Significance
Photosynthesis by cyanobacteria, algae, and plants sustains life on Earth by oxidizing water to the O2 we breathe and by converting CO2 into biomass we eat, burn, or use otherwise. Although O2 production and CO2 reduction are functionally and structurally well separated in photosynthetic organisms, there is a long debated role of CO2/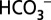 in water oxidation. Here we demonstrate that
in water oxidation. Here we demonstrate that 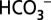 acts as mobile acceptor and transporter of protons produced by photosystem II, and that depletion of
acts as mobile acceptor and transporter of protons produced by photosystem II, and that depletion of 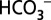 leads to a reversible down-regulation of O2 production. These findings add a previously unidentified component to the regulatory networks in higher plants, algae, and cyanobacteria and conclude the long quest for the function of CO2/
leads to a reversible down-regulation of O2 production. These findings add a previously unidentified component to the regulatory networks in higher plants, algae, and cyanobacteria and conclude the long quest for the function of CO2/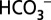 in photosynthetic water oxidation.
in photosynthetic water oxidation.
Keywords: carbon dioxide, bicarbonate, proton release, oxygen evolution, water splitting
Abstract
Cyanobacteria, algae, and plants oxidize water to the O2 we breathe, and consume CO2 during the synthesis of biomass. Although these vital processes are functionally and structurally well separated in photosynthetic organisms, there is a long-debated role for CO2/ in water oxidation. Using membrane-inlet mass spectrometry we demonstrate that
in water oxidation. Using membrane-inlet mass spectrometry we demonstrate that  acts as a mobile proton acceptor that helps to transport the protons produced inside of photosystem II by water oxidation out into the chloroplast’s lumen, resulting in a light-driven production of O2 and CO2. Depletion of
acts as a mobile proton acceptor that helps to transport the protons produced inside of photosystem II by water oxidation out into the chloroplast’s lumen, resulting in a light-driven production of O2 and CO2. Depletion of  from the media leads, in the absence of added buffers, to a reversible down-regulation of O2 production by about 20%. These findings add a previously unidentified component to the regulatory network of oxygenic photosynthesis and conclude the more than 50-y-long quest for the function of CO2/
from the media leads, in the absence of added buffers, to a reversible down-regulation of O2 production by about 20%. These findings add a previously unidentified component to the regulatory network of oxygenic photosynthesis and conclude the more than 50-y-long quest for the function of CO2/ in photosynthetic water oxidation.
in photosynthetic water oxidation.
Oxygenic photosynthesis in cyanobacteria, algae, and higher plants leads to the reduction of atmospheric CO2 to energy-rich carbohydrates. The electrons needed for this process are extracted in a cyclic, light-driven process from water that is split into dioxygen (O2) and protons. This reaction is catalyzed by a penta-µ-oxo bridged tetra-manganese calcium cluster (Mn4CaO5) within the oxygen-evolving complex (OEC) of photosystem II (PSII) (1–4). The possible roles of inorganic carbon, 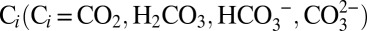 , in this process have been a controversial issue ever since Otto Warburg and Günter Krippahl (5) reported in 1958 that oxygen evolution by PSII strictly depends on CO2 and therefore has to be based on the photolysis of H2CO3 (“Kohlensäure”) and not of water. These first experiments were indirect and, as became apparent later, were wrongly interpreted (6–8). Several research groups followed up on these initial results and identified two possible sites of Ci interaction within PSII (reviewed in refs. 9–12). Functional and spectroscopic studies showed that
, in this process have been a controversial issue ever since Otto Warburg and Günter Krippahl (5) reported in 1958 that oxygen evolution by PSII strictly depends on CO2 and therefore has to be based on the photolysis of H2CO3 (“Kohlensäure”) and not of water. These first experiments were indirect and, as became apparent later, were wrongly interpreted (6–8). Several research groups followed up on these initial results and identified two possible sites of Ci interaction within PSII (reviewed in refs. 9–12). Functional and spectroscopic studies showed that 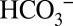 facilitates the reduction of the secondary plastoquinone electron acceptor (QB) of PSII by participating in the protonation of
facilitates the reduction of the secondary plastoquinone electron acceptor (QB) of PSII by participating in the protonation of 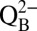 . Binding of
. Binding of 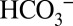 (or
(or 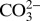 ) to the nonheme Fe between the quinones QA and QB was recently confirmed by X-ray crystallography (3, 13, 14). Despite this functional role at the acceptor side, the very tight binding of
) to the nonheme Fe between the quinones QA and QB was recently confirmed by X-ray crystallography (3, 13, 14). Despite this functional role at the acceptor side, the very tight binding of 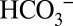 to this site makes it impossible for the activity of PSII to be affected by changing the Ci level of the medium; instead inhibitors such as formate need to be added to induce the acceptor-side effect (15). Consequently, the water-splitting electron-donor side of PSII has also been studied intensively (for recent reviews, see refs. 11 and 12). Although a tight binding of Ci near the Mn4CaO5 cluster is excluded on the basis of X-ray crystallography (3, 14), FTIR spectroscopy (16), and mass spectrometry (17, 18), the possibility that a weakly bound
to this site makes it impossible for the activity of PSII to be affected by changing the Ci level of the medium; instead inhibitors such as formate need to be added to induce the acceptor-side effect (15). Consequently, the water-splitting electron-donor side of PSII has also been studied intensively (for recent reviews, see refs. 11 and 12). Although a tight binding of Ci near the Mn4CaO5 cluster is excluded on the basis of X-ray crystallography (3, 14), FTIR spectroscopy (16), and mass spectrometry (17, 18), the possibility that a weakly bound 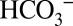 affects the activity of PSII at the donor side remains a viable option (reviewed in refs. 10 and 19).
affects the activity of PSII at the donor side remains a viable option (reviewed in refs. 10 and 19).
In the present study using higher plant PSII membranes, we specifically evaluate a recently suggested role of weakly bound 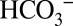 , namely, that it acts as an acceptor for, and transporter of, protons produced by water splitting in the OEC (20–22).
, namely, that it acts as an acceptor for, and transporter of, protons produced by water splitting in the OEC (20–22).
Results
Time-resolved isotope-ratio membrane-inlet mass spectrometry (TR-MIMS) allows simultaneous on-line detection of various dissolved gas molecules with high sensitivity. This method, in which the gas molecules contained in the sample enter the vacuum of the mass spectrometer via pervaporation through a gas-permeable membrane, is therefore ideally suited to test the effect and mechanism of 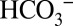 interaction with PSII (23, 24). The TR-MIMS experiments presented below were performed either in on-line or off-line mode. The commonly used on-line approach leads to a strong
interaction with PSII (23, 24). The TR-MIMS experiments presented below were performed either in on-line or off-line mode. The commonly used on-line approach leads to a strong 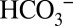 depletion of the samples owing to the consumption of CO2 by the mass spectrometer and the interconversion of all Ci species. In contrast, the off-line approach allows the experiments to be performed at well-defined
depletion of the samples owing to the consumption of CO2 by the mass spectrometer and the interconversion of all Ci species. In contrast, the off-line approach allows the experiments to be performed at well-defined 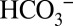 levels (ambient or depleted). The two techniques are illustrated schematically in Fig. 1.
levels (ambient or depleted). The two techniques are illustrated schematically in Fig. 1.
