Abstract
Objective:
Based on the theories of brain reserve and cognitive reserve, we investigated whether larger maximal lifetime brain growth (MLBG) and/or greater lifetime intellectual enrichment protect against cognitive decline over time.
Methods:
Forty patients with multiple sclerosis (MS) underwent baseline and 4.5-year follow-up evaluations of cognitive efficiency (Symbol Digit Modalities Test, Paced Auditory Serial Addition Task) and memory (Selective Reminding Test, Spatial Recall Test). Baseline and follow-up MRIs quantified disease progression: percentage brain volume change (cerebral atrophy), percentage change in T2 lesion volume. MLBG (brain reserve) was estimated with intracranial volume; intellectual enrichment (cognitive reserve) was estimated with vocabulary. We performed repeated-measures analyses of covariance to investigate whether larger MLBG and/or greater intellectual enrichment moderate/attenuate cognitive decline over time, controlling for disease progression.
Results:
Patients with MS declined in cognitive efficiency and memory (p < 0.001). MLBG moderated decline in cognitive efficiency (p = 0.031, ηp2 = 0.122), with larger MLBG protecting against decline. MLBG did not moderate memory decline (p = 0.234, ηp2 = 0.039). Intellectual enrichment moderated decline in cognitive efficiency (p = 0.031, ηp2 = 0.126) and memory (p = 0.037, ηp2 = 0.115), with greater intellectual enrichment protecting against decline. MS disease progression was more negatively associated with change in cognitive efficiency and memory among patients with lower vs higher MLBG and intellectual enrichment.
Conclusion:
We provide longitudinal support for theories of brain reserve and cognitive reserve in MS. Larger MLBG protects against decline in cognitive efficiency, and greater intellectual enrichment protects against decline in cognitive efficiency and memory. Consideration of these protective factors should improve prediction of future cognitive decline in patients with MS.
Cognitive impairment is prevalent in persons with multiple sclerosis (MS), especially cognitive inefficiency and memory decline.1,2 Despite this, many patients with MS are protected against cognitive decline. Indeed, some patients withstand considerable disease burden without cognitive impairment.2–4 Consistent with theories of brain reserve5 and cognitive reserve,6 we have shown in cross-sectional studies that larger maximal lifetime brain growth (MLBG)7 and greater intellectual enrichment7–10 attenuate the negative impact of MS disease burden on cognitive status (for review, see reference 11). There have been only 2 longitudinal studies of reserve in MS.12,13 One study showed that higher intellectual enrichment protected against decline in cognitive efficiency in 91 patients with MS over approximately 4.7 years; however, memory was not evaluated.12 Another study did not find that intellectual enrichment protected against cognitive decline in 35 patients with MS over 1.6 years13; however, on average, patients only declined on 1.4 of 9 neuropsychological measures over this short interval. The theory of cognitive reserve posits that intellectual enrichment moderates/attenuates cognitive decline,6,11 so the effect of enrichment would not be observed in a sample with little to no decline to moderate/attenuate. Neither study investigated the protective effect of brain reserve (MLBG). In the current study, we investigated whether larger MLBG (brain reserve) and greater intellectual enrichment (cognitive reserve) protect against decline in cognitive efficiency and memory in patients with MS over 4.5 years. Based on cross-sectional results,7,8,10 we expected that MLBG would protect against decline in cognitive efficiency but not memory, whereas intellectual enrichment would protect against decline in memory and cognitive efficiency.
METHODS
Subject enrollment.
Subjects were 40 persons with MS14 (28 women) consecutively recruited from the Clinic for Neurology, University of Belgrade, Serbia. Patients were without an exacerbation or corticosteroid use within the 4 weeks preceding baseline or follow-up, and had no history of serious psychiatric illness, learning disability, or other neurologic conditions. Patients underwent baseline and follow-up evaluations 4.5 ± 0.5 years apart. Baseline demographics were as follows: age 43.85 ± 10.60 years; education 13.45 ± 2.89 years; and disease duration 9.57 ± 9.09 years. Median baseline Expanded Disability Status Scale (EDSS) score was 3.5. Baseline phenotypes included clinically isolated syndrome (n = 5), relapsing-remitting MS (RRMS) (n = 17), secondary progressive MS (n = 6), and primary progressive MS (PPMS) (n = 12). The 5 patients with clinically isolated syndrome at baseline were reclassified as RRMS at follow-up; 3 patients with RRMS at baseline converted to secondary progressive MS by follow-up. (Note that our sample consisted of 30% patients with PPMS, which is more than expected within the MS population.)
