Abstract
The influence of central neuronal oscillators on human physiological tremor is controversial. To address this, transcranial alternating current stimulation (TACS) was delivered at peak tremor frequency to 12 healthy volunteers in a 2 × 2 crossover study. Two sites were stimulated [contralateral primary motor cortex (M1), vs ipsilateral cerebellum] while participants performed two types of tasks designed to probe the different manifestations of physiological tremor of the hand–kinetic and postural tremor. Tremor was measured by accelerometry. Cortical coherence with the accelerometry signal was also calculated in the absence of stimulation. The phase synchronization index, a measure of the phase entrainment of tremor, was calculated between stimulation and tremor waveforms. The amplitude modulation of tremor was similarly assessed. There was significant phase entrainment that was dependent both on tremor type and site of stimulation: M1 stimulation gave rise to phase entrainment of postural, but not kinetic, tremor, whereas cerebellar stimulation increased entrainment in both cases. There was no effect on tremor amplitude. Tremor accelerometry was shown to be coherent with the cortical EEG recorded during postural, but not kinetic, tremor. TACS modulates physiological tremor, and its effects are dependent both on tremor type and stimulation site. Accordingly, central oscillators play a significant role in two of the major manifestations of tremor in health.
Keywords: accelerometry, coherence, entrainment, phase synchronization index, physiological tremor, transcranial alternating current stimulation
Introduction
Tremor is an involuntary periodic oscillation of a body part. Not only is it a cardinal symptom in many disorders of movement, it is also exhibited to a more limited extent by all healthy people. The investigation of this so-called “physiological tremor” in healthy volunteers has given insight into the altered physiology that may be responsible for pathological tremors (Lance, 1975; Marsden, 1978, 1984; McAuley and Marsden, 2000; Donaldson et al., 2012) and may elucidate the mechanisms of human neuromuscular control (Matthews, 1972; Stein, 1974).
Physiological tremor is not a homogeneous entity. The existence of a physiological tremor at a peak frequency of ∼7–12 Hz during postural contractions of the outstretched hands is well established (Stiles and Randall, 1967; Marsden et al., 1969; Elble and Randall, 1978; Marsden, 1984; Hömberg et al., 1987; Lakie, 1992; Raethjen et al., 2000). A tremor of similar frequency has also been identified during slow movements of the upper limb (Vallbo and Wessberg, 1993; Wessberg and Vallbo, 1995b) and has been classified as kinetic tremor (Deuschl et al., 1998).
There remains a debate concerning the contributions of central versus peripheral mechanisms to physiological tremor (Herbert, 2012; Brittain and Brown, 2013). Several lines of evidence suggest that physiological tremor is secondary to central oscillatory activity (Conway et al., 1995; Wessberg and Vallbo, 1995a, b, 1996; Elble, 1996; Kakuda et al., 1999; Wessberg and Kakuda, 1999; Duval et al., 2000, 2005;McAuley and Marsden, 2000; Gross et al., 2002; Evans and Baker, 2003; Jaberzadeh et al., 2003; Proudlock and Scott, 2003; Bye and Neilson, 2010). However, mechanical resonance of the limb alone has been shown to be sufficient to produce a characteristic physiological tremor spectrum (Vernooij et al., 2013), and it has been argued that the apparent motor unit synchronization and observations of corticomuscular coherence with tremor are epiphenomenal and a mere consequence of peripheral resonance (Lakie et al., 2012).
Recently, a novel interventional technique using noninvasive brain stimulation over the motor cortex was shown to be capable of reducing tremor amplitude in patients with Parkinson's disease (Brittain et al., 2013). Peripheral tremor recordings were used as a proxy for the central oscillatory tremor network with rhythmic transcranial stimulation delivered to cancel out the tremor oscillations.
Here, we extend this approach to examine postural and kinetic physiological tremor in healthy volunteers. To further explore the neural substrates involved, transcranial alternating current stimulation (TACS; for review, see Herrmann et al., 2013) was applied not only to primary motor cortex (M1), but also to cerebellum. We hypothesized that stimulation over the latter would lead to modulation of physiological tremor, given the putative role of the cerebellum (Welsh et al., 1995) and olivocerebellar system (Llinás, 1991) as a central “clock” driving cortical oscillations, the classical reports of unilateral cerebellar lesions reducing physiological postural tremor in the ipsilateral arm (Holmes, 1917, 1922a,b,c,d), and the identification of a cerebello-thalamo-cortical loop that is thought to be key mediator of physiological kinetic tremor (Gross et al., 2002). We further hypothesized that motor cortical stimulation would be less effective in kinetic, than postural, tremor, given that corticomuscular coherence has been observed in the former, but not in the latter (Marsden et al., 2001).
Materials and Methods
Participants
The study was performed on 12 healthy volunteers (7 males; mean age 34 years, range 19–57 years), all of whom provided informed written consent. One participant was left-handed. The study was approved by the Oxfordshire Research Ethics Committee B, in accordance with the Declaration of Helsinki on the use of human participants in experiments. Each participant completed both experiments, which were performed in the same session.
Experiments
Study design.
The differential effect of rhythmic transcranial stimulation on physiological tremor was studied in a 2 × 2 crossover study. Two stimulation sites were examined (contralateral M1 vs ipsilateral cerebellum) while participants performed two tasks designed to probe the two different manifestations of physiological tremor–namely, kinetic and postural tremor. TACS was applied at each participant's task-dependent peak tremor frequency. Since the stimulation was not forced to align with the ongoing tremor, slow drifts in phase alignment resulted between stimulation and tremor waveforms. Accordingly, this technique permits the evaluation of entrainment and amplitude modulation as a function of phase-alignment between the rhythmic tremor and stimulation signals (Brittain et al., 2013; Helfrich et al., 2014).
