Short statement
A rice ubiquitin ligase plays a role in preventing root meristematic cell death in the nitrogen-triggered pathway that leads to the production of cytokinin and superoxide.
Key words: Cytokinin, nitrogen, reactive oxygen species, rice, root cell death, ubiquitin ligase.
Abstract
Root formation is dependent on meristematic activity and is influenced by nitrogen supply. We have previously shown that ubiquitin ligase, EL5, in rice (Oryza sativa) is involved in the maintenance of root meristematic viability. When mutant EL5 protein is overexpressed to dominantly inhibit the endogenous EL5 function in rice, primordial and meristematic necrosis ia observed. Here, we analysed the cause of root cell death in transgenic rice plants (mEL5) overexpressing EL5V162A, which encodes a partly inactive ubiquitin ligase. The mEL5 mutants showed increased sensitivity to nitrogen that was reflected in the inhibition of root formation. Treatment of mEL5 with nitrate or nitrite caused meristematic cell death accompanied by browning. Transcriptome profiling of whole roots exhibited overlaps between nitrite-responsive genes in non-transgenic (NT) rice plants and genes with altered basal expression levels in mEL5. Phytohormone profiling of whole roots revealed that nitrite treatment increased cytokinin levels, but mEL5 constitutively contained more cytokinin than NT plants and showed increased sensitivity to exogenous cytokinin. More superoxide was detected in mEL5 roots after treatment with nitrite or cytokinin, and treatment with an inhibitor of superoxide production prevented mEL5 roots from both nitrite- and cytokinin-induced meristematic cell death. These results indicate a nitrogen-triggered pathway that leads to changes in root formation through the production of cytokinin and superoxide, on which EL5 acts to prevent meristematic cell death.
Introduction
Root formation affects vigour and the final yield of crop plants. Nitrogen status influences root growth and development (Forde, 2002; Osmont et al., 2007). Inorganic nitrogen is available in the soil as ammonium, nitrite, and nitrate. Nitrate absorbed by roots is reduced to nitrite by nitrate reductase, and then to ammonium by nitrite reductase in roots and shoots. In general, root elongation and lateral root development are stimulated at low-nitrogen concentrations, while high-nitrogen concentrations confer inhibitory effects on root growth. Several phytohormones are involved in the alteration of root growth in response to nitrogen sources (Aloni et al., 2006; Osmont et al., 2007). Arabidopsis (Arabidopsis thaliana) NRT1.1 is the nitrate transporter that also facilitates uptake of auxin, and this transporter is proposed to connect nitrate availability and auxin signalling during changes in root growth (Krouk et al., 2010). Supply of nitrogen leads to increased cytokinin content in roots; exogenous application of cytokinin downregulates nitrogen-uptake-related genes (Sakakibara et al., 2006). Inhibition of root growth in maize (Zea mays) by high nitrate supply is partly attributed to decreased auxin concentrations (Tian et al., 2008) and increased levels of cytokinin in roots (Tian et al., 2005).
Root formation relies on the activity of the root apical meristem (RAM). Cytokinin inhibits root elongation by negatively regulating RAM activity; cytokinin-deficient plants form larger RAMs and more rapidly growing roots (Werner et al., 2003). Ogawa et al. (2011) reported that a rice mutant, rss1 (rice salt sensitive 1), exhibited irreversible growth inhibition under saline conditions; RSS1 contributes to the maintenance of meristematic activity and viability in shoots and roots by regulating the G1–S transition in the cell cycle. However, the ways in which activity or viability of the RAM is maintained under various changes in environmental conditions are largely unknown.
The Arabidopsis Tóxicos en Levadura (ATL) family is a large, plant-specific family of RING-H2-type ubiquitin ligases (E3). ATL genes were found in 24 plant species (including mosses and lycopods), with 91 genes identified in Arabidopsis and 121 in rice (Aguilar-Hernández et al., 2011). CNI1/ATL31 in Arabidopsis is the only ATL family member whose substrate has been identified. CNI1/ATL31 functions in the carbon/nitrogen response for the seedling growth-phase transition (Sato et al., 2009) and ubiquitinates 14-3-3 protein, one function of which is to regulate nitrate reductase activity (Sato et al., 2011).
Rice EL5 is a member of the ATL family, with six copies in the genome (RAP-ID: Os02g0559800–Os02g0561800). The RING-H2-finger domain (or RFD) in EL5 interacts with a rice ubiquitin-conjugating enzyme, UBC5b, and thus is essential for E3 activity (Takai et al., 2002). Mutated RING-H2-finger domains show varying decreases in E3 activity depending on the degree of interaction with UBC5b. Replacement of Trp-165 with Ala results in the complete loss of its E3 activity, whereas replacement of Val-162 with Ala causes a reduction in E3 activity in vitro (Katoh et al., 2005). Overexpression of EL5 in transgenic rice plants and attempts to suppress EL5 by RNAi constructs lead to no evident morphological changes. In contrast, overexpression of mutated EL5 to prevent the endogenous EL5 function inhibits root formation in an E3-activity-dependent manner: rice transformants constitutively expressing EL5W165A exhibit a rootless phenotype when cultured in Murashige and Skoog (MS) medium, whereas plants expressing EL5V162A occasionally develop short crown roots with necrotic lateral roots (Koiwai et al., 2007). The phenotype of these transgenic plants strongly suggests that EL5 preferentially acts on root development by maintaining proliferation and viability of the primordial cells in crown and lateral roots. Moreover, the upregulation of EL5 expression by cytokinin and the similarity between the phenotype of transgenic plants overexpressing the mutated EL5 and that of cytokinin-treated roots in non-transgenic (NT) rice plants imply that EL5 might be involved in the cytokinin action in roots (Koiwai et al., 2007). These previous results prompted us to analyse the cause of root cell death in transgenic rice plants expressing mutated EL5, to elucidate the mechanisms underlying the maintenance of RAM viability during root formation.
Phytohormones influence various cellular processes, including maintenance of the RAM, through production of reactive oxygen species (ROS) and NO (Joo et al., 2001; Pagnussat et al., 2002; Foreman et al., 2003; Jiang et al., 2003; Correa-Aragunde et al., 2004). However, both ROS and NO are implicated in triggering oxidative cell death (Jabs et al., 1996; Delledonne et al., 1998). Because severe necrosis was observed to be centred on the quiescent centre of crown roots in EL5W165A-expressing plants (Koiwai et al., 2007), we hypothesized that phytohormone contents and/or regulation of the generation of ROS or NO are altered in the roots of these plants, which results in meristematic cell death. In the present study, we first found that the presence of nitrogen sources in the culture medium was a causal agent of the rootless phenotype. Thus, we analysed nitrogen-induced root cell death by focusing on the global transcriptome profile, hormone contents, and accumulation of ROS and NO. Through comparative analyses between the mutated EL5-overexpressing roots and NT roots, we propose a mechanism that prevents the inhibitory effects of nitrogen on root formation in rice.
Materials and methods
Plant materials
Rice seeds (Oryza sativa L. japonica ‘Nipponbare BL no. 2’) were provided by Dr Hiroyuki Satoh of the National Institute of Crop Science, Tsukuba, Japan. Transgenic rice plants of the same cultivar expressing EL5V162A (mEL5) were produced as described in Koiwai et al. (2007). Because it was difficult to grow mEL5 transformants to the bearing stage due to their deficient roots and because mEL5 shoots were actively branching, we used regenerated mEL5 shoots that were subcultured for a 3 week interval in MS medium containing 1% (w/v) agar, 25 mg·l−1 hygromycin, and 6.25 mg·l−1 meropen (Dainippon Sumitomo Pharma, Tokyo, Japan) at 28 °C under continuous light (35 µmol·m−2·s−1) in a growth chamber.
Root-formation test
Root-formation tests were performed using shoots that underwent aseptic hydroponic culture in a low-salt culture solution (LSCS) medium [70 µM CaCl2, 36 µM FeSO4, 9 µM MnSO4, 3 µM ZnSO4, 0.2 µM CuSO4, 4.5 µM H3BO4, 0.05 µM Na2MoO4, 7 µM Na2HPO4, 16 µM KCl, 150 µM MgCl2, 3% (w/v) sucrose, and 10mM MES (pH 5.8)], which is a modification of the nutrient solution reported by Sonoda et al. (2003). Husked NT seeds were sterilized in 0.5% (v/v) sodium hypochlorite solution for 30min and then washed five times with distilled water. Seeds were grown in quarter-strength MS medium containing 1% agar at 28 °C under continuous light in a growth chamber until the three-leaf stage. All roots and endosperm tissues were removed and the 12 rootless shoots, whose basal regions were rinsed with distilled water, were placed into holes in a plastic plate that floated on 100ml of LSCS medium with various concentrations of a nitrogen source (Fig. 1A) in a 500ml glass bottle. The shoots were cultured in a growth chamber at 28 °C under continuous light (35 µmol·m−2·s−1). In tests of mEL5 plants, the subcultured rootless shoots described above were rinsed with distilled water and cultured in LSCS medium in the same way as the rootless NT shoots. After 4 days, the length of the longest crown root of each shoot was measured. This crown root-formation test was repeated twice (total n = 22–24 shoots). Statistical analyses (Tukey–Kramer test and Dunnett’s test) were performed using JMP8 software (SAS Institute, Cary, NC, USA).