Standard protocol approvals, registrations, and patient consents.
Approval was received from the local ethical standards committee on human experimentation, and written informed consent was obtained from all subjects participating in the study.
Cognitive functioning.
Cognition was assessed at baseline and follow-up with the Brief Repeatable Battery of Neuropsychological Tests,15 a battery assessing the cognitive domains most affected by MS: cognitive efficiency and memory.1,2 Cognitive efficiency was measured with the Symbol Digit Modalities Test (oral version) and the Paced Auditory Serial Addition Task (3-second version). Norm-referenced15 z scores were calculated for both tasks; mean of these z scores comprised our cognitive efficiency composite. Memory was assessed with the Selective Reminding Test and Spatial Recall Test. Norm-referenced15 z scores were calculated for the Selective Reminding Test (Long Term Storage, Delayed Recall) and Spatial Recall Test (Total Learning, Delayed Recall); mean of these z scores comprised our memory composite. These methods for deriving cognitive efficiency and memory composites are consistent with previous cross-sectional7 and longitudinal2 investigations of cognition in MS.
MRI estimates of MS disease progression.
Using a 1.5-tesla system (Avanto; Siemens, Erlangen, Germany), the following images of the brain were acquired at baseline and follow-up: (1) axial dual-echo turbo spin echo (repetition time = 2,650 milliseconds, echo time = 28–113 milliseconds, echo train length = 5, number of slices = 50, slice thickness = 2.5 mm with no gap, matrix size = 512 × 512, field of view = 250 × 250 mm2); and (2) sagittal 3-dimensional T1-weighted magnetization-prepared rapid-acquisition gradient echo (repetition time = 2,000 milliseconds, echo time = 3.93 milliseconds, inversion time = 1,100 milliseconds, number of slices = 208, slice thickness = 0.9 mm, matrix size = 256 × 224, field of view = 236 × 270 mm2). All scans were positioned following published guidelines.16 T2 lesion volume (LV) was measured on dual-echo scans using a local thresholding segmentation technique (Jim 5.0; Xinapse Systems, www.xinapse.com). Baseline normalized brain volume, as well as baseline and follow-up gray matter volume (GMV), and white matter volume (WMV) were obtained using SIENAX (version 2.6, part of FSL 4.1), after refilling of T1-hypointense lesions with values randomly extracted from a gaussian distribution with means and SDs estimated from the normal-appearing white matter.17 The scaling factor within SIENAX is derived from the transformation that matches the extracted brain and skull to standard space brain and skull images (derived from the MNI152 standard image): values higher than 1 were obtained for heads with small intracranial volume (ICV) and values lower than 1 for ICVs larger than the MNI (Montreal Neurological Institute) atlas. An advantage of this approach is that it does not require that CSF is robustly estimated, being difficult to distinguish between CSF and skull voxels in T1-weighted images. SIENA18 was used to quantify percentage brain volume change (PBVC) from baseline to follow-up, which was our measure of cerebral atrophy progression. Percentage change in T2 LV from baseline to follow-up was calculated using the following formula: (follow-up LV − baseline LV)/baseline LV. PBVC and percentage change in LV were our primary measurements of MS disease progression. We also calculated percentage change in GMV and WMV using the same equation as for LV.
Maximal lifetime brain growth.
ICV is an estimate of MLBG, because brain growth corresponds to increased ICV during development,19 and ICV is strongly correlated with brain size in healthy persons (e.g., r = 0.8620). ICV was used as an estimate of brain reserve in our previous cross-sectional research.7 The aforementioned scaling factor within SIENAX is a measurement of ICV; however, we reversed the direction of values such that larger values represent larger ICV (for ease of presentation). Given that men have larger ICVs than women, as in our sample (t38 = 5.95, p < 0.001), we adjusted ICV measurements for sex. The brain reserve hypothesis states that persons with larger MLBG are protected against cognitive decline despite disease progression, not that larger MLBG slows disease progression. We have previously shown no relationship between ICV and MS disease burden cross-sectionally,7 and we now show no relationship between ICV and cerebral atrophy (PBVC; r = 0.106, p = 0.514) or percentage change in LV (r = 0.081, p = 0.620) longitudinally.
Intellectual enrichment.
