Abstract
Objectives
To compare long-term prostate-specific antigen relapse-free survival outcome and incidence of toxicity for low-risk prostate cancer treated with brachytherapy or intensity-modulated radiotherapy.
Methods
729 consecutive patients were treated with BRT (n=448; prescription dose, 144 Gy) and intensity-modulated radiotherapy alone (n=281; prescription dose, 81 Gy). Prostate-specific antigen relapse-free survival using nadir +2 definitions, and late toxicity using the National Cancer Institute's Common Terminology Criteria for Adverse Events.
Results
Seven-year prostate-specific antigen relapse-free survival for brachytherapy and intensity-modulated radiotherapy was 95% and 89% for low-risk patients (p=0.004). Cox regression analysis demonstrated that brachytherapy was associated with improved prostate-specific antigen relapse-free survival even when adjusted for other variables. Incidence of metastatic disease between treatments was low for both treatment groups. Late grade 2 gastrointestinal toxicities were observed in 5.1% and 1.4% of the brachytherapy and intensity-modulated radiotherapy groups, respectively (p=0.02). There were no significant differences between treatment groups for late grade ≥3 rectal complications (brachytherapy, 1.1%; intensity-modulated radiotherapy, 0%; p=0.19). Late grade 2 urinary toxicities were more often observed for brachytherapy than intensity-modulated radiotherapy (15.6% and 4.3%, respectively; p<0.0001). There were no significant differences between treatment groups for late grade 3 urinary toxicity (brachytherapy, 2.2%; intensity-modulated radiotherapy, 1.4%; p=0.62).
Conclusions
Among low risk prostate cancer patients, 7-year biochemical tumor control is superior for intraoperative-planning brachytherapy compared with high-dose intensity-modulated radiotherapy. While significant toxicities were minimal for both groups, modest but significant increases in grade 2 urinary and rectal symptoms were noted for brachytherapy compared with intensity-modulated radiotherapy.
Keywords: Low dose rate, Iodine-125, Prostate cancer, PSA-relapse–free survival, Brachytherapy, Toxicity
External beam radiotherapy (EBRT) and brachytherapy are each considered acceptable and standard treatment options in the management of localized prostate cancer. Long-term outcomes have demonstrated that for low risk disease, excellent prostate-specific antigen (PSA) relapse-free survival outcomes are observed, and the risk of significant treatment-related morbidities are low for either treatment approach.1-9 Given the lack of randomized trials to further provide guidance for selection of therapy, most physicians and their patients will chose a specific treatment intervention based on personal bias/preference, convenience (favoring brachytherapy), and the presence of baseline urinary obstructive symptoms or larger prostate sizes (favoring EBRT) among other variables.
Over a decade ago we compared the outcomes of EBRT with low-dose-rate permanent interstitial implantation with I-125 for patients with low-risk prostate cancer10. In that report we noted similar biochemical control rates at 5 years for these two treatments, with a higher risk of chronic urinary-related toxicities among patients treated with brachytherapy. Yet, there were several limitations to that analysis, including the fact that EBRT patients were treated to 70.2 Gy instead of the higher dose levels that are routinely employed currently. In addition, brachytherapy patients were treated with a preplanned computed tomography approach, instead of our current approach using real-time intraoperative planning, which has demonstrated more consistent delivery of the radiation dose with less associated morbidities. Thus, the reported differences in efficacy and tolerance profiles between the two treatment interventions may not be applicable with current modes of practices.
There have been few if any outcome comparisons of these two modalities from one institution when optimal high-dose EBRT delivered with intensity-modulated treatment planning has been compared with high-quality permanent seed implantation with optimal delivery of the radiation dose. In the present report we compare the long-term outcomes of 729 patients treated with either high-dose intensity modulated radiotherapy (IMRT) and real-time transrectal ultrasound-guided I-125 prostate implantation with follow-up beyond 10 years after treatment. Biochemical tumor control and survival outcomes are compared between the treatment groups as well as the incidence of late toxicities.
Material and Methods
Between 1993 and 2003, 729 patients with low risk prostate cancer were treated with high-dose conformal EBRT (n=281) or permanent interstitial I-125 implantation (n=448). Pretreatment diagnostic evaluations were performed as previously described.3 Patients were classified low risk according to the definition of the National Comprehensive Cancer Network (NCCN) guidelines (www.nccn.org). The risk group is defined as clinical stages T1-T2a, a Gleason score ≤6, and a pretreatment prostate-specific antigen (PSA) <10 ng/mL. In general, while there was a tendency for patients with greater degree of medical co-morbidities, urinary obstructive symptoms or larger prostates to be treated with IMRT compared to brachytherapy, the decision to be treated with one modality over the other for this low-risk cohort was driven by individual patient preference.
