide (5). It is currently estimated that up to 30% of women with invasive breast cancer will eventually die from it necessitating the need to identify new markers for therapy (6).
Recently, epithelial membrane protein-2 (EMP2) was identified as a novel prognostic indicator in a number of gynecological cancers (7–9). Belonging to the growth arrest specific-3 (GAS3) family of tetraspan proteins, elevated EMP2 levels have been observed in advanced ovarian and endometrial tumors (7, 8), and its expression inversely correlated with endometrial cancer patient survival (9). This effect could be recapitulated through xenograft modeling as endometrial tumors where EMP2 expression was increased showed enhanced tumor growth (10). Furthermore, these tumors showed an addiction to EMP2 expression as they failed to efficiently form when EMP2 levels were suppressed (10, 11). While limited information is known about its direct cellular function, previous studies have implicated EMP2 in the activation of focal adhesion kinase (FAK) and Src kinase in a number of cell types (12–15). Consistent with this putative mechanism, increased EMP2 levels correlated with an increase in cellular migration as well as neoangiogenesis in a FAK and Src dependent manner (10, 11).
Recent studies examining global gene signatures identified upregulation of EMP2 mRNA in breast cancers provided the first hint of dysregulated EMP2 expression in this malignancy (16–18). These studies suggested that EMP2 message positively correlated with advanced disease as well as identified circulating breast tumor cells (17, 19). In the present study, we examined the protein expression and frequency of EMP2 in human invasive breast cancers with an emphasis on triple negative disease. Given its high membrane expression, we hypothesized that EMP2 may serve as novel target for therapy, and consistent with this idea, anti-EMP2 antibody fragments (diabodies) resulted in reduced tumor growth in both endometrial and ovarian cancer models (7, 20). To further the use of anti-EMP2 therapy, we created a novel fully human anti-EMP2 IgG1 antibody and characterized its therapeutic potential. Collectively, our data suggest that anti-EMP2 therapy may be a first in class antibody to potentially treat aggressive breast cancers.
Materials and Methods
Tissue Microarray
Tumor samples were collected as approved and monitored by the UCLA Institutional Review Board, and appropriate permission has been granted for use of the de-identified clinical data. A breast cancer tissue array (TMA) was constructed using archival breast tissue samples from 212 patients who had breast surgery at the UCLA Medical Center between 1995 – 2000 as previously described (21, 22). The samples examined were histopathologies from women who underwent surgery for breast cancer. In this study, 74 cases were examined (Supplemental Table 1) of which on average, each histopathology was represented by at least three cores. Some cases contained multiple histopathologies. For this study, we focused on the categories of normal glandular/ductal epithelium (n=139 spots), ductal hyperplasia (DH; n=35 spots), ductal carcinoma in situ (DCIS; n = 142 spots), invasive ductal carcinoma (IDC; n = 236 spots), and lymph node metastatic lesions (n = 69 spots). The TMA was evaluated for EMP2 expression as described below. A blinded semiquantitative integrated scoring of the intensity and frequency of EMP2 staining was performed by two independent pathologists (RAS, YE) as described previously (21, 22). The following formula (H score) was used to derive the integrated value: [3(%a) + 2(%b) + 1(%c]/100, where a, b, and c is the percentage of cells staining at intensity 3, 2, and 1, respectively. Statistical analyses have been described previously and are presented below (21, 22).
Additional TNBC Cases
Twenty-three TNBC tumors from an additional 23 individuals were stained for EMP2 as detailed below. Tumors were characterized as i) no expression / below the level of detection (H score=0); ii) weak expression (1≤H score>0); and iii) strong expression (H score≥2).
Construction and expression of anti-EMP2 IgG1
We have previously described the construction of anti-EMP2 diabodies (Dbs) KS83 and KS49. For studies described here, we constructed a fully human anti-EMP2 IgG1 antibody. To do this, the Db variable (V) region sequences of KS49 were obtained by PCR (20) and then cloned into the pCR-II-TOPO vector (Life Technologies, Carlsbad, CA). The cloning was confirmed by sequencing.
-
Variable Heavy (VH) sequence:
ATGGCCCAGGTGCAGCTGGTGGAGTCTGGGGGAGGCTTGGTCCAGCCTGGGGGGTCCCTGAGACTCTCCTGTGCAGCCTCTGGATTCACCTTCAGTAGCTATGCTATGCACTGGGTCCGCCAGGCTCCAGGCAAGGGGCTGGAGTGGGTGGCAGTTATATCATATGATGGAAGCAATAAATACTACGCAGACTCCGTGAAGGGCCGATTCACCATCTCCAGAGACAATTCCAAGAACACGCTGTATCTGCAAATGAACAGCCTGAGAGCTGAGGACACGGCTGTGTATTACTGTGCCCGAACAGTGGGAGCTACTGGAGCTTTTGATATCTGGGGCCAAGGGACAATGGTCACCGTCTCG
-
Variable Light (VL) sequence:
GACATCGTGATGACCCAGTCTCCTTCCACCGTGTCTGCTTCTGTAGGAGACAGAGTCATCATCCCTTGCCGGGCCAGTCAGAGTATTGGTAAGTGGTTGGCCTGGTATCAGCAGAAACCAGGGAAAGCCCCAAAACTCCTGATCTATAAGGCGTCTAGTTTAGAAGGTTGGGTTCCATCAAGGTTCAGCGGCAGTGGGTCTGGGACAGAATTCTCTCTCACCATCAGTAGCCTGCAGCCTGACGATTCTGCAACTTATGTCTGTCAACAGTCTCACAATTTCCCTCCCACTTTCGGCGGAGGGACCAAGCTGGAGATCAAACGTGCGGCCGCAGAACAAAAACTCATCTCAGAAGAGGATCTGAATGGGGCCGCA
The sequence contains a functional signal peptide for proper secretion, and they were inserted into the κ light chain and γ1 heavy chain IgG1expression vector using ECORV and SalI or ECORV and NheI sites, respectively. These vectors contain the cytomegalovirus promoter (CMV) and have been shown to secrete functional recombinant antibodies in murine myeloma cells (23). Both expression vectors were provided by Dr. Sheri Morrison (University of California, Los Angeles). The heavy and light chain expression vectors were transfected into CHO-K1 cells as described previously (24). Cells were then screened by ELISA (described below) using goat anti-human IgG (Life Technologies) and goat anti-human κ chain (Sigma-Aldrich). The five highest producing subclones were isolated to use for S-35 biosynthetic antibody labeling and immunoprecipitation with hyperimmune rabbit antihuman IgG (Sigma-Aldrich) and Staph A (IgGSorb, The Enzyme Center, Malden, MA), to validate and select the optimal clone. The best producer was expanded into roller bottles to maximize the secretion of antibodies (25). After 2–3 weeks, supernatants were collected and filtered for purification as below.
Supernatants were passed over a 1.5 mL volume FlexColumn (Thermo Fisher Scientific, Walnut, CA) with 1mL of protein A-Sepharose (Sigma-Aldrich), and bound proteins were eluted with 2 column volumes of 0.2 M citrate buffer (pH 4.5), 3 column volumes of 0.1 M glycine-HCl (pH 2.5) and 2 column volumes of 0.1 M glycine-HCl (pH 2.0), sequentially. The eluted fractions containing the desired antibodies were dialyzed against PBS with Slide-A-Lyzer Dialysis Cassettes (Thermo Fisher Scientific). The final concentration of purified antibodies was measured with Nanodrop 2000 (Thermo Scientific).
Enzyme linked immunosorbent assay (ELISA)
Biotinylated 24 amino acid peptides corresponding to the extracellular loop of human EMP2 (20) were coated onto streptavidin-coated 96-well plates (Roche Applied Science, Indianapolis, IN). ELISA was performed as described previously (20). Specifically, bound antibodies were detected with HRP conjugated goat anti-human IgG (Jackson Immunoresearch, West Grove, PA) and TMB solution (eBioscience, San Diego, CA). Absorbance at 450 nm was determined using a microplate reader Model 550 (Bio-Rad, Hercules, CA).
Flow cytometry
EMP2-positive cells (HEC1a/EMP2, HEC1a,(15) or 4T1 cells) or EMP2-negative cells (EL4 or Ramos) were resuspended at a concentration of 106 cells in 1 mL of cold PBS+0.2% BSA buffer (flow buffer). The cell suspension was centrifuged for 5 min at 500g, at 4°C. Cells were then incubated with 1 µg of recombinant anti-EMP2 IgG1 for 2 h at 4°C. An IgG specific for the hapten dansyl, 5-dimethylamino naphthalene-1-sulfonyl chloride (DNS) was used as a non-targeted antibody negative control (26). Cells were washed three times and then incubated for 30min at 4°C with PE-conjugated goat anti-human IgG (Jackson Immunoresearch). Cells were washed and resuspended in flow buffer. Flow cytometry was immediately performed with a Becton Dickinson FACScan Analytic Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ) in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility.