Vocabulary knowledge is often used as an estimate of lifetime intellectual enrichment in cognitive reserve research,21 because vocabulary is the product of enriching life activities (e.g., education, occupation, reading).22,23 Indeed, vocabulary knowledge is linked to literacy,23 and lifespan research shows that vocabulary at age 53 years is linked to educational and occupational experiences independently of childhood intelligence.22 Vocabulary was measured with the Wechsler Vocabulary Test. Similar to ICV, we have previously shown no relationship between vocabulary and MS disease burden cross-sectionally,10 and here we show no reliable relationships between vocabulary and percentage change in LV (r = 0.096, p = 0.558) or cerebral atrophy (PBVC; r = 0.295, p = 0.064). (Note that PBVC is controlled for in all subsequent analyses, including those investigating the impact of intellectual enrichment.) Consistent with the known relationship between brain size and intelligence,24 intellectual enrichment (estimated with vocabulary) was correlated with MLBG in this sample (r = 0.468, p = 0.002). However, we evaluated whether intellectual enrichment provides reserve against cognitive impairment over and above MLBG, consistent with our previous cross-sectional findings.7 This is an advance relative to previous longitudinal work on cognitive reserve in MS,12,13 which did not control for brain reserve when investigating cognitive reserve.
Statistical analyses.
Cognitive decline and disease progression.
Dependent t tests investigated whether cognitive efficiency and memory declined from baseline to follow-up, and whether MS disease progressed as indicated by changes in LV, brain volumes, and physical disability (EDSS). We next evaluated partial correlations between disease progression (i.e., cerebral atrophy [PBVC]) and decline in cognitive efficiency and memory, controlling for MLBG and intellectual enrichment.
Brain reserve (MLBG).
Repeated-measures analysis of covariance (ANCOVA) investigated whether change in cognitive efficiency from baseline to follow-up (dependent variable) was moderated by MLBG (estimated with ICV), controlling for changes in LV and brain volume (cerebral atrophy). This ANCOVA was repeated for the dependent variable of memory. We expected MLBG to attenuate decline in cognitive efficiency but not memory.
Cognitive reserve (intellectual enrichment).
ANCOVA investigated whether lifetime intellectual enrichment (estimated with vocabulary) independently attenuates change in cognitive efficiency from baseline to follow-up, controlling for changes in LV and brain volume, as well as MLBG (if interaction with MLBG was significant in the previous analysis). This ANCOVA was repeated for the dependent variable of memory. We expected intellectual enrichment to attenuate decline in both cognitive efficiency and memory.
RESULTS
Cognitive decline and disease progression.
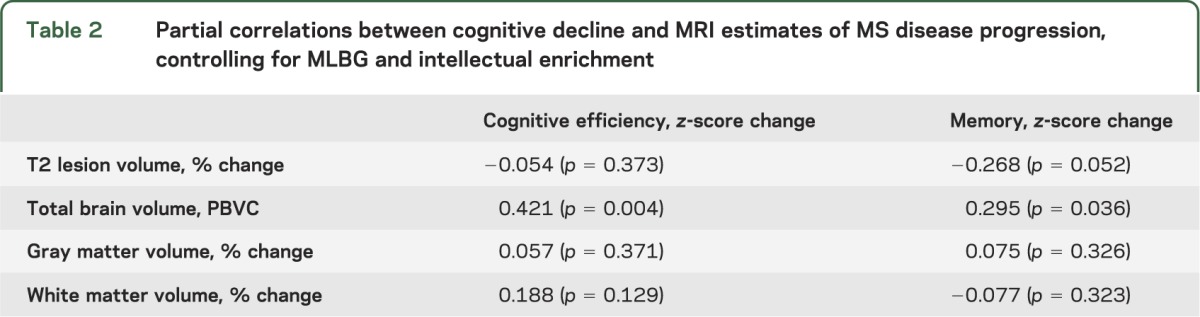
From baseline to follow-up, patients with MS declined in cognitive efficiency and memory, and also showed worsened MS disease progression indicated by increased LV and reduced total normalized brain volume (table 1). Physical disability (EDSS) also worsened. On average, our sample showed a 26.7% increase in LV and a 4.6% reduction in brain volume (mean PBVC = −4.58). As shown (table 2), decline in cognitive efficiency was related to cerebral atrophy (PBVC), and memory decline was related to cerebral atrophy (PBVC) and increased LV. Although we also calculated change in GMV and WMV, only GMV reduced from baseline to follow-up (table 1), and changes in GMV and WMV were unrelated to cognitive decline (table 2). Given these results and previous work supporting the SIENA-derived PBVC value as an appropriate marker of cerebral atrophy in longitudinal work,18 gray matter and white matter were dropped from further analysis. PBVC and percentage change in LV were controlled for in subsequent analyses.
