Background: Cholera toxin (CT) production is induced during anaerobic respiration with trimethylamine N-oxide (TMAO) in Vibrio cholerae.
Results: A bacterial stringent response to nutrient starvation was activated during anaerobic TMAO respiration and influenced CT production.
Conclusion: CT production during anaerobic TMAO respiration is mediated by stringent response in V. cholerae.
Significance: A mechanism of TMAO-stimulated CT production is uncovered.
Keywords: Bacterial Pathogenesis, Cholera Toxin, Respiration, Stress Response, Virulence Factors, Anaerobic Respiration, Stringent Response, Vibrio cholerae
Abstract
As a facultative anaerobe, Vibrio cholerae can grow by anaerobic respiration. Production of cholera toxin (CT), a major virulence factor of V. cholerae, is highly promoted during anaerobic growth using trimethylamine N-oxide (TMAO) as an alternative electron acceptor. Here, we investigated the molecular mechanisms of TMAO-stimulated CT production and uncovered the crucial involvement of stringent response in this process. V. cholerae 7th pandemic strain N16961 produced a significantly elevated level of ppGpp, the bacterial stringent response alarmone, during anaerobic TMAO respiration. Bacterial viability was impaired, and DNA replication was also affected under the same growth condition, further suggesting that stringent response is induced. A ΔrelA ΔspoT ppGpp overproducer strain produced an enhanced level of CT, whereas anaerobic growth via TMAO respiration was severely inhibited. In contrast, a ppGpp-null strain (ΔrelA ΔspoT ΔrelV) grew substantially better, but produced no CT, suggesting that CT production and bacterial growth are inversely regulated in response to ppGpp accumulation. Bacterial capability to produce CT was completely lost when the dksA gene, which encodes a protein that works cooperatively with ppGpp, was deleted. In the ΔdksA mutant, stringent response growth inhibition was alleviated, further supporting the inverse regulation of CT production and anaerobic growth. In vivo virulence of ΔrelA ΔspoT ΔrelV or ΔdksA mutants was significantly attenuated. The ΔrelA ΔspoT mutant maintained virulence when infected with exogenous TMAO despite its defective growth. Together, our results reveal that stringent response is activated under TMAO-stimulated anaerobic growth, and it regulates CT production in a growth-dependent manner in V. cholerae.
Introduction
Vibrio cholerae, the causative agent of the pandemic disease cholera, is a facultative anaerobic bacterium that inhabits aquatic environments, including brackish water and estuaries (1, 2). To acquire pathogenic properties, V. cholerae expresses a variety of virulence factors, including (i) cholera toxin (CT),3 responsible for watery diarrhea and thus dissemination of V. cholerae in nature, and (ii) toxin-co-regulated pilus (TCP), which contributes to the successful colonization in the host intestine (3). To successfully infect a host, pathogens must alter their phenotypic features in order to adapt to the host-specific environment. Mounting evidence suggests that the expression of CT and TCP is influenced by a variety of environmental stimuli, such as pH (4), temperature (4), bile salts (5), bicarbonate (6), cyclic di-GMP (7), and quorum sensing (8). However, signaling cues that stimulate the expression of virulence-associated genes inside the human host are not fully understood.
The oxygen-limiting condition is a distinctive feature of mammalian intestines (9), and it has been suggested that such a condition may serve as a host factor that influences the production of virulence factors in V. cholerae (10–13). Krishnan et al. (10) reported that expression of CT-coding genes was dramatically reduced in anaerobically grown V. cholerae. In contrast, other studies demonstrated that expression of virulence genes was substantially induced under anaerobic conditions through a transcriptional regulation that involved dimerization of AphB, a LysR-type transcriptional activator (12, 13). Together, these divergent results suggest that V. cholerae strains have developed mechanisms that allow active responses to the oxygen-deprived condition, and expression of virulence-associated genes is differentially regulated in an anaerobic environment, a condition that probably mimics host intestine.
Recently, we reported that CT production was remarkably induced when V. cholerae was grown anaerobically using trimethylamine N-oxide (TMAO) as an alternative electron acceptor (11). CT production was not observed when the anaerobic growth was stimulated by other alternative electron acceptors, such as fumarate, DMSO, and nitrate (11), demonstrating the specific effect of TMAO on the anaerobiosis-induced CT production in V. cholerae. Although the level of TMAO in the human intestine is not yet defined, it was reported that gut commensal microbes play a role in the production of TMAO in the murine intestine (14). This result suggests that TMAO is probably present in mammalian intestines, and V. cholerae may take advantage of its availability to support anaerobic respiratory growth in human intestine. Furthermore, this finding also led us to postulate the role of gut microbiota-derived metabolite as a signaling molecule to stimulate V. cholerae virulence.
Stringent response, initially characterized as an adaptive response to nutritional limitation, is one of the global regulatory systems in bacteria, providing a rapid adaptation to variety of growth-inhibiting stresses (15). It is mediated by reorganization of cellular gene transcription, and such a transcriptional response was known to be coordinated by accumulation of intracellular guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp), collectively termed ppGpp (16). In general, stringent response mediates physiological changes at the transcriptional level, often resulting in growth arrest and adaptation to specific stress responses (17). In Gram-negative bacteria, the intracellular level of ppGpp is modulated by RelA (monofunctional synthetase) and SpoT (bifunctional synthetase-hydrolase) (18). Apart from the relA and spoT genes, the novel gene relV has been identified in V. cholerae, the product of which has been shown to be involved in ppGpp synthesis under glucose or fatty acid starvation in a ΔrelA ΔspoT double mutant (19). CgtA, the conserved bacterial GTP-binding protein, is an essential protein and has been shown to interact with SpoT in V. cholerae and Escherichia coli. It has been suggested that CgtA most likely modulates the SpoT function to maintain low ppGpp levels (20, 21). In addition, DksA, an RNA polymerase binding transcription factor, also plays several important roles in modulating stringent response and regulation of other genes in V. cholerae (22, 23).
In this study, we revealed that accumulation of ppGpp was specifically induced in N16961 grown by anaerobic TMAO respiration, a condition leading to robust CT production. We also uncovered a dual role for DksA in regulating anaerobic growth and CT production. This report provides a novel insight into the signaling pathway that may contribute to the activation of CT production during adaptation to host-specific environments.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Condition
All bacterial strains and plasmids used in this study are listed in Table 1. Bacterial cultures were grown at 37 °C in Luria-Bertani medium (LB; 1% (w/v) tryptone, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl), and antibiotics were used in the following concentrations: ampicillin, 100 μg/ml; streptomycin, 200 μg/ml; kanamycin, 50 μg/ml. AKI medium contained 1.5% (w/v) peptone, 0.4% (w/v) yeast extract, 0.5% (w/v) NaCl, and 0.3% (w/v) sodium bicarbonate, and bacterial growth in AKI medium was performed as described previously (24). To support anaerobic growth, TMAO and fumarate (Sigma-Aldrich) were added to the medium (termed LBT and LBF). In order to generate growth curves, an overnight culture was subcultured at a 1:100 dilution in fresh medium and grown at 37 °C in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). Bacterial growth was monitored spectrophotometrically by measuring the optical density at 600 nm every 2 h.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strains or plasmids | Relevant characteristics | Reference or source |
|---|---|---|
| V. cholerae strains | ||
| N16961 | Wild type, O1 serogroup, biotype El Tor | Ref. 60 |
| N16961 PrelA::lacZ fusion | relA promoter lacZ fusion construct | This study |
| N16961 PrelV::lacZ fusion | relV promoter lacZ fusion construct | This study |
| ΔrpoZ | N16961, VC2709 deleted | This study |
| ΔrelAΔspoT | N16961, relA and spoT deleted | Ref. 20 |
| (p)ppGpp° (HH6) | N16961, relA, relV and spoT deleted | Ref. 28 |
| ΔrelAΔspoTΔdksA | N16961, relA, spoT and dksA deleted | This study |
| ΔdksA | N16961, dksA deleted | This study |
| ΔrelA | N16961, relA deleted | Ref. 20 |
| ΔrelV | N16961, relV deleted | Ref. 28 |
| E. coli strains | ||
| SM10/λpir | Kmr thi-1 thr leu tonA lacY supE recA::RP4–2-Tc::Mu pir+, for conjugal transfer | Ref. 61 |
| BW20767 | Km::Tn7 leu-63::IS10 recA1 creC510 hsdR17 endA1 zbf-5 uidA (ΔMlu1)::pir+thi | Ref. 62 |
| Plasmids | ||
| pCVD442 | sacB suicide vector from plasmid pUM24 | Ref. 63 |
| pVIK112 | Suicide vector for lacZ reporter fusion, Kmr | Ref. 64 |
| pTnKGL3 | Suicide vector bearing TnKGL3, Cmr Kmr | Ref. 27 |
| pBAD24 | Ampr, cloning vector | Ref. 65 |
| pDksABAD | pBAD24 carrying the coding sequence of dksA | This study |
Transposon Mutagenesis and Dot Blot Assay
Construction of a transposon insertion mutant library (∼1,200 mutants) was performed following procedures described previously (11). N16961-derived mutants were grown in 96-well plates anaerobically in LBT. After overnight growth, culture supernatants from each well were spotted on a nitrocellulose membrane (Bio-Rad) using a 96-well pin replicator. Membranes were then blocked and probed with a rabbit polyclonal antibody against CT subunit B (Abcam Inc., Cambridge, UK) following the standard Western blot protocol.
