Introduction
Hebbian and homeostatic forms of synaptic plasticity require new gene expression for their persistence (Kandel, 2001; Turrigiano, 2008). For stimulus-induced alterations in transcription to occur, signals must be relayed from synapses to the nucleus (Ch'ng and Martin, 2011; Cohen and Greenberg, 2008). While electrochemical processes permit extremely rapid signaling between subcellular compartments in neurons, soluble signals can also be transported from synapse to nucleus to trigger changes in transcription (Ch'ng and Martin, 2011; Jordan and Kreutz, 2009; Thompson et al., 2004). Inducible transport of transcriptional regulators from synapse to nucleus is a particularly direct way of informing the nucleus about synaptic activity.
The transcription factor CREB plays a central role in many forms of neuronal plasticity (Benito and Barco, 2010; Lonze and Ginty, 2002). Stimuli that induce long-term plasticity activate CREB-mediated transcription by triggering phosphorylation of CREB at serine 133, leading to recruitment of CREB Binding Protein (CBP) and transcriptional activation (Shaywitz and Greenberg, 1999). Montminy and colleagues (Conkright et al., 2003) and Lubow and colleagues (Iourgenko et al., 2003) identified an additional regulator of CREB-mediated transcription in pancreatic beta-islet cells, the CREB-Regulated Transcriptional Co-activator, CRTC (also known as Transducer Of Regulated CREB activity, TORC), whose activity is regulated by nucleocytoplasmic transport. In unstimulated cells, CRTC is phosphorylated (by Salt Inducible Kinase, SIK) and binds to 14-3-3 proteins in the cytoplasm. Calcineurin-dependent dephosphorylation of CRTC triggers its dissociation from 14-3-3 and subsequent translocation into the nucleus. In the nucleus, CRTC binds the bZIP domain of CREB (and other bZIP transcription factors) and, in a manner that is independent of CREB phosphorylation, potently drives downstream gene expression by recruiting TAFII130 and basal transcriptional machinery (Ravnskjaer et al., 2007; Screaton et al., 2004). CRTC nuclear translocation has been found to require coincident calcium and cAMP signaling (Screaton et al., 2004).
Expression of dominant negative forms of CRTC1 in CA1 neurons was reported to block the transcription-dependent late phase of long-term potentiation (LTP) but not the early, transcription-independent phase (Kovacs et al., 2007; Zhou et al., 2006). Conversely, overexpression of CRTC1 in CA1 neurons was found to lower the threshold for induction of late-phase LTP (Zhou et al., 2006). Together, these findings support a critical role for CRTC1 during the transcription-dependent phase of neuronal plasticity.
We followed up on these studies by determining the mechanisms whereby specific types of synaptic stimulation trigger CRTC1 nuclear import from distal synapses to the nucleus and by characterizing the function of this nuclear translocation. We show that CRTC1 localizes to dendrites and spines in electrically silenced rodent hippocampal neurons, and translocates to the nucleus in a calcium- and calcineurin-dependent manner following glutamatergic synaptic transmission. CRTC1 is specifically transported from stimulated synapses, and activity-dependent nuclear translocation occurs only in excitatory neurons. Synaptic stimulation triggers complex changes in CRTC1 phosphorylation, suggesting that the phosphorylation state of CRTC1 may integrate specific types of activity to trigger specific programs of CRE-dependent gene expression. We show that in neurons, elevations in intracellular cAMP are not required for CRTC1 nuclear import, but rather regulate the persistence of CRTC1 nuclear accumulation. siRNA knockdown of CRTC1 reveals that CRTC1 is required for the stimulus-induced regulation of specific CREB target genes in a manner that is independent of CREB (S133) phosphorylation. Together, our data demonstrate that synaptically-driven calcium influx triggers nuclear translocation of CRTC1 while elevations in cAMP regulate the persistence of nuclear CRTC1. In this way, CRTC1 dynamically informs the nucleus about synaptic activity to mediate transcription-dependent forms of plasticity.
Results
CRTC1 localizes to spines and dendrites
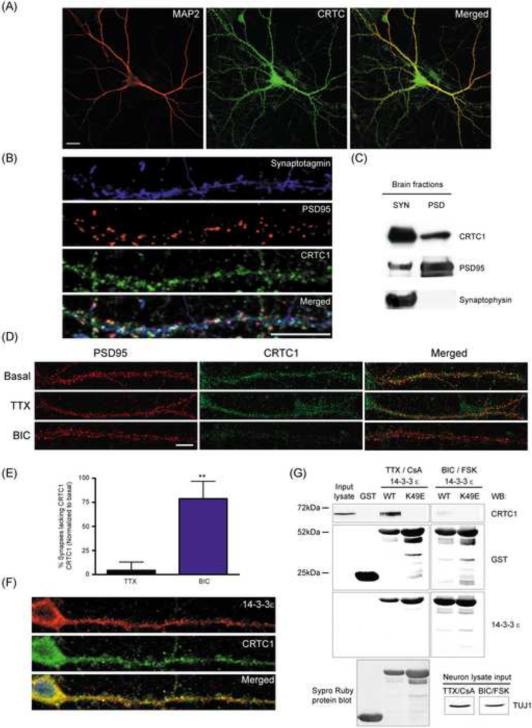
CRTC1 is expressed at high concentrations in the brain (Altarejos et al., 2008; Watts et al., 2011). Using antibodies that specifically recognize CRTC (Fig 1A and S1C) and CRTC1 (Fig 1B-D, S1A-B) to stain cultured rat hippocampal neurons (21 DIV), we detected immunoreactivity in the soma, dendrites and spines (Fig 1A and S1C). Triple labeling with MAP2 (dendrites), synaptotagmin (presynaptic) and PSD95 (excitatory postsynaptic) antibodies revealed localization throughout dendrites and at synapses (Fig 1B). Colocalization analysis revealed that ~99% of PSD95-positive puncta contained CRTC1; this association was further confirmed by a positive Pearson's correlation coefficient (r) (Fig S1D). To further examine the synaptic localization of CRTC1, we fractionated adult rat brain into synaptoneurosome and post-synaptic density (PSD) fractions and found that CRTC1 was present in both (Fig 1C).
Fig 1. Localization of CRTC1 to dendrites and synapses by activity-dependent tethering to 14-3-3.
(A) Rat hippocampal neuron cultures (DIV 14-21) were immunostained with antibodies against panCRTC (green) and MAP2 (red). (B) As in (A) but immunostaining with antibodies specific for CRTC1 (green), synaptotagmin (blue) and PSD95 (red). (C) Mouse brains (5 wks) were fractionated into synaptosomes (SYN) and post-synaptic densities (PSD), and immunoblotted for CRTC1, synaptophysin and PSD95. (D) Cultured hippocampal cultures were treated with TTX (1 μM) or bicuculline (BIC; 40 μM) for 1 h, fixed and stained with CRTC1 and PSD95 antibodies. (E) The percent of PSD95-positive synapses lacking CRTC1 immunoreactivity (** p<0.01 relative to basal control). (F) Hippocampal neurons were immunostained with antibodies against CRTC1 (green) and 14-3-3 ε (red). All scale bars, 10 μm. (G) GST-14-3-3 ε (WT) and binding mutant (K49E) was incubated with protein lysates from hippocampal cultures pretreated with TTX (1 μM) and cyclosporin A (CsA; 5 μM) for 2 h or with bicuculline (40 μM) and forskolin (25 μM) for 15 min. GST pulldowns were immunoblotted with CRTC1, GST, TUJ1 and 14-3-3 ε antibodies, and blots were stained with Sypro Ruby to verify protein concentration and purity.
