Abstract
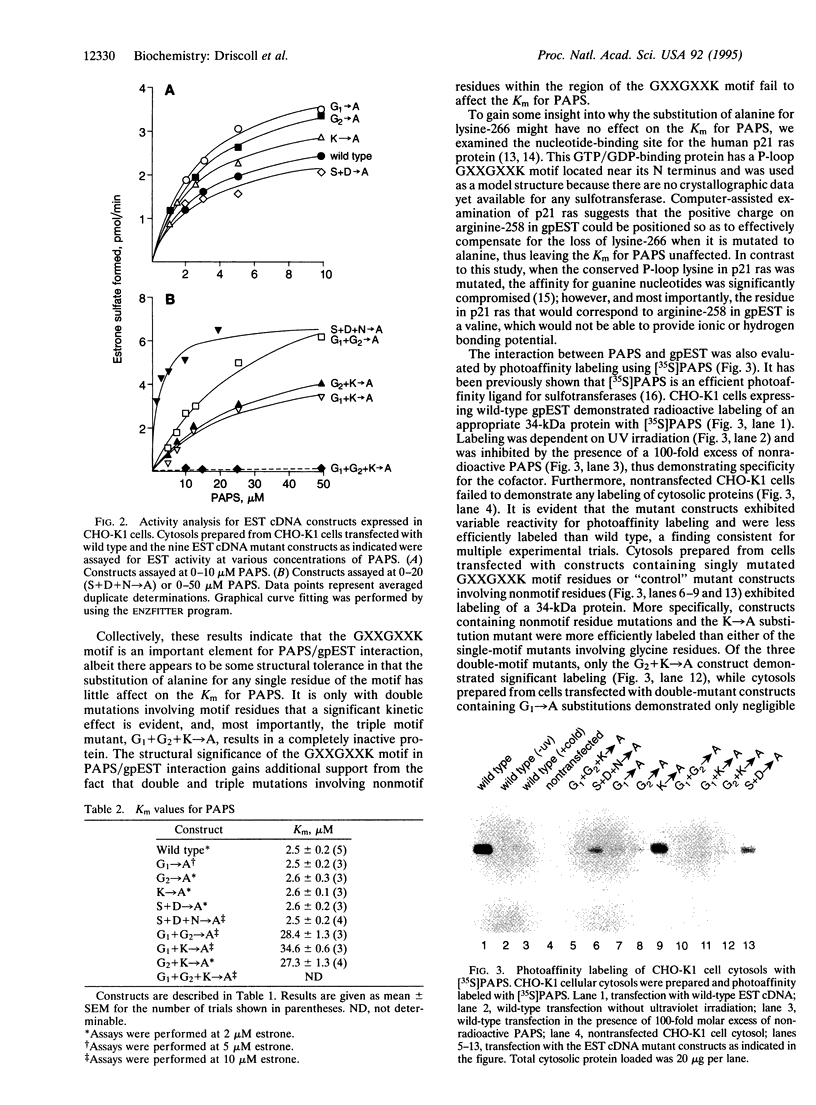
Point mutations were selectively introduced into a cDNA for guinea pig estrogen sulfotransferase (gpEST); each construct was then expressed in Chinese hamster ovary K1 cells. The molecular site chosen for study is a conserved GXXGXXK sequence that resembles the P-loop-type nucleotide-binding motif for ATP- and GTP-binding proteins and is located near the C terminus of all steroid and phenol(aryl) sulfotransferases for which the primary structures are known. Preliminary experiments demonstrated that the GXXGXXK motif is essential for binding the activated sulfonate donor 3'-phosphoadenosine 5'-phosphosulfate (PAPS). The present study was undertaken to ascertain the relative importance of each individual residue of the motif. While the mutation of a single motif residue had little effect on the interaction between gpEST and PAPS as determined by kinetic analysis and photoaffinity labeling, the mutation of any two residues in concert resulted in an approximate 10-fold increase in the Km for PAPS and reduced photoaffinity labeling. The mutation of all three motif residues resulted in an inactive enzyme and complete loss of photoaffinity labeling. Interestingly, several mutants also displayed a striking effect on the Km for the steroid substrate; double mutants, again, demonstrated greater perturbations (8- to 28-fold increase) than did single mutants. Unexpectedly, whereas the mutation of nonmotif residues had a negligible effect on the Km for PAPS, a marked increase in the Km for the estrogen substrate ( > 30-fold) was noted. On the basis of these findings, it is concluded that the sequence GISGDWKN within the C-terminal domain of gpEST represents a critical component of the active site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiba H., Komatsu K., Lee Y. C., Tomizuka T., Strott C. A. The 3'-terminal exon of the family of steroid and phenol sulfotransferase genes is spliced at the N-terminal glycine of the universally conserved GXXGXXK motif that forms the sulfonate donor binding site. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8176–8179. doi: 10.1073/pnas.92.18.8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll W. J., Martin B. M., Chen H. C., Strott C. A. Isolation of two distinct 3-hydroxysteroid sulfotransferases from the guinea pig adrenal. Evidence for 3 alpha-hydroxy versus 3 beta-hydroxy stereospecificity. J Biol Chem. 1993 Nov 5;268(31):23496–23503. [PubMed] [Google Scholar]
- Jones D. H., Howard B. H. A rapid method for site-specific mutagenesis and directional subcloning by using the polymerase chain reaction to generate recombinant circles. Biotechniques. 1990 Feb;8(2):178–183. [PubMed] [Google Scholar]
- Komatsu K., Driscoll W. J., Koh Y. C., Strott C. A. A P-loop related motif (GxxGxxK) highly conserved in sulfotransferases is required for binding the activated sulfate donor. Biochem Biophys Res Commun. 1994 Nov 15;204(3):1178–1185. doi: 10.1006/bbrc.1994.2587. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Oeda T., Lee Y. C., Driscoll W. J., Chen H. C., Strott C. A. Molecular cloning and expression of a full-length complementary DNA encoding the guinea pig adrenocortical estrogen sulfotransferase. Mol Endocrinol. 1992 Aug;6(8):1216–1226. doi: 10.1210/mend.6.8.1406700. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Powers S. P., Miller L. J., Weinshilboum R. M. 3'-Phosphoadenosine-5'-phosphosulfate: photoaffinity ligand for sulfotransferase enzymes. Mol Pharmacol. 1991 Jan;39(1):34–41. [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizuka T., Oeda T., Tamura Y., Yoshida S., Strott C. A. Characterization of guinea pig estrogen sulfotransferase expressed by Chinese hamster ovary cell-K1 stable transfectants. Endocrinology. 1994 Sep;135(3):938–943. doi: 10.1210/endo.135.3.8070389. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnall M. H., Driscoll W. J., Lee Y. C., Strott C. A. Estrogen and hydroxysteroid sulfotransferases in guinea pig adrenal cortex: cellular and subcellular distributions. Endocrinology. 1993 Nov;133(5):2284–2291. doi: 10.1210/endo.133.5.8404682. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Bergold A., Duffel M. W. Affinity labeling of aryl sulfotransferase IV. Identification of a peptide sequence at the binding site for 3'-phosphoadenosine-5'-phosphosulfate. J Biol Chem. 1994 Dec 2;269(48):30313–30319. [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]