Abstract
Increasing evidence indicates that reactive oxygen species (ROS), consisting of superoxide, hydrogen peroxide, and multiple others, do not only cause oxidative stress, but rather may function as signaling molecules that promote health by preventing or delaying a number of chronic diseases, and ultimately extend lifespan. While high levels of ROS are generally accepted to cause cellular damage and to promote aging, low levels of these may rather improve systemic defense mechanisms by inducing an adaptive response. This concept has been named mitochondrial hormesis or mitohormesis. We here evaluate and summarize more than 500 publications from current literature regarding such ROS-mediated low-dose signaling events, including calorie restriction, hypoxia, temperature stress, and physical activity, as well as signaling events downstream of insulin/IGF-1 receptors, AMP-dependent kinase (AMPK), target-of-rapamycin (TOR), and lastly sirtuins to culminate in control of proteostasis, unfolded protein response (UPR), stem cell maintenance and stress resistance. Additionally, consequences of interfering with such ROS signals by pharmacological or natural compounds are being discussed, concluding that particularly antioxidants are useless or even harmful.
1. INTRODUCTION
Mitochondrial metabolism and reactive oxygen species
Mitochondria are important cell organelles that are responsible not only for the conversion of the bulk of nutritive energy, but also exert a major role in aging processes and in the development of age-related diseases. As an inevitable by-product of oxidative phosphorylation (OxPhos), mitochondria generate over 90% of all intracellular reactive oxygen species (ROS), with conversion of 0.15 – 5 % of total oxygen consumed by resting cells (Halliwell and Gutteridge 2007, Chance et al. 1979, Boveris and Chance 1973, St. Pierre et al. 2002). Hence, as main producers of energy and also potentially harmful ROS, mitochondria have a major impact on physiological and pathophysiological processes within the cell.
ROS formation to an extent that exceeds physiological levels and hence causes putative damage is called oxidative stress (Sies 1985). Thus, mitochondrial dysfunction implicating increased oxidative stress has been proposed to be associated with a variety of diseases like diabetes, cancer and neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease (Wiederkehr and Wollheim 2006, Ristow 2006, Fukui and Moraes 2008, Tatsuta and Langer 2008). Beyond that, impairment of mitochondrial activity is supposed to be a major reason for aging (Tatsuta and Langer 2008, Bratic and Larsson 2013, Trifunovic et al. 2004), whereas the role of ROS in this regard is still under debate. On the one hand, ROS have been implicated into cellular damage hence contributing to the aging process. On the other hand, an increasing number of studies linking improvement of mitochondrial capacity to increased lifespan and health span extension. Evidence for this dates back to the 1990s, when essential signaling roles for hydrogen peroxide were established (Barja 1993, Finkel 1998, Sena and Chandel 2012). Hence, it seems that a shift towards oxidative metabolism could delay the onset of age-related diseases and maybe aging itself.
Free radical theories of aging
Increased formation of mitochondrial ROS was postulated to be a major cause of aging in 1956, when Denham Harman introduces his Free Radical Theory of Aging (FRTA) (Harman 1956). According to this concept, increased ROS formation causes an accumulation of damage in the cell within age, resulting in age-related impairment of cellular functions and ultimately death of the cell or the corresponding organism, respectively. Respiratory enzymes, which utilize oxygen to generate readily available energy, were proposed to be the main generators of ROS. Due to the fact that mitochondria are the main intracellular source of ROS, Harman extended his initial FRTA theory to the Mitochondrial Free Radical Theory of Aging (MFRTA) (Harman 1972). Over the last decades, significant research efforts have been invested to prove the MFRTA, however generating inconsistent and conflicting results (Perez et al. 2009). Accordingly, nowadays it seems to be established that enhancement of metabolic rate does not necessarily result in concomitantly increased ROS formation (Lapointe and Hekimi 2010) and that the relationship between ROS levels and aging is not linear (Delaney et al. 2013, Johnson et al. 2001, Lee et al. 2003, Kim and Sun 2007, de Castro et al. 2004). Nevertheless and supporting the MFRTA, a bulk of studies in different organisms found that reduced levels of oxidative stress result in extended lifespan (Harrington and Harley 1988, Phillips et al. 1989, Orr and Sohal 1994, Parkes et al. 1998, Melov et al. 2000, Moskovitz et al. 2001, Bakaev and Lyudmila 2002, Ruan et al. 2002, Ishii et al. 2004, Huang et al. 2006, Zou et al. 2007, Kim et al. 2008, Quick et al. 2008, Dai et al. 2009, Shibamura et al. 2009) and long-lived species seem to produce fewer ROS and accumulate less damage than short-lived organisms (Gredilla et al. 2001, Sanz et al. 2010, Sanz and Stefanatos 2008, Gruber et al. 2008).
As a consequence, ROS-lowering interventions were widely proposed to be a promising strategy to retard aging in humans. In this regard, natural or artificial substances that are able to scavenge ROS, so-called antioxidants, were examined intensively. In contrast to the studies in lower model organisms cited above, several prospective intervention trials did not find any health-promoting effects of antioxidant supplementation. Unexpectedly, most interventional studies found a lack of effects in humans (Greenberg et al. 1994, Liu et al. 1999, Rautalahti et al. 1999, Virtamo et al. 2000, Various 2002, Sacco et al. 2003, Zureik et al. 2004, Czernichow et al. 2005, Czernichow et al. 2006, Cook et al. 2007, Kataja-Tuomola et al. 2008, Sesso et al. 2008, Katsiki and Manes 2009, Lin et al. 2009, Song et al. 2009), whereas others even suggested detrimental effects on human health, for instance promotion of cancer growth or induction of diseases with negative impact on human lifespan (Albanes et al. 1996, Omenn et al. 1996, Vivekananthan et al. 2003, Lonn et al. 2005, Bjelakovic et al. 2007, Ward et al. 2007, Lippman et al. 2009, Schipper 2004, DeNicola et al. 2011, Abner et al. 2011). Consistently, several studies overexpressing antioxidant enzymes in mice failed to exert positive effects on lifespan or associated parameters (Jang et al. 2009, Muller et al. 2007, Perez et al. 2011). Accordingly, several long-lived species were found to have a relatively lower expression of antioxidant genes than short-lived ones (Brown and Stuart 2007, Lopez-Torres et al. 1993, Page et al. 2010, Page and Stuart 2012, Salway et al. 2011). In the fruit fly Drosophila melanogaster, increasing levels of mitochondria-derived ROS were found during aging, but were not altered through interventions that increase longevity (Cocheme et al. 2011). Finally, mice that are heterozygous for Mclk1, coding for a ubiquinone synthesis enzyme, showed both increased mitochondrial ROS production and extended lifespan (Liu et al. 2005).
Mitochondrial hormesis (Mitohormesis): Non-linear responses to increased levels of ROS
These before-mentioned findings fundamentally questioned the FRTA, eventually requiring a modernized view concerning the putative roles of mitochondrial ROS (mtROS) generation. It has been repeatedly shown in recent years that mtROS serve as important signaling molecules mediating both cellular and systemic physiological changes, which has been summarized elsewhere (Finkel 1998, Mittler et al. 2011, Sena and Chandel 2012). Physiological targets for ROS are, for instance, thiol groups on cysteine residues that become oxidized and thereby altering functions of the enzymes in a signaling pathway (Finkel 2012, Rhee et al. 2000, Tonks 2005). However, given the fact that increased levels of oxidative damages do accumulate during the aging process, one interesting new point of view proposes that intrinsic aging is caused by an inadequate response to endogenous ROS signals (Sohal and Orr 2012).
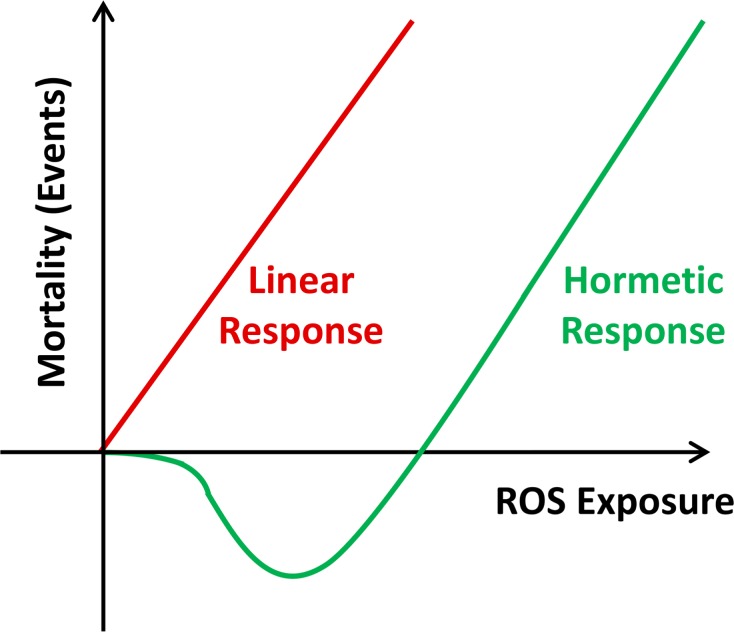
If ROS serve as signaling molecules as outlined above, it appears likely that ROS may also exert specific functions in promoting general health, and specifically lifespan. Since ROS at high doses unquestionably exert detrimental effects on cellular integrity, this insinuates that different levels of ROS, i.e. comparably low versus high amounts, may exert opposite effects on biological outcomes. In a more general sense, this kind of biphasic or non-linear response to potentially harmful substances was named “hormesis” (Southam and Ehrlich 1943). By today, the impact of hormetic effects on aging has been repeatedly proposed, with a range of stressors described (Calabrese and Baldwin 2002, Cypser and Johnson 2002, Rattan 2008, Mattson 2008, Lamming et al. 2004, Yanase et al. 1999). On a hypothetical basis the term was specified to mitochondrial hormesis or mitohormesis in 2006 (Tapia 2006), which after its experimental validation in parallel (Schulz et al. 2007) is repeatedly used in settings where mtROS act as sublethal stressors promoting lifespan, whereas higher doses increase lethality (Figure 1).
FIGURE 1.
Mitochondrial Hormesis (Mitohormesis). While the Free Radical Theory of Aging suggests a linear dose-response relationship between increasing amounts of ROS and oxidative stress on the one hand, and mortality events on the other (red curve), the concept of mitohormesis indicates a non-linear dose-response relationship where low doses of ROS exposure decrease mortality, while higher doses promote mortality.
Aim of the review
We here aim to summarize the wide body of published evidence that refers to the biological relevance of mitohormesis mainly in regards to regulation of stress resistance and longevity, but also affiliated areas of interest. The majority of publications in this regard does not explicitly use the term mitohormesis but rather refers to dose-dependent or non-linear, mtROS-mediated signaling processes that hence reflect typical examples of mitohormesis.
2. CALORIE RESTRICTION (CR)
Calorie restriction (CR), being defined as a 10 – 50% reduction of ad libitum calorie uptake in the absence of malnutrition, is so far the most convincing intervention to delay both aging and the occurrence of age-related diseases in a variety of organisms, as reviewed elsewhere (Fontana et al. 2010). The first observation that laboratory rats maintained on dietary restriction not only showed an increased lifespan, but also seem to be healthier at higher age, dates back to 1935 (McCay et al. 1935). Since then, it has been frequently shown that CR is capable of extending median and maximum lifespan in various species from yeast to mammals (Lin et al. 2004, Lin et al. 2002, Schulz et al. 2007, Iwasaki et al. 1988), insinuating an evolutionarily conserved mechanism, as review elsewhere (Mair and Dillin 2008).
Nevertheless, it is still a matter of debate whether CR prolongs life expectancy in humans too as it is shown that people with average body mass tend to live longest (Berrington de Gonzalez et al. 2010), while CR in humans rather causes severe reduction of body mass (Holloszy and Fontana 2007). However, CR in humans clearly reduces diseases associated with aging, including cardiovascular diseases, cancer, and type 2 diabetes mellitus (DM type 2) (Takemori et al. 2011, He et al. 2012, Harvey et al. 2012, Willette et al. 2012, Ryan et al. 2012) as well as associated risk factors known to promote the before-mentioned diseases (Larson-Meyer et al. 2006, Heilbronn et al. 2006, Lefevre et al. 2009). One study found that CR reduces age-related mortality (which corresponds to only 54% of deaths) in rhesus monkeys, whereas no influence on overall mortality was reported (Colman et al. 2009). In contrast and unlike ad libitum fed control animals, monkeys on CR did not show any age-related impairment in glucose homeostasis, suggesting a reduction of prevalence of metabolic disorders like DM type 2. Another recent study on the same model organism found no changes in mortality following CR, whereas beneficial effects on health and morbidity were clearly observed (Mattison et al. 2012). It should be noted that the two studies have used diets that differed strikingly, also in regards to carbohydrate content. Due to the fact that both studies were not finished by the time this manuscript was prepared, future findings will have to show whether CR may affect overall mortality in these monkeys. Nevertheless, there is suggestive evidence that CR may also prolong life expectancy in primates and ultimately humans (Fontana et al. 2004, Heilbronn et al. 2006, Ingram et al. 2006, Weindruch 2006, Fontana and Klein 2007).
The concept of CR is based on an assumption postulated in the early 20th century, suggesting that there is an inverse correlation between the maximum lifespan of an organism and its metabolized nutritive energy (Rubner 1908). According to this, the Rate of Living hypothesis was formulated soon after by Raymond Pearl, insinuating that an increase in metabolic rate would decrease the lifespan of eukaryotes (Pearl 1928). A possible explanation for this was subsequently proposed within the FRTA by Harman, a hypothesis that became very popular and is frequently cited in aging research up to now (Harman 1956), as it is an explanation for CR which was hypothesized primarily to be a result of reduced oxidative stress and less oxidative cellular damage due to reduced metabolic rate (Sohal and Weindruch 1996).
However, more recent findings on the mechanistic basis of CR are in conflict with the FRTA. For instance, it is unclear whether CR actually does lead to a decrease in metabolic rate, i.e. oxygen consumption and/or heat production. A positive correlation for decreased metabolic rate and increase in longevity is found neither for metazoans like Drosophila and C. elegans, nor is it for mice (Hulbert et al. 2004, Lin et al. 2002, Masoro et al. 1982, Speakman et al. 2002). Rather, it has been reported that CR in C. elegans is associated with an increased metabolic rate (Walker et al. 2005, Schulz et al. 2007) as it is for Drosophila (Magwere et al. 2006, Piper et al. 2005b).
Since increased metabolic rates are necessarily linked to increased mitochondrial metabolism, it appears likely that these lifespan-extending processes may precipitate into increased production of ROS as an inevitable by-product of mitochondrial metabolism, as shown e.g. for glucose restriction (Schulz et al. 2007) and discussed in more detail below, reflecting a CR-associated prime example of mitohormesis.
3. MITOHORMETIC MECHANISMS OF ADAPTIVE RESPONSES
Notably, it was repeatedly reported that CR is capable of inducing stress defense mechanisms, particularly those which are involved in the detoxification of ROS, such as radical-scavenging enzymes and phase I and II biotransformation response enzymes, reflecting a number of putative mitohormetic responses (Koizumi et al. 1987, Semsei et al. 1989, Rao et al. 1990, Pieri et al. 1992, Youngman et al. 1992, Xia et al. 1995, Masoro 1998, Barros et al. 2004, Mahlke et al. 2011, Qiu et al. 2010, Rippe et al. 2010, Sreekumar et al. 2002, Park et al. 2012, Schulz et al. 2007, Zarse et al. 2012, Schmeisser et al. 2013b).