Table 1.
Cognitive decline and disease progression from baseline to follow-up
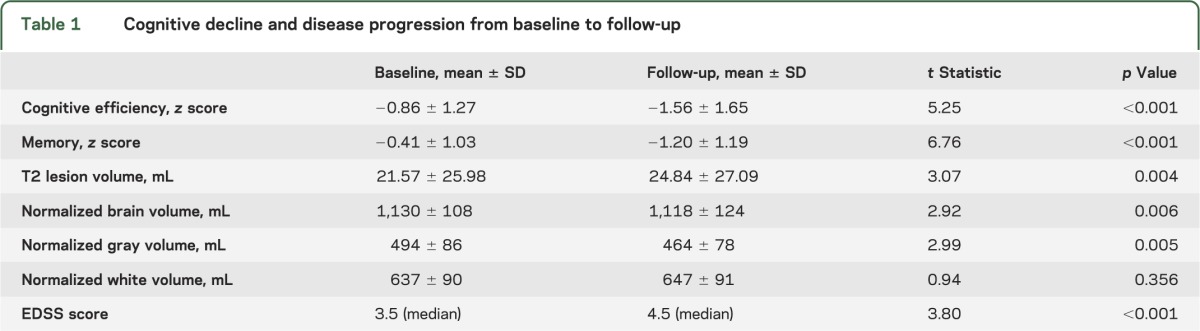
Table 2.
Partial correlations between cognitive decline and MRI estimates of MS disease progression, controlling for MLBG and intellectual enrichment
Maximal lifetime brain growth.
There was an interaction between time (baseline, follow-up) and MLBG on cognitive efficiency (F1,36 = 5.02, p = 0.031, ηp2 = 0.122) whereby larger MLBG protected against decline in cognitive efficiency from baseline to follow-up. To illustrate this protective effect of larger MLBG, we divided the sample into tertiles of low, moderate, and high MLBG. As shown (figure 1A, table e-1 on the Neurology® Web site at Neurology.org), cognitive efficiency declined most precipitously among patients with low MLBG, then moderate MLBG, with least decline in patients with high MLBG. There was no interaction between time (baseline, follow-up) and MLBG on memory (F1,36 = 1.46, p = 0.234, ηp2 = 0.039). That is, there were no reliable differences in memory decline among patients with MS who have low, moderate, or high MLBG (figure 1B, table e-1).
Figure 1. Maximal lifetime brain growth and cognitive decline.
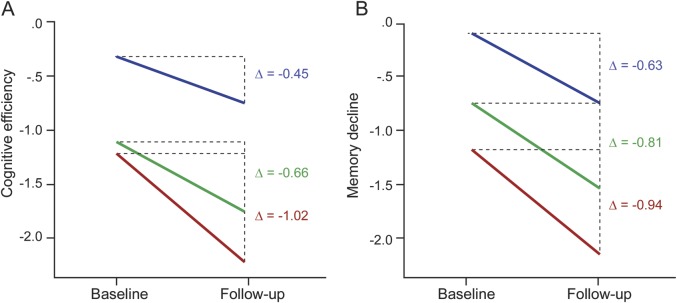
Decline in cognitive efficiency (A) and memory (B) from baseline to follow-up for patients with multiple sclerosis who had low (red), moderate (green), and high (blue) maximal lifetime brain growth. Cognitive efficiency and memory are represented as norm-referenced z scores.15
Intellectual enrichment.
There was an interaction between time (baseline, follow-up) and intellectual enrichment on cognitive efficiency (F1,35 = 5.03, p = 0.031, ηp2 = 0.126) whereby greater lifetime intellectual enrichment protected against decline in cognitive efficiency from baseline to follow-up. To illustrate the protective effect of greater intellectual enrichment, we divided the sample into tertiles of low, moderate, and high intellectual enrichment. As shown (figure 2A, table e-2), cognitive efficiency declined most precipitously among patients with low intellectual enrichment, then moderate intellectual enrichment, with little decline in patients with high intellectual enrichment. There was also an interaction between time (baseline, follow-up) and intellectual enrichment on memory (F1,36 = 4.70, p = 0.037, ηp2 = 0.115) whereby greater intellectual enrichment protected against memory decline. Again, as illustrated (figure 2B, table e-2), memory decline was most prominent among patients with low intellectual enrichment, followed by moderate, and then high enrichment.
