Abstract
Although the origin of autoimmune antibodies to double-stranded DNA is not known, the variable-region structures of such antibodies indicate that they are produced in response to antigen-selective stimulation. In accordance with this, results from experiments using artificial complexes of DNA and DNA-binding polypeptides for immunizations have indicated that DNA may induce these antibodies. Hence, the immunogenicity of DNA in vivo may depend upon other structures or processes that may render DNA immunogenic. We report that in vivo expression of a single DNA-binding protein, the polyoma virus T antigen, is sufficient to initiate production of anti-double-stranded DNA and anti-histone antibodies but not a panel of other autoantigens. Expression of a mutant, non-DNA-binding T antigen did result in strong production of antibodies to the T antigen, but only borderline levels of antibodies to DNA and no detectable antibodies to histones. Nonexpressing plasmid DNA containing the complete cDNA sequence for T antigen did not evoke such immune responses, indicating that DNA by itself is not immunogenic in vivo. The results represent a conceptual advance in understanding a potential molecular basis for initiation of autoimmunity in systemic lupus erythematosus.
Full text
PDF

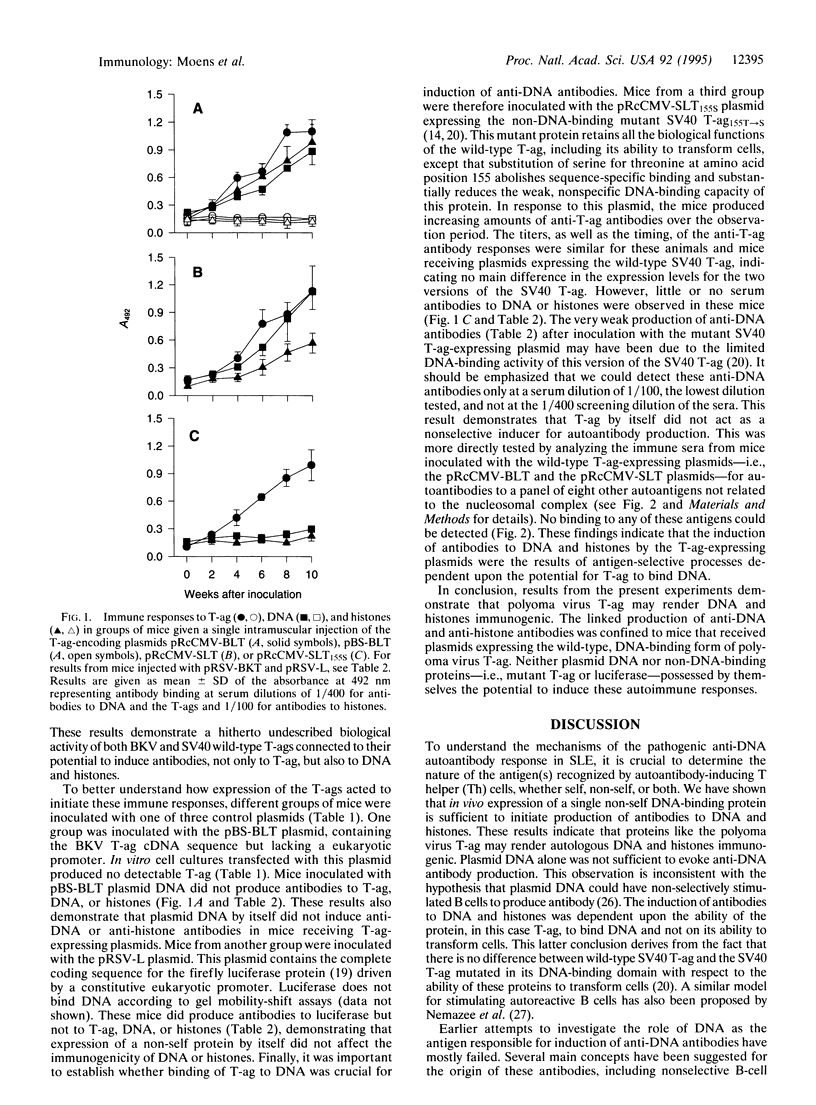


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., de Groot E. R., Feltkamp T. E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975 Jun 30;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- Bondeson K., Rönn O., Magnusson G. Preferred DNA-binding-sites of polyomavirus large T-antigen. Eur J Biochem. 1995 Jan 15;227(1-2):359–366. doi: 10.1111/j.1432-1033.1995.tb20397.x. [DOI] [PubMed] [Google Scholar]
- Brinkman K., Termaat R., Berden J. H., Smeenk R. J. Anti-DNA antibodies and lupus nephritis: the complexity of crossreactivity. Immunol Today. 1990 Jul;11(7):232–234. doi: 10.1016/0167-5699(90)90095-q. [DOI] [PubMed] [Google Scholar]
- Cassill J. A., Subramani S. The late promoter of the human papovavirus BK is contained within the early promoter enhancer region. Virology. 1988 Sep;166(1):175–185. doi: 10.1016/0042-6822(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Hardin J. A. Linked sets of antinuclear antibodies: what do they mean? J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):106–109. [PubMed] [Google Scholar]
- Desai D. D., Krishnan M. R., Swindle J. T., Marion T. N. Antigen-specific induction of antibodies against native mammalian DNA in nonautoimmune mice. J Immunol. 1993 Aug 1;151(3):1614–1626. [PubMed] [Google Scholar]
- Deyerle K. L., Sajjadi F. G., Subramani S. Analysis of origin of DNA replication of human papovavirus BK. J Virol. 1989 Jan;63(1):356–365. doi: 10.1128/jvi.63.1.356-365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Fredriksen K., Dahl B., Traavik T., Rekvig O. P. Inoculation with BK virus may break immunological tolerance to histone and DNA antigens. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8171–8175. doi: 10.1073/pnas.85.21.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaegstad T., Traavik T. BK virus in cell culture: infectivity quantitation and sequential expression of antigens detected by immunoperoxidase staining. J Virol Methods. 1987 May;16(1-2):139–146. doi: 10.1016/0166-0934(87)90038-3. [DOI] [PubMed] [Google Scholar]
- Fredriksen K., Osei A., Sundsfjord A., Traavik T., Rekvig O. P. On the biological origin of anti-double-stranded (ds) DNA antibodies: systemic lupus erythematosus-related anti-dsDNA antibodies are induced by polyomavirus BK in lupus-prone (NZBxNZW) F1 hybrids, but not in normal mice. Eur J Immunol. 1994 Jan;24(1):66–70. doi: 10.1002/eji.1830240111. [DOI] [PubMed] [Google Scholar]
- Hey A. W., Johnsen J. I., Johansen B., Traavik T. A two fusion partner system for raising antibodies against small immunogens expressed in bacteria. J Immunol Methods. 1994 Aug 1;173(2):149–156. doi: 10.1016/0022-1759(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Lin R. H., Mamula M. J., Hardin J. A., Janeway C. A., Jr Induction of autoreactive B cells allows priming of autoreactive T cells. J Exp Med. 1991 Jun 1;173(6):1433–1439. doi: 10.1084/jem.173.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamula M. J., Fatenejad S., Craft J. B cells process and present lupus autoantigens that initiate autoimmune T cell responses. J Immunol. 1994 Feb 1;152(3):1453–1461. [PubMed] [Google Scholar]