CRTC1 undergoes activity-dependent loss from spines and dendrites
To determine whether the dendritic and synaptic localization of CRTC1 was regulated by synaptic activity, we incubated cultures (21 DIV) with the sodium channel blocker tetrodotoxin (TTX, 1 μM, 4 h), which blocks action potentials and thereby silences neuronal cultures, or with the GABAA receptor antagonist bicuculline (40 μM, 1 h), which, by blocking inhibition, drives excitatory synaptic transmission in cultures. As shown in Fig 1D, while TTX did not significantly affect the spine localization of CRTC1, incubation with bicuculline significantly reduced CRTC1 immunoreactivity in spines. Double label immunocytochemistry with antibodies against PSD95 and CRTC1 revealed a 75% increase in the number of PSD95-positive puncta that lack CRTC1 following bicuculline stimulation (Fig 1E).
Since phosphorylated CRTC1 is tethered in the cytoplasm through interactions with 14-3-3 proteins in pancreatic beta islet cells (Screaton et al., 2004), we asked whether dendritic CRTC1 colocalized with a particular 14-3-3 isoform in neurons. Immunocytochemistry with antibodies recognizing 14-3-3 β, γ, ε, σ, η, τ and ζ isoforms revealed that the ε isoform was present in dendritic spines (Fig S1E). Double label experiments revealed striking colocalization between CRTC1 and 14-3-3 ε in dendrites and spines (Fig 1F). Moreover, pull-down experiments with GST-14-3-3 ε revealed an activity-regulated interaction with CRTC1: binding was detected in electrically silenced neurons but dramatically reduced following bicuculline stimulation. Mutation at the binding pocket of 14-3-3 ε (K49E) completely abolished its interaction with CRTC1 (Fig 1G). These findings suggest that CRTC1 undergoes activity-regulated tethering at synapses by binding to 14-3-3 ε.
CRTC1 undergoes activity-dependent nuclear accumulation in excitatory neurons
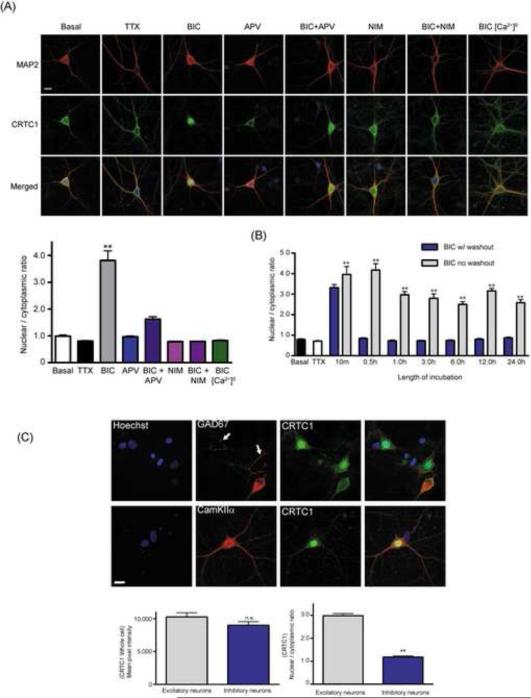
Confocal imaging of the cell body in basal, TTX and bicuculline stimulated cultured neurons revealed that CRTC1 was excluded from the nucleus under basal and TTX conditions, but accumulated in the nucleus following incubation with bicuculline (Fig 2A). Blocking excitatory synaptic transmission with the AMPA receptor antagonist NBQX completely blocked CRTC1 nuclear translocation, while preincubation with the NMDA receptor antagonist APV significantly inhibited nuclear accumulation, indicating that activation of both AMPA and NMDA receptors contributes to CRTC1 synapse to nuclear transport (Fig S2B). Removal of calcium from the extracellular media or inhibition of L-type voltage-gated calcium channels (LVGCC) with nimodipine completely blocked nuclear accumulation of CRTC1, consistent with a requirement for influx of extracellular calcium through LVGCCs (Fig 2A). The ability of bicuculline to drive nuclear accumulation correlated with the synaptic connectivity of the neurons; accumulation was observed after 14 DIV, but not at 7 DIV, when neuronal cultures have fewer synaptic connections (Fig S2A).
Fig 2. Activity-dependent nuclear translocation of CRTC1 in excitatory hippocampal neurons.
(A) Bicuculline (BIC; 40 μM, 1 h) was added to untreated (basal) hippocampal cultures or to hippocampal cultures pretreated with APV (100 μM), nimodipine (NIM; 10 μM) or to cultures in a calcium-free Tyrode's solution. After staining with CRTC1 (green) and MAP2 (red) antibodies and with Hoechst nuclear dye (blue, merged), the nuclear to cytoplasmic ratio of CRTC1 was quantified (** p<0.001 relative to basal). (B) Hippocampal cultures were incubated with bicuculline (BIC; 40 μM) for 10 min before recovery (0.5 h-24 h) in the continued presence or absence of bicuculline. Neurons were fixed and immunostained with CRTC1 and MAP2 antibodies and with Hoechst nuclear dye. The nuclear to cytoplasmic ratio of CRTC1 was quantified (**p<0.001 compared to TTX). (C) Hippocampal cultures were incubated with bicuculline (40 μM) for 1 h, fixed and double-labeled with CRTC1 (green) and GAD67 (red) or CRTC1 (green) and CamKIIα (red) antibodies. White arrows indicate pre-synaptic GAD67 positive puncta in contact with the soma of an excitatory neuron. The total concentrations of somatic CRTC1, and the nuclear to cytoplasmic ratio of CRTC1, were quantified in excitatory and inhibitory neurons (** p<0.001 relative to excitatory neurons; n.s. not significant). All scale bars, 10 μm.
To monitor the persistence of stimulus-induced CRTC1 nuclear translocation, we incubated neurons with bicuculline for 10 min followed by a quick washout and subsequent recovery for 24 h either with or without bicuculline. Twenty min after bicuculline removal, the concentration of CRTC1 in the nucleus returned to basal levels (Fig 2B). CRTC1 remained in the nucleus as long as bicuculline was present, even after 24 h of continuous stimulation (Fig 2B). These results indicate that nuclear CRTC1 dynamically tracks ongoing synaptic glutamatergic activity.
We observed that in 15-20% of our hippocampal cultures, CRTC1 did not translocate to the nucleus following bicuculline stimulation. As shown in Fig 2C, double label experiments with GAD67 to label inhibitory neurons and CamKIIα to label excitatory neurons revealed that although CRTC1 was present in both cell types, bicuculline-induced nuclear translocation occurred exclusively in excitatory neurons.
We next asked whether nuclear CRTC1 resulted from nucleocytoplasmic transport or from new synthesis of CRTC1. Incubation of neurons with the protein synthesis inhibitor emetine prior to and during bicuculline stimulation did not prevent CRTC1 nuclear translocation (Fig S2C), and no change in the total concentration of CRTC1 was observed following TTX or bicuculline stimulation (Fig S2D). Thus, bicuculline induces nuclear import of pre-existing CRTC1.