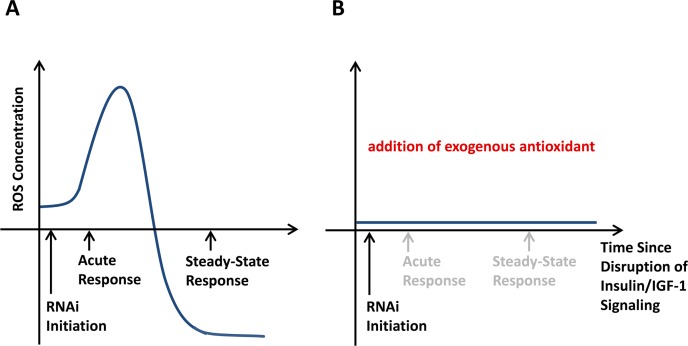
The independent observations of increased mtROS levels on the one hand and the induction of stress defense on the other, notably both in states of CR, raised the possibility that an initial induction of mtROS induce stress defense mechanisms culminating in secondarily decreased mtROS levels, as experimentally shown recently in a time-resolved manner (Zarse et al. 2012): In states of glucose restriction due to a constitutive genetic defect in the insulin/IGF-1 receptor DAF-2, a global decrease of mtROS levels in the steady-state was found. However, when analyzing an acute disruption of the same genetic pathway, a transient increase of mtROS was observed that secondarily induced defense mechanisms, ultimately reducing ROS levels in the steady state (Figure 2A). Blocking the initial ROS signal accordingly abrogated the induction of stress defense, as well as the steady-state reduction of ROS levels (Figure 2B). This indicates that the mitohormetic ROS signal is typically transient, and is reduced or even abolished in the steady-state due to an adaptive up-regulation of antioxidant enzymes and more globally stress defense. In other words, transiently increased ROS levels act to induce a vaccination-like response within the individual cell to lead to reduced ROS levels and better stress defense in the steady state (Figure 3).
FIGURE 2.
Lifespan-promoting ROS signaling can occur transiently and hence requires time-resolved quantification. A) Disruption of the insulin/IGF-1 receptor, named DAF-2, in C. elegans extends lifespan. The constitutive daf-2 mutant exhibits reduced ROS levels. This has led to the conclusion that impairing DAF-2 primarily causes reduced ROS levels. However, as recently published (Zarse et al. 2012), the opposite is the case: When studying the acute effects of an RNAi-mediated daf-2 knockdown, a transient increase in ROS production was observed (“acute response”). As shown in the publication (Zarse et al. 2012), this ROS signal induces various endogenous ROS defense mechanisms that ultimately reduce ROS levels. This leads to a persistent reduction of ROS levels in daf-2 RNAi-treated worms in the steady state. This also exemplifies that quantifying ROS at an inappropriate time point may lead to opposing results: ROS determined during the acute response against RNAi would indicate increased levels, while ROS determined three days later during the steady-state would indicate reduced levels. B) Exogenously added antioxidants prevent the acute induction of a ROS signal (Zarse et al. 2012). The lack of this ROS signal leads to a complete lack of the original adaptive response shown in panel A. This causes higher steady-state ROS levels than in the absence of exogenous antioxidants which only can be explained in the framework of mitohormesis, while the linear dose-response would consider this phenomenon as paradoxical.
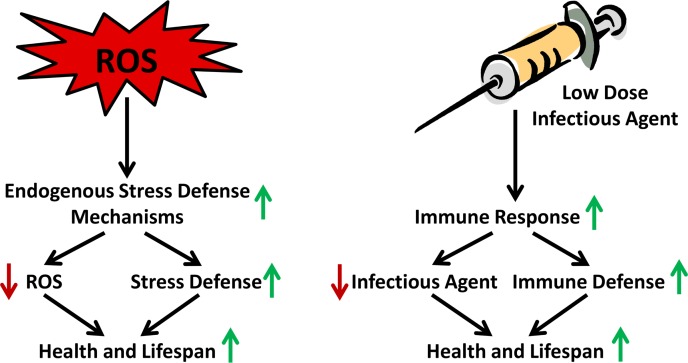
FIGURE 3.
Transiently increased ROS levels cause a vaccination-like adaptive response that promotes endogenous ROS defense capacity. The figure exemplifies the organismal stress response to low-level and/or short-term ROS exposure in comparison to the long-standing vaccination process, where inactive or impaired microbes exert an organismal immune response leading to a long-lasting defense capacity against future infections.
This subsequent decrease in ROS due to an adaptive increase of detoxifying mechanisms has often been misinterpreted as being the primary result of CR, which as outlined above is not the case. Rather, a clear causal relationship between primarily enhanced ROS formation and activation of ROS defense mechanism under conditions of CR was described (Agarwal et al. 2005), which manifests the hypothesis that CR is an essential trigger of mitohormetic mechanisms as shown thereafter (Schulz et al. 2007). Moreover, carbonyl concentrations reflecting oxidative protein damage were found to be increased in the brains of mice shortly after initiation of CR, whereas steady-state concentrations were significantly lower than those of control group (Dubey et al. 1996). Furthermore, levels of F2-isoprostane, reflecting oxidized lipids, were found to be decreased in obese woman under modest calorie restriction after 5 days of intervention (Buchowski et al. 2012). According to this, adaptive response mechanisms seem to be likely the reason for the beneficial effects initiated by CR, which is also supported by more recent research (Schulz et al. 2007, Sharma et al. 2010, Zuin et al. 2010, Rattan and Demirovic 2010, Mesquita et al. 2010), also in a time-resolved manner (Zarse et al. 2012). In rodents that are exposed to CR for instance, an induction of antioxidant defense capacities has been frequently shown (Koizumi et al. 1987, Semsei et al. 1989, Rao et al. 1990, Pieri et al. 1992, Youngman et al. 1992, Xia et al. 1995, Masoro 1998, Barros et al. 2004, Mahlke et al. 2011, Qiu et al. 2010, Rippe et al. 2010, Sreekumar et al. 2002). Additionally, glucose restriction in yeast not only promotes lifespan but also decreases ROS levels although respiration was increased (Barros et al. 2004). In conflict with these findings, subsequent studies using the same models and interventions rather reported an increase in ROS production paralleled by enhanced respiration and elevated antioxidant enzyme activity (Schulz et al. 2007, Sharma et al. 2010, Zuin et al. 2010, Agarwal et al. 2005, Kharade et al. 2005, Piper et al. 2006). This suggests a relationship between increased respiration, ROS generation and the upregulation of ROS defense mechanisms, which in the end mediates longevity. Furthermore, lifespan-extending mutations in C. elegans are commonly associated with increased stress resistance and often also with increased metabolic activity (Lithgow et al. 1995, Vanfleteren and De Vreese 1995, Honda and Honda 1999, Murphy et al. 2003, Houthoofd et al. 2005, Dong et al. 2007).
As mentioned before, CR is able to delay the onset of a broad range of age-related diseases such as cancer, DM type 2, nephropathy, cataracts, hyperlipidemia, and hypertension (Fishbein 1991, Weindruch and Walford 1988). Therefore it seems possible, that the lifespan-extending effect of CR is linked to promotion of mean lifespan due to prevention of life-threatening disorders that reduce longevity. However, additional effects of CR on molecular processes improve cellular functions and therefore improve health-span per se have been observed, for instance the below mentioned activation of NF-E2-Related Factor 2 (NRF2) (Koizumi et al. 1987, Semsei et al. 1989, Rao et al. 1990, Pieri et al. 1992, Youngman et al. 1992, Xia et al. 1995, Masoro 1998, Barros et al. 2004, Mahlke et al. 2011, Qiu et al. 2010, Rippe et al. 2010, Sreekumar et al. 2002, Bishop and Guarente 2007). Another crucial role in CR and aging is attributed to the sirtuins, a conserved family of NAD+-dependent deacetylases as reviewed elsewhere (Baur and Sinclair 2006, Canto and Auwerx 2009) (see also chapter “Sirtuin signaling”).
An important factor regarding the effects of CR could also be thioredoxin, as it is shown to be essential for the lifespan extension in C. elegans under dietary deprivation and knockouts of eat-2, a genetic surrogate of nematodal CR (Fierro-Gonzalez et al. 2011). The oxidoreductase thioredoxin is not only involved in antioxidant response and redox regulation, but also acts as electron donor for metabolic enzymes and prevents aggregation of cytosolic proteins in the cell (Lillig and Holmgren 2007, Berndt et al. 2008). Thioredoxin gene expression is increased through NRF2 binding at the antioxidants responsive elements (AREs) and NRF2 is shown to be activated by ROS (Kim et al. 2001, Papaiahgari et al. 2006).
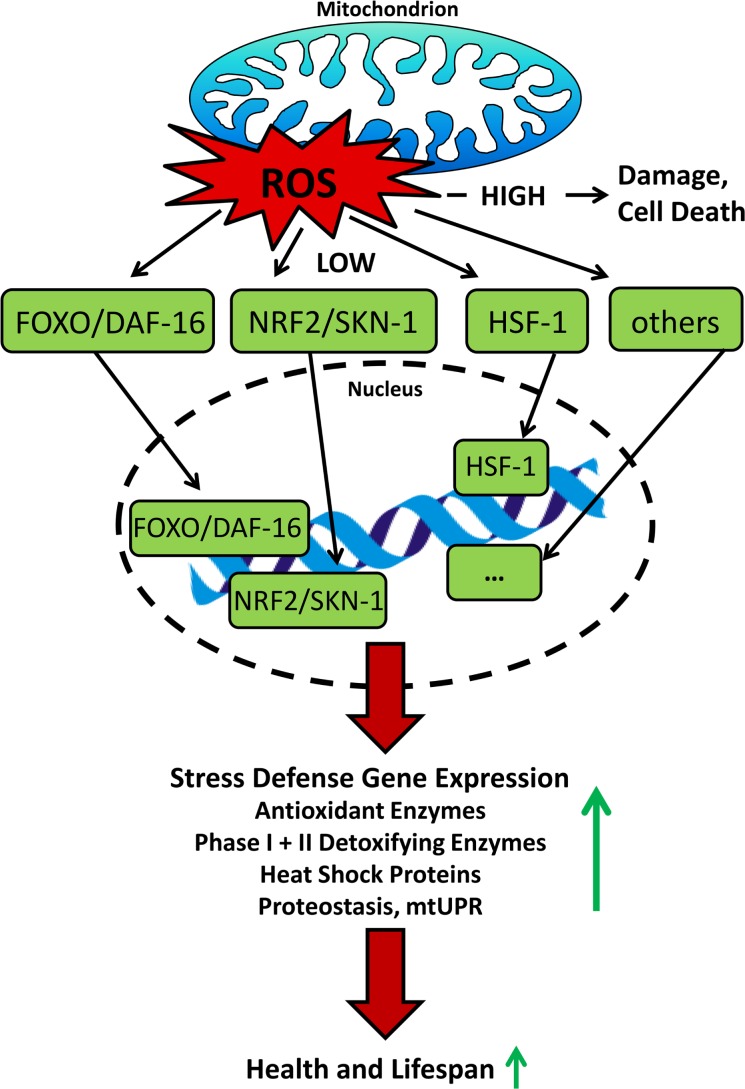
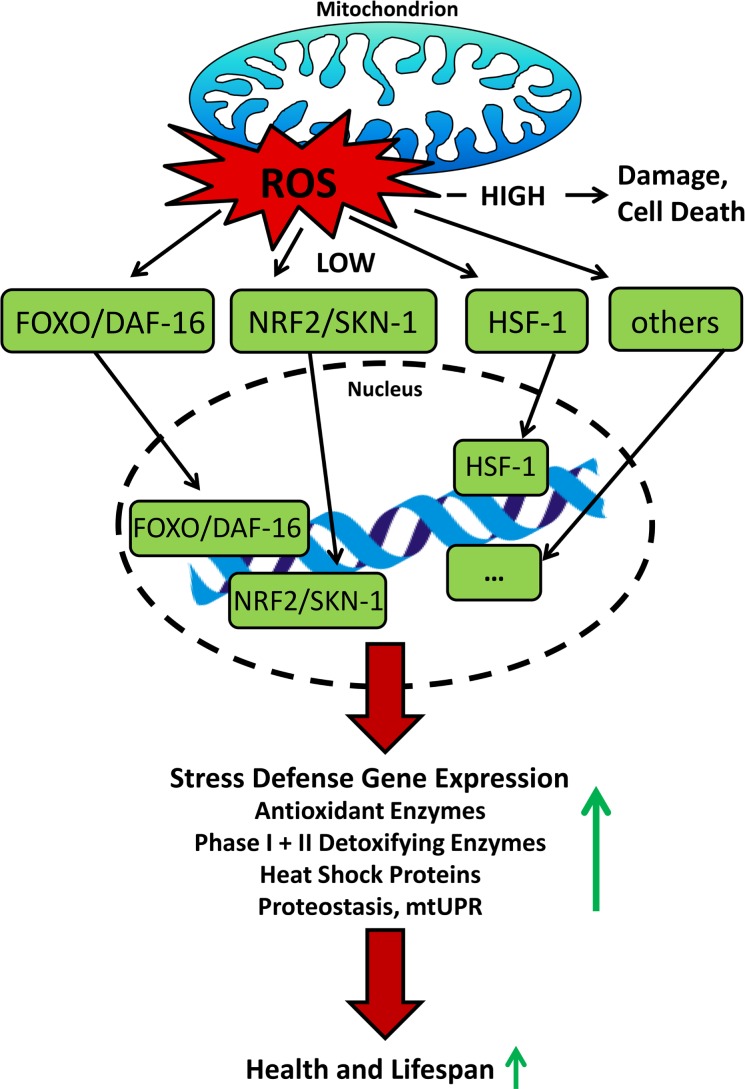
The activation of the transcription factor NRF2 from the leucine zipper family is indeed a crucial pathway to mediate mitohormesis. NRF2 binds to the DNA via AREs, which coordinate a stress response to ROS by boosting the expression of antioxidant proteins and phase I and II detoxification enzymes (Rushmore et al. 1991). Under unstressed conditions, NRF2 is sequestered in the cytoplasm by its specific repressor Kelch-like ECH-Associated Protein 1 (KEAP1), an actin-binding protein, which also targets NRF2 for proteasomal degradation (Itoh et al. 1999). KEAP-1 has redox-sensitive cysteine residues, with which it sensors oxidants and electrophiles, leading to abrogation of the NRF2/KEAP1 complex (Zhang 2006, Itoh et al. 2004). NRF2 then translocates into the nucleus, where it executes its transcriptional regulating functions (Jaiswal 2004) (Figure 4). While the putative functional orthologue of KEAP1 in C. elegans, XREB (Hasegawa and Miwa 2010), has not been examined further so far, the worm NRF2 orthologue SKN-1 similarly responds to oxidative stress by upregulating antioxidant and phase II genes which in the end promotes stress resistance and lifespan extension (An and Blackwell 2003, Bishop and Guarente 2007, Tullet et al. 2008), as it is shown for various other species (Sykiotis and Bohmann 2008, Motohashi and Yamamoto 2004, Leiser and Miller 2010, Lewis et al. 2010).
FIGURE 4.
Overview on how ROS transcriptionally influence stress resistance and lifespan. High levels cause damage resulting in death of the cell and eventually the corresponding organism, whereas low levels are capable of activating transcription factors that mediate adaptive stress response culminating in increased lifespan.
Other transcription factors which are essential for lifespan extension due to various interventions are members of the Forkhead transcription factors (FOX) as well as heat shock factor 1 (HSF-1). FOXOs for example activate a number of target genes involved in cellular stress response and it has been shown, that mitohormetic upregulation of superoxide dismutase and catalase following oxidative stress is FOXO dependent (Kops et al. 2002, Nemoto and Finkel 2002, Brunet et al. 2004), whereas FOXAs are important mediators of development and facilitate the response to CR (Friedman and Kaestner 2006, Panowski et al. 2007). HSF-1 regulates the transcription of heat shock genes that encode proteins (HSPs) in response to heat and other stress, which is linked to protection against diseases and increased lifespan in model organisms (Akerfelt et al. 2010, Anckar and Sistonen 2011). HSPs are also linked to hormetic responses (Cypser and Johnson 2002). Other mechanisms specifically related to the stressors described in the chapters below will be mentioned at the appropriate position within this review (Figure 4).