The postural task involved participants holding a light weight (Fellowes Crystals Gel Flex Rest; 88 × 122 × 18 mm, 181 g) on the palmar surface of their right hand of the outstretched supinated arm, with their fingers splayed. The position adopted was one that was comfortable for the participant at all times during the task. Participants were instructed to maintain the position of their upper limb over four sequential blocks of 90 s, with 1 min of rest in between each block. They were asked to report if they were experiencing fatigue, at which point longer rest periods were introduced as necessary.
The kinetic task involved participants making phasic movements that consisted of rhythmic, self-paced, right index finger flexion and extension through an arc of ∼60° at the metacarpophalangeal joint, at a rate of ∼0.5 Hz for 10 min. The longer recording duration for the kinetic task accounted for the known discontinuous nature of the tremor (Vallbo and Wessberg, 1993; Wessberg and Vallbo, 1995a).
Experiment 1: electroencephalography (without transcranial stimulation).
This served two purposes: (1) to provide an electroencephalogram (EEG) correlate of tremor, replicating the previous findings of Marsden et al. (2001) (see earlier comments), and (2) to act as surrogate data in significance testing (see later). Participants were seated and instructed to maintain vigilance with their eyes open. Electroencephalography was obtained from the scalp (see below) with postural and kinetic tasks performed, as above, in an order that was counterbalanced between participants.
Experiment 2: tremor-frequency TACS.
TACS electrodes were secured to the scalp over contralateral M1 and ipsilateral cerebellum (see below). The kinetic and postural tasks were performed as in Experiment 1, but with TACS delivered at the task-dependent peak frequency of tremor, determined from Experiment 1. Each task was performed twice, once with stimulation applied over contralateral M1, and once with stimulation applied over ipsilateral cerebellum. A 2 min rest break was afforded between each stimulation condition. The order of task and stimulation site (four combinations) was randomly interleaved between, and counterbalanced within, participants.
Transcranial stimulation
Stimulation was performed in accordance with current safety guidelines (Nitsche et al., 2003; Rossi et al., 2009).
Single pulse transcranial magnetic stimulation via a Magstim Super Rapid stimulator was delivered using a figure-of-eight coil applied to the scalp overlying left M1 to locate the motor hotspot that consistently evoked contralateral index finger movement (Rossini et al., 1994).
TACS was delivered through conductive rubber electrodes (neuroConn) enclosed in saline-soaked sponges using a DC-Stimulator Plus (neuroConn). Stimulation electrodes (5 × 7 cm) were placed both over the left motor hotspot and 3 cm lateral to the inion to overlie the right cerebellar hemisphere. These electrodes were secured in place using Velcro straps; a larger reference electrode (5 × 11 cm) was positioned on the contralateral (left) shoulder and secured using hypoallergenic dressing tape. The setup was optimized to ensure that impedance, as measured by the stimulation device, was <10 kΩ. The frequency of the sinusoidal stimulation waveform was matched to each participant's task-dependent peak tremor frequency to the nearest 0.1 Hz (as determined in Spike2, version 7.12b; Cambridge Electronic Design). Stimulation was delivered with no direct current offset at a peak-to-peak amplitude of 2000 μA.
Recordings
EEG and accelerometry measurements were obtained. Following skin preparation with Nuprep gel (Weaver and Company), silver/silver chloride EEG electrodes were placed over: F3, Fz, F4; FC3, FCz, FC4; C3, Cz, C4, and FPz, as per the international 10–20 system of electrode placement (Sharbrough et al., 1991). Electrodes were affixed using Ten20 conductive paste gel (Weaver and Company) and recorded in a common-reference configuration. Additionally, bipolar recordings were taken between the left motor hotspot and FPz (in 10/12 participants). A tri-axial accelerometer (Twente Medical Systems International) was attached onto the dorsum of the index finger of the right hand. The orientation of the accelerometer was fixed across participants, with the z-axis traversing the plane of maximal tremor amplitude perpendicular to the ground. The EEG and accelerometer signals were recorded using a 32-channel Porti amplifier (Twente Medical Systems International) and in-house bespoke software (EditEEG, Dr. A. Pogosyan, Oxford). The signals were sampled at 2048 Hz, amplified (×20 for EEG) and high-pass filtered at 4 Hz.
Data analysis
Accelerometry.
Data were analyzed off-line using MATLAB 8 (version R2013a; The MathWorks). Maximal tremor frequency was extracted following principal component analysis of the filtered tri-axial accelerometer signal. The first principal component was selected and polarity matched to the z-axis of the accelerometer for subsequent analysis. Principal component analysis ensures that the maximal plane of tremor is considered, accounting for any minor variations in the placement of the accelerometer between participants, while polarity matching fixes a common orientation for evaluation across participants. The spectral peak was extracted using Thomson's multitaper method (Thomson, 1982; Percival, 1993), using K = 12 tapers. The signal was then zero-phase bandpass filtered (forward–backward filtering) using a third-order Butterworth filter, centered about the TACS frequency that had been delivered during the task (2 Hz passband). Instantaneous phase and amplitude information were extracted from the derived accelerometer and TACS waveforms via the Hilbert transformation. The amplitude envelope of the derived accelerometer signal was normalized in a task-dependent manner per participant via the Box–Cox transformation (Box and Cox, 1964). Data were segmented and analyzed in 30 s segments to facilitate the construction of confidence intervals.
Data obtained during Experiment 1, when stimulation was not applied, also formed “surrogate” stimulation datasets. Sinusoidal stimulation waveforms were substituted, matched to the TACS frequency that was applied in the stimulation conditions in Experiment 2, for each participant and task. This permitted robust statistics to be formed characterizing the effects of stimulation.