CRTC1 translocate to the nucleus of hippocampal neurons following induction of LTP in organotypic slice cultures
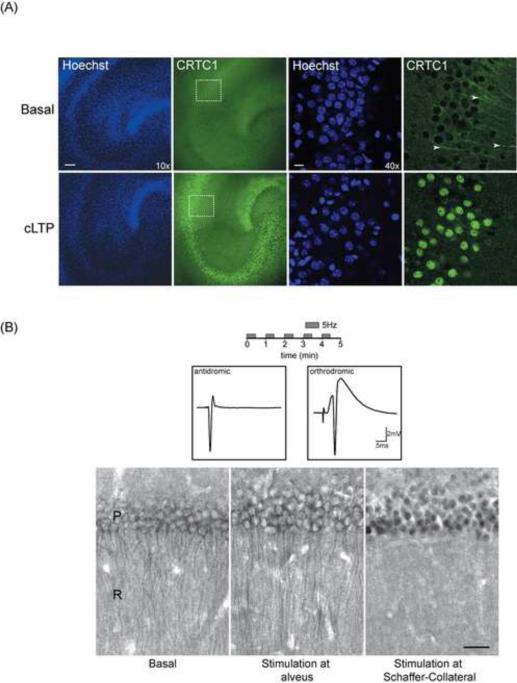
Following up on earlier studies showing nuclear accumulation of CRTC1 in acute hippocampal slices after induction of L-LTP (Zhou et al., 2006), we asked whether we could detect loss of CRTC1 from dendrites and synapses and accumulation in the nucleus following LTP induction in organotypic hippocampal slice cultures (16-18 DIV). To induce chemical LTP, we incubated slice cultures for 60 min in Mg2+-free artificial cerebrospinal fluid (ACSF) supplemented with rolipram, forskolin and picrotoxin (Kopec et al, 2006). As shown in Fig 3A, in unstimulated slice cultures, CRTC1 was present in stratum radiatum dendrites, but was excluded from the nucleus. Following cLTP stimulation, robust nuclear CRTC1 immunoreactivity was detected in all three cell body layers (dentate, CA3 and CA1). Nuclear accumulation was accompanied by a loss of CRTC1 immunoreactivity in MAP2-positive dendrites in the stratum radiatum (Fig 3A; white arrowheads). To complement the studies in organotypic slice cultures, we briefly depolarized neurons with KCl in acute hippocampal slices, which not only triggered nuclear translocation, but also resulted in a loss of immunoreactivity in the stratum radiatum (Fig S3A).
Fig 3. Synapse to nucleus translocation of CRTC1 in organotypic slice cultures and acute hippocampal slice preparations.
(A) Chemical LTP (cLTP) was induced in organotypic hippocampal slice cultures, which were then fixed and immunolabeled with antibodies for CRTC1 (green) and Hoechst nuclear dye (blue). Representative confocal section at 10X magnification (scale bar, 10 μm) and 40X magnification (scale bar, 100 μm) of the CA1 cell body layer. Arrowheads indicate presence of CRTC1 in dendrites. Dashed box indicates CA1 cell body layer shown in high magnification in right panels. (B) Theta pulse stimulation (TPS; 5 trains of 5 Hz stimulation; 30 s duration with 30 s inter-train interval) was delivered to Schaeffer Collateral fibers in the stratum radiatum (orthrodromic stimulation) or directly to the alveus to stimulate the axons of the CA1 pyramidal neurons (antidromic stimulation). Traces show examples of evoked antidromic and postsynaptic responses. After stimulation, slices were collected and immunostained with antibodies specific for CRTC1 in CA1 region of acute hippocampal slices. Scale bar, 30 μm. Panels show an unstimulated control slice (left) and slices where TPS was delivered to the alveus (middle) or to the Schaffer collateral fibers in stratum radiatum (right). P: stratum pyramidale; R: stratum radiatum
CRTC1 nuclear translocation in acute hippocampal slices requires synaptic activity
Zhou et al. (2006) showed that CRTC1 underwent translocation into CA1 pyramidal nuclei in acute hippocampal slices following 4x100 Hz tetanic stimulation, which induces transcription-dependent L-LTP, but not following a single 100 Hz tetanic stimulus, which induces transcription-independent E-LTP. However, these experiments were performed in the presence of bicuculline, which we found was sufficient on its own to drive CRTC1 nuclear import in hippocampal slices (data not shown). To more specifically test the requirement for synaptic activity to drive CRTC1 nuclear translocation in acute hippocampal slices, we stimulated Schaffer Collateral fiber synapses onto CA1 pyramidal cells using multiple trains of theta frequency (5Hz) stimulation. As shown in Fig S3B, this stimulation paradigm triggered nuclear translocation in CA1 neurons but not in CA3 neurons. Because Schaffer Collateral fiber stimulation not only activates synapses onto CA1 pyramidal cells but also triggers antidromic action potentials in CA3 pyramidal cells, this finding suggested that synaptic activation is specifically required for CRTC1 nuclear translocation. To more rigorously test this possibility, we delivered the same pattern of theta frequency stimulation to the alveus to selectively trigger antidromic action potentials in CA1 pyramidal cells in slices in which excitatory synaptic transmission was blocked with the broad-spectrum ionotropic glutamate receptor antagonist kynurenate (3 mM). As shown in Fig 3B, postsynaptic action potentials in the absence of excitatory synaptic transmission failed to induce nuclear translocation of CRTC1. These results indicate that synaptic activity is required to trigger CRTC1 nuclear import, and that neuronal depolarization is not sufficient.
CRTC1 translocates specifically from stimulated synapses to the nucleus
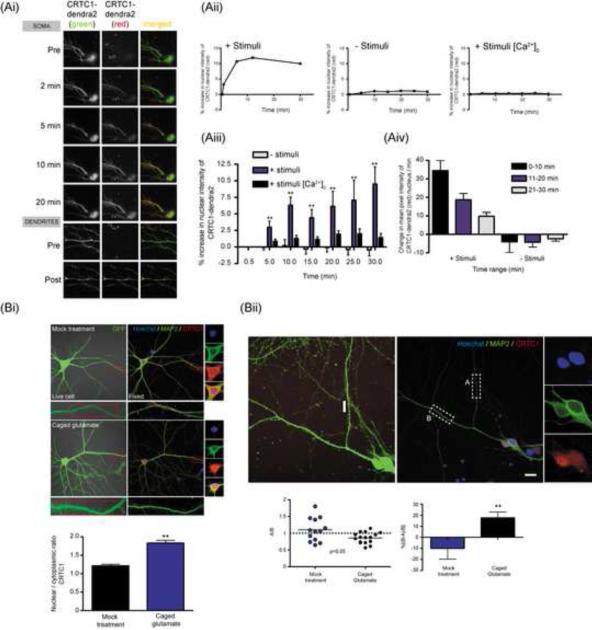
To specifically monitor the transport of CRTC1 from stimulated subsets of synapses to nucleus, we performed two sets of experiments. In the first, we overexpressed CRTC1 fused to the photoconvertible fluorescent protein dendra2 in cultured hippocampal neurons (Fig 4A). In unstimulated neurons, only low levels of CRTC1-dendra2 were detected in the nucleus. A brief UV illumination of distal dendrites (~100-200 μm from soma) converted the dendra2 signal from green to red. Using time-lapse imaging, we followed the accumulation of both the native green and photoconverted signals in the cell body over a period of 30 min post-conversion. Our results revealed that the photoconverted (red) dendritic CRTC1 underwent stimulus-induced translocation into the nucleus (Fig 4Ai-iii). We also observed that the rate of nuclear accumulation of CRTC1-dendra2 is fastest during the first 10 min after stimulation, consistent with stimulus-induced active retrograde transport (Fig 4Aiv).
