Abstract
Background
To best inform evidence-based patient care, it is often desirable to compare competing therapies. We performed a network meta-analysis to indirectly compare low intensity pulsed ultrasonography (LIPUS) with electrical stimulation (ESTIM) for fracture healing.
Methods
We searched the reference lists of recent reviews evaluating LIPUS and ESTIM that included studies published up to 2011 from 4 electronic databases. We updated the searches of all electronic databases up to April 2012. Eligible trials were those that included patients with a fresh fracture or an existing delayed union or nonunion who were randomized to LIPUS or ESTIM as well as a control group. Two pairs of reviewers, independently and in duplicate, screened titles and abstracts, reviewed the full text of potentially eligible articles, extracted data and assessed study quality. We used standard and network meta-analytic techniques to synthesize the data.
Results
Of the 27 eligible trials, 15 provided data for our analyses. In patients with a fresh fracture, there was a suggested benefit of LIPUS at 6 months (risk ratio [RR] 1.17, 95% confidence interval [CI] 0.97–1.41). In patients with an existing nonunion or delayed union, ESTIM had a suggested benefit over standard care on union rates at 3 months (RR 2.05, 95% CI 0.99–4.24). We found very low-quality evidence suggesting a potential benefit of LIPUS versus ESTIM in improving union rates at 6 months (RR 0.76, 95% CI 0.58–1.01) in fresh fracture populations.
Conclusion
To support our findings direct comparative trials with safeguards against bias assessing outcomes important to patients, such as functional recovery, are required.
Abstract
Contexte
Pour mieux orienter les soins fondés sur des données probantes, il est souvent souhaitable de comparer des traitements entre eux. Nous avons procédé à une méta-analyse réseau pour comparer indirectement l’effet des ultrasons pulsés de faible intensité (UPFI) et de l’électrostimulation (ÉS) sur la guérison des fractures.
Méthodes
Nous avons interrogé les listes bibliographiques de revues récentes ayant évalué les UPFI et l’ÉS, en incluant des études publiées jusqu’en 2011 à partir de 4 bases de données électroniques. Nous avons actualisé les interrogations de toutes les bases de données électroniques jusqu’à avril 2012. Les essais admissibles étaient ceux qui incluaient des patients victimes d’une fracture récente ou présentant un retard de soudure ou une non soudure de fracture ayant été assignés aléatoirement aux UPFI ou à l’ÉS ou à un groupe témoin. Deux paires d’examinateurs ont passé en revue indépendamment et en duplicata les titres, les résumés et les textes complets des articles potentiellement admissibles. Ils en ont extrait les données et ont évalué la qualité des études. Nous avons utilisé des techniques de méta-analyse standard et réseau pour synthétiser les données.
Résultats
Parmi les 27 essais admissibles, 15 ont fourni des données pour notre analyse. Chez les patients présentant une fracture récente, les UPFI auraient produit un avantage à 6 mois (risque relatif [RR] 1,17, intervalle de confiance [IC] de 95 % 0,97–1,41). Chez les patients qui présentaient un problème de non soudure ou de retard de soudure osseuse et par rapport aux soins classiques, l’ÉS aurait conféré un avantage sur les taux de soudure osseuse à 3 mois (RR 2,05, IC de 95 % 0,99–4,24), et nous avons noté des preuves de très faible qualité selon lesquelles les UPFI conféreraient un avantage potentiel par rapport à l’ÉS pour ce qui est d’améliorer les taux de soudure osseuse à 6 mois (RR 0,76, IC de 95 % 0,58–1,01) chez les populations dont les fractures étaient récentes.
Conclusion
Pour confirmer nos conclusions, il faudra procéder à des essais comparatifs directs en veillant à écarter tout biais lors de l’évaluation des paramètres importants liés aux patients, tels que le rétablissement fonctionnel.
Fractures are associated with considerable socioeconomic burden1 and may be associated with delayed union and nonunion.2 Delayed union and nonunion can result in loss of function and significant pain and are associated with increased treatment costs and reduced quality of life.2 Factors contributing to delayed union and nonunion include fracture characteristics (e.g., fracture displacement, severity of injury to the soft tissue envelope, infection at the fracture site), iatrogenic factors (e.g., medications, such as anticoagulants, steroids, anti-inflammatory drugs, radiotherapy) and patient characteristics (e.g., vitamin deficiencies, smoking habits).3
The standard care for delayed union and nonunion include nonsurgical (e.g., cast immobilization) and surgical treatment (e.g., external fixation, plating, internal intramedullary nail fixation). Adjunct interventions, such as bone stimulators, are commonly used to facilitate fracture healing. A 2008 survey of 450 Canadian trauma surgeons (79% response rate) showed that 45% of surgeons used bone growth stimulators as part of their treatment strategies for managing fractures.4 Of these, an equal number used low-intensity pulsed ultrasonography (LIPUS) and electrical stimulators (ESTIM).
The US Food and Drug Administration approved LIPUS in 1994 for accelerating fresh fracture healing and in 2000 for the treatment of existing nonunions.5 The technique is non-invasive, and its waves induce micromechanical stress in the fracture site, stimulating molecular and cellular responses involved in fracture healing.6,7 Previous systematic reviews evaluating the effectiveness of LIPUS have suggested a moderate effect on surrogate end points (e.g., reducing time to radiographic union), but inconsistent effects on measures of direct importance to patients, such as return to function.8–11
The use of ESTIM is another noninvasive technique marketed for improving fracture healing. It is believed to affect many cellular pathways, including growth factor synthesis, cytokine production, proteoglycan and collagen, which ultimately stimulate pathways that enhance fracture healing.12–14 Previous systematic reviews evaluating ESTIM for healing existing nonunions concluded that the current evidence is inconsistent — neither showing a significant impact nor confidently rejecting the therapeutic effect of ESTIM.3,15
There have been no comparative studies evaluating LIPUS versus ESTIM for fracture healing. Although the clinical effectiveness for both LIPUS and ESTIM is inconsistent, use of these modalities remains high. In 2012, sales of bone stimulators in the United States were approximately $700 million annually, with a projected growth of 6% per year.16
To best inform evidence-based patient care, it is often desirable to compare competing therapies. Network meta-analysis techniques are powerful approaches that allow for indirect comparison of interventions that have not been directly compared.17,18 The main purpose of this study was to systematically review the LIPUS and ESTIM literature and perform a network meta-analysis of these 2 treatments for accelerating fracture healing in both fresh fracture and nonunion populations.
Methods
Eligibility criteria
All published randomized controlled trials (RCTs) enrolling patients with a fresh fracture or an existing delayed union or nonunion who were randomly assigned to LIPUS or ESTIM as well as a control group were eligible for inclusion in our review and meta-analysis.
Information sources and search
We identified relevant RCTs in any language by examining 2 recent Cochrane systematic reviews evaluating the effectiveness of LIPUS and the effectiveness of ESTIM in fracture healing.3,11 Authors of the LIPUS and ESTIM reviews searched (to November 2011 and April 2011, respectively) the Cochrane Bone, Joint and Muscle Trauma Group Specialized Register, the Cochrane Central Register of Controlled Trials, Medline, Embase, trial registers and reference lists of all eligible articles. We updated these searches to April 2012 to identify additional trials. The medical subject headings used to capture the trials are listed in the Appendix, available at canjsurg.ca.