To quantify the degree of phase entrainment on physiological tremor by TACS, the time-dependent phase difference (ϕt) between accelerometer and TACS time series was derived and regularized (smoothed by 25% of its mean cycling time). Synchronization would imply adjustment of the physiological tremor rhythm to stimulation over time. To quantify this, the phase synchronization index (PSI) was computed for each 30 s segment (Eq. 1) and averaged over segments. By construction, PSI = 0 if the signals are uncoupled and the phase difference uniformly distributed, whereas PSI = 1 when the signals are perfectly synchronized, leading to a constant phase difference. Entrainment likelihood histograms were also constructed by stratifying phase difference into 20 bins, and normalizing to unit sum.
 |
In a similar fashion, the degree of amplitude modulation as a result of TACS was determined by calculating the mean amplitude (following Box–Cox transformation) corresponding to each phase difference bin. Finally, PSIs were derived from the amplitude histograms to quantify the degree of amplitude modulation.
To determine the relative sensitivity of physiological tremor to TACS over M1 versus cerebellum, sensitivity ratios were calculated per participant for each task (Eq. 2).
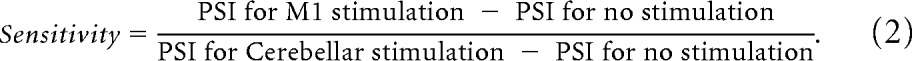 |
Coherence.
Spectral coherence was calculated between the z-axis of the accelerometer and the bipolar EEG electrode overlying the motor hotspot. The presence of a significant coherence peak (p < 0.05 testing the null hypothesis of independence; Halliday et al., 1995) at the frequency of stimulation (± 0.5 Hz) was determined.
Statistics.
Statistical analyses were performed using IBM SPSS Statistics for Windows (version 20.0.0; IBM). Normality of data was examined using the Shapiro–Wilk test. Two-way repeated-measures ANOVA was used to examine the main effects and interaction of Stimulation (three levels: surrogate, M1, and cerebellum) and Task (two levels: kinetic and postural) on PSI and amplitude modulation. Mauchly's test was performed to identify violations of the assumption of sphericity. The significance level was set at p < 0.05. Conditional on a significant F value, post hoc, two-tailed paired samples Student's t tests were performed, corrected for multiple comparisons by the false discovery rate (FDR). Correlation coefficients were calculated using the nonparametric Kendall's tau (τ). Permutation analysis was used to examine whether the observed group split in cortical-tremor coherence was a chance occurrence or not. Unless otherwise stated, arithmetic means are reported ± 1 SEM.
Results
All participants completed the experiments and there were no reported incidences of phosphenes during stimulation or adverse effects following TACS. Figure 1 illustrates exemplar accelerometer and power spectra for one participant. Kinetic tremor, in contrast to postural tremor, demonstrated phasic activity in accordance with finger movements. Peak frequencies in the power spectra of postural and kinetic tremor were not significantly different (8.51 ± 0.40 Hz vs 8.33 ± 0.44 Hz, respectively; t(11) = 0.28, p = 0.787, two-tailed paired Student's t test) and the application of TACS to either contralateral M1 or ipsilateral cerebellum was not associated with a shift in peak tremor frequency (all |t(11)| < 1.49, p > 0.05, two-tailed paired samples Student's t tests).
Figure 1.
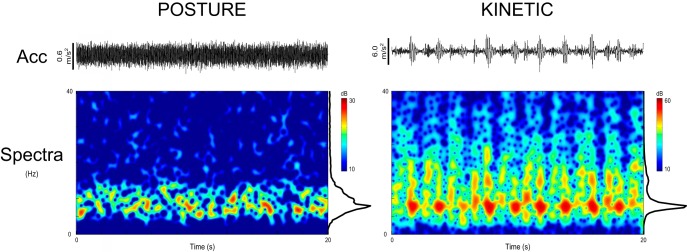
Exemplar accelerometer and time-frequency spectrograms from one participant for postural and kinetic tasks over a 20 s time period. Acc denotes the 4 Hz high-pass filtered z-axis accelerometer signal (hence slow-frequency undulations due to movement are absent). The spectrograms permit visualization of the burst-like structure (“discontinuities”) of the kinetic physiological tremor waveform, in contrast to postural physiological tremor. Note the difference in scale between postural and kinetic tremors.