Figure 2. Intellectual enrichment and cognitive decline.
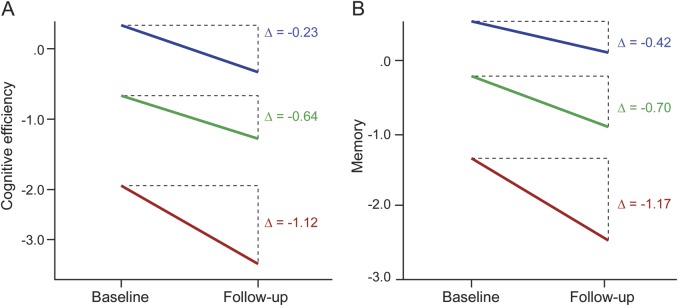
Decline in cognitive efficiency (A) and memory (B) from baseline to follow-up for patients with multiple sclerosis who had low (red), moderate (green), and high (blue) lifetime intellectual enrichment. Cognitive efficiency and memory are represented as norm-referenced z scores.15
Impact of reserve on verbal fluency.
Patients with MS also have verbal fluency deficits (for review and meta-analysis, see reference 25). The only task of the Brief Repeatable Battery not included in the above analyses is Word List Generation (WLG), a test of verbal (phonemic) fluency. Although verbal fluency is likely related to cognitive efficiency, inclusion of WLG within our cognitive efficiency composite differs from methods of previous MS research on reserve7 and cognitive decline.2 Also, WLG data were missing for one subject (n = 39). To be thorough, however, we investigated the impact of reserve on norm-referenced15 z scores for baseline and follow-up verbal fluency. Verbal fluency declined from baseline (mean z = −1.07 ± 0.64) to follow-up (mean z = −1.55 ± 1.06; t38 = 4.95, p < 0.001). The aforementioned ANCOVAs investigating the impact of reserve on cognitive decline were repeated for verbal fluency. First, there was an interaction between time (baseline, follow-up) and MLBG (F1,35 = 11.35, p = 0.002, ηp2 = 0.245) whereby patients with larger MLBG were protected against decline in verbal fluency over time (figure 3A, table e-1). Next, controlling for MLBG, there was an interaction between time (baseline, follow-up) and intellectual enrichment (F1,34 = 23.17, p < 0.001, ηp2 = 0.405) whereby patients with greater lifetime intellectual enrichment were protected against decline in verbal fluency over time (figure 3B, table e-2).
Figure 3. Reserve against decline in verbal fluency.
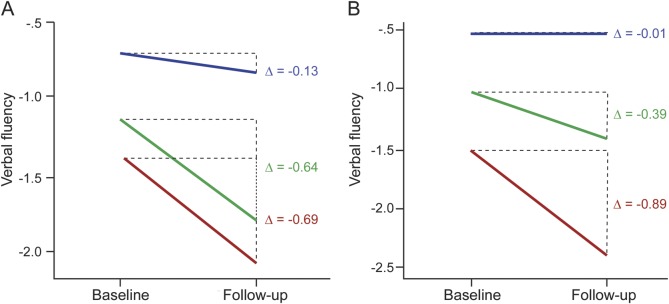
Maximal lifetime brain growth (MLBG) (A) and intellectual enrichment (B) moderate decline in norm-referenced15 verbal fluency z scores from baseline to follow-up. MLBG and intellectual enrichment are presented as low (red), moderate (green), and high (blue).
Supplemental analyses.
The aforementioned analyses controlled for longitudinal change in brain atrophy and LV; however, future disease progression is unpredictable in any given patient. We performed all of these analyses a second time without controlling for change in brain atrophy and LV, with almost no change in the results (table e-3). As such, clinical consideration of MLBG and/or intellectual enrichment may help to predict future cognitive decline (or preservation) even without knowledge of future disease progression, which is clinically unknown/unpredictable.
Differential effect of MS disease progression on cognitive decline.