Study selection
One team consisting of 2 reviewers (S.E., S.B.) screened, independently and in duplicate, titles and abstracts of identified citations. All citations flagged by either reviewer as potentially eligible in the title and abstract screening were reviewed in full text. The same reviewers independently applied the eligibility criteria to the full text of potentially eligible studies. Using the guidelines proposed by Landis and Koch19 for assessing interrater agreement for categorical data, we measured agreement for the full text review stage.
Data collection process and data items
Two pairs of reviewers (S.E. and S.B., and S.E. and B.M.) extracted data, independently and in duplicate, from each eligible study. The data extracted included patient characteristics, intervention and control device details, union rates, and frequency and timing of outcomes. Reviewers resolved disagreements by discussion, and arbitrators (J.W.B. and M.B.) adjudicated unresolved disagreements. We made contact with 1 author directly, as the union rates were not reported in the published study,20 and the author provided this data.
Risk of bias in individual studies
Two pairs of reviewers (S.E. and S.B., and S.E. and B.M.) assessed risk of bias using a modified Cochrane risk of bias instrument.21 Reviewers used modified response options of “definitely yes,” “probably yes,” “probably no” and “definitely no” for each risk of bias component, with “definitely yes” and “probably yes” ultimately assigned low risk of bias and “definitely no” and “probably no” assigned high risk of bias. Reviewers resolved disagreements by discussion, and arbitrators (J.W.B. and M.B.) adjudicated unresolved disagreements.
Summary measures
We completed pooled analyses for every common time point. To compare and pool data across trials for outcomes that measured fracture healing, we calculated risk ratios (RRs) and the associated 95% confidence intervals (CIs).
Synthesis of results
We completed standard meta-analyses to compare LIPUS with the control arm and ESTIM with the control arm. We excluded trials that reported zero events if they were the only trials reporting a given time point, as they would not produce a summary effect and the use of correction factors would provide no additional meaningful data. This resulted in the exclusion of 1 trial from the analysis, as it reported zero events at the end of 4 years.22
The author we contacted for unpublished data provided data for patients with unions (bridging at all 4 cortices by radiographic imaging), possible unions (bridging at 3 cortices) and nonunion (bridging at ≤ 2 cortices). For the purposes of our analyses of dichotomous outcomes, we merged possible union and nonunion into 1 category (nonunion) after consulting with an orthopedic surgeon in our research team in order to be conservative with respect to our treatment effect estimates.
For our network meta-analysis, we used a frequentist approach.17 A network meta-analysis was performed only if 2 conditions were satisfied: 1) the common comparator (control arm) in both trials evaluating LIPUS and trials evaluating ESTIM were considered similar to conduct an indirect comparison of the 2 bone stimulation therapies, and 2) the standard meta-analysis of each bone stimulation therapy versus standard care showed either significant benefit, or the point estimates of the bone stimulation therapies were in opposite directions (e.g., 1 suggesting potential benefit and the other suggesting potential harm).
We used a random-effects approach for our meta-analyses.23,24 We examined heterogeneity using a χ2 test and I2 and Tau2 statistics.25,26 We interpreted heterogeneity using the guidelines proposed by the Cochrane Handbook.27 We generated the following a priori hypothesis to explain variability between studies: studies with greater risk of bias will have larger effects than studies with lower risk of bias. This subgroup analysis was completed only on a risk of bias component × component basis if there was considerable variability within the risk of bias component. On consulting with a methodologist, we performed subgroup analyses only when there were at least 5 studies to avoid high risk of spurious subgroup findings.
We intended to assess publication bias by visually observing asymmetry of the funnel plot for each outcome. As a rule of thumb,28 one should only perform tests for funnel plot asymmetry when there are at least 10 studies included in the meta-analysis. We were underpowered to assess publication bias.
We performed all standard meta-analyses using RevMan software version 5.1.2 and the Canadian Agency for Drugs and Technology in Health indirect comparison software, and we used Microsoft Excel 2011 for our network meta-analyses.
Confidence in estimates
Reviewers (S.E., S.B.), independently and in duplicate, evaluated the quality of the evidence for relevant outcomes analyzed in the network meta-analysis using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.29,30
Results
Study selection
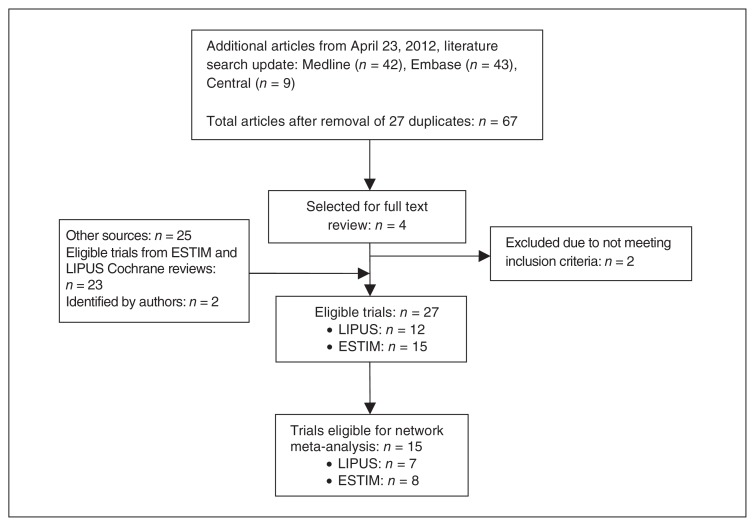
Twelve eligible trials were captured in the LIPUS Cochrane review,11 and 11 in the ESTIM Cochrane review.3 From our search update, we identified an additional 67 potentially eligible studies; we retrieved 4 of them in full text, and 2 of these were eligible for our review (Fig. 1). We also knew of 2 recent trials that were published after our updated search.20,31 Thus, 27 trials were included in our review: 12 evaluating LIPUS32–42 and 15 evaluating ESTIM.20,31,43–55 There was perfect agreement between reviewers in the full text review stage.
Fig. 1.
Study eligibility. ESTIM = electrical stimulation; LIPUS = low-intensity pulsed ultrasonography.
Study characteristics
Table 1 describes the trials included in our review. Eight trials evaluating LIPUS (7 fresh fracture and 1 nonunion populations),32–34,37–39,41 and 7 trials evaluating ESTIM (3 fresh fracture and 5 nonunion populations),43,44,49,52–54 reported union rates as one of their outcomes and were used in the network meta-analyses.
Table 1.