Fig 4. Synapse to nucleus translocation of CRTC1 in hippocampal neurons.
(A-i) Hippocampal neurons were transfected with CRTC1-dendra2 for 14-18 h before incubation in Tyrode’s solution in the presence or absence of calcium. After taking baseline images of CRTC1-dendra2 expression, cultures were incubated with stimuli (Leptomycin B, 10 nM; bicuculline 40 μM; forskolin 25 μM) or were unstimulated (Leptomycin B; 10 nM) and specific dendritic branches expressing the dendra2 construct were photoconverted from green to red with a UV pulse laser. Nuclear red dendra2 signal was imaged every 5 min for 30 min. (A-ii) The percent increase of photoconverted dendra2 signal in the nucleus was quantified as compared to baseline values. (A-iii) Group data (** p<0.001 relative to no stimulation). (A-iv) The rate at which photoconverted red dendra2 fusion protein entered the nucleus was quantified and plotted in 10 min intervals. (B-i) Hippocampal neurons were transduced with lentivirus expressing GFP. Glutamate was uncaged at distal dendrites of GFP-expressing neurons, followed by fixation and staining for MAP2 (green), CRTC1 (red) and Hoechst nuclear dye (blue). The red box indicates region of local uncaging. Neurons were identified after immunocytochemistry and the nuclear to cytoplasmic ratio of CRTC1 was quantified. (B-ii) Hippocampal neurons were treated as in (B-i). The amount of CRTC1 in dendrites adjacent to the region of photo-uncaging of glutamate was quantified (region A). As a control, a randomly selected branch of dendrite adjacent to the area of activation was also selected (region B), and the amount of CRTC1 was quantified. The ratio of region A to region B was determined and plotted as a scatter plot (** p<0.05, paired student t-test). A ratio of 1 indicates equal amounts of CRTC1 in the glutamate uncaged and adjacent control dendrite. Group data of the percent increase of CRTC1 in control relative to the uncaged dendrite for mock and glutamate-uncaged neurons (**p<0.05, paired student t-test). All scale bars, 10 μm.
We next asked whether endogenous CRTC1 underwent synapse to nucleus translocation following local stimulation. To do this, we cultured neurons on gridded coverslips and transduced the neurons with a lentivirus expressing eGFP to visualize the entire dendritic arbor of individual neurons. We locally UV-uncaged MNI glutamate (or vehicle, in controls) at distal dendrites of GFP-expressing neurons, and 10-30 min later fixed and immunolabeled with anti-CRTC1 and MAP2 antibodies. As shown in Fig S4, a brief UV pulse at a distal site uncaged sufficient glutamate to trigger a robust dendritic calcium signal and, as shown in Fig 4B-i, also significantly increased the concentration of CRTC1 in the nucleus as compared to controls. This data indicates that stimulation of distal synapses is sufficient to trigger nuclear accumulation of CRTC1.
Examination of CRTC1 immunoreactivity in dendritic segments of neurons following uncaging revealed that glutamate uncaging resulted in a loss of CRTC1 in local dendritic segments compared to dendrites receiving mock uncaging. Further, loss of dendritic CRTC1 immunoreactivity was branch-specific; local uncaging at one branch triggered a loss of CRTC1 from that branch without changing CRTC1 immunoreactivity in other dendritic branches from the same neuron (Fig 4B-ii). The finding that CRTC1 loss was specific to the site of stimulation even though uncaging produced a much broader depolarization (Fig S4) also demonstrates a requirement for synaptic stimulation, as opposed to depolarization, in CRTC1 nuclear import.
Calcineurin is required for CRTC1 nuclear translocation
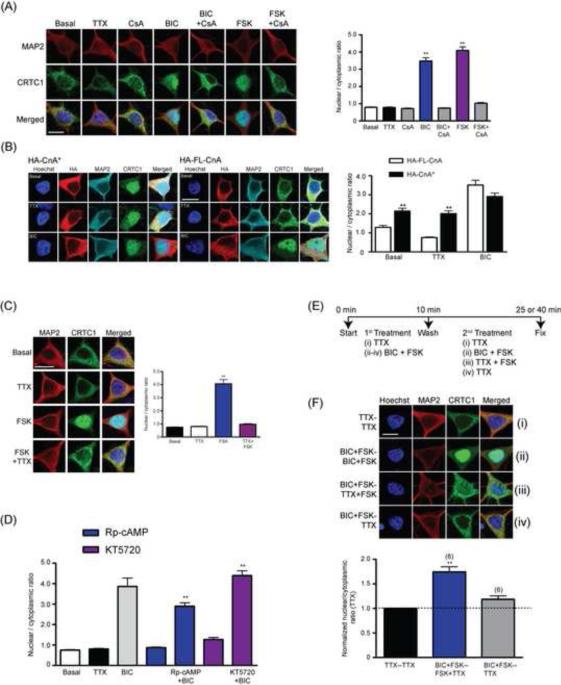
In pancreatic beta-islet cells, CRTC2 dephosphorylation by calcineurin has been shown to trigger its release from 14-3-3 and subsequent CRTC2 translocation into the nucleus (Conkright et al., 2003). We thus asked whether calcineurin was required for bicuculline-induced synapse to nuclear translocation in neurons. As shown in Fig 5A, preincubation of cultured hippocampal neurons with the calcineurin antagonist cyclosporine A (CsA; 30 min; 5 μM) completely blocked nuclear translocation of CRTC1 induced by bicuculline. Moreover, neuronal transfection of a constitutively active calcineurin (HA-CnA*) was sufficient to drive nuclear import of CRTC1 even when the neurons were silenced with TTX. In contrast, overexpression of full-length calcineurin (HA-FL-CnA) was unable to initiate nuclear import of CRTC1 in the absence of neuronal activity (Fig 5B).
Fig 5. Nuclear translocation of CRTC1 requires activation of calcineurin; cAMP regulates the persistence of CRTC1 in the nucleus.
(A) Hippocampal cultures were pre-treated with cyclosporin A (CsA; 5 μM) for 4 h prior to a 1 h stimulation with bicuculline (BIC; 40 μM) or with forskolin (FSK; 25 μM). Neurons were fixed and immunostained with antibodies against MAP2 (red); CRTC1 (green) and Hoechst nuclear dye (blue, merged), and the mean nuclear to cytoplasmic ratio was quantified (** p<0.001 relative to non-stimulated but CsA treated sample) (B) Full length (HA-FLCnA) or constitutively activated calcineurin (HA-CnA*) fused to an HA epitope tag was transiently transfected into hippocampal cultures. After 24 h, transfected cultures were pre-incubated with either TTX (1 μM) for 1 h or with bicuculline (BIC; 40 μM) for 10 min, fixed and immunostained with antibodies against CRTC1 (green), HA (red), MAP2 (Cyan) and the Hoechst nuclear dye (blue, merged). The nuclear to cytoplasmic ratio of CRTC1 was quantified (** p<0.001 when compared to HA-FL-CnA). (C) Hippocampal neurons were stimulated for 10 min with forskolin (FSK; 25 μM) in the presence or absence of TTX (1 μM, 1 hour pretreatment). The nuclear to cytoplasmic ratio of CRTC1 was quantified (** p<0.001 relative to basal level). (D) Hippocampal neurons were pre-treated with Rp-cAMP (0.5 mM), KT5720 (2 μM) for 30 min prior to stimulation with bicuculline (BIC; 40 μM, 10 min). The nuclear to cytoplasmic ratio of CRTC1 was quantified (** p<0.001 relative to no BIC-treated controls). (E) Neurons were stimulated with either BIC + FSK or TTX for 10 min, washed and incubated with media containing TTX alone, BIC + FSK or TTX + FSK for another 15 or 30 min (pooled data). A flow chart and time course of the treatment is included for all four stimulation paradigms (iiv). (F) After fixation and immunostaining, the nuclear to cytoplasmic ratio of CRTC1 was quantified. For all experiments, neurons were immunostained with CRTC1 (green), MAP2 (red) and Hoechst nuclear dye (blue). The normalized nuclear to cytoplasmic ratio of CRTC1 relative to TTX treated samples was plotted on a bar graph for all treatments. The number on top of each bar graph represents the number of independent experiments conducted. (**p<0.01 relative to BIC+FSK–TTX treated sample). All scale bars, 10 μm.