Immunohistochemistry
Immunohistochemistry for human EMP2 expression has been described previously (9). Briefly, following antigen retrieval, slides were incubated with rabbit anti-human EMP2 antisera (1:400) for 1 hour. In some experiments, the anti-EMP2 IgG1 was used to detect EMP2 in normal and tumor tissue; a detailed protocol is provided in the Supplementary Material. Samples were then incubated with biotinylated goat anti-rabbit secondary antibody and then streptavidin horseradish peroxidase from the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). The antibody was detected using the Vector Laboratories DAB substrate kit (Vector Laboratories) following the manufacturer’s protocol. Negative controls included pre-immune serum incubation.
Cell lines and cell culture
Human breast cancer cell lines HS578t, BT-474, SK-BR-3, MCF7, UACC812, BT-20, MDA-MB-231, ZR-75-1 and MDA-MB-468 (American Type Culture Collection; ATCC, Manassas, VA) were cultivated in DMEM medium (Mediatech, Manassas, VA) supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 U/ml streptomycin (all from Life Technologies Life Technologies, Carlsbad, CA). All cell lines were used within 6 months of resuscitation and were characterized by the cell bank. Cells were cultured at 37°C in a humidified 5% CO2. 4T1 cells, a spontaneous mammary tumor syngeneic in BALB/c mice, were obtained from ATCC. 4T1 cells were maintained in RPMI medium supplemented as above, and the cells were infected with firefly luciferase (FLUC) by the JCCC Viral Vector Core Lab. Additionally, HS578t, MDA-MB-231, MDA-MB-468, and SK-BR-3 sublines were prepared which overexpress a EMP2 have been previously described (15). Cell lines bearing an empty expression vector were also produced as described (15). Cell lines were also prepared with reduced EMP2 expression through stable infection using an EMP2 specific shRNA (TRCN0000322911) in pLKO.1-puro (Sigma-Aldrich, St. Louis, MO). Stably infected breast cancer cells containing a non-targeting shRNA control were also created. Cell lines were used within 6 months after selection. Western blot analysis was used to confirm the EMP2 expression levels in each cell line (see below). Cell lines were passaged in our laboratory for less than 6 months.
Western blot analysis
Preparation of breast cancer cell lines or tissue lysates for western blotting has been described previously (27). Cells were lysed in Laemmli buffer, and for EMP2 detection, were treated with N-glycosidase F (New England Biolabs, Beverly, MA) to remove N-link glycosylation (15). Proteins were separated using SDS-PAGE, transferred onto nitrocellulose membrane and blocked in 10% nonfat dry milk in TBS-Tween-20 buffer. Blots were probed using rabbit anti-human EMP2 antisera (1:2000) (28). Proteins were then detected by using a horseradish peroxidase conjugated goat anti-rabbit antibody. As a loading control, β-actin expression was detected using primary monoclonal anti-β actin (Sigma) and secondary horseradish peroxidase-conjugated goat anti-mouse IgG (Amersham, Piscataway, NJ). Bands were visualized using ECL detection reagents (Amersham).
In some experiments, MDA-MB-468 cells were treated for two hours with 100µg/ml of anti-EMP2 IgG1 or control IgG. Cells were then plated to activate FAK and Src and then lysed after 12 hrs (29, 30). Separated proteins were probed using anti-576/577p-FAK (Santa Cruz Biotechnology), anti- total FAK (BD Biosciences), anti-416 p-Src (Cell Signaling, Danvers, MA), anti-total Src (Cell Signaling) or β-actin (Sigma-Aldrich).
Invasion assays
24 well plates with transwell inserts were used to perform the in vitro cell invasion assays. Equivalent numbers (5×103 cells) of MDA-MB-231 breast cancer cells with modified EMP2 levels were added to the top chamber of the transwell, and complete DMEM medium was added to the bottom of the well. Cells were allowed to invade for 6 h at 37°C. The filters were then fixed and stained with 0.1% crystal violet. The invasive cells were visualized using bright-field microscopy. Cells were enumerated by counting 4 random fields per transwell. The experiment was repeated three times, with the data averaged. In some experiments, cells were pretreated with anti-EMP2 IgG1 or control antibodies for 2 hours at 4C.
Antibody-dependent cell-mediated cytotoxicity (ADCC) assays
Peripheral blood mononuclear cells (PBMCs) were isolated from blood using Ficoll-Paque plus (GE Healthcare, Pittsburg, PA) from three volunteers. Blood donors had given informed consent before for obtaining a peripheral venous blood sample for PBMC assays. These experiments were done according to the rules of the Ethical Committee of University of California, Los Angeles. PBMCs were resuspended in DMEM with 10% FCS. SK-BR-3 cells were initially labeled using PKH67 fluorescent dye (Sigma Aldrich) and then plated in 6 well plates. Cells were pre-incubated with anti-EMP2 IgG1 or trastuzumab (anti-HER2/neu; Genentech, San Francisco, CA) as a positive control overnight and then incubated with different ratios of PBMCs for 4–8 hours at 37°C. The percentage of cell death was quantitated by propidium iodide staining using a Becton Dickinson FACScan Analytic Flow Cytometer (Becton Dickinson) at the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility. Experiments were performed in duplicates, normalized to an untreated negative control, and then averaged.
Viability assays
Cells (5 × 104) were placed in triplicate in a 6-well plate (Becton Dickinson) and incubated with anti-EMP2 Db KS83(15, 20), control DbA10(20), anti-EMP2 IgG1, control IgG (Sigma-Aldrich), or a saline control as indicated in the figure legends for 24–96 hours. Cells were then harvested, and the number of viable cells relative to the initial plating (% growth) was determined using a trypan blue exclusion assay. To assess whether resultant cell death was due to apoptosis, cells were harvested and stained with an Annexin V-FITC detection kit according to manufacturer’s instructions (BD Biosciences). Flow cytometry was performed as above.
In order to confirm cell viability on a larger panel of cells, the CellTiter-Glo Luminescent Cell Viability Assay (Promega) was performed according to manufacturer instructions. Cells (5×103) were seeded in 96 well plates and then treated with 0–250 µg/ml anti-EMP2 IgG1 or control antibodies for 3–5 days. The luminescence was quantitated on a Microplate Reader (FLUOstar OPTIMA).
Pharmacokinetic analysis
In order to determine the half life of the anti-EMP2 IgG1 in mice, pharmacokinetic studies were performed. A detailed protocol is described in the Supplementary Methods.
In vivo toxicity
All mouse experiments were performed under protocols approved by the Chancellor’s Animal Research Committee at UCLA, and animals were maintained in accordance with the National Academy of Science Guide for the Care and Use of Laboratory Animals in the Vivarium of UCLA. We tested for potential systemic toxicity by recombinant anti-EMP2 IgG1 in 7-week-old female wild-type (C57BL/6) mice obtained from Jackson Laboratories. At least three animals per group were injected intraperitoneal (i.p.) weekly with 10 mg/kg of anti-EMP2 IgG1 antibody or a control IgG for 7 weeks. In a second experiment, 3 mice per group were treated with sequentially increasing concentrations of antibody beginning at 10mg/kg, then 20mg/kg, and finally 40mg/kg twice a week. Weight was measured weekly. At the end of the time course, mice were euthanized by cervical dislocation. Tissues (kidney, liver, spleen, lung, uterus, heart, ovary, and skin) were collected fixed in formalin, processed, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and analyzed for pathological changes by a pathologist (JB). Complete blood counts and liver enzyme analysis (serum alanine aminotransferase, direct and total bilirubin) were quantified by the UCLA Medical Center Clinical Laboratories.
Mouse xenograft model
To create tumor xenograft models, 4–6-week old female BALB/c nude mice (Charles River, MA) were used for each condition. Briefly, 5×106 MDA-MB-468, 2×106 MDA-MD-231, or 2×107 Ramos cells were suspended in 5% matrigel (BD Biosciences) and injected subcutaneously (s.c.) into the shoulder of female athymic mice. Tumor volume was calculated with the formula: length × width2/2. When tumors reached 4mm3, they were injected intratumor (i.t.) with 1 mg/kg dose of anti-EMP2Db KS83or control Db twice a week as described previously (20). Alternatively, tumors were injected with 3 mg/kg i.t. or between 1–10 mg/kg systemically with anti-EMP2 IgG1 or control IgG (Sigma) weekly as indicated in the figure legends. At the end of each experiment, tumors were isolated, fixed and processed for hematoxylin and eosin staining as previously described (9).
Mouse metastatic model
To create a metastatic model for breast cancer, the spontaneous murine mammary tumor line 4T1 was utilized. 1×1044T1-FLUC cells were injected into the tail vein of BALB/c mice (Charles River), using 9 mice per group. Prior to treatment, the presence of tumors were validated using bioiluminescence. Mice were then treated systemically twice, beginning at day 5, with 10mg/kg anti-EMP2 IgG1 or control IgG. For bioluminescence imaging, mice received an intraperitoneal injection of 150 µl D-luciferin (30 mg/ml). Fifteen minutes after the injection of D-luciferin, the mice were anesthetized with isoflurane/oxygen and placed on the imaging stage. The bioluminescence signals were monitored using an IVIS-200 (Xenogen Corp., Alameda, CA, USA). The data were analyzed using the maximum photon flux emission (photons/second) in the regions of interest. After the final point, mice were euthanized and lungs isolated as above.