4. REDUCTION OF SPECIFIC MACRONUTRIENTS
Macronutrients are carbohydrates, protein and fat (triacylglycerides), which consist of a few different monosaccharides (importantly including glucose), amino acids, and fatty acids, respectively. Metabolism of these macronutrients provides the majority of energy in form of ATP required by an organism. ATP generation out of fatty acids and most amino acids depends on mitochondrial OxPhos and therefore presence and consumption of oxygen. However, only glucose can be metabolized to generate ATP independently of the mitochondria and oxygen, hence without increasing ROS production. Nevertheless, the generation of ATP via OxPhos is considerably more efficient than anaerobic ATP production from glucose or specific amino acids: One mole of glucose metabolized via mitochondrial OxPhos provides 30 moles of ATP, compared to 4 moles of ATP due to exclusively glycolytic breakdown. This implies that restricting glucose may induce OxPhos and mitochondrial metabolism more efficiently than globally restricting calorie uptake.
On one hand, only a few studies have investigated whether it is possible to mimic the CR mediated effect on lifespan by reducing only selected macronutrients, so the evidence available is limited. On the other hand, some studies point out that it might be not the amount of calories affecting health span, but rather the reduction of specific nutrients, as reviewed elsewhere (Fanson et al. 2009, Mair et al. 2005, Piper et al. 2005a).
Studies of fat restriction in invertebrates are lacking, while a restriction of lipids without overall CR in mice does not affect their lifespan (Iwasaki et al. 1988). Feeding a low-carbohydrate/high-fat diet to mice reduced lifespan slightly, at least in comparison to a high-carbohydrate/low-fat diet (Keipert et al. 2011). However, it is likely that fat restriction has less potential to delay the onset of metabolic disorders than carbohydrate restriction (Ryan et al. 2007, Volek et al. 2009).
In mice, it has been shown that a reduced nutritional protein content extent lifespan (Stoltzner 1977, Leto et al. 1976, Fernandes et al. 1976) as it was shown for casein restriction in Drosophila (Min and Tatar 2006). Studies examining the restriction of the essential amino acid and glutathione precursor methionine found it not only to be lifespan extending. Increasing mitochondrial biogenesis and function, energy expenditure, stress resistance, aerobic capacity, insulin sensitivity, glutathione (GSH) and expression of glutathione-S-transferase (GST) as well as a decrease of oxidative stress and cell damage due to adaptive changes in methionine and GSH metabolism were also observed (Zimmerman et al. 2003, Miller et al. 2005, Perrone et al. 2010, Richie et al. 1994, Malloy et al. 2006, Sanz et al. 2006, Perrone et al. 2012, Tsai et al. 2010, Caro et al. 2008). Interestingly, co-treatment with an antioxidant, N-acetylcysteine, blocks some of the health-promoting effects of methionine restriction, accentuating a critical role for ROS with mitohormetic adaption processes in this regard (Elshorbagy et al. 2012, Sanchez-Roman et al. 2012).
As mentioned above, glucose (besides a few amino acids) is the only macronutrient that can be metabolized and generate ATP without producing ROS. In support of the mitohormesis concept, it is documented that glucose restriction initiates health-promoting and lifespan extending effects in rodents and various lower organisms, for instance in Drosophila (Mair et al. 2005) and yeast (Lin et al. 2002). In the latter, studies showed that lifespan extension depends on enhanced respiration and sirtuin activation, which is still a matter of heated debate (Lin et al. 2000, Kaeberlein et al. 2004, Agarwal et al. 2005, Guarente and Picard 2005, Smith et al. 2007, Roux et al. 2009). However, also sirtuin-independent pathways have been discussed (Barros et al. 2004, Roux et al. 2009).
To achieve depletion specifically of glucose metabolism in eukaryotic model organisms such as rodents or C. elegans and hence, to mimic a ketogenic diet (i.e. a very low carbohydrate content) and recapitulate metabolic hallmarks of CR in rodents, the glycolytic inhibitor 2-deoxy-glucose (DOG) is frequently used (Wick et al. 1957, Garriga-Canut et al. 2006, Lane et al. 1998, Ingram et al. 2004). DOG was found to be lifespan extending in C. elegans (Schulz et al. 2007), whereas and unexpectedly, increased mortality in rats was reported following chronic ingestion of DOG (Minor et al. 2010). It should be noted that shuttle mechanism for lactate and alanine may explain differential outcomes of glucose deprivation in metazoans and rodents, which however remains to be evaluated.
Similar as reported in yeast for media-based glucose restriction (Lin et al. 2002), DOG not only extends lifespan in C. elegans, but also increases respiration. However and unlike in S. cerevisiae, in C. elegans the effect seems to be independent of sirtuins. The authors suggested that the underlying mechanisms that lead to increased life expectancy are dependent on the AMP-dependent kinase (AMPK). AMPK acts as a highly conserved key regulator of energy metabolism within a cell, since functionally similar orthologues were found in lower species like flies and worms (Hardie et al. 2006, Apfeld et al. 2004, Greer et al. 2007a, Pan and Hardie 2002). AMPK is activated by metabolic stress like cellular lack of energy, resulting in upregulation of processes that produce energy, such as mitochondrial biogenesis. This leads to a compensation of the energy deficit and likely to additional health-promoting effects (Hardie et al. 2006).
Another approach to reduce glucose content within the cell is the impairment of GLUT-4 glucose transporters. Mice with disruption of GLUT-4 in both muscle and adipose tissue show fasting hyperglycemia, glucose intolerance, increased fatty acid turnover, and utilization. However, lifespan was not affected (Kotani et al. 2004). Strikingly, over-expression of GLUT-4 leading to an increase of cellular glucose does also not affect lifespan, whereas enhanced glucose abundance decreased lifespan significantly in C. elegans (McCarter et al. 2007, Lee et al. 2009b, Schlotterer et al. 2009, Schulz et al. 2007).
In humans, several approaches of varying macronutrients in the diet have been established, especially to lose weight in states of obesity. In this regard, low fat/high carbohydrate diets seem to be as efficient as low carbohydrate/high protein diets. Meta-analyses have shown that reducing carbohydrates may additionally reduce the risk of cardiovascular diseases (Nordmann et al. 2006, Hession et al. 2009, Volek et al. 2009, Wang et al. 2002). Furthermore, the reduction of several inflammation markers in overweight men and women with atherogenic dyslipidemia was reported (Forsythe et al. 2008). It was also shown that a ketogenic diet was capable to lower blood glucose levels in obese diabetic patients more effectively than overall CR (Hussain et al. 2012). In contrast, a Swedish study in over 43.000 middle-aged women found a significant increase in cardiovascular diseases following a ketogenic diet (Lagiou et al. 2012). The authors suggested that the protein source might play an important role and could contribute to this unexpected outcome. Interestingly, it was shown that a short-term low-carbohydrate/high fat intake may increase postprandial plasma glucose, suggesting a decrease in first-phase insulin secretion after the diet has started. However, other studies detect decreased glucose plasma levels on the long run, which could be due to adaptive mechanisms (Nobels et al. 1989, Boden et al. 2005).
5. CALORIE RESTRICTION MIMETICS (CRM)
CRMs are defined as pharmaceutical or naturally occurring compounds that may mimic the metabolic state of CR. Ideally these compounds would allow the organisms to eat normally, i.e. ad libitum, while the metabolic state would reflect reduced caloric uptake. The best studied compound in this regards, DOG has been introduced above. However, due to increased mortality in rats following chronic ingestion of DOG (Minor et al. 2010) this compound meanwhile appears of questionable usefulness. While beyond the immediate scope of this review, it should be noted that DOG as well as CR have been repeatedly shown to exert remarkable neuro-protective effects as reviewed elsewhere (Arumugam et al. 2006).
The phytochemical resveratrol is found to be CR mimetic as it potentially slows aging and certainly delays age-related diseases by activating sirtuins and also mitohormetic responses (Wood et al. 2004, Baur et al. 2006, Rubiolo et al. 2008) also based on a concept named xenohormesis (Howitz and Sinclair 2008). This is possibly linked to the fact that resveratrol may induce mtROS formation (Zini et al. 1999). Interestingly, genetic disruption of cellular mechanisms that target degradation of xenobiotics has been shown to result in lifespan extension in multiple species, suggesting adaptive response processes (Curran and Ruvkun 2007, Smith et al. 2008, Melo and Ruvkun 2012).
More specifically and focusing exclusively on mtROS formation, a recent study in C. elegans found that chemical inhibition of complex I of the mitochondrial electron transport chain (ETC) also mimics CR including increased physical activity and stress resistance, as well as extended lifespan. Interestingly, it was further shown that these complex I inhibitors extend lifespan independent of sirtuins and AMPK, but solely need a transient ROS increase to activate p38 MAP kinase and neuronal NRF2, suggesting that CR extends lifespan by inducing ROS formation (Schmeisser et al. 2013b). Consistently, C. elegans with genetic impairment of complex I, III, and IV are also long-lived (Dillin et al. 2002, Zuryn et al. 2010, Rea et al. 2007). In mice, inhibition of complex IV leads also to lifespan extension as well as elevated fat utilization, increased insulin sensitivity, and increased mitochondrial biogenesis (Deepa et al. 2012). Also, juglone, a known ROS generator and herbicide, has been reported to increase lifespan in low concentrations due to enhanced oxidative stress response (Heidler et al. 2010).
In this regard it is interesting to note that a significant number of pharmaceutically effective compounds, including phytochemicals like resveratrol (Zini et al. 1999), sulforaphane (Singh et al. 2005), niacin (Fukushima 2005), and berberine (Turner et al. 2008), as well as anti-diabetic drugs like metformin (El-Mir et al. 2000) and PPARγ-activating thiazolidinediones (Brunmair et al. 2004b, Nadanaciva et al. 2007), and lastly cholesterol-lowering HMG-CoA-synthase inhibitors (“statins”) (Nadanaciva et al. 2007) and PPARα-activating fibrates (Brunmair et al. 2004a, Nadanaciva et al. 2007), have been found to inhibit mitochondrial complex I of the ETC. Since inhibition of complex I generates a mitochondrial ROS signal, these independent findings insinuate that this group of compounds exerts pleitropic effects, however sharing a common denominator by acting as potential CRMs which however needs further investigation.
6. IMPAIRED INSULIN/IGF-1 SIGNALING (IIS)
Mammalian insulin and IGF-1 (insulin-like growth factor 1) are peptide hormones produced in beta-cells of the pancreas and the liver, respectively. Insulin is a key regulator of glucose metabolism, especially involved in the regulation of cellular glucose uptake, fat metabolism, and food uptake. IGF-1 is produced following growth hormone (GH, a.k.a. somatotropin) mediated stimulation in the liver, and mediates childhood growth and anabolic effects in adults. Furthermore, most of the direct receptor-mediated effects of GH, i.e. IGF-1-independent effects, counteract insulin action. Insulin, IGF-1, and GH bind to different specific receptors to affect cellular functions. With a significantly lower affinity, insulin can activate the IFG-1 receptor, and vice versa.
Mice with impairment of GH or IGF-1 function and signaling show dwarfism and prolonged lifespan, somewhat resembling CR conditions (Quarrie and Riabowol 2004, Brown-Borg et al. 1996). Artificially increasing GH levels abrogates the lifespan extension and leads to elevated body size (Pendergrass et al. 1993, Steger et al. 1993). Furthermore, impairment of the neuronal IGF-1 receptor or heterozygous global disruption of this receptor increases murine lifespan and may be responsible for prevention of neurodegeneration and proteotoxicity (Holzenberger et al. 2003, Kappeler et al. 2008). Conversely, long-term IGF-1 exposure leads to decreased mitochrondrial function and cell viability in human fibroblasts (Bitto et al. 2010).
Impairment of the insulin receptor in humans is linked to insulin resistance, a state which is defined as an inadequate reduction of intra-cellular response to extracellular insulin stimulus (Kahn 1994). One main function of the insulin receptor activation due to extracellular insulin is the translocation of glucose transporter GLUT-4 and hence, glucose uptake in the cell. Accordingly, the outcome of insulin resistance is reduced glucose availability within the cell, which is associated with DM type 2 (Biddinger and Kahn 2006). This disease is linked to decreased lifespan also due to a number of secondary complications, including cardiovascular disease and an increased incidence of cancers (Kannel and McGee 1979, Franco et al. 2007, Coughlin et al. 2004).
Since in mice a global disruption of the insulin receptor knockout leads to embryonic lethality, muscle-specific knock-out mice were established to study the role of insulin receptor signaling. Noteworthy, muscle-tissue is the most relevant tissue in regards to glucose metabolism. Interestingly, those mice showed elevated fat mass, serum triacylglycerides and free fatty acids, but they neither experienced hyperglycemia nor the development of diabetes (Brüning et al. 1998). Moreover, they displayed enhanced glucose uptake in muscle cells in response to exercise like wild type mice do (Wojtaszewski et al. 1999). Lifespan data on those mice are not available, but mice with an adipose tissue-specific knockout have an increased mean and maximum lifespan (Blüher et al. 2003). Furthermore, mice with a global knockout in the insulin receptor substrate 1 (IRS-1) demonstrate increased resistance to several age-related pathologies and are long-lived, as are mice with neuronal knockout of IRS-2 and heterozygous global IRS-2 knockouts (Selman et al. 2008, Page et al. 2013, Taguchi et al. 2007). In addition, there are hints that specific mutations in the insulin receptor might also be associated with longevity in humans (van Heemst et al. 2005, Pawlikowska et al. 2009).
Other than mammals, invertebrates like C. elegans and Drosophila do not have distinct receptors for IGF-1 and insulin, but rather share a common receptor. Impaired insulin/IGF-1 signaling (but not its complete disruption) in invertebrates strikingly extends lifespan (Kimura et al. 1997, Clancy et al. 2001, Tatar et al. 2001).
C. elegans that carry a mutation within daf-2, the worm orthologue of the insulin/IGF-1 receptor, have a lifespan that is twice as long as in wild type worms (Kenyon et al. 1993). Recent studies have shown that the mitochondrial energy production is altered by impairment of DAF-2. Such mutants did not display the typical age-dependent decrease in mitochondria protein and bioenergetics competence, but a higher membrane potential and an increase in ROS, which is interestingly not associated with more, but less damage to mitochondrial DNA and protein (Brys et al. 2010, Zarse et al. 2012). The inhibition of IIS leads to lifespan extension due to changes in gene expression mediated by the FOXO transcription factor DAF-16 (Kenyon 2010). Reduction of IIS followed by lifespan extension and promotion of stress resistance in the worm not only requires DAF-16, it activates also SKN-1 in parallel, which facilitates the above mentioned beneficial adaptive response (Tullet et al. 2008); the same is true for HSF-1 (Kenyon 2005, Chiang et al. 2012). Also, mitochondrial L-proline catabolism plays an important role in that regard since it is upregulated by impaired DAF-2 signaling, which lead to a transient increase in ROS production mediating adaptive response processes to extend lifespan, proving the link between impaired IIS and mitohormesis (Zarse et al. 2012).
Assuming that impairment of the insulin/IGF-1 receptor reduces glucose uptake one could expect that the same lifespan extending mechanisms act here as they occur in regard to glucose restriction or overall CR (see above). In fact, there are several studies proposing shared processes and pathways involved in both interventions (Yechoor et al. 2004, Brooks et al. 2007, Katic et al. 2007, Russell and Kahn 2007, Westbrook et al. 2009, Zarse et al. 2012), while others propose independent mechanisms (Greer et al. 2007a, Lakowski and Hekimi 1998, Bartke et al. 2007, Houthoofd et al. 2003, Min et al. 2008, Bonkowski et al. 2009, Brown-Borg et al. 2002, Clancy et al. 2002).