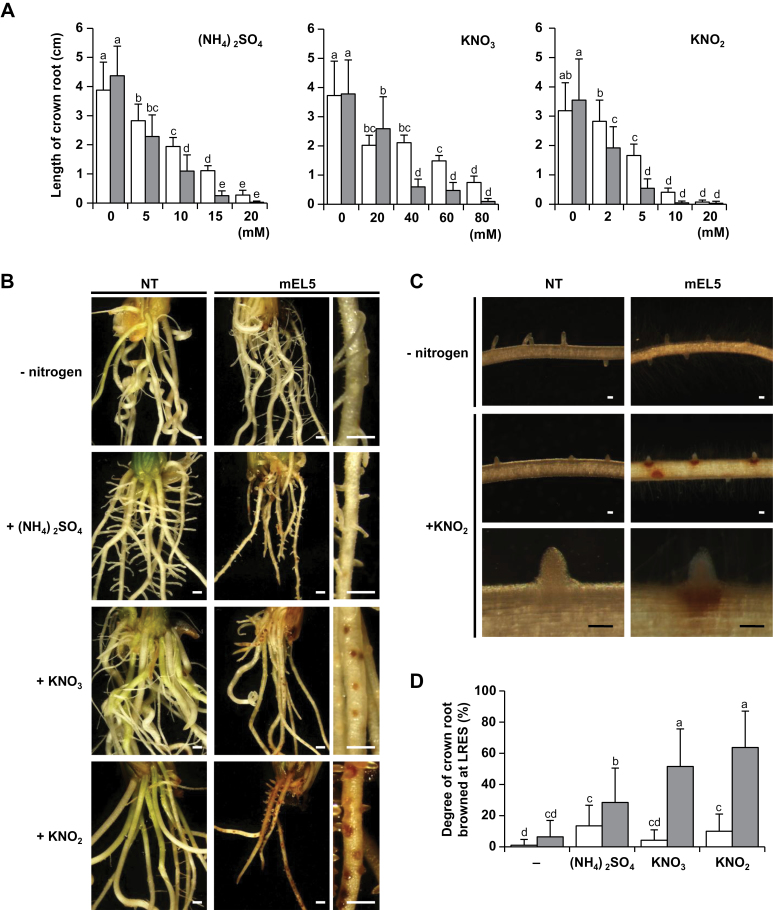
Fig. 1.
Phenotypes of mEL5 roots in response to nitrogen supply. (A) Inhibitory effect of nitrogen on crown root elongation. Average length of the longest crown root of non-transformants (NT, white bars) and transgenic rice plants overexpressing EL5V162A (mEL5, grey bars) formed after 4 days with different concentrations of nitrogen sources is shown. Each treatment included 22–24 roots. Error bars indicate SD. Different letters above the bars indicate significant differences at P < 0.01 (Tukey–Kramer test). (B) Phenotypes of NT and mEL5 roots cultured with different nitrogen sources. Rootless shoots were cultured with 10mM (NH4)2SO4, 15mM KNO3, or 5mM KNO2 for 7 days. Scale bars, 500 µm. (C) Effect of KNO2 on LRESs. Preformed roots were treated with 5mM KNO2 for 48h. Scale bars, 100 µm. (D) Degree of nitrogen-induced browning at LRESs in NT (white bars) and mEL5 (grey bars). Crown roots were grown for 4 days without nitrogen sources and then were treated with 15mM (NH4)2SO4, 40mM KNO3, or 5mM KNO2 for 1 day. The average values of 40 shoots per treatment are shown. Error bars indicate SD. Different letters above the bars indicate significant differences at P < 0.01 (Tukey–Kramer test with inverse sine transformation).
Assays of necrosis at lateral-root-emergence sites
Examination of browning at the lateral-root-emergence site (LRES) was also performed aseptically. Ten rootless shoots per bottle, prepared as mentioned above, were precultured in LSCS medium for 4 days to form crown roots. Roots were then treated with nitrogen and/or cytokinin [trans-zeatin (tZ) or N 6-(2-isopentenyl)-adenine (iP); Nacalai Tesque, Kyoto, Japan] with and without diphenyleneiodonium chloride (DPI) (Toronto Research Chemicals, North York, Canada), or with 2-phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl (PTIO) (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan). Data in Figs 1D, 4B, C, and 5 are presented as the percentage of crown roots per shoot that contained at least one browned LRES. Concentrations of each chemical are indicated in the figure legends. Each assay was repeated four times (total n = 40 shoots). Statistical analyses (Tukey–Kramer test and Dunnett’s test) were performed using JMP8 software.
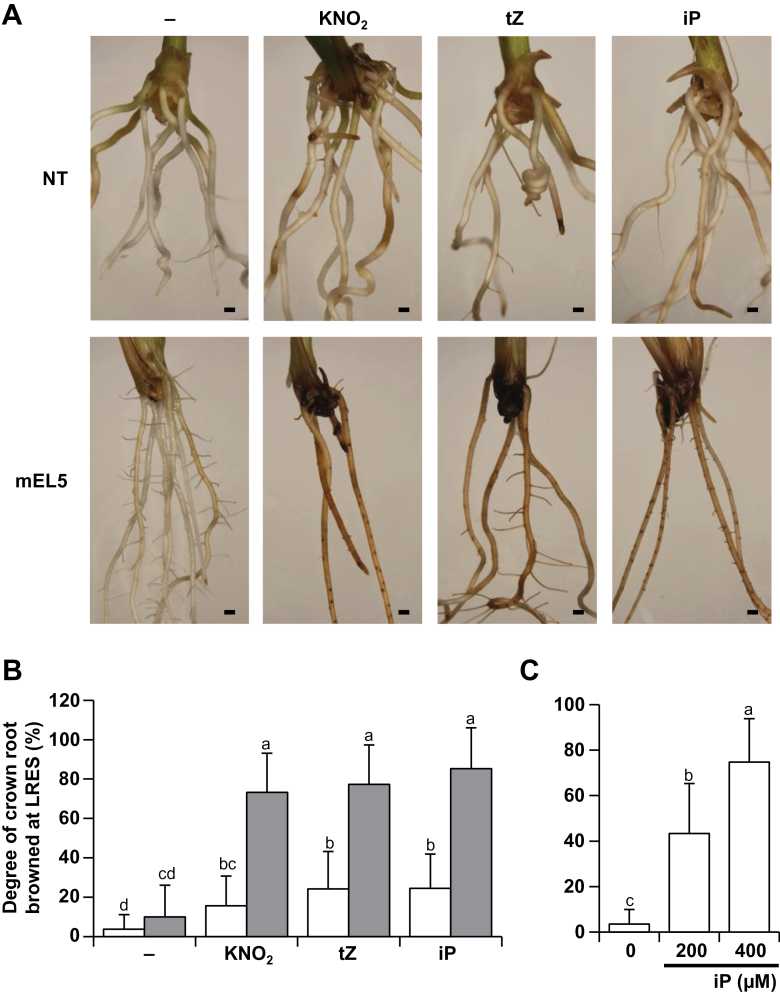
Fig. 4.
Effect of cytokinin on browning of LRESs. (A) Phenotypes of roots of non-transformants (NT) and transgenic rice plants overexpressing EL5V162A (mEL5) treated with 5mM KNO2, 200 µM tZ, or 200 µM iP for 3 days. Scale bars, 1mm. (B) Degree of LRES browning in NT (white bars) and mEL5 (grey bars) after treatment with 5mM KNO2, 200 µM tZ, or 200 µM iP for 3 days. (C) Degree of LRES browning in NT treated with iP for 2 days. Values are averages (n = 40 shoots per treatment) with SD. Different letters above the bars indicate significant differences at P < 0.01 (Tukey–Kramer test with inverse sine transformation).