Description of studies
| Trial | Fracture location | Fracture treatment | Intervention and control therapy duration | No. patients | Age, mean ± SD yr | Type of device (I: intervention; C: control) | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| LIPUS/ESTIM | Control | LIPUS/ESTIM | Control | |||||
| LIPUS trials | ||||||||
|
| ||||||||
| Heckman et al.35 | Tibia | Closed reduction and above-knee casting. An alignment window was placed in the cast at the level of the fracture over the anteromedial aspect of the leg. Reduction of the casting to a below-knee cast; any subsequent splinting and weight bearing status was at the discretion of the clinician. | 20 min/d for 20 wk or until investigator believed fracture was healed sufficiently | 48 | 49 | 36 ± 2.3 | 31 ± 1.8 | I: Unidentified ultrasonography device C: Sham device |
|
| ||||||||
| Kristiansen et al.36 | Radius | Closed reduction and immobilization of the limb in a cast with volar flexion and ulnar deviation. A window was created on the dorsal aspect of the cast overlying the fracture and a retaining alignment fixture was placed in the window. | 20 min/d for 10 wk | 40 | 45 | Not reported | Not reported | I: SAFHS 2A, Exogen Inc. C: Sham device |
|
| ||||||||
| Emami et al.32* | Tibia | Reamed intramedullary nailing. | Started within 3 d after surgery, consisted of 20 min/d for 75 d; for max 25 h | 15 | 17 | 39.9 ± 16.2 | 34.3 ± 14 | I: SAFHS 2A, Exogen Inc. C: Sham device |
|
| ||||||||
| Strauss et al.41* | 5th metatarsal | Short leg cast and weight bearing as tolerated for a mean of 10 d; all were converted to a hinged ankle foot orthosis and continued with weight bearing until fracture union. | 20 min/use, twice daily | 10 | 10 | Not reported | Not reported | I: Adjunctive active treatment with ultrasonography C: Standard care |
|
| ||||||||
| Mayr et al.39* | Scaphoid | Forearm plaster splint was applied. After the swelling subsided a circular [arthrodesis] bandage replaced the splint and stayed on until the fracture was bridged. | 20 min/d | 15 | 15 | 37 ± 14† | 37 ± 14† | I: SAFHS R, Exogen Inc. C: Standard care |
|
| ||||||||
| Leung et al.37* | Tibia | Closed fractures or Gustillo grade 1 or 2 open fractures in the diaphysis underwent fixation with reamed, locked intramedullary nail. Fractures in the metaphysis or Gustillo grade 3 open fractures were treated with an external fixator. All open fractures were treated with emergency débridement and delayed closure. |
20 min/d for 90 d | 16 | 14 | 35.3† | 35.3† | I: Exogen 2000_ device (Exogen, Smith & Nephew Inc.) C: Sham device |
|
| ||||||||
| Rue et al.40 | Tibia | Protected weight bearing, alternative aerobic exercise, calcium and multivitamin supplements. | 20 min/d | 14 | 12 | 18.6 ± 0.8 | 18.4 ± 0.8 | I: Exogen Inc. C: Sham device |
|
| ||||||||
| Handolin et al.33* | Malleolar | Fixed with a 4.5 mm self-reinforced poly-L-lactic acid screw placed anteriorly. The ankle was immobilized for 6 wk with a removable brace. | 20 min/d for 6 wk | 15 | 15 | 41.4 | 39.4 | I: SAFHS 2A, Exogen Inc. C: Sham device |
|
| ||||||||
| Handolin et al.34* | Malleolar | Fixed with 1 anteriorly placed 4.5 mm self-reinforced poly-L-lactic acid, immobilized for 6 wk with a removable brace. | 20 min/d for 6 wk, beginning third postoperative week | 11 | 11 | 37.5 | 45.5 | I: SAFHS 2A; Exogen Inc. C: Sham device |
|
| ||||||||
| Ricardo22 | Scaphoid | Initially immobilized with a long-arm thumb spica cast, followed by a short-arm cast until healing was demonstrated by radiograph. | 20 min/d | 10 | 11 | 26.7† | 26.7 | I: TheraMed 101-B bone-growth stimulator C: Ultrasonography device adjusted to give no signal output across the transducer |
|
| ||||||||
| Lubbert et al.38* | Clavicle | Passive support with a collar and cuff for patients’ convenience as long as needed. | 20 min/d for 28 d | 61 | 59 | Not reported | Not reported | I: Exogen 2000 battery powered main operating unit and a treatment head module transducer (Smith and Nephew Inc.) C: Sham device |
|
| ||||||||
| Yadav et al.42 | Tibia | Managed nonoperatively and prescribed paracetamol and ice packs. | 10 min/d | 39 | 28 | Not reported | Not reported | I: Conventional ultrasonography C: Sham device |
|
| ||||||||
| ESTIM trials | ||||||||
|
| ||||||||
| Barker et al.44* | Tibia | Full leg plaster immobilization, non–weight bearing. | 10 h/d for 24 wk | 9 | 7 | 38.0 ± 18.3 | 29.9 ± 8.0 | I: EM-S; circumferential coils fitted around the cast over fracture site. Pulse frequency: 15Hz. C: Inactive coil |
|
| ||||||||
| Wahlström55 | Extra-articular distal radius | Immobilization alone. | 4 wk (use/d unknown) | 15 | 15 | 61.0 ± 4.3 | 60.8 ± 4.6 | I: Active EM-S; single copper wire placed outside of plaster over fracture. Pulse frequency: 1–1000Hz, Magnetic flux density: 4 gauss. C: As above, without EM-S unit |
|
| ||||||||
| Poli et al.51 | Congenital pseudarthrosis | Surgically treated pseudarthrosis followed by cast immobilization for 3 mo then bracing with progression to weight bearing as tolerated. | 10 h/d for 52 wk | 6 | 6 | Not reported | Not reported | I: Active EM-S; 2 high-impedance coils. Pulse frequency: 75Hz, EMF force: 3.5mV. C: As above, without EM-S unit |
|
| ||||||||
| Borsalino et al.47 | Femoral intertrochant eric wedge osteotomy to treat osteoarthritis | Surgery plus progression to weight bearing as tolerated over 90 d. | 8 h/d for 30 d | 15 | 16 | 56.5 ± 8.8 | 55.1 ± 8.2 | I: Active EM-S; Single coil generating EMF positioned on lateral side of femur. Pulse frequency: 75 Hz; EMF force: 2.5mV or 18 gauss C: Inactive device |
|
| ||||||||
| Sharrard53* | Tibia | Full leg plaster cast with knee at 20–30º. | 12 h/d for 12 wk | 20 | 25 | 34.7 (range 18–84) | 45.4 (range 18–76) | I: EM-S; 2 enclosed external copper wires held over fracture site in Helmboltz configuration. Pulse frequency: 15Hz. C: Sham device |
|
| ||||||||
| Mammi et al.50 | Tibial reduction osteotomy | Postoperation: above-knee cast immobilization and non–weight bearing for 30 d. | 8 h/d for 60 d | 18 | 19 | 62.9 ± 7.9 | 61.1 ± 9.5 | I: Active EM-S unit; EM-S held over cast by Velcro straps. Pulse frequency: 75Hz, EMF force: 3mV C: Sham device |
|
| ||||||||
| Scott et al.52* | Any long bone | Immobilization and weight bearing as tolerated. | 25.4 wk (use/d unknown) | 10 | 11 | 40.6 ± 10.7 | 45.8 ± 20.5 | I: EM-S; 2 stainless steel disks placed over fracture site delivering sine-shaped EMF at frequency of 60000Hz. EMF force: 5–10V C: Sham device |
|
| ||||||||
| Eyres et al.48 | Limb lengthening osteotomies | Surgery plus circular frame with 1mm of distraction/d. | 4 h/d over an average of 24.5 wk | 7 | 6 | 14.4 ± 3.9 | 13.7 ± 2.9 | I: Active EM-S Saddle-shaped coil placed in between proximal and distal fixator pins. Pulse frequency: 15Hz C: Sham device |
|
| ||||||||