7. AMP-DEPENDENT KINASE (AMPK) SIGNALING
AMPK acts as a sensor of available nutrients and hence energy that is regulated by the cellular AMP/ATP ratio and upstream kinases (Hardie et al. 2003). Whenever an energy deficit occurs and concurrently the AMP/ATP ratio rises, AMPK activates catabolic and represses anabolic processes. In other words, being activated by stress that inhibits ATP generation or increases ATP consumption, like glucose starvation or muscle contraction, AMPK inhibits energy consuming pathways and induces ATP-generating processes (Hardie et al. 2003, Salt et al. 1998, Winder and Hardie 1996).
AMPK exists as heterotrimeric complex consisting of a catalytic α-subunit and the regulatory β- and γ-subunits (Kemp et al. 2003). Activation of AMPK requires specific phosphorylation events by upstream kinases such as the serine/threonine protein kinase LKB1 within the catalytic domain of the α-subunit (Woods et al. 2003). Widely expressed, AMPK regulates food uptake in response to nutrient signals and hormones (Minokoshi et al. 2004) and is an important initiator of mitochondrial biogenesis (Zong et al. 2002, Winder et al. 2000), glucose and fatty acid uptake (Barnes et al. 2002, Habets et al. 2009), as well as β-oxidation (Merrill et al. 1997). In C. elegans, AMPK overexpression extends lifespan and is required for the lifespan extension due to impaired insulin/IGF-1 signaling (Apfeld et al. 2004). However, the role of AMPK in CR is less clearly established. The kinase appears to be necessary to mediate lifespan extension due to CR when food limitation starts in middle age employing a pathway that requires DAF-16/FOXO (Greer et al. 2007a). The direct activation of FOXO by AMPK has also been described in mammals (Greer et al. 2007b), linking it to oxidative stress response and therefore potentially mitohormesis.
Accordingly, AMPK is activated by DOG and involved in the induction of mitochondrial metabolism and hence the mitohormetic response (Schulz et al. 2007). Another example for an AMPK-activating substance is metformin, an antidiabetic drug and inhibitor of the mitochondrial complex I (El-Mir et al. 2000), which was found to be lifespan-extending due to AMPK-activation in C. elegans and mice (Onken and Driscoll 2010, Anisimov et al. 2008). Moreover, metformin was shown to promote adaptive processes, which are also involved in CR and oxidative stress response like activation of NRF2/SKN-1, culminating in increased life expectancy (Onken and Driscoll 2010). As described earlier, the CR mimetic resveratrol slows aging and delays age-related diseases by activating, besides sirtuins, also AMPK, again linking it to mitohormetic responses (Zini et al. 1999, Baur et al. 2006). It has been reported that many AMPK activators, including resveratrol and metformin, act by inhibiting mitochondrial function (Hawley et al. 2010). As a consequence of impaired mitochondrial function, the AMP/ATP ratio rises, leading to AMPK activation followed by increased mitochondrial biogenesis, respiration, β-oxidation, and finally increased ROS production (Schulz et al. 2007, Hardie 2011). It has moreover been proposed that ROS themselves are also capable of activating AMPK (Zmijewski et al. 2010, Alexander et al. 2010) and due to this, by acting up- and downstream of AMPK, the stress response may be further amplified. Interestingly, hypoxia has been shown to activate AMPK not by changing the AMP/ATP ratio, but rather by increased ROS production, since the activation is inhibited by antioxidants (Emerling et al. 2009).
However, recent findings suggest that mitochondrial ROS production may be more relevant than AMPK activation in regards to lifespan extension: consistent with the nuo-6 mutation in C. elegans (Yang and Hekimi 2010), inhibiting complex I of the respiratory chain by rotenone and other chemicals generates a ROS signal that extends lifespan in the absence of AMPK, sirtuins, or both (Schmeisser et al. 2013b). This indicates that ROS formation alone, i.e. in the absence of energy sensors, is still capable of promoting longevity. Consistently, it was shown that nematodes lacking AMPK live shorter and die prematurely in the dauer stage since their triglyceride stores are exhausted (Narbonne and Roy 2009, Xie and Roy 2012). The study of Xie and colleagues pointed out an important role for ROS in replacing essential AMPK functions: An increase in hydrogen peroxide activated the transcription factor hypoxiainducible factor 1 (HIF-1; see also chapter “Hypoxia”), which is capable of stimulating key enzymes involved in the biosynthesis of fatty acids, leading to an increased survival of the dauer larvae (Xie and Roy 2012).
Hence, AMPK not only acts as regulator of metabolism, but also may play an important role in ROS signaling and adaptive response processes, which highlights the universal character of mitohormesis within cellular metabolism, whereas ROS signals still promote longevity even in the absence of AMPK.
8. TOR SIGNALING
The so-called “target of rapamycin” (TOR) pathway is known to be another major regulator of life expectancy by sensing nutrient and environmental signals (Pan et al. 2012). The mammalian TOR (mTOR) is a serine/threonine protein kinase and a member of the phosphatidylinositol 3-kinase-related kinase protein family which consists of two functionally distinct multi-protein complexes known as TOR complex 1 (TORC1) and TORC2 (Brunn et al. 1997). Each complex has an accessory protein; for TORC1 it is named regulatory-associated protein of mTOR (RAPTOR), for TORC2 rapamycin-insensitive companion of mTOR (RICTOR) (Hara et al. 2002, Laplante and Sabatini 2012). The major sensor of cellular inputs like nutrients, hormones, energy, and oxidative stress is TORC1, while TORC2 executes regulatory functions concerning cell survival and cytoskeletal polarity (Laplante and Sabatini 2012). TOR signaling has been shown to be regulated by AMPK, suggesting that both nutrient-sensing pathways are key regulators of mitochondrial metabolism (Gwinn et al. 2008).
Impairment of the TOR pathway is shown to be lifespan-extending in various organisms (Kaeberlein et al. 2005, Powers et al. 2006, Jia et al. 2004, Kapahi et al. 2004). The immunosuppressive and antifungal drug rapamycin acts as inhibitor of the TOR pathway as it inhibits TORC1 and is known to extend median and maximum lifespan of C. elegans and mice (Harrison et al. 2009, Robida-Stubbs et al. 2012). Notably, rapamycin not only extends lifespan, but also induces insulin resistance and impaired glucose metabolism (Lamming et al. 2012), again consistent with cellular energy deprivation and subsequent induction of mitohormesis. Similarly, mTOR signaling has been linked to oxidative nutrient metabolism in rodents (Sengupta et al. 2010).
Consistent with this, lifespan extension in yeast due to impaired TOR signaling is promoted by inducing expression of proteins from the respiratory complexes, mitochondrial ETC activity and overall mitochondrial metabolism (Pan and Shadel 2009, Powers et al. 2006, Bonawitz et al. 2007, Pan et al. 2011). In agreement with this, the influence of TOR on mitochondrial biogenesis and turnover is also found in mammals in a strongly tissue-dependent manner. In murine skeleton muscle tissue and cells for instance, rapamycin decreases expression of mitochondrial genes resulting in decreased oxygen consumption (Cunningham et al. 2007), whereas in fatty tissue opposite effects have been observed. Adipocyte-specific disruption of RAPTOR is linked to higher rates of mitochondrial uncoupling followed by enhanced energy expenditure, which protects the mice from gaining weight (Polak et al. 2008). Higher respiration rates and energy expenditure are also linked to increased expression of genes involved in OxPhos and beta-oxidation, especially in older mice (Katic et al. 2007).
There are several protein families like 4E-BP, ATG, and S6K, which modulate mitochondrial biogenesis downstream in the TOR signaling pathway. Consistently, the translational regulator 4E-BP is shown to have strong influence on the expression of OxPhos genes. In Drosophila, CR alters mRNA profiles via TORC1 in a 4E-BP dependent manner (Zid et al. 2009). Furthermore, TOR downstream acting proteins are not only involved in mitochondrial biogenesis, but also mitochondrial quality: ATG-5 mediates the degrading of dysfunctional mitochondria by autophagy (Twig et al. 2008), recently called “mitophagy” (Pua and He 2009). Notably, autophagy has also an important role in response to cellular stress, including starvation and pathogen infection, as well as in IIS mediated lifespan regulation (Kroemer et al. 2010, Levine et al. 2011, Toth et al. 2008, Hansen et al. 2008).
Due to the fact that TOR signaling plays a crucial role in mitochondrial biogenesis and turnover it is consequently involved in regulation of mtROS levels. For example, mouse mitochondria from skeletal muscle cells with impaired ATG-7 exhibit increased production of ROS (Wu et al. 2009b). This negative correlation between TOR signaling and ROS is also observed in yeast, where both tor-1 knock-out and rapamycin treatment caused increased levels of superoxide due to enhanced mitochondrial biogenesis, strikingly coupled with less oxidative damage within the cell (Pan et al. 2011). This research manifests that ROS stimulation due to TOR acts also as mitohormetic stimulus and that mitohormesis is also in this regard the key mediator of lifespan extension (Pan 2011). Impaired TOR signaling through either genetically inhibited TORC1 or the usage of rapamycin is also known to activate SKN-1 and DAF-16 in C. elegans, mediating increased stress resistance and longevity (Robida-Stubbs et al. 2012).
It is assumed that TOR is another key mediator of CR since it has been shown that yeast and Drosophila carrying a deletion in the TOR gene do not benefit of CR in regards to lifespan extension (Kaeberlein et al. 2005, Kapahi et al. 2004). To evidence this hypothesis and point out whether the TOR pathway is involved in CR benefits, further research is strongly needed.
9. SIRTUIN SIGNALING
Sirtuins are NAD+-dependent deacetylases that catalyze the removal of acetyl groups from lysine residues of specifically histones and other proteins. They modulate cell-protective mechanisms such as oxidative stress defense, DNA repair, protein folding, energy utilization, and autophagy (Haigis and Sinclair 2010). The first identified member of this protein family was named silent information regulator 2 (SIR2) (Sinclair et al. 1997, Kaeberlein et al. 1999), giving rise to the term “sirtuins”. By today, seven mammalian orthologues have been found, named SIRT1 to SIRT7 (Blander and Guarente 2004), whereas SIRT1 and SIRT3 are the closest orthologues to SIR2 (Merksamer et al. 2013). Sirtuins are linked to longevity, since over-expression has been shown to extend lifespan in yeast (Kaeberlein et al. 1999) as well as in worms (Tissenbaum and Guarente 2001, Viswanathan and Guarente 2011, Mouchiroud et al. 2013, Ludewig et al. 2013, Schmeisser et al. 2013a) and flies (Rogina and Helfand 2004, Bauer et al. 2009). However, others could not confirm the results in C. elegans and Drosophila (Burnett et al. 2011), whereas one study found sirtuin overexpression only in the fat body of relevance for longevity (Banerjee et al. 2012). Sirtuins were found to be necessary to mediate lifespan extension due to CR (Lin et al. 2000, Guarente and Picard 2005, Boily et al. 2008), whereas others found no such connection (Kaeberlein et al. 2004, Smith et al. 2007, Schulz et al. 2007). In addition, a recent publication pointed out an important role for p53 modulating SIRT1 during CR, as reviewed elsewhere (Tucci 2012).
However, the role of sirtuins in oxidative stress and mitohormetic responses has been implicitly discussed also in this regard (Lin et al. 2000), supported by the observations, that sir2 overexpression rescues the short lifespan phenotype due to hydrogen peroxide treatment in yeast (Oberdoerffer et al. 2008) and that SIRT3 is necessary to mitigate oxidative stress during CR (Someya et al. 2010, Qiu et al. 2010). There is evidence that the mammalian SIRT1 is involved in mediating oxidative stress response, as it directly deacetylates several FOX members (Brunet et al. 2004, Motta et al. 2004, van der Horst et al. 2004). In contracting muscle cells SIRT1 mediates the protection against oxidative stress via enhanced expression of SOD-2 (Pardo et al. 2011). Correspondingly, SIRT2 activates FOXO3a, which promotes resistance to hydrogen peroxide (Wang et al. 2007). Moreover, SIRT1 has been also shown to activate peroxisome-proliferator-activated receptor (PPAR) gamma co-activator-1 alpha (PGC-1α) (Rodgers et al. 2005), a transcriptional co-activator that promotes mitochondrial biogenesis and expression of antioxidant genes including calalase, SOD, and glutathione peroxidase (St. Pierre et al. 2006). Furthermore, SIRT1 suppresses the inducible nitric oxide synthase (iNOS) and thus may decrease cellular ROS levels (Lee et al. 2009a). The catalytic activity of SOD-2 is dependent of the mitochondrial SIRT3, which is also capable of enhancing SOD-2 activity (Qiu et al. 2010). Accordingly, genetic impairment of SIRT3 in mice leads to higher ROS levels, genomic instability, and susceptibility to cancer (Kim et al. 2010), establishing SIRT3 as anticarcinogenic protein by improving stress response, which is supported also by more recent research (Bell et al. 2011, Finley et al. 2011). Consistently, SIRT6 has been shown to promote DNA repair in response to oxidative stress (Mao et al. 2011). Genetic impairment of SIRT6 results in genetic instability and premature aging (Mostoslavsky et al. 2006), whereas overexpression promotes lifespan extension, at least in male mice (Kanfi et al. 2012). Finally, SIRT7 mediates oxidative stress response as well, since cardiomyocytes of SIRT7 knockout mice are more sensitive to hydrogen peroxide treatment (Vakhrusheva et al. 2008, Calabrese et al. 2007).
From a traditional viewpoint, these findings support the notion that sirtuins promote health and lifespan, at least in parts, via increased resistance towards ROS (Webster et al. 2012) and particularly mitohormetic response processes (Merksamer et al. 2013). As outlined in more detail elsewhere (Merksamer et al. 2013), physiological stressors including CR may decrease the activity of antioxidant enzymes like SOD by acetylation processes resulting in hyperacetylation (Hirschey et al. 2010, Hirschey et al. 2011a, Hirschey et al. 2011b, Ozden et al. 2011). The following increase in ROS would activate defense mechanisms against oxidative stress, resulting in lower ROS levels in the steady state. Notably, such stresses have been also shown to increase SIRT3 expression, suggesting subsequently increased deacetylation of SOD and other mitochondrial proteins to counteract chronically increased ROS generation (Hirschey et al. 2010, Merksamer et al. 2013).
Very recently, an alternate mechanism linking sirtuin signaling to ROS-mediated lifespan extension has emerged (Schmeisser et al. 2013a): Sirtuins require NAD+ as a cofactor, and accordingly produce nicotinamide. This product becomes methylated to 1-methylnicotinamide, which itself serves as a substrate for an aldehyde oxidase to produce hydrogen peroxide. The latter acts as a ROS signal to execute sirtuin effects, since disruption of either the methylase or the oxidase fully prevents sirtuin-mediated lifespan extension (Schmeisser et al. 2013a), also implying that sirtuin-mediated deacetylation processes may be of limited relevance regarding lifespan regulation.
10. HYPOXIA
Hypoxia is an environmental state that is characterized by decreased environmental availability of oxygen, which typically leads to reduced mitochondrial respiration rates and a variety of changes on the molecular level (Semenza 2012), notably including increased mtROS production (Kulisz et al. 2002). The master regulator of hypoxia-mediated transcriptional changes is HIF-1, a highly conserved transcription factor that promotes survival under hypoxic stress (Shen and Powell-Coffman 2003, Semenza 2012). Under normal oxygen conditions, the mammalian HIF-1α subunit is hydroxylated and targeted for proteasomal degradation by the von Hippel-Lindau tumor suppressor protein (VHL) (Kim and Kaelin 2003). This signaling pathway seems to be highly conserved, since C. elegans hif-1 and vhl-1 genes encode homologs of HIF-1α subunit and VHL (Shen et al. 2005). However, it was shown that low oxygen atmosphere and decreased respiration is capable of increasing lifespan of C. elegans (Adachi et al. 1998), probably via stabilization of HIF-1 (Lee et al. 2010, Mehta et al. 2009, Zhang et al. 2009). This activation of the hypoxic signaling pathway was found to promote lifespan independently of CR or impaired IIS (Kaeberlein and Kapahi 2009, Mehta et al. 2009) as it is shown that CR due to deprivation of bacteria and genetically induced through mutation in eat-2 increases lifespan in hif-1 knockout nematodes, and impairment of DAF-2 is also able to promote longevity in those animals (Mehta et al. 2009). Notably, it was reported that loss of HIF-1 causes longevity as well (Zhang et al. 2009, Chen et al. 2009). In one study, DAF-16 seems to be essential for lifespan extension, indicating a mechanism similar to reduced IIS (Zhang et al. 2009), whereas others found no such connection, possibly insinuating that HIF-1 acts as a negative regulator of longevity in a pathway upstream of the endoplasmic reticulum (ER) stress response and downstream of CR and TOR signaling (Chen et al. 2009).
