Abstract
Researchers are increasingly focused on the nanoscale level of organization where biological processes take place in living systems. Nanoparticles (NPs, e.g., 1–100 nm diameter) are small forms of natural or manufactured source material whose properties differ markedly from those of the respective bulk forms of the “same” material. Certain NPs have diagnostic and therapeutic uses; some NPs exhibit low-dose toxicity; other NPs show ability to stimulate low-dose adaptive responses (hormesis). Beyond dose, size, shape, and surface charge variations of NPs evoke nonlinear responses in complex adaptive systems. NPs acquire unique size-dependent biological, chemical, thermal, optical, electromagnetic, and atom-like quantum properties. Nanoparticles exhibit high surface adsorptive capacity for other substances, enhanced bioavailability, and ability to cross otherwise impermeable cell membranes including the blood-brain barrier. With super-potent effects, nano-forms can evoke cellular stress responses or therapeutic effects not only at lower doses than their bulk forms, but also for longer periods of time. Interactions of initial effects and compensatory systemic responses can alter the impact of NPs over time. Taken together, the data suggest the need to downshift the dose-response curve of NPs from that for bulk forms in order to identify the necessarily decreased no-observed-adverse-effect-level and hormetic dose range for nanoparticles.
Keywords: nanoparticle, hormesis, nanomedicine, nonlinear dynamics, complex adaptive systems
INTRODUCTION
Nanoparticles (NPs) are very small particles of material that may be natural or manufactured in origin (Buzea et al., 2007; Ju-Nam and Lead, 2008; Merisko-Liversidge and Liversidge, 2011; Roduner, 2006). Sizes typically range from a fraction of one nanometer (nm) in diameter on at least one side up to 100 nanometers (European Commission on the Environment, 2011; International Organization for Standardization, 2005). Although submicron particles between 100–1000 nanometers in size have some advantages toward improving drug delivery (Oyewumi et al., 2010; Stovbun et al., 2012; Wong, 2011), much of the focus of research interest in NPs has been on particles whose size falls below 100 nm. Their small size leads to a large surface area to volume ratio, resulting in variations of properties that differ markedly from those of bulk forms of the “same” material (Buzea et al., 2007; Cao and Wang, 2011; Roduner, 2006).
The range of these altered effects encompasses electromagnetic, thermal, optical, biochemical, and even quantum (in quantum dots, at extremely small particle sizes, typically ranging from 1–10 nm, perhaps as high as 30 nm) properties. Smaller NPs readily cross cell membranes and translocate around the body via blood and lymph (Buzea et al., 2007). Some NPs such as nano-silica or nano-silicon (Demento et al., 2009; Mahony et al., 2013; Petkar et al., 2011; Wang et al., 2012b) and nano-lipid carriers can also serve as immune adjuvants and markedly lower the amount of antigen needed to mount a response in the immune system, e.g., in one study to a dose as low as 2.5 nanograms (Bershteyn et al., 2012; Diwan et al., 2004).
In effect, nanoparticles are often biologically super-potent forms of their source material. For instance, the NP form of an antiretroviral drug in the 3 nanomolar range produced up to a 50-fold reduction in the 50% inhibitory concentration needed, compared with free drug doses (Chaowanachan et al., 2013). Intermittent treatment with the nano-form of the immunosuppressant drug mycophenolic acid improves murine allograft survival time at a dose 1000-fold lower than the bulk form conventional drug (Shirali et al., 2011). By extension, the therapeutic hormetic nanoparticle dose of an otherwise highly toxic source material might fall below the nanomolar level (Raja et al., 2013), e.g., down to picomolar or even lower levels. Some studies further indicate the possibility of sinusoidal hormetic dose-response curves in such a situation (Malarczyk et al., 2011).
Contemporary nanotechnology can generate manufactured nanoparticles in either a top down (e.g., milling, grinding) or bottom up (e.g., nano-silica self-assembly on a structural template) manner (Cho et al., 2011; Cumbo et al., 2013; Ju-Nam and Lead, 2008; Kiel et al., 2012; Merisko-Liversidge and Liversidge, 2011). Reagents and manufacturing parameters such as solvents, dopants, coatings, biosynthetic plant extracts, pH, temperature, and sonication duration and intensity can influence the biological, chemical, electromagnetic, optical and physical properties of the resultant NPs (Cao and Wang, 2011; Pandey et al., 2013; Roduner, 2006). Various nanoparticles will aggregate in the absence of capping agents and/or mechanical dispersion methods (Mudunkotuwa and Grassian, 2011; Pandey et al., 2013; Pham et al., 2007; Tang et al., 2011; Zhang et al., 2012).
APPLICATIONS IN MEDICINE AND TOXICOLOGY
In medicine, NPs have a growing importance for pharmacodiagnostics and therapy (Ahn RW, 2013; Armstead and Li, 2011; Ho and Leong, 2010; Stark, 2011; Yoo et al., 2011) as well as toxicology (Buzea et al., 2007; Winnik and Maysinger, 2013). Exemplar medical applications of nano-forms are the enhanced drug or natural product delivery vehicles with increased bioavailability and cell targeting potential in infectious diseases (Armstead and Li, 2011; Dar et al., 2013) and cancers (Al-Sadoon et al., 2012; Chu et al., 2012; Ghosh et al., 2012; Sayed et al., 2012; Shi et al., 2010b; Wang and Thanou, 2010).
In the emerging area of theranostics, specialized nano-drugs enable more precise targeting of specific cells and/or organs (Vivero-Escoto et al., 2010). For instance, magnetic nanoparticle vehicles can be activated to release their active agent for imaging and/or treatment only after they enter their intended specific cancer cell target (Cole et al., 2011; Ho et al., 2011). The latter approach can take advantage of the nonlinear magnetic behavior of the NPs (Geinguenaud et al., 2012). Nonlinearity of response can derive in part from the unique magnetic or optical properties of certain NPs. For example, near infrared light-activated cell-targeted nanoparticles can augment photothermal ablation therapies (Melancon et al., 2011). However, continuous wave versus nanosecond pulsed laser stimuli can interact with nonlinear absorption processes of gold nanospheres from plasmonic field enhancement to produce different cell death mechanisms in the cancer cell nucleus versus cytoplasm (Huang et al., 2010).
Overall, NPs can more readily enter cancer cells because of the increased vascular leakiness of tumors resulting in passive and/or active uptake processes from ligands on the NP surfaces (Ghosh et al., 2012; Sur et al., 2010). Advantages of nano drug delivery vehicles with targeting include a significant reductions of systemic toxicity in addition to lowering total doses by orders of magnitude (Ahmad et al., 2006; Armstead and Li, 2011; Prakash et al., 2010; Shirali et al., 2011).
In environmental toxicology, high doses of many, though not all, nanoparticles appear to be toxic and potentially contributory to a wide range of diseases, from asthma to autoimmune diseases or atherosclerosis and cancer (Buzea et al., 2007; Winnik and Maysinger, 2013). Even extremely low concentrations of nanoparticles of a given substance can still exert toxic effects on model organisms e.g., 1 nanomolar ceria NPs on C. elegans (Zhang et al., 2011), sublethal adverse effects from 0.02 to 0.20 nanomolar silver NPs or 0.025 to 1.2 nanomolar gold NPs on developing zebrafish embryos (Browning et al., 2009; Lee et al., 2012b; Osborne et al., 2012; Truong et al., 2013), or 20 nanograms/L silver NPs on juvenile salmon (Farmen et al., 2012). Across studies, silver NPs are overall more toxic than gold NPs, but many particle- and organism-related variables affect the specific findings. NP toxicity can derive from activating oxidative stress, inflammatory, immune and even apoptotic pathways as well as genotoxicity in cells (Gualtieri et al., 2011; Sandberg et al., 2012; Shi et al., 2010a; Shi et al., 2010b; Winnik and Maysinger, 2013). Plant-mediated biosynthesis of silver NPs can attenuate toxicity risk in some preparations (Barua et al., 2013). One of the major questions that this heightened sensitivity from NPs asks is whether or not NPs violate the no-observed-adverse-effect level (NOAEL) principle of hormesis or is the NOAEL of NPs simply down-shifted to lower levels and modified by the specific properties of a given NP form than with larger and bulk form particles?
HORMESIS AND NANOPARTICLES
At very low doses below the no-observed-adverse-effect level (NOAEL), previous investigators have documented evidence that some nanoparticles can initiate hormesis (Iavicoli et al., 2010; Nascarella and Calabrese, 2012; Stovbun et al., 2012). Hormesis is the nonlinear dose-response relationship in which low versus high doses of a given agent or stressor can exert effects in opposite directions. If an agent can inhibit function at a high dose, then the hormetic dose will stimulate function, and vice versa. Hormesis is increasingly understood as a dynamic adaptive response or biological plasticity of a complex living system at the level of the whole organism to intermittent mild stressors of various categories (Calabrese, 2013; Calabrese and Mattson, 2011; Iavicoli et al., 2010). Types of stressor categories include physical, chemical, biological, and/or psychological factors (Calabrese and Mattson, 2011; Iavicoli et al., 2010). Calabrese and Mattson (2011) have used hormesis as a quantitative estimate of biological plasticity.
For therapeutic applications, other researchers have proposed that exposing organisms to intermittently-timed hormetic stimuli could shift and shape epigenetic expression toward increased resilience against higher intensity stressors, disease, and aging itself (Stark, 2012; Vaiserman, 2010, 2011). Pickering et al (2013) have emphasized the importance of spacing repeated exposures over time to permit expression of adaptive changes to oxidative stress. The beneficial effects of hormesis may arise from endogenous over-compensatory changes that the cell and organism use to repair or prepare for damage from larger magnitude, adaptively similar external threats from the environment (Stark, 2012; Van Wijk and Wiegant, 2010; Wiegant et al., 2011).
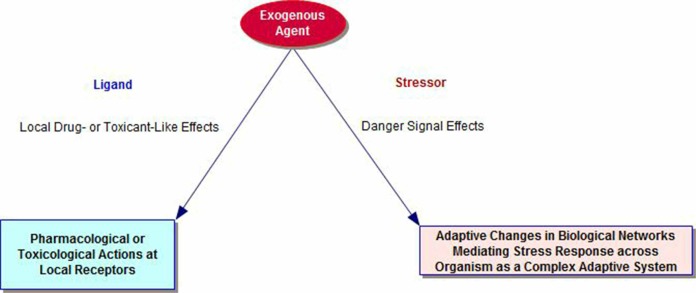
The literature on cross-adaptation, cross-resistance and cross-sensitization includes many examples of overlapping phenomena that emphasize the role of biological plasticity mechanisms and time-dependent mechanisms in the organism. Pathways for the stress response networks involve interactions of metabolic, immune, inflammatory, hormonal, and autonomic functions. Thus, the biology of adaptation is an emergent property of the organism as a whole and distinct from any local chemical effects of the exogenous agent on its specific receptors (see Figure 1). Sub-toxic and sub-lethal stressors initiate adaptive changes that can prepare the organism for future onslaughts from both the original stressor and agents where the physical nature, but not the elicited adaptive response repertoire of the recipient system, differs from that of the original (Milisav et al., 2012). These latter types of responses are sometimes termed cross-adaptation (Hale, 1969) or cross-resistance (Milisav et al., 2012) and, in other cases, heterologous hormesis (Calabrese et al., 2007; Van Wijk and Wiegant, 2010).
FIGURE 1.
Dual Pathways for Exogenous Agents, including Nanoparticles, as Ligands and Stressors.
However, there is a potentially important distinction for a specific agent and its dose or intensity level as a stressor for an individual organism. That is, some agents such as arsenic are relatively toxic and even lethal for most organisms at what pharmacologists consider low doses for their local effects. In that case, looking to a very low dose range might reveal a beneficial hormetic dose range for their adaptation-stimulating effects in most organisms. Thus, it makes sense to look for hormesis at extremely low doses of bulk form arsenic (Raja et al., 2013) and perhaps even lower doses of nano-arsenic trioxide (Ahn et al., 2013). Other nano-materials such as nano-calcium hydroxyapatite may be largely benign across a wider range of dose levels (Zhou and Lee, 2011) and/or can safely deliver nano-forms of less toxic agents for greater bioavailability and clinical benefit than reliance on more toxic bulk form drugs (Chun et al., 2012; Joshi and Muller, 2009; Koning and Krijger, 2007; Lanao et al., 2007; Moulari et al., 2013; Zhao and Feng, 2010).
By analogy, for other types of more benign stressors such as exercise in physiology, the reaction to exercise as a “stressor” will also depend on the pre-established level of physical training and fitness of the individual. In general, exercise is not an inherently toxic event – rather, it is part of the physiological capacity of the organism. Nonetheless, an exercise level that may be moderate for a highly fit person could even be lethal for a poorly-conditioned individual. Many doses of exercise may be fairly benign and initiate adaptive changes consistent with training effects. The “dose” of exercise that is beneficial will thus vary as a function of individual differences in the state of the organism at the time of the exercise. Genetic variations (Rodriguez et al., 2012) and other environmental parameters (Lagisz et al., 2013) can also influence individual differences in dose-response patterns for a variety of events and agents.