Statistical analysis
TMA analyses were performed as previously described (21, 31–34) using the Mann-Whitney test for two-group comparisons. A P value < 0.05 was considered significant. Differences in in vitro phenotypic changes or in vivo tumor growth were evaluated using a two-tailed Student’s unpaired t-test at a 95% confidence level (GraphPad Prism version 3.0; GraphPad Software, La Jolla, CA). P values <0.05 were considered significant.
Supplementary data
The supplementary data include two supplementary figures and five supplementary tables.
Results
EMP2 is upregulated in breast cancers
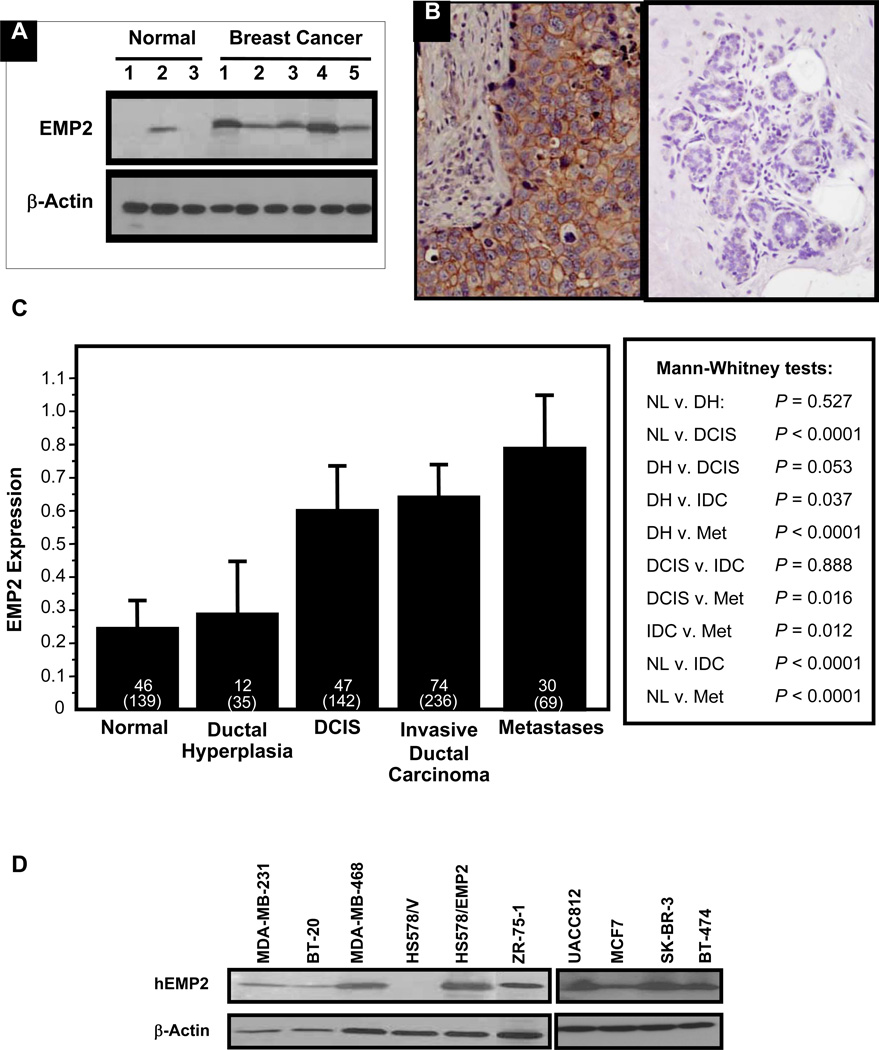
Numerous microarray studies have identified EMP2 mRNA as upregulated in breast cancer (18, 35, 36), and several studies have correlated its expression with advanced disease and metastasis (17, 19). Supplementary Table 1 provides a summary of these studies. However, to date, its protein expression has not been determined in breast cancer. Initially, the protein expression of EMP2 was quantitated by Western blot analysis and immunohistochemistry (IHC) in a small set of 5 flash frozen human invasive ductal carcinoma samples and 3 samples of normal glandular breast epithelium. As shown in Figure 1A, expression of EMP2 (18 kD) was elevated in breast cancer samples compared to normal tissue. To extend and validate these findings, the protein localization of EMP2 was assessed using IHC. Within malignant epithelium, EMP2 was predominantly present at the membrane and/or cytoplasm while minimal/undetectable levels were observed in normal epithelium (Figure 1B).
Figure 1. EMP2 expression is stratified by histologic type and stage.
(A) Western blot analysis was performed on whole tissue homogenates from normal and tumor regions of the breast. (B) Normal and breast cancer tissue were stained for EMP2 expression. Left, A representative patient with high EMP2 staining within a tumor is shown; Right, Representative staining in normal breast. (C) The mean integrated intensity of EMP2 protein expression for each category is shown using bar plots. The error bars represent the standard error of the mean. The top number represents the number of patients and the bottom number in parenthesis represents the total number of spots tested. (D) Expression of EMP2 in a panel of breast cancer cell lines was evaluated by Western blot analysis. HS578/EMP2 cells were generated to stably overexpress EMP2.
We further examined the expression profile of EMP2 in human breast cancer on a population basis using tissue microarray (TMA) technology. The demographic, clinical, and pathology characteristics are shown in Supplementary Table 2 and have been described previously (31–33). Compared to non-malignant glandular and ductal mammary epithelium there was a significant increase in EMP2 expression through disease progression. A significant increase in EMP2 expression was observed during stepwise progression of disease from normal to ductal carcinoma in situ (DCIS) (p<0.0001) as well as between invasive carcinoma and lymph node metastasis (p=0.012; Figure 1C). Within these groups, EMP2 expression was detectable in 63% of samples with invasive carcinoma (n=74) with no correlation observed between EMP2 and hormone status or HER2/neu expression. EMP2 seemingly correlated with tumor progression as EMP2was expressed in 67% of lymph node metastatic lesions (n=30) with its expression correlating with lymphovascular invasion (n=13; p=0.021).
To further confirm EMP2 protein expression within breast tumors, its expression was determined in a panel of breast cancer cell lines (Figure 1D). EMP2 was expressed in 8 out of 9 human cell lines tested by western blot analysis with levels below detection in one cell line (HS578t). Within this panel of cell lines, EMP2 expression did not correlate with hormone and/or HER2/neu status as EMP2 expression was present in both triple negative (MDA-MB-231, MDA-MB-468, BT-20) and triple positive (ZR-75-1) cells. EMP2 also exhibited high expression in all HER2/neu positive cells tested (UACC812, SK-BR-3, BT-474).
As EMP2 was readily detectable within TNBC cell lines, its expression in TNBC tumors from the array was further considered. This group was of particular interest as TNBC is characterized by high recurrence, metastasis, and mortality rates (3, 37). Of the 11 TNBC patients, 8 (73%) had relatively high expression levels of EMP2 while 3 cases had low or non-detectable levels (not shown). To independently validate the expression of EMP2 in TNBC, we further examined its expression in an additional independent set of 23 cases. Concordant with the TMA, 17 of 23 cases (74%) were positive for EMP2 (H score≥1; see Supplementary Table 3).