The theory of reserve posits that the negative relationship between disease progression (i.e., cerebral atrophy) and cognitive decline will be greater in patients with lesser reserve than in those with higher reserve, because patients with higher reserve can withstand more severe disease progression before experiencing cognitive decline. To investigate this, we performed regressions to determine the amount of variance MS disease progression (PBVC, percentage change in LV) explains in decline of cognitive efficiency, memory, and verbal fluency in patients at the highest vs lowest tertiles of MLBG or intellectual enrichment. As shown (table e-4), MS disease progression is much more strongly linked to cognitive decline in patients with smaller MLBG and/or lesser intellectual enrichment (mean R2 = 0.376) than patients with larger MLBG and/or greater intellectual enrichment (mean R2 = 0.045). As such, similar to our cross-sectional observation that disease burden is more strongly correlated with cognition in patients with lower reserve,8 we now show that disease progression is more strongly related to changes in cognition longitudinally among patients with MS who have smaller MLBG and/or lesser intellectual enrichment.
DISCUSSION
Many patients with MS withstand considerable brain atrophy and heavy lesion burdens without cognitive inefficiency or memory impairment.2–4 The theory of brain reserve5 posits that persons with larger MLBG are able to withstand more severe disease burden (and associated brain volume loss) before reaching a critical threshold of brain volume beneath which cognitive decline/impairment emerges. The theory of cognitive reserve6 posits that enriching life experiences are associated with more efficient patterns of neurocognitive processing,26,27 which helps preserve cognition despite neurologic disease. Herein, we provide longitudinal support for these theories of reserve against cognitive impairment, because patients with MS who had larger MLBG and/or greater intellectual enrichment were protected against cognitive decline over 4.5 years. Moreover, MS disease progression (cerebral atrophy, increased lesion load) was much more strongly linked to cognitive decline in patients with lower MLBG and lesser intellectual enrichment than patients with larger MLBG or greater intellectual enrichment. These findings are consistent with cross-sectional work7–10 but further support the theory of reserve in MS by showing protection against cognitive decline longitudinally within patients.
Our findings are also consistent with a previous longitudinal study showing that intellectual enrichment protects against decline in cognitive efficiency over approximately 4.7 years,12 but we extend this by demonstrating protective effects of (1) larger MLBG against decline in cognitive efficiency and verbal fluency, and (2) greater intellectual enrichment against decline in memory and verbal fluency (MLBG, memory, and verbal fluency were not investigated in the previous study12). Our findings are not consistent with a longitudinal study failing to show a protective effect of intellectual enrichment against cognitive decline in 35 patients with MS over a period of 1.6 years,13 although in that study there was very little cognitive decline to protect against. The theory of reserve predicts that persons with greater intellectual enrichment (or larger MLBG) will show less cognitive decline relative to patients with lesser enrichment (or smaller MLBG),6,11 so the interval between baseline and follow-up must be long enough for cognitive decline to occur so that differential decline due to intellectual enrichment or MLBG can be evaluated. With an interval of 4.5 years, we observed decline in cognitive efficiency, memory, and verbal fluency within our sample overall, and we then demonstrated that patients with MS were differentially vulnerable to cognitive decline based on their MLBG and lifetime intellectual enrichment.
Consistent with recent cross-sectional work in MS,7 larger MLBG protected against decline in cognitive efficiency, but not memory. The brain reserve hypothesis has been well supported within the Alzheimer disease/aging literature, but closer examination of these aging studies reveals that larger MLBG is indeed specifically linked to preserved cognitive efficiency, not memory.28–30 Moreover, longitudinal aging studies link cerebral atrophy (loss of brain reserve) to decline in cognitive efficiency, not memory decline.31,32 This specific link between larger MLBG and preserved cognitive efficiency (not memory) may be explained by stronger heritability of MLBG33 and cognitive efficiency34,35 relative to memory,34,35 whereas memory is more influenced by life experience34,35 (i.e., intellectual enrichment). Indeed, intellectual enrichment protected against memory decline within the current sample. (Intellectual enrichment also protected against decline in cognitive efficiency independently of MLBG, and this effect was larger than in our a previous cross-sectional finding.7)
Clinically, it is difficult to predict which patients with MS are at greatest risk of cognitive decline because it is difficult to accurately predict MS disease progression for any given patient. However, cross-sectional3,4 and longitudinal2 correlations between MS disease burden/progression and cognitive status/decline are moderate at best, and our current longitudinal findings show that the link between MS disease progression and cognitive decline differs depending on a patient's MLBG and intellectual enrichment. As such, MLBG and intellectual enrichment are important factors to consider when trying to predict cognitive decline in patients with MS, because patients with lower MLBG and/or lesser intellectual enrichment are at greatest risk of cognitive decline. These at-risk patients can be targeted for early-intervention cognitive rehabilitation, which may help prevent/delay the onset of functional impairment. With few exceptions (e.g., see reference 36), treatments for cognitive impairment in patients with MS consist predominantly of behavioral strategies, which patients must learn and integrate into their daily routines.37,38 As such, patients at risk for cognitive impairment may do well to learn and incorporate these strategies into their lives before cognitive impairment makes such learning and integration more challenging. Also, selective enrollment of at-risk patients for prevention studies should increase statistical power, because differential cognitive decline within the control group is necessary to support effective treatments/interventions.