The unquestionable influences of HIF-1 on aging have initiated several competing hypotheses: One explanation could be that HIF-1 down-regulates mitochondrial activity (Papandreou et al. 2006, Semenza 2011), as this is shown to be lifespan extending through RNAi-mediated knockdown of several mitochondrial proteins (Tormos and Chandel 2010, Rea et al. 2007, Dillin et al. 2002). Alternatively, HIF-1 could act in regards to stress response, like NRF2 or FOXO, especially since it is shown that HIF-1 and DAF-16 share various target genes (McElwee et al. 2004). In mammals, there are also links to TOR signaling and ER unfolded protein response (UPR) with mTOR signaling being reduced by hypoxia and HIF-1 translation being dependent on TOR (Stein et al. 1998, Wouters and Koritzinsky 2008), whereas both hypoxia and TOR are known to activate UPR (Romero-Ramirez et al. 2004).
Interestingly, mitochondrial-derived ROS during hypoxia lead to HIF-1 stabilization in cultured cells (Chandel et al. 1998), as well as activation of c-Jun N-terminal kinase 1 (JNK1), p53, and NF-κB (Chandel et al. 2000a, Chandel et al. 2000b). Moreover, in C. elegans, knockdown of genes encoding respiratory chain components and mutations in such, like clk-1 and isp-1, lead not only to decreased respiration rates, but also to a mild increase in ROS formation, which is responsible for HIF-1 stabilization and longevity of the nematodes (Lee et al. 2010, Yang et al. 2009). Increased ROS levels under hypoxic conditions in C. elegans (Miller et al. 2011, Miller and Roth 2007) as well as in cultured cells (Guzy and Schumacker 2006) occur in a HIF-1 dependent manner. A recent study in C. elegans found also an important role of DAF-16 in this regard, since it is delocalized to the nucleus and necessary to extend lifespan under hypoxic conditions (Leiser et al. 2013). However, in this study, lifespan extension did not require SIR-2.1, AAK-2, SKN-1, or CEP-1, the worms’ orthologues of sirtuins, AMPK, NRF2 and p53, respectively. On the other hand, roles for the sirtuins in HIF-1 deacetylation, AAK-2 in adaption to anoxia, and CEP-1 acting downstream of HIF-1 have been described earlier (Dioum et al. 2009, Zhong et al. 2010, Lim et al. 2010, Leiser and Kaeberlein 2010, Larue and Padilla 2011, Sendoel et al. 2010). As mentioned above, a mild increase in oxidative stress leads to stabilization of HIF-1 followed by increased survival of C. elegans AAK-2/AMPK mutants due to HIF-1-dependent activation of genes involved in fatty acid biosyn-thesis (Xie and Roy 2012). This metabolic adjustment pointed out an important role for HIF-1 and ROS in compensating AMPK functions.
Nevertheless, studies that investigated the influence of hypoxia on mammalian aging are rare. This is not only because the mechanisms according to hypoxia in mammals are much more complex than in lower organisms like C. elegans, but also due to HIF-1α is involved in tumor growth and cancer development, notably also by altering glucose metabolism (Semenza 2012, Semenza et al. 1994). Discovered by its ability to increase erythropoetin production, HIF-1α was associated with the VHL hereditary cancer syndrome, a heterozygous disease characterized by development of various malign tumors in the kidneys, retina, and the central nervous system with an increase in HIF-1α being a negative predictor in metastatic tumors (Wang et al. 1995, Kaelin 2002, Semenza 2010).
However and to our best knowledge, the first evidence that links HIF-1 to longevity and aging per se only dates back to 2009 (Mehta et al. 2009), so future research will properly establish the role of oxygen availability in the aging process and bring hypoxic mechanisms and connections to other pathways to light.
11. TEMPERATURE STRESS
As early as in 1908 it was hypothesized that body temperature may be linked or even determine life expectancy (Loeb 1908). A few years later, the hypothesis was experimentally supported by showing that lowering temperature extends lifespan of poikilothermic Drosophila (Loeb and Northrop 1916). Subsequently, benefits from exposure to lowered temperature regarding lifespan have been shown in other organisms like C. elegans or fish (Klass 1977, Liu and Walford 1966) and notably also in homoeothermic (warm-blooded) animals like rats and mice (Holloszy and Smith 1986, Conti et al. 2006). On the other hand, increasing ambient temperature or mild heat stress are also linked to increased lifespan in various organisms (Shama et al. 1998, Wu et al. 2009a). As mentioned above, HSPs are major regulators of response to heat stress in almost all organisms investigated ranging from bacteria to mammals (Lindquist and Craig 1988, Fargnoli et al. 1990, Udelsman et al. 1993, Lithgow et al. 1995, Rea et al. 2005). HSPs consist of a large number of proteins, often being classified according to their molecular weight: HSP40, HSP60, HSP70, HSP90, HSP110 (with 40, 60, 70, 90, and 110 kilo-daltons in size, respectively) and the small HSPs represent the majority of HSPs (Li and Srivastava 2004). Some HSPs are also known as chaperones, playing crucial roles in the UPR to prevent polypeptides from aggregating into nonfunctional structures (Calderwood et al. 2009, Jazwinski 2005, Parikh et al. 1987), which has also been reported to play a role in lifespan regulation (Calfon et al. 2002, Henis-Korenblit et al. 2010, Yoneda et al. 2004). Transcriptionally regulated by HSF-1, HSPs have been unquestionably linked to hormetic processes (Akerfelt et al. 2010, Cypser and Johnson 2002). For instance, increased expression of HSP-70 family members following activation of HSF-1 due to a variety of stressors leads to protection against the latter, notably including ROS (Westerheide and Morimoto 2005, Raynes et al. 2012). Conversely, HSF-1 depletion shortens lifespan in C. elegans, as overexpression increases longevity and is required for the lifespan extension due to impaired insulin signaling (Hsu et al. 2003). The same study found that DAF-16 is necessary for extending lifespan in hsf-1 overexpressing worms, suggesting that both transcription factors might synergistically act to exert their beneficial effects (Hsu et al. 2003) (Figure 4). Hormetic heat stress is linked to improved mitochondrial function (Shama et al. 1998), which is required for ROS defense (Grant et al. 1997). Notably, long-lived C. elegans daf-2 mutants are resistant to thermal and oxidative stress and display increased expression of various HSPs and antioxidant and drug-metabolizing enzymes (McElwee et al. 2007, Lithgow and Walker 2002).
A mechanism for the observed increased lifespan at low temperatures was recently suggested in a study using C. elegans, pointing out an important role for a member of the transient receptor potential (TRP) family of cationic channels, TRPA-1 (Xiao et al. 2013). TRPA-1 alters its permeability to Ca2+, Na+, and K+ when activated by temperatures around 17°C or lower (Clapham 2003, Story et al. 2003). Worms that lack TRPA-1 have a shorter lifespan when exposed to cold in comparison to wild type animals, whereas overexpression of trpa-1 leads to increased lifespan at 15°C and 20°C, but not under warm (25°C) conditions. These effects were dependent on a calcium influx, which activates the calcium-sensitive protein kinase C (PKC) and the serine/threonine-protein kinase 1 (SGK-1). Interestingly, DAF-16/FOXO has been shown to be necessary to promote longevity in this regard. The TRPA-1 pathway induces nuclear activity of DAF-16, surprisingly without stimulating its nuclear translocation. The well-known fact that calcium influx increases ROS generation in mitochondria, as reviewed elsewhere (Brookes et al. 2004, Csordas and Hajnoczky 2009), insinuates that these ROS act as signal molecules to activate DAF-16 and promote longevity in this regard. Another study reported that hypothermia causes not only calcium influx into mitochondria, but also leads to a redox imbalance caused by an increase in ROS concentration (Brinkkoetter et al. 2008). Thus, mitohormetic processes could be also responsible for the lifespan extension following exposure to cold temperatures.
12. PHYSICAL ACTIVITY
Physical inactivity promotes the onset of a variety of diseases like obesity, cardiovascular disease, DM type 2, and cancer. Consistently, regular physical activity unquestionably exerts beneficial or preventive effects on the above mentioned diseases, and additionally delays depressive symptoms, neurodegeneration (including Alzheimer’s disease), and general aging (Warburton et al. 2006, James et al. 1984, Hu et al. 2001, Brown et al. 2012, Lanza et al. 2008, Manini et al. 2006, Powers et al. 2011). Exercise is not only linked to enhanced mitochondrial biogenesis and oxidative metabolism, but also to increased generation of mtROS (Powers and Jackson 2008, Chevion et al. 2003, Davies et al. 1982, Alessio and Goldfarb 1988, Alessio et al. 1988). Thus, and because of its obvious beneficial effects in regards to health and aging, make it a paradigm of adaptive response processes and finally mitohormesis (Radak et al. 2008, Radak et al. 2005, Ji et al. 2006, Watson 2013). However, similar to physical inactivity, overtraining or excessive exercise represents the other end of the hormesis curve as the adaption process is inhibited, leading to incomplete recovery (Chevion et al. 2003) and resulting in maladaptation and possibly increased risk of diseases (Alessio et al. 1988).
To our knowledge, the first direct evidence that increased ROS production following exercise may act as stimulus to activate mitochondria biogenesis and mediates potential health-beneficial effects dates back to 1982 (Davies et al. 1982). An indirect clue was already given in 1971 with an antioxidant, namely vitamin E, causing unfavorable effects on the endurance performance of swimmers (Sharman et al. 1971). Since then, a bulk of studies (in most cases inadvertently) proved the hypothesis that ROS are required for the health-promoting effects of physical activity, causing an increase in antioxidant defense mechanisms and with this, prolong health span and mean lifespan (Crawford and Davies 1994, Davies 1986, Kim et al. 1996, Marzatico et al. 1997, Balakrishnan and Anuradha 1998, Ji et al. 2006, Powers and Lennon 1999, Niess et al. 1999, Hollander et al. 2001, Higuchi et al. 1985, Gomez-Cabrera et al. 2008b, Quintanilha 1984, Vincent et al. 1999, Boveris and Navarro 2008).
One of the main changes due to regular physical activity is the increase in mitochondria energy metabolism. Exercise activates PGC-1α, which is capable of controlling mitochondrial gene expression via NRF1 and the mitochondrial transcription factor A (TFAM). This mediates enhanced replication of mitochondrial DNA, leading to increased mitochondrial biogenesis and efficient muscle contraction (Nikolaidis and Jamurtas 2009, Akimoto et al. 2005, Baar 2004, Arbogast and Reid 2004). Furthermore, PGC-1 promotes the response to oxidative stress through activation of NRF2 and induction of antioxidant enzyme expression (St. Pierre et al. 2006). Another important point is the massive consumption of ATP followed by an increase in AMP, which activates AMPK, leading again to induction of PGC-1 and enhanced mitochondrial biogenesis (Bergeron et al. 2001, Atherton et al. 2005). This increase in mitochon -drial metabolism leads to enhanced oxygen consumption in muscle fibers followed by lower intracellular oxygen tension during exercise, promoting ROS generation (Franco et al. 1999, Puntschart et al. 1996). There are also other so-called contraction-induced changes that stimulate ROS production in muscle, for instance increased CO2 tension, decreased cellular pH, and rise in muscle temperature (Arbogast and Reid 2004). The main source of ROS during exercise is probably skeletal muscle (Davies et al. 1982, Powers and Jackson 2008), but other tissues such as heart, lungs, and blood are also likely to be important contributors (Powers and Jackson 2008, Nikolaidis and Jamurtas 2009). On cellular level, mtROS were considered to be the predominant fraction of ROS produced during physical activity over decades (Koren et al. 1983, Davies et al. 1982), whereas recent research pointed out also important roles for nicotin-amide adenine dinucleotide phosphate (NADPH) oxidase, phospholipase A2, and xanthine oxidase (Powers et al. 2011).
ROS signals caused by a single bout of exercise only already activate antioxidant defense enzymes like mitochondrial SOD and inducible nitric oxide synthase (iNOS) (Hemmrich et al. 2003, Hollander et al. 2001). Regular exercise leads to proper adaptation to oxidative stress due to upregulation of diverse SODs, catalase, HSPs, and glutathione peroxidase (Powers and Lennon 1999, Leeuwenburgh and Heinecke 2001, Franco et al. 1999, Puntschart et al. 1996). The second line of antioxidant response which includes repair systems is important to minimize the damaging effects of ROS and is also activated through regular physical activity (Crawford and Davies 1994, Davies 1986), assigning important roles for proteasomal degradation and DNA repair enzymes (Radak et al. 2000, Radak et al. 1999, Radak et al. 2003).
Correspondingly, there is convincing evidence that supplementation of antioxidants is useless (Gey et al. 1970, Keren and Epstein 1980, Maughan 1999, Theodorou et al. 2011, Yfanti et al. 2010) or even harmful for athletes, potentially abolishing the beneficial effects on endurance performance, immune status, muscle development, and prevention of diseases (Gomez-Cabrera et al. 2008a, Strobel et al. 2011, Ristow et al. 2009, Marshall et al. 2002, Khassaf et al. 2003). For instance, athletes supplementing vitamin C and E did not display an induction of insulin sensitivity and endogenous antioxidant defense regulators due to exercise as seen in the control group (Ristow et al. 2009). It was shown that enhanced mitochondrial biogenesis and with this, increased respiration and ROS generation according to physical activity is prevented by co-treatment with antioxidants, leading to the inhibition of the beneficial mitohormetic response (Gomez-Cabrera et al. 2008a, Strobel et al. 2011, Kang et al. 2009, Fischer et al. 2006, Ristow et al. 2009). Furthermore, studies proved the harmful effect of antioxidants in regards to performance as it has shown to delay the recovery process (Close et al. 2006, Jackson 2008). Hence, supplementation of antioxidants should not be recommended to healthy athletes due to evidence that antioxidants have counter-productive effects on performance, health, and the onset of diseases.
13. OUTLOOK
All the above mentioned interventions are able to promote health-and lifespan in a variety of model organisms via mitohormetic processes (Figure 5). Future research will have to show whether these interventions will be capable of slowing aging and prolonging health span also in humans, in case it is not been shown yet. However, it seems unquestionable that the hypothesis of mitohormesis is, at least in parts, suitable to explain how the aging process could be beneficially influenced. Of course, mitohormesis cannot be considered in isolation to understand aging, which is to describe unfortunately beyond the topic and space limitations of this review. Notably, recent evidence suggests that stem cell aging is linked to impaired ROS signaling, i.e. that low levels of ROS production may prevent stem cell decline (Owusu-Ansah and Banerjee 2009, Owusu-Ansah et al. 2008, Morimoto et al. 2013). Given the eminent role of stem cell maintenance in the prevention of aging, it will be interesting to see whether the emerging link to increased ROS levels can be expanded. Secondly, ROS-mediated nitric oxide-signaling appears to be an increasingly expanding field of mitochondrial biology and disease control (D’Antona et al. 2010, Nisoli et al. 2005). Moreover, processes like proteostasis and mitochondrial UPR exert, while beyond the scope of this review, significant links to ROS-dependent signaling events, suggesting an overarching ROS-triggered mechanism in cellular and systemic quality control (Taylor and Dillin 2013, Balch et al. 2008). Furthermore, it should be emphasized that ROS signaling is a rather established mechanism in plant biology research (Mittler et al. 2011), which could not possibly be covered in the current review.