Construction of the anti-EMP2 IgG1
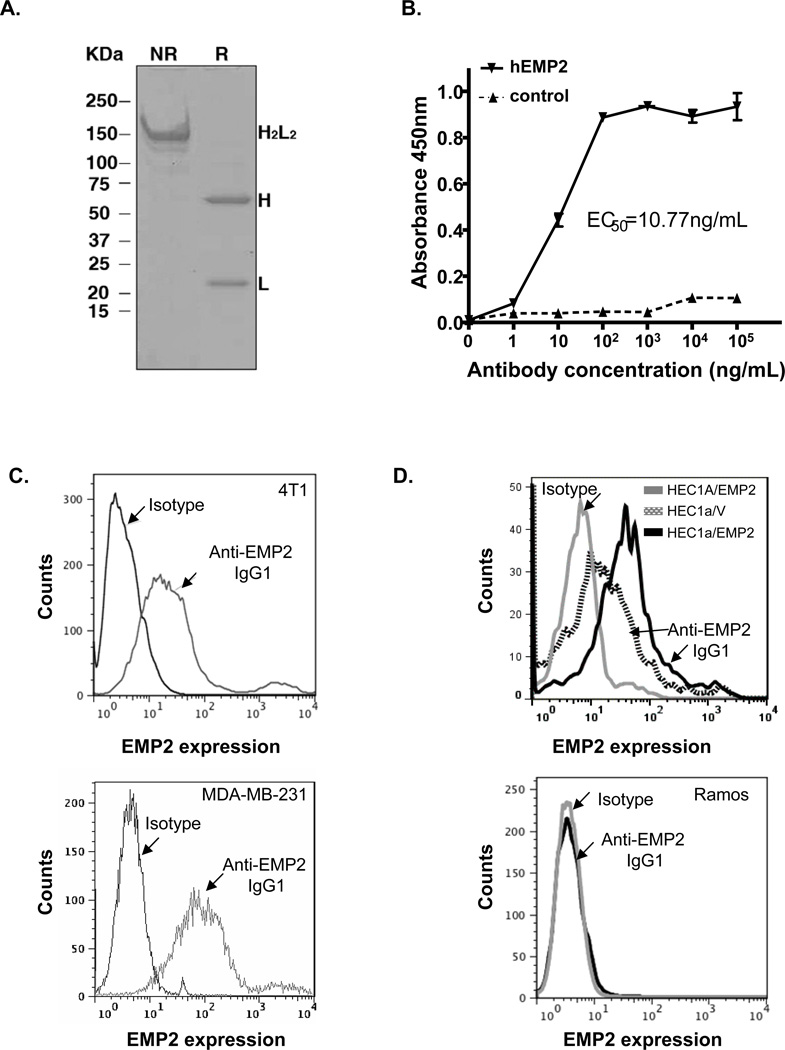
In prior studies, we demonstrated the efficacy of recombinant anti-EMP2 Db-mediated therapy in ovarian and endometrial cancers (7, 20). As EMP2 is widely expressed in breast cancer, we were prompted to assess the utility of targeting EMP2 in this malignancy as well. As Dbs tend to have a short half-life in vivo (T1/2 = 6 hours; (38), we constructed a fully human anti-EMP2 IgG1 antibody to determine if we could improve the therapeutic potential of targeting EMP2 using the VH and VL genes from the anti-EMP2 Db clone KS49 (20). Anti-EMP2 IgG1 antibody was validated through sequencing (data not shown) and analyzed by SDS-PAGE under non-reducing (NR) and reducing (R) conditions (Figure 2A).
Figure 2. Characterization of Recombinant Anti-EMP2 IgG1.
(A) The anti-EMP2 IgG1 antibody was analyzed by SDS-PAGE under non-reducing (NR) and reducing (R) conditions. The full antibody structure is visualized under non-reducing conditions through a band around 150 kDa. This corresponds to the expected molecular weight for the anti-EMP2 IgG1. Under reducing conditions both the heavy chain (~60 kDa) and light chain (~20 kDa) are visualized. (B) Binding of the anti-EMP2 IgG1 to a human EMP2 peptide by ELISA. The sensitivity was confirmed using flow cytometric analysis of the anti-EMP2 IgG1 binding to (C) Binding of anti-EMP2 IgG1 to both murine and human EMP2 by flow cytometry. Top panel, Murine EMP2 was detected on the surface of murine mammary tumors (4T1 cells) or on MDA-MB-231 TNBC cells (bottom panel). (D) The sensitivity and specificity of the anti-EMP2 IgG1 was determined by flow cytometry. Top panel, The sensitivity of the anti-EMP2 IgG1 to alternations in EMP2 expression was confirmed using HEC1a/EMP2 to endogenous levels of EMP2 (HEC1a/V). Bottom panel, The specificity was confirmed using an EMP2 negative cell line, human Ramos lymphoma cells.
To characterize the specificity of theanti-EMP2 IgG1, its binding to an EMP2 peptide as well as to native protein was measured. Using a human EMP2 peptide, serial dilutions of the native antibody revealed an EC50 of 10.8 ng/ml (Figure 2B). To further determine the binding characteristics of the anti-EMP2 IgG1, its binding to native EMP2 was assessed using flow cytometry. The anti-EMP2 IgG1 recognized both murine EMP2 present on 4T1 cells (Figure 2C, top panel) and human EMP2 on the TNBC cell line MDA-MB-231 (Figure 2C, bottom panel). Finally, the sensitivity and specificity of the antibody was confirmed. Anti-EMP2 IgG1 detected a difference in surface expression between HEC-1A wild type and HEC-1A/EMP2 cells (Figure 2D, top panel), which express a two to four-fold increase in total EMP2 levels (39). Moreover, the binding of the antibody was specific as it did not bind the EMP2 negative cell line Ramos (Figure 2D, bottom panel).
To further confirm the binding specificity of the anti-EMP2 IgG1, the antibody was biotinylated and tested by immunohistochemistry on tissue with known EMP2 expression (40). The anti-EMP2 IgG1 antibody recognized EMP2 on normal lung alveolar cells as well as on breast tumors. This binding was specific as peptide from the second extracellular loop of EMP2 blocked antigen recognition (Supplementary Figure 1).
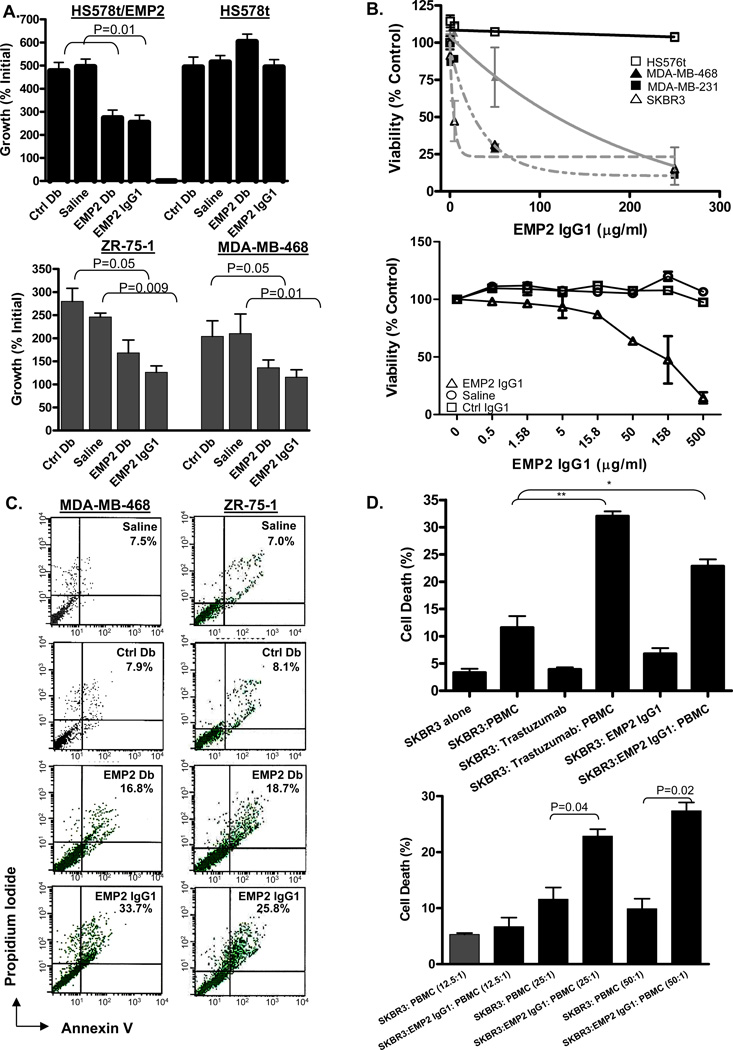
Anti-EMP2 treatment induces cell death in vitro
The therapeutic potential of the anti-EMP2 IgG1 was initially compared to the anti-EMP2 Db. ZR-75-1, MDA-MB-468, HS578/V (the HS578t EMP2-negative TNBC cell line bearing an empty expression vector) and HS578t/EMP2 (bearing an EMP2 expression vector) (15) were incubated in vitro with molar equivalent amounts of anti-EMP2 Db, non-immune Ctrl Db, anti-EMP2 IgG1, or a saline control for 72 hours as described previously and monitored for viability (7, 20). Treatment of ZR-75-1, MDA-MB-468, and HS578/EMP2 cells with either anti-EMP2 Db or anti-EMP2 IgG1 resulted in ~35%–50% loss of viability compared to the control Db or saline control (Figure 3A). Treatment of HS578/V (EMP2-negative) cells with the anti-EMP2 Db or anti-EMP2 IgG1 caused no loss in viability, indicating that the effect was dependent on EMP2 expression (Figure 3A).
Figure 3. Anti-EMP2 IgG1 antibodies and diabodies induce cell death.