Construction of Mutants and Promoter-lacZ Fusion Strains
V. cholerae mutants were created by allele replacement as described previously (25, 26). The 500-base pair flanking sequences located at both ends to introduce mutation were amplified by PCR with the primers listed in Table 2. Constructions of single-copy PrelA::lacZ and PrelV::lacZ transcriptional fusions were performed as described previously (11).
TABLE 2.
Primers used in this study
| Gene name | Direction | Primer sequence (5′–3′)a |
|---|---|---|
| Cloning | ||
| relA promoter fusion | Forward | ATATCGAATTCCTAAAGTACTGCTCGACCCAGC |
| relA promoter fusion | Reverse | GAATTGTCGACCATTGATATCGTCCTAATAATTGTATTTTT |
| relV promoter fusion | Forward | ATATCGAATTCAATACAGACAGACTATGCGATTGGT |
| relV promoter fusion | Reverse | GAATTGTCGACCATTCACTCTCCTTAGCTTGCG |
| rpoZ left | Forward | CTCAAGAGCTCTCGGGTGTGGATTGAAAACACAC |
| rpoZ left | Reverse | GGTATGGATCCAGCGTCTTGAACTGTTACGCGTG |
| rpoZ right | Forward | CTCAAGGATCCCTGGCTGCAGTTAGCAGCATCAT |
| rpoZ right | Reverse | GGTATGAGCTCTTCTAAGGTTTCACGGGCAATGC |
| dksA left | Forward | AATTCGAGCTCCGGTCATGAAAATAGCCGTATCGAT |
| dksA left | Reverse | GATATGTCGACCATACAGATCTCCTACTAACCCTAGTCAACTGC |
| dksA right | Forward | AATTCGTCGACCTCGACAAGATCAAAGAGGAGGATTTC |
| dksA right | Reverse | GATATGAGCTCTGCACTGGCAGTAGTAAGCTTGACC |
a Restriction enzyme recognition sequences are underlined.
CT ELISA and β-Galactosidase Assays
CT production was measured using V. cholerae culture supernatants by an GM1-enzyme-linked immunosorbent assay (GM1-ELISA) as described previously (4). Purified CT subunit B (List Biological Laboratories, Inc., Campbell, CA) was used to provide a standard curve, and phosphate-buffered saline (PBS) was used as a negative control. Rabbit polyclonal anti-CT subunit B (Abcam Inc., Cambridge, UK) and anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc., Dallas, TX) were used for detection. A β-galactosidase activity assay was performed as described previously (27).
Determination of Intracellular ppGpp Concentration by TLC
Intracellular ppGpp levels were measured as described previously with slight modification (28). To detect ppGpp concentrations in anaerobically grown cultures, bacterial strains cultured aerobically in LB were diluted into various media supplemented with 100 μCi/ml [32P]orthophosphate (PerkinElmer Life Sciences) and grown statically inside the anaerobic chamber for 8 h. The cultures were extracted with 19 m formic acid. After centrifugation to remove cell debris, supernatants were spotted on a polyethyleneimine-coated TLC plate (Merck). The plate was developed in 1.5 m KH2PO4 (pH 3.5) and visualized by autoradiography.
Infant Mouse Infection
Infant mice (∼5–6 days old, Central Lab Animal Inc., Seoul) were orogastrically infected with 50 μl of aerobically grown V. cholerae strains (2 × 106 cells). Prior to infection, bacterial strains were suspended in LB or LB + 100 mm TMAO to a final cell density of 4 × 107 cfu/ml. Viability of mice was checked every 3 h, and the fluid accumulation ratio was calculated as intestine weight/remaining body weight. After 16 h of infection, the entire intestine was removed and homogenized in 5 ml of PBS. The number of viable cells was determined by enumerating colony numbers of serially diluted gut homogenates. An LB-containing streptomycin agar plate was used for cfu counting. Animal experiments were approved by the committee on the ethics of animal experiments of the Yonsei University College of Medicine (permit number 2011-0166).
Flow Cytometry Analysis
The average protein or DNA content per single bacterial cell was measured following procedures described previously (29).
Statistical Analysis
Data are expressed as mean ± S.D. An unpaired Student's t test was used to analyze the data. A p value of <0.05 was considered statistically significant. All experiments were repeated for reproducibility.
RESULTS
Identification of a V. cholerae Mutant Strain That Produced a Higher Level of CT during Anaerobic TMAO Respiration
Our previous studies demonstrated that CT production is specifically and dramatically induced, whereas V. cholerae O1 serotype strains were grown anaerobically in the presence of TMAO (11). To better define the molecular basis of this finding, we performed a genetic screen looking for mutants that exhibited altered ability to produce CT under the same condition. A transposon insertion mutant library of N16961 was constructed, and ∼1,200 mutants were screened for their ability to produce CT by a dot blot analysis using an antibody against CT subunit B. Whereas most of the mutants produced similar levels of CT when compared with that produced by wild type strain N16961, four mutants produced elevated levels of CT in our initial primary screen (data not shown). Each candidate clone was rigorously retested by subsequent ELISA, and a mutant that harbors a transposon insertion in the VC2709 gene was finally validated. The VC2709 gene encodes the ω subunit of RNA polymerase (annotated as rpoZ) (30, 31), which has been proposed to interact with major microbial metabolites, guanosine 5′-diphosphate 3′-diphosphate (ppGpp) or guanosine pentaphosphate (pppGpp) (collectively termed ppGpp) (31). We then proceeded to make an in-frame deletion mutant of rpoZ (Table 1). As shown in Fig. 1, the mutant defective in rpoZ produced a >2-fold higher level of CT than its parental strain when grown anaerobically in the presence of 50 mm TMAO. Consistent with our previous observations, CT production was only minimal when the mutant was grown in LB. There was a small but statistically significant increase in CT production when the mutant was grown in LB supplemented with fumarate (Fig. 1).
FIGURE 1.