Phase entrainment of physiological tremor is dependent on tremor type and site of stimulation
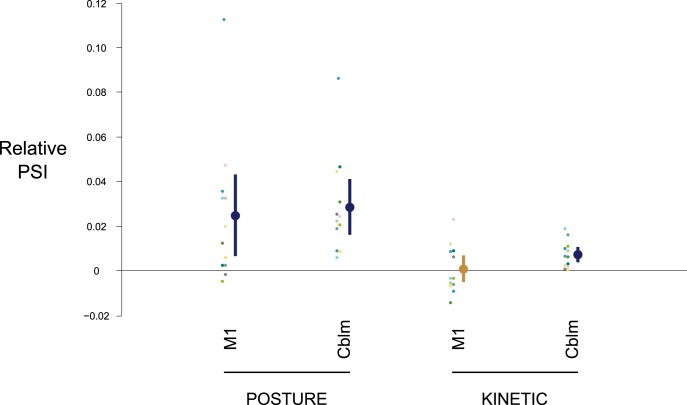
Figure 2 shows representative phase difference histograms (between tremor and TACS) normalized as probability distributions for one participant for postural and kinetic tasks. The extent of entrainment was quantified by PSI, which conformed to a Normal distribution at the group level (p > 0.05, Shapiro–Wilk test), and Mauchly's test did not show any violation of the assumption of sphericity. A two-way repeated-measures ANOVA showed significant main effects of Task (F(1,11) = 23.86, p < 0.001) and Stimulation (F(2,22) = 9.35, p = 0.001), with a significant Task × Stimulation interaction (F(2,22) = 4.93, p = 0.017; Fig. 3). Post hoc two-tailed, paired samples Student's t tests revealed an effect of M1 stimulation on postural, but not kinetic, tremor relative to surrogates (Posture: t(11) = 2.64, p = 0.023, PSI = 0.053 ± 0.009; Kinetic: t(11) = 0.27, p = 0.789, PSI = 0.019 ± 0.003; Surrogates: PSIposture = 0.029 ± 0.004, PSIkinetic = 0.018 ± 0.003). In contrast, stimulation over ipsilateral cerebellum was less selective, increasing entrainment in both the postural and kinetic tasks (Posture: t(11) = 4.46, p = 0.001, PSI = 0.057 ± 0.007; Kinetic: t(11) = 4.09, p = 0.002, PSI = 0.025 ± 0.004; Surrogates: PSIposture = 0.029 ± 0.004, PSIkinetic = 0.018 ± 0.003). Indeed, TACS over M1 and cerebellum increased entrainment in postural tremor by 126 ± 62 and 133 ± 43%, respectively, compared with the surrogate dataset; similarly, TACS over cerebellum increased entrainment in kinetic tremor by 49 ± 16%. Intra-individual variability of PSI is illustrated in Figure 3.
Figure 2.
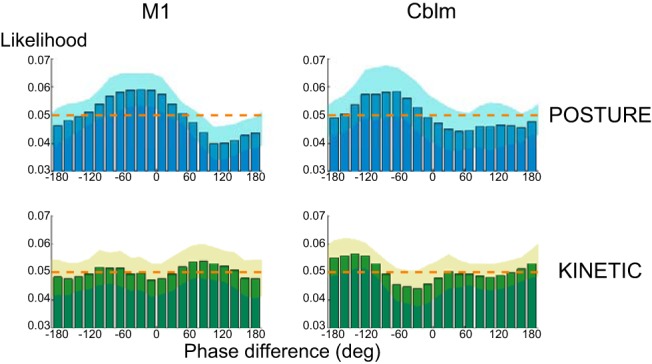
Exemplar likelihood histograms of phase difference between tremor and stimulation signals normalized as probability distributions for one participant for Posture and Kinetic tasks. The shaded regions depict intrasubject pointwise 95% confidence intervals derived from averaging the 30 s segments (see Materials and Methods). The dashed orange line demarcates the uniform distribution expected in the absence of entrainment; phase synchronization (entrainment) is shown by the presence of a peak in the distribution. Thus, for this participant, the histograms show that entrainment capacity following TACS is greater for postural, than kinetic, tremor. M1, contralateral primary motor cortex; Cblm, ipsilateral cerebellum.
Figure 3.
Scatter plot of group mean PSI (larger dots colored dark blue or gold) showing the influence of TACS on entrainment with respect to stimulation site (M1, contralateral primary motor cortex vs Cblm, ipsilateral cerebellum) and type of physiological tremor (postural vs kinetic tremor). The ordinate reflects the pairwise differences in PSI relative to surrogate datasets (see Materials and Methods). The vertical error bars span the 95% confidence interval for the groups. Dark blue coloring denotes significant comparisons shown by post hoc two-tailed, paired samples Student's t tests (p < 0.05, corrected for multiple comparisons by the FDR). The smaller dots depict individual participant's (color-coded) mean PSI.
The mean preferred phases for entrainment calculated from the derived PSI values as a result of TACS over the cerebellum were −100.1° (for postural tremor) and −65.6° (for kinetic tremor); similarly, stimulation over M1 for postural tremor led to a mean preferred phase of −49.7°.
Stimulation has no effect on the amplitude of physiological tremor
The extent of amplitude modulation was similarly examined; the data conformed to a Normal distribution at the group level (p > 0.05, Shapiro–Wilk test) and Mauchly's test did not show any violation of the assumption of sphericity. Two-way repeated-measures ANOVA showed no main effects of Task (F(1,11) = 0.068, p = 0.799) or Stimulation (F(2,22) = 3.033, p = 0.069), and there was no Task × Stimulation interaction (F(2,22) = 0.732, p = 0.492). Thus, there was no significant modulation of either postural or kinetic tremor amplitude by TACS.
The relative site-dependent effects of TACS in postural tremor are independent of those in kinetic physiological tremor
To address the possibility that the two stimulation sites might reflect the same response but with different sensitivities to stimulation, sensitivity ratios were calculated per participant (see Materials and Methods). Correlation analysis showed that there was no significant correlation between the sensitivities for postural and kinetic tasks, suggesting that the relative effects of stimulation between the two sites in the postural task were independent of the relative effects of stimulation between the two sites in the kinetic task (p < 0.05, Shapiro–Wilk test, therefore Kendall's τ was calculated: τ = −0.12, p = 0.58).
Cortical coherence in physiological tremor is a feature of postural but not kinetic tremor
In 6 of10 participants, coherence analysis demonstrated significant coupling at the frequency of stimulation (which was, by design, matched to the peak tremor frequency) between the EEG signal over the contralateral motor hotspot, and the accelerometer recording of the tremor during the postural task. In contrast, none of these 10 participants demonstrated coherence during the kinetic task. Given two groups with 10 observations in each, the probability of all six significant values falling into the same group can be computed through combinatorial statistics. In this instance, permutation analysis revealed that the probability of observing all six significant coherence values within the same group is p = 0.011. Figure 4 shows exemplar spectra and coherence plots from one participant.