(A) Top, HS578 cells do not express any endogenous EMP2 expression, and HS578/EMP2 cells were engineered to overexpress EMP2. 60 µg/ml anti-EMP2 IgG1, 20 µg/ml anti-EMP2Db, control Db, or a vehicle control were added to the above cells for 72 hours. Cell growth, calculated as a percent of the initial cells plated, was determined using trypan blue exclusion, and values represent results from 3 independent experiments (±SEM). Bottom Panel, ZR-75-1 and MBA-MD-468 cells were treated for 72 hours as above to determine cell growth. Values are averages (±SEM, n=3). (B) SK-BR-3, MDA-MB-468, MDA-MB-231, and HS578t cells (top panel) or 4T1 cells (bottom panel) were incubated with increasing concentrations of anti-EMP2 IgG1 or control antibodies for up to 5 days. Cellular viability was assessed by the CellTiter-Glo Luminescent Cell Viability Assay. (C) EMP2 diabodies and IgG1 promote apoptosis. ZR-75-1 (top panels) and MBA-MD-468 (bottom panels) cells were incubated with 20 µg/mL anti-EMP2 Db, 20 µg/mL control Db, 60 µg/ml anti-EMP2 IgG1, or 60 µg/mL control IgG. Cells were washed and stained with Annexin V and propidium iodide. Staining is expressed as the % Annexin V, propidium iodide positive cells above the isotype control. The experiment was repeated three times with similar results. A representative graph is shown. (D) In vitro anti-EMP2 IgG1 mediated ADCC against SK-BR-3 tumor cells. Top, SK-BR-3 cells were labeled with PKH67. Cells were mixed with freshly isolated PBMCs and/or 100µg/ml trastuzumab or anti-EMP2 IgG1 (EMP2) at an effector/target ratio of 25:1. Bottom, SK-BR-3 cells were labeled with PKH67 and mixed at effector/target ratios of 12.5:1, 25:1, and 50:1 and/or 100 µg/ml anti-EMP2 IgG1. The percentage of killed cells was calculated as described in the Materials and Methods.
To determine the sensitivity of a panel of breast cancer cells to EMP2 treatment, cells were treated with varying concentrations of anti-EMP2 IgG1 or control IgG (Figure 3B). A dose-dependent reduction in cell viability was observed after 4 days in SK-BR-3, MDA-MB-231, and MDA-MB-468 cells. The respective sensitivity (IC50) of the cell lines to anti-EMP2 IgG1 ranged from 2µg/ml to 140µg/ml for SK-BR-3 and MDA-MB-468, respectively. Importantly, no change in cellular viability was measured in EMP2-negative HS578t cells even at high concentrations of the antibody. As anti-EMP2 IgG1 recognized murine EMP2, we determined if the antibody could elicit a similar response in the 4T1 mouse mammary tumor line. 4T1 cells were susceptible to anti-EMP2 IgG1 with an IC50 of 108µg/ml.
To determine if cell death occurred via apoptosis, cells were incubated with anti-EMP2Db or IgG1 (or appropriate controls) and then stained for Annexin V and by propidium iodide. As shown in Figure 2C, incubation of ZR-75-1 or MDA-MB-468 cells with anti-EMP2 Db or anti-EMP2 IgG1, induced apoptosis within 48 hours (Figure 3C). Such an effect was not seen with the corresponding negative controls.
Anti-EMP2 IgG1 mediates ADCC against breast cancer cells
While antibody treatment was able to directly induce apoptosis in EMP2-expressing breast cancer cells, we were curious as to whether such treatment might also elicit ADCC. We tested this effect in vitro using freshly isolated PBMCs and incubated them with the HER2-positive SK-BR-3 cell line. As shown in Figure 3D (top and bottom panel), anti-EMP2 IgG1 treatment elicited a significant increase in cell death compared to the PBMC treatment alone. This ADCC effect was similar to that produced with trastuzumab.
Anti-EMP2 antibodies reduce tumor growth in vivo
To translate our in vitro data, we initially performed pharmacokinetic and toxicity studies to estimate a dosing schedule. Using C57B/6 mice, serum concentrations of trastuzumab and anti-EMP2 IgG1 after a single dose of 10 mg/kg were similar when measured over 1 week. For both antibodies, <5% of the antibody remained in circulation after 7 days (Supplementary Figure 2). We previously have shown that weekly injections of EMP2Db demonstrated no detectable systemic toxicity or adverse host effects (7, 20). Likewise, upon extensive testing with weekly injections of the full length anti-EMP2 IgG1 in C57B/6 mice (10 mg/kg) for seven weeks or injections with increasing concentrations of antibody ranging from 10mg/kg to 40mg/kg, no indication of systemic or tissue-specific damage or toxicity was observed (Supplementary Table 4 and 5, respectively). Comparison of weight, tissue histology, and pharmacological parameters revealed no significant differences compared to control antibody treatment.
To determine the effectiveness of anti-EMP2 IgG1in vivo, two mouse systems, a xenograft model and an orthotopic model of breast cancer metastasis, were utilized.
Subcutaneous xenograft tumor model
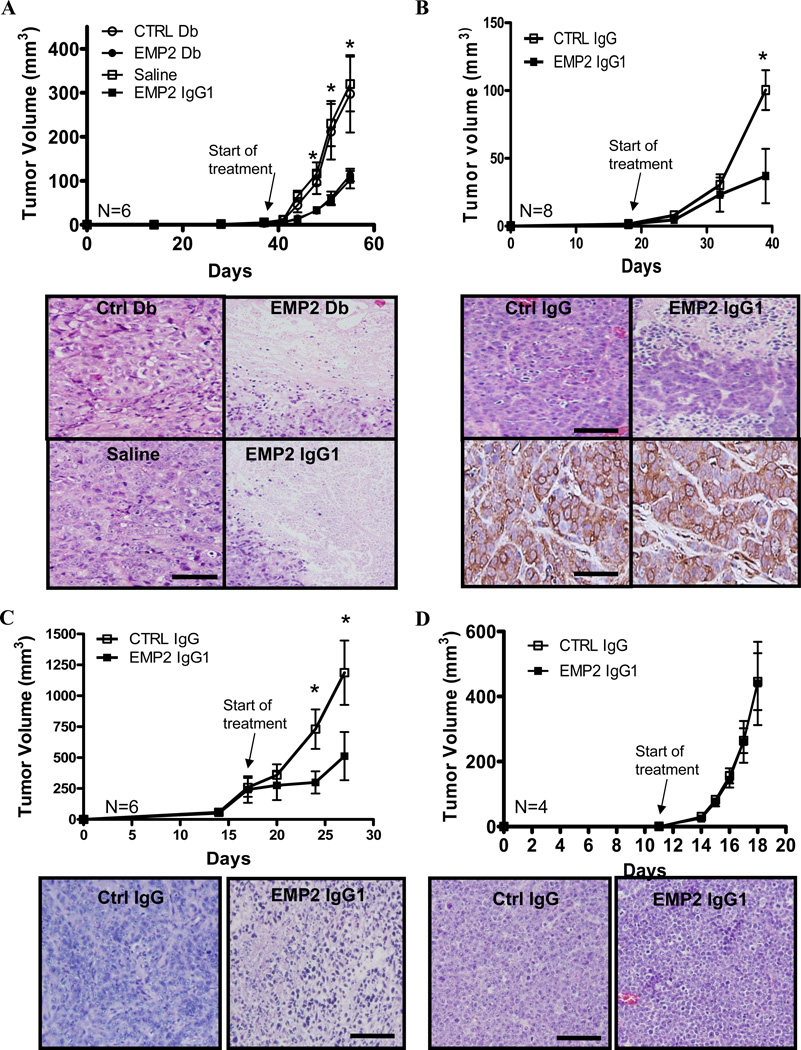
Two triple negative cell lines were tested for their susceptibility to anti-EMP2 treatment. Initially, we tested a xenograft model involving the triple-negative cell line, MDA-MB-468. Cells (2 × 106) were injected subcutaneously into nude BALB/c mice, and when tumors reached 4 mm2 in size, anti-EMP2 Db (1 mg/kg, 2× / week) or the anti-EMP2 IgG1 (3 mg/kg; 1×/week) were injected directly into xenografts. A negative control Db or saline were used as control treatments at the same dose and timing of treatment. As shown in Figure 4A, treatment of tumors with anti-EMP2 Db or anti-EMP2 IgG1 resulted in significant reduction of tumor growth by day 15 compared to controls. Upon histological examination, tumors treated with anti-EMP2 Db or IgG1had extensive areas of necrosis in contrast with tumors treated with non-immune reagents (Figure 4A, bottom panel). To confirm that the ability of anti-EMP2 IgG1 to limit tumor growth was not dependent on localized injection, tumors were created from the MDA-MB-468 cell line as above but treated systemically. Anti-EMP2 IgG1 reduced tumor size (Figure 4B) with extensive necrosis visible throughout the tumor (Figure 4B, bottom panel).
Figure 4. Targeting EMP2 reduces tumor load.
