Abstract
The psychostimulants amphetamine and methylphenidate (MPD / Ritalin) are the drugs most often used to treat attention deficit hyperactivity disorder (ADHD). In addition, students of all ages take these drugs to improve academic performance but also abuse them for pleasurable enhancement. In addition, other psychostimulants such 3,4 methylenedioxymethamphetamine (MDMA / ecstasy) are used / abused for similar objectives. One of the experimental markers for the potential of a drug to produce dependence is its ability to induce behavioral sensitization and cross sensitization with other drugs of abuse. The objective of this study is to use identical experimental protocols and behavioral assays to compare in female rats the effects of amphetamine, MPD and MDMA on locomotor activity and to determine if they induce behavioral sensitization and/or cross sensitization with each other. The main findings of this study are 1. Acute amphetamine, MPD and MDMA all elicited increases in locomotor activity. 2. Chronic administration of an intermediate dose of amphetamine or MPD elicited behavioral sensitization. 3. Chronic administration of MDMA elicited behavioral sensitization in some animals and behavioral tolerance in others. 4. Cross sensitization between MPD and amphetamine was observed. 5. MDMA did not show either cross sensitization or cross tolerance with amphetamine. In conclusion, these results suggest that MDMA act by different mechanisms compared to MPD and amphetamine.
Index words: Ritalin, Amphetamine, Ecstasy, locomotor, cross-sensitization, female rats
1. Introduction
Psychostimulant abuse has become a tremendous problem, especially in adolescents and young adults. The psychostimulants amphetamine and methylphenidate (MPD) have been the gold standard for decades in psychotherapy for treating attention deficit hyperactivity disorder (ADHD) (Challman and Lipsky, 2000; Gaytan et al., 1997, 1999; Robinson and Becker 1986). These drugs mainly target the dopamine transporter (DAT) leading to an increase in extracellular dopamine levels and to a lesser degree block the norepinephrine and serotonin transporters (Challman and Lipsky, 2000; Giorgetti et al., 2001; Kalivas et al., 1993; Nestler, 2001; Wolf, 1998). Although it is still unclear how amphetamine and MPD affect cognition and attention improvement, it was postulated that the drug acts to modulate sensory processes at the motive circuit (Pierce and Kalivas, 1997). The drug 3,4-methylenedioxymethamphetamine (MDMA/ecstasy) is one of the most popular recreational psychoactive drugs (Atkin et al.; 2009, Meyer and Maurer, 2010; Modi, et al., 2006). MDMA produces stimulant-like effects that lead to a heightened response to sensory stimulation (Martin et al., 1995). The consequences of MDMA use include neurodegeneration of the serotonergic and catecholaminergic pathways (Gudelsky and Nash, 1996; James et al., 2010; Ricuarte and McCann, 2001). Multiple administration of psychostimulants results in the initiation and intensification of biochemical and behavioral manifestations that lead to dependence on the drug and behavioral sensitization. Behavioral sensitization refers to a phenomenon whereby the repeated use of the psychostimulant produces a progressive augmentation of the subjective behavioral response (Dafny and Yang, 2006; Kalivas and Stewart, 1991; Robinson and Berridge, 1993; Vanderschuren et al., 1999) It was reported that MPD, amphetamine and MDMA elicit behavioral sensitization (Gaytan et al., 1997, 1999; Modi et al., 2006; Yang et al., 2003, 2007, 2010) One of the side effects produced by chronic use of psychostimulants as well as a measurable indication of the drug's liability is the development of behavioral sensitization and cross sensitization between two or more of these drugs (Aizenstein et al., 1990; Ball et. al., 2009; Callaway and Geyer, 1992; Dafny and Yang, 2006, Yang et al., 2003). Behavioral sensitization is manifest as a long lasting hypersensitivity to the drug following repetitive psychostimulant administration (Kalivas and Stewart, 1991; Wolf,, 1998). It has been suggested that behavioral sensitization represents an enduring alteration of drug response (Kalivas and Stewart, 1991; Kalivas et al., 1998) and has been used as an experimental model of drug craving (Kalivas et al., 1998; Wolf, 1998). Cross sensitization is defined as hyper-responsiveness to one psychostimulant after pre-exposure to a different psychostimulant (Aizenstein et al, 1990; Dafny and Yang, 2006). Drugs such as amphetamine and cocaine are known to be drugs of abuse and produce sensitization and cross sensitization with each other (Aizenstein et al, 1990; Bonate et al, 1997; Brandon et al, 2001). By contrast, current studies dispute whether the psychostimulants methylphenidate (MPD) and 3,4-methylenedioxymethamphetamine (MDMA) elicit sensitization (Atkin et al., 2009; Modi et al, 2006) or whether they cross sensitized with other psychostimulants. There are no studies comparing the acute and chronic effects of all three drugs using the same experimental method and technology and whether there is cross-sensitization between them. The objectives of this study are to compare the effects of amphetamine, MPD, and MDMA on behavioral activity in adult female Sprague-Dawley rats and to determine if they cause sensitization and cross-sensitize with each other.
2. Material and Methods
2.1 Animals
Adult female (N=50) Sprague-Dawley (SD) rats weighing about 180-190g were purchased from Harlan Laboratories and used as subject. All rats were housed in Plexiglass cages, two to a cage, with food and water made available ad libitum for 5 to 7 days for acclimation. The rats were held in a room at an ambient temperature of 21± 2°C, a relative humidity of 37-42%, and a 12/12-h light/dark cycle. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used.
2.2 Drugs
Three psychostimulants were used as follows: 0.6 mg/kg amphetamine, 2.5 mg/kg methylphenidate hydrochloride (MPD), and 5.0 mg/kg 3,4- methylenedioxymethamphetamine (MDMA). These dosages were shown in our previous dose response studies to elicit behavioral sensitization (Gaytan et al, 1997; 1998; Modi et al., 2006 Yang et al, 2003, 2006). The doses were calculated as the free base, and were dissolved in 0.9% saline, equalized to 0.8 cc according to weight of the animal, and administered intraperitoneally in the morning. All injections were given in test cages in the morning.
In preliminary experiments, using an identical experimental protocol (Table 1), female rats were kept four per cage from postnatal (P) day 40 to P80 with the intention that all the female rats would cycle on the same day. Vaginal smears (Dafny and Terkel, 1990) were taken prior to daily MPD injections from P60 to P80. It was found that the MPD treatment did not alter the estrous cycle. In this study, all experiments started on the proestrous day.
Table 1.
describes the experimental protocol used for methylphenidate (MPD), amphetamine, ecstasy (MDMA) in the table marked D and the saline (Sal) control group. Exp Day-the experiment lasted 12 days as follows. On experimental day 1 (ED 1) recordings were obtained following saline injection (Sal). In ED2 to 7, MPD or amphetamine or MDMA (D) or Sal was given. On ED 8 to 10 no injections were given [washout (W)]. On ED 11, the same treatment as given on EDs 2 to 7 was administered. On ED 12, a different drug either MPD or amphetamine was given to test for cross-sensitization (XD).
| Exp Day | 1* | 2* | 3 | 4 | 5 | 6 | 7* | 8* | 9 | 10 | 11* | 12* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection | Sal | D | D | D | D | D | D | W | W | W | D | XD |
| Injection | Sal | Sal | Sal | Sal | Sal | Sal | Sal | W | W | W | Sal | Sal |
Sal-saline; D-drug; W-washout; XD-cross-sensitization
-indicates the recording day (i.e. ED1, 2, 7, 8, 11, and 12)
2.3 Apparatus
Each animal was tested in a computerized open field animal activity monitoring system (AccuScan Instruments, Columbus, OH) before and after single and repetitive (chronic) daily drug administration. Rats were tested for behavioral locomotion in activity chambers after 20-30 min of acclimation to their test cage. The activity chambers consist of clear acrylic open field boxes (40.5×40.5×31.5 cm) fitted with two rows of infrared motion sensors that can detect interruption of each infrared beam at a frequency of 100 Hz. Any interruption of the infrared sensor counted as an activity score. Cumulative counts were compiled and downloaded every ten min for two h using VERSAMAX data collection software (AccuScan Instruments, Columbus, OH) that separated the sensor interruptions into different locomotor indices. (Algahim et al., 2009; Gaytan et al., 1997, 1999, 2000; Lee et al., 2009; Yang et al., 2003, 2006, 2007)
The locomotor indices evaluated in this study are: horizontal activity (HA), a measurement of the overall locomotor activity in the lowest tier detected by total beam interruptions during an individual sample; vertical activity (VA), a record of the vertical sensor beam interruptions, a measure of the amount of rearing; number of stereotypies (NOS), a measurement of repetitive or stereotyped behavior detected by having at least one second intervals between repetitive episodes; and, total distance (TD) in cm, a measurement of ambulatory activity during a given sample.