| Betti et al.46 | Femoral neck | Three cannulated screws followed by non-descriptive rehabilitation program. | 8 h/d for 90 d | 30 | 35 | 67.1 ± 6.0 Compliant patients only |
68.7 ± 6.0 | I: Active EM-S unit; commercially available pulsed EM-S unit. Pulse frequency: 75Hz, EMF force 3.5mV C: Sham device |
|
| ||||||||
| Simonis et al.54* | Tibia | Fibular osteotomy and external fixator application with compression; non–weight bearing. | 14 h/d for 26 wk | 18 | 16 | 31.7 ± 14.6 | 32.3 ± 16.3 | I: EM-S; pulsed EM-s via 2 external coils in contact with skin held by crepe bandage. Pulse frequency: 23.3Hz, EMF force: 150V, Field intensity: 6A. C: Sham device |
|
| ||||||||
| Beck et al.45 | Tibial stress fracture | Rest from activity plus calcium supplementation (500 mg/d calcium carbonate). | 15 h/d until stress fracture clinically healed | 22 | 21 | Not reported | Not reported | I: Active EM-S; commercially available stimulator unit delivering sinusoidal wave via 2 water-based gel electrodes. Pulse frequency 60Hz, EMF Force: 3–6V, Current: 5–10mA. C: Inactive device |
|
| ||||||||
| Faldini et al.49* | Femoral neck | 3 cannulated screws followed by progressive weight bearing routine starting at 30 d. | 8 h/d for 90 d | 30 | 35 | 67.1 ± 6 compliant; 71.6 ± 3.1 non-compliant | 67.4 ± 6.9 compliant; 69.8 ± 5.2 non-compliant | I: Active EM-S unit; commercially available pulsed EM-S unit. Pulse frequency 75Hz, Peak Value: 2mT C: Sham device |
|
| ||||||||
| Adie et al.43* | Tibia | Nonoperative or operative (Intramedullary nail, plate, or ex-fix) treatment with surgeon dictated rehabilitation. | 10 h/d for 12 wk | 106 | 112 | 38.5 | 39.7 | I: Active EM-S unit; commercially available pulsed EM-S unit (EBI Bone Healing System, Biomet Inc. C: Inactive device |
|
| ||||||||
| Hanneman et al.20* | Acute scaphoid | Immobilization in a forearm cast with the first metacarpal and both phalanges immobilized. | 24 h/d | 24 | 29 | 44.3 | 37.7 | I: PEMF (Ossatec) bone growth stimulator C: Disabled PEMF bone growth stimulator (same device as intervention) |
|
| ||||||||
| Shi et al.31* | Femur, tibia, humerus and radius/ulna | Surgical reduction and fixation of the fracture. | 8 h/d | 31 | 27 | 41 ± 14.5 | 38.4 ± 11.6 | I: Electromagnetic field delivered through a coil (Orthopulse II, OSSATEC) C: Sham signal generator from the same manufacturer |
EMF = electromagnetic force; ESTIM = electrical stimulation; LIPUS = low intensity pulsed ultrasonography; PEMF = pulsed electromagnetic field therapy; SAFHS = Sonic Accelerated Fracture Healing System; SD = standard deviation.
Analyzed quantitatively in the indirect comparison analysis.
Only the age for the full sample was reported, and was thus assumed to be the same for both intervention and control arm.
Risk of bias within studies
Fig. 2 and Table 2 present the risk of bias within included studies.
Fig. 2.
Risk of bias within included studies; (+) denotes low risk of bias; (−) denotes high risk of bias.
Table 2.
Risk of bias within included studies
| Trial | Concealment of allocation | Blinding of patients | Blinding of health care providers | Blinding of data collectors | Blinding of outcome assessors | Blinding of data analysts | Incomplete outcome data, total % | Other bias |
|---|---|---|---|---|---|---|---|---|
| LIPUS trials | ||||||||
| Heckman et al.35 | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 31% | Study funded by Exogen Inc., which produces ultrasonography devices |
| Kristiansen et al.36 | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 28% | None identified |
| Emami et al.32* | Definitely yes | Definitely yes | Probably no | Definitely yes | Definitely yes | Probably no | 3% | None identified |
| Strauss et al.41* | Probably no | Definitely no | Probably no | Probably no | Probably no | Probably no | NR | None identified |
| Mayr et al.39* | Probably no | Definitely no | Probably no | Probably no | Definitely yes | Probably no | 0% | None identified |
| Leung et al.37* | Definitely no | Probably no | Probably no | Probably no | Probably yes | Probably no | 0% | None identified |
| Rue et al.40 | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 0% | 40 participants originally enrolled, but on 26 tibial fractures were analyzed |
| Handolin et al.33* | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 5% | None identified |
| Handolin et al.34* | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 0% (3 mo) 47% (18 mo) |
None identified |
| Ricardo22* | Probably yes | Probably yes | Definitely yes | Probably yes | Probably yes | Probably no | 0% | None identified |
| Lubbert et al.38* | Definitely yes | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 16% | None identified |
| Yadav et al.42 | Probably no | Definitely yes | Probably yes | Probably yes | Probably yes | Probably no | NR | None identified |
| ESTIM trials | ||||||||
| Barker et al.44* | Probably no | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 6% | Evidence that control group may have been exposed to some active EM-S fields |
| Wahlström55 | Probably no | Definitely no | Definitely no | Definitely no | Definitely no | Probably no | 6% | None evident |
| Poli et al.51 | Probably no | Definitely no | Definitely no | Definitely no | Definitely no | Probably no | 0% | None evident |
| Borsalino et al.47 | Probably no | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 3% | None evident |
| Sharrard53* | Probably yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely no | 12% | Younger patients in active group with less comminution |
| Mammi et al.50 | Probably no | Definitely yes | Definitely yes | Probably yes | Definitely yes | Probably no | 7% | None evident |
| Scott et al.52* | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 9% | None evident |
| Eyres et al.48 | Probably no | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 0% | Multiple limbs analyzed in individually randomized patients |
| Betti et al.46 | Probably no | Definitely yes | Probably no | Probably no | Probably no | Probably no | 16% | Most outcomes only report on subset of compliant patients |
| Simonis et al.54* | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 0% | More smokers in control group (81% to 44%) |
| Beck et al.45 | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 14% | None evident |
| Faldini et al.49* | Probably no | Definitely yes | Definitely yes | Probably yes | Probably yes | Probably no | 16% | Most outcomes only report on a subset of compliant patients |
| Adie et al.43* | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 16% | No intention to treat analysis |
| Hanneman et al.20* | Probably yes | Probably yes | Definitely yes | Probably yes | Probably yes | Probably no | 4% | None evident |
| Shi et al.31* | Definitely yes | Probably yes | Definitely yes | Definitely yes | Definitely yes | Probably no | 9% | None evident |
ESTIM = electrical stimulation; LIPUS = low intensity pulsed ultrasonography; NR = not reported.