Figure 4.
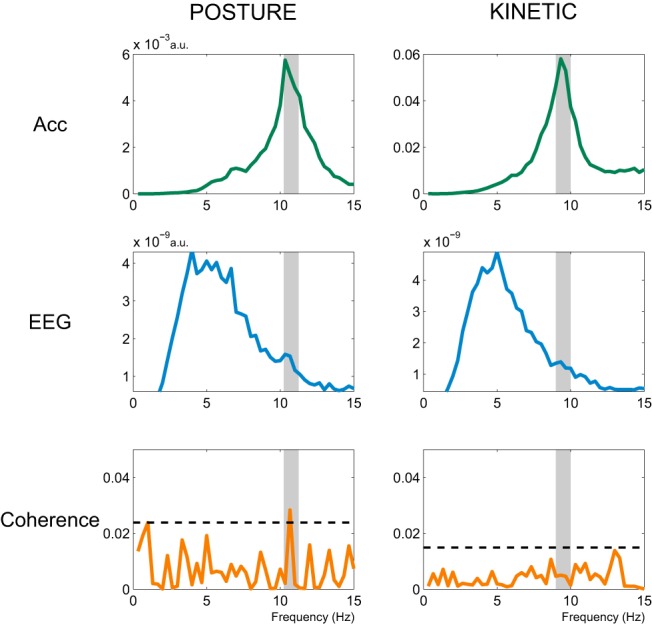
Exemplar power spectra (arbitrary units) of the z-axis of the accelerometer (green trace) and of M1 EEG (blue trace), together with spectral coherence between EEG and Acc (orange trace) for one participant for both postural and kinetic tasks. Both the accelerometer and EEG signals were 4 Hz high-pass filtered. The shaded region represents the ±0.5 Hz coherence window centered on the selected TACS frequency (see Materials and Methods). There is a significant peak in coherence at the TACS frequency in postural, but not kinetic, tremor, as illustrated by the orange trace crossing the horizontal black dashed line marking the threshold of p < 0.05 for testing the null hypothesis of independence.
Discussion
Human physiological tremor proved susceptible to TACS, with the level of entrainment achieved dependent on motor task and stimulation site. Specifically, stimulation over M1 gave rise to phase entrainment of postural, but not kinetic, tremor, whereas cerebellar stimulation increased entrainment in both cases.
Our results support previous studies that suggest a key, albeit not exclusive, role of central oscillators in physiological tremor. Thus, M1 stimulation modulated the phase of postural tremor. In addition, 6 of10 participants in the present study demonstrated cortical coherence with the peripheral tremor in the postural task, a level consistent with that which has been previously described (Ushiyama et al., 2011). Ozen et al. (2010) showed in rats that modulation by TACS was more effective when the underlying cortex exhibited endogenous slow-wave oscillations, and the presence of coherent cortical and tremor oscillations suggests that this may have also been the case in the present study. In contrast, TACS over M1 did not entrain kinetic tremor. This is consistent with earlier work that adopted magnetoencephalography (MEG) to examine the correspondence between cortical oscillations and postural versus kinetic tremor, as measured by electromyography (Marsden et al., 2001). This, too, demonstrated cortical involvement in physiological postural tremor, but not in kinetic tremor. Finally, epicortical recordings from grid electrodes over M1 made simultaneously with recordings of postural tremor in patients with epilepsy also confirm coherence between oscillatory activity at the two levels, with M1 leading tremor activity at an interval compatible with conduction in fast pyramidal pathways (Raethjen et al., 2002).
Stimulation of the cerebellum, on the other hand, modulated the phase of both postural and kinetic physiological tremor. This was a relatively stronger and more consistent effect than for M1 stimulation. A conceptual, yet parsimonious, explanation of these findings is that M1 and cerebellum interact in patterning postural tremor. The mean phase differences that elicited maximal entrainment with stimulation at the two sites of intervention raise the possibility that the cerebellar system might even drive M1, which then provides descending tremorogenic output in physiological postural tremor (Fig. 5). Although such a hypothesis needs to be directly tested, further support for it is the fact that physiological postural tremor is lost after thalamic lesions focused on the posterior portion of the ventral lateral nucleus, and believed to include the cerebello-thalamo-cortical pathway (Duval et al., 2000, 2005). In contrast, TACS of the cerebellum was able to modulate the phase of kinetic tremor, whereas stimulation of M1 could not. Similarly, none of the participants demonstrated coherence between EEG and kinetic tremor. Accordingly, it seems plausible that the cerebellar system provides a tremorogenic output that is not relayed via M1 in physiological kinetic tremor (Fig. 5). Other studies have reported coherence between motor cortical activity and kinetic tremor, but these need not necessarily suggest that M1 is involved on the efferent side of tremor generation. This question–of directionality in kinetic tremor–has proved contentious. Studies in monkeys suggest that coupling between motor cortex and kinetic tremor may be afferent in nature (given that the cortical activity lagged the tremor), as determined by both phase and directed coherence (Williams et al., 2009), while some MEG investigations in humans suggest that M1 activity leads kinetic tremor (Gross et al., 2002). Regardless, our interventional experiments indicate that the motor cortex is differentially involved, at least in relative terms, in postural and kinetic physiological tremor. This conclusion is further supported by the observation that the relative sensitivities of entrainment over the two stimulation sites differed between postural and kinetic tremors.
