Abstract
Tissue engineering offers the possibility for soft tissue reconstruction and augmentation without autologous grafting or conventional synthetic materials. Two critical challenges have been addressed in a number of recent studies: a biology challenge of angiogenesis and an engineering challenge of shape maintenance. These two challenges are inter-related and are effectively addressed by integrated bioengineering strategies. Recently, several integrated bioengineering strategies have been applied to improve bioengineered adipose tissue grafts, including internalized microchannels, delivery of angiogenic growth factors, tailored biomaterials and transplantation of precursor cells with continuing differentiation potential. Bioengineered soft tissue grafts are only clinically meaningful if they are vascularized, maintain shape and dimensions, and remodel with the host. Ongoing studies have begun to demonstrate the feasibility towards an ultimate goal to generate vascularized soft tissue grafts that maintain anatomically desirable shape and dimensions.
Keywords: Adipocytes, Pre-adipocytes, Stem cells, Tissue engineering, Biomaterials, Wound healing, Regenerative medicine, Breast reconstruction, Facial reconstruction
1. Introduction
Soft tissue defects result from congenital anomalies, chronic diseases, tumor resection or trauma. Reconstruction of subcutaneous soft tissue represents critical challenges for contemporary medical practice, and yet is substantially under-addressed by the scientific community. Currently, plastic surgeons utilize autologous tissue grafts or synthetic materials for reconstruction of tissue defects or augmentation of soft tissue [1,2]. Autologous soft tissue grafts are common practice in light that allografts, xenografts and synthetic materials have complications such as pathogen transmission, increased infection rates, and immune rejection issues. Various levels of clinical success have been reported on the use of autologous soft tissue grafts [3–8]. However, donor site morbidity continues to be an intrinsic drawback of autologous grafting that neither the surgeon, nor the patient desires to endure. Furthermore, patients may not have an adequate tissue source for autologous grafting.
Synthetic materials such as silicone or saline implants for breast reconstruction or augmentation have the advantage of endless supply and have been documented to replace missing soft tissues with various levels of clinical success. However, synthetic prostheses have drawbacks such as rupture, leakage, capsular contracture, ectopic mineralization, dislocation and suboptimal biocompatibility [9]. Certain synthetic materials interfere with the detection of breast cancer [10]. Furthermore, a lack of natural feel and abnormal tissue rippling of synthetic prostheses are discouraging prospects. Other synthetic materials used for facial augmentation or prostheses suffer from drawbacks such as poor integration with native tissue, lack of remodeling ability, and an unnatural appearance.
Bioengineered soft tissue grafts are anticipated to circumvent some of the drawbacks associated with autologous tissue grafting or synthetic materials. However, bioengineered soft tissue grafts face two critical challenges: (1) suboptimal angiogenesis or revascularization as primarily a biology challenge, and (2) gradual loss of shape and dimensions as primarily an engineering challenge. In this review, we discuss several strategies that have begun to address these two critical barriers in the generation of clinically relevant soft tissue grafts: suboptimal angiogenesis and loss of shape and dimensions.
2. Key biology challenge: suboptimal angiogenesis of soft tissue grafts
Adipose tissue is traditionally viewed as no more than a reservoir for energy storage. This incomplete viewpoint has been revised to consider adipose tissue as metabolically active [11]. Like other metabolically active structures, the adipose tissue has a high demand for vascularity. Slow revascularization of autologous tissue grafts is a barrier for survival and viability of current soft tissue reconstruction procedures [12,13]. Multiple studies have shown that autologous tissue grafts resorb up to 60% within 6 months following transplantation [14–20]. These statistics are a vivid reminder to tissue engineers with respect to the challenge of angiogenesis in bioengineered soft tissue grafts. A classic limitation of cell survival in tissue constructs is nutrient and oxygen diffusion [22]. Vascular diffusion is limited to 200 microns in regenerating tissue [21–26]. In a recent survey conducted by the Editors-in-Chief of Tissue Engineering, inadequate angiogenesis is the top priority to engineer virtually any tissue, and yet remains the area of least progress in the past decade [29]. This deficiency applies to the engineering of adipose tissue grafts.
3. Bioengineering strategies for vascularizing soft tissue grafts
A limited number of meritorious studies have recognized the issue of inadequate angiogenesis in the field of adipose tissue engineering. A pre-adipocyte cell line, 3T3-F442A cells, has been injected subcutaneously in nude mice to generate fat pads [32,33]. Neovascularization in the fat pad is derived from the host mice and not induced by 3T3-F442A cells [32,33]. The endothelial cells of the newly formed blood vessels are from the host mice instead of differentiation from the injected 3T3-F442A cells [32,33], indicating the critical role played by host vasculature and endothelium. Up to date, most bioengineered adipose tissue grafts still need to be scaled up, and scaling up is associated with an inevitable need for angiogenesis [22,23,26–28].