Analyzed quantitatively in the indirect comparison analysis.
Effect of LIPUS on rate of fracture union
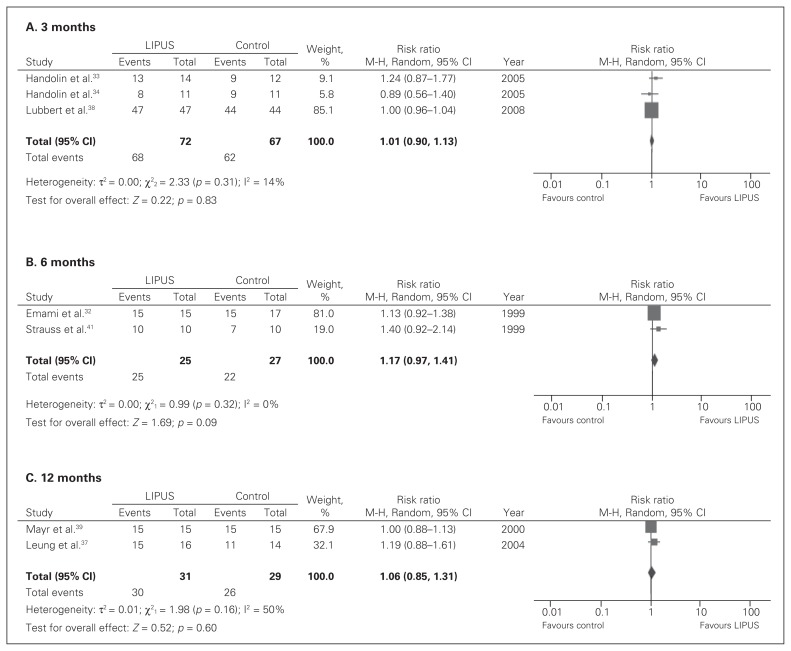
In patients with a fresh fracture, very low-quality evidence showed that LIPUS, when compared with standard care, had no significant effects on improving rates of healing at 3 months (RR 1.01, 95% CI 0.90–1.13; Fig. 3A), 6 months (RR 1.17, 95% CI 0.97–1.41; Fig. 3B) or 12 months (RR 1.06, 95% CI 0.85–1.31; Fig. 3C).
Fig. 3.
Fracture union rates in low intensity pulsed ultrasound (LIPUS) versus control for fresh fractures at 3, 6 and 12 months; (A) 3 months; (B) 6 months; (C) 12 months; events refer to those who had a fracture union. CI = confidence interval.
Effect of ESTIM on rate of fracture union
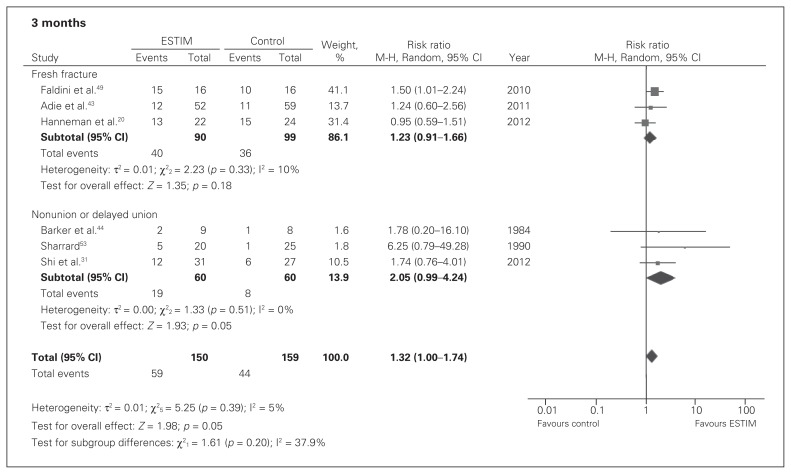
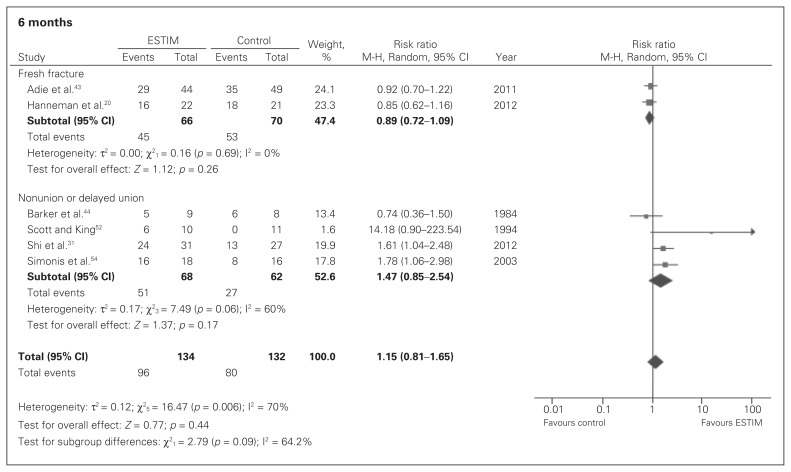
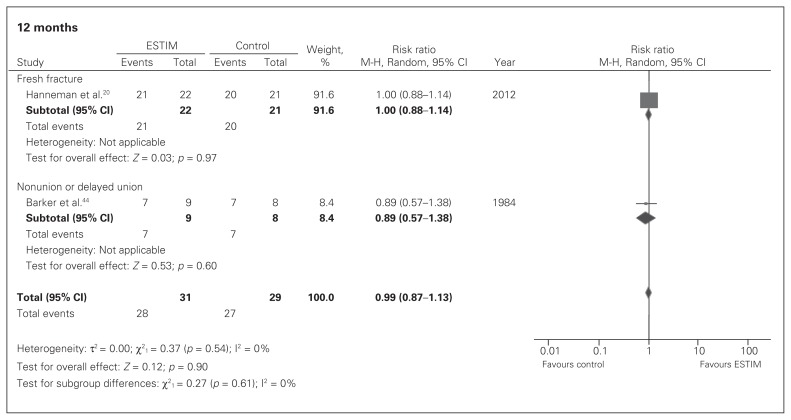
In patients with a fresh fracture, very low-quality evidence showed no significant effects in union rates between ESTIM and standard care at 3 months (RR 1.23, 95% CI 0.91–1.66; Fig. 4), 6 months (RR 0.89, 95% CI 0.72–1.09; Fig. 5) or 12 months (RR 1.00, 95% CI 0.88–1.14; Fig. 6).
Fig. 4.
Fracture union rates in electrical stimulation (ESTIM) versus control for fresh fracture and nonunion populations at 3 months; events refer to those who had a fracture union. CI = confidence interval.
Fig. 5.