Elevations in intracellular cAMP increase the persistence of nuclear CRTC1
Nuclear translocation of CRTC2 has been reported to require coincident elevations in calcium and cAMP in non-neuronal cells (Screaton et al., 2004), triggering coincident calcineurin activation and SIK inactivation. To study the role of cAMP during CRTC1 translocation in neurons, we briefly incubated dissociated cultures with forskolin (25 μM, 10 min) to activate adenylyl cyclase. Since forskolin also increases excitability of cultured hippocampal neurons (Hoffman and Johnston, 1998), we performed these experiments in the presence or absence of TTX. As shown in Fig 5C, while forskolin induces nuclear accumulation of CRTC1, it does so only in the presence of neuronal activity; TTX-silenced neurons did not undergo forskolin-induced CRTC1 nuclear translocation (Fig 5C). Our studies also indicate that forskolin-induced CRTC1 nuclear translocation requires calcineurin (Fig 5A), extracellular calcium (Fig S5A) and LVGCC (Fig S5B). We next asked whether the cAMP-PKA pathway was required for CRTC1 nuclear translocation during bicuculline-induced synaptic activation by incubating neurons in pharmacological agents that block adenylyl cyclase activity (SQ22536; 20 μM), antagonize PKA (KT5720; 2 μM) or competitively inhibit cAMP (Rp-cAMP; 0.5 mM). As shown in Figs 5D and S5C, none of these agents blocked the nuclear accumulation of CRTC1 in neurons induced by bicuculline, indicating that increases in cAMP are not required for CRTC1 nuclear import.
cAMP blocks the rephosphorylation of CRTC1 by inhibiting AMPK or SIK (Conkright et al., 2003; Mair et al., 2011; Screaton et al., 2004). This suggested to us that while increases in cAMP might not be required for the initial import of CRTC1 from synapse to nucleus, they might increase the persistence of nuclear CRTC1. We tested this idea by stimulating neurons briefly with bicuculline and forskolin for 10 min, followed by a quick washout and incubation in TTX and forskolin for another 15 to 30 min (Fig 5E). As shown in Fig 5F, addition of forskolin (in the presence of TTX) following the initial bicuculline stimulation prolongs the nuclear accumulation of CRTC1, presumably by preventing the rephosphorylation CRTC1. To examine the specific role of AMPK or SIK in this experiment, we stimulated neurons with bicuculline, and then allowed the neurons to recover in the presence of dorsomorphin dihydrochloride (DM; 20 μM), an inhibitor of both AMPK and SIK kinase activity (Sasaki et al., 2011). DM prolonged CRTC1 presence in the nucleus (Fig S5D), consistent with AMPK or SIK rephosphorylating CRTC1 and promoting rapid nuclear export.
CRTC1 undergoes differential patterns of regulated phosphorylation and dephosphorylation
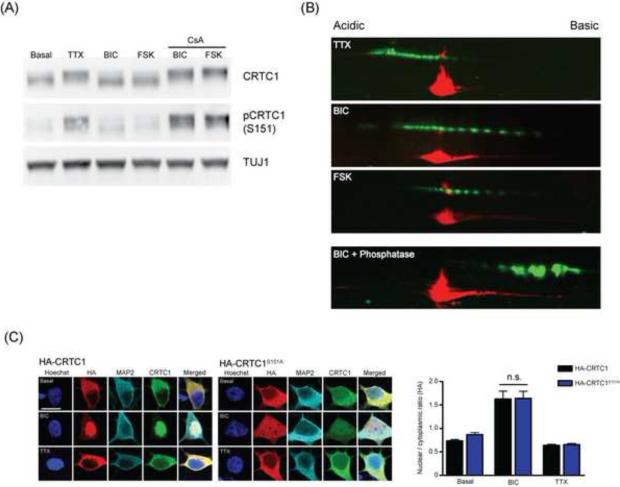
To gain further insight into the mechanisms whereby stimulation triggers CRTC1 translocation from synapse to nucleus, we performed immunoblots of cultured neurons silenced with TTX (1 μM; 1 h) or stimulated with either bicuculline (40 μM; 10 min) or forskolin (25 μM; 10 min). As shown in Fig 6A, these experiments revealed large, activity-dependent shifts in the molecular weight (MW) of the protein. When neuronal cultures were silenced with TTX, CRTC1 was ~10-15 kDa larger in MW than it was in neuronal cultures that were stimulated with either bicuculline or forskolin.
Fig 6. Activity triggers complex changes in CRTC1 phosphorylation.
(A) Cultured hippocampal neurons were incubated with TTX (1 μM), bicuculline (BIC; 40 μM), forskolin (FSK; 25 μM) in the presence or absence of cyclosporin A (CsA; 5 μM). After 10 min, neuronal cultures were lysed, separated by SDS-PAGE and immunoblotted with antibodies against TUJ1, CRTC1 or phosphorylated CRTC1-S151 (pCRTC1). (B) Cultured hippocampal neurons were stimulated as described in (A) and lysates were subjected to two-dimensional gel electrophoresis and immunoblotted with antibodies against CRTC1 (green) and TUJ1 (red). (C) Neurons were transiently transfected with either full length HA-tagged CRTC1 (HA-CRTC1) or CRTC1 bearing a point mutation converting serine 151 to alanine (HA-CRTC1S151A). After 12 h, transfected neurons were pre-incubated with either TTX (1 μM) for 1 h or with bicuculline (BIC; 40 μM) for 10 min before fixation and immunostaining with antibodies to CRTC1 (green), HA (red), MAP2 (Cyan) and Hoechst nuclear dye (blue); The nuclear to cytoplasmic ratio of HA immunostaining was quantified (n.s. not significant); scale bar, 10 μm.
The coding region of mouse CRTC1 contains 146 serine, threonine and tyrosine residues (approximately 1 in 4.3 residues). Based on this and previous work on CRTC2 (Screaton et al., 2004), we reasoned that the MW shift might be due to phosphorylation of CRTC1. Incubation of lysates with calf intestinal phosphatase shifted CRTC1 to a much lower MW, suggesting that the shifts in MW resulted primarily from regulated phosphorylation and dephosphorylation (Fig S6B). We further used a CRTC1 antibody that specifically recognizes the phosphorylated serine residue at S151 (Fig S6A). This antibody primarily detected only bands that were higher in MW, which likely correspond to phosphorylated CRTC1 (Fig 6A and S6A). When lysates were incubated with the calcineurin inhibitor cyclosporine A, neither bicuculline nor forskolin induced a decrease in MW, or a dephosphorylation of serine 151, consistent with calcineurin-dependent dephosphorylation of CRTC1 in response to stimulation (Fig 6A). To complement our studies in cell culture, we also analyzed the hippocampal acute slice after theta pulse stimulation of the Schaeffer collateral (stimulation as described in fig 3B) via Western blots and observed a significant reduction in the levels of CRTC1 that was phosphorylated at serine 151 (Fig S6C).