Certain NPs pose an additional challenge for determination of the no-observed-adverse-effect level (NOAEL) cut-off. In a complex nonlinear paradox, lower doses of NPs can sometimes increase rather than decrease the toxicity of a given source material as a function of the surface properties of the particles themselves. That is, in the absence of surface modifications to prevent spontaneous agglomeration from close physical interactions of highly reactive NPs in concentrated colloidal liquids (Bagwe et al., 2006; Clark et al., 2010; Sur et al., 2012), higher concentrations or doses can favor NP agglomeration. The resultant nano-aggregates as a whole then can hide or quench the originally hyperreactive surfaces of their smaller NP “parts” (Mudunkotuwa and Grassian, 2011). Consequently, the specific larger agglomerated NP form is less toxic at a higher concentration than a lower dose of smaller, but well-dispersed NPs, e.g., NPs of PbS or copper.
For environmental toxicology, various forms of agitation, together with natural dilution factors that reduce concentration in the marine environment, for example, may disperse such agglomerates (Bourdineaud et al., 2013; Rodrigues et al., 2013; Ruan and Jacobi, 2012; Tang et al., 2011; Zhang et al., 2012). In such scenarios, the dispersion at a lower concentration re-exposes the hyperreactive surfaces of the smaller NPs and enhances their toxic potential (Mudunkotuwa and Grassian, 2011).
For nanomedicine applications, it is possible to take advantage of such issues by adding nontoxic capping agents (Singh et al., 2013) and/or to time the use of sonication or vortexing prior to administration. Such a strategy can determine more systematically the nanoparticle size, shape, and surface chemistry. That is, certain capping agents, e.g., sugars or polysaccharides, can markedly reduce metal NP toxicity. Moreover, sonication will mechanically disperse any larger nanostructures that may have formed as a result of aging, agglomeration, and/or Ostwald ripening of the NPs in colloidal solution. In contrast, longer shelf storage over time could permit resumption of aging effects and thermodynamically-based development of increasingly larger nanostructures (Gautam et al., 2013; Liu et al., 2007). Thus, recency of sonication in solution can affect experimental and clinical findings (Bel Haaj et al., 2013; Liu et al., 2009; Murdock et al., 2008; Tang et al., 2011).
Such issues become critical for evaluating NP effects in medicine and toxicology, when size, shape, and surface chemistry interact with dose to determine nonlinear response patterns (Mudunkotuwa and Grassian, 2011). For example, it is possible to reduce macrophage toxicity of antibacterial silver nanoparticles by coating the surfaces with chitosan (Jena et al., 2012). Chitosan is a fibrous sugar extracted from shellfish outer skeletons. NP characteristics also contribute to unique challenges for controlling inter-experiment variability with NPs such as fullerene C60 nanoparticles (Chang and Vikesland, 2013).
What the data on nanomaterials raise for the discussion of hormesis is an analogous need to readjust our thinking about what constitutes a low dose, or more, precisely, a hormetic dose. Beyond the identity of the source material, the specific sizes, shapes, and surface charges of the NPs in air or water become significant factors interacting with dose. Hormesis researchers may need a type of sliding scale for defining very low hormetic doses where adverse events do not occur and yet beneficial adaptive changes can develop. First, the super-potent reactivity of small nanoparticles lowers the dose range for both toxic and, if relevant, therapeutic effects from a pharmacological perspective (Armstead and Li, 2011). Second, nanoparticles of the “same” material at a given low dose can exert either toxic or benign effects, depending in part on the size and surface reactivity of the particles (Lee et al., 2012b; Mudunkotuwa and Grassian, 2011; Murdock et al., 2008; Winnik and Maysinger, 2013).
Third, the coating or dopants on the surfaces of nanoparticles can also markedly change an otherwise toxic particle into a benign one or to acquire modified actions (Das et al., 2013; McKibbin et al., 2013; Rowe et al., 2013; Sur et al., 2012; Sur et al., 2010; Thurber et al., 2012; Tripathi et al., 2009; Van Hoecke et al., 2011). Fourth, the state of the recipient system cell or organism as a complex adaptive system (CAS) at the time of exposure is another modifying variable in the intensity and even direction of the response to nano and bulk form materials (Antelman and Caggiula, 1996; Bell and Schwartz, 2013; Browning et al., 2009; Lee et al., 2012b; Shi et al., 2010b). Finally, NP forms of various source materials, including animal venoms, calcium phosphate, and nanocrystalline fullerene can exert marked toxicity for cancer cells but spare healthy cells (Al-Sadoon et al., 2012; Harhaji et al., 2007; Shi et al., 2010b).
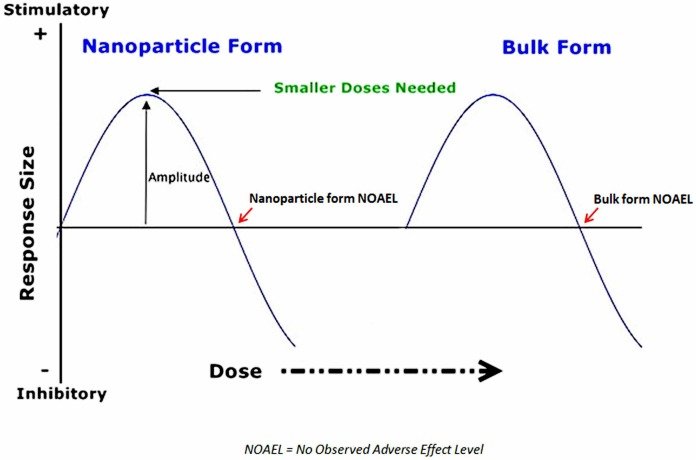
Thus, for nanoparticles, nonlinear hormetic responses are no longer a function of merely specific low doses. It is necessary to take into account the variable toxicity of a given specific nanoparticle based on its potential to change size, and hence, surface charge and related direct effects. The NPs then interact with individual differences in organisms’ ever-changing dynamical state of adaptive resilience to the lower dose ranges of nanoparticles in general. In general, the shift of the toxic dose range for nanoparticle forms of environmental toxicants toward lower doses is also orders of magnitude below that for bulk forms of the “same” materials. By extension, the no-observed-adverse-event-level (NOAEL) for nanoparticles of typically toxic agents must be very low to accommodate the range of various NP sizes and surface chemistries that may emerge within a given environmental context, far below the more fixed NOAEL for bulk forms of the same material (Figure 2). The potential for multiple particle-related, environment-related, and recipient-related factors to contribute variance in determining the NOAEL in each study of a given nanoparticle form make it much more difficult to define an appropriate metric for what constitutes a “low dose” or a hormetic dose.
FIGURE 2.
Left Shift of NOAEL and Hormetic Dose Range for Nanoparticles. Reprinted with permission (Bell and Schwartz, 2013).
Dose frequency also plays a role in adaptive phenomena. Nano-drugs persist inside cells longer than do conventional bulk form drugs (Ahmad and Khuller, 2008; Ahmad et al., 2006; Armstead and Li, 2011; Shirali et al., 2011). Even for bulk forms, with overly frequent dosing of an agent, the direction of the response can reverse when the nature of the response depends on the biological plasticity and metaplasticity mechanisms of adaptation to the agent as a stressor rather than on the mechanisms of its local effects on specific receptors (Abraham, 2008; Antelman and Caggiula, 1996; Antelman et al., 1992; Antelman et al., 2000; Pincus and Metten, 2010). Such data suggest the potential role of pulsed versus continuous dosing in mobilizing compensatory adaptive responses to an exogenous stressor or agent (Milisav et al., 2012; Shirali et al., 2011; Stark, 2012).
In addition, the nature of adaptive responses, as opposed to local lig-and-receptor responses, is that the emergent result is increased resistance to the original stressor and cross-resistance or cross-adaptation to other stressors that can affect similar adaptive pathways. In pharmacology and physiology research outside toxicology, many empirical examples of cross-adaptation, cross-resistance and cross-sensitization are documented (Antelman et al., 1992; Antelman et al., 2000; Hale, 1969; Milisav et al., 2012). For instance, hypoxia cross-adapts with cold or hot temperatures (Banti et al., 2008; Launay et al., 2006; Lunt et al., 2010; Ning and Chen, 2006); stress cross-sensitizes with amphetamine (Antelman et al., 1980); sucrose cross-sensitizes with amphetamine or cocaine (Avena and Hoebel, 2003; Gosnell, 2005); formaldehyde cross-sensitizes with cocaine (Sorg et al., 2001; Sorg et al., 1998); heat shock, sodium arsenite, and cadmium chloride can cross-sensitize with one another depending on their heat shock protein activation patterns (Wiegant et al., 1998).
The inference from such evidence is that an external agent at various doses is a salient biological stressor for the cell or organism as a complex adaptive system in addition to the local, receptor-specific actions (Bell and Schwartz, 2013). Given the data showing the ability of sub-toxic doses of NPs to initiate cellular stress responses, e.g., oxidative stress (Tang et al., 2010; Winnik and Maysinger, 2013), cytokine and exosome release and other intercellular signaling events (Andersson-Willman et al., 2012; Beloribi et al., 2012; Demento et al., 2009; Ristorcelli et al., 2009; Zhu et al., 2012a; Zhu et al., 2012b), there are a number of potentially fruitful, albeit challenging directions for future research into biological mechanisms for NP-induced adaptive and hormetic responses (Demirovic and Rattan, 2013).
UNIQUE FEATURES OF NANOPARTICLES
Nonlinearity from Nanoparticle Properties: Beyond Hormetic Dose-Response Relationships
With nanoparticles, the nonlinearity of responses by cells and organisms may involve even more complexity than with bulk form materials. In contrast with conventional bulk forms of drugs, chemicals, herbs, and other materials, nanoscale forms vary in their effects as a function of not only the dose size, but also seemingly minor variations in their individual particle or aggregate sizes, shapes, and surface charges. Trace “contaminants,” “dopants,” and coatings on the surfaces of nanoparticles can also markedly change their properties, effects and level of toxicity at a given size (Isoda et al., 2011; Kaur and Tikoo, 2012; Kumar et al., 2012; Rowe et al., 2013; Sun et al., 2012; Sur et al., 2012; Van Hoecke et al., 2008; Wang et al., 2012a). The environmental medium in which the NPs interact also modify their toxic or beneficial potential (Kaur and Tikoo, 2012; Mudunkotuwa and Grassian, 2011; Zhang et al., 2012; Zhu et al., 2006).
More highly charged surfaces often lead to greater NP toxicity (Truong et al., 2013; Winnik and Maysinger, 2013). As a result, nanoparticles can evoke nonlinear response patterns from not only hormetic low dose-stimulatory response relationships, but also their inherent physico-chemical properties. That is, a given dose of NPs from the “same” source material can elicit lethal effects for one size nanoparticle, whereas the same dose at another size does not (Browning et al., 2009; Lee et al., 2012b).
In experimental cancer studies, certain sources of nanoparticles and certain sizes of those NPs are more toxic to cancer cells in vitro than to normal cells, e.g., calcium phosphate NPs (Shi et al., 2010b). Enhanced intracellular access in “leaky” blood vessels supporting cancer cells, the inherently reactive surface properties of the NPs inside the cells, and different endogenous biological mechanisms may contribute to the differential cell type toxicity. Size-dependent responses and cell-specific interactions are a widespread phenomenon for NPs (Harhaji et al., 2007; Jiang et al., 2008; Kim et al., 2012; Lankoff et al., 2012).
In the real world environment, NP exposures encompass a wide range of particle sizes and shapes. Some NPs are crudely formed from uncontrolled environmental events that yield, irregular sizes, shapes and properties. Early laboratory methods for making nanoparticles involved prolonged grinding and milling procedures from bulk source materials (top-down methods), which make NPs with many structural irregularities and defects (DeCastro and Mitchell, 2002). Contemporary manufactured nanoparticles necessarily attain more consistent and defect-free shapes and sizes with more precise technological procedures like photo-lithography or bottom-up template synthesis methods (Ju-Nam and Lead, 2008).
In the manufacturing realm, nanotechnologists are also now using plants and other more natural biological agents to biosynthesize “green” silver and gold nanoparticles (Daisy and Saipriya, 2012; Das et al., 2013; Hudecova et al., 2012; Snitka et al., 2012; Suriyakalaa et al., 2012). Biologically synthesized silver and gold NPs have the advantage that the residual amounts of the plant extract adsorb onto the final NP surface and can enhance therapeutic effects while reducing toxicity (Tripathi et al., 2009; Umashankari et al., 2012). Some investigators include plant extracts, phytochemicals and antioxidants in their manufacturing methods to change surface properties and thereby reduce the cellular toxicity potential of certain metal NPs, e.g., silver NPs (Du et al., 2012; Hudecova et al., 2012; Lee et al., 2012a; Mittal et al., 2013; Osborne et al., 2012; Park et al., 2012; Suriyakalaa et al., 2012; Tournebize et al., 2012).