Fracture union rates in electrical stimulation (ESTIM) versus control for fresh fracture and nonunion populations at 6 months; events refer to those who had a fracture union. CI = confidence interval.
Fig. 6.
Fracture union rates in electrical stimulation (ESTIM) versus control for fresh fracture and nonunion populations at 12 months; events refer to those who had a fracture union. CI = confidence interval.
In patients with an existing nonunion or delayed union, very low-quality evidence showed that ESTIM, when compared with standard care, had a suggested nonsignificant benefit on union rates at 3 months (RR 2.05, 95% CI 0.99–4.24; Fig. 4) but no significant effect at 6 months (RR 1.47, 95% CI 0.85–2.54; Fig. 5) or 12 months (RR 0.89, 95% CI 0.57–1.38; Fig. 6).
Network meta-analysis of LIPUS and ESTIM on fresh fracture union rates
Results from the network meta-analysis showed that in patients with a fresh fracture, there was a potential non-significant benefit with LIPUS at 6 months (RR 0.76, 95% CI 0.58–1.01).
Confidence in estimates
Using the GRADE system, we rated the confidence in our estimates for the network meta-analysis to be very low (Table 3).
Table 3.
GRADE evidence profile: LIPUS versus ESTIM for improvement in union rates in patients with fresh fractures
| Quality assessment | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Participants (studies) follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | No. of patients | Risk ratio (95% CI) | Risk difference (95% CI) |
| 91 (4 RCTs) | Serious due to lack of reporting allocation concealment and blinding of personnel | Undetected | Indirect comparison | Serious due to less than optimal population size | Undetected | Very low due to risk of bias, indirectness and imprecision | 66 ESTIM; 25 LIPUS | 0.76 (0.58 to 1.01) | −0.25 (−0.47 to 0.03) |
CI = confidence interval; ESTIM = electrical stimulation; GRADE = Grading of Recommendations Assessment, Development and Evaluation; LIPUS = low intensity pulsed ultrasonography; RCT = randomized controlled trial.
Discussion
Summary of findings
We found that neither LIPUS nor ESTIM (compared with standard care) were effective in improving union rates at 3, 6 or 12 months in fresh fracture populations. However, the estimates suggest a potential but nonsignificant benefit of LIPUS at 6 months. In patients with a delayed union or nonunion, ESTIM showed a borderline significant effect in improving union rates (compared with standard care) at 3 months, but not at 6 or 12 months. Data were not available to compare LIPUS with standard care in nonunion populations.
Our network meta-analysis suggested a potential but nonsignificant benefit with an average of 24% greater union rate using LIPUS at 6 months in fresh fracture populations. The wide CIs around the estimates of effect suggest considerable uncertainty about relative effects.
Our findings are consistent with those of 2 recent Cochrane reviews that showed no significant difference over standard care for either LIPUS or ESTIM in improving union rates.3,11 Our review adds 4 additional trials and, to our knowledge, presents the first network analysis to indirectly compare LIPUS and ESTIM for fracture healing.
The strengths of our study include a comprehensive and transparent search strategy, independent and duplicate eligibility assessment and data extraction, use of standard meta-analytic techniques to assess the effectiveness of LIPUS and ESTIM separately in both fresh fracture and nonunion populations, and use of network meta-analysis approaches to obtain our estimates of the improvement in union rates for the comparison between the 2 bone stimulation devices in fresh fracture populations.
Network meta-analysis has been gaining considerable attention for its ability to evaluate interventions that have never been directly compared.56 However, it is important to be cautious of the inferences made from network meta-analyses. A recent article in the Journal of American Medical Association provides guidance for readers to assess the strength of inferences and credibility of a network meta-analysis.57 A critical appraisal of our study using these guidelines is presented in Table 4. Based on these criteria, our review addresses a sensible clinical question for the network meta-analysis and includes all relevant studies. However, we were limited in our comparisons, and given the lack of direct comparisons, we were unable to verify whether the results would have been consistent between direct and indirect comparisons.
Table 4.
Critical appraisal of the network meta-analysis57
| Appraisal question | Response |
|---|---|
| A. Are the results of the study valid? | |
| Did the review explicitly address a sensible clinical question? | The use of LIPUS and ESTIM for fracture healing is equal and high, but there have been no comparative studies evaluating their effect on fracture healing. |
| Was the search for relevant studies exhaustive? | We searched previous Cochrane reviews, the Cochrane Bone, Joint and Muscle Trauma Group Specialized Register, the Cochrane Central Register of Controlled Trials, Medline, Embase, trial registers and reference lists of articles. |
| Were there major biases in the primary studies? | Most studies did not report allocation concealment, some did not report blinding of personnel, and none reported blinding of data analysts. |
| B. What are the results? | |
| What was the amount of evidence in the network? | 15 trials: 7 evaluating LIPUS and 8 evaluating ESTIM. The total sample size for each comparison was less than 200 patients. |
| Were the results similar from study to study? | Heterogeneity was undetected. |
| Were the results consistent in direct and indirect comparisons? | There were no direct comparisons, thus we were unable to measure consistency. |
| What were the overall treatment effects and their uncertainty, and how did the treatments rank? | No comparisons showed a significant effect. There may be a potential but non-significant benefit using LIPUS at 6 mo for fresh fracture populations. The confidence intervals around the effects were wide. |
| Were the results robust to sensitivity assumptions and potential biases? | We did not complete sensitivity analyses, owing to being underpowered. |
| C. How can I apply the results to patient care? | |
| Were all outcomes important to patients considered? | We compared all comparable outcomes for fracture healing. There were no available data to compare outcomes important to patients (e.g., return to work, functional recovery). |
| Were all potential treatment options considered? | We compared only LIPUS and ESTIM. This makes up 93% of bone stimulators used.4 |
| Are any postulated subgroup effects credible? | We were unable to carry out subgroup analyses owing to being underpowered for each comparison. |
| What is the overall quality and what are limitations of the evidence? | The confidence in our estimates was very low (see Table 3). Limitations: fracture union rate, our primary outcome, is a surrogate for functional recovery. Previous reviews evaluating the effectiveness of LIPUS that assessed time to fracture healing have shown a significant benefit; however, we were unable to pool estimates of time to fracture healing given that the limited data. |
ESTIM = electrical stimulation; LIPUS = low intensity pulsed ultrasonography.