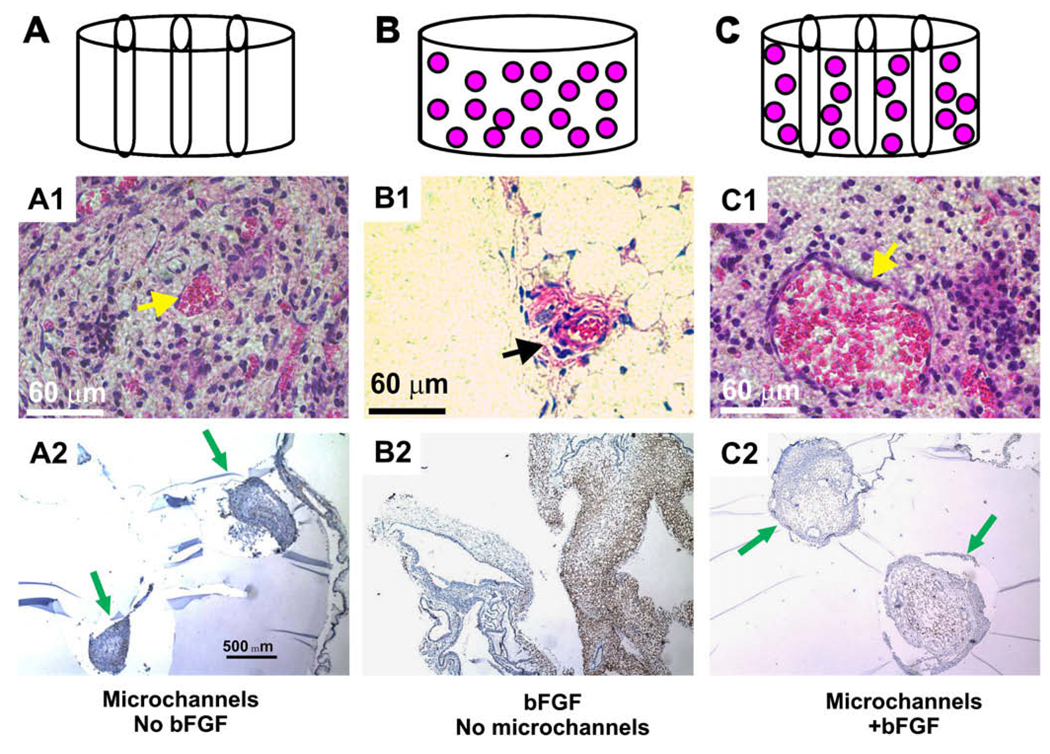
In a recent study, Stosich et al. [23] demonstrated that an integrated biology and engineering approach generated vascularized adipose tissue grafts. A hydrogel, poly(ethylene glycol) diacrylate (MW 3400; Nektar, Huntsville, AL) is advantageous as a scaffold for adipose tissue engineering because of its specificity for shape and dimensions, and also its ability to accommodate cell growth and differentiation in vivo [25,26]. However, a number of hydrogels, such as PEGDA, are not angio-inductive or not even permissive for vascular ingrowth. In Stosich et al. [23], a combined biophysical and bioactive approach was used to modify PEGDA. Microchannels were created in PEGDA to serve as conduits for vascular ingrowth, with or without basic fibroblast growth factor (bFGF) that acted as an active angiogenic growth factor. A total of four PEG hydrogel configurations were fabricated: (1) PEG hydrogel alone, (2) a total of three microchannels were fabricated in the photopolymerized PEG hydrogel (Fig. 1A with permission from Tissue Engineering); (3) 0.5 µg/µL bFGF was adsorbed in PEG hydrogel without microchannels (Fig. 1B); and (4) a combination of 0.5 µg/µL bFGF and microchannels in PEG hydrogel (Fig. 1C). Modified PEG hydrogel samples were implanted in the subcutaneous pockets of SCID mice for up to the tested 12 weeks.
Fig. 1.
In vivo implantation of PEG hydrogel. PEG hydrogel was fabricated in four configurations: PEG alone, PEG with bFGF, microchanneled PEG, or both bFGF-adsorbed and microchanneled PEG. (A) PEG hydrogel molded into 6 × 4 mm (diameter × height) cylinder (without either bFGF or microchannels). (B) PEG hydrogel with three microchannels. (C) PEG hydrogel cylinder with adsorbed 0.5 mg/mL bFGF and three microchannels. Following in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the harvested PEG hydrogel samples showed distinct histological features. (A1) PEG hydrogel with microchannels but without bFGF showed host tissue infiltration primarily in the lumen of microchannels, and scarcely in the rest of PEG hydrogel. The infiltrating host tissue includes erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (A2) VEGF was immunolocalized only to host-derived tissue within the lumen of microchannels, indicating the vascular nature of the infiltrating host tissue. Arrows point to microchannels and the infiltrating host tissue. (B1) PEG hydrogel with bFGF but without microchannels showed apparently random and isolated islands of infiltrating host tissue (arrow). The infiltrating host tissue includes vascular structures with erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (B2) VEGF was immunolocalized to host-derived tissue within PEG hydrogel (without microchannels). (C1) PEG hydrogel with both microchannels and bFGF showed host tissue infiltration only in the lumen of microchannels, but scarcely in the rest of the PEG hydrogel. The infiltrating host tissue includes vascular structures with erythrocyte-filled blood vessels that are lined by endothelial cells (arrow). (C2) VEGF was immunolocalized only to host-derived tissue within the lumen of microchannels. Since no cells were delivered in any of the PEG hydrogel samples, tissue infiltration following in vivo implantation is derived from the host. Arrows point to microchannels.