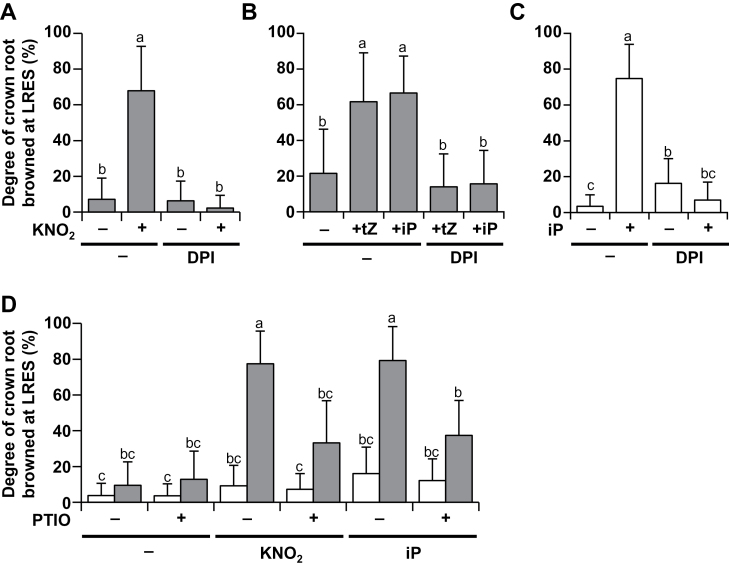
Fig. 5.
Effects of an inhibitor of superoxide generation and an NO scavenger on browning of LRESs. (A) Degree of LRES browning in mEL5 after treatment with 5mM KNO2 and/or 20 µM DPI for 1 day. (B) Degree of LRES browning in mEL5 after treatment with 100 µM tZ or 100 µM iP and/or 20 µM DPI for 2 days. (C) Degree of LRES browning in NT after treatment with 400 µM iP and/or 20 µM DPI for 2 days. (D) Degree of LRES browning in NT (white bars) and mEL5 (grey bars) after treatment with 5mM KNO2, 200 µM iP, and/or 100 µM PTIO for 1 day. Roots were grown for 4 days without nitrogen sources and then were simultaneously treated with each chemical. Values are averages (n = 40 shoots per treatment) with SD. Different letters above the bars indicate significant differences at P < 0.01 (Tukey–Kramer test with inverse sine transformation). NT, non-transformants; mEL5, transgenic rice plants overexpressing EL5V162A.
Microarray analysis
Twelve rootless shoots per bottle were precultured for 4 days in 100ml of LSCS medium to form roots, after which 1mM KNO2 was added. After 12h, whole roots were collected from 10 of the 12 treated plants per bottle and were immediately frozen in liquid nitrogen and homogenized using Micro Smash (Tomy Seiko, Tokyo, Japan). Three sets of preparations were repeated for each experimental condition. Total RNA was isolated using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). Microarray experiments were performed using Rice 4×44 K Microarray RAP-DB (G2519F#15241; Agilent, Santa Clara, CA, USA) as described in Takehisa et al. (2012).
The processed raw signal intensity of all probes (45,151) was subjected to 75th-percentile normalization with Subio platform software with the basic plugin (Subio, Kagoshima, Japan) and transformed to a log2 scale. A total of 24235 probes (corresponding to 17003 genes) were extracted from 12 microarray data sets after ANOVA normalization. Fold change was calculated as the ratio of log2-transformed signal intensity between the untreated NT control and the treatment. We used 1.0 and −1.0 as the critical points for declaring upregulation and downregulation, respectively. Boxplot analysis was performed using Origin 8.6 software (OriginLab, Northampton, MA, USA).
Quantification of phytohormones
One bottle containing 12 rootless shoots was prepared for each experimental condition and precultured for 4 days in 100ml LSCS medium to form roots. Then, 1mM KNO2 was added, and after 6h whole roots from 10 of the 12 treated plants were collected from each bottle and immediately frozen in liquid nitrogen. High-throughput, comprehensive plant-hormone analysis for indole-3-acetic acid (IAA), abscisic acid (ABA), jasmonic acid (JA), jasmonoyl-isoleucine (JA-Ile), salicylic acid (SA), tZ, and iP was performed using liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) as described previously (Yoshimoto et al., 2009). A series of these tests was repeated 8–15 times. Statistical analyses of phytohormone contents were performed with the Tukey–Kramer test using JMP8 software.
Detection of ROS
Nitro blue tetrazolium chloride (NBT; Nacalai Tesque) staining was performed as described by Dunand et al. (2007) with modifications. Roots from 20 shoots were prepared for each treatment and the experiments were repeated two times. Ten rootless shoots per bottle were precultured in LSCS medium for 4 days to form roots, and were then treated with 5mM KNO2 or 100 µM iP with and without 20 µM DPI for 12h. Roots were collected and floated on 10% Triton X-100 for 5min. After washing twice with 100 µM phosphate buffer (pH 7.4), samples were immersed in 2mM NBT solution (pH 7.4) for 30min.
Quantitative RT-PCR analysis
Twelve rootless shoots per bottle were precultured for 4 days in 100ml LSCS to form roots. Whole roots from 10 of the 12 treated plants were collected from each bottle and total RNA was isolated. Trace amounts of DNA were removed by treatment with RQ1 RNase-Free DNase (Promega, Madison, WI, USA); cDNA was prepared from 0.5 µg of total RNA in 10 µl of reaction mixture with a PrimeScript RT reagent Kit (Takara Bio, Otsu, Japan). Quantitative PCR was performed using Mx3000P (Agilent) with 2-µl cDNA solution and a SYBR Premix Ex Taq II Kit (Takara Bio) in 20 µl of reaction mixture. Sets of primers used for amplifying EL5 and rice ubiquitin gene rub1 (the control) are shown in Table S1. The initial denaturation step was performed for 10min at 95 °C, followed by cycles of 30 s at 95 °C, 60 s at 55 °C, and 30 s at 72 °C. Relative EL5 expression was calculated using the 2−∆∆Ct method as described in Livak et al. (2001). The experiment was repeated three times, and the representative data are shown in Fig. 7A.
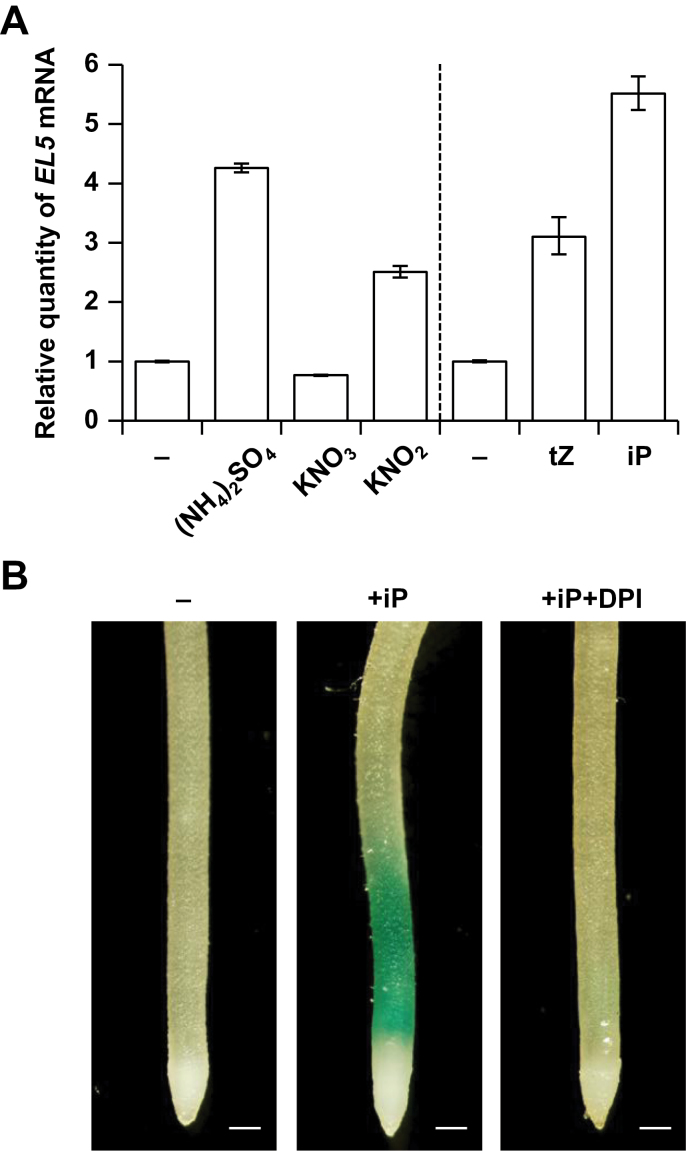
Fig. 7.
Expression analysis of EL5. (A) Quantitative RT-PCR analysis of EL5 expression in non-transformants. Total RNA was extracted from whole roots treated with 15mM (NH4)2SO4, 40mM KNO3, 5mM KNO2, 100 µM iP, or 100 µM tZ for 12h. Quantities relative to the EL5 mRNA level in untreated roots are presented with SE (three technical repeats). (B) GUS staining assay of transgenic rice plants carrying EL5p-GUS. After rooting for 4 days, crown roots were treated with 100 µM iP with and without 20 µM DPI for 12h. Two independent transgenic lines showed similar results and the representative dataset is presented. Scale bars, 200 µm.