2.4 Experimental Design
After five to seven days of acclimation to the test cages, animals were tested for locomotor activity (Table 1). Animals were divided into five groups: 1) saline control (N=8); 2) chronic MPD (2.5 mg/kg) administration following a single amphetamine dose (N=8) on experimental day 12; 3) chronic amphetamine (0.6 mg/kg) administration following a single MPD (2.5 mg/kg) dose on experimental day 12 (N=8); 4) chronic amphetamine(N=8) administration followed by single MDMA (5.0 mg/kg); and 5) chronic MDMA (N=18) administration followed by a single amphetamine (0.6 mg/kg) dose on experimental day 12 (Table 1). Baseline activity following 0.8 cc of 0.9% saline was recorded at experimental day 1 (ED1) to establish the baseline control measurement. Then, drug naïve animals were injected with either saline, or amphetamine, or MPD, or MDMA for six consecutive days (ED2 to ED7). The drug's effect on locomotor behavior was tested (recorded) on the first and last day of consecutive drug administration, respectively i.e. ED2 and ED7 (Table 1). Next, the animals were deprived of drug for three days, (ED8 to ED10), as a washout period. Then animals were re-challenged with the same drug given on ED2 and locomotor behavior was recorded post-drug injection on ED 11. On ED 12, all animals in the MPD and MDMA groups were injected with 0.6 mg/kg of amphetamine, and all animals in the amphetamine group were injected with 2.5 mg/kg MPD, to test for the occurrence of cross-sensitization between these drugs (Table 1). All recordings started in the morning immediately after injection and lasted for 120 min.
2.5 Data Analysis
Repeated measure analysis of variance (ANOVA) was used to determine statistically significant differences among various days and drugs. Bonferroni adjusted pairwise comparisons were used as post-hoc tests to compare between the different groups for all ANOVA that produced significant results. All tests were considered significant at P<0.05 for all comparisons. The recordings following saline injection on experimental day 1 (ED1) represent the control, baseline activity (Gaytan et. al., 1997, 1998, 1999, 2000; Lee et al., 2009; Yang et. al., 2003, 2006, 2007). The recordings on ED 2 compared to ED1 expresses the acute effect of the drug. Comparison of the data obtained on ED7 to the data obtained on ED2 indicates whether sensitization was induced, while comparing data obtained on ED11 with data obtained on ED2 shows whether sensitization had expressed (Algahim et al, 2009; Lee et al, 2009; Pierce and Kalivas, 1997; Yang et al, 2006). Comparing the activity of ED12 with that seen after a single injection in drug naïve animals (control data) indicates whether cross sensitization occurred between the drug that was given to the naïve animal and the drug that was given on ED12 (Yang et al, 2003).
Four locomotor indices were used to study the acute and chronic effects of locomotor activity as follows: horizontal activity (HA), total distance (TD) travelled, vertical activity (VA) and number of sterotypies (NOS). The data were presented in two forms: a sequential temporal (line) graph and a bar histogram. The line graphs summarize the temporal response to the treatment in 10-min bins for 120 min post-injection for experimental days 1, 2, 7, and 11. The y-axis on the temporal graph represents the absolute count change from baseline measured in counts per ten min. The x-axis represents the sequential min post treatment. Values are presented as ± S.E.M. with #P<0.05 used to calculate the acute effect of the drug (ED2 compared to ED1), *P<0.05 calculates if the chronic effect of the drug induced tolerance or sensitization (ED7 compared to ED2) and ^P<0.05 shows whether tolerance or sensitization is expressed after 3 washout days (ED11 compared to ED2). A difference of two or more consecutive data points (i.e. 20min) was considered a significant change due to the treatment (Gaytan et al., 1997; Yang et al., 2003, 2007). The bar histograms represent the two h total activity post-drug injection on each experimental day. Values of the bar histograms are presented as mean ± S.E.M. with the same comparisons as those in the temporal graphs.
3. Results
The saline control group was used to determine the effect of handling and the injection procedure. Mild increases in locomotion and licking at the injection site for about four to five min post injection were observed and then the activity returned to baseline. All recordings post saline injection (EDs 1 to 7; 11 and 12) exhibited similar activities (Fig. 1) with minor non-significant fluctuation similar to our previously reported 42 consecutive time controls (no injection) and saline controls (Yang et al., 2006). Since ED 1 activity post saline injection was similar to that obtained post saline injection, on all the other experimental days. ED1 post saline injection activity in each group (amphetamine, MPD, and MDMA) was used as control for the other experimental days. Moreover, since in the drug groups ED1 activity is recorded post saline injection, this activity (ED1 post saline) was used as control for the drug acute effect on ED2.
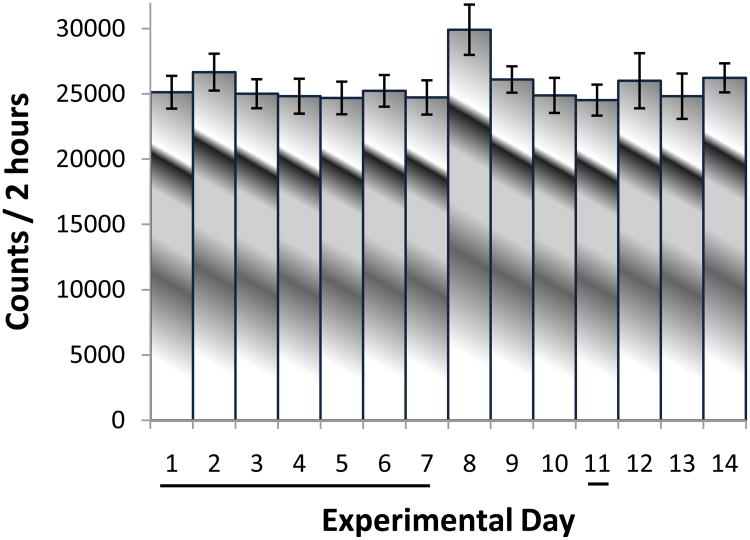
Fig. 1.
The figure summarizes (N=8) the horizontal activity / 2h following seven consecutive days, i.e., experimental days 1 to 7 and 11 and washout days at experimental days 8 to 10 and 12 to 14 and shows that over the 14 consecutive days, the animals exhibit similar level of activity with non-significant fluctuation. The black underline in the experimental days indicates the days after saline injection.
3.1.1 Acute effects of Methylphenidate (MPD)-Comparing ED 2 to ED 1
Fig. 2 summarizes the locomotor responses post-injection of 2.5 mg/kg MPD for all the experimental days (N=8). Comparing the activity of ED2 after MPD treatment to ED1 after saline injection represents the acute effect. There was a significant increase (F1, 25 = 4.67; F1, 15 = 6.97; F1, 24 = 4.38; F1, 23 = 4.92; P< 0.05) in activity on ED2 as compared to ED1 in all four motor indices as shown in the histograms. The temporal graph demonstrates that the increases in horizontal activity and number of stereotypies were observable within the first ten min post injection and persisted for the duration of one hour, while the increases in vertical activity started within twenty min post injection and lasted for fifty min post injection. Total distance traveled was increased at twenty min post injection and lasted to sixty min post injection.
Fig. 2.
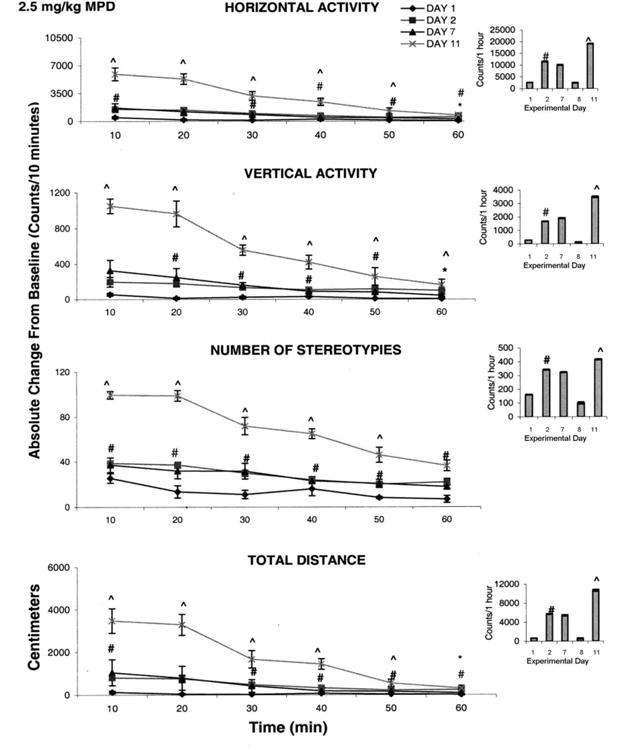
This summarizes (N=8) the effect of 2.5 mg/kg methylphenidate (MPD) on four locomotor indices. On the left side are the sequential graphs of 10 minute temporal activity post injection and on the right side are the histograms of total activity under the temporal graph. # symbol indicates significant (P< 0.05) difference between ED 1 vs. ED 2 and symbol ^ indicates significant (P<0.05) difference between ED 2 vs. ED 11.