In vivo implanted PEG hydrogel samples with bFGF and/or microchannels are well integrated with surrounding host tissue. PEG hydrogel with microchannels but without bFGF demonstrated host tissue infiltration only in the lumen of microchannels, but not the rest of PEG (Fig. 1A1). This is verified by immunolocalization of VEGF to host-derived tissue in the lumen of microchannels in PEG hydrogel, and the scarcity of host tissue infiltration in the rest of the PEG hydrogel (between microchannels) (Fig. 1A2), indicating that the infiltrated host tissue is vascularized. In contrast, PEG hydrogel adsorbed with bFGF but without microchannels demonstrated apparently random and isolated areas of host tissue infiltration, but nonetheless consisted of blood vessel-like structures as shown in Fig. 1B1. Immunolocalization of VEGF again was positive in the infiltrating host tissue, indicating its vascular nature (Fig. 1B2). Interestingly, PEG hydrogel with both microchannels and bFGF demonstrated host tissue ingrowth only in microchannels, but scarcely in the rest of PEG hydrogel between microchannels (Fig. 1C1). The infiltrating host tissue was also immunolocalized positively to VEGF (Fig. 1C2). PEG hydrogel lacking both microchannels and bFGF showed no host tissue infiltration (data not shown), which is consistent with our previous reports showing a lack of host tissue infiltration into PEG hydrogel [26]. Given that no cells are delivered in PEG hydrogel with or without bFGF and/or microchannels in this experiment, any and all infiltrating tissue into PEG hydrogel must be host-derived.
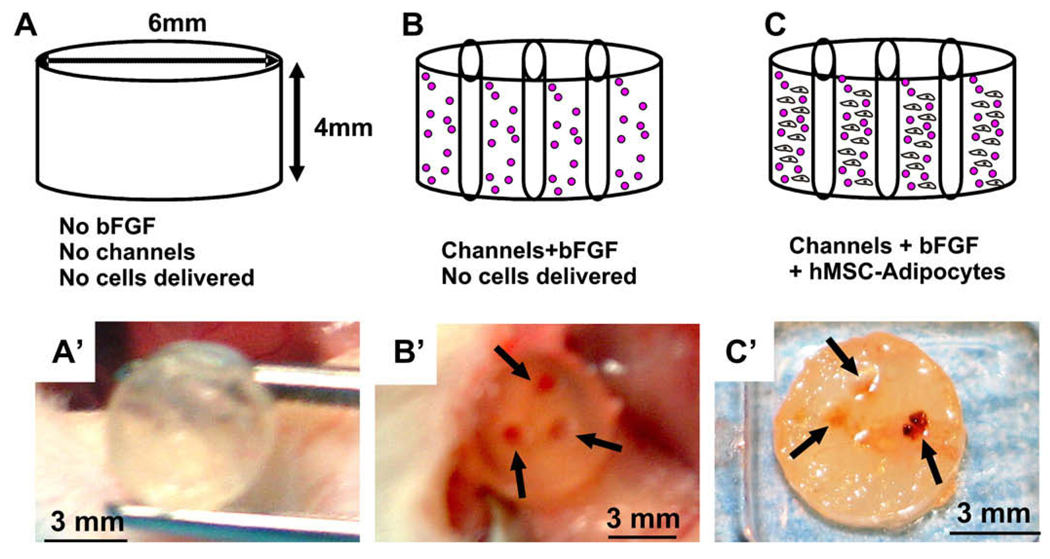
Furthermore, human mesenchymal stem cells (hMSCs) differentiated into adipogenic cells, and when seeded in PEG hydrogel with built-in microchannels and conjugated bFGF generated vascularized adipose tissue. As in our previous studies [25,26], PEG hydrogel was not permissive to cell infiltration (Fig. 2A,A’). However, PEG hydrogel with engineered microchannels and adsorbed bFGF showed not only darker color, but also three red circles in the transverse plane (Fig. 2B,B’). PEG hydrogel with both microchannels and bFGF, and seeded with hMSC-derived adipogenic cells, showed not only darker color, but also red circles (Fig. 2C,C’). Upon histological and immunohistochemical examination, PEG hydrogel encapsulating hMSC-derived adipogenic cells with built-in microchannels and bFGF showed islands of tissue formation (Fig. 3A). Multiple islands of the engineered tissue were Oil-red O positive, shown as a representative in Fig. 3B, suggesting the presence of engineered adipogenesis. Anti-VEGF antibody showed positive staining in the interstitial tissue (Fig. 3C), and anti-WGA lectin antibody was localized to the vicinity of engineered adipose tissue (Fig. 3D). Basic fibroblast growth factor (bFGF) is one of several highly potent pro-angiogenic growth factors. It will not be of surprise if other pro-angiogenic growth factors have similar effects. These findings suggest that an integrated bioengineering approach such as physical microchannels and bioactive factors promotes vascularized adipogenesis in vivo.