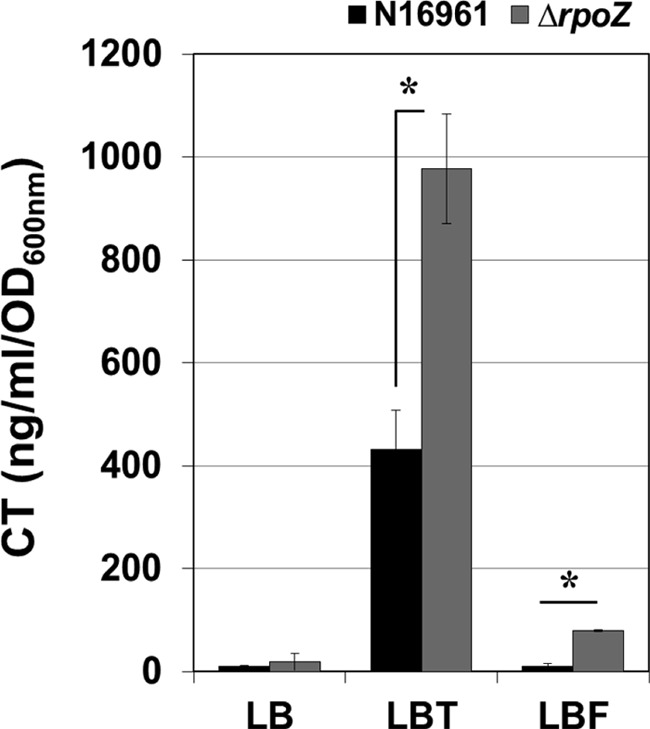
CT production was increased in ΔrpoZ mutant during anaerobic TMAO respiration. Wild type N16961 and ΔrpoZ deletion mutant were inoculated in LB, LBT (LB containing 50 mm TMAO), or LBF (LB containing 50 mm fumarate), respectively, and grown statically inside an anaerobic chamber for 16 h. Culture supernatants were harvested, and the CT level was quantified by ELISA. *, p < 0.05 versus CT levels produced from wild type N16961. Three independent experiments were performed, and values of mean ± S.D. (error bars) are displayed in each bar.
In the V. cholerae N16961 genome, the rpoZ gene is clustered as an operon with a downstream gene spoT (VC2710) encoding a bifunctional enzyme that can both synthesize and hydrolyze ppGpp, with its dominant role in ppGpp hydrolysis (17). Because ppGpp is deeply involved in the transcriptional regulation when bacterial cells encounter stresses, such as nutrient starvation, it is often called a “stringent response” regulator (32). Therefore, overproduction of CT due to disruption of the rpoZ-spoT locus led us to hypothesize that stringent response might be involved in CT production during anaerobic TMAO respiration in V. cholerae.
Bacterial Growth Is Impaired during Anaerobic TMAO Respiration
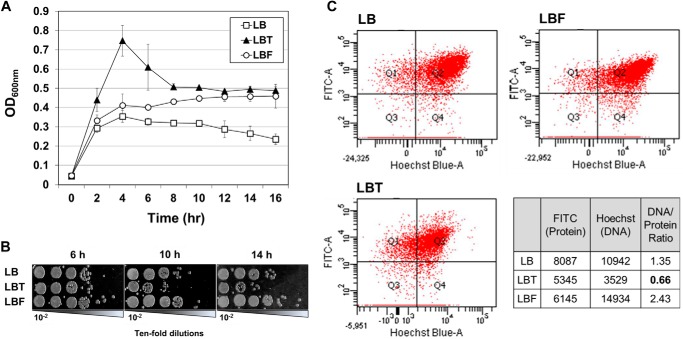
Stringent response in bacterial cells leads to cell cycle arrest and thus growth inhibition (33). To explore how bacterial growth is affected during anaerobic TMAO respiration, we monitored growth-related properties of V. cholerae cells. When N16961 was grown anaerobically in plain LB, only marginal growth was observed (Fig. 2A). A600 values were reached at ∼0.35 after 4 h of growth and gradually decreased for the rest of the experimental period. When grown in LB supplemented with fumarate, A600 values kept increasing, and the final A600 values were ∼2 times higher than those achieved upon anaerobic growth in LB (Fig. 2A). This result clearly suggests that using fumarate as an alternative electron acceptor supports anaerobic growth of V. cholerae. It is noteworthy that anaerobic growth of N16961 in LB medium supplemented with TMAO was divided into two distinct phases. Initial growth was fairly robust during the first 4 h, and a marked decrease in A600 was observed (Fig. 2A). Such a unique growth pattern in TMAO-stimulated growth was also reflected in viable cell counting. The number of viable cells decreased during the anaerobic growth by TMAO respiration (Fig. 2B). cfu after 14 h of growth decreased >10-fold when compared with that after 6 h of growth. In contrast, the number of viable cells either increased or kept constant at the 6, 10, and 14 h time points in LBF or in LB (Fig. 2B). This suggests that TMAO-stimulated anaerobic growth was accompanied by loss of cell viability, and bacterial cells were probably under significant stress.
FIGURE 2.
N16961 grown with anaerobic TMAO respiration produced severe growth defects. A, growth curves of N16961 in LB (open squares), LBT (black triangles), or LBF (open circles). An aerobically grown preculture of N16961 was diluted in each medium and grown anaerobically for 16 h. Aliquots of each culture were withdrawn at designated times, and A600 values (means ± S.D. (error bars), n = 3) were plotted for growth curves. B, viability changes of N16961 during anaerobic growth in each medium. Aliquots of each culture were sampled at 6, 10, and 14 h postinoculation and serially diluted for cfu counting. C, double-fluorescent dot plot analysis of N16961. Bacterial cells (∼10,000 cells) grown anaerobically in LB, LBT, or LBF for 16 h were labeled with Hoechst 33258 and FITC. Each spot in the plots represent a single bacterial cell with intensities derived from Hoechst 33258 (x axis, DNA) and FITC (y axis, proteins). The table shows average fluorescent intensities of N16961 grown in each condition, and the DNA/protein ratios were calculated by dividing the average Hoechst 33258 intensity by the FITC intensity.
Bacterial cells undergoing stringent response were reported to experience DNA replication arrest (33). Therefore, we sought to examine whether DNA replication occurred normally in LBT-grown N16961. To address this issue, we compared DNA/protein ratios of N16961 cells grown anaerobically in LB, LBT, or LBF. The average protein or DNA content per single cell was estimated by measuring average fluorescent intensities of FITC or Hoechst 33258 that labeled cellular proteins and chromosomal DNA, respectively. As shown in Fig. 2C, the average Hoechst intensity in LBT-grown N16961 was significantly lower than those of LB- or LBF-grown cells. The mean FITC intensity, however, was only mildly decreased in the same cells, yielding a DNA/protein ratio of 0.66. The ratios were 1.35 and 2.43 in LB- and LBF-grown N16961. This result suggests that there was DNA replication arrest during anaerobic TMAO respiration.
Intracellular Level of ppGpp Was Increased during Anaerobic TMAO Respiration
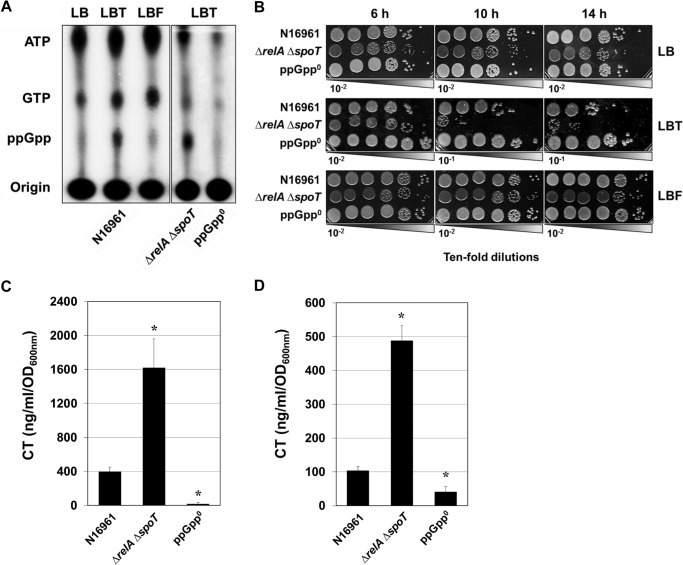
Accumulation of ppGpp is a hallmark of bacterial cells undergoing stringent response (34). To elucidate whether CT production specifically induced by anaerobic TMAO respiration is associated with stringent response, we measured the intracellular level of ppGpp in N16961 grown in diverse culture media under anaerobic conditions. For clear comparisons, we also used N16961-derived ΔrelA ΔspoT double and ΔrelA ΔrelV ΔspoT triple deletion mutant strains. The ΔrelA ΔspoT double mutant was determined to produce an elevated level of ppGpp due to (i) the activity of RelV, another enzyme involved in ppGpp synthesis in V. cholerae, and (ii) the absence of SpoT that degrades ppGpp (28). The triple mutant lacking all enzymes for ppGpp synthesis was found to produce no ppGpp (28). Higher levels of ppGpp accumulation were clearly observed in N16961 grown anaerobically by TMAO respiration in our polyethyleneimine-TLC assay (Fig. 3A). The accumulation of ppGpp was not observed in N16961 grown in LB or in LB containing fumarate, further demonstrating the specific induction of ppGpp synthesis under the TMAO respiration condition. As expected, the intensity of the spot corresponding to ppGpp was significantly increased in the ΔrelA ΔspoT double mutant, whereas the spot was not detected in the ΔrelA ΔrelV ΔspoT ppGpp null mutant (Fig. 3A). These data strongly suggest that a stringent response was indeed induced during anaerobic TMAO respiration in N16961.