For more general sustainable NP manufacturing, natural plant materials such as English ivy, certain native plants from India, the traditional Chinese herb Cuscuta chinensis, glycyrrhizic acid from radix glycyrrhizae, guar gum, and rice husk also can also release their own organic nanoparticles of various sizes under appropriate conditions (Barve and Chaughule, 2013; Burris et al., 2012; Im et al., 2011; Lenaghan et al., 2013; Salavati-Niasari et al., 2012; Soumya et al., 2010; Wang et al., 2013; Yen et al., 2008). Beyond plant sources, a combination of ball-milling and ultrasound can reduce other organic material sources such as waste eggshells into calcium carbonate nanoparticles (Hassan et al., 2013).
A rationale has been that all of the reagents used in making NPs will adsorb onto the large charged surface area to one degree or another and serve to “dope” or coat and thus modify the primary silver, gold, or silicon NP. Different solvents at different concentrations, for example, result in NPs of the “same” material with different characteristics and sizes (Abbasi and Morsali, 2012; Cao and Wang, 2011; Rao et al., 2005; Yang et al., 2011; Yoo et al., 2006). As a result, the adsorbed materials can change the surface charge and properties of the NPs and thereby, their biological, therapeutic or toxic potential (Lu et al., 2011; Sur et al., 2010). The use of nontoxic or less toxic natural source reagents and materials could reduce toxic waste from NP manufacturing and potentially improve safety of nanomedicine products.
Studies on manufacturing also highlight the need for scrutiny of sample preparation and analytic methods in NP studies (Chikramane et al., 2012). It is important to keep in mind that the precise laboratory conditions in which researchers examine effects of a nanoparticle preparation may influence the findings. In addition to any added solvents and reagents, basic manufacturing parameters such as intensity, duration, and the timing and extent of sonication will affect dispersion of nanoparticles that otherwise aggregate into larger particles with aging (Abbasi and Morsali, 2012; Murdock et al., 2008; Ruan and Jacobi, 2012; Song et al., 2012; Tang et al., 2011). Variations in temperature and pH will also modify the properties of the nanoparticle samples in solution (Abbasi and Morsali, 2012; Rao et al., 2005). Interactions with serum albumin lead to differential agglomeration and sizing of nanostructures during biological experiments (Song et al., 2012; Tantra et al., 2010).
Even making reliable NP concentrations for research purposes is potentially confounded by variations in additional nanostructures that might get into solution from sonication or vortexing agitation of the materials in liquid within different glassware or polymer containers (Betts et al., 2013; Ives et al., 2010; Liu et al., 2012). Taken together, the data indicate the possibility of meaningful interactions between dose size, particle size, sample preparation and testing parameters, and state of the cells or organism at the time of administration in the expression of specific effects.
Quantum Properties of Nanoparticles
Smaller NP sizes also introduce quantum mechanical considerations into the problem for trying to evaluate nonlinear dose-response relationships in a conventional cause-effect medical model (Berec, 2012; Gupta and Wiggers, 2011; Roduner, 2006; Yao and Hughes, 2009). For example, in nano-optics and nano-electronics, the smallest NPs (quantum dots) exhibit the ability to manifest macro quantum entanglement (Berec, 2012; Yao and Hughes, 2009), quantum coherence (Chudnovsky and Friedman, 2000; Hatef et al., 2012), and quantum confinement (Gupta and Wiggers, 2011; Hannah et al., 2012; Kleps et al., 2010) phenomena. Some nanotechnologists take advantage of quantum confinement, for instance, to generate tunable quantum dot NPs with specific optical properties (Biju et al., 2010; Kang et al., 2011; Luther et al., 2011; Troia et al., 2009).
Few medical researchers consider the implications of quantum effects of NPs on biological function (Stovbun et al., 2012). However, some investigators are using the ability of quantum confinement of electrons by different sizes of small NPs (quantum dots, with their atom-like properties) to yield different wavelength colors inside cells for specialized diagnostic imaging methods (Browning et al., 2009; Huang et al., 2012; Lee et al., 2012b; McGuinness et al., 2011; Shalchian et al., 2005; Wang and Chen, 2011). Scientists working at the nanoscale point out the fact that biological processes occur at the nanoscale and sub-nanoscale level. Lloyd recently noted, for instance: “Nature is the great nano-technologist. The chemical machinery that powers biological systems consists of complicated molecules structured at the nanoscale and sub-nanoscale. At these small scales, the dynamics of the chemical machinery is governed by the laws of quantum mechanics” (Lloyd, 2011).
The literature contains only a few papers on the role of quantum physics in biological systems (Davies, 2004). Given limited evidence of the quantum mechanical properties of nanoparticles and their possible role in biological effects of NPs, much research lies ahead to understand the full potential effects of nanoparticles on living organisms. Nonetheless, at least some smaller sized NPs may act within the worlds of both conventional physics and quantum mechanical phenomena, making characterization of their dose-response relationships and mechanisms even more difficult to determine in a reproducible manner.
Still, it is important to realize that the biological effects of nanoparticles may change the scientific rules by which medical studies of nanoparticles are done. Clinical research on NPs could differ in design and even reliability from those for bulk forms of materials. The atom-like properties of very small nanoparticles may force quantum physics into the discussion of their therapeutic and toxicological effects in biology and medicine.
Interactions of NPs with the Organism as a Complex Adaptive System or Network
In the laboratory, technological advances now permit following the random walk of single nanoparticles through an individual organism, revealing the limitations to using averaged or ensemble data for evaluating specific nanoparticle effects (Browning et al., 2009; Lee et al., 2012b). The latter issue of individual variability in NPs and individual differences in responses of each organism may hamper efforts to rely on the proposed average magnitude and response distributions for assessing hormesis from nanoparticles (Nascarella and Calabrese, 2012). Awareness of these factors, however, can reduce the risk of overly broad assumptions or generalizations about the effects of NPs in living systems, including relative to hormesis.
So far, this paper has alluded to the complex nonlinear dynamics and network organization of the organism as another factor in modifying the nature, magnitude and direction of nanoparticle effects (Sugarman et al., 2013). What are the implications of considering the interaction of the nanostructured material with an individual organism? In his text, Introduction to Nanoscience, Lindsay (Lindsay, 2010) commented: “Nanoscience is where atomic physics converges with the physics and chemistry of complex systems.”
Current thinking suggests that the adaptive response nature of hormesis is a manifestation of biological plasticity (Calabrese and Mattson, 2011). As a result, the dose-response observations reflect emergent interactions of a given mild stressor or low dose agent with a specific organism in a particular dynamic state, modified by genetics and past experiences. Biological metaplasticity, or the plasticity of plasticity can reverse directionality of responses as a function of past adaptations around a set point compatible with maintaining homeostatic balance (Abraham, 2008; Antelman and Caggiula, 1996). Moreover, for in vivo studies, NPs may yield very different findings from in vitro experiments (Clift et al., 2011; Lu et al., 2011). Using intact organisms may ultimately be necessary to understand when and how NPs might cause hormesis and other nonlinear responses, e.g., stochastic resonance (Chen et al., 2012; McDonnell and Abbott, 2009).
Complete organisms and intact cells are each complex adaptive systems (CAS) at different levels of scale. CASs are interconnected, interactive and interdependent networks of self-organized components. The specialized components in turn generate emergent properties at the higher levels of organization not seen in the individual parts (Pincus and Metten, 2010). Furthermore, a CAS can change behaviors over time at different time scales, with a range between order and chaos that adapts nonlinearly to changes in the environment.
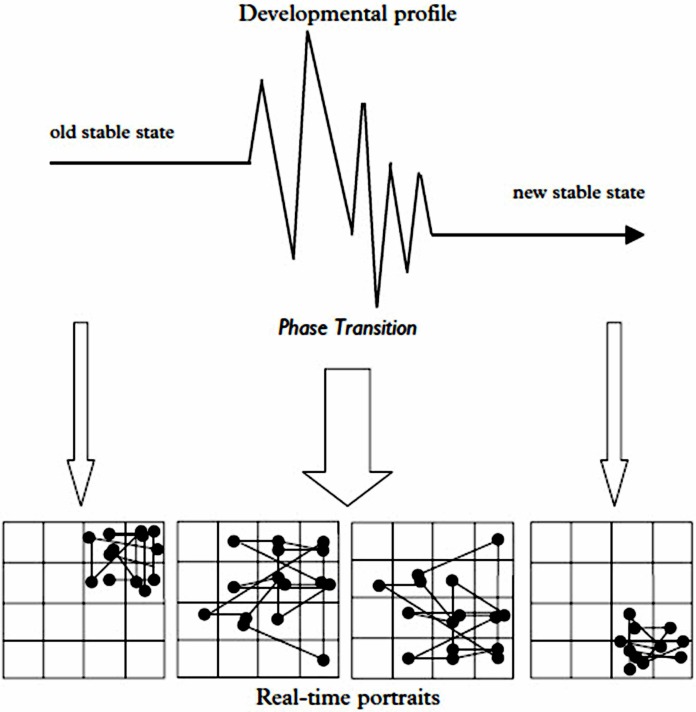
The result observed can vary, revealing degrees of resilience from the capacity for adjusting intrinsic flexibility and stability in the behaviors of the complex system. The dynamic self-organized “goal” for a CAS is to optimize the organism or cell’s fitness within a given environment to the extent possible within the current state and meta-flexibility of the system (Pincus and Metten, 2010). The nature, magnitude, and direction of the change can be difficult to predict in a CAS, especially at critical points of dynamical instability (Hollenstein, 2007; Malarczyk et al., 2011; Sugarman et al., 2013). In addition, Figure 3 illustrates some time-dependent variables in a living CAS, e.g., developmental state of the recipient organism and the frequency of repeated exposures that can influence the adaptive changes and even the direction of the observed responses, apart from the dose itself.
FIGURE 3A.
Time-Dependent Interactions of Host and Environment that Can Affect the Initiation and Direction of Responses in a Nonlinear Dynamical System such as a Living Organism. Developmental phase transition in an adolescent human being can destabilize system dynamics and lead to subsequent self-reorganization of interpersonal interaction dynamics over time. Reprinted with permission (Hollenstein, 2007).
Some researchers in nonlinear dynamical systems (NDS) propose that disease and aging reflect losses of complexity in the dynamics of the organism (Costa, 2002, 2007; Costa et al., 2002; Fredrickson and Losada, 2005; Goldberger et al., 2002; Losada, 1999; Losada and Heaphy, 2004). With regard to high doses of nanoparticles and other small particles from air pollution, many believe that such toxic level exposures promote disease, as reflected in a loss of complexity in physiological measures such as heart rate variability (Shannahan et al., 2012).
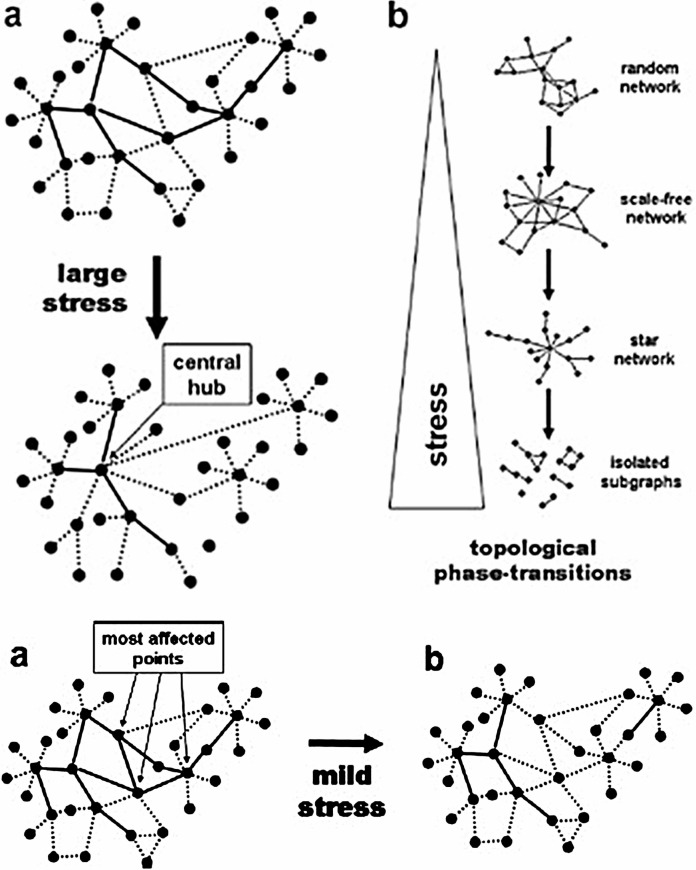
However, studies on the effects of lower, subtoxic NP doses reveal individual variability in the effects of the agent on different organisms receiving the “same” exposure. Stressing a CAS and observing how it responds to the stressor in spatially and temporally remote areas of function can reveal the larger capacity for resilience of the individual organism (Bar-Yam, 1997; Bar-Yam and Epstein, 2004). Cause-effect relationships in CAS tend to be indirect rather than direct. Overwhelming intensities of stress can completely disrupt a complex biochemical network, for instance, whereas lesser levels of stress may simply induce a self-reorganization of function to cope with the effects (Mihalik and Csermely, 2011; Szalay et al., 2007) (Figure 4).