Fig. 2.
In vivo implantation of bFGF and microchanneled PEG hydrogel loaded with adipogenic cells derived from hMSCs. Diagrams (top row) and corresponding representative photographs at the time of harvest of in vivo samples. (A) PEG hydrogel molded into 6 × 4 mm (width × height) cylinder (without either bFGF or microchannels). (B) PEG hydrogel cylinder with 0.5 mg/mL bFGF and three microchannels, but without the delivery of cells. (C) PEG hydrogel cylinder loaded with 0.5 mg/mL bFGF and three microchannels, in addition to the encapsulation of adipogenic cells that have been derived from human mesenchymal stem cells at a cell seeding density of 3 × 106 cells/mL. Following in vivo implantation subcutaneously in the dorsum of immunodeficient mice, the harvested PEG hydrogel samples showed distinct histological features. (A’) PEG hydrogel cylinder without either microchannels or bFGF showed somewhat transparent appearance. (B’) PEG hydrogel cylinder with both bFGF and three microchannels, but without delivered cells, showed darker color and a total of three openings of microchannels (arrows) that are confirmed to be areas of host cell infiltration histologically. (C’) PEG hydrogel cylinder with both microchannels and bFGF in addition to encapsulated hMSC-derived adipogenic cells showed the opening of microchannels (red color and pointed with arrows) that are confirmed to be areas of host cell infiltration histologically in Fig. 3.
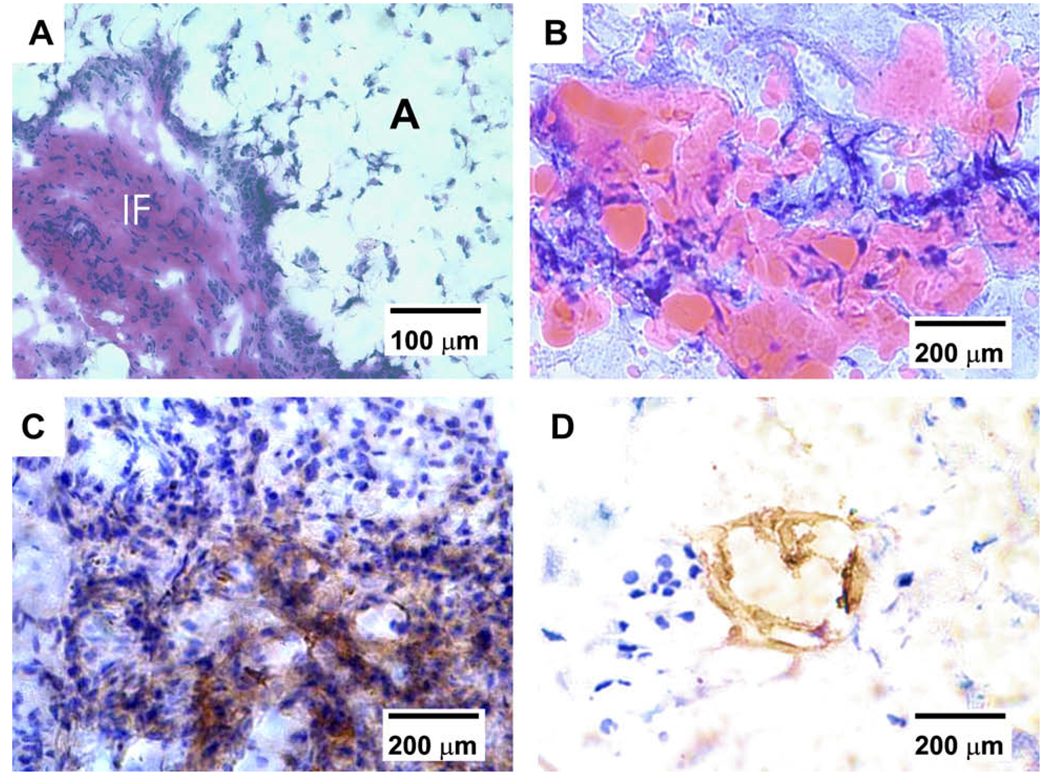
Fig. 3.