Limitations
Our study has limitations. First, for our pooled network meta-analysis, we used fracture union rates as our outcome. This is a surrogate for functional recovery and improvements in union rates may not necessarily translate to commensurate improvements in function.58 Only 5 trials evaluating LIPUS32,34,37,38,40 and no trials evaluating ESTIM reported functional outcomes. Second, fracture union rates may fail to take into account faster healing if the difference in fracture healing appears between reported time points. We had limited data to pool estimates of time to fracture healing in our network meta-analysis. Previous reviews evaluating the effectiveness of LIPUS that assessed time to radiographic fracture healing have shown a significant benefit.11 The 1 trial that evaluated the effectiveness of ESTIM on time to fracture healing showed no difference in time to fracture healing between ESTIM and standard care.20 Thus, it is possible that there is a difference in fracture healing time between LIPUS and ESTIM for fresh fracture populations; if a difference exists, it may be between 3 and 6 months. Third, although we intended to compare LIPUS and ESTIM in patients with an existing nonunion or delayed union, we were able to perform a network meta-analysis only for fresh fracture populations owing to the lack of available data for nonunion populations. This stresses the importance of evaluating the effectiveness of LIPUS in patients with delayed unions or nonunions on union rates, given that current recommendations support its use for this population albeit with no evidence from RCTs. The 1 trial that evaluated the effect of LIPUS on time to fracture healing in nonunion populations was different than typical nonunion populations, as the study included a surgical treatment designed to address nonunions but also administered ultra-sonography to increase bone graft uptake.22 Fourth, we analyzed only studies that provided union rates in our standard and network meta-analyses. We were unable to analyze other eligible studies that did not provide the outcome data. Thus, this may be introducing a potential bias, as there is a possibility that there could be differences between trials that reported and did not report the outcome of interest. Fifth, the confidence in our estimates was very low, as we rated down for risk of bias, imprecision and indirectness using the GRADE system for rating quality of evidence per outcome.59 Finally, our findings provide inferences on the comparative effectiveness of LIPUS and ESTIM that, to our knowledge, have never been directly evaluated in clinical trials. A head-to-head comparison would provide more credibility to our findings.57 However, as bone stimulator trials typically rely on manufacturers to supply both treatment and sham devices, this would involve agreement between industry competitors to collaborate and require investigators to implement comprehensive strategies to minimize any bias that may be introduced as a result.
Conclusion
The current evidence suggests that there may not be significant difference between LIPUS, ESTIM and standard care in improving union rates. There may, however, be a potential benefit to using LIPUS at 6 months for fresh fractures and ESTIM for nonunion populations at 3 months. The evidence in this area is extremely weak, as patient-reported outcomes are not reported, sample sizes are very small, and no direct comparisons of bone stimulation devices exist. Large head-to-head trials with safeguards against bias that assess outcomes inportant to patients (e.g., return to function) are required to confirm or refute the role of bone stimulation devices for fracture healing in either fresh fracture or nonunion populations.
Acknowledgements
We thank Dr. Gordon Guyatt for his intellectual contributions on network meta-analyses and subgroup analyses, and Ms. Rachel Couban for updating the systematic search.
Footnotes
Competing interests: J.W. Busse is the co-principal investigator of an industry-partnered trial to explore the effect of low-intensity pulsed ultrasonography on fracture healing: Trial to Re-evaluate Ultrasound in the Treatment of Tibial Fractures (TRUST). The TRUST trial is registered at clinicaltrials.gov (NCT00667849). No other competing interests declared.
Contributors: S. Ebrahim and M. Bhandari designed the study. S. Ebrahim, B. Mollon and S. Bance acquired the data, which S. Ebrahim, J.W. Busse and M. Bhandari analyzed. S. Ebrahim wrote the article, which all authors reviewed and approved for publication.
References
- 1.Friedlaender GE. Osteogenic protein-1 in treatment of tibial nonunions: current status. Surg Technol Int. 2004;13:249–52. [PubMed] [Google Scholar]
- 2.Aaron RK, Ciombor DM, Simon BJ. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop Relat Res. 2004;(419):21–9. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Griffin XL, Costa ML, Parsons N, et al. Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database Sys Rev. 2011;(4):CD008471. doi: 10.1002/14651858.CD008471.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Busse JW, Morton E, Lacchetti C, et al. Current management of tibial shaft fractures: a survey of 450 Canadian orthopedic trauma surgeons. Acta Orthop. 2008;79:689–94. doi: 10.1080/17453670810016722. [DOI] [PubMed] [Google Scholar]
- 5.Rubin C, Bolander M, Ryaby JP, et al. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83-A:259–70. doi: 10.2106/00004623-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Watson T, Young SR. Therapeutic ultrasound. In: Watson T, editor. Electrotherapy evidence-based practice. Edinburgh (UK): Churchill Livingstone; 2008. [Google Scholar]
- 7.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384–98. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Bashardoust Tajali S, Houghton P, Macdermid JC, et al. Effects of low-intensity pulsed ultrasound therapy on fracture healing: a systematic review and meta-analysis. Am J Phys Med Rehabil. 2012;91:349–67. doi: 10.1097/PHM.0b013e31822419ba. [DOI] [PubMed] [Google Scholar]
- 9.Mundi R, Petis S, Kaloty R, et al. Low-intensity pulsed ultrasound: Fracture healing. Indian J Orthop. 2009;43:132–40. doi: 10.4103/0019-5413.50847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse JW, Kaur J, Mollon B, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ. 2009;338:b351. doi: 10.1136/bmj.b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin XL, Smith N, Parsons N, et al. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Database Syst Rev. 2012;(2):CD008579. doi: 10.1002/14651858.CD008579.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Aaron RK, Boyan BD, Ciombor DM, et al. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;(419):30–7. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ciombor DM, Aaron RK. The role of electrical stimulation in bone repair. Foot Ankle Clin. 2005;10:579–93. doi: 10.1016/j.fcl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Spadaro JA. Mechanical and electrical interactions in bone remodeling. Bioelectromagnetics. 1997;18:193–202. [PubMed] [Google Scholar]
- 15.Mollon B, da Silva V, Busse JW, et al. Electrical Stimulation for Long-Bone Fracture-Healing: A Meta-Analysis of Randomized Controlled Trials. J Bone Joint Surg Am. 2008;90:2322–30. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 16.Wachovia Capital Markets. Equity research: bone growth stimulation. Personal communication. 2012.