3.1.2 Chronic administration of MPD-Induction Phase-Comparing ED7 to ED2
The recordings obtained on ED7 were compared to those obtained on ED2 in order to determine whether tolerance or sensitization was induced (see Table 1). The increase in activity elicited by the initial MPD injection remained the same during five consecutive injections, i.e. ED 2 to ED7 (Fig. 1). There was no significant attenuation or further increase in activity on ED7 compared to ED2 on any of the locomotor indices studied. This comparison suggests that neither tolerance nor sensitization was initiated during the six consecutive days of MPD administration.
3.1.3 Chronic effects of MPD-Expression Phase; Comparing ED11 to ED2
Fig. 2 also summarizes the activities of the four locomotor indices obtained after chronic MPD administration. Comparison between ED11 and ED2. Comparison between ED11 and ED2 shows that the same MPD dose given at at ED2 and again at ED11 produced a further increase (F2, 32 = 2.31; F2, 32 = 6.24; F2, 52 = 3.67; and F2, 27 = 4.38; P<0.05) in all four locomotor activities (Fig. 2 histograms). Furthermore, the duration of the increases in horizontal activity, total distance, and number of stereotypies persisted for fifty min post injection while the increase in vertical activity was significant (F1, 32 = 6.29; ^P<0.05) for the entire hour post injection (Fig. 1 temporal graphs).
3.2.1 Acute Effects of Amphetamine-Comparing ED2 to ED1
Fig. 3 summarizes (N=8) the effects of injection of 0.6 mg/kg i.p. amphetamine on the locomotor responses for all the experimental days. Acute injection of amphetamine elicited a significant increase (F1, 25 = 4.95; F1, 15 = 6.83; F1, 23 = 5.02; and F1, 15 = 4.16; #P<0.05) in activity on ED2 compared to ED1 in all four motor indices (Fig. 3 histograms). The increases in horizontal activity and stereotypic activity started shortly post injection and endured for the entire two hour recording session (Fig. 3 temporal graph), while the total distance travelled increased significantly (F2, 32 = 6.15; #P<0.05) at 20 min following amphetamine (0.6mg/kg) injection and lasted up to ninety min post injection. The significant (F2, 32 = 2.47; P<0.05) increase in vertical activity started at sixty min after injection and lasted for eighty min (Fig. 3 temporal graphs). In general, the increases in the four locomotor indices following an acute dose of 0.6 mg/kg amphetamine elicited effects on locomotion similar to those elicited by 2.5 mg/kg MPD.
Fig. 3.
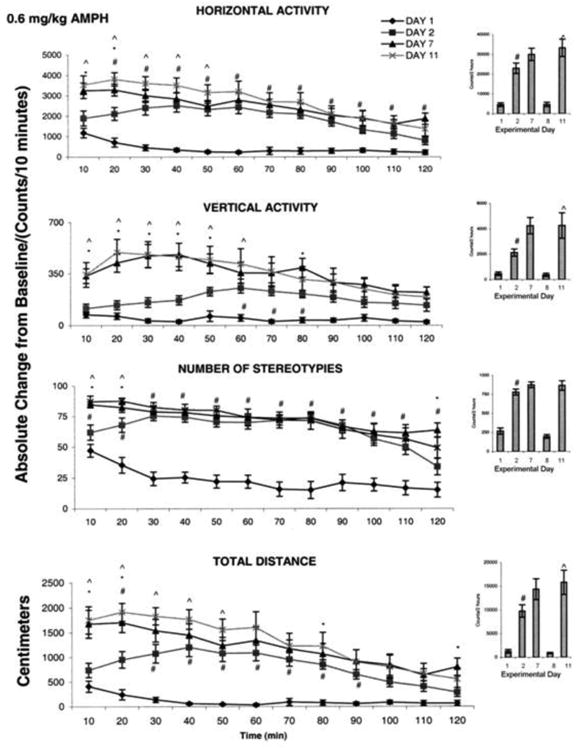
This figure summarizes (N=8) the effect of 0.6 mg/kg amphetamine on four locomotor indices. On the left side are the sequential graphs of 10 minute temporal activity post injection and on the right side are the histograms of total activity under the temporal graph. # symbol indicates significant (P< 0.05) difference comparing the activity on experimental day ED 1 vs. ED 2; ^ symbol indicates significant (P< 0.05) difference between ED 11 vs. ED 2.
3.2.2 Chronic Effects of Amphetamine-Induction Phase- Comparing ED7 to ED2
Amphetamine injection on ED7 elicited increases in locomotor activity similar to those elicited on ED2 (Fig. 3 histograms). The temporal graphs show that horizontal activity, total distance, and number of stereotypies exhibited a significant (F3, 51 = 3.91; F3, 51 = 3.87; F3, 51 = 3.80; *P<0.05) increase at twenty min post 0.6 mg/kg amphetamine injection at ED7 compared to the effects of the same amphetamine dose injected on ED2, while the significant increase in vertical activity lasted about eighty min (Fig. 3 temporal graphs). This significant increase shown in the temporal graph indicates that behavioral sensitization was initiated at ED7. The data was skewed in the histogram that sum the activity for 2 h. i.e. this behavioral sensitization lasted for a short time.
3.2.3 Chronic Effects of Amphetamine-Expression Phase-Comparing ED11 to ED2
There was a significant (F2, 51 = 3.63; F2, 51 = 4.52; F2, 27 = 3.41; F2, 27 = 4.04; ^P<0.05) increase in horizontal activity, total distance, and vertical activity on ED11 post amphetamine injection compared to the same amphetamine dose on ED2; but this dose did not elicit a significant increase in number of sterotypies (Fig. 3 histograms). Significant increases (F2, 27 = 4.44; F2, 27 = 4.46; P< 0.05) in horizontal activity and total distance traveled started immediately following amphetamine administration and lasted for fifty min post injection, while the increases in vertical activity lasted for sixty min post injection (Fig. 3 temporal graphs). The stereotypies activity increased immediately following amphetamine administration and lasted for twenty min. Analysis of the hourly and the temporal recordings indicated that all four locomotor indices following 0.6 mg/kg amphetamine administration expressed behavioral sensitization.
3.3.1 Acute Effects of 3,4-methylenedioxymethamphetamine (MDMA)-Comparing ED2 to ED1
Fig. 4 summarizes (N=18) the locomotor activity post injection of 5.0 mg/kg MDMA for ED1 and ED2. MDMA elicited a significant (F1, 23 = 11.62; F1, 25 = 14.61; P< 0.05) increase in a horizontal activity and total distance on ED2 compared to ED1, while the vertical activity exhibited significant (F1, 15 = 5.55; P< 0.05) decrease on ED2 post MDMA injection compared to ED1 (Fig. 4 histogram); stereotypies activity failed to increase significantly following MDMA (5.0 mg/kg) administration. The 10 min temporal graphs show that there was a significant (F1, 25 = 15.46; P< 0.05) increase in horizontal activity that began at thirty min post injection and persisted for the entire two h. The decrease in vertical activity and increase in number of stereotypies started immediately post MDMA injection and each response lasted for twenty min post injection (Fig. 4 temporal). Furthermore, the significant (F1, 23 = 4.92; P< 0.05) increase in total distance began at ten min post injection and lasted for one hundred ten min post injection. This observation (from the temporal graphs) shows that the dose of 5.0 mg/kg MDMA elicited an increase in horizontal activity, sterotypies and total distance traveled while decreasing the vertical activity.
Fig. 4.
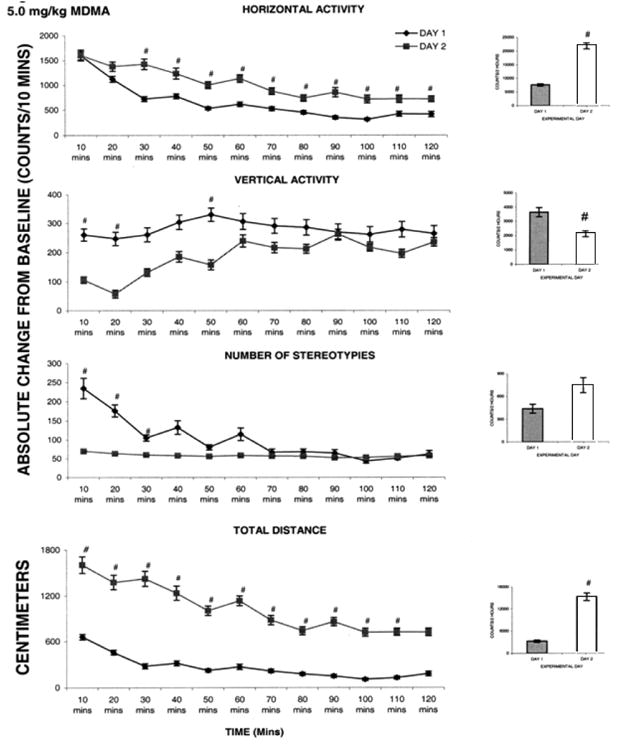
This figure summarizes (N=8) the acute effect of 5.0 mg/kg MDMA on four locomotor indices. On the left side are the sequential graphs of 10 minute temporal activity post injection and on the right side are the histograms of total activity under the temporal graph. # symbol indicates significant (P< 0.05) difference comparing the activity on experimental day ED 1 vs. ED 2.