FIGURE 5.
A non-exhaustive overview on lifespan-extending interventions linked to mitohormetic ROS signaling. As outlined in the text of the current review, a number of apparently diverse interventions lead to a mitohormetic response mechanism, insinuating that distinct molecular pathways culminate in a common mechanistic denominator by promoting a ROS-dependent stress response.
Related to the theory of mitohormesis is the Epigenetic oxidative redox shift (EORS) theory of aging, proposing a metabolic shift away from the use of mitochondrial energy towards reliance on glycolysis as a cause of aging. This is due to epigenetic mediators influencing histone deacetylases as well as histone acetylases and DNA methyltransferases (Brewer 2010, Ghosh et al. 2012). The shift in the oxidized direction of relevant oxidants and reductants, such as cysteine/cystine or GSH/GSSG occurs with aging and is initiated by low demands of mitochondrial produced energy. The low energy demand is caused by low physical or mental activity, initiating a vicious cycle of oxidized signaling molecules, transcription factors, membrane receptors, and epigenetic transcriptional regulators. This results in the inability to respond to energy demands and stress, leading to typical age accompaniments like cell death and organ failure. Notably, this EORS occurs upstream of the commonly observed increase in ROS damage to macromolecules (Brewer 2010, Ghosh et al. 2012).
Another interesting approach which further extends the mitohormesis concept is the Redox stress hypothesis of aging (Sohal and Orr 2012), according to which ROS have essential functions in regulating protein activity. The theory distinguishes between young and old organisms, proposing that in the first part of life thiol redox potential is high, whereas ROS generation is relatively low. In older organisms, ROS formation increases, which would lead to a pro-oxidized shift in redox state and overoxidation of thiols, resulting in loss of sensitivity and coordination among the regulatory processes, a progressive decline of function and finally death. Notably and like mitohormesis, the hypothesis relegates cumulative damages through ROS and antioxidant defense to an auxiliary status, with little impact on altering redox-sensitive signaling (Sohal and Orr 2012).
Taken together, mitohormesis unifies a significant number of lifespan-regulating molecular pathways, and may, dependent on additional scientific evidence, become a common denominator in aging research.
Acknowledgments
The authors apologize to all colleagues whose work could not be cited solely due to the lack of space. We thank many members of the Ristow lab for interesting discussions regarding the topics outlined in this review. Funding sources: German Research Association (DFG) Graduate School of Adaptive Stress Response (#1715); Jena Centre for Systems Biology of Ageing (JenAge) funded by the German Ministry for Education and Research (Bundesministerium für Bildung und Forschung; support code BMBF 0315581); European Foundation for the Study of Diabetes (EFSD).
REFERENCES
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci. 2011;4:158–70. doi: 10.2174/1874609811104020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi H, Fujiwara Y, Ishii N. Effects of oxygen on protein carbonyl and aging in Caenorhabditis elegans mutants with long (age-1) and short (mev-1) life spans. J Gerontol A Biol Sci Med Sci. 1998;53:B240–4. doi: 10.1093/gerona/53a.4.b240. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sharma S, Agrawal V, Roy N. Caloric restriction augments ROS defense in S. cerevisiae by a Sir2p independent mechanism. Free Radic Res. 2005;39:55–62. doi: 10.1080/10715760400022343. [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–93. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64:1333–6. doi: 10.1152/jappl.1988.64.4.1333. [DOI] [PubMed] [Google Scholar]
- Alessio HM, Goldfarb AH, Cutler RG. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am J Physiol. 1988;255:C874–7. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 2010;107:4153–8. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, Semenchenko AV. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–73. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase aak-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–9. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–78. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–8. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63:269–73. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- Bakaev VV, Lyudmila MB. Effect of ascorbic acid on longevity in the nematoda Caenorhabditis elegans. Biogerontology. 2002;3(suppl1):12–16. [Google Scholar]
- Balakrishnan SD, Anuradha CV. Exercise, depletion of antioxidants and antioxidant manipulation. Cell Biochem Funct. 1998;16:269–75. doi: 10.1002/(SICI)1099-0844(1998120)16:4<269::AID-CBF797>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2:1485–91. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–42. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Barja G. Oxygen radicals, a failure or a success of evolution? Free Radic Res Commun. 1993;18:63–70. doi: 10.3109/10715769309147343. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–8. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Morris SN, Chang C, Flatt T, Wood JG, Helfand SL. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging (Albany NY) 2009;1:38–48. doi: 10.18632/aging.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–96. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–6. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Berndt C, Lillig CH, Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta. 2008;1783:641–50. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–9. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–58. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–9. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Bitto A, Lerner C, Torres C, Roell M, Malaguti M, Perez V, Lorenzini A, Hrelia S, Ikeno Y, Matzko ME, McCarter R, Sell C. Long-term igf-I exposure decreases autophagy and cell viability. PLoS ONE. 2010;5:e12592. doi: 10.1371/journal.pone.0012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–11. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, Xuan J, Evans M, Harper ME, McBurney MW. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS ONE. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007;5:265–77. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–9. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–7. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp Gerontol. 2010;45:173–9. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkkoetter PT, Song H, Losel R, Schnetzke U, Gottmann U, Feng Y, Hanusch C, Beck GC, Schnuelle P, Wehling M, van der Woude FJ, Yard BA. Hypothermic injury: the mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem. 2008;22:195–204. doi: 10.1159/000149797. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brooks NL, Trent CM, Raetzsch CF, Flurkey K, Boysen G, Perfetti MT, Jeong YC, Klebanov S, Patel KB, Khodush VR, Kupper LL, Carling D, Swenberg JA, Harrison DE, Combs TP. Low utilization of circulating glucose after food withdrawal in Snell dwarf mice. J Biol Chem. 2007;282:35069–77. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood) 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;8:864–74. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- Brown MF, Stuart JA. Correlation of mitochondrial superoxide dismutase and DNA polymerase beta in mammalian dermal fibroblasts with species maximal lifespan. Mech Ageing Dev. 2007;128:696–705. doi: 10.1016/j.mad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–69. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Brunmair B, Lest A, Staniek K, Gras F, Scharf N, Roden M, Nohl H, Waldhausl W, Furnsinn C. Fenofibrate impairs rat mitochondrial function by inhibition of respiratory complex I. J Pharmacol Exp Ther. 2004a;311:109–14. doi: 10.1124/jpet.104.068312. [DOI] [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004b;53:1052–9. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- Brys K, Castelein N, Matthijssens F, Vanfleteren JR, Braeckman BP. Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans. BMC Biol. 2010;8:91. doi: 10.1186/1741-7007-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Hongu N, Acra S, Wang L, Warolin J, Roberts LJ., 2nd Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS ONE. 2012;7:e47079. doi: 10.1371/journal.pone.0047079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–5. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–8. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21:91–7. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging—a mini-review. Gerontology. 2009;55:550–8. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–31. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro P, Gomez J, Lopez-Torres M, Sanchez I, Naudi A, Jove M, Pamplona R, Barja G. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008;9:183–96. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–20. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000a;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000b;165:1013–21. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion S, Moran DS, Heled Y, Shani Y, Regev G, Abbou B, Berenshtein E, Stadtman ER, Epstein Y. Plasma antioxidant status and cell injury after severe physical exercise. Proc Natl Acad Sci U S A. 2003;100:5119–23. doi: 10.1073/pnas.0831097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 Regulators DDL-1/2 Link Insulin-like Signaling to Heat-Shock Responses and Modulation of Longevity. Cell. 2012;148:322–34. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–6. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Close GL, Ashton T, Cable T, Doran D, Holloway C, McArdle F, MacLaren DP. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br J Nutr. 2006;95:976–81. doi: 10.1079/bjn20061732. [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carre JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–50. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–8. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- Crawford DR, Davies KJ. Adaptive response and oxidative stress. Environ Health Perspect. 1994;102(Suppl 10):25–8. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–62. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–40. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Curran SP, Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3:e56. doi: 10.1371/journal.pgen.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57:B109–14. doi: 10.1093/gerona/57.3.b109. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Bertrais S, Blacher J, Galan P, Briancon S, Favier A, Safar M, Hercberg S. Effect of supplementation with antioxidants upon long-term risk of hypertension in the SU.VI.MAX study: association with plasma antioxidant levels. J Hypertens. 2005;23:2013–8. doi: 10.1097/01.hjh.0000187259.94448.8a. [DOI] [PubMed] [Google Scholar]
- Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–9. doi: 10.1093/ajcn/84.1.394. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial bio-genesis in middle-aged mice. Cell Metab. 2010;12:362–72. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ. Intracellular proteolytic systems may function as secondary antioxidant defenses: an hypothesis. J Free Radic Biol Med. 1986;2:155–73. doi: 10.1016/s0748-5514(86)80066-6. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- de Castro E, Hegi de Castro S, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med. 2004;37:139–45. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Pulliam D, Hill S, Shi Y, Walsh ME, Salmon A, Sloane L, Zhang N, Zeviani M, Viscomi C, Musi N, Van Remmen H. Improved insulin sensitivity associated with reduced mitochondrial complex IV assembly and activity. FASEB J. 2013;27:1371–80. doi: 10.1096/fj.12-221879. [DOI] [PubMed] [Google Scholar]
- Delaney JR, Ahmed U, Chou A, Sim S, Carr D, Murakami CJ, Schleit J, Sutphin GL, An EH, Castanza A, Fletcher M, Higgins S, Jelic M, Klum S, Muller B, Peng ZJ, Rai D, Ros V, Singh M, Wende HV, Kennedy BK, Kaeberlein M. Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell. 2013;12:156–66. doi: 10.1111/acel.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–93. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR., 3rd Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science. 2007;317:660–3. doi: 10.1126/science.1139952. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–97. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, Plummer JD, Orentreich DS, Orentreich N, Refsum H, Perrone CE. Effect of taurine and N-acetylcysteine on methionine restriction-mediated adiposity resistance. Metabolism. 2013;62:509–17. doi: 10.1016/j.metabol.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free Radic Biol Med. 2009;46:1386–91. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Perez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–23. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Fargnoli J, Kunisada T, Fornace AJ, Jr, Schneider EL, Holbrook NJ. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990;87:846–50. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976;73:1279–83. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Gonzalez JC, Gonzalez-Barrios M, Miranda-Vizuete A, Swoboda P. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem Biophys Res Commun. 2011;406:478–82. doi: 10.1016/j.bbrc.2011.02.079. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–53. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–28. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer CP, Hiscock NJ, Basu S, Vessby B, Kallner A, Sjoberg LB, Febbraio MA, Pedersen BK. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol. 2006;100:1679–87. doi: 10.1152/japplphysiol.00421.2005. [DOI] [PubMed] [Google Scholar]
- Fishbein LE. Biological effects of dietary restriction. New York: Springer-Verlag; 1991. [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, Kraemer WJ, Feinman RD, Volek JS. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radical Biology and Medicine. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007;167:1145–51. doi: 10.1001/archinte.167.11.1145. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–28. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Moraes CT. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31:251–6. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T. Niacin Metabolism and Parkinson’s Disease. Environ Health Prev Med. 2005;10:3–8. doi: 10.1265/ehpm.10.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–7. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Gey GO, Cooper KH, Bottenberg RA. Effect of ascorbic acid on endurance performance and athletic injury. JAMA. 1970;211:105. [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Barnett AJ, Brewer GJ. A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci. 2012;32:5821–32. doi: 10.1523/JNEUROSCI.6192-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008a;87:142–9. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic Biol Med. 2008b;44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Grant CM, MacIver FH, Dawes IW. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:219–22. doi: 10.1016/s0014-5793(97)00592-9. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Sanz A, Lopez-Torres M, Barja G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J. 2001;15:1589–91. doi: 10.1096/fj.00-0764fje. [DOI] [PubMed] [Google Scholar]
- Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007a;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007b;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing—where do we stand? Front Biosci. 2008;13:6554–79. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–82. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–19. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets DD, Coumans WA, El Hasnaoui M, Zarrinpashneh E, Bertrand L, Viollet B, Kiens B, Jensen TE, Richter EA, Bonen A, Glatz JF, Luiken JJ. Crucial role for LKB1 to AMPKalpha2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochim Biophys Acta. 2009;1791:212–9. doi: 10.1016/j.bbalip.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Sirtuins in Aging and Age-Related Diseases. In: Masoro EJ, Austad SN, editors. Handbook of the Biology of Aging. AbeBooks, Academic Press; 2010. [Google Scholar]
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. fourth ed. Oxford University Press; 2007. [Google Scholar]
- Hansen M, Chandra A, Mitic LL, Onken B, Driscoll M, Kenyon C. A Role for Autophagy in the Extension of Lifespan by Dietary Restriction in C. elegans. PLoS Genet. 2008;4:e24. doi: 10.1371/journal.pgen.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–6. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase: development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–7. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech Ageing Dev. 1988;43:71–8. doi: 10.1016/0047-6374(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AE, Lashinger LM, Otto G, Nunez NP, Hursting SD. Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression. Mol Carcinog. 2013;52:997–1006. doi: 10.1002/mc.21940. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Miwa J. Genetic and cellular characterization of Caenorhabditis elegans mutants abnormal in the regulation of many phase II enzymes. PLoS ONE. 2010;5:e11194. doi: 10.1371/journal.pone.0011194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XY, Zhao XL, Gu Q, Shen JP, Hu Y, Hu RM. Calorie restriction from a young age preserves the functions of pancreatic beta cells in aging rats. Tohoku J Exp Med. 2012;227:245–52. doi: 10.1620/tjem.227.245. [DOI] [PubMed] [Google Scholar]
- Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 2010;11:183–95. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmrich K, Suschek CV, Lerzynski G, Kolb-Bachofen V. iNOS activity is essential for endothelial stress gene expression protecting against oxidative damage. J Appl Physiol. 2003;95:1937–46. doi: 10.1152/japplphysiol.00419.2003. [DOI] [PubMed] [Google Scholar]
- Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107:9730–5. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–6. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–5. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Huang JY, Schwer B, Verdin E. SIRT3 Regulates Mitochondrial Protein Acetylation and Intermediary Metabolism. Cold Spring Harb Symp Quant Biol. 2011a;76:267–77. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Jr, Kahn CR, Verdin E. SIRT3 Deficiency and Mitochondrial Protein Hyperacetylation Accelerate the Development of the Metabolic Syndrome. Mol Cell. 2011b;44:177–90. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflugers Arch. 2001;442:426–34. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–12. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory”. J Appl Physiol. 1986;61:1656–60. doi: 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–93. [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38:947–54. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Fidalgo MA, Hoogewijs D, Braeckman BP, Lenaerts I, Brys K, Matthijssens F, De Vreese A, Van Eygen S, Munoz MJ, Vanfleteren JR. Metabolism, physiology and stress defense in three aging Ins/IGF-1 mutants of the nematode Caenorhabditis elegans. Aging Cell. 2005;4:87–95. doi: 10.1111/j.1474-9726.2005.00150.x. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–91. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–5. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15:1187–94. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Clancy DJ, Mair W, Braeckman BP, Gems D, Partridge L. Metabolic rate is not reduced by dietary-restriction or by lowered insulin/IGF-1 signalling and is not correlated with individual lifespan in Drosophila melanogaster. Exp Gerontol. 2004;39:1137–43. doi: 10.1016/j.exger.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Hussain TA, Mathew TC, Dashti AA, Asfar S, Al-Zaid N, Dashti HM. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition. 2012;28:1016–21. doi: 10.1016/j.nut.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–23. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Roth GS, Lane MA, Ottinger MA, Zou S, de Cabo R, Mattison JA. The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies. Biogerontology. 2006;7:143–8. doi: 10.1007/s10522-006-9013-2. [DOI] [PubMed] [Google Scholar]