FIGURE 3.
Accumulation of intracellular ppGpp is specifically induced during anaerobic TMAO respiration, and CT production is regulated by the level of intracellular ppGpp. A, intracellular nucleotides were detected by using TLC analysis. Wild type N16961 was grown in LB, LBT, or LBF, with [32P]orthophosphate for 8 h. The ΔrelA ΔspoT double mutant and ΔrelA ΔspoT ΔrelV triple mutant (ppGpp null mutant, termed ppGpp°) were grown in LBT with [32P]orthophosphate for 8 h. Cellular extracts were prepared and analyzed in TLC. B, strains indicated at the left were grown in LB, LBT, and LBF, and aliquots were sampled for cfu counting. Experimental conditions were identical to those described in the legend to Fig. 2B. C, the levels of CT produced in N16961, ΔrelA ΔspoT, and ppGpp° mutants. Strains were grown in LBT for 16 h, and culture supernatants were assayed for CT ELISA. Experimental conditions were identical to those described in the legend to Fig. 1. D, the level of CT produced in N16961, ΔrelA ΔspoT, and ppGpp° mutants. Strains were grown in AKI conditions, as described under “Experimental Procedures,” and culture supernatants were assayed for CT ELISA. Error bars, S.D.
Next, we examined how bacterial growth of V. cholerae strains that produced varying amounts of ppGpp was influenced during anaerobic TMAO respiration. When grown in LB, there were no differences in growth between the three strains. In addition, there was no significant difference in viability among strains (Fig. 3B, top). In fumarate-supplemented anaerobic culture, better growth was invariably observed in all of the tested strains, and the number of viable cells was ∼10-fold larger than that detected after anaerobic growth in LB (Fig. 3B, bottom). Importantly, however, a strain-to-strain difference in viability was observed during anaerobic growth with TMAO. The ΔrelA ΔspoT double mutant that produced a higher level of ppGpp exhibited a severe growth defect, whereas the ppGpp null mutant grew significantly better than its parental strain N16961 (Fig. 3B, middle). These results strongly suggest that the degree of anaerobic growth by TMAO respiration may be inversely correlated with the amount of intracellular ppGpp in V. cholerae.
Our results suggest that both CT production and stringent response were activated during anaerobic TMAO respiration. These findings strongly suggest that robust CT production during TMAO respiration occurs in association with the stringent response. To provide further evidence for this notion, we measured the levels of CT produced in N16961, ΔrelA ΔspoT double (i.e. ppGpp overproducer strain) and ΔrelA ΔrelV ΔspoT triple (i.e. ppGpp null strain) mutants by ELISA. CT production in the ΔrelA ΔspoT double mutant was significantly increased, and the level was ∼1600 ng/ml/A600, which was ∼4 times higher than that produced in wild type N16961 (Fig. 3C). Importantly, CT production was completely abrogated in the ppGpp null mutant (Fig. 3C). Together, these results clearly demonstrate that CT production during anaerobic TMAO respiration is promoted in proportion to the degree of ppGpp accumulation in V. cholerae. To further validate the effect of ppGpp on the CT production, we assessed the CT production in AKI conditions. As shown in Fig. 3D, the level of CT produced in the ΔrelA ΔspoT mutant was ∼5 times higher than in the wild type strain. Likewise, the ppGpp null strain produced a reduced level of CT under AKI conditions.
RelV-mediated Stringent Response Plays a More Important Role in CT Production during Anaerobic TMAO Respiration
Next, we investigated whether TMAO-stimulated ppGpp production was reflected in the transcriptional activation of relA and relV, genes involved in ppGpp synthesis. Although SpoT is a bifunctional enzyme, it has only a minimal activity for ppGpp synthesis, and RelA and RelV are the primary ppGpp synthases during stringent response conditions in V. cholerae (28). Therefore, we constructed PrelA::lacZ and PrelV::lacZ reporter fusions and assessed the promoter activity of each gene by measuring β-galactosidase activity. Consistent with the TLC ppGpp quantification assay results, both the PrelA::lacZ and PrelV::lacZ activities were detected at the highest levels during anaerobic TMAO respiration, with the relV gene promoter showing higher activity than the relA gene promoter (Fig. 4A). It is of note that relV promoter activity was ∼18-fold and ∼6-fold increased during TMAO respiration when compared with the activities measured in N16961 cells grown in LB or LBF, respectively. In contrast, only an ∼1.7-fold increase in the relA promoter activity was detected in N16961 grown by TMAO respiration, compared with those in LB or LBF-grown N16961. Importantly, CT production was considerably decreased in ΔrelV but not in the ΔrelA mutant. The levels of CT produced in the ΔrelV and ΔrelA mutants were ∼25 ng/ml/A600 and ∼220 ng/ml/A600, respectively (Fig. 4B). Together, these findings suggest that although both of the two ppGpp synthases (RelA and RelV) are known to participate in the production of the stringent response regulator, RelV plays a more pivotal role in stringent response-mediated CT production under the anaerobic TMAO respiration condition.
FIGURE 4.
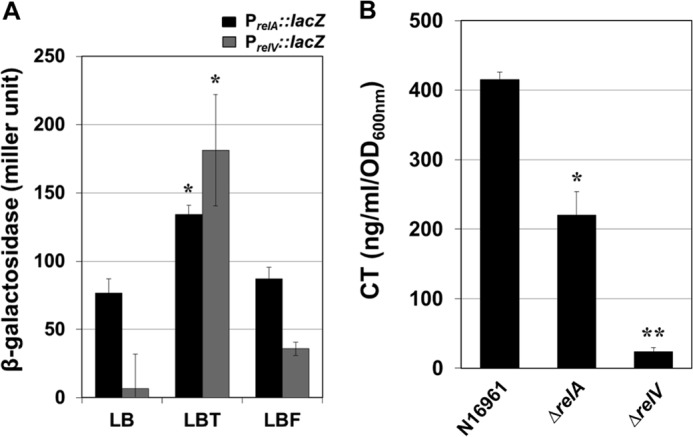
Transcription of relV gene is highly induced during anaerobic TMAO respiration, and a V. cholerae mutant defective in relV gene exhibited a severe defect in CT production. A, promoter activity of relA and relV genes in N16961 grown anaerobically for 8 h in LB, LBT, or LBF was measured. N16961 reporter strains harboring each chromosomal lacZ reporter fusion were assayed in triplicate for β-galactosidase activity. Values of means ± S.D. (error bars) are presented. *, p < 0.05 versus β-galactosidase activity in LB-grown cultures. B, the levels of CT produced in N16961, ΔrelA, and ΔrelV mutants. Strains were grown anaerobically in LBT for 16 h, and culture supernatants were assayed for CT ELISA. Experimental conditions were identical to those described in the legend to Fig. 1. *, p < 0.05 versus CT produced in N16961; **, p < 0.001 versus CT produced in N16961.