3.3.2 Chronic Effects of MDMA-Induction Phase-Comparing ED7 to ED2
There was no significant increase or decrease in activity on ED 7 compared to ED2 after injection of MDMA. This dose of MDMA did not induce either significant tolerance or sensitization in any of the locomotor indices after six daily injections (data not shown).
3.3.3 Chronic Effects of MDMA-Expression Phase-Comparing ED11 to ED2
Activities measured following 5.0 mg/kg MDMA injection on ED11 were compared to those observed on ED2 (Table 1). No significant difference was observed between the activities recorded for these two experimental days. This lack of significance was due to the large standard error in this group (data not shown), suggesting that the number of animals should be increased. In spite of an increase to N=18, the standard error was large and the statistical test indicated that the drug had no effect. However, observations during the experiment showed that the drug exerts a significant effect on the animals' behavior. Therefore, each rat was statistically analyzed separately instead of evaluating all eighteen rats as one group. This evaluation revealed that eight out of the eighteen rats tested with 5.0 mg/kg MDMA on ED11 elicited significant (F1, 25 = 14.52; P< 0.05) increases in locomotion, while ten exhibited a significant (F1, 23 = 11.62; P< 0.05) decrease in activity in comparison to ED2 (Fig. 5 and 6 respectively).
Fig. 5.
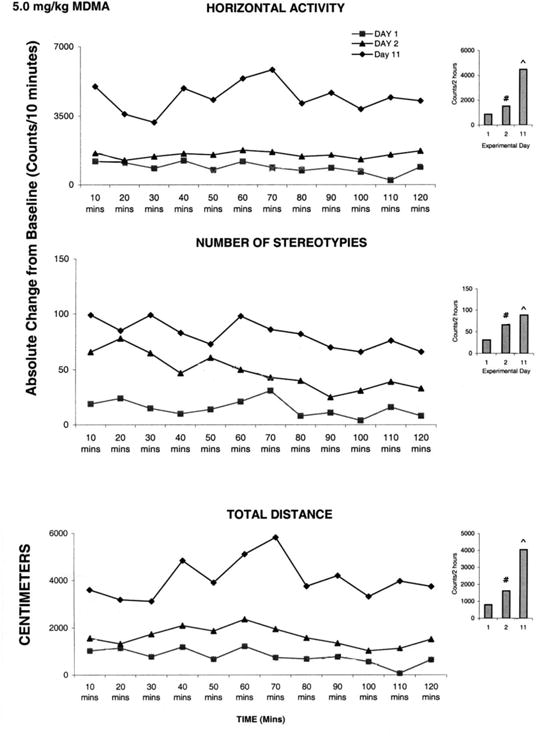
This figure summarizes the acute and chronic effect of 5.0 mg/kg MDMA in animals exhibiting behavioral sensitization (For details, see text). On the left side are the sequential graphs of 10 minute temporal activity post injection and on the right side are the histograms of total activity under the temporal graph. # symbol indicates significant (P< 0.05) difference comparing the activity on experimental day ED 1 vs. ED 2; ^ symbol indicates significant (P< 0.05) difference between ED 11 vs. ED 2.
Fig. 6.
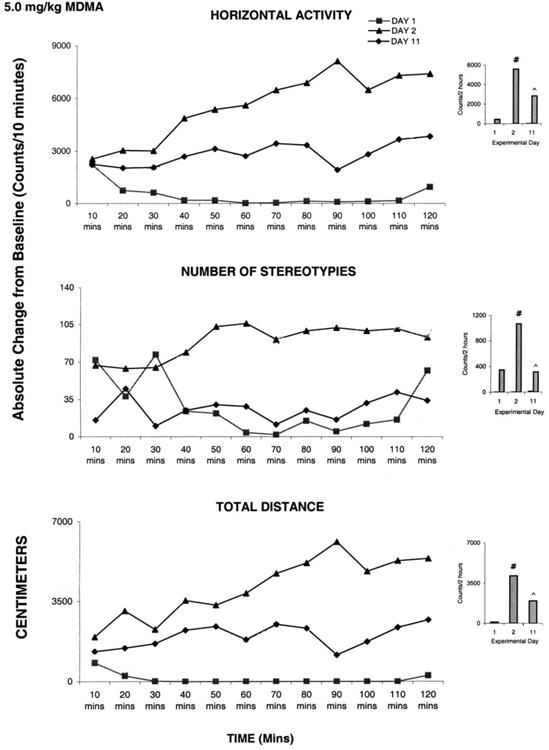
This figure summarizes the acute and chronic effect of 5.0 mg/kg MDMA in animals exhibiting behavioral sensitization (For details, see text). On the left side are the sequential graphs of 10 minute temporal activity post injection and on the right side are the histograms of total activity under the temporal graph. # symbol indicates significant (P< 0.05) difference comparing the activity on experimental day ED 1 vs. ED 2; ^ symbol indicates significant (P< 0.05) difference between ED 11 vs. ED 2.
Fig. 5 shows data from one animal that exhibits a significant (F2, 32 = 2.48; P<0.05) increase in activity following rechallenge MDMA administration at ED11 compared to the ED2 initial MDMA injection. MDMA elicited significant (F2, 32 = 2.46; F2, 32 = 6.29; F2, 27 = 3.50; P< 0.05) increases in horizontal activity, number of stereotypies, and total distance traveled on ED 2, and further significant (F2, 32 = 6.33; F2, 27 = 3.48; F2, 27 = 4.15; P< 0.05) increases on ED11 compared to ED2. This increase in locomotion on ED11 compared to ED2 indicates that this MDMA dose elicits behavioral sensitization. Similar observations were obtained from the other seven animals.
The other ten rats exhibited significant (F2, 51 = 3.64; F2, 27 = 3.51, F2, 27 = 5.02; F2, 51 = 3.27; P< 0.05) decreases in locomotor activity on ED11 compared to ED2. Fig. 6 shows representative data from an animal from this group. There was a significant (F1, 25 = 4.68; F1, 25 = 14.02; F1, 15 = 6.69; P< 0.05) increase in locomotion on ED2 compared to ED1, and a significant (F2, 32 = 6.30; F2, 27 = 3.52; F2, 25 = 4.37; F2, 27 = 3.48; P< 0.05) decrease in locomotor activity on ED11 compared to ED2, which was interpreted as behavioral tolerance.
3.4 Is there cross sensitization between MPD and Amphetamine?
In preliminary experiments, six control groups were used to record the activity following injection of either MPD, amphetamine, or MDMA on ED2 compared to ED1 (saline)(N=6), and another three groups treated with saline for 4 days and at ED12 treated either with MPD, amphetamine or MDMA (N=6). The activity post injection of the ED2 group was similar to that of animals treated with drug at ED12. To minimize the number of animals, we used the ED1 recording (Table1) as control. Fig. 7a compares data obtained from drug naïve animals (N=8) that received MPD on ED2 with data obtained from those animals who received MPD on ED12 after having received amphetamine on ED2-7 followed by 3 days washout and then another dose of amphetamine on ED11. The response to MPD in those animals previously treated with amphetamine was significantly (F10, 142 = 4.82; F10, 131 = 33.34; F 10, 87 = 2.5; P<0.05) greater compared to the response to MPD in naïve animals in three of the four locomotor indices. This observation indicates that cross sensitization between MPD and amphetamine was obtained.
Fig. 7.
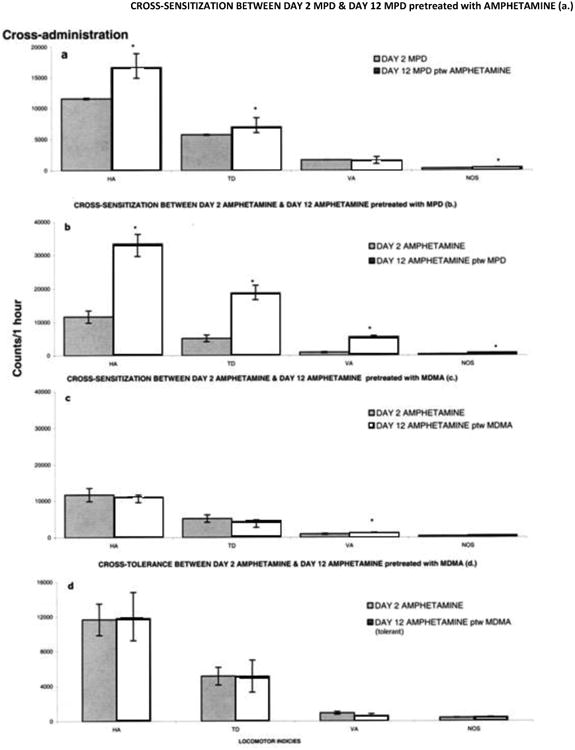
This figure summarizes all the experimental groups to show whether cross-sensitization between NPD, Amphetamine and MDMA is expressed. * symbol indicates significant difference between the group, i.e., cross-sensitization is expressed. HA-horizontal activity, TD-total distance traveled, VA-vertical activity and NOS-number of stereotypic activity. (For details, see text).