EBRT Group
In general patients were treated with a five-field intensity-modulated conformal treatment plan as has been previously described.3 Treatment was delivered with 15 MV x-rays in daily fractions of 1.8 Gy to the prescription dose of 81 Gy. The volume of the planning target volume (PTV) receiving at least 95% of the prescription dose was at least 90% for patients treated to the prescription dose of 81 Gy and the maximum PTV dose never exceeded 111% of the prescribed dose. The median IPS urinary score was 7 (range 1-29). Prior to radiotherapy, a total of 89 patients (32%) were treated with short-course (3-month) androgen-deprivation therapy (ADT). In general short-course ADT was given to decrease the size of enlarged prostate glands prior to radiotherapy. ADT was routinely discontinued at the completion of radiotherapy.
Brachytherapy Group
Our prostate implantation procedure using a real-time intraoperative-planned approach has been previously described.11 An intraoperative planning system that incorporated a genetic optimization algorithm was used to achieve the optimal seed-loading pattern for delivery of the prescription dose and maintained established dose constraints for the surrounding normal tissue structures. Patients were treated with I-125 monotherapy to a prescribed dose of 144 Gy. A postimplantation computed tomography scan was performed several hours after the brachytherapy procedure for the purpose of dosimetric evaluation. The median V100 (volume of prostate receiving 100% of the prescription dose) was 96% and the median D90 (dose to 90% of the prostate) was 170 Gy.
In general, patients with gland sizes >50 cm3 (n=138; 31%) were pretreated for 3 months with short-term neo-adjuvant ADT to achieve prostate volume reduction prior to the initiation of therapy. The median prostate volume at the time of implantation was 37 cm3 (range, 10-91 cm3). The median IPS urinary score was 5 (range 1-27). The patient characteristics of both treatment groups are shown in Table 1.
Table 1. Patient Characteristics.
| Patient Demographics |
Brachytherapy (n=448)a | EBRT (n=281)b | P Valuec | |
|---|---|---|---|---|
|
| ||||
| No.(%) | No.(%) | |||
| Age | <65 | 188 (42.0) | 86 (30.6) | 0.002 |
| ≥65 | 260 (58.0) | 195 (69.4) | ||
|
| ||||
| PreTX PSA | <4 | 93 (20.8) | 43 (15.3) | 0.08 |
| ≥4 | 355 (79.2) | 238 (84.7) | ||
|
| ||||
| T-stage | T1c | 365 (81.5) | 197 (70.1) | 0.001 |
| T2a | 83 (18.5) | 84 (29.9) | ||
|
| ||||
| NeoHT | No | 310 (69.2) | 192 (68.3) | 0.81 |
| Yes | 138 (30.8) | 89 (31.7) | ||
Median follow-up time, 77 months.
Median follow-up time, 76 months.
Used Fisher's exact test.
Follow-up evaluations after treatment were performed at intervals of 3 to 6 months for 5 years and yearly thereafter. The overall median follow-up time was 77 months (range, 1-11 years). The median follow-up times for the IMRT and brachytherapy groups were 76 and 77 months, respectively. Late toxicity was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events toxicity scale version 3.0. A PSA relapse was defined according to the Houston definition (absolute nadir plus 2 ng/mL dated at the call).12 None of the patients received postirradiation ADT or other anticancer therapy before documentation of a PSA relapse. Distributions of PSA relapse-free survival times were calculated according to the product-limit (Kaplan-Meier) method. Differences between time-adjusted incidence rates were evaluated using the Mantel log-rank test for censored data.
Results
As demonstrated in Figure 1 the 7-year PSA relapse-free survival (PRFS) outcome was 95% for brachytherapy compared with 89% for the IMRT group (P=0.004). The multivariate analyses for predictors of PSA relapse are included in Table 2. These data indicate that brachytherapy-based treatment was significantly superior to EBRT for improved PRFS outcomes even when adjusted for the other variables. The use of neo-adjuvant ADT had no impact on long-term biochemical outcome in this low-risk patient population.
Figure 1.