DksA Is Necessary for Stringent Response-mediated CT Production during Anaerobic TMAO Respiration
The stringent response regulator ppGpp binds to RNA polymerase (RNAP), and the resultant complex selectively regulates the gene transcription to relieve the stress induced by nutrient limitation (35). DksA (VC0596, 17.2 kDa) binds to RNAP and facilitates the effects of ppGpp on the transcriptional control in bacterial cells (22, 31, 36–38). Recently, DksA has also been suggested to play a critical role in regulating V. cholerae virulence (22). To test whether CT production elicited by stringent response during anaerobic TMAO respiration was also affected by the deficiency of DksA, we constructed a dksA in-frame deletion mutant. TMAO-stimulated CT production was completely abolished by dksA gene deletion (Fig. 5A). Similar to wild type N16961, no CT was produced in the ΔdksA mutant grown in LB or LBF (Fig. 5A). When the DksA was overproduced from pDksABAD by an arabinose-inducible promoter in the ΔdksA mutant, CT production was restored (Fig. 5B). Furthermore, no CT was produced even in the ΔrelA ΔspoT double mutant that produced an elevated level of CT, when the dksA gene was additionally deleted (Fig. 5B), suggesting that DksA is absolutely required for the CT production during TMAO respiration. The capability to produce CT was partially restored by overexpression of the dksA gene from the pDksABAD plasmid in the ΔrelA ΔspoT ΔdksA mutant (Fig. 5B). Interestingly, we observed that the growth defect, observed in N16961 during anaerobic TMAO respiration, was somewhat diminished by dksA gene deletion. Although the sharp decrease in A600 values was initiated after 4 h of culture in N16961, the increase in A600 of the ΔdksA mutant culture persisted for a longer time period before A600 values declined (Fig. 5C). At the 6 h time point, A600 of the ΔdksA mutant culture was ∼0.95, whereas that of the wild type culture was ∼0.65 (Fig. 5C). Attenuation of the growth defect by dksA gene deletion occurred more evidently in the ΔrelA ΔspoT mutant. As shown in Fig. 5D, the ΔrelA ΔspoT ΔdksA triple mutant grew significantly better than the ppGpp-overproducing ΔrelA ΔspoT mutant. When dksA gene was overexpressed by an arabinose-inducible promoter, the growth of the triple mutant was not as robust as that of the identical mutant harboring the empty plasmid. These results suggest that (i) CT production is closely related with growth inhibition that inevitably ensues during anaerobic TMAO respiration and (ii) DksA-mediated transcriptional regulation actively participates in the process leading to the growth inhibition of the ppGpp overproducer (i.e. ΔrelA ΔspoT double mutant).
FIGURE 5.
Effects of dksA gene deletion on stringent response-mediated CT production and anaerobic growth of V. cholerae. A, CT production of ΔdksA mutant. N16961 and the ΔdksA mutant were grown anaerobically in LB, LBT, and LBF for 16 h. Culture supernatants were harvested and assayed for CT ELISA. Three independent experiments were performed, and values of means ± S.D. (error bars) are displayed in each bar. *, p < 0.001 versus CT levels produced in N16961. B, effects of deletion or overexpression of the dksA gene on CT production. The indicated strains were anaerobically grown in LB supplemented with 50 mm TMAO with 0.1% (w/v) l-arabinose (black bars) as an inducer or without l-arabinose (white bars) for 16 h. The levels of CT production were determined by CT ELISA. Three independent experiments were performed, and values of means ± S.D. are displayed in each bar. *, p < 0.05 versus CT levels from ΔdksA mutant or the mutant harboring empty plasmid, pBAD24; **, p < 0.02 versus CT levels from ΔrelA ΔspoT mutant; ***, p < 0.05 versus CT levels from ΔrelA ΔspoT mutant harboring pBAD24. C and D, growth curves of the indicated strains in LBT. Aerobically grown preculture of each strain was diluted in LBT and grown anaerobically for 16 h.
In Vivo Virulence of Stringent Response Mutants Is Attenuated
Our in vitro results demonstrate that CT production during anaerobic TMAO respiration is mediated by stringent response. Finally, we examined the effect of stringent response on the modulation of in vivo virulence by utilizing an infant mouse infection model. To this end, we infected 5–6-day-old infant mice with the ppGpp overproducer strain, the ppGpp null strain, and the ΔdksA mutant resuspended in LB medium containing 100 mm TMAO and monitored the fluid accumulation ratio, mouse mortality, and bacterial cell numbers recovered from mouse intestine. There was a significant increase of the fluid accumulation ratio in mice infected with wild type N16961, whereas it was significantly decreased in mice infected with the ppGpp null strain (ΔrelA ΔrelV ΔspoT) or the ΔdksA mutant (Fig. 6A). This is in line with our in vitro findings that no CT was produced in each of these two strains. Contrary to our expectation, fluid accumulation induced by the ΔrelA ΔspoT double mutant that produced a significantly elevated level of CT in the presence of TMAO was lower than what was observed in wild type-infected mice (Fig. 6A). This is probably due to the defective growth of the mutant during anaerobic TMAO respiration (Fig. 3B). Consistent with fluid accumulation results, mice were susceptible to infection with N16961 (Fig. 6B). All of the N16961-infected mice perished after the 24-h experimental period. However, mice infected with ΔrelA ΔrelV ΔspoT or ΔdksA mutant remained alive for 24 h despite TMAO in the inoculum (Fig. 6B). Because the fluid accumulation ratios were significantly decreased in mice infected with ΔrelA ΔrelV ΔspoT or ΔdksA mutant compared with that in wild type-infected mice, we anticipated that the capability of these mutants to colonize the mouse intestine would be impaired. To address this, we measured how many viable cells of each strain were recovered from the intestine of infected mice. When compared with N16961, ΔrelA ΔrelV ΔspoT and ΔdksA mutant strains were significantly less capable of colonizing the mouse intestine. The degree of intestinal colonization by the ΔrelA ΔspoT double mutant was lower than that of wild type but was relatively higher than the levels of colonization by ΔrelA ΔrelV ΔspoT or ΔdksA deletion mutant (Fig. 6C). Together, these results suggest that stringent response induced by anaerobic TMAO respiration can modulate not only CT production but also the strain's capability to colonize host intestine in vivo.
FIGURE 6.
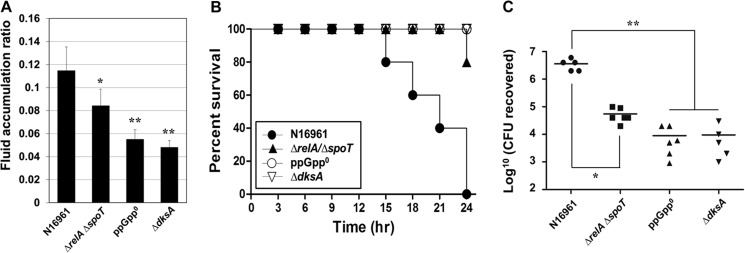
Effects of stringent response on in vivo virulence and colonization. A, infant mice (n = 6) were infected with 50 μl of each V. cholerae strain (2 × 106 cells) suspended in LB supplemented with 100 mm TMAO. After 24 h, mice were sacrificed, and the entire intestines were extracted for weight measurement. Mice that died prior to the planned time of sacrifice were used for the measurement right after death. The fluid accumulation ratio was calculated as described under “Experimental Procedures.” Values of means ± S.D. (error bars) are displayed in each bar. *, p < 0.05; **, p < 0.01 versus fluid accumulation ratio from infection with N16961. B, survival rate of mice infected with the indicated strains (2 × 106 cells) was monitored every 3 h. The percentage survival in each group was plotted as Kaplan-Meier curves. C, mice were infected with the indicated bacterial strains (2 × 106 cells) suspended in LB supplemented with 100 mm TMAO, and intestinal colonization was measured after 16 h by counting the number of viable bacterial cells recovered from the mouse intestine. The solid horizontal lines represent the geometric mean values. *, p < 0.001; **, p < 0.002 versus cfu from infection with wild type N16961. Each symbol shows the value obtained from each mouse.