GUS staining analysis
An EL5 promoter fragment (2131bp) was amplified from rice genomic DNA using the primers shown in Table S1, and subcloned into pGEM-T vector (Promega). The EL5 promoter fragment was cloned into the NcoI site of a binary vector pSMAHdN627-M2GUS (Hakata et al., 2010). The resulting plasmid carrying EL5p-GUS was introduced into rice (Nipponbare) via Agrobacterium-mediated transformation (Toki et al., 2006). Ten rootless shoots (T5 generation) per bottle were precultured in LSCS medium for 4 days to form roots, and were then treated with 100 µM iP with and without 20 µM DPI for 12h. Detached roots were treated with 10% Triton X-100 for 5min and immersed in a solution containing 0.5 mg·ml−1 X-Gluc (Nacalai Tesque), 0.5mM potassium ferricyanide, 0.5mM potassium ferrocyanide, and 100mM NaPO4 (pH 7.0), and incubated at 37 °C for 24h.
Results
Crown root growth of mEL5 is hypersensitive to nitrogen
First, we found that mEL5 (the EL5V162A-expressing plants) formed crown roots without necrosis when cultured in nutrient-free conditions. Hydroponic culture of mEL5 shoots in modified MS medium that contained no nitrogen source resulted in crown root formation, indicating that nitrogen is an inhibitory factor in MS media for root formation in mEL5.
To examine the effects of nitrogen sources on crown root formation in mEL5, rootless shoots of mEL5 and NT were aseptically hydrocultured with various concentrations of (NH4)2SO4, KNO3, or KNO2, and the length of the most elongated crown root was compared. The mEL5 formed crown roots comparable in length to those of NT when cultured without a nitrogen source (Fig. 1A). Although root growth in both mEL5 and NT was inhibited as nitrogen concentration increased, inhibition of mEL5 roots occurred at lower nitrogen concentrations compared to NT (Fig. 1A). On the other hand, the inhibition of crown root elongation by KNO2 in transgenic plants overexpressing the wild-type EL5 was similar to that in NT (Fig. S1).
Nitrate forms of nitrogen induce more severe necrosis at LRESs in mEL5
Culture of the rootless mEL5 shoots with (NH4)2SO4 diminished crown and lateral root growth, but browning at the LRES did not occur frequently (Fig. 1B). On the other hand, culture with KNO3 or KNO2 led to browning of the outer cell layers of crown roots at the LRES and prospective LRES, which was followed by termination of lateral root growth. These phenotypes enabled visual evaluation of the response of mEL5 roots to nitrogen, and were almost identical to the phenotype that resulted when mEL5 was cultured in MS medium (Koiwai et al., 2007).
Browning of cells around the LRES and termination of lateral root growth in mEL5 also occurred when the preformed crown roots were treated with KNO2 (Fig. 1C). Microscopic observation of mEL5 root sections revealed that cell death accompanied by browning occurred in the meristematic cells of crown and lateral roots in addition to the outer cell layers of crown roots at the LRES (Koiwai et al., 2007). Thus, to describe the effects of treatment with the various chemicals, we considered the number of roots with browned LRES as an indicator of meristematic cell death in the following experiments. The degree of LRES browning in mEL5 was significantly increased by treatment with (NH4)2SO4, KNO3, and KNO2, with the most severe browning induced by KNO3 and KNO2 (Fig. 1D). Treatment with KNO3 and KNO2 resulted in similar phenotypes in mEL5 roots but KNO2 led to a clearer phenotype at lower concentrations; thus, we used KNO2 to further investigate the function of EL5 in response to nitrogen during root formation. In the analyses that follow, the degree of browning observed in mEL5 roots was regarded as ‘not browned’ (untreated roots) or ‘significantly browned’ (roots treated with 5mM KNO2).
Overexpression of EL5V162A affects the expression of nitrite-responsive genes
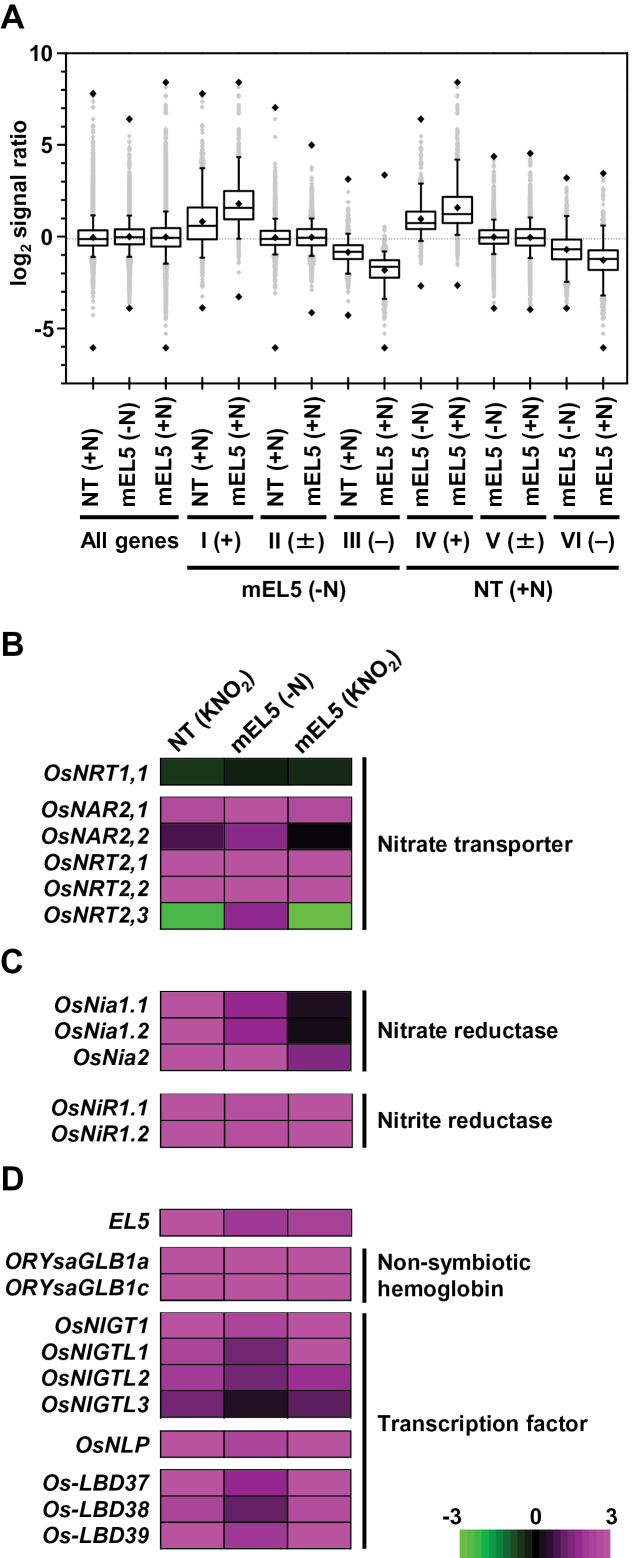
To characterize the gene expression in mEL5 roots, we performed microarray analyses of whole roots treated with 1mM KNO2 for 12h. For the 17003 genes selected as described in the Materials and methods, the statistical distribution of changes in expression levels compared to untreated NT roots (designated as the fold changes) was analysed using boxplot diagrams (Fig. 2A, Fig. S2A). We first verified that the distribution of fold changes of the 17003 genes was not significantly different among the three data sets: KNO2-treated NT and mEL5 roots, and untreated mEL5 roots; the median log2 values of the fold changes were nearly zero. Next, we classified the fold changes of the 17003 genes in untreated mEL5 roots into three groups—group I, upregulated genes; group II, unchanged genes; and group III, downregulated genes—and then plotted them according to the fold changes after KNO2 treatment of NT and mEL5 roots. The median log2 values of the fold changes in group I genes in KNO2-treated NT and mEL5 roots increased significantly, while those in group III genes in the same treatments decreased significantly. The expression of these genes was more strongly affected by KNO2-treatment in mEL5 roots than in NT roots.
Fig. 2.