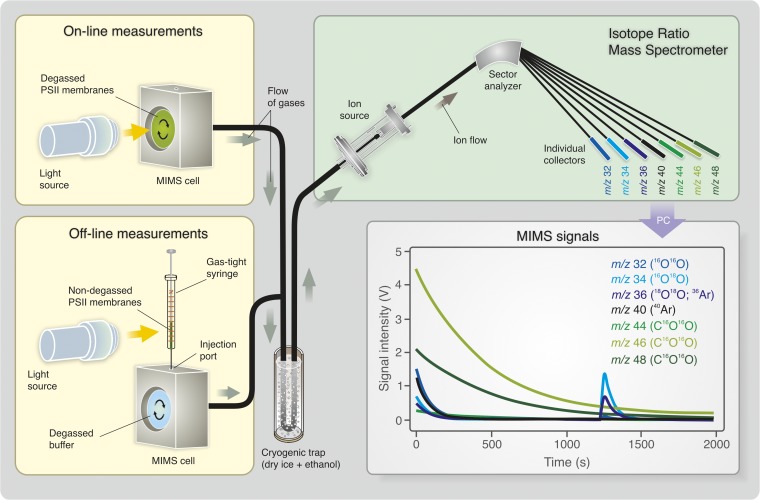
Fig. 1.
Schematic view of the on-line and off-line TR-MIMS setup. For on-line experiments PSII membranes were loaded into the home-built MIMS cell and degassed for 20–40 min until stable baselines for all detected gases were reached (Lower Right). This leads to a nearly complete depletion of the samples of Ci. The sample was then illuminated with xenon flashes or by continuous white light from a slide projector. In off-line measurements the PSII membranes were illuminated inside of gas-tight syringes, that is, at well-defined Ci levels (ambient or depleted), and then injected into buffer of identical composition and pH that was thoroughly degassed in the MIMS cell.
Effects of Mild 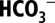 Depletion on PSII Activity.
Depletion on PSII Activity.
To confirm that 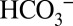 is of functional relevance for photosynthetic water splitting under our mild
is of functional relevance for photosynthetic water splitting under our mild 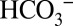 -depletion conditions we monitored O2 evolution of PSII by off-line TR-MIMS at ambient
-depletion conditions we monitored O2 evolution of PSII by off-line TR-MIMS at ambient 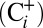 and Ci-depleted conditions
and Ci-depleted conditions 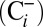 . In these experiments an approximately 20-fold reduction in Ci levels was achieved by bubbling sample solutions, electron acceptors, and
. In these experiments an approximately 20-fold reduction in Ci levels was achieved by bubbling sample solutions, electron acceptors, and 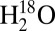 (97.6%) with CO2-depleted air inside a sealed septum vial (25). The sample suspensions were then transferred into gas-tight syringes and illuminated with white light for 10 s to induce O2 formation by water splitting in PSII. The O2 production was assayed by injecting the sample into the MIMS cell that contained a buffer of the same composition and pH, but extremely low dissolved gas concentration owing to the constant removal of gas by the mass spectrometer via the membrane inlet. Comparison of the
(97.6%) with CO2-depleted air inside a sealed septum vial (25). The sample suspensions were then transferred into gas-tight syringes and illuminated with white light for 10 s to induce O2 formation by water splitting in PSII. The O2 production was assayed by injecting the sample into the MIMS cell that contained a buffer of the same composition and pH, but extremely low dissolved gas concentration owing to the constant removal of gas by the mass spectrometer via the membrane inlet. Comparison of the 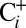 and
and 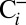 traces in Fig. 2 shows that the O2 yield of the
traces in Fig. 2 shows that the O2 yield of the 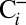 sample is 20% lower than that of the
sample is 20% lower than that of the 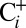 sample. Reversibility of the effect was established by exposing
sample. Reversibility of the effect was established by exposing 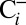 samples for 20 min to air before illumination in the syringe (
samples for 20 min to air before illumination in the syringe (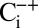 samples). A similar inhibition was achieved if the Ci depletion was performed by bubbling with N2, and a rapid (≤2 min) recovery was seen after Ci was added in absence of O2 in form of NaHCO3 powder (Fig. S1). These experiments confirm that HCO3− enhances the activity of PSII.
samples). A similar inhibition was achieved if the Ci depletion was performed by bubbling with N2, and a rapid (≤2 min) recovery was seen after Ci was added in absence of O2 in form of NaHCO3 powder (Fig. S1). These experiments confirm that HCO3− enhances the activity of PSII.
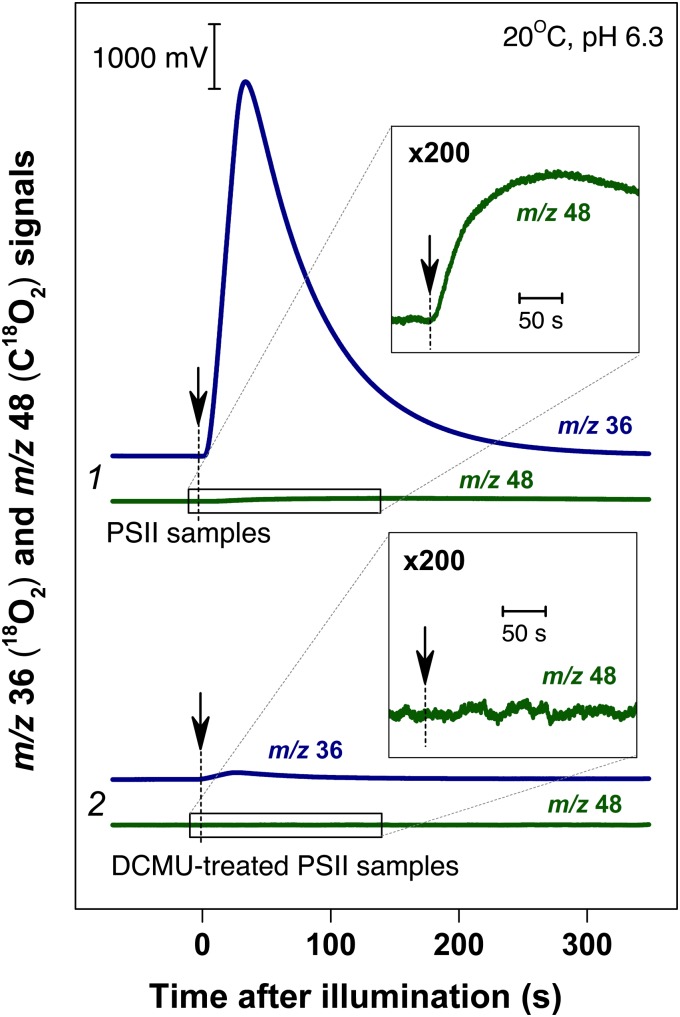
Fig. 2.
Dependence of water splitting in PSII on the Ci concentration of the medium. PSII membranes were illuminated with continuous white light of a slide projector for 10 s inside of gas-tight syringes and then injected into the MIMS cell. The 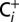 trace was obtained at ambient Ci-concentration, whereas the
trace was obtained at ambient Ci-concentration, whereas the 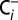 trace was recorded at a 20-times-reduced Ci concentration that was reached by purging PSII sample with CO2 absorber (PCDA220M; Puregas). The reversibility was demonstrated by exposing a
trace was recorded at a 20-times-reduced Ci concentration that was reached by purging PSII sample with CO2 absorber (PCDA220M; Puregas). The reversibility was demonstrated by exposing a 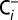 sample for 20 min to air (
sample for 20 min to air (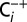 trace). The final chlorophyll concentration was 50 µg (Chl) ml−1, the
trace). The final chlorophyll concentration was 50 µg (Chl) ml−1, the 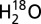 -enrichment was 10%, and the medium contained 1 mM MES (pH 6.3), 15 mM NaCl, and 2 mM K3[Fe(CN)6] and 0.25 mM 2-phenyl-p-benzoquinone (dissolved in DMSO) as electron acceptors. The average of two to three repeats is presented.