3.5 Is there cross sensitization between amphetamine and MPD?
Fig. 7b compares data obtained from drug naïve animals that received amphetamine on ED2 with data obtained from those animals who received amphetamine on ED12 (N=8) after having received MPD on ED2-7 followed by washout and then MPD on ED11. The response to amphetamine in those animals previously treated with MPD was significantly (F10, 87 = 14.26; F10, 131 = 3.03; F10, 87 = 3.01; F10, 87 = 2.89; P< 0.05) greater compared to the response to MPD in naïve animals in all four indices.
These comparisons indicate that the 2.5 mg/kg dose of MPD resulted in cross-sensitization with the 0.6 mg/kg dose of amphetamine and the 0.6 mg/kg dose of amphetamine resulted in cross-sensitization with the 2.5 mg/kg dose of MPD.
3.6 Is there cross-sensitization between amphetamine and MDMA?
a. In animals expressing behavioral sensitization to MDMA
Fig. 7c compares data obtained from those naïve animals that received amphetamine on ED2 with data obtained from those animals who received amphetamine on ED12 after having received MDMA on ED2-7 followed by washout and then displayed sensitization to MDMA on ED11 (N=8). There were no significant differences in horizontal activity, total distance, or number of stereotypies, while the vertical activity exhibited a significant (F10, 87 = 13.83; P< 0.05) further increase in activity.
b. In animals expressing behavioral tolerance to MDMA
Fig. 7d compares data obtained from those naïve animals that received amphetamine on ED2 with data obtained from those animals who received amphetamine on ED12 after having received MDMA on ED2-7 followed by washout and then displayed tolerance to MDMA on ED11 (N=10). There were no significant changes in activity between these two groups. These comparisons indicate that amphetamine did not cross-sensitize or cross-tolerate with MDMA in animals exhibiting tolerance to multiple MDMA administration.
4. Discussion
Most of the reports on psychostimulants (MPD, amphetamine, and MDMA) used only one of them and moreover each used different experimental procedures, different doses, different drug schedules, etc. making it difficult to compare the actions of MPD, amphetamine, and MDMA. The objective of this study was to use the same experimental protocol to study the effects of acute and chronic administration of MPD, amphetamine, and MDMA as well as to determine if there was cross sensitization between them.
Pyschostimulants affect mainly the central nervous system brain sites known as the reward circuit. The reward circuit includes the ventral tegmental area, nucleus accumbence, prefrontal cortex, and other sites which are collectively termed the “motive circuit” (Pierce and Kalivas, 1997). These structures are believed to be involved in the induction and expression of behavioral sensitization using catecholaminergic, glutaminergic, and serotonergic pathways. This circuit acts also as an interface between limbic and motor systems.
Most experiments use male animals; however, the reactions and mechanisms involved in metabolizing psychostimulants may differ between sexes. Studies involving psychostimulants such as amphetamine and cocaine have shown that females become more seriously dependent to psychostimulants than their male counterparts by expressing a more rapid and robust behavioral response to acute and chronic administration (Anderson and Teicher, 2000; Booze et al., 1999). Therefore, in this study, females were used to better observe behavioral sensitization (Booze et al., 1999).
4.1 MPD
Methylphenidate (MPD) has become a highly prescribed drug in past years. In many instances, MPD is used to treat children and young adults with attention deficit hyperactivity disorder (ADHD) over long periods of time (Accardo and Blondis, 2001; Challman and Lipsky, 2000; Garland, 1998). These long term treatment regimens can elicit some structural and physiological alteration in the developing brain of a child (Andersen and Teicher, 2000; Levin and Kleber, 1995). Behavioral investigation on MPD is necessary because it mirrors the pharmacological properties of other addictive stimulants such as cocaine and amphetamine (Gerasimov et al, 2000; Volkow et al, 1999). Therefore, there should be great concern when prescribing Ritalin® to young children who are still developing neuronal processes.
The initial administration of 2.5 mg/kg MPD elicited an increase in activity on ED2 in comparison to saline controls on ED1, indicating an acute effect. Chronic MPD administration elicited further increases in activity on ED11 in comparison to ED2 indicating that behavioral sensitization was evident. Similar observations were obtained using male rats (Gaytan et. al., 1997, 1999, 2000; Lee et al., 2009; Yang et. al., 2003, 2006, 2007). MPD binds the dopamine transporter (DAT) and causes dopamine to remain active in the synaptic cleft for a longer time (Volkow et al., 1999). It has been suggested that increases in synaptic dopamine concentration may contribute to the acute effect of MPD (Sagvolden and Sergeant, 1998).
Considerable evidence suggests that the chronic effect of MPD may occur at the molecular level. There are reports of increased levels of chromosomal abnormalities in children prescribed MPD (Biederman et al., 2009; El-Zein et al, 2005). Furthermore, it has been suggested that these chronic effects could be due to altered transcriptional activation of immediate early genes (IEG) such as c-fos (Yanol and Steiner, 2005) through intracellular changes involving second messenger systems. It has been reported that MPD administration in cats caused an increase in the density of cortical cells expressing c-fos (Lin et al 1996). C-fos is the most frequently activated IEG after external or internal stimuli (Lin et al 1996). Second messenger systems are suggested to activate third messenger systems, therefore, c-fos has been considered as a third messenger system triggering long term cell reaction cascades (Morgan and Curran, 1991, 1989; Sheng and Greenberg, 1990). Once post-synaptic dopamine receptors have been activated due to the continuous stimulation of dopamine, receptor-linked channels promote intracellular changes through second messenger molecules. The G protein is “turned on” as it binds to guanosine triphosphate (GTP), displacing guanosine diphosphate (GDP), and thereby activating adenylate cyclase which generates the second messenger cyclic AMP (cAMP). cAMP then triggers an enzymatic cascade activating the c-fos gene. In addition, expression of IEG's in striatal and cortical dopamine targets has been suggested to mediate drug dependency (Hughes and Dragunow, 1995).
One explanation for why the chronic administration of MPD led to increased motor activity on ED11 in comparison to ED2 (Fig. 2) is that dopamine activated dopamine receptors for longer periods of time due to an increase in the number of dopamine receptors or an increased responsiveness of the dopamine receptors. These increases could activate motor nuclei, more specifically, the D1 receptors in the nucleus accumbence and caudate nucleaus which could have led to further increases in locomotion i.e. expression of sensitization (Wolf et al., 1993, 1994). Alternatively, presynaptic dopamine autoreceptors may have prevented any further dopamine from being released from the presynaptic cell due to its inhibitory nature (Shi et al., 2004; Yang et al., 2006). D2 receptor subsensitivity activation in the ventral tegmental area is another possible explanation for the induction of sensitization (Yang et al., 2006) and could be part of the underlying mechanism behind enhanced activity in day 11 in all the locomotor activities studied.
The current findings are similar to other reports of increased locomotor responses following MPD administration in male Sprague Dawley rats (Gaytan et al., 1997; Yang et al., 2003, 2006, 2007,2010). However, the results of our study are in contrast to others reporting that rats fail to exhibit sensitization after chronic exposure to MPD (Izenwasser et al.,1999; Kuczenski and Segal, 2002). Methodology is an important factor to consider when determining reasons for inconsistencies. The different observations can be explained due to differences in experimental procedures such as the route of administration e.g. oral (Kuczenski and Segal, 2002), different doses of MPD (Izenwasser et al., 1999), different times of drug administration and different number of washout days. Gaytan et al., (2000) reported higher total distance activity during the dark phase than the light phase, and reported that behavioral sensitization was observed only when the drug was administered during the day time. Factors such as time of day, route of administration, and dose of MPD all affect whether sensitization will be elicited or not.
The present observations provide evidence that systemic MPD administration to rats produced behavioral sensitization to itself, suggesting that MPD has the potential to elicit dependence. Moreover, today's adolescent and adult students around the world, take prescription drug Ritalin (MPD) for cognitive enhancement and recreation, using different routes and times for its administration. Some articles claim that “we should welcome new methods of improving our brain function” (Nature Online, December, 2008), but our findings caution that one must be aware of and prepared for new unknown outcomes.