DISCUSSION
It remains elusive how V. cholerae CT production is induced inside the human intestine. Adaptation of bacterial pathogens to the host-specific environments is accompanied by reprogramming of cellular processes that often result in both reduced growth and enhanced virulence. One of the methods that bacteria use to coordinate gene expression with the environment is the stringent response (18, 32), and considerable attention has been paid to elucidate virulence regulation of V. cholerae in association with stringent response (22, 23, 28). It is relatively well known that the expression of CT coding genes is controlled, in part, by quorum sensing, a gene regulatory system that involves cell density-dependent production of and response to autoinducer molecules (8, 39). However, the mechanism of quorum sensing-dependent CT production was defined using strains grown in AKI medium, an in vitro culture condition known to be permissive for CT production (24), and CT was not produced under non-AKI culture conditions even at low cell density, a condition that activates expression of virulence-related genes. This result suggests that quorum sensing may not be the sole determinant for virulence regulation during the infectious process in the human intestine and, therefore, requires further exploration of environmental cues that regulate V. cholerae virulence in vivo.
Our previous work demonstrated that CT production was markedly induced during anaerobic TMAO respiration (11). This finding is significant because the human intestine was proposed to be an anaerobic environment (9), and TMAO is probably produced by commensal microbes residing in the human intestine. Three recent papers showed that the TMAO level was decreased when mice were treated with antibiotics, and the TMAO level was restored when gut microbes were recovered from the antibiotic-treated mice (14, 40, 41). These results clearly demonstrate that gut microbes play a vital role in the production of TMAO. In these studies, however, TMAO was measured in serum, and it is unclear how much TMAO is actually present in the mammalian intestine. It will definitely be a future direction to measure the level of TMAO in mammalian intestine and evaluate its effect on V. cholerae in vivo virulence.
Although an E. coli ΔrelA ΔspoT double mutant is unable to produce ppGpp, a V. cholerae mutant defective in both genes is still able to produce ppGpp (23). This phenotype is due to an additional ppGpp synthase in V. cholerae, RelV (19). The level of ppGpp accumulation was higher in the ΔrelA ΔspoT double mutant when compared with that in wild type N16961 (Fig. 3A). This result is due to the presence of active RelV, because a ΔrelA ΔspoT ΔrelV triple mutant was determined to produce no ppGpp. Compared with RelA and SpoT, RelV is a small protein (i.e. 259 amino acids) with a single synthetase domain, and it does not contain the hydrolase domain found in RelA and SpoT. It has a structural similarity with small alarmone synthetase proteins, such as RelP, RelQ, YjbM, and YwaC, identified in Gram-positive bacterial species (42, 43). It remains to be elucidated why V. cholerae possesses two different ppGpp synthase enzymes and how the expression of each gene is regulated under differential conditions. Interestingly, our results in Fig. 4 showed that transcription of the relV gene was significantly induced during anaerobic TMAO respiration. Expression of the relA gene, however, was fairly high in all of the tested conditions and only mildly increased in response to anaerobic TMAO respiration. Moreover, the ΔrelV single mutant was more defective than ΔrelA mutant in CT production. These data strongly suggest that relA gene expression may occur constitutively and that transcription of the relV gene is induced under specific conditions, such as anaerobic TMAO respiration. Notably, production of YwaC, a small alarmone synthetase in Bacillus subtilis, was induced by alkaline shock, and such induction resulted in transient accumulation of ppGpp (42). Likewise, accumulation of ppGpp was observed in Pseudomonas aeruginosa under high pH conditions (44). These results suggest that ppGpp biosynthesis may be induced by alkaline stress. Consistent with this finding, we found that the pH of the culture broth increased during anaerobic TMAO respiration of N16961 (data not shown). This was probably due to the reduction of TMAO to trimethylamine, and a pH increase was not detected in LB or LBF culture. Furthermore, pH of the AKI medium is 8.2–8.5 due to the presence of 0.3% (w/v) NaHCO3, and our results demonstrated that CT production of the ΔrelA ΔspoT mutant, in which RelV-mediated accumulation of ppGpp was apparent, was significantly increased when grown in AKI conditions. Therefore, it will be interesting to explore whether RelV-mediated ppGpp production would be specifically induced under basic conditions.
Because the accumulation of intracellular ppGpp leads to cessation of bacterial growth, the function of SpoT is important in keeping the ppGpp level low. We initially identified a mutant in which the rpoZ gene was disrupted by transposon insertion as one that produced higher levels of CT than wild type during anaerobic TMAO respiration. Although the rpoZ gene constitutes an operon with the spoT gene in E. coli (30, 45) and V. cholerae, the physiological role of RpoZ in bacterial stringent response is not clearly defined. Two previous studies using E. coli show that RpoZ is involved in regulation of intracellular ppGpp levels. Chatterji et al. (30) demonstrated that the expression of relA was considerably decreased by rpoZ gene deletion. In addition, the ΔrpoZ mutant exhibited a slow growth phenotype, and it was proposed to be due to the elevated level of ppGpp (45). These reports indicate that functional RpoZ is necessary to keep the concentration of stringent response regulator, ppGpp, at the basal level. The aforementioned phenotypes of the ΔrpoZ mutant, however, are probably caused by a polar effect on its downstream gene, spoT (45), and not by an apparent effect of rpoZ gene deletion. In the ΔrpoZ mutant that we constructed, an in-frame deletion of the rpoZ gene was introduced, and the spoT gene was left intact. In addition, normal expression of the spoT gene was confirmed in our ΔrpoZ mutant by quantitative RT-PCR analysis (data not shown), suggesting that apart from the downstream polar effect, rpoZ gene deletion may indeed influence bacterial stringent response and CT production during anaerobic TMAO respiration. Further work is required to provide molecular details with regard to the role of RpoZ in the stringent response in V. cholerae.
Here, we also examined the function of DksA, which acts as a co-regulator with ppGpp in CT production during anaerobic TMAO respiration. Together with ppGpp, DksA can control transcriptional regulation of physiologically important genes either by a direct or indirect mechanism. In the direct mechanism, DksA can stabilize the ppGpp-RNAP complex by binding to a channel of RNAP, and transcription of target genes can occur, albeit with decreased efficiency, without DksA (35). In the indirect model, DksA plays a critical role in promoting the interaction of the ppGpp-RNAP complex with alternative σ-factors (46–48). Our data showed that the CT production was completely abolished by dksA gene deletion in both WT and ΔrelA ΔspoT double mutant (Fig. 5B). Therefore, stringent response-mediated ctxAB gene expression is predicted to be controlled by the indirect mechanism, known as σ-factor competition. This hypothesis was supported by previous studies demonstrating that recruitment of alternative σ-factors (σ54, σ38, σ28, and σE) affected survival and virulence under starvation and other stress conditions in V. cholerae (22, 49, 50).
Our data clearly demonstrated that the deletion of the dksA gene provided a positive impact on bacterial cell growth and negatively regulated CT production (Fig. 5). The negative influence of DksA on bacterial growth has been well studied. In an E. coli strain defective in DksA protein, transcription of the fis operon, expression of which was known to actively occur during exponential growth, was extended into the stationary phase (51). In addition, P. aeruginosa DksA represses rRNA gene expression under both actively growing and stringent response conditions (52). Based on our results, the effect of dksA gene deletion was significantly more evident in the ppGpp-overproducing ΔrelA ΔspoT double mutant than in the wild type strain, further suggesting that the role of DksA is amplified under the condition of ppGpp accumulation.
In V. cholerae, genes encoding TCP (toxin-co-regulated pilus), involved in intestinal colonization and genes coding for CT are co-regulated by ToxT, a major virulence-associated transcription factor (53). Intestinal colonization was substantially decreased in ppGpp0 and ΔdksA V. cholerae mutants. Whether the defective colonization of these two mutants can be ascribed to the reduced synthesis of TCP, the loss of ability to persist in mouse intestine, or elevated susceptibility to host immunity remains to be determined. Based on our previous study, however, transcription of tcpP and toxT genes was greatly up-regulated when N16961 was grown anaerobically with TMAO, a condition that activates stringent response (11). Therefore, it is reasonable to postulate that the bacterial capability of colonizing the mouse intestine is also increased when stringent response occurs. It is of particular interest that stringent response positively regulates biofilm formation, a virulence mechanism often involved in persistent colonization (28, 54, 55). When stringent response was inactivated, the resistant nature of bacterial biofilm was significantly compromised in P. aeruginosa, an opportunistic human pathogen (55).