Transcriptome profiling of KNO2-treated roots. (A) Boxplot analysis of microarray data (log2 signal ratio of values of treated vs untreated non-transformant roots). Boxes represent data between the 25th and 75th percentiles. Whiskers (error bars) above and below the boxes indicate the 90th and 10th percentiles, and black rhombic marks above, within, and below the boxes represent maximum, average, and minimum log2 signal ratios, respectively. Lines inside the boxes represent the median. +N, treated with KNO2; –N, untreated; +, upregulated (log2 signal ratio ≥ 1.0); ±, not changed (between –1.0 and 1.0); −, downregulated (≤ –1.0). I, II, III: fold changes of the 17003 genes in untreated roots of transgenic rice plants overexpressing EL5V162A (mEL5) were classified into group I, upregulated genes; group II, unchanged genes; and group III, downregulated genes. IV, V, VI: fold changes of the 17003 genes in KNO2-treated non-transformant (NT) roots were classified into group IV, upregulated genes; group V, unchanged genes; and group VI, downregulated genes. (B–D) Gene expression profiles. Expression profile of (B) nitrate transporter genes; (C) nitrogen reductase genes; and (D) nitrogen-induced genes. Data for EL5 consisted of signals from a probe that specifically detected transcripts of endogenous EL5. Magenta and green colours indicate upregulated and downregulated expression, respectively. The colour scale (representing the ratio of the average log2 value to the same value of untreated NT) is shown at the bottom right. The RAP-ID and Probe-ID for each gene are listed in Table S2.
Similarly, we classified the fold changes of the 17003 genes in KNO2-treated NT roots into three groups: groups IV, V, and VI (Fig. 2A). The median log2 values of the fold changes in group IV and group VI genes increased and decreased significantly, respectively, even in the untreated mEL5 roots, and gene expression was further altered after KNO2 treatment. Heat maps with hierarchical clustering of genes that showed changes in expression levels in untreated mEL5 roots (Fig. S2B, C) and in KNO2-treated NT roots (Fig. S2D, E) also exhibited overlaps between nitrite-responsive genes in NT roots and genes with altered expression in untreated mEL5 roots.
The overlapped genes included several that code for high-affinity nitrate transporters or nitrogen assimilation, and nitrate-responsive genes including non-symbiotic haemoglobin genes (Ohwaki et al., 2005), but not ammonium transporter genes (Fig. 2B–D, Fig. S3). The endogenous EL5 was also a nitrite-inducible gene and its expression was elevated in untreated mEL5 roots (Fig. 2D). In addition, KNO2 treatment increased the expression of pathogenesis-related genes (Fig. S4) and decreased the expression of photosynthesis-related genes (Fig. S5), most of which changed in mEL5 roots before KNO2 treatment. In other words, the expression levels of 73% of nitrite-responsive pathogenesis-related genes (49/67) and 100% of nitrite-responsive photosynthesis-related genes (28/28) were constitutively upregulated and downregulated, respectively, in mEL5. These results indicate that overexpression of EL5V162A activates portions of nitrite-signalling pathways that lead to changes in gene expression, and that mEL5 roots retain competence to respond to nitrite.
Overexpression of EL5V162A affects basal levels of phytohormones
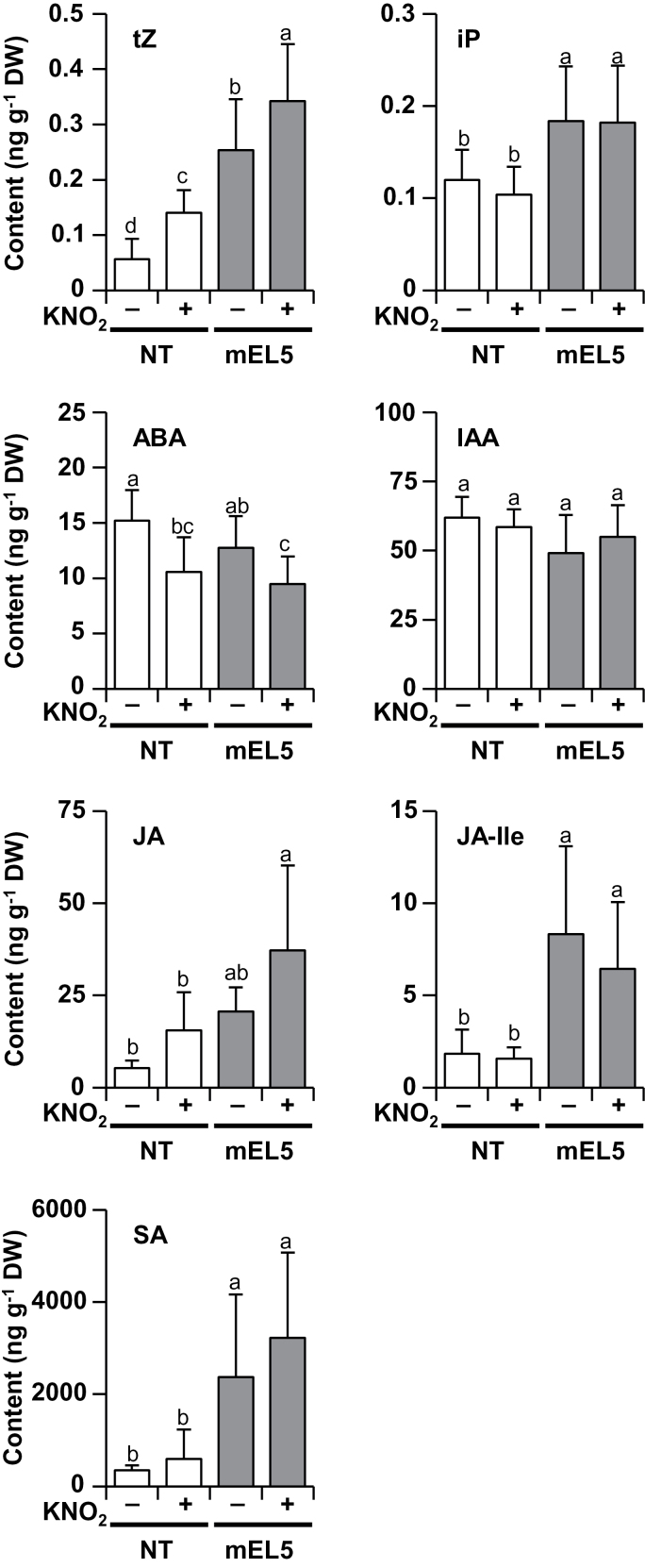
Next, we quantified the levels of phytohormones in whole roots (Fig. 3). Under KNO2 treatment, significant increases and decreases in the contents of tZ and ABA, respectively, were detected. The mEL5 roots contained significantly more tZ compared to NT roots, but ABA content was not affected by EL5V162A overexpression. In addition, mEL5 contained significantly more JA, JA-Ile, SA, and iP than NT. IAA content was not affected by either KNO2 treatment or by EL5V162A overexpression.
Fig. 3.
Effect of KNO2 treatment and EL5 function on phytohormone contents. Roots were grown for 4 days without nitrogen sources and then treated with 1mM KNO2 for 6h. The contents of tZ, iP, ABA, IAA, JA, JA-Ile, and SA in whole roots are presented. Error bars indicate SD. Different letters above the bars indicate significant differences at P < 0.05 (n = 8–15 independent treatments; Tukey–Kramer test). DW, dry weight; NT, non-transformants; mEL5, transgenic rice plants overexpressing EL5V162A.
Consistent with the phytohormone profile, microarray analysis revealed that KNO2 treatment increased the expression of a cytokinin biosynthesis gene (OsIPT4), a cytokinin-inactivation gene (OsCKX3), and type-A cytokinin-response regulator genes; however, in mEL5 roots, the expression of these genes was elevated prior to KNO2 treatment (Fig. S6).
Excess cytokinin causes cell death at the LRESs
To examine the possibility that the elevated basal level of cytokinin in mEL5 roots caused the LRES browning after KNO2 treatment, effects of cytokinin on the induction of browning were examined. Treatment with 200 µM tZ or iP alone induced LRES browning in mEL5 roots to the same extent as treatment with 5mM KNO2 alone (Fig. 4A, B). The LRES browning caused by cytokinin was dose-dependent (Fig. S7A). Excess cytokinin also caused LRES browning in NT roots (Fig. 4C). Moreover, concomitant treatment of NT roots with 5mM KNO2 and 200 µM tZ or iP significantly increased LRES browning (Fig. S7B). These results indicate that cytokinin is a causal agent of LRES browning in rice.
Elimination of ROS prevents roots from nitrite- and cytokinin-induced cell death
ROS are widely known to cause cell death in tandem with NO, which is a reduced product of nitrate and nitrite. To clarify the contribution of ROS and NO to nitrite- and cytokinin-induced cell death, we examined the effects of a superoxide-generation inhibitor, DPI, and a NO scavenger, PTIO. Concomitant treatment with 20 µM DPI suppressed LRES browning in mEL5 treated with KNO2, tZ, and iP (Fig. 5A, B). DPI also prevented iP-induced browning at the LRES in NT roots (Fig. 5C). Concomitant treatment with 100 µM PTIO also suppressed LRES browning induced by KNO2 or iP treatment in mEL5 (Fig. 5D). These results suggest that both ROS and NO are involved in nitrite- and cytokinin-induced cell death.