4.2 Amphetamine
Amphetamine is classified as a Schedule II drug in the United States. Popular brands including Adderall® have been used to treat narcolepsy and inattention (Weinshenker et. al., 2002). Amphetamine is a psychostimulant that affects the central nervous system and shares similar behavioral and neuropharmacological properties with MPD (Mayorga et al.,1999). It is used also to treat ADHD patients.
Results from our current and previous behavioral studies indicate that repeated administration of 0.6 mg/kg amphetamine elicits augmented locomotor responses in rats (Gaytan et al., 1998, 1999; Tang et al., 2009). Similar to the effects seen with MPD, there were dose dependent increases in all four indicies of activity on ED2 in comparison to ED1 (see Table 1). This was interpreted as eliciting an acute effect. The re-challenge injection after three washout days elicited an increase in activity on ED11 in comparison to ED2 (Fig. 2). This was interpreted as behavioral sensitization. The results obtained are similar to those reported by others (Gaytan et al., 1997; Sripada et al., 1998, Tang, 2009). Amphetamine binds to presynaptic membranes, converting all monoamine transporters, particularly dopamine and norepinephrine (Weinshenker et al., 2002) into open dopamine and norepinephrine channels, thus allowing efflux of dopamine and norepinephrine (Vanderschuren et al., 1999 a and b). The acute rise in locomotor activity is proposed to results from increases in dopamine and norepinephrine that elicit neural changes. Dopaminergic ventral tegmental area neurons are interconnected to nucleus accumbence, prefrontal cortex, basal ganglia and also innervates other limbic system nuclei (Pierce and Kalivas, 1997). This circuit regulates ambulatory activity and is activated after amphetamine administration (Wolf, 1998). It has also been postulated that sensitization to amphetamine is mediated by the excess of norepinephrine and dopamine in the synaptic cleft that activates D1 receptors (Bjijou et al., 1996; Vezina 1993; Vezina and Stewart, 1993). Furthermore sensitization to amphetamine was enhanced when SKF-38393, a D1 agonist, was administered to amphetamine pretreated rats (Pierce and Kalivas, 1996).
It was suggested that the induction of behavioral sensitization to amphetamine occurs at glutamatergic synapses of ventral tegmental area dopamine neurons (Kalivas and Stewart, 1991; Pert, 1998). This suggestion is based on experiments that showed a glutamate antagonist, such as MK-801, blocked the induction of sensitization to amphetamine (Pacchioni et al., 2002). Moreover, destruction of medial prefrontal cortex glutamatergic afferents blocked both the induction and the expression of sensitization following chronic amphetamine administration (Cador et al. 1999; Tang et al, 2009). These observations suggest that glutamatergic inputs are necessary to amphetamine effect not only for the induction phase, but also for the expression phase as well.
Molecular changes in the reward pathway due to chronic administration of amphetamine, or any other stimulant, can result in changes in gene transcription and RNA and/or protein synthesis (Nestler, 2001). This suggests that chronic administration of amphetamine may increase the expression of transcription factors, such as those regulating AMPA receptor expression, and could lead to the enhancement of the rewarding effects of amphetamine. The induction of sensitization to amphetamine has been attributed to a transient increase in the AMPA receptors' responsiveness in ventral tegmental area dopamine neurons (Giorgeti et al, 2001) due to extracellular dopamine remaining in the synaptic cleft.
In summary, increases in locomotor activity following amphetamine administration can be attributed to the drug-induced changes in several neurochemical mechanisms including elevation of dopamine and norepinephrine levels. Amphetamine has been suggested to produce behavioral sensitization following chronic application via neural and physiological mechanisms affecting the projecting excitatory transmitters onto D1, glutamate, and AMPA receptors at the motive circuit (Bardo and Bevins, 2000).
4.3 MDMA
Using identical experimental protocols as described above for MPD and amphetamine the acute effects of MDMA elicited significant increases in locomotive activity. By contrast, the chronic effects showed no significant change in behavior compared to the initial MDMA injection due to a high standard error. This led us to increase the number of animals. Despite the increase in numbers of animals, similar observations were obtained. Once again, acute MDMA caused a significant increase in locomotion while chronic MDMA show in some animals further increases in activity and in other animals had the opposite effect i.e. decreases in activity and the average of all animals showing no significant effect. Therefore, each animal was statistically evaluated separately. This evaluation showed that chronic MDMA elicited behavioral sensitization in about 44% of the animals and tolerance in about 56% of the animals. This observation is consistent with other reports since some researchers report sensitization following MDMA administration (Atkins et al., 2009; Ball et. al., 2009; Biezonski et. al., 2009; Dafters 1995; Kalivas et al., 1998a; Modi et al., 2006), some report tolerance following MDMA administration (Baumann et. al., 2008, Baumann et. al., 2009; Jones et al., 2010; Marston et al., 1999), and others find a complex spectrum of behavior with neither sensitization or tolerance developed after MDMA administration (Baumann et. al., 2008; McNamara et al., 1995). MDMA exhibited mixed behavioral results, unlike MPD and amphetamine (Cole and Summall, 2003). Since tolerance and sensitization are experimental indicators for the liability of a drug, these results indicate a likely propensity for the development of dependence on MDMA.
4.4 How to interpret these two different observations? Tolerance and sensitization to the same dose
Pharmacologically, 5-HT is a key indicator of acute effects of MDMA (Kalivas et al., 1998). MDMA causes an overall serotonergic depletion in nerve terminal endings over time, especially in the cortex and hippocampus (Atkins et al., 2009; Capela et. al., 2009; McNamara et al., 1995) due to the rise in extracellular 5-HT after MDMA administration. MDMA also inhibits 5-HT and 5-HT1 receptor synthesis (Callaway and Geyer, 1992 a and b; Callaway et al., 1990; Trickelbank et al., 1986), and can produce a decrease in 5-HT's metabolite, 5-hydroxyindoleacetic acid (5-HIAA), and its rate limiting enzyme, for 5-HT synthesis, tryptophan hydroxylase. Furthermore, pretreatment with the 5-HT1B receptor agonist RU24969 increases the release of presynaptic 5-HT during MDMA administration thereby enhancing locomotor activating effects (Callaway and Geyer, 1992 a and b; Oberlander et al., 1987, 1986). This indicates that 5-HT plays a major role in the development of sensitization to MDMA. It has also been postulated that dopamine plays a role in the acute effect of MDMA and in MDMA treatment leading to sensitization (Koch and Gallaway, 1997; Gold et al., 1988; Modi et al., 2006). Blocking 5-HT transporters with a 5-HT2 receptor antagonist attenuated extracellular dopamine (Kalivas et al., 1998b) which correlates with hyperactivity attributable to the co-activation of dopamine releasing neurons and presynaptic 5-HT in the ventral tegmental area, nucleus accumbence, and prefrontal cortex neurons. Moreover, 5-HT2 receptor subtypes are localized on dopamine releasing neurons (Gudelsky and Nash, 1996; Karler et al., 1995) providing even further evidence for dopamine co-activation along with 5-HT.
Although dopamine can contribute to motor activating effects, dopamine can also have severe effects on serotonin nerve terminals. Dopamine causes the 5-HT axon terminal to become shriveled up and damaged. It takes up to two weeks to replenish serotonin levels after the initial MDMA doses and even longer after larger doses (Quinton and Yamamoto, 2006). The chronic effects of MDMA can cause substantial loss of serotonin reuptake transporters (Quinton and Yamamoto. 2006) causing irreversible degeneration of serotonin nerve terminals (Quinton and Yamamoto, 2006). Chronic MDMA administration regulates transcription of c-fos and egr-1 genes (Shirayama et al., 2000 and Stephenson et al., 1999) which play a role in converting acute neural stimulation into chronic cellular changes in Neuroplasticity (Herrera and Robertson, 1996 and Thiriet et al., 2002). Over stimulation of postsynaptic receptors due to chronic drug administration leads to increased cAMP and calcium signaling cascades, which can result in increased transcription of these immediate early genes [IEGs] (Thiriet et al., 2002; Vaccarino et al., 1993). Therefore, MDMA elicits both an acute and chronic effect as reported by others (Atkins et al., 2009; Modi et al., 2006; Shenk et al., 2003) due to co-activation of dopamine and 5-HT releasing neurons, 5-HT1 and 5-HT2 receptor interference and chronic neuroadaptations due to transcriptional changes. Since repetitive MDMA administration results in sensitization or tolerance to itself, it has the potential to elicit dependence.