Histological and immunohistochemical characterization of vascularized adipose tissue from human mesenchymal stem cells. (A) Hematoxylin and eosin staining revealed IF tissue interposing between foam-like space labeled with A for adipose tissue. The presence of adipose tissue is confirmed in (B), showing substantial Oil red O positive staining in PEG hydrogel encapsulating hMSC-derived adipogenic cells, in addition to bFGF and built-in microchannels. In contrast, there is no evidence of adipogenesis in PEG hydrogel with bFGF and built-in microchannels, despite the seeding of hMSCs but without adipogenic differentiation. (C) Positive immunolocalization of VEGF antibody in the IF tissue interposing areas of adipogenesis, indicating the presence of vascular supply. (D) Positive immunolocalization of lectin WGA in the interstitial fibrous tissue interposing areas of adipogenesis, serving as further indication of the presence of vascular endothelial cells.
4. Engineering challenge: shape and dimension maintenance
Reconstructive and plastic surgeries are as much about the art and science of surgical practice as about shape and dimensions. Hypothetically, a successfully bioengineered kidney, when realized, does not necessarily need to have the precise shape as the patient’s normal kidney so long as the engineered kidney functions in vivo. In contrast, soft tissue defects must be restored to the original or esthetic shape and dimensions, in addition to having appropriate physiological functions. Current soft tissue reconstruction procedures suffer from post-operative volume reduction or shrinkage [30]. Volume reduction can be as severe as up to 70% over time [30]. In fact, “post-operative volume reduction” of soft tissue grafts is identified as a key issue among other complications of soft tissue reconstruction procedures [30].
During morphogenesis, shape and dimensions are specified by genetic patterning. In soft tissue reconstruction, the functional role of patterning of shape and dimension can be assumed by biomaterials. A variety of biomaterials have been used as cell carriers for adipose tissue engineering. As injectable materials, several growth factor carriers such as polylactide glycolic acid (PLGA)/polyethylene glycol (PEG) microspheres and gelatin microspheres in Matrigel have been reported to generate adipose tissues in animal models. However, most scaffold biomaterials alone without seeded adipogenic cells lack an appreciable adipogenic activity when transplanted in vivo [19,31–33]. Biodegradable polymers such as PLGA and PGA also showed the formation of neovascularization and mature adipocytes within the implants in 2–5 weeks post implantation [18,19]. However, the amount of adipose tissue may decrease with increasing scaffold degradation down and both the adipose tissue and the scaffold are resorbed completely by 5–12 months after implantation [18,19].
As three-dimensional carriers, nonwoven meshes or sponges of polylactide glycolic acid (PLGA), polyglycolic acid (PGA), hyaluronan, and collagen-I have been evaluated using ex vivo expanded preadipocytes ([19,31,33]). Adipose tissue formation correlates positively with pore size. For example, open hyaluronan sponges with interconnecting pores of 50–340 µm allow deeper penetration of adipocytes than either collagen sponges or nonwoven hyaluronan meshes. Porous scaffolds appear to allow vascularization and cell penetration but prevent the conversion of preadipocytes into mature adipocytes, probably due to a lack of contact inhibition that is required for adipogenic differentiation.
Besides rigid biopolymer carriers, soft and deformable hydrogels such as fibrin, Matrigel, and polyethylene glycol diacrylate (PEGDA) have been used for adipose tissue regeneration [26]. Fibrin seeded with preadipocytes shows volume retention up to 1-year post implantation (Schoeller et al. 2001; Wechselberger et al. 2002). But fibrin matrix alone does not provide sufficient mechanical stability to withstand in vivo compressive forces for volume-stable adipose tissue formation [32,33]. Matrigel, which is comprised largely of laminin, collgen-IV, entactin and perlecan, appears to be an effective substrate for angiogenesis as well as pre-adipocyte proliferation and differentiation in vitro and in vivo. However, Matrigel is restricted to experimental use because it is derived from murine sarcoma [32,33]. Our group previously demonstrated that PEGDA hydrogel is an effective biomaterial for adipose tissue engineering. PEGDA photoencapsulated with hMSC-derived adipocytes showed newly formed fat tissue as well as adipogenic marker gene expressions in 4 weeks postimplantation [24,25,29]. Importantly, the engineered adipose tissue in PEGDA retains the original shape and dimensions 12 weeks after in vivo implantation. These results demonstrate the roles played by biomaterials in defining the shape and dimension of adipose tissue grafts.