-enrichment was 10%, and the medium contained 1 mM MES (pH 6.3), 15 mM NaCl, and 2 mM K3[Fe(CN)6] and 0.25 mM 2-phenyl-p-benzoquinone (dissolved in DMSO) as electron acceptors. The average of two to three repeats is presented.
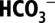 Acts as Proton Acceptor.
Acts as Proton Acceptor.
According to a recent proposal, the activating role of 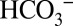 on PSII demonstrated above may be caused by the ability of
on PSII demonstrated above may be caused by the ability of 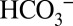 to act as a base for protons produced by photosynthetic water oxidation (20–22). If this proposal is correct, then the transiently formed H2CO3 should decay according to Reaction 1 into water and CO2:
to act as a base for protons produced by photosynthetic water oxidation (20–22). If this proposal is correct, then the transiently formed H2CO3 should decay according to Reaction 1 into water and CO2:
The CO2 should then be released from PSII simultaneously with O2 and must be detectable with TR-MIMS, because a maximum of four CO2 per O2 can be expected in the absence of any other proton acceptors. Fig. 3 displays the results obtained by using the conventional on-line approach, that is, by illuminating PSII suspensions inside the MIMS cell by a series of 50 saturating xenon flashes fired at 2 Hz. Because all mass peaks give the same results, Fig. 3 displays for clarity only the traces at mass-to-charge ratios of m/z 36 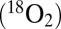 and m/z 48
and m/z 48 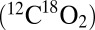 , which detect CO2 and O2 with about the same high sensitivity (see Fig. S2 for the other traces). Under these conditions only light-induced O2 evolution, and no CO2 formation, is seen (Fig. 3, experiment 1). However, a 200-fold amplification of the CO2 trace reveals a very small light-induced rise in the m/z 48 trace (Fig. 3, Upper Inset). Although small, the effect was reproducible and a light-induced heating artifact could be excluded by the inhibition of PSII turnover with the herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (Fig. 3, experiment 2). The more than 200-times smaller amplitude of the light-induced CO2 production vs. O2 evolution by PSII explains in part why the CO2 formation by PSII was not reported previously. However, this very small CO2 production may also call into question its biological relevance.
, which detect CO2 and O2 with about the same high sensitivity (see Fig. S2 for the other traces). Under these conditions only light-induced O2 evolution, and no CO2 formation, is seen (Fig. 3, experiment 1). However, a 200-fold amplification of the CO2 trace reveals a very small light-induced rise in the m/z 48 trace (Fig. 3, Upper Inset). Although small, the effect was reproducible and a light-induced heating artifact could be excluded by the inhibition of PSII turnover with the herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (Fig. 3, experiment 2). The more than 200-times smaller amplitude of the light-induced CO2 production vs. O2 evolution by PSII explains in part why the CO2 formation by PSII was not reported previously. However, this very small CO2 production may also call into question its biological relevance.
Fig. 3.
Simultaneous on-line TR-MIMS measurements of O2 and CO2 production by PSII. O2 (blue traces) and CO2 (green traces) evolution of dark-adapted spinach PSII membranes [0.3 mg (Chl)⋅mL−1] induced by 50 saturating flashes at 2 Hz (trace 1). The arrows indicate the start of illumination. The inset displays the CO2 evolution with 200-fold amplification. Trace 2 shows the same measurements after inhibition of PSII with the herbicide DCMU (80 µM). The amplitudes of the traces for CO2 and O2 can be directly compared, because our set-up detects both gases with nearly equal sensitivity. The measurements were performed in a buffer containing 3 mM MES (pH 6.3) in the presence of 1 mM K3[Fe(CN)6] as exogenous electron acceptor. Final H218O enrichment was 10%. One representative result out of two to three repeats is presented.
During the above on-line TR-MIMS experiments the samples need to be degassed in the MIMS cell for 20–40 min to reach a stable baseline. This leads to an almost complete removal of dissolved CO2 from the solution (see data in Fig. 1). Owing to the interconversion of all Ci species (Reaction 1) this also results in very low concentrations of 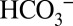 in the sample suspension (17), which may significantly reduce light-induced CO2 formation by PSII compared with in vivo conditions. We therefore performed off-line TR-MIMS experiments in which the dark-adapted PSII membranes were illuminated at ambient levels of CO2/
in the sample suspension (17), which may significantly reduce light-induced CO2 formation by PSII compared with in vivo conditions. We therefore performed off-line TR-MIMS experiments in which the dark-adapted PSII membranes were illuminated at ambient levels of CO2/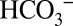 in gas-tight syringes with 100 xenon flashes (2 Hz). For the detection of the light-induced gas formation the samples were then injected rapidly (<3 s) after the illumination into the MIMS cell containing the same buffer (but degassed). Fig. 4 displays the light-minus-dark difference signals of the
in gas-tight syringes with 100 xenon flashes (2 Hz). For the detection of the light-induced gas formation the samples were then injected rapidly (<3 s) after the illumination into the MIMS cell containing the same buffer (but degassed). Fig. 4 displays the light-minus-dark difference signals of the 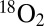 and
and 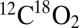 gases (see original traces in Fig. S3). Under these more natural conditions, the CO2 evolution reaches up to 20% of the O2 evolution and can be clearly seen at the same sensitivity scale as O2.
gases (see original traces in Fig. S3). Under these more natural conditions, the CO2 evolution reaches up to 20% of the O2 evolution and can be clearly seen at the same sensitivity scale as O2.
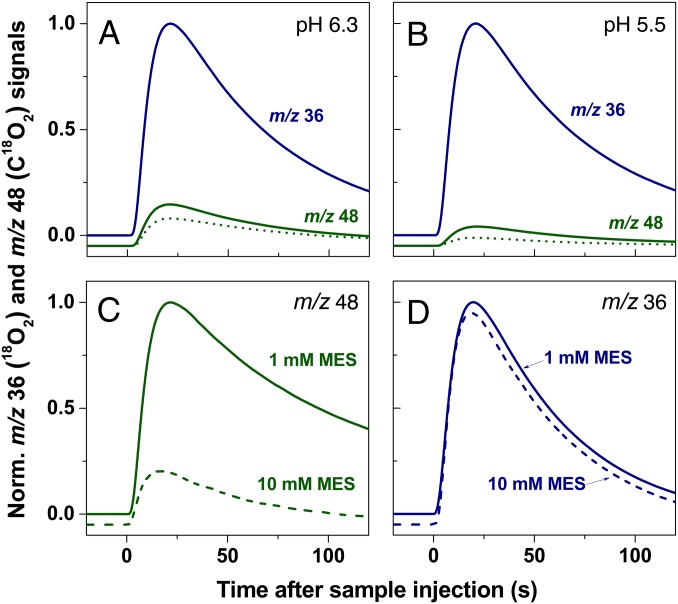
Fig. 4.
Simultaneous off-line TR-MIMS measurements of O2 and CO2 production by PSII. Dark-adapted PSII membranes (1 mg Chl/mL) were illuminated inside of a gas tide syringe with 100 xenon flashes and then injected into the MIMS cell either as fast as possible with our current set up (<3 s, solid lines) or after 1 min (dotted line). Displayed are the light-minus-dark difference signals of O2 (blue traces) and CO2 (green traces), normalized to the amplitude of O2 release (for original traces see Fig. S3). The amplitudes of the traces for CO2 and O2 can be directly compared, because our set-up detects both gases with nearly equal sensitivity. Conditions: (A) 1 mM MES, pH 6.3; (B) 1 mM MES, pH 5.5; (C and D) 1 mM MES (solid lines) or 10 mM MES (dashed lines), pH 6.3. In addition to MES, the medium contained 15 mM NaCl, 15% 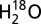 , and 2 mM K3[Fe(CN)6] as electron acceptor. Zero levels are off set for clarity of presentation. In all panels the average of two to three repeats is presented.