- 17.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 18.Mills EJ, Bansback N, Ghement I, et al. Multiple treatment comparison meta-analyses: a step forward into complexity. Clin Epidemiol. 2011;3:193–202. doi: 10.2147/CLEP.S16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 20.Hannemann PF, Göttgens KW, van Wely BJ, et al. The clinical and radiological outcome of pulsed electromagnetic field treatment for acute scaphoid fractures: a randomised double-blind placebo-controlled multicentre trial. J Bone Joint Surg Br. 2012;94:1403–8. doi: 10.1302/0301-620X.94B10.28844. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, editors. Cochrane handbook for systematic reviews of interventions (version 5.0.2) Oxford (UK): Cochrane Collaboration; 2009. Chapter 8: Risk of bias. [Google Scholar]
- 22.Ricardo M. The effect of ultrasound on the healing of muscle-pediculated bone graft in scaphoid non-union. Int Orthop. 2006;30:123–7. doi: 10.1007/s00264-005-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montori V, Ioannidis J, Cook DJ, et al. Fixed-effects and random-effects models. In: Guyatt G, Rennie D, Meade MO, et al., editors. Users’ guides to the medical literature: A manual for evidence-based clinical practice. 2nd ed. New York (NY): McGraw-Hill; 2008. pp. 555–562. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thompson SG, editors. Cochrane handbook for systematic reviews of interventions (version 5.0.2) Oxford (UK): Cochrane Collaboration; 2009. Chapter 952: Identifying and measuring heterogeneity. [Google Scholar]
- 28.Higgins JPT, Thompson SG, editors. Cochrane handbook for systematic reviews of interventions (version 5.0.2) Oxford (UK): Cochrane Collaboration; 2009. Chapter 10: Addressing reporting biases. [Google Scholar]
- 29.Atkins D, Best D, Briss PA, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–7. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: rating the quality of evidenced–introduction. J Clin Epidemiol. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Shi H-F, Xiong J, Chen Y-X, et al. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled study. BMC Musculoskelet Disord. 2013;14:35. doi: 10.1186/1471-2474-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emami A, Petren-Mallmin M, Larsson S. No effect of low intensity ultrasound on healing time of intramedullary fixed tibial fractures. J Orthop Trauma. 1999;13:252–7. doi: 10.1097/00005131-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Handolin L, Kiljunen V, Arnala I, et al. The effect of low intensity ultrasound and bioabsorbable self-reinforced poly-L-lactide screw fixation on bone in lateral malleolar fractures. Arch Orthop Trauma Surg. 2005;125:317–21. doi: 10.1007/s00402-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 34.Handolin L, Kiljunen V, Arnala I, et al. Effect of ultrasound therapy on bone healing of lateral malleolar fractures of the ankle joint fixed with bioabsorbable screws. J Orthop Sci. 2005;10:391–5. doi: 10.1007/s00776-005-0901-0. [DOI] [PubMed] [Google Scholar]
- 35.Heckman JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing by non-invasive, low-intesity pulsed ultrasound. J Bone Joint Surg Am. 1997;79:961–73. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kristiansen TK, Ryaby JP, McCabe J, et al. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: a multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961–73. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Leung KS, Lee WS, Tsui HF, et al. Complex tibial fracture outcomes following treatment with low-intensity pulsed ultrasound. Ultrasound Med Biol. 2004;30:389–95. doi: 10.1016/j.ultrasmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Lubbert PHW, van der Rijt RHH, Hoorntje LE, et al. Low-intensity pulsed ultrasound (LIPUS) in fresh clavicle fractures: a multi-centre double blind randomised controlled trial. Injury. 2008;39:1444–52. doi: 10.1016/j.injury.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Mayr E, Rudzki MM, Rudzki M, et al. Does pulsed low-intensity ultrasound accelerate healing of scaphoid fractures? Handchir Mikrochir Plast Chir. 2000;32:115–22. doi: 10.1055/s-2000-19253. [DOI] [PubMed] [Google Scholar]
- 40.Rue JP, Armstrong DW, III, Frassica FJ, et al. The effect of pulsed ultrasound in the treatment of tibial stress fractures. Orthopedics. 2004;27:1192–5. doi: 10.3928/0147-7447-20041101-18. [DOI] [PubMed] [Google Scholar]
- 41.Strauss E, Ryaby JP, McCabe J. Treatment of Jones’ fractures of the foot with adjunctive use of low-pulsed ultrasound stimulation. J Orthop Trauma. 1999;13:310. [Google Scholar]
- 42.Yadav YK, Salgotra KR, Banerjee A. Role of ultrasound therapy in the healing of tibial stress fractures. MJAFI. 2008;64:234–6. doi: 10.1016/S0377-1237(08)80101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adie S, Harris IA, Naylor JM, et al. Pulsed electromagnetic field stimulation for acute tibial shaft fractures: a multicenter, double-blind, randomized trial. J Bone Joint Surg Am. 2011;93:1569–76. doi: 10.2106/JBJS.J.00869. [DOI] [PubMed] [Google Scholar]
- 44.Barker AT, Dixon RA, Sharrard WJ, et al. Pulsed magnetic field therapy for tibial non-union. Interim results of a double-blind trial. Lancet. 1984;1:994–6. doi: 10.1016/s0140-6736(84)92329-8. [DOI] [PubMed] [Google Scholar]
- 45.Beck BR, Matheson GO, Bergman G, et al. Do capacitively coupled electric fields accelerate tibial stress fracture healing? A randomized controlled trial. Am J Sports Med. 2008;36:545–53. doi: 10.1177/0363546507310076. [DOI] [PubMed] [Google Scholar]
- 46.Betti E, Marchetti S, Cadossi R, et al. Effect of stimulation by low-frequency pulsed electromagnetic fields in subjects with fracture of the femoral neck. In: Bersani F, editor. Electricity and magnetism in biology and medicine. New York (NY): Kluwer Academic/Plenum; 1999. pp. 853–855. [Google Scholar]
- 47.Borsalino G, Bagnacani M, Bettati E, et al. Electrical stimulation of human femoral intertrochanteric osteotomies. Double-blind study. Clin Orthop Relat Res. 1988;(237):256–63. [PubMed] [Google Scholar]
- 48.Eyres KS, Saleh M, Kanis JA. Effect of pulsed electromagnetic fields on bone formation and bone loss during limb lengthening. Bone. 1996;18:505–9. doi: 10.1016/8756-3282(96)00070-1. [DOI] [PubMed] [Google Scholar]
- 49.Faldini C, Cadossi M, Luciani D, et al. Electromagnetic bone growth stimulation in patients with femoral neck fractures treated with screws: prospective randomized double-blind study. Curr Orthop Pract. 2010;21:282–7. [Google Scholar]
- 50.Mammi GI, Rocchi R, Cadossi R, et al. The electrical stimulation of tibial osteotomies. Double-blind study. Clin Orthop Relat Res. 1993;(288):246–53. [PubMed] [Google Scholar]
- 51.Poli G, Dal Monte A, Cosco F. Treatment of congenital pseudarthrosis with endomedullary nail and low frequency pulsing electromagnetic fields: a controlled study. Electromagn Biol Med. 1985;4:195–209. [Google Scholar]
- 52.Scott G, King JB. A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J Bone Joint Surg Am. 1994;76:820–6. doi: 10.2106/00004623-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br. 1990;72:347–55. doi: 10.1302/0301-620X.72B3.2187877. [DOI] [PubMed] [Google Scholar]
- 54.Simonis RB, Parnell EJ, Ray PS, et al. Electrical treatment of tibial non-union: a prospective, randomised, double-blind trial. Injury. 2003;34:357–62. doi: 10.1016/s0020-1383(02)00209-7. [DOI] [PubMed] [Google Scholar]
- 55.Wahlström O. Stimulation of fracture healing with electromagnetic fields of extremely low frequency (EMF of ELF) Clin Orthop Relat Res. 1984;(186):293–301. [PubMed] [Google Scholar]
- 56.Salanti G, Schmid CH. Research Synthesis Methods special issue on network meta-analysis: introduction from the editors. Res Syn Meth. 2012;3:69–70. doi: 10.1002/jrsm.1050. [DOI] [PubMed] [Google Scholar]
- 57.Mills EJ, Ioannidis JPA, Thorlund K, et al. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308:1246–53. doi: 10.1001/2012.jama.11228. [DOI] [PubMed] [Google Scholar]
- 58.Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9:171–9. doi: 10.1586/erp.09.7. [DOI] [PubMed] [Google Scholar]
- 59.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]