5. Bioengineering strategies for anatomically shaped soft tissue grafts
Despite the clinical importance of shape and dimensions in soft tissue reconstruction, little has been reported on the use of anatomically shaped adipose grafts. A number of meritorious studies have reported on the maintenance of shape and dimensions of adipose tissue grafts [24–26]. The next pending issue is how to create biocompatible scaffolds with not only anatomically specific shape, but also internal characteristics that accommodate adipose tissue genesis. Recently, a number of layer-by-layer fabrication approaches have demonstrated that biocompatible materials can be fabricated with not only anatomically defined shape, but also internal structures that facilitate cell seeding and angiogenesis. For example, a specialized rapid prototyping system (Bioplotter™, EnvisionTec, Germany) enables the design and fabrication of anatomically shaped scaffolds with varying internal architectures, thereby allowing precise control over pore size, porosity, interconnectivity of pores, permeability, and stiffness. Bioplotter™ technique, including 3D dispensing in a liquid medium, is applied to fabricate scaffolds using a variety of synthetic and native materials including melts, solutions, pastes, thermosets, filled polymers or reactive oligomers. Thus bioplotted 3D scaffolds by rapid prototyping have tremendous potential to generate clinically relevant soft tissue grafts with anatomical shape and optimized internal microstructure for metabolite diffusion and vascularization.
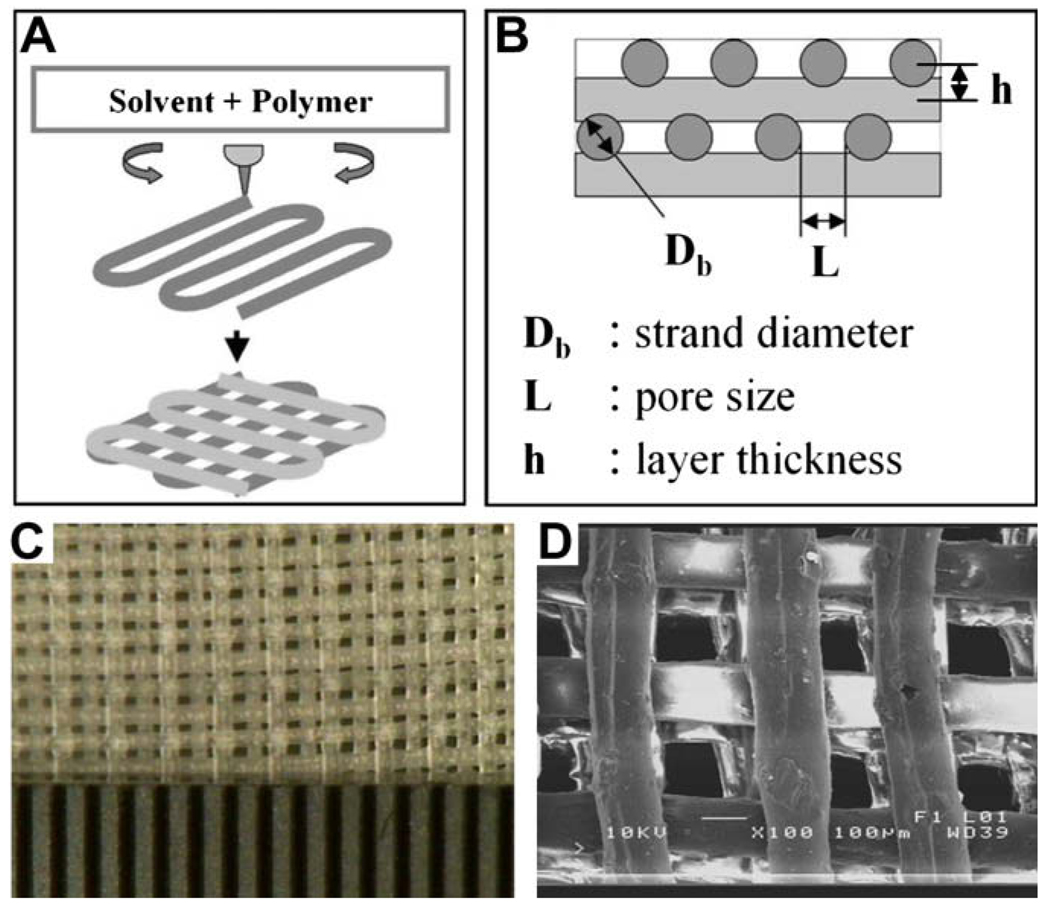
The architectural information of a target tissue or tissue defect needed for a layer-by-layer fabrication process for soft tissue scaffold can be obtained by imaging such as laser scanning or magnetic resonance imaging (MRI). Then the 3D information of tissue defect is used to design a scaffold as CAD file format and transferred to the Bioplotter™ system with a computer-guided 3D dispensing of a variety of biomaterials. The selected biomaterials can be melted by heat or dissolved in solvents and then dispensed layer-by-layer on a collecting plate or in a liquid medium, depending on the material properties. Bioplotting™ may be applied to fabricate a 3D anatomically shaped scaffold for soft tissue reconstruction not only with structural polymers such as PLGA, PGA, PLA, and PCL, but also with a number of hydrogels such as alginate and agarose. Bioplotting™ may also be utilized to develop a 3D shaped plastic mold that can be used for various hydrogels such as collagen gel [34], hyaluronan-based gel [35], and silk-based gel [36,37]. We have recently fabricated a hydrogel with internal interlaid strands and microchannels. To produce bioplotted hydrogels, polymers were partially gelated with sufficient viscosity, and bioplotted layer by layer. Polyethylene oxide (PEO) was dissolved in methyl ethyl and bioplotted with an input CAD file specifying the geometry of the scaffold. Fig. 4 shows a typical structure that has been plotted and fabricated using PEO. The schematics of the bioplotting technique and the adjustable parameters for the bioplotter are shown in Fig. 4A and B. A photomicrograph of the 3D-plotted PEO hydrogel with interlaid strands and interconnecting microchannels is shown in Fig. 4C. Scanning electron microscopy (SEM) image of a bioplotted PEO hydrogel with interlaid strands and interconnecting microchannels is shown in Fig. 4D. Bioplotting and other rapid prototyping approaches have previously been applied in the fabrication of hard tissue such as bone scaffolds. Our current effort demonstrates that soft tissue reconstruction may also benefit from layer-by-layer fabrication approaches.