(A) MDA-MB-468 cells were injected s.c. into nude BALB/c female mice. Top panel, Treatments were started when tumors reached 4mm3 (Day 38). Tumors were injected i.t. with molar equivalent amounts of Db (1 mg/kg), IgG1 antibody (3 mg/kg), or the indicated controls. Mice were injected twice a week, and tumor volume was monitored using calipers. Tumor volume values are averages (±SEM, n = 6 per group) with anti-EMP2 Db and anti-EMP2 IgG1 treatment compared with control Db and sterile saline, respectively. At day 56, tumors were excised, formalin fixed, and paraffin embedded. Bottom panel, Tumor histology was assessed by hemotoxylin and eosin staining. A representative image is shown on the right. (B) MDA-MB-468 cells were injected into nude BALB/c mice as above. Top panel, tumors were treated when they reached 4mm3 (Day 18), and mice were systemically treated with 10mg/kg anti-EMP2 IgG1 or control IgG every week. Bottom panels, at day 38, tumor histology was assessed by hematoxylin and eosin staining as well as for residual EMP2 expression following treatment. N=6 per group. (C) MDA-MB-231 cells were injected into the mammary pad of BALB/c nude mice. When tumors reached ~250mm3 (Day 16), mice were systemically injected with 10 mg/kg anti-EMP2 IgG1 or control IgG twice weekly. Representative images of Day 27 tumors stained with hematoxylin and eosin, right. N = 6 per group. (D) Ramos cells were injected into s.c. into nude BALB/c mice. As tumors grow rapidly, they were treated systemically twice in the week (Day 0 and 3) with 10 mg/kg anti-EMP2 IgG1 or control IgG. Representative hematoxylin and eosin staining at day 28 is shown on the right. In all experiments: *, p<0.05, comparison by Student’s t-test; image magnification, 20×; scale bar, 100µm.
We also examined the efficacy of anti-EMP2 IgG1 treatment on larger tumors (~200mm3) derived from another triple negative breast cancer cell line, MDA-MB-231. Treatment with anti-EMP2 IgG1 significantly reduced tumor load by 50% compared to control IgG treatment (Figure 4C) with tumors exhibiting pronounced necrosis (Figure 4C, bottom panel). To validate that the effects of the anti-EMP2 IgG1 treatment were dependent on EMP2 expression, xenografts were created from the EMP2 negative B lymphoma cell line Ramos. Injection of anti-EMP2 IgG1 or controlIgG1 showed no difference in tumor load (Figure 4D) or in tumor histology (Figure 4D, bottom panel).
Metastastic tumor model
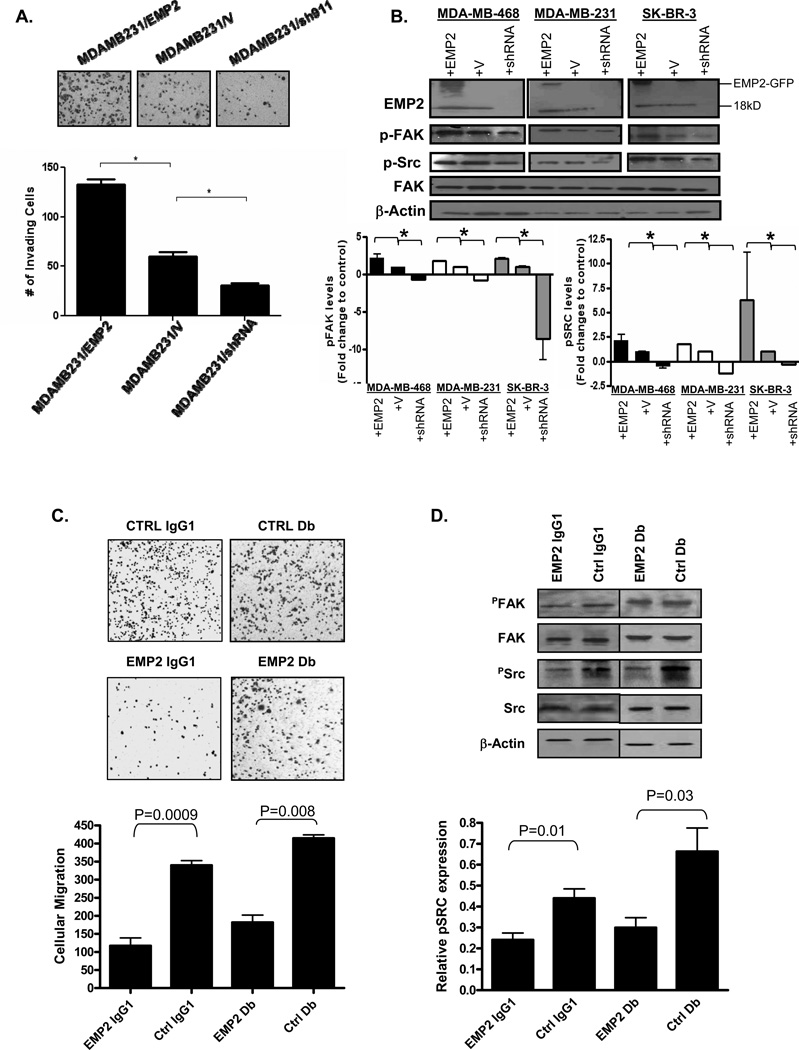
Ninety percent of cancer-related mortality is caused by metastases formed by disseminated primary tumor cells at distant anatomic sites (41). Previous studies have suggested that EMP2 expression promotes FAK and Src activation, resulting in an increase in cellular migration and invasion (11). To validate that a similar effect occurs in breast cancer, MDA-MB-231 breast cancer cells were engineered to overexpress EMP2 or express a shRNA vector to knockdown expression. Similar to that observed in other models, EMP2 expression augmented cell migration while shRNA knockdown reduced this effect (Figure 5A). To correlate this change in migration with FAK and Src activation, a panel of breast cancer cells were tested for FAK and Src activation 24 hours after plating. Consistent with effects seen in endometrial cancer, upregulation of EMP2 promoted a significant increase in the activation of FAK and Src while the reciprocal effects were observed when EMP2 expression was reduced (Figure 5B).
Figure 5. EMP2 promotes cellular migration andanti-EMP2 IgG1 antibodies and diabodies suppress this effect.
(A) MDA-MB-231 cells were engineered to overexpress EMP2 (MDAMB231/EMP2), express a vector control (MDAMB231/V) or reduce its expression via shRNA lentiviral vectors (MDAMB231/shRNA). Equivalent numbers of cells were plated into transwells and enumerated after 6 hours. The experiment was repeated three times and the results averaged. *, p<0.05 as determined by Student’s unpaired t-test. (B) MDA-MB-231, MDA-MB-468, and SK-BR-3 cells were engineered to overexpress or reduce EMP2 expression as above. Cells were plated for 12 hours and FAK and Src activation were assessed by western blot analysis. N=3; *, p<0.05, Student’s unpaired t-test. (C) 60 µg/ml anti-EMP2 IgG1 or control IgG or 20 µg/ml anti-EMP2 or control Db were added to MDA-MB-468 cells for 1 hr. The number of cells that migrated through the transwell was measured. Top panel: cells were visualized using crystal violet. Bottom panel: averaged number of migrated cells from 3 experiments. (D) 60 µg/ml anti-EMP2 IgG1 or control IgG was added to MDA-MB-468 cells for 1 hr. Alternatively, 20 µg/ml anti-EMP2 or control Db were added. Cells were then plated for 12 hrs and lysed. Proteins were separated by SDS-PAGE and probed using the indicated antibodies. The experiment was repeated three times, and representative image is shown. Bottom panel, Activated pSRC expression from three experiments was quantified using Image J, and the data was normalized relative to total Src levels. Treatment with anti-EMP2 IgG1 or anti-EMP2 Db significantly reduced pSRC expression. Values are averages (±SEM).
In order to determine if EMP2 antibodies inhibit migrationin vitro, MDA-MB-231 cells were treated with non-toxic doses of anti-EMP2 Db or IgG1 with their appropriate antibody controls. Two hour treatment with either anti-EMP2 Db or IgG1 inhibited transwell migration compared to the controls (Figure 5C). When the mechanism behind this action was investigated, we observed that anti-EMP2 Db or IgG1 significantly reduced Src phosphorylation (Figure 5D). A modest reduction in FAK phosphorylation was also observed, but this effect was not significant (data not shown).