, and 2 mM K3[Fe(CN)6] as electron acceptor. Zero levels are off set for clarity of presentation. In all panels the average of two to three repeats is presented.
To compare the CO2/O2 ratios at different 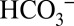 concentrations in the sample buffer, we performed these off-line TR-MIMS experiments at pH 6.3 (Fig. 4A) and at pH 5.5 (Fig. 4B), which approximately corresponds to the luminal pH in photosynthetic organisms under illumination (26, 27). At pH 6.3 the CO2 and
concentrations in the sample buffer, we performed these off-line TR-MIMS experiments at pH 6.3 (Fig. 4A) and at pH 5.5 (Fig. 4B), which approximately corresponds to the luminal pH in photosynthetic organisms under illumination (26, 27). At pH 6.3 the CO2 and 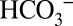 concentrations are about equal. When changing the pH of the solution to pH 5.5 under conditions that allow equilibration with air (open system), the concentration of dissolved CO2 in the medium remains unchanged compared with pH 6.3, but the
concentrations are about equal. When changing the pH of the solution to pH 5.5 under conditions that allow equilibration with air (open system), the concentration of dissolved CO2 in the medium remains unchanged compared with pH 6.3, but the 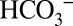 concentration is reduced about six times (28) (SI Text and Table S1). Comparison of Fig. 4 A and B shows that the CO2/O2 ratio is more than two times lower at pH 5.5 compared with pH 6.3. Although the relative CO2 evolution by PSII is not directly proportional to the
concentration is reduced about six times (28) (SI Text and Table S1). Comparison of Fig. 4 A and B shows that the CO2/O2 ratio is more than two times lower at pH 5.5 compared with pH 6.3. Although the relative CO2 evolution by PSII is not directly proportional to the 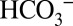 concentration, these data follow the trend expected if CO2 is produced according to Reaction 1.
concentration, these data follow the trend expected if CO2 is produced according to Reaction 1.
The idea that 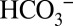 acts as proton acceptor was probed in air-saturated media by comparing light-induced CO2 evolution at 10-fold different MES buffer concentrations (1 mM and 10 mM; Fig. 4C). MES at 200 mM was previously used to detect the proton release pattern of PSII by isotope-editing FTIR spectroscopy (29) and is therefore known to be a good proton acceptor for PSII that can outcompete
acts as proton acceptor was probed in air-saturated media by comparing light-induced CO2 evolution at 10-fold different MES buffer concentrations (1 mM and 10 mM; Fig. 4C). MES at 200 mM was previously used to detect the proton release pattern of PSII by isotope-editing FTIR spectroscopy (29) and is therefore known to be a good proton acceptor for PSII that can outcompete 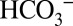 in its suspected role. A strongly reduced CO2 and the same O2 production were observed at 10 mM MES compared with 1 mM MES (Fig. 4 C and D), confirming this proposal. We note that this result excludes Warburg-type mechanisms (30) in which
in its suspected role. A strongly reduced CO2 and the same O2 production were observed at 10 mM MES compared with 1 mM MES (Fig. 4 C and D), confirming this proposal. We note that this result excludes Warburg-type mechanisms (30) in which 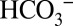 acts directly or indirectly as a substrate of PSII, because for such mechanisms a buffer capacity independent CO2/O2 ratio is expected.
acts directly or indirectly as a substrate of PSII, because for such mechanisms a buffer capacity independent CO2/O2 ratio is expected.
The competition between buffer and 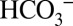 for protons is further illustrated by the dotted traces in Fig. 4 A and B. They show that at 1 mM MES the CO2/O2 ratios are significantly lower if the PSII suspension is not injected rapidly (1–3 s) after illumination into the MIMS cells, but after waiting for some time (∼1 min). This phenomenon reflects the back-conversion (Reaction 1) of CO2 into
for protons is further illustrated by the dotted traces in Fig. 4 A and B. They show that at 1 mM MES the CO2/O2 ratios are significantly lower if the PSII suspension is not injected rapidly (1–3 s) after illumination into the MIMS cells, but after waiting for some time (∼1 min). This phenomenon reflects the back-conversion (Reaction 1) of CO2 into 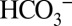 by equilibration with water and proton acceptors such as side chains of proteins and lipids head groups of the sample or with MES according to their respective pKa values (6.15 for MES and 6.38 for
by equilibration with water and proton acceptors such as side chains of proteins and lipids head groups of the sample or with MES according to their respective pKa values (6.15 for MES and 6.38 for 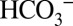 at 20 °C) and the respective free base concentrations
at 20 °C) and the respective free base concentrations 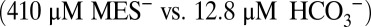 . It is noted that the O2 levels do not change during the 1-min waiting time (Fig. S4). The CO2 signals in Fig. 4 are therefore lower estimates of the initial production. We further illustrate this point in Fig. S5, where by using an automated flash-injection system we were able to observed CO2/O2 ratios >0.5 at 1 ms between illumination and injection. Importantly, the time-dependent equilibration shows that
. It is noted that the O2 levels do not change during the 1-min waiting time (Fig. S4). The CO2 signals in Fig. 4 are therefore lower estimates of the initial production. We further illustrate this point in Fig. S5, where by using an automated flash-injection system we were able to observed CO2/O2 ratios >0.5 at 1 ms between illumination and injection. Importantly, the time-dependent equilibration shows that 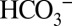 accepts protons produced by water splitting at sites within PSII that are not equally well accessible for the larger MES buffer molecules (i.e., that the CO2 production is primarily a PSII-specific effect, rather than caused by a simple pH change of the medium).
accepts protons produced by water splitting at sites within PSII that are not equally well accessible for the larger MES buffer molecules (i.e., that the CO2 production is primarily a PSII-specific effect, rather than caused by a simple pH change of the medium).
Discussion
 Interaction with PSII in Vitro.
Interaction with PSII in Vitro.
The data in this study were obtained with isolated PSII membranes from spinach. This made it possible to study the effects of 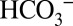 on PSII activity without the need to consider indirect effects from related processes such as the carbon sequestration mechanisms (CCMs) operational in many algae and cyanobacteria (31). Our results clearly demonstrate that illumination of higher-plant PSII membranes with visible light induces both O2 and CO2 evolution, and that the yield of CO2 depends both on the concentrations of
on PSII activity without the need to consider indirect effects from related processes such as the carbon sequestration mechanisms (CCMs) operational in many algae and cyanobacteria (31). Our results clearly demonstrate that illumination of higher-plant PSII membranes with visible light induces both O2 and CO2 evolution, and that the yield of CO2 depends both on the concentrations of 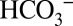 and MES in the sample suspension. Quantitation of the signals in Fig. 4A reveals the production of about four CO2 molecules per PSII (Methods). Therefore, we can exclude the possibility that the light-induced CO2 production is due to the release of
and MES in the sample suspension. Quantitation of the signals in Fig. 4A reveals the production of about four CO2 molecules per PSII (Methods). Therefore, we can exclude the possibility that the light-induced CO2 production is due to the release of 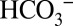 from its binding site at the nonheme iron. Collectively our data demonstrate conclusively that exchangeable
from its binding site at the nonheme iron. Collectively our data demonstrate conclusively that exchangeable 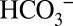 acts as a base for protons produced by PSII during water splitting.
acts as a base for protons produced by PSII during water splitting.