FIGURE 4.
Effects of Different Levels of Stress on Functional Network Organization of a Complex Adaptive System. Reprinted with permission (Szalay et al., 2007). Note: Solid and dotted lines represent strong and weak (high and low affinity) links, respectively.
For instance, with silver NP exposures at sub-lethal doses, some developing zebrafish organisms remain healthy whereas others develop deformities (Lee et al., 2012b). Those with the deformities show an NP size-dependent effect, with larger numbers of larger versus smaller size silver NPs accumulating inside the surviving deformed versus healthy individuals, at the same given molar concentration. Such data support the likely interactions of subtoxic nanoparticle doses and sizes with the state of the organisms at the time of exposure. The dynamical state of the individual CAS here would translate into the variable ability of blood vessel integrity and cell membranes either to allow or to block entry of the damaging larger-sized nanoparticles inside the cells, e.g., (Shi et al., 2010b).
In the therapeutic realm, harnessing low doses of certain sized nanoparticles as hormetic stimuli may be useful. It is instructive to look at research on using low level discrete, well-timed stimuli to mobilize widespread changes in function of the overall organism. For example, adding a low level, subsensory noise applied to the feet of elderly individuals can improve the complexity of sway fluctuations in their postural balance (Costa, 2007). Adding noise in the latter case enhances the ability to detect otherwise age-weakened sensory signals within the organism. On the other hand, applying discrete pulsed electrical stimuli with precise magnitude and timing for the individual’s diseased state (i.e., which is emergent biological “noise”) can disrupt and normalize cardiac arrhythmias or experimental epileptic seizure activity in the brain (Coffey, 1998; Garfinkel et al., 1992; Schiff et al., 1994).
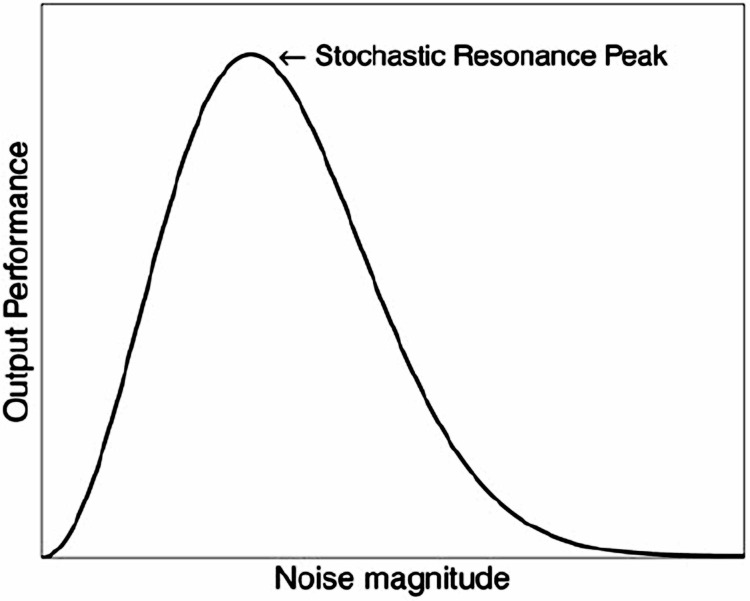
One underlying theory for the clinical benefits of introducing a small or weak signal within a larger endogenous noise in a CAS is stochastic resonance (SR) (Casado-Pascual et al., 2003; Czaplicka et al., 2013; Kelty-Stephen and Dixon, 2013; Korn and Faure, 2003; Krawiecki et al., 2000; Magalhaes and Kohn, 2011; McDonnell and Abbott, 2009; Pinamonti et al., 2012; Torres and Ruiz, 1996). SR is a phenomenon which involves the ability of a small signal to exert noise-enhanced amplified effects when given in the background of the much larger noise to a nonlinear complex system (Figure 5) (McDonnell and Abbott, 2009).
FIGURE 5.
Nature of Stochastic Resonance (SR) as a Model Nonlinear Process for Noise-Enhanced Small Signals. Typical SR Curve of Output Performance versus Input Noise Magnitude for Complex Systems Capable of Stochastic Resonance. Reprinted under the Creative Commons License with Attribution (McDonnell and Abbott, 2009).
Certain nanoparticles, e.g., carbon nanotube transistors, can evoke this type of noise-amplified response to a weak signal in a non-living complex system (Lee et al., 2006). In biological systems, previous studies have demonstrated stochastic resonance in sensory systems. SR is involved, for example, in crayfish detection of incident pressure waves from predators as well as in human visual perception and balance control (Moss et al., 2004). To further explore SR-related phenomena, Lee et al (2010) showed coherence resonance or self-synchronization at an optimal noise level, in transport of single ions through the interior of a 500 micrometer long carbon nanotube. The latter observations involved increases in throughput of the nanopore by a factor of 100 (Lee et al., 2010).
SR is a testable hypothesis as one way in which a pulsed dose of a salient, low dose agent or nanoparticle might initiate the cascades of amplified biological signaling reported in hormesis (Calabrese, 2013). The “noise” in an adaptive living system might be a pattern of dysfunctions manifesting as a disease, toxicity, or aging (Soti and Csermely, 2007). Then the therapeutic strategy could be either (a) to add noise to enhance sensory detection capacity in an aging individual (Costa et al., 2007) or (b) to introduce a salient mild hormetic signal into the pre-existing systemic noise of disease to trigger a reversal of direction toward health (Stark, 2012; Torres and Ruiz, 1996; Van Wijk and Wiegant, 2011; Yu et al., 2013).
SR may play a role in the aging process and in anti-aging interventions. Soti and Csermely (2007) have proposed that aging leads to increased noise in the functional cellular biochemical networks. The noise grows via cumulative damage to weak biochemical network links involving chaperone proteins such as heat shock proteins. Both aging and disease can induce a loss of complexity in the nonlinear dynamics of a complex adaptive system across levels of organizational scale, including cell systems, physiological systems, and whole organisms (Costa, 2007; Costa et al., 2005; Fredrickson and Losada, 2005; Goldberger, 1996; Goldberger et al., 2002; Hollenstein, 2007; Pincus and Metten, 2010; Soti and Csermely, 2003, 2007).
However, well-timed mild hormetic stressors from certain nanoparticles in low doses and certain particle sizes could serve as one type of small salient SR signal embedded in the larger noise to trigger beneficial recovery of complexity in the system (Stark, 2012; Sugarman et al., 2013). Modulation of heat shock proteins offers a potential biological mechanism (Soti and Csermely, 2006) by which to reverse age- or disease-related loss of complexity in the adaptive networks of cells. In hormesis research, one intervention strategy involves postconditioning hormesis to elicit therapeutic effects in heat shock protein activation patterns from mild (low dose) hormetic environmental stimuli (Van Wijk and Wiegant, 2010; Wiegant et al., 2011). Previous studies have already shown the capacity of various nanoparticles at toxic doses to modulate heat shock protein activation patterns (Farmen et al., 2012; Foldbjerg et al., 2012; Lim et al., 2012; Richert et al., 2012; Siddiqi et al., 2012; Zhao et al., 2012).
It is not always necessary to use low doses of the same specific stressor that may have caused deterioration. In the adaptive stress response networks, the phenomenon of cross-adaptation or cross-resistance (Hale, 1969; Milisav et al., 2012) could permit selection of a heterologous, cross-adapted stressor to serve as the hormetic stimulus. Newer evidence suggests that low doses of certain salient cross-adapted nanoparticles could act as such postconditioned hormetic stressors (Bell and Schwartz, 2013). It may also be possible, as Vaiserman has proposed, to use intermittent mild hormetic stressors to initiate preconditioned hormesis and adaptive changes for preventive purposes (Vaiserman, 2010, 2011). Nonetheless, identifying the optimal conditions for beneficial shaping of health- and longevity-promoting exposures remains a challenge (Pickering et al., 2013).
For low doses of small nanoparticles to act via stochastic resonance, e.g., in hormesis, they would need to take advantage of endogenous amplification processes possible within the individual as a nonlinear complex adaptive system (McDonnell and Abbott, 2009). Notably, the NPs would need to arrive as a discrete properly-timed, pulsed low intensity signal rather than at continuous dosing levels (Antelman et al., 2000; Casado-Pascual et al., 2003; Kelty-Stephen and Dixon, 2013). While speculative, the concept of stochastic resonance in complex adaptive systems could add a new layer of discovery to advance our understanding of the circumstances in which the effects of hormesis might be utilized for prevention or treatment of disease.
CONCLUSIONS
In conclusion, for the therapeutic application of hormesis with NPs (Iavicoli et al., 2010; Nascarella and Calabrese, 2012), additional considerations beyond traditional dose explanations likely come into play to understand nonlinear responses. Numerous interacting factors related to nanoparticle size, shape, and surface charge, in addition to the material composition and low dose, determine the net effects of particular nanoparticles in a given study. The NPs then interact with individual differences in the dynamical state of the cells and organisms as complex adaptive systems to generate emergent nonlinear effects.
The time-dependent, multifactorial and individualized nature of adaptive phenomena raises significant questions about the most appropriate experimental designs on NP hormesis. Careful characterization of nanoparticles used in a given study and the pre-treatment dynamical state of the individual recipient organisms or cells may lessen the risk of generating confusing and even irreproducible findings in this field (Chang and Vikesland, 2013; Vijayaraghavan and Nalini, 2010; Xia et al., 2009). Even relying on averaged versus individualized data may be misleading (Browning et al., 2009; Huang et al., 2012; Lee et al., 2012b).
Implications of the available literature for future studies on NPs and hormesis include:
Because of enhanced biological potencies of NPs, it is necessary to look for significant down-shifting to the left along the x-axis for dose levels below the no-observed-adverse-effect-level (NOAEL) where hormesis is more likely to occur. That is, in some cases, hormetic doses of some NPs may sometimes occur at levels below 1 nanomolar concentration. However, since NP size and surface chemistries can vary in a given environment, the cut-off levels for their direct and indirect therapeutic and/or toxic effects may also vary accordingly.
Because of the potential interactions of small particle size and low dose with the state of the recipient complex adaptive system, developing multifactorial models for biological plasticity mechanisms of hormesis may be particularly important with nanoscale materials.
For therapeutic applications of hormesis using NPs, dosing regimens may need to involve discrete pulsed rather than continuous administration of the low doses of specific sized NPs in order to take advantage of biological signaling and nonlinear stochastic resonance. Timing of NPs can affect the nature and direction of the response (Hossu et al., 2010; Jonasson et al., 2013; Vesterdal et al., 2010). The amplified effects of small pulsed signals in the context of a larger noisy signal can produce large magnitude, clinically significant change in an individual as a complex adaptive system (Costa, 2007; Ichiki and Tadokoro, 2013; McDonnell and Abbott, 2009; Pinamonti et al., 2012; Soti and Csermely, 2007).
The past decade has seen an explosion of research and discovery in nanoscience, nanotechnology, and nanomedicine. The potential of interacting therapeutic nanoparticles with hormetic dose treatment strategies is largely unexplored. Tools from systems biology (Abu-Asab et al., 2011), network analysis (Farkas et al., 2011) and nonlinear dynamical systems (Pincus and Metten, 2010) may facilitate this direction for future research. The evidence supports the importance of exploratory and hypothesis-driven studies in this area.
FIGURE 3B.
Overly frequent repetitions of a low dose environmental stressor (e.g., experimental cocaine pretreatments in an animal during a sensitization protocol study of brain striatal dopamine efflux evoked by eliciting dose of cross-sensitized amphetamine) leads to reversal of direction in observed responses over consecutive repetitive exposures. Reprinted with permission (Antelman et al., 1997).