- Ishii N, Senoo-Matsuda N, Miyake K, Yasuda K, Ishii T, Hartman PS, Furukawa S. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev. 2004;125:41–6. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–13. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Gleiser CA, Masoro EJ, McMahan CA, Seo EJ, Yu BP. Influence of the restriction of individual dietary components on longevity and age-related disease of Fischer rats: the fat component and the mineral component. J Gerontol. 1988;43:B13–21. doi: 10.1093/geronj/43.1.b13. [DOI] [PubMed] [Google Scholar]
- Jackson MJ. Free radicals generated by contracting muscle: By-products of metabolism or key regulators of muscle function? Free Radic Biol Med. 2008;44:132–41. doi: 10.1016/j.freeradbiomed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- James DE, Kraegen EW, Chisholm DJ. Effect of exercise training on whole-body insulin sensitivity and responsiveness. J Appl Physiol. 1984;56:1217–22. doi: 10.1152/jappl.1984.56.5.1217. [DOI] [PubMed] [Google Scholar]
- Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci. 2009;64:1114–25. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski SM. The retrograde response links metabolism with stress responses, chromatin-dependent gene activation, and genome stability in yeast aging. Gene. 2005;354:22–7. doi: 10.1016/j.gene.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–35. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S, Tedesco P. Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans. Exp Gerontol. 2001;36:1609–17. doi: 10.1016/s0531-5565(01)00144-9. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kapahi P. The hypoxic response and aging. Cell Cycle. 2009;8:2324. doi: 10.4161/cc.8.15.9126. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–80. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–6. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Banting Lecture: Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–84. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–21. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kang C, O’Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappeler L, De Magalhaes Filho CM, Dupont J, Leneuve P, Cervera P, Perin L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. doi: 10.1371/journal.pbio.0060254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataja-Tuomola M, Sundell JR, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- Katic M, Kennedy AR, Leykin I, Norris A, McGettrick A, Gesta S, Russell SJ, Bluher M, Maratos-Flier E, Kahn CR. Mitochondrial gene expression and increased oxidative metabolism: role in increased lifespan of fat-specific insulin receptor knock-out mice. Aging Cell. 2007;6:827–39. doi: 10.1111/j.1474-9726.2007.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr. 2009;28:3–9. doi: 10.1016/j.clnu.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Keipert S, Voigt A, Klaus S. Dietary effects on body composition, glucose metabolism, and longevity are modulated by skeletal muscle mitochondrial uncoupling in mice. Aging Cell. 2011;10:122–36. doi: 10.1111/j.1474-9726.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–8. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–4. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of aging. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Keren G, Epstein Y. The effect of high dosage vitamin C intake on aerobic and anaerobic capacity. J Sports Med Phys Fitness. 1980;20:145–8. [PubMed] [Google Scholar]
- Kharade SV, Mittal N, Das SP, Sinha P, Roy N. Mrg19 depletion increases S. cerevisiae lifespan by augmenting ROS defence. FEBS Lett. 2005;579:6809–13. doi: 10.1016/j.febslet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549:645–52. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Takahashi M, Shimizu T, Shirasawa T, Kajita M, Kanayama A, Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech Ageing Dev. 2008;129:322–31. doi: 10.1016/j.mad.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Kim JD, McCarter RJ, Yu BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging (Milano) 1996;8:123–9. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- Kim W, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein: new insights into oxygen sensing and cancer. Curr Opin Genet Dev. 2003;13:55–60. doi: 10.1016/s0959-437x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Kim Y, Sun H. Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell. 2007;6:489–503. doi: 10.1111/j.1474-9726.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276:18399–406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–6. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–29. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Weindruch R, Walford RL. Influences of dietary restriction and age on liver enzyme activities and lipid peroxidation in mice. J Nutr. 1987;117:361–7. doi: 10.1093/jn/117.2.361. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Koren A, Sauber C, Sentjurc M, Schara M. Free radicals in tetanic activity of isolated skeletal muscle. Comp Biochem Physiol B. 1983;74:633–5. doi: 10.1016/0305-0491(83)90241-9. [DOI] [PubMed] [Google Scholar]
- Kotani K, Peroni OD, Minokoshi Y, Boss O, Kahn BB. GLUT4 glucose transporter deficiency increases hepatic lipid production and peripheral lipid utilization. J Clin Invest. 2004;114:1666–75. doi: 10.1172/JCI21341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1324–9. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. Bmj. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–6. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–9. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–43. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiologic effects of calorie restriction. J Anti-Aging Medicine. 1998;1:327–336. [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–42. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue BL, Padilla PA. Environmental and Genetic Preconditioning for Long-Term Anoxia Responses Requires AMPK in Caenorhabditis elegans. PLoS ONE. 2011;6:e16790. doi: 10.1371/journal.pone.0016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, Park JW, Kwon KB, Park BH. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-kappaB signaling pathway. Diabetes. 2009a;58:344–51. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans lifespan via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–6. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by down-regulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009b;10:379–91. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–8. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8:829–38. doi: 10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–13. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-Span Extension From Hypoxia in Caenorhabditis elegans Requires Both HIF-1 and DAF-16 and Is Antagonized by SKN-1. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Kaeberlein M. A role for SIRT1 in the hypoxic response. Mol Cell. 2010;38:779–80. doi: 10.1016/j.molcel.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 Signaling: a Mechanism for Cellular Stress Resistance in Long-lived Mice. Mol Cell Biol. 2010;30:871–84. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto S, Kokkonen GC, Barrows CH., Jr Dietary protein, life-span, and biochemical variables in female mice. J Gerontol. 1976;31:144–8. doi: 10.1093/geronj/31.2.144. [DOI] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a Guardian of Healthspan and Gatekeeper of Species Longevity. Integr Comp Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Srivastava P. Heat-shock proteins. Curr Protoc Immunol. 2004. Appendix 1, Appendix 1T. [DOI] [PubMed]
- Lillig CH, Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–78. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta-carotene supplementation and cancer risk: A randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–6. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–8. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002;123:765–71. doi: 10.1016/s0047-6374(01)00422-5. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended lifespan conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92:7540–4. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RK, Walford RL. Increased growth and lifespan with lowered ambient temperature in the annual fish, Cynolebis Adloffi. Nature. 1966;212:1277–1278. [Google Scholar]
- Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA. 1999;282:1073–5. doi: 10.1001/jama.282.11.1073. [DOI] [PubMed] [Google Scholar]
- Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–34. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. Über den Temperaturkoeffizienten für die Lebensdauer kaltblütiger Tiere und über die Ursache des natürlichen Todes. Arch. Ges. Physiol. 1908;124:411–426. [Google Scholar]
- Loeb J, Northrop JH. Is There a Temperature Coefficient for the Duration of Life? Proc Natl Acad Sci U S A. 1916;2:456–7. doi: 10.1073/pnas.2.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev. 1993;70:177–99. doi: 10.1016/0047-6374(93)90047-u. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR Signaling in Growth Control and Disease. Cell. 2012;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig AH, Izrayelit Y, Park D, Malik RU, Zimmermann A, Mahanti P, Fox BW, Bethke A, Doering F, Riddle DL, Schroeder FC. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc Natl Acad Sci U S A. 2013;110:5522–5527. doi: 10.1073/pnas.1214467110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, Goodall S, Skepper J, Mair W, Brand MD, Partridge L. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J Gerontol A Biol Sci Med Sci. 2006;61:36–47. doi: 10.1093/gerona/61.1.36. [DOI] [PubMed] [Google Scholar]
- Mahlke MA, Cortez LA, Ortiz MA, Rodriguez M, Uchida K, Shigenaga MK, Lee S, Zhang Y, Tominaga K, Hubbard GB, Ikeno Y. The anti-tumor effects of calorie restriction are correlated with reduced oxidative stress in ENU-induced gliomas. Pathobiol Aging Age Relat Dis. 2011;1 doi: 10.3402/pba.v1i0.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–54. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy VL, Krajcik RA, Bailey SJ, Hristopoulos G, Plummer JD, Orentreich N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5:305–14. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- Manini TM, Everhart JE, Patel KV, Schoeller DA, Colbert LH, Visser M, Tylavsky F, Bauer DC, Goodpaster BH, Harris TB. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–9. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–6. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Scott KC, Hill RC, Lewis DD, Sundstrom D, Jones GL, Harper J. Supplemental vitamin C appears to slow racing greyhounds. J Nutr. 2002;132:1616S–21S. doi: 10.1093/jn/132.6.1616S. [DOI] [PubMed] [Google Scholar]
- Marzatico F, Pansarasa O, Bertorelli L, Somenzini L, Della Valle G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J Sports Med Phys Fitness. 1997;37:235–9. [PubMed] [Google Scholar]
- Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–6. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc Natl Acad Sci U S A. 1982;79:4239–41. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–21. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ. Nutritional ergogenic aids and exercise performance. Nutrition Research Reviews. 1999;12:255–80. doi: 10.1079/095442299108728956. [DOI] [PubMed] [Google Scholar]
- McCarter R, Mejia W, Ikeno Y, Monnier V, Kewitt K, Gibbs M, McMahan A, Strong R. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62:1059–70. doi: 10.1093/gerona/62.10.1059. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, Selman C, Withers DJ, Thornton JM, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–43. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–8. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of Conserved C. elegans Genes Engages Pathogen- and Xenobiotic-Associated Defenses. Cell. 2012;149:452–66. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–9. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 2013;5:144–50. doi: 10.18632/aging.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–12. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–8. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Budde MW, Roth MB. HIF-1 and SKN-1 Coordinate the Transcriptional Response to Hydrogen Sulfide in Caenorhabditis elegans. PLoS ONE. 2011;6:e25476. doi: 10.1371/journal.pone.0025476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:20618–22. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–25. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Tatar M. Restriction of amino acids extends lifespan in Drosophila melanogaster. Mech Ageing Dev. 2006;127:643–6. doi: 10.1016/j.mad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minor RK, Smith DL, Jr, Sossong AM, Kaushik S, Poosala S, Spangler EL, Roth GS, Lane M, Allison DB, de Cabo R, Ingram DK, Mattison JA. Chronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in rats. Toxicol Appl Pharmacol. 2010;243:332–9. doi: 10.1016/j.taap.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–9. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12:774–86. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A. 2001;98:12920–5. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–29. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–57. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–83. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nadanaciva S, Dykens JA, Bernal A, Capaldi RA, Will Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol Appl Pharmacol. 2007;223:277–87. doi: 10.1016/j.taap.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–4. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- Niess AM, Dickhuth HH, Northoff H, Fehrenbach E. Free radicals and oxidative stress in exercise—immunological aspects. Exerc Immunol Rev. 1999;5:22–56. [PubMed] [Google Scholar]
- Nikolaidis MG, Jamurtas AZ. Blood as a reactive species generator and redox status regulator during exercise. Arch Biochem Biophys. 2009;490:77–84. doi: 10.1016/j.abb.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Nobels F, van Gaal L, de Leeuw I. Weight reduction with a high protein, low carbohydrate, calorie-restricted diet: effects on blood pressure, glucose and insulin levels. Neth J Med. 1989;35:295–302. [PubMed] [Google Scholar]
- Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Jr, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135:907–18. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–30. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–41. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–61. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 2011;3:102–7. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MM, Richardson J, Wiens BE, Tiedtke E, Peters CW, Faure PA, Burness G, Stuart JA. Antioxidant enzyme activities are not broadly correlated with longevity in 14 vertebrate endotherm species. Age (Dordr) 2010;32:255–70. doi: 10.1007/s11357-010-9131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MM, Stuart JA. Activities of DNA base excision repair enzymes in liver and brain correlate with body mass, but not lifespan. Age (Dordr) 2012;34:1195–209. doi: 10.1007/s11357-011-9302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MM, Withers DJ, Selman C. Longevity of insulin receptor substrate1 null mice is not associated with increased basal antioxidant protection or reduced oxidative damage. Age (Dordr) 2013;35:647–58. doi: 10.1007/s11357-012-9395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J. 2002;367:179–86. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. Mitochondria, reactive oxygen species, and chronological aging: A message from yeast. Exp Gerontol. 2011;46:847–52. doi: 10.1016/j.exger.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Pan Y, Nishida Y, Wang M, Verdin E. Metabolic Regulation, Mitochondria and the Life-Prolonging Effect of Rapamycin: A Mini-Review. Gerontology. 2012;58:524–30. doi: 10.1159/000342204. [DOI] [PubMed] [Google Scholar]
- Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13:668–78. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Shadel GS. Extension of chronological life span by reduced TOR signaling requires down-regulation of Sch9p and involves increased mitochondrial OXPHOS complex density. Aging (Albany NY) 2009;1:131–45. doi: 10.18632/aging.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–5. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Pardo PS, Mohamed JS, Lopez MA, Boriek AM. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem. 2011;286:2559–66. doi: 10.1074/jbc.M110.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh VS, Morgan MM, Scott R, Clements LS, Butow RA. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987;235:576–80. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators Inflamm. 2012;2012:984643. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–4. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner A, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–72. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. The rate of living. Being an account of some experimental studies on the biology of life duration. New York: Alfred Knopf; 1928. pp. 183–185. [Google Scholar]
- Pendergrass WR, Li Y, Jiang D, Wolf NS. Decrease in cellular replicative potential in “giant” mice transfected with the bovine growth hormone gene correlates to shortened life span. J Cell Physiol. 1993;156:96–103. doi: 10.1002/jcp.1041560114. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–14. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VI, Cortez LA, Lew CM, Rodriguez M, Webb CR, Van Remmen H, Chaudhuri A, Qi W, Lee S, Bokov A, Fok W, Jones D, Richardson A, Yodoi J, Zhang Y, Tominaga K, Hubbard GB, Ikeno Y. Thioredoxin 1 Overexpression Extends Mainly the Earlier Part of Life Span in Mice. J Gerontol A Biol Sci Med Sci. 2011;66:1286–99. doi: 10.1093/gerona/glr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone CE, Malloy VL, Orentreich DS, Orentreich N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp Gerontol. 2013;48:654–60. doi: 10.1016/j.exger.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism. 2010;59:1000–11. doi: 10.1016/j.metabol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Campbell SD, Michaud D, Charbonneau M, Hilliker AJ. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989;86:2761–5. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri C, Falasca M, Marcheselli F, Moroni F, Recchioni R, Marmocchi F, Lupidi G. Food restriction in female Wistar rats: V. Lipid peroxidation and antioxidant enzymes in the liver. Arch Gerontol Geriatr. 1992;14:93–9. doi: 10.1016/0167-4943(92)90010-2. [DOI] [PubMed] [Google Scholar]
- Piper MD, Mair W, Partridge L. Counting the calories: the role of specific nutrients in extension of life span by food restriction. J Gerontol A Biol Sci Med Sci. 2005a;60:549–55. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Piper MD, Skorupa D, Partridge L. Diet, metabolism and lifespan in Drosophila. Exp Gerontol. 2005b;40:857–62. doi: 10.1016/j.exger.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Piper PW, Harris NL, MacLean M. Preadaptation to efficient respiratory maintenance is essential both for maximal longevity and the retention of replicative potential in chronologically ageing yeast. Mech Ageing Dev. 2006;127:733–40. doi: 10.1016/j.mad.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–84. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc Nutr Soc. 1999;58:1025–33. doi: 10.1017/s0029665199001342. [DOI] [PubMed] [Google Scholar]
- Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–50. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Pua HH, He YW. Mitophagy in the little lymphocytes: an essential role for autophagy in mitochondrial clearance in T lymphocytes. Autophagy. 2009;5:745–6. doi: 10.4161/auto.5.5.8702. [DOI] [PubMed] [Google Scholar]
- Puntschart A, Vogt M, Widmer HR, Hoppeler H, Billeter R. Hsp70 expression in human skeletal muscle after exercise. Acta Physiol Scand. 1996;157:411–7. doi: 10.1046/j.1365-201X.1996.512270000.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–7. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Quarrie JK, Riabowol KT. Murine models of life span extension. Sci Aging Knowledge Environ. 2004;2004:re5. doi: 10.1126/sageke.2004.31.re5. [DOI] [PubMed] [Google Scholar]
- Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging. 2008;29:117–28. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Quintanilha AT. Effects of physical exercise and/or vitamin E on tissue oxidative metabolism. Biochem Soc Trans. 1984;12:403–4. doi: 10.1042/bst0120403. [DOI] [PubMed] [Google Scholar]
- Radak Z, Apor P, Pucsok J, Berkes I, Ogonovszky H, Pavlik G, Nakamoto H, Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–33. doi: 10.1016/s0024-3205(02)02476-1. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–5. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Radak Z, Kaneko T, Tahara S, Nakamoto H, Ohno H, Sasvari M, Nyakas C, Goto S. The effect of exercise training on oxidative damage of lipids, proteins, and DNA in rat skeletal muscle: evidence for beneficial outcomes. Free Radic Biol Med. 1999;27:69–74. doi: 10.1016/s0891-5849(99)00038-6. [DOI] [PubMed] [Google Scholar]
- Radak Z, Sasvari M, Nyakas C, Pucsok J, Nakamoto H, Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys. 2000;376:248–51. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- Rao G, Xia E, Nadakavukaren MJ, Richardson A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr. 1990;120:602–9. doi: 10.1093/jn/120.6.602. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SI, Demirovic D. Hormesis as a Mechanism for the Anti-Aging Effects of Calorie Restriction. In: Everitt AV, Rattan SIS, Couteur DG, Cabo RD, editors. Calorie Restriction, Aging and Longevity. Springer; Netherlands: 2010. pp. 233–245. [Google Scholar]
- Rautalahti MT, Virtamo JR, Taylor PR, Heinonen OP, Albanes D, Haukka JK, Edwards BK, Karkkainen PA, Stolzenberg-Solomon RZ, Huttunen J. The effects of supplementation with alpha-tocopherol and beta-carotene on the incidence and mortality of carcinoma of the pancreas in a randomized, controlled trial. Cancer. 1999;86:37–42. [PubMed] [Google Scholar]
- Raynes R, Leckey BD, Jr, Nguyen K, Westerheide SD. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J Biol Chem. 2012;287:29045–53. doi: 10.1074/jbc.M112.353714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–8. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–7. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–12. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M. Oxidative metabolism in cancer growth. Curr Opin Clin Nutr Metabol. 2006;9:339–345. doi: 10.1097/01.mco.0000232892.43921.98. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Nat Acad Sci. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR Signaling and Rapamycin Influence Longevity by Regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–24. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–8. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–7. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Roux AE, Leroux A, Alaamery MA, Hoffman CS, Chartrand P, Ferbeyre G, Rokeach LA. Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet. 2009;5:e1000408. doi: 10.1371/journal.pgen.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Tang XD, Chen ML, Joiner ML, Sun G, Brot N, Weissbach H, Heinemann SH, Iverson L, Wu CF, Hoshi T. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc Natl Acad Sci U S A. 2002;99:2748–53. doi: 10.1073/pnas.032671199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Rubner M. III. Das Wachstumsproblem und die Lebensdauer des Menschen und einiger Säugetiere vom energetischen Standpunkt aus betrachtet. In: Rubner M, editor. Das Problem der Lebensdauer und seine Beziehungen zum Wachstum und der Ernährung. Munich and Berlin: R. Oldenbourg; 1908. pp. 127–208. [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266:11632–9. [PubMed] [Google Scholar]
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–91. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302:E145–52. doi: 10.1152/ajpendo.00618.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Abbasi F, Lamendola C, Carter S, McLaughlin TL. Serum alanine amino-transferase levels decrease further with carbohydrate than fat restriction in insulin-resistant adults. Diabetes Care. 2007;30:1075–80. doi: 10.2337/dc06-2169. [DOI] [PubMed] [Google Scholar]
- Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–72. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533–9. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salway KD, Page MM, Faure PA, Burness G, Stuart JA. Enhanced protein repair and recycling are not correlated with longevity in 15 vertebrate endotherm species. Age (Dordr) 2011;33:33–47. doi: 10.1007/s11357-010-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roman I, Gomez A, Perez I, Sanchez C, Suarez H, Naudi A, Jove M, Lopez-Torres M, Pamplona R, Barja G. Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology. 2012;13:399–411. doi: 10.1007/s10522-012-9384-5. [DOI] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ayala V, Portero-Otin M, Pamplona R, Barja G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–73. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- Sanz A, Fernandez-Ayala DJ, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in drosophila. Aging (Albany NY) 2010;2:200–3. doi: 10.18632/aging.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Stefanatos RK. The mitochondrial free radical theory of aging: a critical view. Curr Aging Sci. 2008;1:10–21. doi: 10.2174/1874609810801010010. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schlotterer A, Kukudov G, Bozorgmehr F, Hutter H, Du X, Oikonomou D, Ibrahim Y, Pfisterer F, Rabbani N, Thornalley P, Sayed A, Fleming T, Humpert P, Schwenger V, Zeier M, Hamann A, Stern D, Brownlee M, Bierhaus A, Nawroth P, Morcos M. C. elegans as model for the study of high glucose mediated lifespan reduction. Diabetes. 2009;58:2450–6. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, Schmeisser S, Schuster S, Pfeiffer AF, Guthke R, Platzer M, Hoppe T, Cohen HY, Zarse K, Sinclair DA, Ristow M. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013a;9:693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser S, Priebe S, Groth M, Monajembashi S, Hemmerich P, Guthke R, Platzer M, Ristow M. Neuronal ROS Signaling Rather Than AMPK/Sirtuin-Mediated Energy Sensing Links Dietary Restriction to Lifespan Extension. Mol Metab. 2013b;2:92–102. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–18. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63. [PubMed] [Google Scholar]
- Semsei I, Rao G, Richardson A. Changes in the expression of superoxide dismutase and catalase as a function of age and dietary restriction. Biochem Biophys Res Commun. 1989;164:620–5. doi: 10.1016/0006-291x(89)91505-2. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendoel A, Kohler I, Fellmann C, Lowe SW, Hengartner MO. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature. 2010;465:577–83. doi: 10.1038/nature09141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–4. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–33. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shama S, Lai CY, Antoniazzi JM, Jiang JC, Jazwinski SM. Heat stress-induced life span extension in yeast. Exp Cell Res. 1998;245:379–88. doi: 10.1006/excr.1998.4279. [DOI] [PubMed] [Google Scholar]
- Sharma PK, Agrawal V, Roy N. Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. Age (Dordr) 2011;33:143–54. doi: 10.1007/s11357-010-9169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman IM, Down MG, Sen RN. The effects of vitamin E and training on physiological function and athletic performance in adolescent swimmers. Br J Nutr. 1971;26:265–76. doi: 10.1079/bjn19710033. [DOI] [PubMed] [Google Scholar]
- Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J Biol Chem. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- Shen C, Powell-Coffman JA. Genetic analysis of hypoxia signaling and response in C elegans. Ann N Y Acad Sci. 2003;995:191–9. doi: 10.1111/j.1749-6632.2003.tb03222.x. [DOI] [PubMed] [Google Scholar]
- Shibamura A, Ikeda T, Nishikawa Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: effects of oral supplementation with antioxidants on the nematode lifespan. Mech Ageing Dev. 2009;130:652–5. doi: 10.1016/j.mad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative Stress. London: Academic Press; 1985. Oxidative Stress: Introductory Remarks. [Google Scholar]
- Sinclair DA, Mills K, Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277:1313–6. doi: 10.1126/science.277.5330.1313. [DOI] [PubMed] [Google Scholar]
- Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–24. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- Smith DL, Jr, McClure JM, Matecic M, Smith JS. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the Sirtuins. Aging Cell. 2007;6:649–62. doi: 10.1111/j.1474-9726.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- Smith ED, Tsuchiya M, Fox LA, Dang N, Hu D, Kerr EO, Johnston ED, Tchao BN, Pak DN, Welton KL, Promislow DE, Thomas JH, Kaeberlein M, Kennedy BK. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 2008;18:564–70. doi: 10.1101/gr.074724.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–55. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell. 2010;143:802–12. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009;90:429–37. doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam CM, Ehrlich J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–24. [Google Scholar]
- Speakman JR, Selman C, McLaren JS, Harper EJ. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132:1583S–97S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;283:E38–43. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- St. Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- St. Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Steger RW, Bartke A, Cecim M. Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18:3112–9. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzner G. Effects of life-long dietary protein restriction on mortality, growth, organ weights, blood counts, liver aldolase and kidney catalase in Balb/C mice. Growth. 1977;41:337–48. [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Med Sci Sports Exerc. 2011;43:1017–24. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–72. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Takemori K, Kimura T, Shirasaka N, Inoue T, Masuno K, Ito H. Food restriction improves glucose and lipid metabolism through Sirt1 expression: a study using a new rat model with obesity and severe hypertension. Life Sci. 2011;88:1088–94. doi: 10.1016/j.lfs.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: ‘Mitohormesis’ for health and vitality. Med Hypotheses. 2006;66:832–43. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–10. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–14. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–47. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y, Jamurtas AZ. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr. 2011;93:1373–83. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–70. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Tormos KV, Chandel NS. Inter-connection between mitochondria and HIFs. J Cell Mol Med. 2010;14:795–804. doi: 10.1111/j.1582-4934.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–8. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Tsai CW, Lin AH, Wang TS, Liu KL, Chen HW, Lii CK. Methionine restriction up-regulates the expression of the pi class of glutathione S-transferase partially via the extra-cellular signal-regulated kinase-activator protein-1 signaling pathway initiated by glutathione depletion. Mol Nutr Food Res. 2010;54:841–50. doi: 10.1002/mnfr.200900083. [DOI] [PubMed] [Google Scholar]
- Tucci P. Caloric restriction: is mammalian life extension linked to p53? Aging (Albany NY) 2012;4:525–34. doi: 10.18632/aging.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–38. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DE, Hu LH, Li J, Ye JM. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–8. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udelsman R, Blake MJ, Stagg CA, Li DG, Putney DJ, Holbrook NJ. Vascular heat shock protein expression in response to stress. Endocrine and autonomic regulation of this age-dependent response. J Clin Invest. 1993;91:465–73. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–10. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–9. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Vanfleteren JR, De Vreese A. The gerontogenes age-1 and daf-2 determine metabolic rate potential in aging Caenorhabditis elegans. FASEB J. 1995;9:1355–61. doi: 10.1096/fasebj.9.13.7557026. [DOI] [PubMed] [Google Scholar]
- Various. Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:23–33. [Google Scholar]
- Vincent HK, Powers SK, Demirel HA, Coombes JS, Naito H. Exercise training protects against contraction-induced lipid peroxidation in the diaphragm. Eur J Appl Physiol Occup Physiol. 1999;79:268–73. doi: 10.1007/s004210050505. [DOI] [PubMed] [Google Scholar]
- Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, Huttunen JK, Hartman AM, Hietanen P, Maenpaa H, Koss L, Nordling S, Heinonen OP. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland) Cancer Causes Control. 2000;11:933–9. doi: 10.1023/a:1026546803917. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–23. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- Walker G, Houthoofd K, Vanfleteren JR, Gems D. Dietary restriction in C. elegans: from rate-of-living effects to nutrient sensing pathways. Mech Ageing Dev. 2005;126:929–37. doi: 10.1016/j.mad.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–14. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kaneko T, Wang PY, Sato A. Decreased carbohydrate intake is more important than increased fat intake in the glucose intolerance by a low-carbohydrate/high-fat diet. Diabetes Res Clin Pract. 2002;55:61–3. doi: 10.1016/s0168-8227(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. Can Med Ass J (CMAJ) 2006;174:801–9. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens. 2007;25:227–34. doi: 10.1097/01.hjh.0000254373.96111.43. [DOI] [PubMed] [Google Scholar]
- Watson JD. Antioxidant antidote. New Scientist. 2013:28–29. [Google Scholar]
- Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med. 2012;52:281–90. doi: 10.1016/j.freeradbiomed.2011.10.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. Will dietary restriction work in primates? Biogerontology. 2006;7:169–71. doi: 10.1007/s10522-006-9007-0. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Illinois: Charles C Thomas Pub Ltd; 1988. [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64:443–51. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280:33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957;224:963–9. [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147:2643–9. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, Sridharan A, Allison DB, Anderson R, Voytko ML, Kemnitz JW, Weindruch RH, Johnson SC. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 2012;61:1036–42. doi: 10.2337/db11-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest. 1999;104:1257–64. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–64. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- Wu D, Cypser JR, Yashin AI, Johnson TE. Multiple mild heat-shocks decrease the Gompertz component of mortality in Caenorhabditis elegans. Exp Gerontol. 2009a;44:607–12. doi: 10.1016/j.exger.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging (Albany NY) 2009b;1:425–37. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia E, Rao G, Van Remmen H, Heydari AR, Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr. 1995;125:195–201. doi: 10.1093/jn/125.2.195. [DOI] [PubMed] [Google Scholar]
- Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZ. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–17. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Roy R. Increased Levels of Hydrogen Peroxide Induce a HIF-1-dependent Modification of Lipid Metabolism in AMPK Compromised C. elegans Dauer Larvae. Cell Metab. 2012;16:322–35. doi: 10.1016/j.cmet.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Yanase S, Hartman PS, Ito A, Ishii N. Oxidative stress pretreatment increases the X-radiation resistance of the nematode Caenorhabditis elegans. Mutat Res. 1999;426:31–9. doi: 10.1016/s0027-5107(99)00079-2. [DOI] [PubMed] [Google Scholar]
- Yang W, Hekimi S. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell. 2010;9:433–47. doi: 10.1111/j.1474-9726.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- Yang YY, Gangoiti JA, Sedensky MM, Morgan PG. The effect of different ubiquinones on lifespan in Caenorhabditis elegans. Mech Ageing Dev. 2009;130:370–6. doi: 10.1016/j.mad.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor VK, Patti ME, Ueki K, Laustsen PG, Saccone R, Rauniyar R, Kahn CR. Distinct pathways of insulin-regulated versus diabetes-regulated gene expression: an in vivo analysis in MIRKO mice. Proc Natl Acad Sci U S A. 2004;101:16525–30. doi: 10.1073/pnas.0407574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH, Lykkesfeldt J, Rose AJ, Fischer CP, Pedersen BK. Antioxidant Supplementation Does Not Alter Endurance Training Adaptation. Med Sci Sports Exerc. 2010;42:1388–95. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci. 2004;117:4055–66. doi: 10.1242/jcs.01275. [DOI] [PubMed] [Google Scholar]
- Youngman LD, Park JY, Ames BN. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992;89:9112–6. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired insulin/IGF1-signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS ONE. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–93. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–60. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Exp Gerontol. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]
- Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
- Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285:33154–64. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S, Sinclair J, Wilson MA, Carey JR, Liedo P, Oropeza A, Kalra A, de Cabo R, Ingram DK, Longo DL, Wolkow CA. Comparative approaches to facilitate the discovery of prolongevity interventions: effects of tocopherols on lifespan of three invertebrate species. Mech Ageing Dev. 2007;128:222–6. doi: 10.1016/j.mad.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuin A, Carmona M, Morales-Ivorra I, Gabrielli N, Vivancos AP, Ayte J, Hidalgo E. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. EMBO J. 2010;29:981–91. doi: 10.1038/emboj.2009.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetiere P, Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Thromb Vasc Biol. 2004;24:1485–91. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- Zuryn S, Kuang J, Tuck A, Ebert PR. Mitochondrial dysfunction in Caenorhabditis elegans causes metabolic restructuring, but this is not linked to longevity. Mech Ageing Dev. 2010;131:554–61. doi: 10.1016/j.mad.2010.07.004. [DOI] [PubMed] [Google Scholar]