Special pathways or channels have been discussed for guiding protons away from the water-splitting Mn4CaO5 cluster (32, 33). We show that 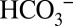 can compete for protons from water splitting with up to 300 times higher MES− concentration (at 10 mM MES) and that the CO2 yield observed is time-dependent (Fig. 4 and Fig. S5). We therefore propose that
can compete for protons from water splitting with up to 300 times higher MES− concentration (at 10 mM MES) and that the CO2 yield observed is time-dependent (Fig. 4 and Fig. S5). We therefore propose that 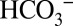 is able to penetrate more deeply into PSII to accept protons than MES molecules, which are not present in vivo. Inspection of the 1.9-
is able to penetrate more deeply into PSII to accept protons than MES molecules, which are not present in vivo. Inspection of the 1.9-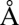 PSII crystal structure (3ARC code) reveals that
PSII crystal structure (3ARC code) reveals that 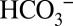 should indeed be able to penetrate easily into the entrance regions of all postulated channels; some channels are even wide and flexible enough to allow glycerol molecules to penetrate far inside (32, 33). In contrast, access to these channels for the even larger MES molecules would be more restricted. Once
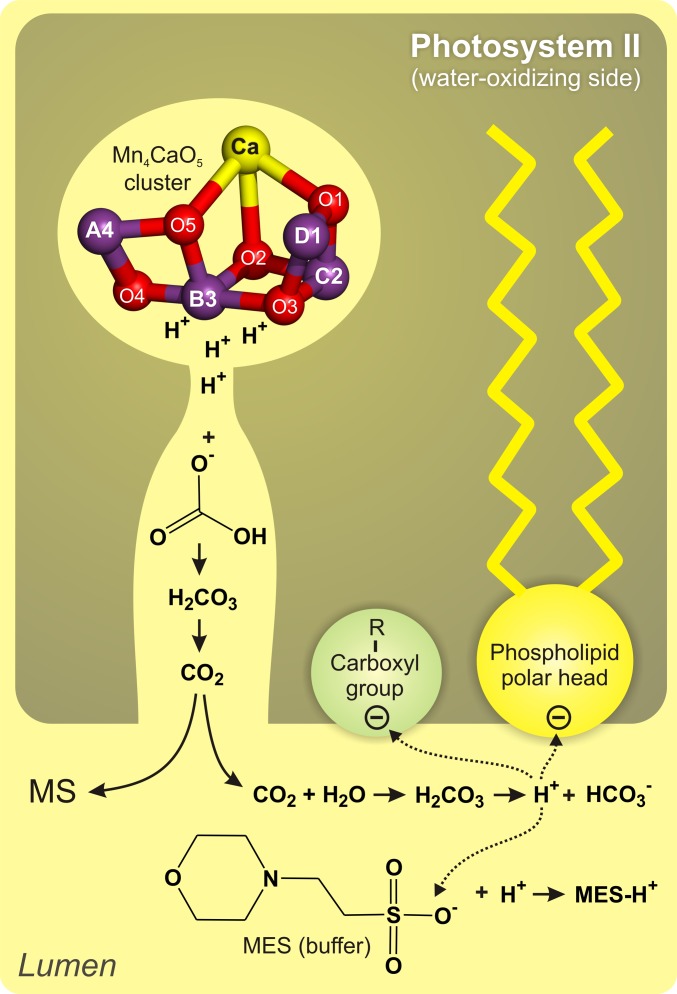
should indeed be able to penetrate easily into the entrance regions of all postulated channels; some channels are even wide and flexible enough to allow glycerol molecules to penetrate far inside (32, 33). In contrast, access to these channels for the even larger MES molecules would be more restricted. Once 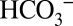 accepts a proton it decomposes according to Reaction 1, and the CO2 is released from the channel and is either detected by MIMS or converted back to
accepts a proton it decomposes according to Reaction 1, and the CO2 is released from the channel and is either detected by MIMS or converted back to 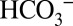 by equilibration with water and buffer groups in the lumen, or with the artificially added MES buffer. This is illustrated schematically in Fig. 5. These equilibration processes likely lead to the delayed detection of CO2 compared with O2 that is visible in the on-line experiment displayed in Fig. 3.
by equilibration with water and buffer groups in the lumen, or with the artificially added MES buffer. This is illustrated schematically in Fig. 5. These equilibration processes likely lead to the delayed detection of CO2 compared with O2 that is visible in the on-line experiment displayed in Fig. 3.
Fig. 5.
Schematic representation of the function of 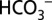 in PSII.
in PSII.
We suggest that owing to the comparatively weak binding in the channels, which is required for the proposed function as mobile proton acceptor and carrier, 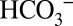 does not have a high enough occupancy at any particular site to be observable in the crystal structures. It is noted that in vivo the buffer capacity of the lumen of chloroplasts was estimated to be 0.8–1.0 mM (pH 6.4–8.1) and suggested to be due mostly to stationary groups such as proteins and lipid head groups (34), and not to mobile buffers such as MES added here at low concentrations to minimize the bulk pH changes. In vivo the role of
does not have a high enough occupancy at any particular site to be observable in the crystal structures. It is noted that in vivo the buffer capacity of the lumen of chloroplasts was estimated to be 0.8–1.0 mM (pH 6.4–8.1) and suggested to be due mostly to stationary groups such as proteins and lipid head groups (34), and not to mobile buffers such as MES added here at low concentrations to minimize the bulk pH changes. In vivo the role of 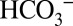 for the activity of PSII may therefore be even more pronounced than observed during the experiments shown in Fig. 2.
for the activity of PSII may therefore be even more pronounced than observed during the experiments shown in Fig. 2.
Biological Relevance.
Higher plants take up CO2 for carbon fixation by Rubisco via opening their stomata. Therefore, they do not possess special CCMs. In contrast, algae and cyanobacteria acquire CO2 in the form of 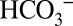 from water, where its concentration is often very low. This requires CCMs that consist of several transporters to channel
from water, where its concentration is often very low. This requires CCMs that consist of several transporters to channel 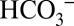 through the various membranes, and of carbonic anhydrases that allow for efficient
through the various membranes, and of carbonic anhydrases that allow for efficient 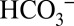 /CO2 interconversion (31, 35). The discovery of the PSII-bound Cah3 carbonic anhydrase (cia3 gene product) in Chlamydomonas reinhartii (22, 36, 37) raised the question of whether this carbonic anhydrase together with
/CO2 interconversion (31, 35). The discovery of the PSII-bound Cah3 carbonic anhydrase (cia3 gene product) in Chlamydomonas reinhartii (22, 36, 37) raised the question of whether this carbonic anhydrase together with 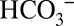 are required for PSII function, or whether they are part of the CCM mechanism that provides the nearby Rubisco with CO2. Despite numerous experiments this point remained unresolved (21, 22, 35, 38). Although PSII membranes isolated from spinach also possess some carbonic anhydrase activity (6, 8), there is presently no evidence for a carbonic anhydrase directly connected to higher-plant PSII, and there is also no need for a CCM function (discussed above). Our finding that under continuous illumination the activity of PSII is nevertheless dependent on the
are required for PSII function, or whether they are part of the CCM mechanism that provides the nearby Rubisco with CO2. Despite numerous experiments this point remained unresolved (21, 22, 35, 38). Although PSII membranes isolated from spinach also possess some carbonic anhydrase activity (6, 8), there is presently no evidence for a carbonic anhydrase directly connected to higher-plant PSII, and there is also no need for a CCM function (discussed above). Our finding that under continuous illumination the activity of PSII is nevertheless dependent on the 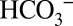 concentration of the buffer therefore demonstrates that
concentration of the buffer therefore demonstrates that 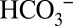 directly affects the activity of PSII. It is noted that the ability of
directly affects the activity of PSII. It is noted that the ability of 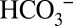 to capture protons will affect the H+/e− ratio owing to the loss of some CO2 into the stroma, unless this is prevented by subsequent reformation of
to capture protons will affect the H+/e− ratio owing to the loss of some CO2 into the stroma, unless this is prevented by subsequent reformation of 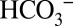 via equilibration with stationary buffers in the lumen, which is strongly accelerated by carbonic anhydrases.
via equilibration with stationary buffers in the lumen, which is strongly accelerated by carbonic anhydrases.