REFERENCES
- Abbasi AR, Morsali A. Influence of solvents on the morphological properties of AgBr nano-structures prepared using ultrasound irradiation. Ultrason Sonochem. 2012;19:540–545. doi: 10.1016/j.ultsonch.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abu-Asab MS, Chaouchi M, Alesci S, Galli S, Laassri M, Cheema AK, Atouf F, VanMeter J, Amri H. Biomarkers in the age of omics: time for a systems biology approach. OMICS. 2011 2011 Mar;15(3):105–12. doi: 10.1089/omi.2010.0023. Epub 2011 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Khuller GK. Alginate-based sustained release drug delivery systems for tuberculosis. Expert Opin Drug Deliv. 2008;5:1323–1334. doi: 10.1517/17425240802600662. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Pandey R, Sharma S, Khuller GK. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J Chest Dis Allied Sci. 2006;48:171–176. [PubMed] [Google Scholar]
- Ahn RW, Barrett SL, Raja MR, Jozefik JK, Spaho L. Nano-Encapsulation of Arsenic Trioxide Enhances Efficacy against Murine Lymphoma Model while Minimizing Its Impact on Ovarian Reserve In Vitro and In Vivo. PLoS ONE. 2013;8:e58491. doi: 10.1371/journal.pone.0058491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadoon MK, Abdel-Maksoud MA, Rabah DM, Badr G. Induction of Apoptosis and Growth Arrest in Human Breast Carcinoma Cells by a Snake (Walterinnesia aegyptia) Venom Combined With Silica Nanoparticles: Crosstalk Between Bcl2 and Caspase 3. Cell Physiol Biochem. 2012;30:653–665. doi: 10.1159/000341446. [DOI] [PubMed] [Google Scholar]
- Andersson-Willman B, Gehrmann U, Cansu Z, Buerki-Thurnherr T, Krug HF, Gabrielsson S, Scheynius A. Effects of subtoxic concentrations of TiO(2) and ZnO nanoparticles on human lymphocytes, dendritic cells and exosome production. Toxicol Appl Pharmacol. 2012;264:94–103. doi: 10.1016/j.taap.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR. Oscillation follows drug sensitization: implications. Critical Reviews in Neurobiology. 1996;10:101–117. doi: 10.1615/critrevneurobiol.v10.i1.50. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR, Gershon S, Edwards DJ, Austin MC, Kiss S, Kocan D. Stressor-induced oscillation. A possible model of the bidirectional symptoms in PTSD. Annals of the New York Academy of Sciences. 1997;821:296–304. doi: 10.1111/j.1749-6632.1997.tb48288.x. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Caggiula AR, Knopf S, Kocan DJ, Edwards DJ. Amphetamine or haloperidol 2 weeks earlier antagonized the plasma corticosterone response to amphetamine; evidence for the stressful/foreign nature of drugs. Psychopharmacology. 1992;107:331–336. doi: 10.1007/BF02245157. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Levine J, Gershon S. Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Molecular Psychiatry. 2000;5:350–356. doi: 10.1038/sj.mp.4000721. [DOI] [PubMed] [Google Scholar]
- Armstead AL, Li B. Nanomedicine as an emerging approach against intracellular pathogens. Int J Nanomedicine. 2011;6:3281–3293. doi: 10.2147/IJN.S27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia. Pharmacol Biochem Behav. 2003;74:635–639. doi: 10.1016/s0091-3057(02)01050-x. [DOI] [PubMed] [Google Scholar]
- Bagwe RP, Hilliard LR, Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357–4362. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant Cell Environ. 2008;31:1029–1037. doi: 10.1111/j.1365-3040.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- Bar-Yam Y. Dynamics of Complex Systems. Perseus Books; Reading, MA: 1997. [Google Scholar]
- Bar-Yam Y, Epstein IR. Response of complex networks to stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4341–4345. doi: 10.1073/pnas.0400673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S, Konwarh R, Bhattacharya SS, Das P, Devi KS, Maiti TK, Mandal M, Karak N. Non-hazardous anticancerous and antibacterial colloidal ‘green’ silver nanoparticles. Colloids Surf B Biointerfaces. 2013;105:37–42. doi: 10.1016/j.colsurfb.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Barve R, Chaughule R. Size-dependent in vivo/in vitro results of homoeopathic herbal extracts. Journal of Nanostructure in Chemistry. 2013;3:18. [Google Scholar]
- Bel Haaj S, Magnin A, Petrier C, Boufi S. Starch nanoparticles formation via high power ultrasonication. Carbohydrate polymers. 2013;92:1625–1632. doi: 10.1016/j.carbpol.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Bell IR, Schwartz GE. Adaptive network nanomedicine: an integrated model for homeopathic medicine. Frontiers in Bioscience (Scholar Ed) 2013;5:685–708. doi: 10.2741/s400. [DOI] [PubMed] [Google Scholar]
- Beloribi S, Ristorcelli E, Breuzard G, Silvy F, Bertrand-Michel J, Beraud E, Verine A, Lombardo D. Exosomal Lipids Impact Notch Signaling and Induce Death of Human Pancreatic Tumoral SOJ-6 Cells. PLoS One. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berec V. Quantum entanglement and spin control in silicon nanocrystal. PLoS One. 2012;7:e45254. doi: 10.1371/journal.pone.0045254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershteyn A, Hanson MC, Crespo MP, Moon JJ, Li AV, Suh H, Irvine DJ. Robust IgG responses to nanograms of antigen using a biomimetic lipid-coated particle vaccine. J Control Release. 2012;157:354–365. doi: 10.1016/j.jconrel.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts JN, Johnson MG, Rygiewicz PT, King GA, Andersen CP. Potential for metal contamination by direct sonication of nanoparticle suspensions. Environ Toxicol Chem. 2013;32:889–893. doi: 10.1002/etc.2123. [DOI] [PubMed] [Google Scholar]
- Biju V, Itoh T, Ishikawa M. Delivering quantum dots to cells: bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem Soc Rev. 2010;39:3031–3056. doi: 10.1039/b926512k. [DOI] [PubMed] [Google Scholar]
- Bourdineaud JP, Rossignol R, Brethes D. Zebrafish: a model animal for analyzing the impact of environmental pollutants on muscle and brain mitochondrial bioenergetics. Int J Biochem Cell Biol. 2013;45:16–22. doi: 10.1016/j.biocel.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Browning LM, Lee KJ, Huang T, Nallathamby PD, Lowman JE, Xu XH. Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale. 2009;1:138–152. doi: 10.1039/b9nr00053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris JN, Lenaghan SC, Zhang M, Stewart CN. Nanoparticle biofabrication using English ivy (Hedera helix) J Nanobiotechnology. 2012;10:41. doi: 10.1186/1477-3155-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormetic mechanisms. Critical Reviews in Toxicology. 2013;43:580–606. doi: 10.3109/10408444.2013.808172. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Wang Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications. 2nd Edition. World Scientific; New Jersey: 2011. [Google Scholar]
- Casado-Pascual J, Gomez-Ordonez J, Morillo M, Hanggi P. Subthreshold stochastic resonance: rectangular signals can cause anomalous large gains. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68:061104. doi: 10.1103/PhysRevE.68.061104. [DOI] [PubMed] [Google Scholar]
- Chang X, Vikesland PJ. Uncontrolled Variability in the Extinction Spectra of C60 Nanoparticle Suspensions. Langmuir. 2013;29:9685–9693. doi: 10.1021/la401583v. [DOI] [PubMed] [Google Scholar]
- Chaowanachan T, Krogstad E, Ball C, Woodrow KA. Drug Synergy of Tenofovir and Nanoparticle-Based Antiretrovirals for HIV Prophylaxis. PLoS One. 2013;8:e61416. doi: 10.1371/journal.pone.0061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Powell BA, Mortimer M, Ke PC. Adaptive Interactions between Zinc Oxide Nanoparticles and Chlorella sp. Environ Sci Technol. 2012;46:12178–12185. doi: 10.1021/es303303g. [DOI] [PubMed] [Google Scholar]
- Chikramane PS, Kalita D, Suresh AK, Kane SG, Bellare JR. Why Extreme Dilutions Reach Non-zero Asymptotes: A Nanoparticulate Hypothesis Based on Froth Flotation. Langmuir. 2012;28:15864–15875. doi: 10.1021/la303477s. [DOI] [PubMed] [Google Scholar]
- Cho GB, Choi SY, Noh JP, Jeon YM, Jung KT, Nam TH. Dependence of milling time on electrochemical properties of nano Si electrodes prepared by ball-milling. J Nanosci Nanotechnol. 2011;11:6262–6265. doi: 10.1166/jnn.2011.4332. [DOI] [PubMed] [Google Scholar]
- Chu SH, Feng DF, Ma YB, Li ZQ. Hydroxyapatite nanoparticles inhibit the growth of human glioma cells in vitro and in vivo. Int J Nanomedicine. 2012;7:3659–3666. doi: 10.2147/IJN.S33584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudnovsky EM, Friedman JR. Macroscopic Quantum Coherence in a Magnetic Nanoparticle Above the Surface of a Superconductor. Physical Review Letters. 2000;85:5206–5209. doi: 10.1103/PhysRevLett.85.5206. [DOI] [PubMed] [Google Scholar]
- Chun YS, Bisht S, Chenna V, Pramanik D, Yoshida T, Hong SM, de Wilde RF, Zhang Z, Huso DL, Zhao M, Rudek MA, Stearns V, Maitra A, Sukumar S. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 2012;33:2242–2249. doi: 10.1093/carcin/bgs248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RJ, Dang MK, Veinot JG. Exploration of organic acid chain length on water-soluble silicon quantum dot surfaces. Langmuir. 2010;26:15657–15664. doi: 10.1021/la102983c. [DOI] [PubMed] [Google Scholar]
- Clift MJ, Gehr P, Rothen-Rutishauser B. Nanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternative. Arch Toxicol. 2011;85:723–731. doi: 10.1007/s00204-010-0560-6. [DOI] [PubMed] [Google Scholar]
- Coffey DS. Self-organization, complexity, and chaos: the new biology for medicine. Nature Medicine. 1998;4:882–885. doi: 10.1038/nm0898-882. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Yang VC, David AE. Cancer theranostics: the rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011;29:323–332. doi: 10.1016/j.tibtech.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy to distinguish physiologic and synthetic RR time series. Comput Cardiol. 2002;29:137–140. [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Physical Review E. 2005;71:1–18. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89 doi: 10.1103/PhysRevLett.89.068102. 068102:068101-068104. [DOI] [PubMed] [Google Scholar]
- Costa M, Priplata AA, Lipsitz LA, Wu Z, Huang NE, Goldberger AL, Peng CK. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbo A, Lorber B, Corvini PF, Meier W, Shahgaldian P. A synthetic nanomaterial for virus recognition produced by surface imprinting. Nat Commun. 2013;4:1503. doi: 10.1038/ncomms2529. [DOI] [PubMed] [Google Scholar]
- Czaplicka A, Holyst JA, Sloot PM. Noise enhances information transfer in hierarchical networks. Sci Rep. 2013;3:1223. doi: 10.1038/srep01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine. 2012;7:1189–1202. doi: 10.2147/IJN.S26650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MA, Ingle A, Rai M. Enhanced antimicrobial activity of silver nanoparticles synthesized by Cryphonectria sp. evaluated singly and in combination with antibiotics. Nanomedicine. 2013;9:105–110. doi: 10.1016/j.nano.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Das S, Das J, Samadder A, Bhattacharyya S, Das D, Khuda-Bukhsh AR. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids and Surfaces B: Biointerfaces. 2013;101:325–336. doi: 10.1016/j.colsurfb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Davies PC. Does quantum mechanics play a non-trivial role in life? Biosystems. 2004;78:69–79. doi: 10.1016/j.biosystems.2004.07.001. [DOI] [PubMed] [Google Scholar]
- DeCastro CL, Mitchell BS. Nanoparticles from mechanical attrition. In: Baraton MI, editor. Synthesis, Functionalization, and Surface Treatment of Nanoparticles. American Scientific Publisher; Valencia, CA: 2002. pp. 1–15. [Google Scholar]
- Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirovic D, Rattan SI. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp Gerontol. 2013;48:94–98. doi: 10.1016/j.exger.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv. 2004;1:405–412. doi: 10.2174/1567201043334597. [DOI] [PubMed] [Google Scholar]
- Du L, Miao X, Jiang Y, Jia H, Tian Q, Shen J, Liu Y. An effective strategy for the synthesis of biocompatible gold nanoparticles using danshensu antioxidant: prevention of cytotoxicity via attenuation of free radical formation. Nanotoxicology. 2013;7:94–300. doi: 10.3109/17435390.2011.653415. [DOI] [PubMed] [Google Scholar]
- European Commission on the Environment Nanomaterials, Definition of nanomaterials. 2011. Available at http://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm.