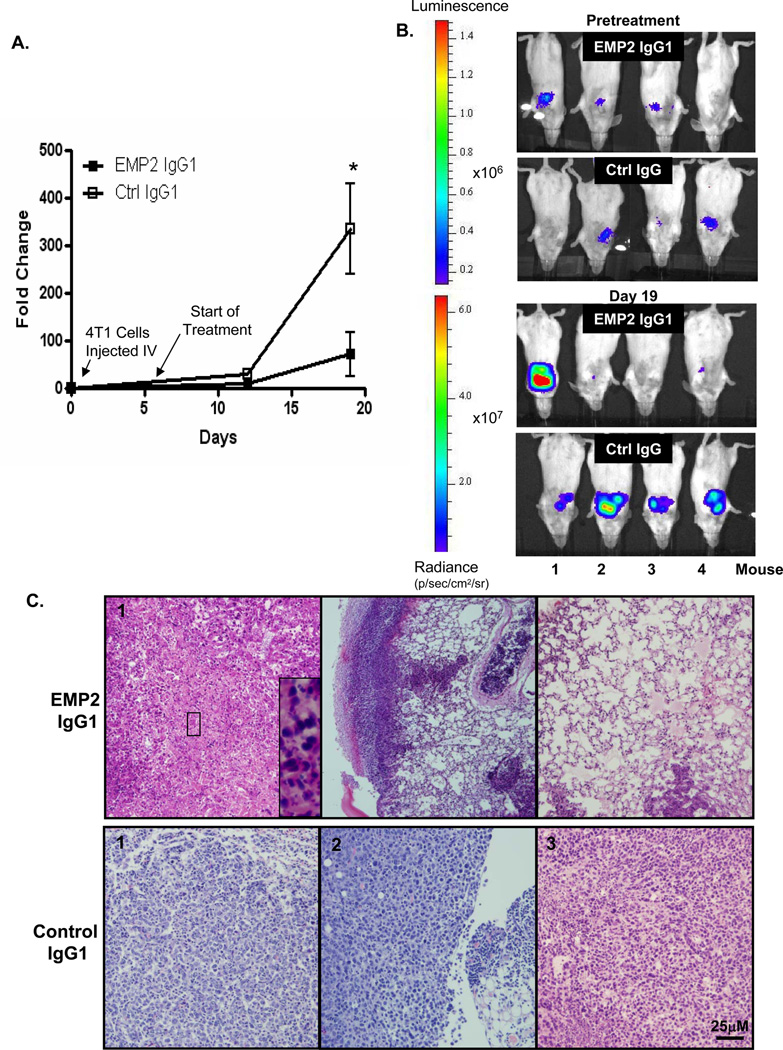
To determine the putative efficacy of anti-EMP2 IgG1 onmetastatic tumorsin vivo, 4T1/Fluc cells were injected intravenously into immunocompetent, syngeneic BALB/c hosts. Consistent with previous reports, these cells rapidly seed within the lungs, creating a model of late stage metastatic disease (41, 42). Starting at day 5 post cell injection, mice were treated systemically twice with10 mg/kg, and at day 18, all mice were euthanized for further analyses. Biophotonic imaging of the animals prior to antibody treatment and after treatment reveal that anti-EMP2 IgG1 treatment slowed tumor growth with 6 of 8 (75%) mice following anti-EMP2 IgG1 treatment showing an exponential decrease in tumor load by imaging (Figures 6A and B). When tumors were removed and evaluated by immunohistochemistry, all mice treated with anti-EMP2 IgG1 showed an increase in necrosis throughout the tumor (Figure 6C). Importantly, the normal mouse lung parenchyma and stroma showed no pathology due to anti-EMP2 IgG1 therapy.
Figure 6. Anti-EMP2 IgG1 antibodies reduce tumor load in a 4T1 lung metastatic model.
(A) 4T1-FLUC cells were injected into the tail vein of nude BALB/c mice. Mice were treated systemically with 10mg/kg EMP2 IgG1 or Ctrl IgG twice weekly, and tumor load was monitored. N=9per group; *, comparison by Student’s t-test p=0.02. (B) 5 days after cell injection but prior to treatment, luciferase activity was monitored showing localization of cells into the lungs of all mice. Following three treatments with anti-EMP2 IgG1 or Ctrl IgG (Day 18), tumor load was measured by bioluminescence. (C) At day 18, all mice were euthanized, and lungs were examined by hematoxylin and eosin staining. Representative images from four mice are shown. Image magnification: 20×; insets, 140× magnification; scale bar, 25µm.
Discussion
In this study we examined the expression levels of EMP2 in breast cancer and found that it was expressed in 63% of invasive ductal carcinomas tested with low to minimal expression in normal mammary glandular and ductal cells. Of significance, greater than 70% of TNBC cases from two independent cohorts of patients expressed EMP2. This is of particular interest given that TNBC, while accounting for 20–30% of all breast cancer cases, is associated with high mortality rates as these patients show a high recurrence of residual disease within 3 years (43). Moreover, high EMP2 expression was observed in lymph node metastatic lesions. Given that an estimated 90% of deaths due to breast cancer are a consequence of metastatic disease, the identification of a new molecular target to specifically target these types of tumors is of high importance. Importantly, our results are concordant with several studies showing that EMP2 mRNA is upregulated in breast cancer and that its expression correlates with advanced and metastatic disease. Thus, its expression profile and localization on the plasma membrane make EMP2 an attractive target for passive immunotherapy with recombinant monoclonal antibodies.
Of note, treatment of proliferating EMP2-expressing malignant cells with anti-EMP2 IgG1 promoted cell death with resultant reductions in tumor load in both human xenograft and murine metastatic models. Our results suggest that anti-EMP2 IgG1 elicits cell death both directly as well as through an ADCC response. Although the exact mechanism for direct cytotoxicity is still being elucidated, the known cell biology of EMP2 may provide insight. Previous studies have shown that EMP2 associates with and regulates the localization and activity of select integrin pairs (39, 44), and it has been shown to promote the phosphorylation of both FAK and Src in a number of normal and malignant cells (11, 12). In this study, we show that in breast cancer EMP2 levels correlate with FAK and Src activation and promote invasion in vitro, and treatment with anti-EMP2 Db or IgG1 reduces phosphorylation of Src within 12 hours of administration. This may be important as groups have linked Src activation to breast cancer survival, metastasis, as well as Trastuzumab resistance (45–47). Correspondingly, several groups have shown that a reduction in Src leads to programmed cell death in breast cancer (48). Hence, the reduction in Src activation by anti-EMP2 IgG1 may provide a plausible explanation for the cell death elicited by the antibody.
The anti-EMP2 IgG1 cross-reacts with human and murine EMP2, and we have previously shown using normal human tissue arrays and murine sections that both species have a similar tissue distribution (40). Although EMP2 is not ubiquitously expressed, it is present in a number of discrete locations in mice and humans such as normal lung type 1 pneumocytes (49), the retinal pigmented epithelium of the eye (50), and secretory epithelium of the endometrium (9). Nevertheless, in healthy mice injected with 10 mg/kg weekly of anti-EMP2 IgG1 for up to 2 months showed no obvious adverse effects as gaged by animal weight and health, tissue histology, and the release of liver serum proteins. Moreover, treatment of up to 40 mg/kg also did not elicit any measurable toxicity. Indeed, to date we have found no evidence of toxicity following chronic systemic treatment with anti-EMP2 IgG1in vivo.
Over the last two decades, the use of monoclonal antibodies for cancer therapy has achieved considerable success. In breast cancer, trastuzumab has changed the standard of care for women with cancers overexpressing HER2/neu, and remains one of the few FDA approved options for women with metastatic breast cancer (51, 52). Our findings offer proof-of-concept for the use of anti-EMP2 IgG1as an effective therapy for breast cancer. While anti-EMP2 IgG1 reduced tumor load between 50–80% in all three breast cancer models tested in vivo, we recognize that like all targeted therapies, anti-EMP2 IgG1 may need to be used in combination with other drugs. While we are currently investigating the best combinations of drugs to pair with anti-EMP2 IgG1, studies suggest that its mRNA expression in breast cancer is retained following standard chemotherapy (19). Thus, the biologic properties and expression profile of EMP2, elucidated by the preclinical studies presented previously and here, suggest that this fully human, full length antibody has the potential to be a first-in-class, effective therapeutic for the treatment of EMP2-dependent cancers.
Conclusions
These results present compelling evidence that EMP2 is highly expressed in triple negative breast cancer as well as in other breast cancer variants. Antibodies and antibody fragments to EMP2 reduce tumor load in animal models, suggesting that EMP2 is a novel therapeutic target in TNBC and other forms of breast cancer.
Supplementary Material
Acknowledgements
We thank Lin Lin for helpful discussions on the ADCC experiments and Dr. Sheri Morrison (University of California, Los Angeles) for providing the antibody expression vectors. Gustavo Helguera is a member of the National Council for Scientific and Technological Research (CONICET), Argentina.
Grant Support
The work is supported by the Early Detection Research Network NCI CA-86366 (L. Goodglick), a Charles Drew University/UCLA National Institutes of Health grant (U54-CA-143931) (M. Wadehra), R01 CA163971 (M. Wadehra), K01-CA138559 (T.R. Daniels-Wells), P30 CA016042 (University of California at Los Angeles Jonsson Comprehensive Cancer Center Flow Cytometry Core), NCRR and NCATS UL1TR000124 (UCLA Clinical Translational Science Institute), the Stein Oppenheimer Seed Grant (M. Wadehra), ANPCyT-FONARSEC PICT-PRH 2008–00315 (G.F. Helguera), CONICET PIP N° 114-2011-01-00139 (G.F. Helguera) and UBACYT N° 200-2011-02-00027 (G.F. Helguera).
Abbreviations
- ctrl
control
- Db
diabody
- HR
hormone receptor
- FAK
focal adhesion kinase
- EMP2
epithelial membrane protein-2
- TMA
tissue microarray
- TNBC
triple-negative breast cancer
- ADCC
antibody dependent cell-mediated cytotoxicity
- i.t.
intratumor
- VL
variable light
- VH
variable heavy
- FLUC
firefly luciferase
Footnotes
Conflicts of Interest
M.W., L.K.G, and J.B. hold patents on the EMP2 IgG1 antibody presented in this work. They are also founders of Paganini Biopharma. No other authors have competing interests.