Although 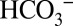 cannot be viewed as a cofactor of water oxidation in PSII, our experiments do clearly demonstrate that it affects its activity. As such, a feedback loop can be envisioned between CO2 fixation in the stroma and water oxidation at the luminal side of PSII: At high CO2 levels in leaves the gas will be taken up into the alkaline stroma of the chloroplasts and trapped by conversion into
cannot be viewed as a cofactor of water oxidation in PSII, our experiments do clearly demonstrate that it affects its activity. As such, a feedback loop can be envisioned between CO2 fixation in the stroma and water oxidation at the luminal side of PSII: At high CO2 levels in leaves the gas will be taken up into the alkaline stroma of the chloroplasts and trapped by conversion into 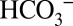 . Stromal carbonic anhydrases convert it back into CO2 when the residual CO2 is consumed by Rubisco for carbon fixation, or when it gets lost by diffusion into the lumen, where it will be also partially trapped as
. Stromal carbonic anhydrases convert it back into CO2 when the residual CO2 is consumed by Rubisco for carbon fixation, or when it gets lost by diffusion into the lumen, where it will be also partially trapped as 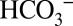 . This leaching of CO2 through the thylakoid membrane into the lumen allows PSII to “sense” the Ci level in the stroma. At high Ci levels in the chloroplasts many electrons can be used for CO2 fixation, and
. This leaching of CO2 through the thylakoid membrane into the lumen allows PSII to “sense” the Ci level in the stroma. At high Ci levels in the chloroplasts many electrons can be used for CO2 fixation, and 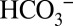 in the lumen activates PSII so that it produces electrons at maximum efficiency. In contrast, at low CO2/
in the lumen activates PSII so that it produces electrons at maximum efficiency. In contrast, at low CO2/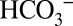 levels fewer electrons are needed, and the down-regulation of PSII by 20% (or more) helps to mitigate an overreduction of the plastoquinone pool, which reduces the risk of producing reactive oxygen species that are known to be damaging to enzymes involved in photosynthesis (39–41). Future studies will be needed to elucidate whether such a feedback loop is indeed operational.
levels fewer electrons are needed, and the down-regulation of PSII by 20% (or more) helps to mitigate an overreduction of the plastoquinone pool, which reduces the risk of producing reactive oxygen species that are known to be damaging to enzymes involved in photosynthesis (39–41). Future studies will be needed to elucidate whether such a feedback loop is indeed operational.
Summary
The light-induced CO2 formation by PSII and the dependence of CO2 evolution on the concentrations of Ci and added buffer in the medium demonstrate conclusively that 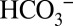 acts as mobile proton acceptor of PSII. The data in Fig. 2 show that the lack of this component leads under “stress” (continuous strong light) to reduced oxygen evolution by PSII (21, 22). Because strictly alternating electron and proton removals from the OEC seem to be an inherent and crucial part of the mechanism of water oxidation in PSII (42, 43), it seems very likely that this function of
acts as mobile proton acceptor of PSII. The data in Fig. 2 show that the lack of this component leads under “stress” (continuous strong light) to reduced oxygen evolution by PSII (21, 22). Because strictly alternating electron and proton removals from the OEC seem to be an inherent and crucial part of the mechanism of water oxidation in PSII (42, 43), it seems very likely that this function of 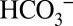 developed early during evolution and can be found in all O2-evolving organisms (44). This dependence of PSII activity on
developed early during evolution and can be found in all O2-evolving organisms (44). This dependence of PSII activity on 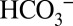 concentration may also allow for a feedback regulation with the Calvin–Benson–Bassham cycle, which uses the electrons and protons from water splitting for CO2 reduction to biomass.
concentration may also allow for a feedback regulation with the Calvin–Benson–Bassham cycle, which uses the electrons and protons from water splitting for CO2 reduction to biomass.
Methods
Sample Preparation.
PSII membranes (“BBY” type) were prepared from fresh leaves of Spinacia (S.) oleracea following protocols described earlier (45, 46). Typical rates of O2 evolution for spinach PSII membranes were 400–500 µmol (O2)⋅mg (Chl)−1⋅h−1. After isolation, the PSII membranes were frozen by dropping small aliquots into liquid nitrogen. The samples were then stored at –80 °C until used. Before the measurements, the samples were thawed in the dark on ice and diluted to the desired concentrations with MES medium containing 200–400 mM sucrose, 35 mM NaCl, and 1–10 mM MES/NaOH at pH 6.3 (or 5.5).
TR-MIMS Measurements.
TR-MIMS measurements were performed with an isotope ratio mass spectrometer (FinniganPlus XP; Thermo) that was connected via a dry ice/C2H5OH cooling trap to a home-built membrane-inlet cell of 150- or 175-µL volume (depending on stir bar used) (47). The sample in the cell was separated from the vacuum (3 × 10−8 bar) of the mass spectrometer via a 1-cm-diameter inlet that was covered by a 25-µm-thick silicon membrane (MEM-213; Mempro) resting on a porous Teflon support (Ø 10 mm). In the mass spectrometer the gases were ionized by electron impact and separated by a magnetic sector field into a seven-cup Faraday detector array for simultaneous detection of 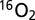 (m/z 32),
(m/z 32), 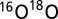 (m/z 34),
(m/z 34), 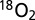 and 36Ar (m/z 36), 40Ar (m/z 40),
and 36Ar (m/z 36), 40Ar (m/z 40), 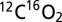 (m/z 44),
(m/z 44), 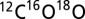 (m/z 46), and
(m/z 46), and 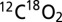 (m/z 48). The simultaneous recording of argon (m/z 40) served as a control. The MIMS cell was thermostated to 20 °C. During the measurements the sample suspensions were stirred constantly at high speed with a magnetic stir bar. Before the assays the sample suspensions (or buffers) were degassed for 20–40 min until an only slightly sloping baseline was reached. On-line monitoring of light-induced O2 and CO2 evolutions (Fig. 3) was done by illumination of the dark-adapted PSII membranes inside the MIMS cell with a train of 50 short saturating flashes (2 Hz) provided by xenon flash lamp (LS-1130–4, ∼5-µs half-width; Perkin–Elmer). Off-line measurements of light-induced O2 and CO2 evolutions were performed by excitation of the dark-adapted PSII membranes inside a gas-tight 250-µL Hamilton syringe and subsequent injection of the illuminated sample into the cell filled with the thoroughly degassed buffer solution of the same composition and pH. For the experiments displayed in Fig. 4 50-µL PSII sample suspensions [1 mg (Chl)⋅mL−1] were illuminated with 100 xenon flashes (PS 302, 2 Hz, light pack FY-604 ∼15-µs half-width; EG&G). For controls, 50-µL aliquots of dark-adapted PSII sample suspensions were injected into the MIMS cell (Fig. S3). The light-induced O2 and CO2 signals were calibrated by the injection of defined volumes of air-equilibrated buffer (284 µM O2 and 15.4 µM CO2 at 20 °C, pH 5.0) into the same medium (www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html and http://co2now.org/current-co2/co2-now/). The CO2/PSII ratio was determined by dividing the calibrated CO2 concentration by the PSII reaction center (RC) concentration, assuming 250 Chl/RC. The analysis of the MIMS spectra was performed by using Origin software.