- Farkas IJ, Korcsmaros T, Kovacs IA, Mihalik A, Palotai R, Simko GI, Szalay KZ, Szalay-Beko M, Vellai T, Wang S, Csermely P. Network-based tools for the identification of novel drug targets. Sci Signal. 2011;4:pt3. doi: 10.1126/scisignal.2001950. [DOI] [PubMed] [Google Scholar]
- Farmen E, Mikkelsen HN, Evensen O, Einset J, Heier LS, Rosseland BO, Salbu B, Tollefsen KE, Oughton DH. Acute and sub-lethal effects in juvenile Atlantic salmon exposed to low mug/L concentrations of Ag nanoparticles. Aquat Toxicol. 2012;108:78–84. doi: 10.1016/j.aquatox.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Foldbjerg R, Irving ES, Hayashi Y, Sutherland D, Thorsen K, Autrup H, Beer C. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs225. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Losada MF. Positive affect and the complex dynamics of human flourishing. American Psychologist. 2005;60:678–686. doi: 10.1037/0003-066X.60.7.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel A, Spano ML, Ditto WL, Weiss JN. Controlling cardiac chaos. Science. 1992;257:1230–1235. doi: 10.1126/science.1519060. [DOI] [PubMed] [Google Scholar]
- Gautam S, Dubey P, Gupta MN. A facile and green ultrasonic-assisted synthesis of BSA conjugated silver nanoparticles. Colloids Surf B Biointerfaces. 2013;102:879–883. doi: 10.1016/j.colsurfb.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Geinguenaud F, Souissi I, Fagard R, Motte L, Lalatonne Y. Electrostatic assembly of a DNA superparamagnetic nano-tool for simultaneous intracellular delivery and in situ monitoring. Nanomedicine. 2012;8:1106–1115. doi: 10.1016/j.nano.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Choudhury ST, Ghosh S, Mandal AK, Sarkar S, Ghosh A, Saha KD, Das N. Nanocapsulated curcumin: oral chemopreventive formulation against diethylnitrosamine induced hepatocellular carcinoma in rat. Chem Biol Interact. 2012;195:206–214. doi: 10.1016/j.cbi.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Goldberger AL. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet. 1996;347:1312–1314. doi: 10.1016/s0140-6736(96)90948-4. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–26. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Gualtieri M, Skuland T, Iversen TG, Lag M, Schwarze P, Bilanicova D, Pojana G, Refsnes M. Importance of agglomeration state and exposure conditions for uptake and pro-inflammatory responses to amorphous silica nanoparticles in bronchial epithelial cells. Nanotoxicology. 2012;6:700–712. doi: 10.3109/17435390.2011.604441. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wiggers H. Freestanding silicon quantum dots: origin of red and blue luminescence. Nanotechnology. 2011;22:055707. doi: 10.1088/0957-4484/22/5/055707. [DOI] [PubMed] [Google Scholar]
- Hale HB. Cross-adaptation. Environmental Research. 1969;2:423–434. doi: 10.1016/0013-9351(69)90013-9. [DOI] [PubMed] [Google Scholar]
- Hannah DC, Yang J, Podsiadlo P, Chan MK, Demortiere A, Gosztola DJ, Prakapenka VB, Schatz GC, Kortshagen U, Schaller RD. On the origin of photoluminescence in silicon nanocrystals: pressure-dependent structural and optical studies. Nano Lett. 2012;12:4200–4205. doi: 10.1021/nl301787g. [DOI] [PubMed] [Google Scholar]
- Harhaji L, Isakovic A, Raicevic N, Markovic Z, Todorovic-Markovic B, Nikolic N, Vranjes-Djuric S, Markovic I, Trajkovic V. Multiple mechanisms underlying the anticancer action of nanocrystalline fullerene. Eur J Pharmacol. 2007;568:89–98. doi: 10.1016/j.ejphar.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Hassan TA, Rangari VK, Rana RK, Jeelani S. Sonochemical effect on size reduction of CaCO3 nanoparticles derived from waste eggshells. Ultrason Sonochem. 2013;20:1308–1315. doi: 10.1016/j.ultsonch.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Hatef A, Sadeghi SM, Singh MR. Coherent molecular resonances in quantum dot-metallic nanoparticle systems: coherent self-renormalization and structural effects. Nanotechnology. 2012;23:205203. doi: 10.1088/0957-4484/23/20/205203. [DOI] [PubMed] [Google Scholar]
- Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Acc Chem Res. 2011;44:875–882. doi: 10.1021/ar200090c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YP, Leong KW. Quantum dot-based theranostics. Nanoscale. 2010;2:60–68. doi: 10.1039/b9nr00178f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein T. State space grids: analyzing dynamics across development. International Journal of Behavioral Development. 2007;31:384–396. [Google Scholar]
- Hossu M, Ma L, Chen W. Nonlinear enhancement of spontaneous biophoton emission of sweet potato by silver nanoparticles. J Photochem Photobiol B. 2010;99:44–48. doi: 10.1016/j.jphotobiol.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Huang T, Browning LM, Xu XH. Far-field photostable optical nanoscopy (PHOTON) for real-time super-resolution single-molecular imaging of signaling pathways of single live cells. Nanoscale. 2012;4:2797–2812. doi: 10.1039/c2nr11739h. [DOI] [PubMed] [Google Scholar]
- Huang X, Kang B, Qian W, Mackey MA, Chen PC, Oyelere AK, El-Sayed IH, El-Sayed MA. Comparative study of photothermolysis of cancer cells with nuclear-targeted or cytoplasm-targeted gold nanospheres: continuous wave or pulsed lasers. J Biomed Opt. 2010;15:058002. doi: 10.1117/1.3486538. [DOI] [PubMed] [Google Scholar]
- Hudecova A, Kusznierewicz B, Runden-Pran E, Magdolenova Z, Hasplova K, Rinna A, Fjellsbo LM, Kruszewski M, Lankoff A, Sandberg WJ, Refsnes M, Skuland T, Schwarze P, Brunborg G, Bjoras M, Collins A, Miadokova E, Galova E, Dusinska M. Silver nanoparticles induce pre-mutagenic DNA oxidation that can be prevented by phytochemicals from Gentiana asclepiadea. Mutagenesis. 2012;27:759–769. doi: 10.1093/mutage/ges046. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Calabrese EJ, Nascarella MA. Exposure to nanoparticles and hormesis. Dose Response. 2010;8:501–517. doi: 10.2203/dose-response.10-016.Iavicoli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki A, Tadokoro Y. Relation between optimal nonlinearity and non-Gaussian noise: enhancing a weak signal in a nonlinear system. Phys Rev E Stat Nonlin Soft Matter Phys. 2013;87:012124. doi: 10.1103/PhysRevE.87.012124. [DOI] [PubMed] [Google Scholar]
- Im YB, Wahab R, Ameen S, Kim YS, Yang OB, Shin HS. Synthesis and characterization of high-purity silica nanosphere from rice husk. J Nanosci Nanotechnol. 2011;11:5934–5938. doi: 10.1166/jnn.2011.4386. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization ISO/TC 229 Nanotechnologies, Standardization in the field of nanotechnologies. 2005. Available at http://www.iso.org/iso/iso_tech-nical_committee?commid=381983.
- Isoda K, Hasezaki T, Kondoh M, Tsutsumi Y, Yagi K. Effect of surface charge on nano-sized silica particles-induced liver injury. Pharmazie. 2011;66:278–281. [PubMed] [Google Scholar]
- Ives JA, Moffett JR, Arun P, Lam D, Todorov TI, Brothers AB, Anick DJ, Centeno J, Namboodiri MA, Jonas WB. Enzyme stabilization by glass-derived silicates in glass-exposed aqueous solutions. Homeopathy. 2010;99:15–24. doi: 10.1016/j.homp.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Jena P, Mohanty S, Mallick R, Jacob B, Sonawane A. Toxicity and antibacterial assessment of chitosancoated silver nanoparticles on human pathogens and macrophage cells. Int J Nanomedicine. 2012;7:1805–1818. doi: 10.2147/IJN.S28077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- Jonasson S, Gustafsson A, Koch B, Bucht A. Inhalation exposure of nano-scaled titanium dioxide (TiO2) particles alters the inflammatory responses in asthmatic mice. Inhal Toxicol. 2013;25:179–191. doi: 10.3109/08958378.2013.770939. [DOI] [PubMed] [Google Scholar]
- Joshi MD, Muller RH. Lipid nanoparticles for parenteral delivery of actives. Eur J Pharm Biopharm. 2009;71:161–172. doi: 10.1016/j.ejpb.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ju-Nam Y, Lead JR. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. 2008;400:396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kang Z, Liu Y, Lee ST. Small-sized silicon nanoparticles: new nanolights and nanocatalysts. Nanoscale. 2011;3:777–791. doi: 10.1039/c0nr00559b. [DOI] [PubMed] [Google Scholar]
- Kaur J, Tikoo K. Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles. Food Chem Toxicol. 2012;51C:1–14. doi: 10.1016/j.fct.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Kelty-Stephen DG, Dixon JA. Temporal correlations in postural sway moderate effects of stochastic resonance on postural stability. Hum Mov Sci. 2013;32:91–105. doi: 10.1016/j.humov.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Kiel S, Grinberg O, Perkas N, Charmet J, Kepner H, Gedanken A. Forming nanoparticles of water-soluble ionic molecules and embedding them into polymer and glass substrates. Beilstein J Nanotechnol. 2012;3:267–276. doi: 10.3762/bjnano.3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100:1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- Kleps I, Ignat T, Miu M, Craciunoiu F, Trif M, Simion M, Bragaru A, Dinescu A. Nanostructured silicon particles for medical applications. J Nanosci Nanotechnol. 2010;10:2694–2700. doi: 10.1166/jnn.2010.1419. [DOI] [PubMed] [Google Scholar]
- Koning GA, Krijger GC. Targeted multifunctional lipid-based nanocarriers for image-guided drug delivery. Anticancer Agents Med Chem. 2007;7:425–440. doi: 10.2174/187152007781058613. [DOI] [PubMed] [Google Scholar]
- Korn H, Faure P. Is there chaos in the brain? II. Experimental evidence and related models. [Review] [343 refs] Comptes Rendus Biologies. 2003;326:787–840. doi: 10.1016/j.crvi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Krawiecki A, Sukiennicki A, Kosinski RA. Stochastic resonance and noise-enhanced order with spatiotemporal periodic signal. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;62:7683–7689. doi: 10.1103/physreve.62.7683. [DOI] [PubMed] [Google Scholar]
- Kumar V, Kumari A, Guleria P, Yadav SK. Evaluating the toxicity of selected types of nanochemicals. Rev Environ Contam Toxicol. 2012;215:39–121. doi: 10.1007/978-1-4614-1463-6_2. [DOI] [PubMed] [Google Scholar]
- Lagisz M, Hector KL, Nakagawa S. Life extension after heat shock exposure: Assessing meta-analytic evidence for hormesis. Ageing Res Rev. 2013;12:653–60. doi: 10.1016/j.arr.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Lanao JM, Briones E, Colino CI. Recent advances in delivery systems for anti-HIV1 therapy. J Drug Target. 2007;15:21–36. doi: 10.1080/10611860600942178. [DOI] [PubMed] [Google Scholar]
- Lankoff A, Arabski M, Wegierek-Ciuk A, Kruszewski M, Lisowska H, Banasik-Nowak A, Rozga-Wijas K, Wojewodzka M, Slomkowski S. Effect of surface modification of silica nanoparticles on toxicity and cellular uptake by human peripheral blood lymphocytes in vitro. Nanotoxicology. 2013;7:235–50. doi: 10.3109/17435390.2011.649796. [DOI] [PubMed] [Google Scholar]
- Launay JC, Besnard Y, Guinet-Lebreton A, Savourey G. Acclimation to intermittent hypobaric hypoxia modifies responses to cold at sea level. Aviat Space Environ Med. 2006;77:1230–1235. [PubMed] [Google Scholar]
- Lee CW, Yen FL, Huang HW, Wu TH, Ko HH, Tzeng WS, Lin CC. Resveratrol Nanoparticle System Improves Dissolution Properties and Enhances the Hepatoprotective Effect of Resveratrol through Antioxidant and Anti-Inflammatory Pathways. J Agric Food Chem. 2012a;60:4662–4671. doi: 10.1021/jf2050137. [DOI] [PubMed] [Google Scholar]
- Lee CY, Choi W, Han JH, Strano MS. Coherence resonance in a single-walled carbon nanotube ion channel. Science. 2010;329:1320–1324. doi: 10.1126/science.1193383. [DOI] [PubMed] [Google Scholar]
- Lee I, Liu X, Chongwu Z, Kosko B. Noise-Enhanced Detection of Subthreshold Signals With Carbon Nanotubes. Nanotechnology, IEEE Transactions on. 2006;5:613–627. [Google Scholar]
- Lee KJ, Browning LM, Nallathamby PD, Desai T, Cherukuri PK, Xu XH. In vivo quantitative study of sized-dependent transport and toxicity of single silver nanoparticles using zebrafish embryos. Chem Res Toxicol. 2012b;25:1029–1046. doi: 10.1021/tx300021u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenaghan SC, Burris JN, Chourey K, Huang Y, Xia L, Lady B, Sharma R, Pan C, Lejeune Z, Foister S, Hettich RL, Stewart CN, Jr, Zhang M. Isolation and chemical analysis of nanoparticles from English ivy (Hedera helix L.) J R Soc Interface. 2013;10:20130392. doi: 10.1098/rsif.2013.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DH, Jang J, Kim S, Kang T, Lee K, Choi IH. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials. 2012;33:4690–4699. doi: 10.1016/j.biomaterials.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Lindsay SM. Introduction to Nanoscience. Oxford: 2010. [Google Scholar]