Stringent response is also required for full virulence in many other human pathogens. A relA-defective mutant of Mycobacterium tuberculosis exhibited decreased ability to survive under anaerobic and starvation conditions (56) and in a guinea pig infection model (57). Virulence of a ppGpp null mutant of Salmonella typhimurium was significantly attenuated, and the mutant was tested for its potential as a live vaccine (58). Likewise, stringent response was shown to play roles in host cell invasion and intracellular survival in Campylobacter jejuni, a food-borne pathogen (59). Together, these findings show that the ability to mount a stringent response is necessary to cope with hostile host environments and establish successful infection.
In conclusion, we revealed that a stringent response is induced during anaerobic growth using TMAO as an alternative electron acceptor, and such an induction is deeply involved in CT production. Moreover, we proposed a mechanistic link that couples the bacterial capability to produce CT with DksA-controlled anaerobic growth (summarized in Fig. 7). Inside the human intestine, V. cholerae encounters unfavorable conditions that are expected to trigger bacterial stringent response. Such a response is anticipated to help V. cholerae increase both survival fitness and virulence potential. We hope that results presented in the current study stimulate future investigations to better understand the in vivo pathogenesis of V. cholerae and devise better strategies to alleviate the virulence of this clinically important human pathogen.
FIGURE 7.
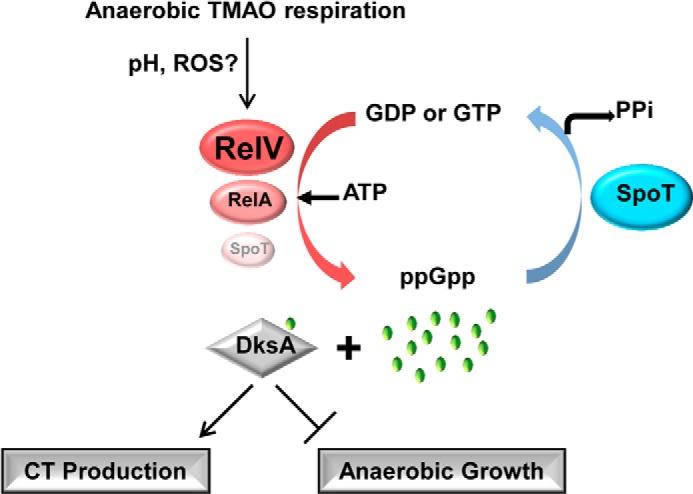
Potential mechanisms of stringent response-mediated CT production during anaerobic TMAO respiration in V. cholerae. Anaerobic TMAO respiration stimulates the production of ppGpp in V. cholerae. RelV probably plays a dominant role in ppGpp biosynthesis under this particular condition. Reactive oxygen species (ROS), specifically generated during anaerobic TMAO respiration (11) or pH increase due to the action of TMAO reductase (see “Discussion”), can be considered as contributing factors to the induction of stringent response. DksA protein in conjunction with its cofactor ppGpp is required for the CT production, and DksA/ppGpp inhibits the anaerobic growth of V. cholerae. CT production is only active when stringent response-mediated growth inhibition occurs.
This work was supported by National Research Foundation (NRF) of Korea Grant 2011-0016210, funded by the Korean Government (MEST). This work was also supported by Basic Science Research Program Grant 2013R1A6A3A01064850, through the NRF of Korea, funded by the Ministry of Education.
- CT
- cholera toxin
- TCP
- toxin-co-regulated pilus
- TMAO
- trimethylamine N-oxide
- RNAP
- RNA polymerase.
REFERENCES
- 1. Faruque S. M., Albert M. J., Mekalanos J. J. (1998) Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62, 1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sikora A. E. (2013) Proteins secreted via the type II secretion system: smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog. 9, e1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matson J. S., Withey J. H., DiRita V. J. (2007) Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 75, 5542–5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gardel C. L., Mekalanos J. J. (1994) Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol. 235, 517–526 [DOI] [PubMed] [Google Scholar]
- 5. Hung D. T., Mekalanos J. J. (2005) Bile acids induce cholera toxin expression in Vibrio cholerae in a ToxT-independent manner. Proc. Natl. Acad. Sci. U.S.A. 102, 3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abuaita B. H., Withey J. H. (2009) Bicarbonate induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect. Immun. 77, 4111–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tischler A. D., Camilli A. (2005) Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect. Immun. 73, 5873–5882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu J., Miller M. B., Vance R. E., Dziejman M., Bassler B. L., Mekalanos J. J. (2002) Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 99, 3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colgan S. P., Taylor C. T. (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishnan H. H., Ghosh A., Paul K., Chowdhury R. (2004) Effect of anaerobiosis on expression of virulence factors in Vibrio cholerae. Infect. Immun. 72, 3961–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee K. M., Park Y., Bari W., Yoon M. Y., Go J., Kim S. C., Lee H. I., Yoon S. S. (2012) Activation of cholera toxin production by anaerobic respiration of trimethylamine N-oxide in Vibrio cholerae. J. Biol. Chem. 287, 39742–39752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacikova G., Lin W., Skorupski K. (2010) The LysR-type virulence activator AphB regulates the expression of genes in Vibrio cholerae in response to low pH and anaerobiosis. J. Bacteriol. 192, 4181–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Z., Yang M., Peterfreund G. L., Tsou A. M., Selamoglu N., Daldal F., Zhong Z., Kan B., Zhu J. (2011) Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc. Natl. Acad. Sci. U.S.A. 108, 810–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z., Klipfell E., Bennett B. J., Koeth R., Levison B. S., Dugar B., Feldstein A. E., Britt E. B., Fu X., Chung Y. M., Wu Y., Schauer P., Smith J. D., Allayee H., Tang W. H., DiDonato J. A., Lusis A. J., Hazen S. L. (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magnusson L. U., Farewell A., Nyström T. (2005) ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13, 236–242 [DOI] [PubMed] [Google Scholar]
- 16. Potrykus K., Cashel M. (2008) (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 [DOI] [PubMed] [Google Scholar]
- 17. Dalebroux Z. D., Swanson M. S. (2012) ppGpp: magic beyond RNA polymerase. Nat. Rev. Microbiol. 10, 203–212 [DOI] [PubMed] [Google Scholar]
- 18. Dalebroux Z. D., Svensson S. L., Gaynor E. C., Swanson M. S. (2010) ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74, 171–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das B., Pal R. R., Bag S., Bhadra R. K. (2009) Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol. Microbiol. 72, 380–398 [DOI] [PubMed] [Google Scholar]
- 20. Raskin D. M., Judson N., Mekalanos J. J. (2007) Regulation of the stringent response is the essential function of the conserved bacterial G protein CgtA in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 104, 4636–4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang M., Sullivan S. M., Wout P. K., Maddock J. R. (2007) G-protein control of the ribosome-associated stress response protein SpoT. J. Bacteriol. 189, 6140–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pal R. R., Bag S., Dasgupta S., Das B., Bhadra R. K. (2012) Functional characterization of the stringent response regulatory gene dksA of Vibrio cholerae and its role in modulation of virulence phenotypes. J. Bacteriol. 194, 5638–5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pal R. R., Das B., Dasgupta S., Bhadra R. K. (2011) Genetic components of stringent response in Vibrio cholerae. Indian J. Med. Res. 133, 212–217 [PMC free article] [PubMed] [Google Scholar]
- 24. Iwanaga M., Yamamoto K., Higa N., Ichinose Y., Nakasone N., Tanabe M. (1986) Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30, 1075–1083 [DOI] [PubMed] [Google Scholar]
- 25. Philippe N., Alcaraz J. P., Coursange E., Geiselmann J., Schneider D. (2004) Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51, 246–255 [DOI] [PubMed] [Google Scholar]