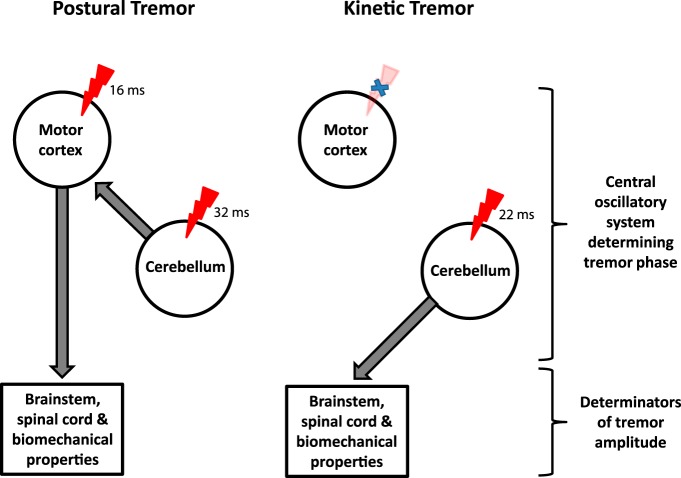
Figure 5.
A hypothesized unifying framework elucidating physiological tremor. This schema distinguishes the role of central oscillatory circuits involving motor cortex and cerebellum on tremor phase, from the role of brainstem, spinal cord, and biomechanics on tremor amplitude. The red symbol marks the application of TACS to M1 or cerebellum. This causes significant phase entrainment that depends both on tremor type and site of stimulation: M1 stimulation causes phase entrainment of postural, but not kinetic, tremor, whereas cerebellar stimulation increases entrainment in both types of physiological tremor. TACS has no effect on the amplitude of physiological tremor, suggesting a dominant role of factors downstream of central oscillators in modulating physiological tremor amplitude. The numbers relate to the calculated conduction delays using the mean preferred phase angles for a stimulation induced effect on tremor phase.
Our data also tend to refute the hypothesis that physiological tremor, whether postural or kinetic, is exclusively mediated by peripheral and/or spinal mechanisms (Joyce and Rack, 1974; Lakie et al., 1986, 2012; Vernooij et al., 2013). This hypothesis posits that physiological tremor is either the consequence of broad-band forcing of a nonlinear, resonant system, and that synchronous central input is not required (Vernooij et al., 2013), or that it reflects frequency components in the band of the firing rates of last-recruited, large motor units (Christakos et al., 2006), or that local oscillatory reflex loops induce synchronous motor unit activity at the level of the spinal cord (Lippold, 1970; Christakos et al., 2006). However, our results demonstrate the phase of peripheral tremor can be modulated by TACS over the cerebellum and, in the case of the postural variety, M1. This implies modulation of a central oscillator circuit. Interaction of a TACS-induced oscillatory drive with a spinal reflex-supported tremor circuit at the level of the anterior horn cell is made unlikely, because of the absence of a change in tremor amplitude coincident with the modulation of phase that was observed.
As described above, the effects of TACS at either site were solely confined to entrainment of tremor phase. This contrasts with findings in pathological rest tremor in Parkinson's disease, where TACS over M1 led to significant suppression of tremor amplitude, with more modest effects on phase entrainment (Brittain et al., 2013). The implication is that tremor phase, and, by inference, frequency (insofar as changes in phase entail changes in instantaneous frequency), and tremor amplitude can be independently modulated. This notion is gaining credence in parkinsonian tremors (Helmich et al., 2012), and, in the case of physiological tremor, suggests that factors affecting tremor amplitude predominate downstream of our sites of intervention (Fig. 5). Thus, TACS over M1 and cerebellum can interact with circuits elaborating tremor frequency and phase, as evidenced by phase entrainment, but has no effect on those circuits controlling the amplitude of physiological tremor. For example, in the case of physiological postural tremor, a descending oscillatory drive of relatively fixed strength might be gain modulated at the level of the spinal cord, perhaps according to factors governed by peripheral feedback.
We must acknowledge a few limitations of our study. First, current flow from cortical and cerebellar electrodes is not well localized (Bikson et al., 2012). However, animal and modeling studies on the effects of transcranial stimulation have demonstrated neural modulation below the stimulation pads (Ozen et al., 2010; Bikson et al., 2012). Indeed, even modeling of our less common cerebellar montage suggests targeted containment of significant current flow within the cerebellar hemispheres (Parazzini et al., 2014). Still, it is wise to consider our targeting of the motor cortex and cerebellum as approximate, rather than precise. Second, our use of a bipolar EEG montage may have acted to underestimate cortical-tremor coherence (Mima and Hallett, 1999). Even so, it allowed us to reaffirm the relatively selective pattern of cortical-tremor coherence previously described in the literature (Marsden et al., 2001). Third, it is possible that stimulation might have led to after-effects on subsequent stimulation conditions, despite our interleaving of 2 min rest periods. This effect was minimized at the group level by randomization and counterbalancing of the order of task and stimulation. Finally, there are caveats associated with translating phase shifts between stimulation and tremor signals into timing delays. These have previously been highlighted in the form of the “constant phase shift plus constant time lag model” (Mima and Hallett, 1999). Here, under the assumption of a linear system, phase delays relate to both time lags and the constant phase shift due to the shape of signal features, both of which are independent of frequency. Time lags include not only conduction delays in descending pathways, but also delays related to synaptic transmission and integration in the brain and spinal cord. Note that our analysis used the shape-invariant stimulation waveform, given by the reference (sinusoidal) TACS signal, which precisely relates to the actual induced population activity and hence has no constant phase shift; conversely, the accelerometer signal was bandpass filtered so that the timing of tremor cycles (and not necessarily their precise shape, which tends to be lost during such filtering) is given precedence. Accordingly, our computed phase differences should relate to timing delays. However, what comprises such timing delays is not trivial, as this is a composite of conduction delays and those necessary for synaptic transmission and integration in principal neurons and local interneuronal circuits, whether excitatory, inhibitory, or mixed in nature. Therefore, further research is needed to confirm or refute the hypothetical schema proposed in Figure 5, given that it is largely based on considerations of relating timing.