(m/z 48). The simultaneous recording of argon (m/z 40) served as a control. The MIMS cell was thermostated to 20 °C. During the measurements the sample suspensions were stirred constantly at high speed with a magnetic stir bar. Before the assays the sample suspensions (or buffers) were degassed for 20–40 min until an only slightly sloping baseline was reached. On-line monitoring of light-induced O2 and CO2 evolutions (Fig. 3) was done by illumination of the dark-adapted PSII membranes inside the MIMS cell with a train of 50 short saturating flashes (2 Hz) provided by xenon flash lamp (LS-1130–4, ∼5-µs half-width; Perkin–Elmer). Off-line measurements of light-induced O2 and CO2 evolutions were performed by excitation of the dark-adapted PSII membranes inside a gas-tight 250-µL Hamilton syringe and subsequent injection of the illuminated sample into the cell filled with the thoroughly degassed buffer solution of the same composition and pH. For the experiments displayed in Fig. 4 50-µL PSII sample suspensions [1 mg (Chl)⋅mL−1] were illuminated with 100 xenon flashes (PS 302, 2 Hz, light pack FY-604 ∼15-µs half-width; EG&G). For controls, 50-µL aliquots of dark-adapted PSII sample suspensions were injected into the MIMS cell (Fig. S3). The light-induced O2 and CO2 signals were calibrated by the injection of defined volumes of air-equilibrated buffer (284 µM O2 and 15.4 µM CO2 at 20 °C, pH 5.0) into the same medium (www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html and http://co2now.org/current-co2/co2-now/). The CO2/PSII ratio was determined by dividing the calibrated CO2 concentration by the PSII reaction center (RC) concentration, assuming 250 Chl/RC. The analysis of the MIMS spectra was performed by using Origin software.
CO2/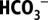 -Depletion Procedure and Monitoring of Ci Levels.
-Depletion Procedure and Monitoring of Ci Levels.
Ci was removed from the PSII sample suspension containing 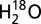 and electron acceptors by means of 30-min flushing with CO2-free air that was generated by a Puregas PCDA220M instrument, in which compressed air is directed through a desiccant chamber containing an adsorbent material for CO2.This Ci-depletion procedure led to a ∼20-fold decrease of the CO2 level (∼0.8 µM) without decreasing the oxygen level compared with samples containing ambient CO2 and O2 levels owing to exposure to air (
and electron acceptors by means of 30-min flushing with CO2-free air that was generated by a Puregas PCDA220M instrument, in which compressed air is directed through a desiccant chamber containing an adsorbent material for CO2.This Ci-depletion procedure led to a ∼20-fold decrease of the CO2 level (∼0.8 µM) without decreasing the oxygen level compared with samples containing ambient CO2 and O2 levels owing to exposure to air (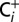 media; ∼15.4 µM). To avoid contamination of Ci-depleted
media; ∼15.4 µM). To avoid contamination of Ci-depleted 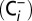 media with atmospheric CO2, the depletion procedure and all following sample handling and incubation steps were performed inside sealed septum vials. This procedure resulted in our
media with atmospheric CO2, the depletion procedure and all following sample handling and incubation steps were performed inside sealed septum vials. This procedure resulted in our 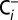 -depleted PSII membranes. For reversibility measurements the
-depleted PSII membranes. For reversibility measurements the 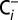 -depleted suspension of PSII membranes (∼80 µL) was incubated for 20 min in air. For a better equilibration with atmospheric CO2, the sample suspensions were continuously stirred in a septum vial with an opened cap.
-depleted suspension of PSII membranes (∼80 µL) was incubated for 20 min in air. For a better equilibration with atmospheric CO2, the sample suspensions were continuously stirred in a septum vial with an opened cap.
The CO2 and O2 concentrations in CO2-depleted air were determined by monitoring the oxygen and carbon dioxide content of the 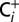 and
and 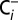 sample solutions. For this, the 50-µL aliquots of the
sample solutions. For this, the 50-µL aliquots of the 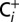 and
and 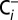 media were injected into degassed MES buffer (pH 6.3) in the MIMS cell.
media were injected into degassed MES buffer (pH 6.3) in the MIMS cell.
Supplementary Material
Acknowledgments
We thank Govindjee, A. Stemler, and V. V. Klimov for many stimulating discussions on bicarbonate effects over the years, G. Han for help with PSII sample preparation, U. Sauer and E. Sauer-Eriksson for discussions on the accessibility of PSII channels for HCO3−, W. Junge for valuable comments on the manuscript, and the Max Planck Institute for Chemical Energy Conversion for the loan of the isotope ratio mass spectrometer. Financial support was provided by Vetenskapsrådet (J.M. and G.S.), Energimyndigheten (J.M.), Kempe Stiftelse (J.M. and G.S.), the Strong Research Environment Solar Fuels (Umeå University), and the Artificial Leaf Project Umeå (Knut and Alice Wallenberg Foundation).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323277111/-/DCSupplemental.
References
- 1.Lubitz W, Reijerse EJ, Messinger J. Solar water-splitting into H2 and O2: Design principles of photosystem II and hydrogenases. Energy Environ Sci. 2008;1:15–31. [Google Scholar]
- 2.Yano J, et al. Where water is oxidized to dioxygen: Structure of the photosynthetic Mn4Ca cluster. Science. 2006;314(5800):821–825. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umena Y, Kawakami K, Shen J-R, Kamiya N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature. 2011;473(7345):55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]
- 4.Dau H, Zaharieva I. Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc Chem Res. 2009;42(12):1861–1870. doi: 10.1021/ar900225y. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O, Krippahl G. Hill-Reaktionen. Z Naturforsch B. 1958;13B(8):509–514. [PubMed] [Google Scholar]
- 6.Clausen J, Beckmann K, Junge W, Messinger J. Evidence that bicarbonate is not the substrate in photosynthetic oxygen evolution. Plant Physiol. 2005;139(3):1444–1450. doi: 10.1104/pp.105.068437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radmer R, Ollinger O. Isotopic composition of photosynthetic O2 flash yields in the presence of H218O and HC18O3- FEBS Lett. 1980;110(1):57–61. [Google Scholar]
- 8.Hillier W, et al. Quantitative assessment of intrinsic carbonic anhydrase activity and the capacity for bicarbonate oxidation in photosystem II. Biochemistry. 2006;45(7):2094–2102. doi: 10.1021/bi051892o. [DOI] [PubMed] [Google Scholar]
- 9.Stemler AJ. The bicarbonate effect, oxygen evolution, and the shadow of Otto Warburg. Photosynth Res. 2002;73(1-3):177–183. doi: 10.1023/A:1020447030191. [DOI] [PubMed] [Google Scholar]
- 10.Van Rensen JJS, Klimov VV. In: Bicarbonate interactions. Photosystem II The Light-Driven Water: Plastoquinone Oxidoreductase, Advances in Photosynthesis and Respiration. Wydrzynski T, Satoh K, editors. Vol 22. Dordrecht: Springer; 2005. pp. 329–346. [Google Scholar]
- 11.Shevela D, Eaton-Rye JJ, Shen J-R, Govindjee Photosystem II and the unique role of bicarbonate: A historical perspective. Biochim Biophys Acta. 2012;1817(8):1134–1151. doi: 10.1016/j.bbabio.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 12.McConnell IL, Eaton-Rye JJ, Van Rensen JJS. Regulation of photosystem II electron transport by bicarbonate. In: Eaton-Rye JJ, Tripathy BC, Sharkey TD, editors. Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation. Dordrecht: Springer; 2012. pp. 475–500. [Google Scholar]