We next examined the activity-dependent phosphorylation status of CRTC1 using two-dimensional gel electrophoresis. As shown in Fig 6B, in TTX-silenced cultures, CRTC1 ran as a series of discrete spots (green) clustered towards the acidic pH3 isoelectric point, and running at approximately 75 kDa. As a reference, we co-stained the two-dimensional gels with antibodies that detect the neuron-specific class III beta tubulin (TUJ1; 55 kDa; pI 4.88, red). Ten minutes of stimulation with bicuculline triggered a dramatic shift in CRTC1 immunoreactivity towards the more basic, pH 11 isoelectric point, and a decrease in MW. Ten minutes of stimulation with forskolin also triggered a shift towards more basic and lower molecular weight spots, although the extent of dephosphorylation was not as great as with bicuculline stimulation. When lysates were incubated with λ phosphatase, CRTC1 immunoreactivity converged on a cluster of spots closer to pH 11 isoelectric point. The complete loss of phospho-MAP kinase immunoreactivity following incubation with λ phosphatase treatment demonstrates the efficacy of the dephosphorylation (Fig S6D). Together, these data indicate that CRTC1 undergoes a complex change in phosphorylation in response to stimuli. The finding that phosphatase treatment of lysates did not collapse CRTC1 to a single spot indicates that, while dephosphorylation accounts for the majority of change in pI and MW following stimulation, CRTC1 likely undergoes additional post-translational modifications (Liu et al., 2008, Jeong et al., 2011).
Phosphorylation of S151 by SIK has been reported to be necessary for 14-3-3 binding and cytoplasmic anchoring in non-neuronal cells (Screaton et al., 2004). As described above, we found that stimulation of hippocampal neurons with bicuculline or forskolin triggered calcineurin-dependent S151 dephosphorylation (Fig 6A). To test whether S151 dephosphorylation was sufficient to drive nuclear translocation, we generated a HA-tagged CRTC1 mutant in which S151 was changed to an alanine (CRTC1S151A), and thus could not be phosphorylated. This mutant localizes constitutively to the nucleus in mouse hypothalamic GT1-7 cells (Altarejos et al., 2008). However, when expressed in primary cultured hippocampal neurons, the CRTC1S151A mutant was excluded from the nucleus in basal or TTX-silenced neurons, but underwent bicuculline-induced translocation into the nucleus (Fig 6D). These results indicate that elevations in intracellular calcium and cAMP trigger dephosphorylation of S151 in CRTC1, but, consistent with the 2D gel analysis, which reveals that multiple residues undergo regulated dephosphorylation, S151 dephosphorylation on its own is not sufficient for nuclear import in neurons.
CRTC1 is required for induction of specific CREB gene targets
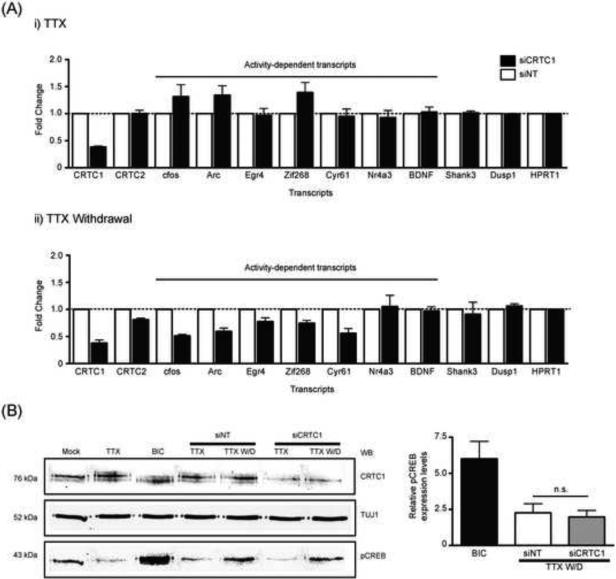
To address the function of activity-dependent CRTC1 nuclear translocation, we reduced CRTC1 expression in hippocampal neurons with CRTC1 siRNAs (Fig S7A). We then used an established protocol to study induction of CREB-induced gene expression in cultured hippocampal neurons, in which cultures are silenced with TTX for 6 hours, and TTX is then withdrawn, leading to robust action potential firing and induction of several immediate-early genes (Saha et al. 2011). This protocol also triggered nuclear translocation of CRTC1 (Fig S7B). As shown in Fig 7A and Fig S7C, in cultures in which CRTC1 concentrations were reduced by ~60%, the induction of five CREB target genes, including cfos, arc, egr4, zif268 and cyr61 was significantly reduced compared to cultures receiving non-targeting siRNAs. In contrast, the induction of other CREB target genes, including nr4a3, BDNF, shank3 and dusp1, was not affected by the reduction in CRTC1 concentrations at the time point examined (30 min after TTX withdrawal). We also asked whether silencing of CRTC1 had any effect on CREB phosphorylation. As shown in Fig 7B, TTX withdrawal induced equivalent CREB phosphorylation at serine133 in cultures treated with CRTC1 or nontargeting siRNAs. That CRTC1 knockdown inhibited the induction of cfos, arc, egr4, zif268 and cyr61 mRNAs without any effects on CREB phosphorylation indicates that CRTC1 nuclear translocation, rather than serine 133 phosphorylation, is critical to the stimulus-induced expression of these CREB target genes.
Fig 7. CRTC1 is required for activity-dependent induction of specific CREB target genes.
(A) Hippocampal neuron cultures (2-3 wk.) were incubated with Accell siRNA to CRTC1 (siCRTC1) or a non-targeted control (siNT). After 48 h of siRNA treatment, neurons were incubated with TTX (1 μM) for 6 h, TTX was withdrawn and the neurons were incubated in media lacking TTX for 30 min. As controls, half the siRNA treated neurons were continuously maintained in TTX for an additional 30 min. Quantitative PCR was carried out to examine the concentrations of activity-dependent transcripts. A bar graph showing the relative fold change of these transcripts between the siCRTC1-and siNT-treated neurons were plotted for both (i) TTX-treated and (ii) TTX-withdrawal conditions. (B) Mouse hippocampal neurons (2-3 wk.) were incubated with Accell siRNA and treated as described above in (A). A third set of cultures were stimulated with bicuculline (40 μM; 10 min). Neurons were lysed and analyzed by immunoblotting for CRTC1, TUJ1 and pCREB (S133). The relative concentration of pCREB was plotted (n.s. not significant).
Discussion
The results of our studies indicate that the transcriptional regulator CRTC1 undergoes activity-dependent trafficking from dendrites and synapses to the nucleus in hippocampal neurons. CRTC1 nuclear accumulation is tightly coupled to stimulation, with synaptic activity rapidly triggering translocation of CRTC1 from synapse to nucleus and with CRTC1 remaining localized in the nucleus as long as excitatory synaptic activity or cAMP levels remain elevated. These data indicate that nuclear accumulation of CRTC1 is a sensitive monitor of synaptic and neuromodulatory activity that dynamically informs the nucleus about activity received at synapses. Since the nuclear translocation does not require any transcription or translation, it is also a very rapid marker of activity.