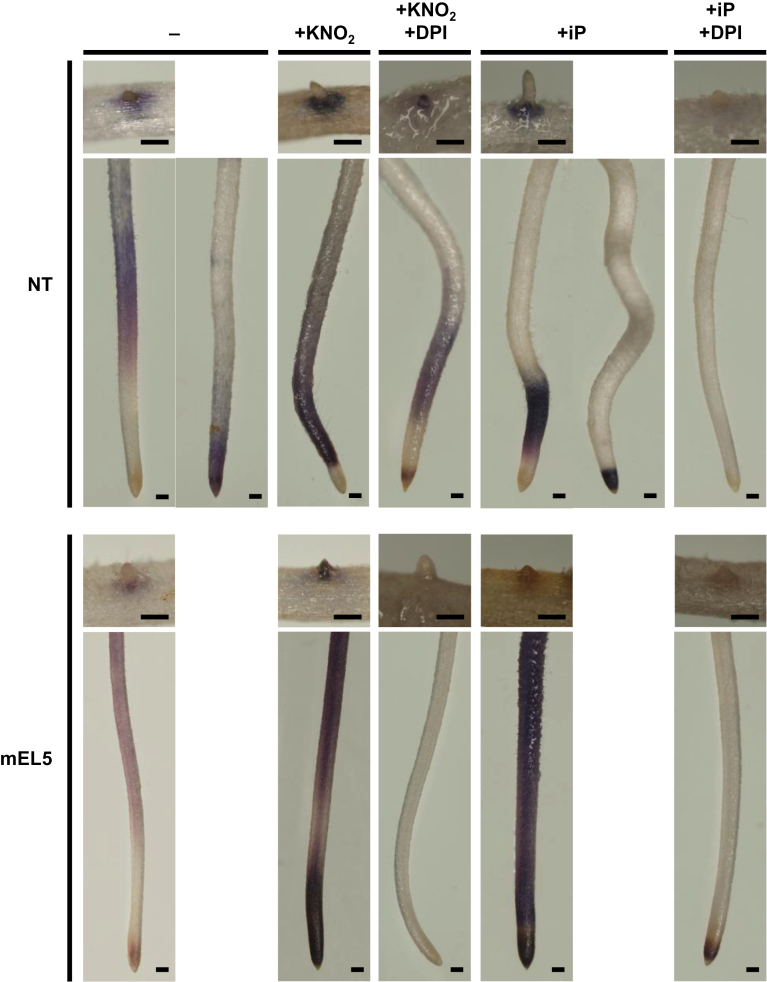
Next, we observed ROS and NO production in roots to examine the possibility that abnormal production of oxidants may cause nitrite- and cytokinin-induced cell death in mEL5 roots. Accumulation of superoxide as detected with NBT was evident at root tips and LRES of untreated crown roots in NT and mEL5, although two different staining patterns were observed in NT (Fig. 6). Both KNO2 and iP treatments increased superoxide accumulation in crown roots, which was inhibited by concomitant treatment with DPI. The mEL5 roots accumulated more superoxide than NT roots, particularly in crown root tips, supporting the hypothesis that ROS are a causal agent of nitrite- and cytokinin-induced cell death in mEL5 roots. The regions that reacted with NBT differed between mEL5 and NT, and they did not necessarily coincide with LRES that were intensely browned by nitrite or cytokinin treatment. On the other hand, KNO2 treatment led to NO production, but there was no significant difference between NT and mEL5 in quantity of NO in the culture medium (Fig. S8A) or any evident differences in the localization of NO (Fig. S8B).
Fig. 6.
Micrographs of crown roots and LRESs stained with NBT. Roots were grown for 4 days without nitrogen sources and then treated with 1mM KNO2 or 100 µM iP with and without 20 µM DPI for 12h. Scale bars, 200 µm. NT, non-transformants; mEL5, transgenic rice plants overexpressing EL5V162A.
EL5 expression is induced by nitrogen and cytokinin
We analysed EL5 expression in NT roots after treatment with various nitrogen sources and cytokinin (Fig. 7A). KNO2 and (NH4)2SO4 clearly induced EL5 expression, and tZ and iP also induced EL5 expression. GUS-staining analysis of transgenic rice plants carrying EL5 promoter-GUS fusion revealed that GUS activity was induced by iP at the elongation zone of crown roots. The iP-induced GUS staining was suppressed by concomitant treatment with DPI (Fig. 7B), suggesting the involvement of superoxide in the iP-induced EL5 expression. Induction of GUS activity was not observed after treatment with KNO2, probably because KNO2 treatment induced a lower level of EL5 expression (Fig. 7A) as well as superoxide generation in the broader region (Fig. 6) compared to the iP treatment.
Discussion
Nitrogen is a causal agent of the rootless phenotype of mEL5
EL5 plays a pivotal role as ubiquitin ligase in crown and lateral root formation by maintaining viability of the RAM (Koiwai et al., 2007). In the present study, we revealed that nitrogen sources, especially nitrate forms, cause RAM death accompanied by browning in plants expressing mutated EL5. The mEL5 roots showed increased sensitivity to all nitrogen sources tested, although the location and degree of browning differed according to the nitrogen source (Fig. 1). These differences might be a result of differences in the locus and/or amount of incorporation of the nitrogen form, or the quantity of NO generated after the treatment.
We performed most of the characterizations using nitrite, which produced more rapid and clearer effects than nitrate, but nitrate and nitrite caused similar LRES browning. Similarity in signalling between nitrate and nitrite has been reported in Arabidopsis. Transcriptome analysis of Arabidopsis roots indicated that nitrite is a more potent signal than nitrate for regulating gene expression, and almost all the pathways and processes induced by nitrate are also induced by nitrite (Wang et al., 2007); Wang et al. (2007) thus proposed that the nitrate-sensing system in Arabidopsis roots also recognizes nitrite. Our data suggest that nitrate and nitrite act via the same pathway to induce LRES browning.
Cytokinin-mediated high-nitrogen effects on root formation
Phytohormone profiling of whole roots revealed that mEL5 contains more cytokinin than NT, and nitrite treatment increased cytokinin levels in both mEL5 and NT roots (Fig. 3). Supplementation with nitrate has been shown to induce cytokinin accumulation in the roots of barley (Hordeum vulgare) (Samuelson and Larsson, 1993), maize (Sakakibara et al., 1998), Arabidopsis (Takei et al., 2001), wheat (Triticum aestivum) (Garnica et al., 2010), and rice (Yoshida and Oritani, 1974), indicating that this is a common reaction in plants. Our microarray data suggest that OsIPT4 is responsible for the increased level of cytokinin in roots by overexpression of EL5V162A and in response to nitrite (Fig. S6).
High levels of cytokinin induce apoptosis in plant-cultured cells (Mlejnek and Procházka, 2002; Carimi et al., 2003). We hypothesized that the higher basal levels of cytokinin, which were further increased after nitrite treatment, led to higher sensitivity to nitrogen and thus to RAM death in mEL5. This idea is supported by the finding that mEL5 was hypersensitive to cytokinin compared to NT as reflected in cell death at the LRES, and simultaneous treatment with nitrite and cytokinin also induced LRES browning in NT (Fig. 4, Fig. S7). Our observations are consistent with the finding that overproduction of cytokinin in rice plants by overexpression of OsIPT leads to the rootless phenotype (Sakamoto et al., 2006). These results strongly suggest that cytokinin levels should be properly regulated to maintain RAM viability when root formation is altered in response to nitrogen.
We also examined effects of JA and SA on the induction of LRES browning, but, unlike tZ and iP, there were no differences between mEL5 and NT (data not shown). This result suggests that, with the exception of cytokinin, elevated levels of phytohormones in mEL5 are not responsible for the mEL5 phenotype in which the RAM is easily damaged by nitrite. Recently, Novák et al. (2013) reported that conditional overproduction of cytokinin by dexamethasone-induced bacterial ipt expression in tobacco (Nicotiana tabacum) plants caused ROS production in chloroplasts and leaf cell death accompanied by increased levels of JA, SA, and ABA. These researchers speculated that oxidative membrane damage in chloroplasts elevates the content of these stress-related phytohormones (Novák et al., 2013). Likewise, in mEL5 roots, an increase in basal cytokinin levels might have secondary effects on JA and SA levels.