Patients with MS who have lower MLBG should be encouraged to pursue a brain-healthy lifestyle to preserve the brain reserve they currently possess. Indeed, higher cardiorespiratory fitness may help preserve brain volume and cognitive efficiency,39 and preliminary data show that aerobic exercise training may result in improved memory, increased hippocampal volume, and increased hippocampal functional connectivity in patients with MS.40 Finally, patients with MS should also be encouraged to engage in intellectual enrichment (e.g., reading, hobbies, etc.), because previous research7–10,12 and the current findings suggest that greater intellectual enrichment protects against cognitive decline. However, randomized controlled trials of intellectual enrichment are necessary to causally evaluate the contribution of intellectual enrichment to cognitive preservation. In addition, future research in larger samples is needed to (1) investigate whether greater intellectual enrichment can counteract the negative effect of smaller MLBG, and vice versa, and (2) examine whether the protective impact of larger MLBG or greater intellectual enrichment against cognitive decline differs across MS phenotypes (e.g., RRMS vs PPMS).
Supplementary Material
GLOSSARY
- ANCOVA
analysis of covariance
- EDSS
Expanded Disability Status Scale
- GMV
gray matter volume
- ICV
intracranial volume
- LV
lesion volume
- MLBG
maximal lifetime brain growth
- MS
multiple sclerosis
- PBVC
percentage brain volume change
- PPMS
primary progressive multiple sclerosis
- RRMS
relapsing-remitting multiple sclerosis
- WLG
Word List Generation
- WMV
white matter volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
James F. Sumowski, PhD, drafted the manuscript for content, contributed to the study concept and design and analysis/interpretation of the data, and performed statistical analyses. Maria A. Rocca, MD, assisted in drafting the manuscript for content and analysis/interpretation of data, as well as acquisition of data and study supervision/coordination. Victoria M. Leavitt, PhD, assisted in drafting the manuscript for content and contributed to the interpretation of the data. Jelena Dackovic, MD, contributed to neuropsychological assessment. Sarlota Mesaros, MD, PhD, and Jelena Drulovic, MD, contributed to the study concept and design and analysis/interpretation of the data, and performed clinical evaluations. John DeLuca, PhD, assisted in drafting the manuscript for content. Massimo Filippi, MD, assisted in drafting the manuscript for content and interpretation of data, as well as acquisition of data, study supervision, and obtaining funding.
STUDY FUNDING
This study was partially supported by a grant from the Ministry of Science, Republic of Serbia (project number 175031). Dr. Sumowski was funded in part by the NIH (R00 HD060765).
DISCLOSURE
J. Sumowski reports no disclosures relevant to the manuscript. M. Rocca received speaker honoraria from Biogen Idec and Serono Symposia International Foundation and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. V. Leavitt and J. Dackovic report no disclosures relevant to the manuscript. S. Mesaros has received speaker grants form Merck Serono SA and travel grants from Bayer Schering Pharma. J. Drulovic has received research grant support from Bayer Schering Pharma, and has received speaker honoraria from Merck Serono SA and Bayer Schering Pharma. J. DeLuca received salary support through compensation to the Kessler Foundation Research Center from Biogen Idec. He is also a consultant for Biogen. M. Filippi serves on scientific advisory boards for Teva Pharmaceutical Industries and Genmab A/S; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Genmab A/S, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Genmab A/S, Merck Serono, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, and the Jacques and Gloria Gossweiler Foundation (Switzerland). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–1151 [DOI] [PubMed] [Google Scholar]
- 2.Deloire MS, Ruet A, Hamel D, et al. MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 2011;76:1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict RH, Bruce JM, Dwyer MG, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 2006;63:1301–1306 [DOI] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, Benedict RH, et al. The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 2010;75:2121–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 1993;7:273–295 [Google Scholar]
- 6.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460 [PubMed] [Google Scholar]
- 7.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve in multiple sclerosis: what you've got and how you use it. Neurology 2013;80:2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumowski JF, Chiaravalloti N, Wylie G, Deluca J. Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc 2009;15:606–612 [DOI] [PubMed] [Google Scholar]