The relationship between CREB and CRTC1 in establishing long-term memory
Studies in multiple systems have uncovered a central role for CREB-dependent transcription in the conversion of short-term to long-term plasticity and memory (Silva et al., 1998; Kauffman et al., 2010; but see also Balschun et al., 2003; Perazzona et al., 2004). Previous studies have focused primarily on activation of CREB by phosphorylation at serine 133 (pCREB133), and pCREB133 immunoreactivity is often used as a proxy for long-term plasticity and memory. Increasing evidence, however, indicates that CREB phosphorylation at serine 133 does not always correspond to transcriptional activation, raising the question of whether additional means of activating CRE-driven transcription operate during plasticity and memory (Bito et al., 1996; Impey at al, 1998; Kornhauser et al., 2002). Transcriptional activation mediated by CRTC nuclear import, which can dramatically increase CRE-driven gene expression in the absence of serine 133 phosphorylation, provides one such mechanism (Conkright et al., 2003; Iourgenko et al., 2003; Screaton et al., 2004).
How the phosphorylation state of CREB relates to CRTC1-induced transcriptional activation following stimulation in neurons remains unclear. One possibility is that distinct states of CREB phosphorylation, on serine 133 as well as other residues (Kornhauser et al., 2002), coupled with CRTC1 activation, may allow CREB to transcribe specific subsets of genes in response to distinct stimuli. Our data showing that CRTC1 undergoes an elaborate pattern of regulated phosphorylation and dephosphorylation at multiple residues (Fig 6) suggests a degree of complexity that could contribute significantly to diverse transcriptional responses. Thus, distinct stimuli may elicit distinct patterns of CRTC1 phosphorylation to allow recruitment of distinct bZIP transcription factors, thereby conferring selectivity of CREB-mediated gene expression to generate distinct programs of gene activation. It will be of great interest to map out the specific residues that undergo regulated changes in phosphorylation, and to then determine how phosphorylation/dephosphorylation of each site alters downstream gene expression.
Synapse-to-nuclear trafficking of CRTC1 in neurons
In addition to potentially contributing to the specificity of CREB-dependent transcriptional responses, activity-dependent synapse to nucleus translocation of CRTC1 may preserve spatial information about the initial site of stimulation. Thus, stimuli that lead to CREB phosphorylation do so by activating second messenger cascades that spread throughout the cell. In this mode of signaling, information about the spatial location of the originating stimulus is lost. However, stimuli that promote CRTC1 translocation from synapse to nucleus do so by triggering loss of CRTC1 specifically from stimulated synapses. Our experiments using local glutamate uncaging followed by CRTC1 immunocytochemistry (Fig 4B) indicate that the loss of CRTC1 is confined to the stimulated dendrite. Future experiments aimed at resolving the loss of CRTC1 from individual synapses may provide insight into the nature of the unit of stimulation that is required for long-term changes in synaptic efficacy.
cAMP regulates CRTC1 nuclear persistence
Our experiments indicate that elevations in intracellular cAMP, in the absence of neuronal activity, are not sufficient to trigger CRTC1 nuclear translocation in neurons (Fig 5C). Moreover, inhibiting cAMP function in cultured neurons did not block bicuculline-induced nuclear translocation of CRTC1 (Fig 5D and Fig S5C), strongly arguing that the cAMP-PKA pathway is not necessary for the initial translocation of CRTC1 to nucleus. Forskolin-induced CRTC1 nuclear translocation in dissociated cultures likely results from cAMP-induced increases in neuronal excitability (Hoffman and Johnston, 1998; Madison and Nicoll, 1986). Our findings stand in contrast to several other published reports that indicate that cAMP can induce translocation in a calcineurin-independent mechanism (Bittinger et al., 2004). We demonstrate that in neurons, cAMP regulates CRTC1 nuclear persistence, rather than CRTC1 nuclear import (Fig 5F). We propose that cAMP, by inactivating SIK and/or AMP kinases (Katoh et al., 2006; Katoh et al., 2004), prevents the rapid rephosphorylation of CRTC1, which in turn prolongs CRTC1 nuclear accumulation.
This observation has important implications about the function of neuromodulators such as dopamine and norepinephrine, both of which elevate intracellular cAMP, in long-term memory formation. Our findings suggest that synaptic stimuli activate calcineurin to trigger the nuclear translocation of synaptic CRTC1. CRTC1 remains in the nucleus as long as synaptic stimulation persists, but its nuclear persistence can be maintained in the absence of activity if cAMP levels are elevated. Relevant to this hypothesis, norepinephrine and dopamine concentrations are elevated in the hippocampus for up to 5 hours following strong tetanic stimulation (Neugebauer et al., 2009). From a learning perspective, this would imply that activation of modulatory neurotransmission following a stimulus increases the transcriptional changes induced by that stimulus. This idea is supported by a wealth of literature showing that emotional arousal, acting through neuromodulators like norepinephrine and dopamine, enhance long-term memory formation (McGaugh, 2006; Rossato et al., 2009; Navakkode et al., 2007; O'Dell et al., 2010). Nuclear translocation of CRTC1 in response to glutamatergic synaptic activity, followed by maintenance of CRTC1 in the nucleus in response to neuromodulatory neurotransmission, provides a molecular mechanism for these observations.
The finding that siRNA knockdown of CRTC1 in cultured hippocampal neurons inhibits the induction of specific CREB targets in response to TTX withdrawal, including cfos, arc, Egr4, zif268 and cyr61 (Fig 7A and Fig S7C) indicates that CRTC1 has a critical function in the transcriptional response to neuronal activity. These changes are particularly remarkable because the siRNA knockdown is incomplete (reduces levels of CRTC1 to ~40%). Moreover, we found that CREB phosphorylation at serine 133 following TTX withdrawal was not altered by CRTC1 knockdown (Fig 7B), indicating that CREB phosphorylation on its own is insufficient to drive full expression of specific CRE-containing genes, and that activity-dependent CRTC1 nuclear translocation is required.
Taken together, the results of our studies raise the possibility that excitatory synaptic activity and neuromodulators contribute to dynamic changes in gene expression in mechanistically distinct ways, with synaptic glutamatergic stimulation triggering nuclear import of CRTC1 and neuromodulators regulating its duration in the nucleus. Given the complexity of the stimulus-induced changes in CRTC1 phosphorylation, it is likely that many other types of neuronal activity might differentially influence CRTC1-dependent gene expression and thereby trigger distinct types of CREB-dependent memory over distinct time domains.
Experimental Procedures
Neuron culture and pharmacological treatments
Rodent hippocampal neurons were cultured for 2-4 weeks as described in supplemental experimental procedures. All pharmacological manipulations of neurons are also described in supplemental experimental procedures.
Antibodies
All primary, secondary antibodies, and protocols for immunoassays are detailed in the supplemental experimental procedures.
Plasmids and neuronal transfection
Transfections were done using calcium phosphate precipitation (Jiang and Chen, 2006, see supplemental procedures). Plasmids, cloning and PCR site-directed mutagenesis are described in supplemental experimental procedures.