- 13.Cox N, et al. The semiquinone-iron complex of photosystem II: structural insights from ESR and theoretical simulation; evidence that the native ligand to the non-heme iron is carbonate. Biophys J. 2009;97(7):2024–2033. doi: 10.1016/j.bpj.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guskov A, et al. Recent progress in the crystallographic studies of photosystem II. ChemPhysChem. 2010;11(6):1160–1171. doi: 10.1002/cphc.200900901. [DOI] [PubMed] [Google Scholar]
- 15.Govindjee Bicarbonate-reversible inhibition of plastoquinone reductase in photosystem II. Z Naturforsch. 1993;C 48(3-4):251–258. [Google Scholar]
- 16.Aoyama C, Suzuki H, Sugiura M, Noguchi T. Flash-induced FTIR difference spectroscopy shows no evidence for the structural coupling of bicarbonate to the oxygen-evolving Mn cluster in photosystem II. Biochemistry. 2008;47(9):2760–2765. doi: 10.1021/bi702241t. [DOI] [PubMed] [Google Scholar]
- 17.Shevela D, Su JH, Klimov V, Messinger J. Hydrogencarbonate is not a tightly bound constituent of the water-oxidizing complex in photosystem II. Biochim Biophys Acta. 2008;1777(6):532–539. doi: 10.1016/j.bbabio.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Ulas G, Olack G, Brudvig GW. Evidence against bicarbonate bound in the O2-evolving complex of photosystem II. Biochemistry. 2008;47(10):3073–3075. doi: 10.1021/bi8000424. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta J, Ananyev GM, Dismukes GC. Photoassembly of the water-oxidizing complex in photosystem II. Coord Chem Rev. 2008;252(3-4):347–360. doi: 10.1016/j.ccr.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananyev G, Nguyen T, Putnam-Evans C, Dismukes GC. Mutagenesis of CP43-arginine-357 to serine reveals new evidence for (bi)carbonate functioning in the water oxidizing complex of Photosystem II. Photochem Photobiol Sci. 2005;4(12):991–998. doi: 10.1039/b507519j. [DOI] [PubMed] [Google Scholar]
- 21.Shutova T, et al. The photosystem II-associated Cah3 in Chlamydomonas enhances the O2 evolution rate by proton removal. EMBO J. 2008;27(5):782–791. doi: 10.1038/emboj.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villarejo A, et al. A photosystem II-associated carbonic anhydrase regulates the efficiency of photosynthetic oxygen evolution. EMBO J. 2002;21(8):1930–1938. doi: 10.1093/emboj/21.8.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckmann K, Messinger J, Badger MR, Wydrzynski T, Hillier W. On-line mass spectrometry: Membrane inlet sampling. Photosynth Res. 2009;102(2-3):511–522. doi: 10.1007/s11120-009-9474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shevela D, Messinger J. Studying the oxidation of water to molecular oxygen in photosynthetic and artificial systems by time-resolved membrane-inlet mass spectrometry. Front Plant Sci. 2013;4:473. doi: 10.3389/fpls.2013.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevela D, et al. Efficiency of photosynthetic water oxidation at ambient and depleted levels of inorganic carbon. Photosynth Res. 2013;117(1-3):401–412. doi: 10.1007/s11120-013-9875-5. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa K, Cruz JA, Kanazawa A, Kramer DM. The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta. 2007;1767(10):1233–1244. doi: 10.1016/j.bbabio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Tikhonov AN, et al. Spin-probes designed for measuring the intrathylakoid pH in chloroplasts. Biochim Biophys Acta. 2008;1777(3):285–294. doi: 10.1016/j.bbabio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Stumm W, Morgan JJ. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. 3rd Ed. New York: Wiley; 1995. p. 1040. [Google Scholar]
- 29.Suzuki H, Sugiura M, Noguchi T. Monitoring proton release during photosynthetic water oxidation in photosystem II by means of isotope-edited infrared spectroscopy. J Am Chem Soc. 2009;131(22):7849–7857. doi: 10.1021/ja901696m. [DOI] [PubMed] [Google Scholar]
- 30.Metzner H. Photosynthetic Oxygen Evolution. London: Academic; 1978. [Google Scholar]
- 31.Badger MR, Price GD. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J Exp Bot. 2003;54(383):609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 32.Gabdulkhakov A, et al. Probing the accessibility of the Mn(4)Ca cluster in photosystem II: Channels calculation, noble gas derivatization, and cocrystallization with DMSO. Structure. 2009;17(9):1223–1234. doi: 10.1016/j.str.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Ho FM. Uncovering channels in photosystem II by computer modelling: Current progress, future prospects, and lessons from analogous systems. Photosynth Res. 2008;98(1-3):503–522. doi: 10.1007/s11120-008-9358-2. [DOI] [PubMed] [Google Scholar]
- 34.Junge W, Ausländer W, McGeer AJ, Runge T. The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta. 1979;546(1):121–141. doi: 10.1016/0005-2728(79)90175-0. [DOI] [PubMed] [Google Scholar]
- 35.Van Hunnik E, Sultemeyer D. A possible role for carbonic anhydrase in the lumen of chloroplast thylakoids in green algae. Funct Plant Biol. 2002;29(2-3):243–249. doi: 10.1071/PP01196. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson J, et al. A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998;17(5):1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Hunnik E, et al. Identification and localization of a thylakoid-bound carbonic anhydrase from the green algae Tetraedron minimum (Chlorophyta) and Chlamydomonas noctigama (Chlorophyta) Planta. 2001;212(3):454–459. doi: 10.1007/s004250000418. [DOI] [PubMed] [Google Scholar]
- 38.Hanson DT, Franklin LA, Samuelsson G, Badger MR. The Chlamydomonas reinhardtii cia3 mutant lacking a thylakoid lumen-localized carbonic anhydrase is limited by CO2 supply to rubisco and not photosystem II function in vivo. Plant Physiol. 2003;132(4):2267–2275. doi: 10.1104/pp.103.023481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Pospísil P. Production of reactive oxygen species by photosystem II. Biochim Biophys Acta. 2009;1787(10):1151–1160. doi: 10.1016/j.bbabio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Rutherford AW, Osyczka A, Rappaport F. Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O(2) FEBS Lett. 2012;586(5):603–616. doi: 10.1016/j.febslet.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 42.Siegbahn PEM. Water oxidation in photosystem II: Oxygen release, proton release and the effect of chloride. Dalton Trans. 2009;45(45):10063–10068. doi: 10.1039/b909470a. [DOI] [PubMed] [Google Scholar]
- 43.Klauss A, Haumann M, Dau H. Alternating electron and proton transfer steps in photosynthetic water oxidation. Proc Natl Acad Sci USA. 2012;109(40):16035–16040. doi: 10.1073/pnas.1206266109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dismukes GC, et al. The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proc Natl Acad Sci USA. 2001;98(5):2170–2175. doi: 10.1073/pnas.061514798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berthold DA, Babcock GT, Yocum CF. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981;134:231–234. [Google Scholar]
- 46.Ono T-A, Inoue Y. Mn-preserving extraction of 33-, 24- and 16-kDa Proteins from O2-evolving PS II particles by divalent salt-washing. FEBS Lett. 1983;164(2):255–260. [Google Scholar]
- 47.Messinger J, Badger MR, Wydrzynski T. Detection of one slowly exchanging substrate water molecule in the S3 state of photosystem II. Proc Natl Acad Sci USA. 1995;92(8):3209–3213. doi: 10.1073/pnas.92.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.