Fig. 4.
A soft hydrogel with internal microchannels fabricated with interlaid strands and interconnecting microchannels. Polyethylene oxide (PEO) was dissolved in methyl ethyl and bioplotted with an input CAD file specifying the geometry of internal microchannels. (A, B) The schematics of the bioplotting technique and the adjustable parameters for the bioplotter. (C) Photomicrograph of the 3D-bioplotted PEO hydrogel with interlaid strands and interconnecting microchannels. (D) Scanning electron microscopy (SEM) image of a bioplotted PEO hydrogel with interlaid strands and interconnecting microchannels.
6. Concluding remarks: bioengineered soft tissue grafts for clinical applications
The following strategies have emerged from a number of important reports that contribute to the field of adipose tissue regeneration. These strategies are by no means exclusive and are outlined here with the anticipation that further innovative approaches will be devised by all those who are interested in the clinically relevant subfield of adipose tissue engineering.
Creating microchannels in scaffolds. Microchannels should be of sufficient pore size to serve as conduits for cell seeding and vascular ingrowth and are interconnected by physical passages or allow diffusion of soluble factors.
Delivery of angiogenic growth factors. Previous work has shown that angiogenic growth factors may initiate or augment host-derived vascularization. Growth factors can be either exogenously delivered or host-derived. Multiple angiogenic growth factors may be necessary to induce host-derived endothelial sprouting and subsequent blood vessel maturation.
Fabrication of blood vessel analogs that are either free standing or embedded in scaffolds. This approach likely may require microsurgery for anastomosis with native vasculature.
Identify existing polymer biomaterials that maintain the shape and dimension of the growing adipose tissue graft whilst transplanted or host-derived adipogenic cells proliferate, and accumulate intracellular lipid vacuoles.
Utilize porous capsular materials that may define the boundary of bioengineered adipose tissue grafts. This approach has yet to be experimentally verified.
Fabricate novel polymer biomaterials or enable existing polymer biomaterials with high fidelity that maintain shape and dimensions.
Volume reduction and poor vascularization are two long-standing barriers for soft tissue grafting in plastic and reconstructive surgeries. The same two barriers have impaired the development of bioengineered soft tissue grafts. Several recent studies have begun to address these challenges with novel approaches including internalized microchannels, delivery of angiogenic growth factors, tailored biomaterials and transplantation of precursor cells with continuing differentiation potential. Bioengineered soft tissue grafts are not clinically meaningful unless they are vascularized, maintain shape and dimensions, and remodel with the host. Successful fabrication of bioengineered soft tissue grafts for clinical applications may depend upon a modularized approach to fabricate repetitive units of vascularized, shape-maintaining scaffolds, with each unit not exceeding the general upper limit of diffusion (~200 µm). Each module will contain microchannels and/or bioactive cues. We submit that integrated bioengineering strategies are critical to address the biology challenge of inadequate vascularization and the engineering challenge of volume reduction. It is foreseeable that bioengineered grafts will replace autologous grafting and conventional synthetic materials in plastic and reconstructive surgeries in the coming years.
Acknowledgments
We thank Lauren Feldman and Fen Guo for administrative assistance. This research was supported by NIH Grant EB006261 to J.J.M.