In summary, physiological tremor has proven differentially susceptible to transcranial stimulation over the motor cortex and cerebellum in a task-dependent manner. The results provide support for the hypothesis that physiological tremor reflects activity in central oscillators and that oscillators supporting postural and kinetic physiological tremor can be distinguished. The present study is also the first to demonstrate that tremor can be entrained by TACS over the cerebellum, with a potency that at least matches that of stimulation over M1. Together, these observations encourage further work using TACS to explore the neurobiology of tremor both in health and disease.
Footnotes
This work was funded by the Medical Research Council, Rosetrees Trust, and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. A.R.M. is supported by a NIHR Medical Academic Clinical Fellowship. The work was carried out in the NIHR Cognitive Health Clinical Research Facility, Oxford.
The authors declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci. 2012;43:176–183. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J Roy Stat Soc B. 1964;26:211–252. [Google Scholar]
- Brittain JS, Brown P. The many roads to tremor. Exp Neurol. 2013;250:104–107. doi: 10.1016/j.expneurol.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye RT, Neilson PD. The BUMP model of response planning: intermittent predictive control accounts for 10 Hz physiological tremor. Hum Mov Sci. 2010;29:713–736. doi: 10.1016/j.humov.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Christakos CN, Papadimitriou NA, Erimaki S. Parallel neuronal mechanisms underlying physiological force tremor in steady muscle contractions of humans. J Neurophysiol. 2006;95:53–66. doi: 10.1152/jn.00051.2005. [DOI] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- Donaldson IM, Marsden CD, Schneider SA, Bhatia KP. Marsden's book of movement disorders. New York: Oxford UP; 2012. [Google Scholar]
- Duval C, Panisset M, Bertrand G, Sadikot AF. Evidence that ventrolateral thalamotomy may eliminate the supraspinal component of both pathological and physiological tremors. Exp Brain Res. 2000;132:216–222. doi: 10.1007/s002210000358. [DOI] [PubMed] [Google Scholar]
- Duval C, Strafella AP, Sadikot AF. The impact of ventrolateral thalamotomy on high-frequency components of tremor. Clin Neurophysiol. 2005;116:1391–1399. doi: 10.1016/j.clinph.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Elble RJ. Central mechanisms of tremor. J Clin Neurophysiol. 1996;13:133–144. doi: 10.1097/00004691-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Randall JE. Mechanistic components of normal hand tremor. Electroencephalogr Clin Neurophysiol. 1978;44:72–82. doi: 10.1016/0013-4694(78)90106-2. [DOI] [PubMed] [Google Scholar]
- Evans CM, Baker SN. Task-dependent intermanual coupling of 8 Hz discontinuities during slow finger movements. Eur J Neurosci. 2003;18:453–456. doi: 10.1046/j.1460-9568.2003.02751.x. [DOI] [PubMed] [Google Scholar]
- Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A. The neural basis of intermittent motor control in humans. Proc Natl Acad Sci U S A. 2002;99:2299–2302. doi: 10.1073/pnas.032682099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/S0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. 2014;24:333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert R. Shaking when stirred: mechanisms of physiological tremor. J Physiol. 2012;590:2549. doi: 10.1113/jphysiol.2012.232876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci. 2013;7:279. doi: 10.3389/fnhum.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain. 1917;40:461–535. doi: 10.1093/brain/40.4.461. [DOI] [Google Scholar]
- Holmes G. The Croonian lectures on the clinical symptoms of cerebellar disease and their interpretation. Lancet. 1922a;200:111–115. doi: 10.1016/S0140-6736(00)94859-1. [DOI] [Google Scholar]
- Holmes G. The Croonian Lectures on the clinical symptoms of cerebellar disease and their interpretation. Lancet. 1922b;200:59–65. doi: 10.1016/S0140-6736(01)12307-X. [DOI] [Google Scholar]
- Holmes G. The Croonian lectures on the clinical symptoms of cerebellar disease and their interpretation. Lancet. 1922c;199:1231–1237. doi: 10.1016/S0140-6736(01)33076-3. [DOI] [Google Scholar]
- Holmes G. The Croonian lectures on the clinical symptoms of cerebellar disease and their interpretation. Lancet. 1922d;199:1177–1182. doi: 10.1016/S0140-6736(00)55081-8. [DOI] [Google Scholar]
- Hömberg V, Hefter H, Reiners K, Freund HJ. Differential effects of changes in mechanical limb properties on physiological and pathological tremor. J Neurol Neurosurg Psychiatry. 1987;50:568–579. doi: 10.1136/jnnp.50.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaberzadeh S, Brodin P, Flavel SC, O'Dwyer NJ, Nordstrom MA, Miles TS. Pulsatile control of the human masticatory muscles. J Physiol. 2003;547:613–620. doi: 10.1113/jphysiol.2003.030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce GC, Rack PM. The effects of load and force on tremor at the normal human elbow joint. J Physiol. 1974;240:375–396. doi: 10.1113/jphysiol.1974.sp010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda N, Nagaoka M, Wessberg J. Common modulation of motor unit pairs during slow wrist movement in man. J Physiol. 1999;520:929–940. doi: 10.1111/j.1469-7793.1999.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakie M. Is essential tremor physiological? In: Findley LJ, Koller WC, editors. Handbook of tremor disorders. New York: Marcel Dekker; 1992. [Google Scholar]