- 26. Bari W., Lee K. M., Yoon S. S. (2012) Structural and functional importance of outer membrane proteins in Vibrio cholerae flagellum. J. Microbiol. 50, 631–637 [DOI] [PubMed] [Google Scholar]
- 27. Yoon S. S., Mekalanos J. J. (2006) 2,3-butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect. Immun. 74, 6547–6556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He H., Cooper J. N., Mishra A., Raskin D. M. (2012) Stringent response regulation of biofilm formation in Vibrio cholerae. J. Bacteriol. 194, 2962–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee K. M., Go J., Yoon M. Y., Park Y., Kim S. C., Yong D. E., Yoon S. S. (2012) Vitamin B12-mediated restoration of defective anaerobic growth leads to reduced biofilm formation in Pseudomonas aeruginosa. Infect. Immun. 80, 1639–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chatterji D., Ogawa Y., Shimada T., Ishihama A. (2007) The role of the ω subunit of RNA polymerase in expression of the relA gene in Escherichia coli. FEMS Microbiol. Lett. 267, 51–55 [DOI] [PubMed] [Google Scholar]
- 31. Vrentas C. E., Gaal T., Ross W., Ebright R. H., Gourse R. L. (2005) Response of RNA polymerase to ppGpp: requirement for the ω subunit and relief of this requirement by DksA. Genes Dev. 19, 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jain V., Kumar M., Chatterji D. (2006) ppGpp: stringent response and survival. J. Microbiol. 44, 1–10 [PubMed] [Google Scholar]
- 33. Ferullo D. J., Lovett S. T. (2008) The stringent response and cell cycle arrest in Escherichia coli. PLoS Genet. 4, e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatterji D., Ojha A. K. (2001) Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4, 160–165 [DOI] [PubMed] [Google Scholar]
- 35. Perederina A., Svetlov V., Vassylyeva M. N., Tahirov T. H., Yokoyama S., Artsimovitch I., Vassylyev D. G. (2004) Regulation through the secondary channel: structural framework for ppGpp-DksA synergism during transcription. Cell 118, 297–309 [DOI] [PubMed] [Google Scholar]
- 36. Nakanishi N., Abe H., Ogura Y., Hayashi T., Tashiro K., Kuhara S., Sugimoto N., Tobe T. (2006) ppGpp with DksA controls gene expression in the locus of enterocyte effacement (LEE) pathogenicity island of enterohaemorrhagic Escherichia coli through activation of two virulence regulatory genes. Mol. Microbiol. 61, 194–205 [DOI] [PubMed] [Google Scholar]
- 37. Kolmsee T., Delic D., Agyenim T., Calles C., Wagner R. (2011) Differential stringent control of Escherichia coli rRNA promoters: effects of ppGpp, DksA and the initiating nucleotides. Microbiology 157, 2871–2879 [DOI] [PubMed] [Google Scholar]
- 38. Paul B. J., Barker M. M., Ross W., Schneider D. A., Webb C., Foster J. W., Gourse R. L. (2004) DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118, 311–322 [DOI] [PubMed] [Google Scholar]
- 39. Kovacikova G., Skorupski K. (2002) Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46, 1135–1147 [DOI] [PubMed] [Google Scholar]
- 40. Tang W. H., Wang Z., Levison B. S., Koeth R. A., Britt E. B., Fu X., Wu Y., Hazen S. L. (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koeth R. A., Wang Z., Levison B. S., Buffa J. A., Org E., Sheehy B. T., Britt E. B., Fu X., Wu Y., Li L., Smith J. D., DiDonato J. A., Chen J., Li H., Wu G. D., Lewis J. D., Warrier M., Brown J. M., Krauss R. M., Tang W. H., Bushman F. D., Lusis A. J., Hazen S. L. (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nanamiya H., Kasai K., Nozawa A., Yun C. S., Narisawa T., Murakami K., Natori Y., Kawamura F., Tozawa Y. (2008) Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol. Microbiol. 67, 291–304 [DOI] [PubMed] [Google Scholar]
- 43. Lemos J. A., Lin V. K., Nascimento M. M., Abranches J., Burne R. A. (2007) Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol. Microbiol. 65, 1568–1581 [DOI] [PubMed] [Google Scholar]
- 44. Boes N., Schreiber K., Schobert M. (2008) SpoT-triggered stringent response controls usp gene expression in Pseudomonas aeruginosa. J. Bacteriol. 190, 7189–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gentry D. R., Burgess R. R. (1989) rpoZ, encoding the ω subunit of Escherichia coli RNA polymerase, is in the same operon as spoT. J. Bacteriol. 171, 1271–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brown L., Gentry D., Elliott T., Cashel M. (2002) DksA affects ppGpp induction of RpoS at a translational level. J. Bacteriol. 184, 4455–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jishage M., Kvint K., Shingler V., Nyström T. (2002) Regulation of σ factor competition by the alarmone ppGpp. Genes Dev. 16, 1260–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bernardo L. M., Johansson L. U., Skärfstad E., Shingler V. (2009) σ54-promoter discrimination and regulation by ppGpp and DksA. J. Biol. Chem. 284, 828–838 [DOI] [PubMed] [Google Scholar]
- 49. Yildiz F. H., Schoolnik G. K. (1998) Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding Y., Davis B. M., Waldor M. K. (2004) Hfq is essential for Vibrio cholerae virulence and downregulates σ expression. Mol. Microbiol. 53, 345–354 [DOI] [PubMed] [Google Scholar]
- 51. Mallik P., Paul B. J., Rutherford S. T., Gourse R. L., Osuna R. (2006) DksA is required for growth phase-dependent regulation, growth rate-dependent control, and stringent control of fis expression in Escherichia coli. J. Bacteriol. 188, 5775–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perron K., Comte R., van Delden C. (2005) DksA represses ribosomal gene transcription in Pseudomonas aeruginosa by interacting with RNA polymerase on ribosomal promoters. Mol. Microbiol. 56, 1087–1102 [DOI] [PubMed] [Google Scholar]
- 53. Klose K. E. (2001) Regulation of virulence in Vibrio cholerae. Int. J. Med. Microbiol. 291, 81–88 [DOI] [PubMed] [Google Scholar]
- 54. Balzer G. J., McLean R. J. (2002) The stringent response genes relA and spoT are important for Escherichia coli biofilms under slow-growth conditions. Can. J. Microbiol. 48, 675–680 [DOI] [PubMed] [Google Scholar]
- 55. Nguyen D., Joshi-Datar A., Lepine F., Bauerle E., Olakanmi O., Beer K., McKay G., Siehnel R., Schafhauser J., Wang Y., Britigan B. E., Singh P. K. (2011) Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Primm T. P., Andersen S. J., Mizrahi V., Avarbock D., Rubin H., Barry C. E., 3rd. (2000) The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182, 4889–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klinkenberg L. G., Lee J. H., Bishai W. R., Karakousis P. C. (2010) The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J. Infect. Dis. 202, 1397–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Na H. S., Kim H. J., Lee H. C., Hong Y., Rhee J. H., Choy H. E. (2006) Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24, 2027–2034 [DOI] [PubMed] [Google Scholar]
- 59. Gaynor E. C., Wells D. H., MacKichan J. K., Falkow S. (2005) The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol. Microbiol. 56, 8–27 [DOI] [PubMed] [Google Scholar]
- 60. Fullner K. J., Mekalanos J. J. (1999) Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67, 1393–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller V. L., Mekalanos J. J. (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metcalf W. W., Jiang W., Daniels L. L., Kim S. K., Haldimann A., Wanner B. L. (1996) Conditionally replicative and conjugative plasmids carrying lacZ α for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35, 1–13 [DOI] [PubMed] [Google Scholar]
- 63. Donnenberg M. S., Kaper J. B. (1991) Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59, 4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kalogeraki V. S., Winans S. C. (1997) Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188, 69–75 [DOI] [PubMed] [Google Scholar]
- 65. Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]