- Marion T. N., Tillman D. M., Jou N. T. Interclonal and intraclonal diversity among anti-DNA antibodies from an (NZB x NZW)F1 mouse. J Immunol. 1990 Oct 1;145(7):2322–2332. [PubMed] [Google Scholar]
- Messina J. P., Gilkeson G. S., Pisetsky D. S. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991 Sep 15;147(6):1759–1764. [PubMed] [Google Scholar]
- Moens U., Sundsfjord A., Flaegstad T., Traavik T. BK virus early RNA transcripts in stably transformed cells: enhanced levels induced by dibutyryl cyclic AMP, forskolin and 12-O-tetradecanoylphorbol-13-acetate treatment. J Gen Virol. 1990 Jul;71(Pt 7):1461–1471. doi: 10.1099/0022-1317-71-7-1461. [DOI] [PubMed] [Google Scholar]
- Mohan C., Adams S., Stanik V., Datta S. K. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993 May 1;177(5):1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D., Russell D., Arnold B., Haemmerling G., Allison J., Miller J. F., Morahan G., Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991 Aug;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Radic M. Z., Mascelli M. A., Erikson J., Shan H., Shlomchik M., Weigert M. Structural patterns in anti-DNA antibodies from MRL/lpr mice. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):933–946. doi: 10.1101/sqb.1989.054.01.108. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Fredriksen K., Brannsether B., Moens U., Sundsfjord A., Traavik T. Antibodies to eukaryotic, including autologous, native DNA are produced during BK virus infection, but not after immunization with non-infectious BK DNA. Scand J Immunol. 1992 Sep;36(3):487–495. doi: 10.1111/j.1365-3083.1992.tb02964.x. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Fredriksen K., Hokland K., Moens U., Traavik T., Krishnan M. R., Marion T. Molecular analyses of anti-DNA antibodies induced by polyomavirus BK in BALB/c mice. Scand J Immunol. 1995 Jun;41(6):593–602. doi: 10.1111/j.1365-3083.1995.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. The specificity of human autoantibodies that react with both cell nuclei and plasma membranes: the nuclear antigen is present on core mononucleosomes. J Immunol. 1979 Dec;123(6):2673–2681. [PubMed] [Google Scholar]
- Simmons D. T., Upson R., Wun-Kim K., Young W. Biochemical analysis of mutants with changes in the origin-binding domain of simian virus 40 tumor antigen. J Virol. 1993 Jul;67(7):4227–4236. doi: 10.1128/jvi.67.7.4227-4236.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Wun-Kim K., Young W. Identification of simian virus 40 T-antigen residues important for specific and nonspecific binding to DNA and for helicase activity. J Virol. 1990 Oct;64(10):4858–4865. doi: 10.1128/jvi.64.10.4858-4865.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar B. D. Antibodies to DNA. CRC Crit Rev Biochem. 1986;20(1):1–36. doi: 10.3109/10409238609115899. [DOI] [PubMed] [Google Scholar]
- Stollar B. D. The origin and pathogenic role of anti-DNA autoantibodies. Curr Opin Immunol. 1989;2(4):607–612. doi: 10.1016/0952-7915(90)90019-d. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Hara K., Kajioka J., Nagaki D. Isolation of BK virus from a patient with systemic lupus erythematosus (SLE). Microbiol Immunol. 1979;23(11):1131–1132. doi: 10.1111/j.1348-0421.1979.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Tillman D. M., Jou N. T., Hill R. J., Marion T. N. Both IgM and IgG anti-DNA antibodies are the products of clonally selective B cell stimulation in (NZB x NZW)F1 mice. J Exp Med. 1992 Sep 1;176(3):761–779. doi: 10.1084/jem.176.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. J., DeLucia A. L., Tegtmeyer P. Sequence-specific binding of simian virus 40 A protein to nonorigin and cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2631–2638. doi: 10.1128/mcb.4.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z. Q., Spitalnik S., Tran M., Wunner W. H., Cheng J., Ertl H. C. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994 Feb 15;199(1):132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]