Synaptosomes and PSD fractionation and 2D gels
Synaptosomes and PSDs were prepared from adult rats (Sprague Dawley) and mice (C57/Bl6) as previously described (Jeffrey et al., 2009). Fluorescent signals from immunoblots were detected using the Odyssey Imaging System (LI-COR). Detailed protocols for 2D gels are described in the supplemental experimental procedures.
Microscopes and imaging
A Marianas spinning disc confocal microscope attached to a Photometrics Evolve camera (Intelligent Imaging Innovation, Denver, CO) was used for live microscopy and single plane quantification of nucleocytoplasmic intensity. For high resolution imaging of subcellular compartments, we used a scanning confocal LSM 700 (Zeiss, Thornwood, NY). For live cell microscopy protocols, please refer to supplemental experimental procedures.
Hippocampal acute slice and organotypic culture studies
Organotypic Slice Cultures
Organotypic hippocampal slices were prepared as previously described (Johnson and Buonomano, 2007), and chemical LTP was induced as described in the supplemental experimental procedures.
Acute Slices
Standard techniques approved by the UCLA IACUC were used to prepare acute hippocampal slices from 8- to 16- week-old C57-Bl6 mice as previously described (Delgado and O'Dell T, 2005). Slices were maintained at 30 °C in an interface chamber (Fine Science Tools, Foster City, CA) and recovered for at least 2 h before each experiment while being continuously perfused (2-3 ml/min) with oxygenated (95% O2 / 5% CO2) ACSF (124 mM NaCl, 4.4 mM KCl, 25 mM Na2HCO3, 1 mM NaH2PO4, 1.2 mM MgSO4, 2 mM CaCl2, and 10 mM glucose). Protocols for stimulation are described in supplemental experimental procedures.
Statistical Analysis
Statistical significance was analyzed by one-way analysis of variance (ANOVA) and post-hoc Bonferroni's multiple comparison test (Prism Graphpad, La Jolla, CA) unless otherwise noted.
Supplementary Material
Research highlights.
CRTC1 undergoes regulated, calcium-dependent nuclear transport from stimulated synapses
cAMP increases the persistence of nuclear CRTC1
Neuronal activity induces complex changes in CRTC1 phosphorylation
CRTC1 is required for stimulus-induced activation of select CREB targets
Acknowledgements
We thank members of the Carew and Martin lab for helpful discussions and C. Alberini, K. Olofsdotter-Otis, V. Ho, C. Houser and L. Zipursky for critical reading of the manuscript. We thank M. Chin for advice on qPCR experiments, M. DeSalvo for processing tissue samples, and Z. Peng and C. Houser for immunohistochemistry of acute slices. The work was supported by a NARSAD Young Investigator Award (to THC), NIH R01 MH077022 (to KCM) and R01 MH609197 (to TJO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altarejos JY, Goebel N, Conkright MD, Inoue H, Xie J, Arias CM, Sawchenko PE, Montminy M. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nat Med. 2008;14:1112–1117. doi: 10.1038/nm.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bittinger MA, McWhinnie E, Meltzer J, Iourgenko V, Latario B, Liu X, Chen CH, Song C, Garza D, Labow M. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Martin KC. Synapse-to-nucleus signaling. Curr Opin Neurobiol. 2011;21:345–352. doi: 10.1016/j.conb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Delgado JY, O'Dell T J. Long-term potentiation persists in an occult state following mGluR-dependent depotentiation. Neuropharmacology. 2005;48:936–948. doi: 10.1016/j.neuropharm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–82. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, et al. Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey RA, Ch'ng TH, O'Dell TJ, Martin KC. Activity-dependent anchoring of importin alpha at the synapse involves regulated binding to the cytoplasmic tail of the NR1-1a subunit of the NMDA receptor. J Neurosci. 2009;29:15613–15620. doi: 10.1523/JNEUROSCI.3314-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Cohen DE, Cui L, Wupinski A, Savas JN, Mazzulli JR, Yates JR, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nature Med. 2011;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Chen G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 2006;1:695–700. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. Development and plasticity of spontaneous activity and Up states in cortical organotypic slices. J Neurosci. 2007;27:5915–5925. doi: 10.1523/JNEUROSCI.0447-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Kreutz MR. Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci. 2009;32:392–401. doi: 10.1016/j.tins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Takemori H, Lin XZ, Tamura M, Muraoka M, Satoh T, Tsuchiya Y, Min L, Doi J, Miyauchi A, et al. Silencing the constitutive active transcription factor CREB by the LKB1-SIK signaling cascade. Febs J. 2006;273:2730–2748. doi: 10.1111/j.1742-4658.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Takemori H, Min L, Muraoka M, Doi J, Horike N, Okamoto M. Salt-inducible kinase-1 represses cAMP response element-binding protein activity both in the nucleus and in the cytoplasm. Eur J Biochem. 2004;271:4307–4319. doi: 10.1111/j.1432-1033.2004.04372.x. [DOI] [PubMed] [Google Scholar]
- Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 2010;8:e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhauser JM, Cowan CW, Shaywitz AJ, Dolmetsch RE, Griffith EC, Hu LS, Haddad C, Xia Z, Greenberg ME. CREB transcriptional activity in neurons is regulated by multiple, calcium-specific phosphorylation events. Neuron. 2002;34:221–233. doi: 10.1016/s0896-6273(02)00655-4. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dentin R, Chen D, Hedrick S, Ravnskjaer K, Schenk S, Milne J, Meyers DJ, Cole P, Yates J, 3rd, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456:269–273. doi: 10.1038/nature07349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J Physiol. 1986;372:245–259. doi: 10.1113/jphysiol.1986.sp016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Neugebauer F, Korz V, Frey JU. Modulation of extracellular monoamine transmitter concentrations in the hippocampus after weak and strong tetanization of the perforant path in freely moving rats. Brain Res. 2009;1273:29–38. doi: 10.1016/j.brainres.2009.03.055. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors. Cell Signal. 2010;22:728–736. doi: 10.1016/j.cellsig.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazzona B, Isabel G, Preat T, Davis RL. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24:8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, 3rd, Montminy M. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. Embo J. 2007;26:2880–2889. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Saha RN, Wissink EM, Bailey ER, Zhao M, Fargo DC, Hwang JY, Daigle KR, Fenn JD, Adelman K, Dudek SM. Rapid activity-induced transcription of Arc and other IEGs relies on poised RNA polymerase II. Nat Neurosci. 2011;14:848–56. doi: 10.1038/nn.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Takemori H, Yagita Y, Terasaki Y, Uebi T, Horike N, Takagi H, Susumu T, Teraoka H, Kusano K, et al. SIK2 is a key regulator for neuronal survival after ischemia via TORC1-CREB. Neuron. 2011;69:106–119. doi: 10.1016/j.neuron.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–48. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Thompson KR, Otis KO, Chen DY, Zhao Y, O'Dell TJ, Martin KC. Synapse to nucleus signaling during long-term synaptic plasticity; a role for the classical active nuclear import pathway. Neuron. 2004;44:997–1009. doi: 10.1016/j.neuron.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Liu Y, Aguilera G. The Distribution of messenger RNAs Encoding the Three Isoforms of the Transducer Of Regulated CREB Activity (TORC) in The Rat Forebrain. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wu H, Li S, Chen Q, Cheng XW, Zheng J, Takemori H, Xiong ZQ. Requirement of TORC1 for late-phase long-term potentiation in the hippocampus. PLoS ONE. 2006;1:e16. doi: 10.1371/journal.pone.0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.