- Liu D, Wu Q, Chen H, Chang PR. Transitional properties of starch colloid with particle size reduction from micro- to nanometer. J Colloid Interface Sci. 2009;339:117–124. doi: 10.1016/j.jcis.2009.07.035. [DOI] [PubMed] [Google Scholar]
- Liu L, Randolph TW, Carpenter JF. Particles shed from syringe filters and their effects on agitation-induced protein aggregation. J Pharm Sci. 2012;101:2952–2959. doi: 10.1002/jps.23225. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kathan K, Saad W, Prudhomme RK. Ostwald ripening of B-carotene nanoparticles. Physical Review Letters. 2007;98:1–4. doi: 10.1103/PhysRevLett.98.036102. [DOI] [PubMed] [Google Scholar]
- Lloyd S. Quantum coherence in biological systems. Journal of Physics: Conference Series International Symposium “Nanoscience and Quantum Physics 2011”. 2011:012037. [Google Scholar]
- Losada M. The complex dynamics of high performance teams. Mathematical Computer Modelling. 1999;30:179–192. [Google Scholar]
- Losada M, Heaphy E. The role of positivity and connectivity in the performance of business teams: a nonlinear dynamics model. American Behavioral Scientist. 2004;47:740–765. [Google Scholar]
- Lu X, Tian Y, Zhao Q, Jin T, Xiao S, Fan X. Integrated metabonomics analysis of the size-response relationship of silica nanoparticles-induced toxicity in mice. Nanotechnology. 2011;22:055101. doi: 10.1088/0957-4484/22/5/055101. [DOI] [PubMed] [Google Scholar]
- Lunt HC, Barwood MJ, Corbett J, Tipton MJ. ‘Cross-adaptation’: habituation to short repeated cold-water immersions affects the response to acute hypoxia in humans. J Physiol. 2010;588:3605–3613. doi: 10.1113/jphysiol.2010.193458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JM, Jain PK, Ewers T, Alivisatos AP. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nat Mater. 2011;10:361–366. doi: 10.1038/nmat3004. [DOI] [PubMed] [Google Scholar]
- Magalhaes FH, Kohn AF. Vibratory noise to the fingertip enhances balance improvement associated with light touch. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011;209:139–151. doi: 10.1007/s00221-010-2529-3. [DOI] [PubMed] [Google Scholar]
- Mahony D, Cavallaro AS, Stahr F, Mahony TJ, Qiao SZ, Mitter N. Mesoporous Silica Nanoparticles Act as a Self-Adjuvant for Ovalbumin Model Antigen in Mice. Small. 2013;9:3138–46. doi: 10.1002/smll.201300012. [DOI] [PubMed] [Google Scholar]
- Malarczyk E, Pazdzioch-Czochra M, Graz M, Kochmanska-Rdest J, Jarosz-Wilkolazka A. Nonlinear changes in the activity of the oxygen-dependent demethylase system in Rhodococcus erythropolis cells in the presence of low and very low doses of formaldehyde. Nonlinear Biomed Phys. 2011;5:9. doi: 10.1186/1753-4631-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MD, Abbott D. What Is Stochastic Resonance? Definitions, Misconceptions, Debates, and Its Relevance to Biology. PLoS Comput Biol. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness LP, Yan Y, Stacey A, Simpson DA, Hall LT, Maclaurin D, Prawer S, Mulvaney P, Wrachtrup J, Caruso F, Scholten RE, Hollenberg LC. Quantum measurement and orientation tracking of fluorescent nanodiamonds inside living cells. Nat Nanotechnol. 2011;6:358–363. doi: 10.1038/nnano.2011.64. [DOI] [PubMed] [Google Scholar]
- McKibbin SR, Scappucci G, Pok W, Simmons MY. Epitaxial top-gated atomic-scale silicon wire in a three-dimensional architecture. Nanotechnology. 2013;24:045303. doi: 10.1088/0957-4484/24/4/045303. [DOI] [PubMed] [Google Scholar]
- Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc Chem Res. 2011;44:947–956. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63:427–440. doi: 10.1016/j.addr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Mihalik A, Csermely P. Heat shock partially dissociates the overlapping modules of the yeast protein-protein interaction network: a systems level model of adaptation. PLoS Comput Biol. 2011;7:e1002187. doi: 10.1371/journal.pcbi.1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milisav I, Poljsak B, Suput D. Adaptive response, evidence of cross-resistance and its potential clinical use. Int J Mol Sci. 2012;13:10771–10806. doi: 10.3390/ijms130910771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–56. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clin Neurophysiol. 2004;115:267–281. doi: 10.1016/j.clinph.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Moulari B, Beduneau A, Pellequer Y, Lamprecht A. Nanoparticle targeting to inflamed tissues of the gastrointestinal tract. Curr Drug Deliv. 2013;10:9–17. doi: 10.2174/1567201811310010004. [DOI] [PubMed] [Google Scholar]
- Mudunkotuwa IA, Grassian VH. The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit. 2011;13:1135–1144. doi: 10.1039/c1em00002k. [DOI] [PubMed] [Google Scholar]
- Murdock RC, Braydich-Stolle L, Schrand AM, Schlager JJ, Hussain SM. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 2008;101:239–253. doi: 10.1093/toxsci/kfm240. [DOI] [PubMed] [Google Scholar]
- Nascarella MA, Calabrese EJ. A method to evaluate hormesis in nanoparticle dose-responses. Dose Response. 2012;10:344–354. doi: 10.2203/dose-response.10-025.Nascarella. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning XH, Chen SH. Mild hypothermic cross adaptation resists hypoxic injury in hearts: a brief review. Chin J Physiol. 2006;49:213–222. [PubMed] [Google Scholar]
- Osborne OJ, Johnston BD, Moger J, Balousha M, Lead JR, Kudoh T, Tyler CR. Effects of particle size and coating on nanoscale Ag and TiO(2) exposure in zebrafish (Danio rerio) embryos. Nanotoxicology. 2012;7:1315–1324. doi: 10.3109/17435390.2012.737484. [DOI] [PubMed] [Google Scholar]
- Oyewumi MO, Kumar A, Cui Z. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Thakur M, Shah R, Oza G, Mewada A, Sharon M. A comparative study of economical separation and aggregation properties of biologically capped and thiol functionalized gold nanoparticles: Selecting the eco-friendly trojan horses for biological applications. Colloids Surf B Biointerfaces. 2013;109:25–31. doi: 10.1016/j.colsurfb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Park Y, Noh HJ, Han L, Kim HS, Kim YJ, Choi JS, Kim CK, Kim YS, Cho S. Artemisia capillaris extracts as a green factory for the synthesis of silver nanoparticles with antibacterial activities. J Nanosci Nanotechnol. 2012;12:7087–7095. doi: 10.1166/jnn.2012.6575. [DOI] [PubMed] [Google Scholar]
- Petkar KC, Chavhan SS, Agatonovik-Kustrin S, Sawant KK. Nanostructured materials in drug and gene delivery: a review of the state of the art. Crit Rev Ther Drug Carrier Syst. 2011;28:101–164. doi: 10.1615/critrevtherdrugcarriersyst.v28.i2.10. [DOI] [PubMed] [Google Scholar]
- Pham KN, Fullston D, Sagoe-Crentsil K. Surface modification for stability of nano-sized silica colloids. J Colloid Interface Sci. 2007;315:123–127. doi: 10.1016/j.jcis.2007.06.064. [DOI] [PubMed] [Google Scholar]
- Pickering AM, Vojtovich L, Tower J, KJ AD. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic Biol Med. 2013;55:109–118. doi: 10.1016/j.freeradbiomed.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinamonti G, Marro J, Torres JJ. Stochastic resonance crossovers in complex networks. PLoS One. 2012;7:e51170. doi: 10.1371/journal.pone.0051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus D, Metten A. Nonlinear dynamics in biopsychosocial resilience. Nonlinear Dynamics Psychol Life Sci. 2010;14:353–380. [PubMed] [Google Scholar]
- Prakash DJ, Arulkumar S, Sabesan M. Effect of nanohypericum (Hypericum perforatum gold nanoparticles) treatment on restraint stress induced behavioral and biochemical alteration in male albino mice. Pharmacognosy Res. 2010;2:330–334. doi: 10.4103/0974-8490.75450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja WK, Satti J, Liu G, Castracane J. Dose Response of MTLn3 Cells to Serial Dilutions of Arsenic Trioxide and Ionizing Radiation. Dose Response. 2013;11:29–40. doi: 10.2203/dose-response.11-025.Raja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KS, El-Hami K, Kodaki T, Matsushige K, Makino K. A novel method for synthesis of silica nanoparticles. J Colloid Interface Sci. 2005;289:125–131. doi: 10.1016/j.jcis.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Richert LE, Servid AE, Harmsen AL, Rynda-Apple A, Han S, Wiley JA, Douglas T, Harmsen AG. A virus-like particle vaccine platform elicits heightened and hastened local lung mucosal antibody production after a single dose. Vaccine. 2012;30:3653–3665. doi: 10.1016/j.vaccine.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- Rodrigues MT, Ajayan PM, Silva GG. Fast vortex-assisted self-assembly of carbon nanoparticles on an air-water interface. J Phys Chem B. 2013;117:6524–6533. doi: 10.1021/jp4014114. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Snoek LB, Riksen JA, Bevers RP, Kammenga JE. Genetic variation for stress-response hormesis in C. elegans lifespan. Exp Gerontol. 2012;47:581–587. doi: 10.1016/j.exger.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Roduner E. Size matters: why nanomaterials are different. Chemical Society Reviews. 2006;35:583–592. doi: 10.1039/b502142c. [DOI] [PubMed] [Google Scholar]
- Rowe DJ, Jeong JS, Mkhoyan KA, Kortshagen UR. Phosphorus-doped silicon nanocrystals exhibiting mid-infrared localized surface plasmon resonance. Nano Lett. 2013;13:1317–1322. doi: 10.1021/nl4001184. [DOI] [PubMed] [Google Scholar]
- Ruan B, Jacobi M. Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Research Letters. 2012;7:127. doi: 10.1186/1556-276X-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavati-Niasari M, Javidi J, Dadkhah M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb Chem High Throughput Screen. 2012;16:458–62. doi: 10.2174/1386207311316060006. [DOI] [PubMed] [Google Scholar]
- Sandberg WJ, Lag M, Holme JA, Friede B, Gualtieri M, Kruszewski M, Schwarze PE, Skuland T, Refsnes M. Comparison of non-crystalline silica nanoparticles in IL-1beta release from macrophages. Part Fibre Toxicol. 2012;9:32. doi: 10.1186/1743-8977-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed D, Al-Sadoon MK, Badr G. Silica Nanoparticles Sensitize Human Multiple Myeloma Cells to Snake (Walterinnesia aegyptia) Venom-Induced Apoptosis and Growth Arrest. Oxidative medicine and cellular longevity. 2012;2012:386286. doi: 10.1155/2012/386286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Jerger K, Duong DH, Chang T, Spano ML, Ditto WL. Controlling chaos in the brain. Nature. 1994;370:615–620. doi: 10.1038/370615a0. [DOI] [PubMed] [Google Scholar]
- Shalchian M, Grisolia J, Assayag GB, Coffin H, Atarodi SM, Claverie A. Room-temperature quantum effect in silicon nanoparticles obtained by low-energy ion implantation and embedded in a nanometer scale capacitor. Applied Physics Letters. 2005;86:163111–163113. [Google Scholar]
- Shannahan JH, Kodavanti UP, Brown JM. Manufactured and airborne nanoparticle cardiopulmonary interactions: a review of mechanisms and the possible contribution of mast cells. Inhal Toxicol. 2012;24:320–339. doi: 10.3109/08958378.2012.668229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang JH, Jiang M, Zhu LH, Tan HQ, Lu B. Synergistic genotoxicity caused by low concentration of titanium dioxide nanoparticles and p,p’-DDT in human hepatocytes. Environ Mol Mutagen. 2010a;51:192–204. doi: 10.1002/em.20527. [DOI] [PubMed] [Google Scholar]
- Shi Z, Huang X, Liu B, Tao H, Cai Y, Tang R. Biological response of osteosarcoma cells to size-controlled nanostructured hydroxyapatite. J Biomater Appl. 2010b;25:19–37. doi: 10.1177/0885328209339396. [DOI] [PubMed] [Google Scholar]
- Shirali AC, Look M, Du W, Kassis E, Stout-Delgado HW, Fahmy TM, Goldstein DR. Nanoparticle delivery of mycophenolic acid upregulates PD-L1 on dendritic cells to prolong murine allograft survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:2582–2592. doi: 10.1111/j.1600-6143.2011.03725.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi NJ, Abdelhalim MA, El-Ansary AK, Alhomida AS, Ong WY. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J Neuroinflammation. 2012;9:123. doi: 10.1186/1742-2094-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Jagannathan R, Khandelwal P, Abraham PM, Poddar P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory investigation. Nanoscale. 2013;5:1882–1893. doi: 10.1039/c2nr33776b. [DOI] [PubMed] [Google Scholar]