4.5 Cross-sensitization
Cross-sensitization between two psychostimulants indicates that similar neural mechanisms underly the two drugs (Dafny and Yang, 2006). It has been postulated that pre-exposure to one psychostimulant will lead to an increased sensitivity and subsequent vulnerability to abuse of other psychostimulants (Brandon et al., 2001; Kalivas et al., 1998b; Torres-Reveron and Dow-Edward, 2005). This experimental procedure is also used to verify if an unknown drug is considered to have the potential to elicit dependence by comparing it with a drug known to elicit dependence (Dafny and Yang, 2006). Amphetamine is known to elicit dependence while researchers are still debating whether MPD has the potential to cause dependence. If MPD exhibits behavioral sensitization or tolerance and cross-sensitization with amphetamine and vice versa, it will strongly indicate that MPD has the potential to elicit dependence. Children diagnosed with ADHD have been treated with MPD and amphetamine over extended periods of time (Garland, 1998; Robin, 1999). Since sensitization and cross-sensitization is an experimental behavioral indicator for the development of dependence in animals (Kalivas and Duffy, 1998; Robinson and Berridge, 1993) previous exposure to either MPD or amphetamine may heighten an already augmented locomotor response leading to cross sensitization. In this study, animals pretreated with MPD cross-sensitized to a challenge dose of amphetamine and animals pretreated with amphetamine cross-sensitized to a challenge dose of MPD. These results are similar to those reported by Yang et al., (2003) using male rats. Both MPD and amphetamine are indirect dopamine agonists although they facilitate dopamine in the dopaminergic pathway by different mechanisms. MPD blocks the dopamine transporter from re-uptake of dopamine, leading to increased amounts of extracellular dopamine. On the other hand, amphetamine stimulates the release of synaptic dopamine into the synapse, thus causing increased extracellular dopamine. Therefore, cross sensitization between these two drugs provides a further indication of the propensity of each drug to influence other forms of drug use.
Neither cross-sensitization or cross-tolerance occurred between MDMA and amphetamine, as also reported by others (Modi et al., 2006; Cole et al., 2003). A possible explanation for the failure of MDMA to cross-sensitize to amphetamine is its differing primary mode of action. Although MDMA is an amphetamine derivative, the two act by different neuronal mechanisms. MDMA primarily activates the sertonergic pathway, and utilizes the dopaminergic pathway secondarily, while amphetamine primarily activates the dopaminergic pathway. However, Callaway and Geyer (1992 a and b) reported that pretreatment with MDMA elicits motor activating effects similar to the effects elicited by amphetamine. Discrepancies can be attributed to, but are not limited to, differences in methodology such as time and the dose of administration, age and sex of the subjects, route of drug administration, etc. Reports that Ecstasy “tablets” contain other drugs of abuse such as amphetamine (Milroy et al., 1996) and ephedrine (Baggot et al., 2000) suggest that additional studies on this issue are needed.
5. Conclusion
This study investigates the acute and chronic effects of MPD, amphetamine, and MDMA using the same experimental procedure to provide comparisons of the effects of these drugs on locomotor behavior. In summary, acute MPD, amphetamine and MDMA all elicited increases in locomotor activity, repetitive application of MPD and amphetamine elicited behavioral sensitization, while the same dose of MDMA elicited sensitization in some animals and tolerance in others Cross-sensitization between amphetamine and MPD when rats were pretreated with MPD and vice versa were obtained. However, MDMA did not cross-sensitize or cross-tolerate with amphetamine suggesting that MDMA and amphetamine act via different mechanisms.
Acknowledgments
The authors wish to thank NIDA for the gift of MDMA, Mallincrodkt for the gift of MPD, and D. Wood for help with manuscript preparation. This research was supported in part by the NIH F31 DA 14441 and R01-DA 027222.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accardo P, Blondis TA. What's All the Fuss About Ritalin? The Journal of Pediatrics. 2001;138:6–9. doi: 10.1067/mpd.2001.111505. [DOI] [PubMed] [Google Scholar]
- Aizenstein ML, Segal DS, Kuczenski R. Repeated Amphetamine and Fencamfamine: Sensitization and Reciprocal Cross-Sensitization. Neuropsychopharmacology. 1990;3:283–290. [PubMed] [Google Scholar]
- Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague Dawley rats. Pharmacol Biochem Behav. 2009;92:93–99. doi: 10.1016/j.pbb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Atkins K, Burks T, Swann AC, Dafny N. MDMA (ecstasy) modulates locomotor and prefrontal cortex sensory evoked activity. Brain Res. 2009;1302:175–182. doi: 10.1016/j.brainres.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Baggot M, Heifets B, Jones RT, Mendelson J, Sferios E, Zehnder J. Chemical analysis of ecstasy pills. JAMA: the journal of the American Medical Association. 2000;284:2190–2191. doi: 10.1001/jama.284.17.2190. [DOI] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fontenberry E, Rebec GV. Sensitizing regimens of (+/-) 3-4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is co-related with dialysate levels of serotonin and dopamine in rat brain. Pharmacol Biochem Behav. 2008;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high doses binges. Neuroscience. 2008;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, Mick E, Monuteaux MC, Faraone SV. Familial risk analyses of attention deficit hyperactivity disorder and substance abuse disorders. Am J Psychiatry. 2008;147:107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- Biezonski DK, Courtemanche AB, Hong SB, Piper BJ, Meyer JS. Repeated adolescent MDMA (“Ecstasy”) exposure in rats increases behavioral and neuroendocrine responses to a 5-HT2A/2C agonist. Brain Res. 2009;1252:87–93. doi: 10.1016/j.brainres.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Bjijou Y, Stinus L, Le Moal M, Cador M. Evidence for selective involvement of dopamine D1 receptors of the ventral tegmental area in the behavioral sensitization induced by intra-ventral tegmental area injection of D-Amphetamine. J Pharmacol Exp Ther. 1996;277:1177–1187. [PubMed] [Google Scholar]
- Bonate PL, Swann A, Silverman PB. Context-dependent cross-sensitization between cocaine and amphetamine. Life Sciences. 1997;60:L1–L7. doi: 10.1016/s0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wood ML, Welch MA, Berry S, Mactutus CF. Estrous cyclicity and behavioral sensitization in female rats following repeated intravenous cocaine administration. Pharmacol Biochem Behav. 1999;64:605–610. doi: 10.1016/s0091-3057(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Cador M, Bjijou Y, Cailhol S, Stinus L. D-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortexventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Stimulant effects of 3,4 methylenedioxymethamphetamine in the nucleus accumbens of rat. Eur J Pharmacol. 1992a;214:45–51. doi: 10.1016/0014-2999(92)90094-k. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Geyer MA. Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine 1B agonist. J Pharmacol Exp Ther. 1992b;263:318–326. [PubMed] [Google Scholar]
- Camarini R, Frussa-Filho R, Monteiro MG, Calil HM. MK-801 blocks the development of behavioral sensitization to ethanol. Alcohol Clin Exp Res. 2000;24:285–290. [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR, O'Shea E, Marsden CA. Effects of MDMA exposure on the conditioned place preference produced by other drugs of abuse. Psychopharmacology (Berl) 2003;166:383–390. doi: 10.1007/s00213-002-1374-x. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Altered States: the clinical effects of Ecstasy. Pharmacology & therapeutics. 2003;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Dafny Nachum, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Research Bulletin. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiological Behavior. 1995;58:877–882. doi: 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- EI-Zein RA, Abdel-Rahman SZ, Hay MJ, Lopez MS, Bondy ML, Morris DL, Legator MS. Cytogenic effects in children treated with methylphenidate. Cancer Letters. 2005;230:284–291. doi: 10.1016/j.canlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Garland EJ. Intranasal Abuse of Prescribed Methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:573–574. doi: 10.1097/00004583-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Gaytan 0, AI-rahim S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Science. 1997;61:L101–L107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- Gaytan 0, Swann A, Dafny N. Time-dependent differences in the rat's motor response to amphetamine. Pharmacol Biochem Behav. 1998;59:459–67. doi: 10.1016/s0091-3057(97)00438-3. [DOI] [PubMed] [Google Scholar]
- Gaytan 0, Lewis C, Swann A, Dafny D. Diurnal differences in amphetamine sensitization. Eur J Pharmacol. 1999;374:1–9. doi: 10.1016/s0014-2999(99)00243-5. [DOI] [PubMed] [Google Scholar]
- Gaytan 0, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res 2000. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marstellar D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methyphenidate administration: A microdialysis and locomotor activity study. The journal of pharmacology and experimental therapeutics. 2000;295:51–57. [PubMed] [Google Scholar]
- Giorgetti M, Hotsenpiller G, Ward P, Teppen T, Wolf ME. Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats. J Neurosci. 2001;21:6362–6369. doi: 10.1523/JNEUROSCI.21-16-06362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4- methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4methylenedioxymethamphetamine: implications for serotonin-dopamine interactions. J Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hughes P, Dragunow M. Induction of immediate-early and the control of neurotransmitter-regulated gene transmission within the nervous system. Pharmacol Rev. 1995;47:133–178. [PubMed] [Google Scholar]
- Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. European Journal of Pharmacology. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Jones K, Brennan KA, Colussi-Mas J, Schenk S. Tolerance to 3,4 methylenedioxymeth-amphetamine is associated with impaired serotonin release. Addict Biol. 2010;15:289–298. doi: 10.1111/j.1369-1600.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behavioural Pharmacology. 1993;4:315–334. [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, White SR. MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology. 1998;18:6. doi: 10.1016/S0893-133X(97)00195-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. Journal of Psychopharmacology. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Thai LH, Bedingfield JB. The dopaminergic, glutamergic, GABAergic bases for the action of amphetamine and cocaine. Brain Research. 1995;671:100–104. doi: 10.1016/0006-8993(94)01334-e. [DOI] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induce dopamine release in vivo: role of endogenous serotonin. J Neural Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of Amphetamine-Induced Stereotyped Behaviors During the Acute Response: Role of D 1 and D2 Dopamine Receptors. Brain Research. 2002;822:164–174. doi: 10.1016/s0006-8993(99)01149-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology. 2009;57:201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Attention deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harvard review of psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- Lin JS, Hou Y, Jouvet M. Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci USA. 1996;93:14128–14133. doi: 10.1073/pnas.93.24.14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston HM, Reid ME, Lawrence JA, Olverman, Butcher SP. Behavioural analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology (Berl) 1999;144:67–76. doi: 10.1007/s002130050978. [DOI] [PubMed] [Google Scholar]
- Martin PR, Lavinger DM, Breese GR. Alcohol and other abuse substances. In: Munson PL, editor. Prin Of Pharmacol. 1995. pp. 441–442. [Google Scholar]
- Mayorga AJ, Popke EJ, Fogle CM, Paule MG. Similar effects of amphetamine and methylphenidate on the performance of complex operant tasks in rats. Behavioural brain research. 1999;109:59–68. doi: 10.1016/s0166-4328(99)00165-5. [DOI] [PubMed] [Google Scholar]
- McNamara MG, Kelly JP, Leonard BE. Some behavioural and neurochemical aspects of subacute (+/-)3,4-methylenedioxymethamphetamineetamjne administration in rats. Pharmacol Biochem Behav. 1995;52:479–484. doi: 10.1016/0091-3057(95)00206-c. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metab. 2010;11:468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with “ecstasy” and “eve” misuse. Journal of clinical pharmacology. 1996;49:149–153. doi: 10.1136/jcp.49.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi GM, Yang PB, Swann AC, Dafny N. Chronic exposure to MDMA (Ecstasy) elicits behavioral sensitization in rats but fails to induce cross sensitization to other psychostimulants. Behavioral and Brain Functions: BBF. 2006;2:1–12. doi: 10.1186/1744-9081-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long term plasticity underlying addiction. Nature Reviews Neuroscience. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Oberlander C, Blaquiere B, Pujol JF. Distinct functions for dopamine and serotonin in locomotor behaviour: Evidence using the 5-HTI agonist RU 24969 in globus pallidus-lesioned rats. Neurosci Lett. 1986;67:113–118. doi: 10.1016/0304-3940(86)90382-4. [DOI] [PubMed] [Google Scholar]
- Oberlander C, DeMassey Y, Verdu A, Van de V, Bardelay C. Tolerance to the 5-HT1 agonist RU24969 and effects on dopaminergic behavior. Eur J Pharmacol. 1987;139:205–214. doi: 10.1016/0014-2999(87)90253-6. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Gioino G, Assis A, Cancela LM. A Single exposure to restraint stress induces behavioral and neurochemical sensitization to stimulating effect of amphetamine: involvement of NMDA receptors. Annals of the New York Academy of Sciences. 2002;965:233–246. doi: 10.1111/j.1749-6632.2002.tb04165.x. [DOI] [PubMed] [Google Scholar]
- Pert A. Neurobiological substrates underlying conditioned effects of cocaine. Adv Pharmacol. 1998;42:991–995. doi: 10.1016/s1054-3589(08)60913-8. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Born B, Adams M, Kalivas PW. Repeated intra-ventral tegmental administration of SKF-38393 induces behavioral and neurochemical sensitization to a subsequent cocaine challenge. 1. J Pharmacol Exp Ther. 1996;278:384–392. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A Circuitry Model of the Expression of Behavioral Sensitization to Amphetamine-Like Psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Souza-Formigoni ML, Fornari RV, Nobrega JN, Oliveira MG. Is behavioral sensitization to ethanol associated with contextual conditioning in mice? Behavioral Pharmacology. 2003;14:129–136. doi: 10.1097/00008877-200303000-00004. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and Consequences of Methamphetamine and MDMA Toxicity. The AAPS Journal. 2006;8 doi: 10.1007/BF02854904. Article 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD. Assessing long-term effects of MDMA (ecstasy) Lancet. 2001;358:1831–1832. doi: 10.1016/S0140-6736(01)06880-5. [DOI] [PubMed] [Google Scholar]
- Robin AL. Attention deficit/hyperactivity disorder in adolescents. Common pediatric concerns. Pediatric clinics of North America. 1999;46:1027–1038. doi: 10.1016/s0031-3955(05)70170-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Research. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit hyperactivity disorder from brain dysfunctions to behaviour. Behavioural Brain Research. 1998;94:1–10. [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing of dopamine neurons. Neuropsychopharmacology. 2004;2:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Hashimoto K, Iyo M, Watanabe K, Higuchi T, Minabe Y. 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy)-induced egr-1 mRNA in rat brain: pharmacological manipulation. Eur J Pharmacol. 2000;402:215–222. doi: 10.1016/s0014-2999(00)00521-5. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sripada S, Gaytan O, AI-rahim S, Swann A, Dafny N. Dose-related effects of MK-801 on acute and chronic methylphenidate administration. Brain Res. 1998;814:78–85. doi: 10.1016/s0006-8993(98)01035-x. [DOI] [PubMed] [Google Scholar]
- Stephenson CP, Hunt GE, Topple AN, McGregor IS. The distribution of 3,4-Methylenedioxymethamphetamine “Ecstasy” -induced c-fos expression in rat brain. Neuroscience. 1999;92:1011–1023. doi: 10.1016/s0306-4522(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Tang A, Wanchoo SJ, Swann AC, Dafny N. Psychostimulant treatment for ADHD is modulated by prefrontal cortex manipulation. Brain Res Bull. 2009;80:353–358. doi: 10.1016/j.brainresbull.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Ladenheim B, McCoy MT, Cadet JL. Analysis of Ecstasy (MDMA)-induced transcriptional responses in the rat cortex. FASEB J. 2002;16:1887–1894. doi: 10.1096/fj.02-0502com. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Repeated administration of methylphenidate in young, adolescent, and mature rats affects the response to cocaine later in adulthood. Psychopharmacology (Berl) 2005;181:38–47. doi: 10.1007/s00213-005-2221-7. [DOI] [PubMed] [Google Scholar]
- Trickelbank MD, Middlemiss DN, Neill J. Pharmacological analysis of the behavioural and thermoregulatory effects of the putative 5-HT1 receptor agonist, RU24969, in the rat. Neuropharmacology. 1986;25:877–886. doi: 10.1016/0028-3908(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1993;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of Amphetamine and morphine. Brain Research. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Hayward MD, Le HN, Hartigan DJ, Duman RS, Nestler EJ. Induction of immediate early genes by cyclic AMP in primary cultures of neurons from rat cerebral cortex. Brain Res Mol. Brain Res. 1993;19:76–82. doi: 10.1016/0169-328x(93)90151-e. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ, Schoffelmeer AN. A single exposure to amphetamine is sufficient to induce long-term behavioral neuroendocrine, and neurochemical sensitization in rats. J Neurosci. 1999;19:9579–9586. doi: 10.1523/JNEUROSCI.19-21-09579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Mulder AH, DeVries TJ. Dopaminergic mechanisms mediating the long-term expression of locomotor sensitization following pre-exposure to morphine or amphetamine. Psychopharmacology (Berl) 1999;143:244–253. doi: 10.1007/s002130050943. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding S, Hitzemann R, Pappas N. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:L7–L12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Miller NS, Blizinsky K, Laughlin ML, Palmiter D. Mice with chronic norepinehrine deficiency resemble amphetamine sensitized animals. Proc Natl Acad Sci USA. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Progress in neurobiology. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Nassar R, Brooderson RJ, Khansa MR. Differential development of autoreceptor subsensitivity and enhanced dopamine release during amphetamine sensitization. J Pharmacol Exp Ther. 1993;264:249–255. [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic pretreatment with methylphenidate induces cross-sensitization with amphetamine. Life Sci. 2003;73:2899–2911. doi: 10.1016/s0024-3205(03)00673-8. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology. 2006;51:546–556. doi: 10.1016/j.neuropharm.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Methylphenidate treated at the test-cage – dose dependent sensitization or tolerance depend on the behavioral assay used. Crit Rev Neurobiol. 2007;19:59–77. doi: 10.1615/critrevneurobiol.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Psychostimulants given in adolescence modulate their effects in adulthood using the open field and the wheel-running assays. Brain Res Bull. 2010;82:208–217. doi: 10.1016/j.brainresbull.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]