- Lakie M, Walsh EG, Wright GW. Passive mechanical properties of the wrist and physiological tremor. J Neurol Neurosurg Psychiatry. 1986;49:669–676. doi: 10.1136/jnnp.49.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakie M, Vernooij CA, Osborne TM, Reynolds RF. The resonant component of human physiological hand tremor is altered by slow voluntary movements. J Physiol. 2012;590:2471–2483. doi: 10.1113/jphysiol.2011.226449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW. A physiological approach to clinical neurology. London: Butterworth; 1975. [Google Scholar]
- Lippold OC. Oscillation in the stretch reflex arc and the origin of the rhythmical, 8–12 C-S component of physiological tremor. J Physiol. 1970;206:359–382. doi: 10.1113/jphysiol.1970.sp009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. The noncontinuous nature of movement execution. In: Humphrey DR, Freund HJ, editors. Motor control: concepts and issues. Chichester, UK: John Wiley; 1991. pp. 223–242. [Google Scholar]
- Marsden CD. The mechanisms of physiological tremor and their significance for pathological tremors. Basel: Karger; 1978. [Google Scholar]
- Marsden CD. Origins of normal and pathological tremor. In: Findley LJ, Capildeo R, editors. Movement disorders: tremor. London: MacMillan; 1984. pp. 37–84. [Google Scholar]
- Marsden CD, Meadows JC, Lange GW, Watson RS. The relation between physiological tremor of the two hands in healthy subjects. Electroencephalogr Clin Neurophysiol. 1969;27:179–185. doi: 10.1016/0013-4694(69)90171-0. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Brown P, Salenius S. Involvement of the sensorimotor cortex in physiological force and action tremor. Neuroreport. 2001;12:1937–1941. doi: 10.1097/00001756-200107030-00033. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian muscle receptors and their central actions. London: Edward Arnold; 1972. [Google Scholar]
- McAuley JH, Marsden CD. Physiological and pathological tremors and rhythmic central motor control. Brain. 2000;123:1545–1567. doi: 10.1093/brain/123.8.1545. [DOI] [PubMed] [Google Scholar]
- Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol. 1999;16:501–511. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2222. doi: 10.1016/S1388-2457(03)00235-9. author reply 2222–2223. [DOI] [PubMed] [Google Scholar]
- Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci. 2010;30:11476–11485. doi: 10.1523/JNEUROSCI.5252-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014;125:577–584. doi: 10.1016/j.clinph.2013.09.039. [DOI] [PubMed] [Google Scholar]
- Percival DB. Spectral analysis for physical applications: multitaper and conventional univariate techniques. New York: Cambridge UP; 1993. [Google Scholar]
- Proudlock FA, Scott J. Tremor in the human hand following peripheral nerve transection and reinnervation. Brain Res. 2003;989:238–245. doi: 10.1016/S0006-8993(03)03377-8. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Pawlas F, Lindemann M, Wenzelburger R, Deuschl G. Determinants of physiologic tremor in a large normal population. Clin Neurophysiol. 2000;111:1825–1837. doi: 10.1016/S1388-2457(00)00384-9. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Dümpelmann M, Wenzelburger R, Stolze H, Pfister G, Elger CE, Timmer J, Deuschl G. Corticomuscular coherence in the 6–15 Hz band: is the cortex involved in the generation of physiologic tremor? Exp Brain Res. 2002;142:32–40. doi: 10.1007/s00221-001-0914-7. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH. Non invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. American Electroencephalographic Society guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1991;8:200–202. doi: 10.1097/00004691-199104000-00007. [DOI] [PubMed] [Google Scholar]
- Stein RB. Peripheral control of movement. Physiol Rev. 1974;54:215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- Stiles RN, Randall JE. Mechanical factors in human tremor frequency. J Appl Physiol. 1967;23:324–330. doi: 10.1152/jappl.1967.23.3.324. [DOI] [PubMed] [Google Scholar]
- Thomson DJ. Spectrum estimation and harmonic-analysis. Proc IEEE. 1982;70:1055–1096. doi: 10.1109/PROC.1982.12433. [DOI] [Google Scholar]
- Ushiyama J, Suzuki T, Masakado Y, Hase K, Kimura A, Liu M, Ushiba J. Between-subject variance in the magnitude of corticomuscular coherence during tonic isometric contraction of the tibialis anterior muscle in healthy young adults. J Neurophysiol. 2011;106:1379–1388. doi: 10.1152/jn.00193.2011. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol. 1993;469:673–691. doi: 10.1113/jphysiol.1993.sp019837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij CA, Reynolds RF, Lakie M. A dominant role for mechanical resonance in physiological finger tremor revealed by selective minimization of voluntary drive and movement. J Neurophysiol. 2013;109:2317–2326. doi: 10.1152/jn.00926.2012. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Kakuda N. Single motor unit activity in relation to pulsatile motor output in human finger movements. J Physiol. 1999;517:273–285. doi: 10.1111/j.1469-7793.1999.0273z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Vallbo AB. Coding of pulsatile motor output by human muscle afferents during slow finger movements. J Physiol. 1995a;485:271–282. doi: 10.1113/jphysiol.1995.sp020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Vallbo AB. Human muscle spindle afferent activity in relation to visual control in precision finger movements. J Physiol. 1995b;482:225–233. doi: 10.1113/jphysiol.1995.sp020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J, Vallbo AB. Pulsatile motor output in human finger movements is not dependent on the stretch reflex. J Physiol. 1996;493:895–908. doi: 10.1113/jphysiol.1996.sp021432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Coherence between motor cortical activity and peripheral discontinuities during slow finger movements. J Neurophysiol. 2009;102:1296–1309. doi: 10.1152/jn.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]