References
- 1.Higgins MJ, Baselga J. Targeted therapies for breast cancer. The Journal of Clinical Investigation. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol. 2010;33:637–645. doi: 10.1097/COC.0b013e3181b8afcf. [DOI] [PubMed] [Google Scholar]
- 3.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 4.Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Maresh EL, Soslow RA, Alavi M, Mah V, Zhou Q, et al. Epithelial membrane protein-2 is a novel therapeutic target in ovarian cancer. Clin Cancer Res. 2010;16:3954–3963. doi: 10.1158/1078-0432.CCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habeeb O, Goodglick L, Soslow RA, Rao RG, Gordon LK, Schirripa O, et al. Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer. 2010;116:4718–4726. doi: 10.1002/cncr.25259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadehra M, Natarajan S, Seligson DB, Williams CJ, Hummer AJ, Hedvat C, et al. Expression of epithelial membrane protein-2 is associated with endometrial adenocarcinoma of unfavorable outcome. Cancer. 2006;107:90–98. doi: 10.1002/cncr.21957. [DOI] [PubMed] [Google Scholar]
- 10.Gordon LK, Kiyohara M, Fu M, Braun J, Chan AM, Goodglick L, et al. EMP2 regulates angiogenesis in endometrial cancer cells through induction of VEGF. Oncogene. 2013 doi: 10.1038/onc.2012.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu M, Rao R, Sudhakar D, Hogue CP, Rutta Z, Morales S, et al. Epithelial Membrane Protein-2 Promotes Endometrial Tumor Formation through Activation of FAK and Src. PLoS One. 2011;6:e19945. doi: 10.1371/journal.pone.0019945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Morales SA, Mareninov S, Coulam P, Wadehra M, Goodglick L, Braun J, et al. Functional consequences of interactions between FAK and epithelial membrane protein 2 (EMP2) Invest Ophthalmol Vis Sci. 2009;50:4949–4956. doi: 10.1167/iovs.08-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales SA, Mareninov S, Prasad P, Wadehra M, Braun J, Gordon LK. Collagen gel contraction by ARPE-19 cells is mediated by a FAK-Src dependent pathway. Exp Eye Res. 2007;85:790–798. doi: 10.1016/j.exer.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Morales SA, Mareninov S, Wadehra M, Zhang L, Goodglick L, Braun J, et al. FAK activation and the role of epithelial membrane protein 2 (EMP2) in collagen gel contraction. Invest Ophthalmol Vis Sci. 2009;50:462–469. doi: 10.1167/iovs.07-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadehra M, Forbes A, Pushkarna N, Goodglick L, Gordon LK, Williams CJ, et al. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. Dev Biol. 2005;287:336–345. doi: 10.1016/j.ydbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 16.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 17.Obermayr E, Sanchez-Cabo F, Tea MK, Singer CF, Krainer M, Fischer MB, et al. Assessment of a six gene panel for the molecular detection of circulating tumor cells in the blood of female cancer patients. BMC Cancer. 2010;10:666. doi: 10.1186/1471-2407-10-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, et al. Isolation and Molecular Profiling of Bone Marrow Micrometastases Identifies TWIST1 as a Marker of Early Tumor Relapse in Breast Cancer Patients. Clinical Cancer Research. 2007;13:5001–5009. doi: 10.1158/1078-0432.CCR-07-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimazaki K, Lepin EJ, Wei B, Nagy AK, Coulam CP, Mareninov S, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367–7377. doi: 10.1158/1078-0432.CCR-08-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon NK, Seligson DB, Chia D, Elshimali Y, Sulur G, Li A, et al. Higher expression levels of 14-3-3 sigma in ductal carcinoma in situ of the breast predict poorer outcome. Cancer Biomark. 2009;5:215–224. doi: 10.3233/CBM-2009-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presson AP, Yoon NK, Bagryanova L, Mah V, Alavi M, Maresh EL, et al. Protein expression based multimarker analysis of breast cancer samples. BMC Cancer. 2011;11:230. doi: 10.1186/1471-2407-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864–2871. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helguera G, Penichet ML. Antibody-cytokine fusion proteins for the therapy of cancer. Methods Mol Med. 2005;109:347–374. doi: 10.1385/1-59259-862-5:347. [DOI] [PubMed] [Google Scholar]
- 25.Qu Z, Griffiths GL, Wegener WA, Chang C-H, Govindan SV, Horak ID, et al. Development of humanized antibodies as cancer therapeutics. Methods. 2005;36:84–95. doi: 10.1016/j.ymeth.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SL, Wims L, Wallick S, Tan L, Oi VT. Genetically engineered antibody molecules and their application. Ann N Y Acad Sci. 1987;507:187–198. doi: 10.1111/j.1749-6632.1987.tb45801.x. [DOI] [PubMed] [Google Scholar]
- 27.Habeeb O, Goodglick L, Soslow RA, Rao RG, Gordon LK, Schirripa O, et al. Epithelial membrane protein-2 expression is an early predictor of endometrial cancer development. Cancer. 2010 doi: 10.1002/cncr.25259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial Membrane Protein-2 is expressed in discrete anatomical regions of the eye. Experimental and Molecular Pathology. 2003;74:106–112. doi: 10.1016/s0014-4800(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 29.Colello D, Mathew S, Ward R, Pumiglia K, LaFlamme SE. Integrins Regulate Microtubule Nucleating Activity of Centrosome through Mitogen-activated Protein Kinase/Extracellular Signal-regulated Kinase Kinase/Extracellular Signal-regulated Kinase (MEK/ERK) Signaling. Journal of Biological Chemistry. 2012;287:2520–2530. doi: 10.1074/jbc.M111.254128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, et al. Shp2 Regulates Src Family Kinase Activity and Ras/Erk Activation by Controlling Csk Recruitment. Molecular cell. 2004;13:341–355. doi: 10.1016/s1097-2765(04)00050-4. [DOI] [PubMed] [Google Scholar]
- 31.Yoon NK, Maresh EL, Elshimali Y, Li A, Horvath S, Seligson DB, et al. Elevated MED28 expression predicts poor outcome in women with breast cancer. BMC Cancer. 2010;10:335. doi: 10.1186/1471-2407-10-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon NK, Maresh EL, Shen D, Elshimali Y, Apple S, Horvath S, et al. Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol. 2010;41:1794–1801. doi: 10.1016/j.humpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Mah V, Seligson DB, Li A, Marquez DC, Wistuba II, Elshimali Y, et al. Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Langerød A, Ji Y, Nowels KW, Nesland JM, Tibshirani R, et al. Different Gene Expression Patterns in Invasive Lobular and Ductal Carcinomas of the Breast. Molecular Biology of the Cell. 2004;15:2523–2536. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X-J, Dahiya S, Richardson E, Erlander M, Sgroi D. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Research. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–636. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holliger P, Prospero T, Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90:6444–6448. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadehra M, Forbes A, Pushkarna N, Goodglick L, Gordon LK, Williams CJ, et al. Epithelial membrane protein-2 regulates surface expression of alphavbeta3 integrin in the endometrium. DevBiol. 2005;287:336–345. doi: 10.1016/j.ydbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Fu M, Brewer S, Olafsen T, Wu A, Gordon L, Said J, et al. Positron Emission Tomography Imaging of Endometrial Cancer Using Engineered Anti-EMP2 Antibody Fragments. Molecular Imaging and Biology. 2013;15:68–78. doi: 10.1007/s11307-012-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotech. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Hortobagyi GN. Toward Individualized Breast Cancer Therapy: Translating Biological Concepts to the Bedside. The Oncologist. 2012;17:577–584. doi: 10.1634/theoncologist.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadehra M, Iyer R, Goodglick L, Braun J. The tetraspan protein epithelial membrane protein-2 interacts with beta1 integrins and regulates adhesion. J Biol Chem. 2002;277:41094–4100. doi: 10.1074/jbc.M206868200. [DOI] [PubMed] [Google Scholar]
- 45.Burgess DJ. Breast cancer: SRC hits the mark. Nat Rev Cancer. 2011;11:314–315. doi: 10.1038/nrc3062. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Huang W-C, Li P, Guo H, Poh S-B, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang XHF, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent Bone Metastasis in Breast Cancer Tied to Src-Dependent Survival Signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D, et al. Inhibition of Protein Kinase c-Src Reduces the Incidence of Breast Cancer Metastases and Increases Survival in Mice: Implications for Therapy. Journal of Pharmacology and Experimental Therapeutics. 2006;318:161–172. doi: 10.1124/jpet.106.102004. [DOI] [PubMed] [Google Scholar]
- 49.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, et al. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 50.Wadehra M, Sulur GG, Braun J, Gordon LK, Goodglick L. Epithelial membrane protein-2 is expressed in discrete anatomical regions of the eye. Exp Mol Pathol. 2003;74:106–112. doi: 10.1016/s0014-4800(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 51.Nahta R, Yu D, Hung M-C, Hortobagyi GN, Esteva FJ. Mechanisms of Disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Prac Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 52.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.