References
- 1.Walgenbach KJ, Shestak KC. Breast Dis. 2002;16:73–77. doi: 10.3233/bd-2002-16111. [DOI] [PubMed] [Google Scholar]
- 2.Garfein ES, Orgill DP, et al. Clin. Plast. Surg. 2003;30:485–498. doi: 10.1016/s0094-1298(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 3.Erol OO, Spira M. Plast. Reconstr. Surg. 1990;86:510–518. doi: 10.1097/00006534-199009000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Kononas TC, Bucky LP, et al. Plast. Reconstr. Surg. 1993;91:763–768. doi: 10.1097/00006534-199304001-00001. [DOI] [PubMed] [Google Scholar]
- 5.Butler DL, Awad HA. Clin. Orthop. Relat. Res. 1999;367(Suppl.):S324–S332. doi: 10.1097/00003086-199910001-00031. [DOI] [PubMed] [Google Scholar]
- 6.Eppley BL. Plast. Reconstr. Surg. 1999;104:1761–1783. doi: 10.1097/00006534-199911000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Hart D. Plast. Surg. Nurs. 2003;23:55–63. 72. doi: 10.1097/00006527-200323020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Miller MJ, Patrick CW., Jr Clin. Plast. Surg. 2003;30:91–103. doi: 10.1016/s0094-1298(02)00071-8. (vi) [DOI] [PubMed] [Google Scholar]
- 10.Boon Yin K, Najimudin N, Muhammad TS. Biochem. Biophys. Res. Comm. 2008;371:177–179. doi: 10.1016/j.bbrc.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Marler JJ, Guha A, et al. Plast. Reconstr. Surg. 2000;105:2049–2058. doi: 10.1097/00006534-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Stosich MS, Mao JJ. Sem. Plast. Surg. 2005;19:251–260. [Google Scholar]
- 13.Katz AJ, Llull R, et al. Clin. Plast. Surg. 1999;26:587–603. [PubMed] [Google Scholar]
- 14.Patrick CW, Jr, Chauvin PB, et al. Tissue Eng. 1999;5:139–151. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 15.Alster TS, West TB. Plast. Reconstr. Surg. 2000;105:2515–2525. doi: 10.1097/00006534-200006000-00034. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz HP, Hedrick MH, et al. Plast. Reconstr. Surg. 2000;105:2467–2481. doi: 10.1097/00006534-200006000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Patrick CW., Jr Anat. Rec. 2001;263:361–366. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 18.Patrick CW, Jr, Zheng B, et al. Tissue Eng. 2002;8:283–293. doi: 10.1089/107632702753725049. [DOI] [PubMed] [Google Scholar]
- 19.Beahm EK, Walton RL, et al. Clin. Plast. Surg. 2003;30:547–558. doi: 10.1016/s0094-1298(03)00072-5. [DOI] [PubMed] [Google Scholar]
- 20.Rouwkema J, Rivron NC, van Blitterswijk CA. Trends Biotechnol. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Koike N, Fukumura D, Gralla O, Au P, Schechner JS, Jain RK. Nature. 2004;428:138–139. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 22.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Nat. Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 23.Stosich MS, Bastian B, Marion NW, Clark PA, Reilly G, Mao JJ. Tissue Eng. 2007;13:2881–2890. doi: 10.1089/ten.2007.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alhadlaq A, Tang M, et al. Tissue Eng. 2005;11:556–566. doi: 10.1089/ten.2005.11.556. [DOI] [PubMed] [Google Scholar]
- 25.Stosich MS, Mao JJ. Plast. Reconstr. Surg. 2007;119:71–83. doi: 10.1097/01.prs.0000244840.80661.e7. (discussion 84–85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Nat. Biotechnol. 2005;23(7):821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 27.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 28.Koike T, Vernon RB, Hamner MA, Sadoun E, Reed MJ. J. Cell Biochem. 2002;86(4):748–758. doi: 10.1002/jcb.10257. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PC, Mikos AG, Fisher JP, Jansen JA. Tissue Eng. 2007;13:2827–2837. doi: 10.1089/ten.2007.0335. [DOI] [PubMed] [Google Scholar]
- 30.Coleman SR. Clin. Plast. Surg. 1997;24:347–367. [PubMed] [Google Scholar]
- 31.Fischbach C, Spruss T, et al. Exp. Cell Res. 2004;300:54–64. doi: 10.1016/j.yexcr.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Hemmrich K, von Heimburg D. Expert Rev. Med. Devices. 2006;3:635–645. doi: 10.1586/17434440.3.5.635. [DOI] [PubMed] [Google Scholar]
- 33.Neels JG, Thinnes T, Loskutoff DJ. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 34.Vashi AV, Keramidaris E, et al. Biomaterials. 2008;29:573–579. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Hemmrich K, Van de Sijpe K, et al. J. Surg. Res. 2008;144:82–88. doi: 10.1016/j.jss.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Mauney JR, Nguyen T, et al. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousefi AM, Gauvin C, Sun L, DiRaddo RW, Fernandes J. Polym. Eng. Sci. 2007;47:608–618. [Google Scholar]