- Snitka V, Naumenko DO, Ramanauskaite L, Kravchenko SA, Snopok BA. Generation of diversiform gold nanostructures inspired by honey’s components: Growth mechanism, characterization, and shape separation by the centrifugation-assisted sedimentation. J Colloid Interface Sci. 2012;386:99–106. doi: 10.1016/j.jcis.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Song L, Yang K, Jiang W, Du P, Xing B. Adsorption of bovine serum albumin on nano and bulk oxide particles in deionized water. Colloids Surf B Biointerfaces. 2012;94:341–346. doi: 10.1016/j.colsurfb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Tschirgi ML, Swindell S, Chen L, Fang J. Repeated formaldehyde effects in an animal model for multiple chemical sensitivity. Annals of the New York Academy of Sciences. 2001;933:57–67. doi: 10.1111/j.1749-6632.2001.tb05814.x. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Willis JR, See RE, Hopkins B, Westberg HH. Repeated low-level formaldehyde exposure produces cross-sensitization to cocaine: possible relevance to chemical sensitivity in humans. Neuropsychopharmacology. 1998;18:385–394. doi: 10.1016/S0893-133X(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Aging and molecular chaperones. Exp Gerontol. 2003;38:1037–1040. doi: 10.1016/s0531-5565(03)00185-2. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Pharmacological modulation of the heat shock response. Handb Exp Pharmacol. 2006:417–436. doi: 10.1007/3-540-29717-0_17. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Aging cellular networks: chaperones as major participants. Exp Gerontol. 2007;42:113–119. doi: 10.1016/j.exger.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Soumya RS, Ghosh S, Abraham ET. Preparation and characterization of guar gum nanoparticles. Int J Biol Macromol. 2010;46:267–269. doi: 10.1016/j.ijbiomac.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Stark M. The sandpile model: optimal stress and hormesis. Dose Response. 2012;10:66–74. doi: 10.2203/dose-response.11-010.Stark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark WJ. Nanoparticles in biological systems. Angew Chem Int Ed Engl. 2011;50:1242–1258. doi: 10.1002/anie.200906684. [DOI] [PubMed] [Google Scholar]
- Stovbun SV, Kiselev AV, Zanin AM, Kalinina TS, Voronina TA, Mikhailov AI, Berlin AA. Effects of physicochemical forms of phenazepam and Panavir on their action at ultra-low doses. Bull Exp Biol Med. 2012;153:455–458. doi: 10.1007/s10517-012-1739-z. [DOI] [PubMed] [Google Scholar]
- Sugarman J, Tsai S, Santamaria P, Khadra A. Quantifying the importance of pMHC valency, total pMHC dose and frequency on nanoparticle therapeutic efficacy. Immunol Cell Biol. 2013;91:350–359. doi: 10.1038/icb.2013.9. [DOI] [PubMed] [Google Scholar]
- Sun H, Chen X, Chen D, Dong M, Fu X, Li Q, Liu X, Wu Q, Qiu T, Wan T, Li S. Influences of surface coatings and components of FePt nanoparticles on the suppression of glioma cell proliferation. Int J Nanomedicine. 2012;7:3295–3307. doi: 10.2147/IJN.S32678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur I, Altunbek M, Kahraman M, Culha M. The influence of the surface chemistry of silver nanoparticles on cell death. Nanotechnology. 2012;23:375102. doi: 10.1088/0957-4484/23/37/375102. [DOI] [PubMed] [Google Scholar]
- Sur I, Cam D, Kahraman M, Baysal A, Culha M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology. 2010;21:175104. doi: 10.1088/0957-4484/21/17/175104. [DOI] [PubMed] [Google Scholar]
- Suriyakalaa U, Antony JJ, Suganya S, Siva D, Sukirtha R, Kamalakkannan S, Pichiah PB, Achiraman S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf B Biointerfaces. 2012;102C:189–194. doi: 10.1016/j.colsurfb.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Szalay MS, Kovacs IA, Korcsmaros T, Bode C, Csermely P. Stress-induced rearrangements of cellular networks: Consequences for protection and drug design. FEBS Lett. 2007;581:3675–3680. doi: 10.1016/j.febslet.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Tang C, Zhou T, Yang J, Zhang Q, Chen F, Fu Q, Yang L. Wet-grinding assisted ultrasonic dispersion of pristine multi-walled carbon nanotubes (MWCNTs) in chitosan solution. Colloids Surf B Biointerfaces. 2011;86:189–197. doi: 10.1016/j.colsurfb.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Tang M, Zhang T, Xue Y, Wang S, Huang M, Yang Y, Lu M, Lei H, Kong L, Yuepu P. Dose dependent in vivo metabolic characteristics of titanium dioxide nanoparticles. J Nanosci Nanotechnol. 2010;10:8575–8583. doi: 10.1166/jnn.2010.2482. [DOI] [PubMed] [Google Scholar]
- Tantra R, Tompkins J, Quincey P. Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension. Colloids Surf B Biointerfaces. 2010;75:275–281. doi: 10.1016/j.colsurfb.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Thurber A, Wingett DG, Rasmussen JW, Layne J, Johnson L, Tenne DA, Zhang J, Hanna CB, Punnoose A. Improving the selective cancer killing ability of ZnO nanoparticles using Fe doping. Nanotoxicology. 2012;6:440–452. doi: 10.3109/17435390.2011.587031. [DOI] [PubMed] [Google Scholar]
- Torres JL, Ruiz MAG. Stochastic resonance and the homeopathic effect. British Homoeopathic Journal. 1996;85(3):134–140. [Google Scholar]
- Tournebize J, Boudier A, Joubert O, Eidi H, Bartosz G, Maincent P, Leroy P, Sapin-Minet A. Impact of gold nanoparticle coating on redox homeostasis. Int J Pharm. 2012;438:107–116. doi: 10.1016/j.ijpharm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Chandrasekaran N, Raichur AM, Mukherjee A. Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. J Biomed Nanotechnol. 2009;5:93–98. doi: 10.1166/jbn.2009.038. [DOI] [PubMed] [Google Scholar]
- Troia A, Giovannozzi A, Amato G. Preparation of tunable silicon q-dots through ultrasound. Ultrason Sonochem. 2009;16:448–451. doi: 10.1016/j.ultsonch.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Truong L, Tilton SC, Zaikova T, Richman E, Waters KM, Hutchison JE, Tanguay RL. Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology. 2013;7:192–201. doi: 10.3109/17435390.2011.648225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umashankari J, Inbakandan D, Ajithkumar TT, Balasubramanian T. Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquatic biosystems. 2012;8:11. doi: 10.1186/2046-9063-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman AM. Hormesis, adaptive epigenetic reorganization, and implications for human health and longevity. Dose Response. 2010;8:16–21. doi: 10.2203/dose-response.09-014.Vaiserman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman AM. Hormesis and epigenetics: is there a link? Ageing Res Rev. 2011;10:413–421. doi: 10.1016/j.arr.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Van Hoecke K, De Schamphelaere KA, Ramirez-Garcia S, Van der Meeren P, Smagghe G, Janssen CR. Influence of alumina coating on characteristics and effects of SiO2 nanoparticles in algal growth inhibition assays at various pH and organic matter contents. Environ Int. 2011;37:1118–1125. doi: 10.1016/j.envint.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Van Hoecke K, De Schamphelaere KA, Van der Meeren P, Lucas S, Janssen CR. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area. Environ Toxicol Chem. 2008;27:1948–1957. doi: 10.1897/07-634.1. [DOI] [PubMed] [Google Scholar]
- Van Wijk R, Wiegant FA. Postconditioning hormesis and the homeopathic Similia principle: molecular aspects. Hum Exp Toxicol. 2010;29:561–565. doi: 10.1177/0960327110369860. [DOI] [PubMed] [Google Scholar]
- Van Wijk R, Wiegant FA. Postconditioning hormesis and the similia principle. Front Biosci (Elite Ed) 2011;3:1128–1138. doi: 10.2741/e316. [DOI] [PubMed] [Google Scholar]
- Vesterdal LK, Folkmann JK, Jacobsen NR, Sheykhzade M, Wallin H, Loft S, Moller P. Pulmonary exposure to carbon black nanoparticles and vascular effects. Part Fibre Toxicol. 2010;7:33. doi: 10.1186/1743-8977-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan K, Nalini SP. Biotemplates in the green synthesis of silver nanoparticles. Biotechnol J. 2010;5:1098–1110. doi: 10.1002/biot.201000167. [DOI] [PubMed] [Google Scholar]
- Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]
- Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol Res. 2010;62:90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bao Y, Ahire J, Chao Y. Co-encapsulation of Biodegradable Nanoparticles with Silicon Quantum Dots and Quercetin for Monitored Delivery. Advanced healthcare materials. 2012a;2:459–66. doi: 10.1002/adhm.201200178. [DOI] [PubMed] [Google Scholar]
- Wang T, Jiang H, Zhao Q, Wang S, Zou M, Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int J Pharm. 2012b;436:351–358. doi: 10.1016/j.ijpharm.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Wang W, Luo M, Fu Y, Wang S, Efferth T, Zu Y. Glycyrrhizic acid nanoparticles inhibit LPS-induced inflammatory mediators in 264.7 mouse macrophages compared with unprocessed glycyrrhizic acid. Int J Nanomedicine. 2013;8:1377–1383. doi: 10.2147/IJN.S37788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen L. Quantum dots, lighting up the research and development of nanomedicine. Nanomedicine. 2011;7:385–402. doi: 10.1016/j.nano.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Wiegant FA, Prins HA, Van Wijk R. Postconditioning hormesis put in perspective: an overview of experimental and clinical studies. Dose Response. 2011;9:209–224. doi: 10.2203/dose-response.10-004.Wiegant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegant FA, Spieker N, van Wijk R. Stressor-specific enhancement of hsp induction by low doses of stressors in conditions of self- and cross-sensitization. Toxicology. 1998;127:107–119. doi: 10.1016/s0300-483x(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Winnik FM, Maysinger D. Quantum Dot Cytotoxicity and Ways To Reduce It. Acc Chem Res. 2013;46:672–680. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- Wong TW. Oral fast-release solid dispersion-paradigm shift to nanoparticles. Recent Pat Drug Deliv Formul. 2011;5:227–243. doi: 10.2174/187221111797200542. [DOI] [PubMed] [Google Scholar]
- Xia Y, Xiong Y, Lim B, Skrabalak SE. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed Engl. 2009;48:60–103. doi: 10.1002/anie.200802248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang AL, Yang RQ, Yuan GJ, Shi YL. Investigation of the enhancement fluorescence of ethanol doped SiO2 nanoparticles. J Nanosci Nanotechnol. 2011;11:9717–9720. doi: 10.1166/jnn.2011.5299. [DOI] [PubMed] [Google Scholar]
- Yao P, Hughes S. Macroscopic entanglement and violation of Bell’s inequalities between two spatially separated quantum dots in a planar photonic crystal system. Opt Express. 2009;17:11505–11514. doi: 10.1364/oe.17.011505. [DOI] [PubMed] [Google Scholar]
- Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Nanoparticles formulation of Cuscuta chinensis prevents acetaminophen-induced hepatotoxicity in rats. Food Chem Toxicol. 2008;46:1771–1777. doi: 10.1016/j.fct.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44:863–874. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- Yoo JW, Yun DS, Kim HJ. Influence of reaction parameters on size and shape of silica nanoparticles. J Nanosci Nanotechnol. 2006;6:3343–3346. doi: 10.1166/jnn.2006.006. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang J, Du J, Deng B, Wei X, Liu C. Effects of time delay on the stochastic resonance in small-world neuronal networks. Chaos. 2013;23:013128. doi: 10.1063/1.4790829. [DOI] [PubMed] [Google Scholar]
- Zhang H, He X, Zhang Z, Zhang P, Li Y, Ma Y, Kuang Y, Zhao Y, Chai Z. Nano-CeO2 exhibits adverse effects at environmental relevant concentrations. Environ Sci Technol. 2011;45:3725–3730. doi: 10.1021/es103309n. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen CS, Schwehr K, Quigg A, Chin WC, Santschi PH, Spurgin J, Jiang Y. Aggregation, Dissolution and Stability of Quantum Dots in Marine Environments: The Importance of Extracellular Polymeric Substances. Environ Sci Technol. 2012;46:8764–72. doi: 10.1021/es301000m. [DOI] [PubMed] [Google Scholar]
- Zhao L, Feng SS. Enhanced oral bioavailability of paclitaxel formulated in vitamin E-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J Pharm Sci. 2010;99:3552–3560. doi: 10.1002/jps.22113. [DOI] [PubMed] [Google Scholar]
- Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR, Tang X, Niu G, Jin L, Varela-Ramirez A, Zhang JY, Gardea-Torresdey JL. Stress Response and Tolerance of Zea mays to CeO(2) Nanoparticles: Cross Talk among H(2)O(2), Heat Shock Protein, and Lipid Peroxidation. ACS Nano. 2012;6:9615–22. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769–2781. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Zhu M, Li Y, Shi J, Feng W, Nie G, Zhao Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small. 2012a;8:404–412. doi: 10.1002/smll.201101708. [DOI] [PubMed] [Google Scholar]
- Zhu M, Tian X, Song X, Li Y, Tian Y, Zhao Y, Nie G. Nanoparticle-Induced Exosomes Target Antigen-Presenting Cells to Initiate Th1-Type Immune Activation. Small. 2012b;8:2841–2848. doi: 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- Zhu S, Oberdorster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res. 2006;62(Suppl):S5–9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]