- 9.Sumowski JF, Wylie GR, Gonnella A, et al. Premorbid cognitive leisure independently contributes to cognitive reserve in multiple sclerosis. Neurology 2010;75:1428–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sumowski JF, Wylie GR, Chiaravalloti N, DeLuca J. Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 2010;74:1942–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumowski JF, Leavitt VM. Cognitive reserve in multiple sclerosis. Mult Scler 2013;19:1122–1127 [DOI] [PubMed] [Google Scholar]
- 12.Benedict RH, Morrow SA, Weinstock Guttman B, et al. Cognitive reserve moderates decline in information processing speed in multiple sclerosis patients. J Int Neuropsychol Soc 2010;16:829–835 [DOI] [PubMed] [Google Scholar]
- 13.Amato MP, Razzolini L, Goretti B, et al. Cognitive reserve and cortical atrophy in multiple sclerosis: a longitudinal study. Neurology 2013;80:1728–1733 [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boringa JB, Lazeron RH, Reuling IE, et al. The brief repeatable battery of neuropsychological tests: normative values allow application in multiple sclerosis clinical practice. Mult Scler 2001;7:263–267 [DOI] [PubMed] [Google Scholar]
- 16.Miller DH, Barkhof F, Berry I, et al. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry 1991;54:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging 2010;32:223–228 [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489 [DOI] [PubMed] [Google Scholar]
- 19.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000;216:672–682 [DOI] [PubMed] [Google Scholar]
- 20.Mori E, Hirono N, Yamashita H, et al. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer's disease. Am J Psychiatry 1997;154:18–24 [DOI] [PubMed] [Google Scholar]
- 21.Stern Y. Cognitive reserve. Neuropsychologia 2009;47:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol 2003;25:614–624 [DOI] [PubMed] [Google Scholar]
- 23.Stanovich KE, Cunningham AE. Where does knowledge come from? Specific associations between print exposure and information acquisition. J Educ Psychol 1993;85:211–229 [Google Scholar]
- 24.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci 2010;11:201–211 [DOI] [PubMed] [Google Scholar]
- 25.Henry JD, Beatty WW. Verbal fluency deficits in multiple sclerosis. Neuropsychologia 2006;44:1166–1174 [DOI] [PubMed] [Google Scholar]
- 26.Stern Y, Habeck C, Moeller J, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 2005;15:394–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain 2010;133:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLullich AM, Ferguson KJ, Deary IJ, et al. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology 2002;59:169–174 [DOI] [PubMed] [Google Scholar]
- 29.Tisserand DJ, Bosma H, Van Boxtel MP, Jolles J. Head size and cognitive ability in nondemented older adults are related. Neurology 2001;56:969–971 [DOI] [PubMed] [Google Scholar]
- 30.Farias ST, Mungas D, Reed B, et al. Maximal brain size remains an important predictor of cognition in old age, independent of current brain pathology. Neurobiol Aging 2012;33:1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabbitt P, Mogapi O, Scott M, et al. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. Neuropsychology 2007;21:684–695 [DOI] [PubMed] [Google Scholar]
- 32.Kramer JH, Mungas D, Reed BR, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology 2007;21:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain 1997;120:257–269 [DOI] [PubMed] [Google Scholar]
- 34.Pedersen NL, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychol Sci 1992;3:346–353 [Google Scholar]
- 35.McClearn GE, Johansson B, Berg S, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science 1997;276:1560–1563 [DOI] [PubMed] [Google Scholar]
- 36.Sumowski JF, Chiaravalloti N, Erlanger D, et al. L-amphetamine improves memory in MS patients with objective memory impairment. Mult Scler 2011;17:1141–1145 [DOI] [PubMed] [Google Scholar]
- 37.Amato MP, Langdon D, Montalban X, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol 2013;260:1452–1468 [DOI] [PubMed] [Google Scholar]
- 38.Sumowski JF, Leavitt VM, Cohen A, et al. Retrieval practice is a robust memory aid for memory-impaired patients with MS. Mult Scler 2013;19:1943–1946 [DOI] [PubMed] [Google Scholar]
- 39.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic fitness is associated with gray matter volume and white matter integrity in multiple sclerosis. Brain Res 2010;1341:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leavitt VM, Cirnigliaro C, Cohen A, et al. Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase Epub 2013 Oct 4 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