ROS are generated during cytokinin-induced apoptosis in cultured cells of Arabidopsis and tobacco (Mlejnek et al., 2003; Carimi et al., 2005), but whether ROS mediates the apoptosis has not been reported. In the present study, we demonstrated that ROS was required for both nitrite- and cytokinin-induced cell death. An NBT staining assay demonstrated that both nitrite and cytokinin treatment increased superoxide accumulation in roots, and this effect was greater in mEL5 roots than in NT roots. These results strongly suggest that increased accumulation of ROS in mEL5 helps to explain the higher sensitivity of mEL5 to nitrite and cytokinin. Therefore, EL5 might function to regulate ROS production under inhibitory nitrogen conditions. In agreement with this proposed function for EL5, the regions in which ROS were detected in NT roots corresponded to the regions in which the expression of EL5 was induced by cytokinin. OsPUB15 encodes U-box-type ubiquitin ligase, and its T-DNA insertional mutant, ospub15, which contains higher levels of ROS and oxidized proteins, shows a rootless phenotype similar to that of mEL5 (Park et al., 2011). Thus, both OsPUB15 and EL5 probably function to protect the RAM from oxidative damage. However, the steady-state ROS level in ospub15 was elevated (Park et al., 2011) while that in mEL5 was similar to NT. In addition, the expression level of OsPUB15 was not affected in mEL5 or by nitrite treatment in our microarray analysis. Therefore, unlike OsPUB15, which is generally involved in tolerance to oxidative damage under conditions including high salt and drought, our data suggest that EL5 plays a role in tolerance to oxidative damage caused by high nitrogen supply.
Carimi et al. (2005) reported that NO mediates cytokinin-induced programmed cell death in cultured Arabidopsis cells. In tobacco, NO can be produced from nitrite by nitrate reductase and from the root-specific plasma-membrane-bound nitrite-NO reductase (Stöhr et al., 2001). Indeed, we observed NO accumulation after nitrite treatment in the medium cultured with rice roots (Fig. S8A). An NO scavenger suppressed nitrite- and cytokinin-induced cell death in mEL5 (Fig. 5D), indicating the involvement of NO in the cell death. However, there were no significant differences between NT and mEL5 in quantity or location of NO production after nitrite treatment (Fig. S8). Thus, we consider that NO is a trigger of, but not a direct factor in, nitrite- and cytokinin-induced cell death in rice roots; however, we did not detect NO accumulation after cytokinin treatment in our experimental conditions. There was a discrepancy between the loci associated with necrosis and those stained with NBT after nitrite and cytokinin treatment at the LRES. We speculate that other cytotoxic compounds, formed by the reaction with superoxide and/or NO but undetectable by NBT, might execute the LRES cell death.
EL5 affects the nitrogen-cytokinin signal pathway
Transcriptome profiling of roots revealed that overexpression of EL5V162A had a profound effect on gene expression. Boxplot analyses demonstrated that the expression profiles of untreated mEL5 roots significantly resembled those of nitrite-treated NT roots (Fig. 2A, Fig. S2). These results strongly suggest that reduction of EL5 function causes activation of nitrogen signalling despite the absence of a nitrogen source. Recently, transcription factors that regulate nitrogen signalling have been identified in rice and Arabidopsis (LOB, Rubin et al., 2009; Albinsky et al., 2010; NIGT1, Sawaki et al., 2013; NLP6 and NLP7, Castaings et al., 2009; Konishi and Yanagisawa, 2013; Marchive et al., 2013). Expression of genes in the same family as these transcription factor genes was induced in rice roots after nitrite treatment, but in mEL5 their expression levels were elevated prior to nitrite treatment (Fig. 2D), which probably contributed to the wide range of alteration in gene expression shown in untreated mEL5 roots.
In contrast with cytokinin, ABA content was deduced after nitrite treatment (Fig. 3). Generally, ABA signalling is mediated by ROS and NO (Gayatri et al., 2013). Thus, it is possible that the reduction of ABA content is an upstream event in the nitrite-cytokinin signal pathway. However, basal levels of ABA-related gene expression, ABA content, and their alteration patterns after nitrite treatment were similar in mEL5 and NT roots (Fig. 3, Fig. S9). We also examined the effect of ABA on the induction of LRES browning in mEL5; simultaneous treatment with nitrite and ABA did not negate the browning (data not shown). In addition, the expression of EL5 was induced by cytokinin but not by ABA (Koiwai et al., 2007). These facts suggest that the pathway leading to the reduction of ABA content is independent of nitrite-induced cytokinin synthesis.
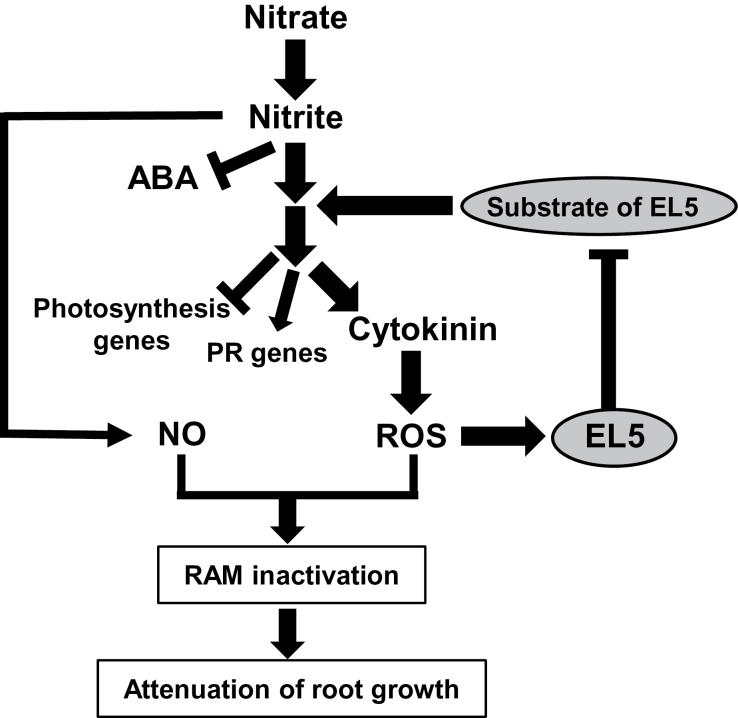
Taken together, these data strongly suggest that EL5 acts on the nitrogen-signalling pathway(s) leading to cytokinin synthesis, transcriptional induction of nitrogen-related and pathogenesis-related genes, and suppression of photosynthesis-related gene expression; the data also suggest that EL5 does not act on the pathway leading to suppression of ABA synthesis. Because EL5 is involved in root formation as ubiquitin ligase (Koiwai et al., 2007), we conclude that EL5 affects the nitrate-cytokinin signal pathway via ubiquitination of the putative target protein, which leads to alteration of RAM vigour through ROS generation (Fig. 8). Identification of the target protein would elucidate the biological significance and molecular mechanism of its additive action to the effects of nitrogen.
Fig. 8.
Model of the effects of high concentrations of nitrogen on root growth and EL5 function, derived from the present comparative studies between non-transformants and transgenic rice plants overexpressing EL5V162A (mEL5). PR genes, pathogenesis-related genes.
Supplementary material
Supplementary material is available at JXB online.
Supplementary Fig. S1. Inhibitory effect of nitrogen on crown root elongation in transgenic rice plants overexpressing EL5.
Supplementary Fig. S2. Effect of KNO2 treatment and EL5 function on gene expression in whole roots.
Supplementary Fig. S3. Expression profiles of representative nitrogen-related genes.
Supplementary Fig. S4. Expression profiles of representative pathogenesis-related genes.
Supplementary Fig. S5. Expression profiles of representative photosynthesis-related genes.
Supplementary Fig. S6. Expression profiles of representative genes for biosynthesis, inactivation, and response of cytokinin.
Supplementary Fig. S7. Effect of cytokinin on browning at lateral root emergence sites (LRES).
Supplementary Fig. S8. Accumulation of NO after nitrite treatment.
Supplementary Fig. S9. Expression profiles of representative abscisic acid (ABA)-related genes.
Supplementary Table S1. Oligonucleotide primers used in this work.
Supplementary Table S2. RAP-ID and Probe-ID list of the nitrogen-related genes shown in Fig. 2.
Funding
This work was supported by Grants-in-Aids for Foundation Research (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan [grant number 20380030], and by a grant from the Japan Society for the Promotion of Science initiated by the Council for Science and Technology Policy [grant number GS028].
Supplementary Material
Acknowledgements
We specially thank Ms. Emi Nakajima for assistance with all assays. We also thank Dr. Yoshiaki Nagamura and Ms. Ritsuko Motoyama of NIAS for their support in the microarray analyses, Dr. Takeshi Nishimura of NIAS for helpful discussion, Dr. Chise Suzuki of NILGS for statistical analysis, and Ms. Hiroko Mochizuki and Ms. Kyoko Iwasaki for daily assistance.
Glossary
Abbreviations:
- ABA
abscisic acid
- ATL
Arabidopsis Tóxicos en Levadura
- DPI
diphenyleneiodonium chloride
- IAA
indole-3-acetic acid
- iP
N 6-(2-isopentenyl)-adenine
- JA
jasmonic acid
- JA-Ile
jasmonoyl-isoleucine
- LRES
lateral-root-emergence site
- LSCS
low-salt culture solution
- MS medium
Murashige and Skoog medium
- NBT
nitro blue tetrazolium chloride
- NT
non-transgenic
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl
- RAM
root apical meristem
- ROS
reactive oxygen species
- SA
salicylic acid
- tZ
trans-zeatin.
References
- Aguilar-Hernández V, Aguilar-Henonin L, Guzmán P. 2011. Diversity in the architecture of ATLs, a family of plant ubiquitin-ligases, leads to recognition and targeting of substrates in different cellular environments. PLoS One 6, e23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinsky D, Kusano M, Higuchi M, et al. 2010. Metabolomic screening applied to rice FOX Arabidopsis lines leads to the identification of a gene-changing nitrogen metabolism. Molecular Plant 3, 125–142 [DOI] [PubMed] [Google Scholar]
- Aloni R, Aloni E, Langhans M, Ullrich CI. 2006. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany 97, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carimi F, Zottini M, Costa A, Cattelan I, De Michele R, Terzi M, Lo Schiavo F. 2005. NO signalling in cytokinin-induced programmed cell death. Plant, Cell & Environment 28, 1171–1178 [Google Scholar]
- Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F. 2003. Cytokinins: new apoptotic inducers in plants. Planta 216, 413–421 [DOI] [PubMed] [Google Scholar]
- Castaings L, Camargo A, Pocholle D, et al. 2009. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. The Plant Journal 57, 426–435 [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. 2004. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218, 900–905 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. 1998. Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588 [DOI] [PubMed] [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C. 2007. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist 174, 332–341 [DOI] [PubMed] [Google Scholar]
- Forde BG. 2002. Local and long-range signaling pathways regulating plant responses to nitrate. Annual Review of Plant Biology 53, 203–224 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446 [DOI] [PubMed] [Google Scholar]
- Garnica M, Houdusse F, Zamarreño AM, Garcia-Mina JM. 2010. The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. Journal of Plant Physiology 167, 1264–1272 [DOI] [PubMed] [Google Scholar]
- Gayatri G, Agurla S, Raghavendra AS. 2013. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Frontiers in Plant Science 4, article 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakata M, Nakamura H, Iida-Okada K, et al. 2010. Production and characterization of a large population of cDNA-overexpressing transgenic rice plants using Gateway-based full-length cDNA expression libraries. Breeding Science 60, 575–585 [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. 1996. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273, 1853–1856 [DOI] [PubMed] [Google Scholar]
- Jiang DJ, Jiang JL, Tan GS, Huang ZZ, Deng HW, Li YJ. 2003. Demethylbellidifolin inhibits adhesion of monocytes to endothelial cells via reduction of tumor necrosis factor alpha and endogenous nitric oxide synthase inhibitor level. Planta Medica 69, 1150–1152 [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS. 2001. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiology 126, 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S, Tsunoda Y, Murata K, Minami E, Katoh E. 2005. Active site residues and amino acid specificity of the ubiquitin carrier protein-binding RING-H2 finger domain. Journal of Biological Chemistry 280, 41015–41024 [DOI] [PubMed] [Google Scholar]
- Koiwai H, Tagiri A, Katoh S, Katoh E, Ichikawa H, Minami E, Nishizawa Y. 2007. RING-H2 type ubiquitin ligase EL5 is involved in root development through the maintenance of cell viability in rice. The Plant Journal 51, 92–104 [DOI] [PubMed] [Google Scholar]
- Konishi M, Yanagisawa S. 2013. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nature Communications 4, 1617. [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A. 2013. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nature Communications 4, 1713. [DOI] [PubMed] [Google Scholar]
- Mlejnek P, Doležel P, Procházka S. 2003. Intracellular phosphorylation of benzyladenosine is related to apoptosis induction in tobacco BY-2 cells. Plant, Cell & Environment 26, 1723–1735 [Google Scholar]
- Mlejnek P, Procházka S. 2002. Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215, 158–166 [DOI] [PubMed] [Google Scholar]
- Novák J, Pavlu J, Novák O, Nozková-Hlavácková V, Spundová M, Hlavinka J, Koukalová S, Skalák J, Cerny M, Brzobohatý B. 2013. High cytokinin levels induce a hypersensitive-like response in tobacco. Annals of Botany 112, 41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa D, Abe K, Miyao A, et al. 2011. RSS1 regulates the cell cycle and maintains meristematic activity under stress conditions in rice. Nature Communications 2, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwaki Y, Kawagishi-Kobayashi M, Wakasa K, Fujihara S, Yoneyama T. 2005. Induction of class-1 non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured rice cells. Plant and Cell Physiology 46, 324–331 [DOI] [PubMed] [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. 2007. Hidden branches: developments in root system architecture. Annual Review of Plant Biology 58, 93–113 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. 2002. Nitric oxide is required for root organogenesis. Plant Physiology 129, 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Yi J, Yoon J, Cho LH, Ping J, Jeong HJ, Cho SK, Kim WT, An G. 2011. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. The Plant Journal 65, 194–205 [DOI] [PubMed] [Google Scholar]
- Rubin G, Tohge T, Matsuda F, Saito K, Scheible WR. 2009. Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. The Plant Cell 21, 3567–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, Suzuki M, Takei K, Deji A, Taniguchi M, Sugiyama T. 1998. A response-regulator homologue possibly involved in nitrogen signal transduction mediated by cytokinin in maize. The Plant Journal 14, 337–344 [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Takei K, Hirose N. 2006. Interaction between nitrogen and cytokinin in regulation of metabolism and development. Trends in Plant Science 11, 440–448 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, Sato Y, Matsuoka M. 2006. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice. Plant Physiology 142, 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson ME, Larsson CM. 1993. Nitrate regulation of zeatin riboside levels in barley roots - Effects of inhibitors of N-assimilation and comparison with ammonium. Plant Science 93, 77–84 [Google Scholar]
- Sato T, Maekawa S, Yasuda S, et al. 2009. CNI1/ATL31, a RING-type ubiquitin ligase that functions in the carbon/nitrogen response for growth phase transition in Arabidopsis seedlings. The Plant Journal 60, 852–864 [DOI] [PubMed] [Google Scholar]
- Sato T, Maekawa S, Yasuda S, Domeki Y, Sueyoshi K, Fujiwara M, Fukao Y, Goto DB, Yamaguchi J. 2011. Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis. The Plant Journal 68, 137–146 [DOI] [PubMed] [Google Scholar]
- Sawaki N, Tsujimoto R, Shigyo M, Konishi M, Toki S, Fujiwara T, Yanagisawa S. 2013. A nitrate-inducible GARP family gene encodes an auto-repressible transcriptional repressor in rice. Plant and Cell Physiology 54, 506–517 [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Ikeda A, Saiki S, von Wirén N, Yamaya T, Yamaguchi J. 2003. Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant and Cell Physiology 44, 726–734 [DOI] [PubMed] [Google Scholar]
- Stöhr C, Strube F, Marx G, Ullrich WR, Rockel P. 2001. A plasma membrane-bound enzyme of tobacco roots catalyses the formation of nitric oxide from nitrite. Planta 212, 835–841 [DOI] [PubMed] [Google Scholar]
- Takai R, Matsuda N, Nakano A, Hasegawa K, Akimoto C, Shibuya N, Minami E. 2002. EL5, a rice N-acetylchitooligosaccharide elicitor-responsive RING-H2 finger protein, is a ubiquitin ligase which functions in vitro in co-operation with an elicitor-responsive ubiquitin-conjugating enzyme, OsUBC5b. The Plant Journal 30, 447–455 [DOI] [PubMed] [Google Scholar]
- Takehisa H, Sato Y, Igarashi M, et al. 2012. Genome-wide transcriptome dissection of the rice root system: implications for developmental and physiological functions. The Plant Journal 69, 126–140 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Taniguchi M, Sugiyama T. 2001. Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator. Plant and Cell Physiology 42, 85–93 [DOI] [PubMed] [Google Scholar]
- Tian Q, Chen F, Liu J, Zhang F, Mi G. 2008. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. Journal of Plant Physiology 165, 942–951 [DOI] [PubMed] [Google Scholar]
- Tian Q, Chen F, Zhang F, Mi G. 2005. Possible involvement of cytokinin in nitrate-mediated root growth in maize. Plant Soil 277, 185–196 [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H. 2006. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. The Plant Journal 47, 969–976 [DOI] [PubMed] [Google Scholar]
- Wang R, Xing X, Crawford N. 2007. Nitrite acts as a transcriptome signal at micromolar concentrations in Arabidopsis roots. Plant Physiology 145, 1735–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Onckelen HV, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Oritani T. 1974. Studies on Nitrogen Metabolism in Crop Plants. XIII. Effects of nitrogen top-dressing on cytokinin content in the root exudate of rice plant. Proceedings of the Crop Science Society of Japan 43, 47–51 [Google Scholar]
- Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K. 2009. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. The Plant Cell 21, 2914–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.