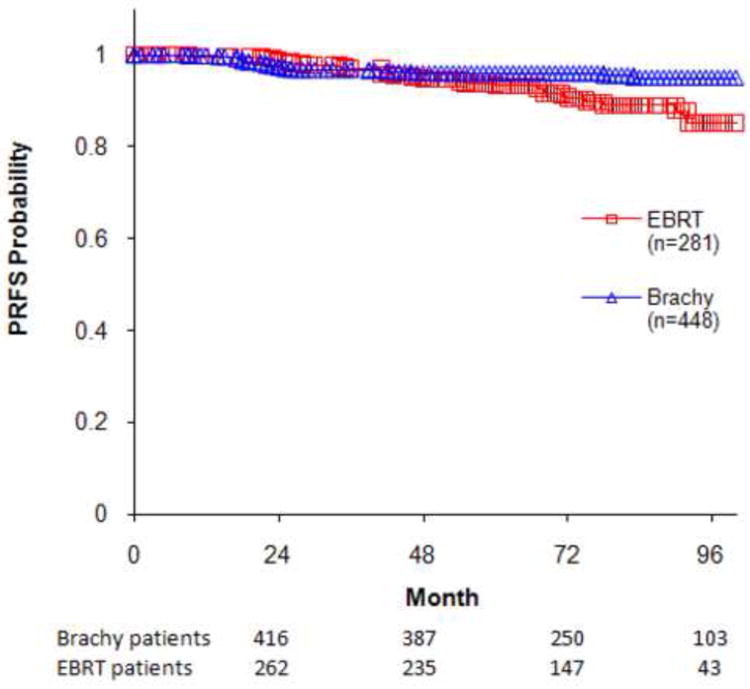
PSA Relapse-Free Survival for Favorable Risk Patients; the 7-year PRFS for Brachytherapy Compared with IMRT Patients Were 95% and 89%, Respectively; P=0.004.
Table 2. Univariate and Multivariate Analyses for Predictors of PSA Relapse.
| PSA Relapse | ||||
|---|---|---|---|---|
|
| ||||
| Factor | Univariate | Multivariate | ||
|
| ||||
| HR | P Value | HR | P Value | |
| Mode (Brachy vs EBRT) | 0.43 | 0.005 | 0.416 | 0.004 |
| Pre-treatment PSA | 1.18 | 0.027 | 1.18 | 0.025 |
| Age (continuous) | 0.966 | 0.09 | 0.955 | 0.025 |
| Age (>65 vs <=65) | 0.767 | 0.37 | ||
| HT (1 vs 0) | 0.783 | 0.47 | ||
Post-treatment nadir PSA levels were significantly lower among brachytherapy patients than EBRT patients. Among the 310 patients treated with brachytherapy alone (without ADT), the median nadir PSA value was 0.1 ng/mL compared with 0.6 ng/mL among the 192 patients treated with EBRT alone (P<0.0001 based on a Wilcoxon rank sum test). Interestingly, the time to reach nadir was twice as long in the brachytherapy group compared with EBRT patients (43 months vs 23.5 months, P<0.0001 based on a Wilcoxon rank sum test).
The 7-year distant metastases-free survival (DMFS) outcome was 100% for brachytherapy compared with 99.2% for the EBRT group (there were no distant metastases among the brachytherapy group, only 2 cases in the EBRT group).
Table 3 summarizes the late-toxicity outcomes of patients in the brachytherapy and IMRT treatment groups. While the overall incidence of toxicity was low in both groups, there was a greater incidence of late grade 2 urinary and rectal toxicities in the brachytherapy group compared with IMRT patients. Among the brachytherapy group the incidence of grade 2 late urinary toxicities was 15.6% compared with 4.3% for the IMRT group (P<0.0001, based on binomial test). In addition grade 2 late rectal toxicity manifested as rectal bleeding was more often noted in the brachytherapy group than in IMRT patients (5.1% vs 1.4%, respectively; P=0.018). The incidence of grade 3 urinary and rectal toxicities was low in both groups and not significantly different between groups.
Table 3. Late Toxicity Outcomes.
p-values are based on binomial tests looking at the overall number of events in each group
| Treatment/Toxicities | Grade | Brachytherapy (n=448) | EBRT (n=281) | P-value |
|---|---|---|---|---|
| Gastrointestinal late toxicities, No. (%) | 2 | 23 (5.1%) | 4 (1.4%) | 0.018 |
| 3 | 5 (1.1%) | 0 (0.0%) | 0.19 | |
| Genitourinary late toxicities, No. (%) | 2 | 70 (15.6%) | 12 (4.3%) | <0.0001 |
| 3 | 10 (2.2%) | 4 (1.4%) | 0.62 |
Among the patients who were potent prior to therapy (defined as ability to achieve erection sufficient for sexual intercourse), brachytherapy patients (n=350) developed post-treatment impotence in 35% compared with 44% of the IMRT cohort (n=185) (P=0.04 based on a two sample proportion test). In this cohort of patients who were potent prior to therapy, the average age was 65 years for the brachytherapy group and 66 years for the EBRT group. A trend for increased incidence of erectile dysfunction was observed among IMRT patients treated with ADT (51% treated with ADT compared to 41% without ADT, P=0.09). However, the negative impact of ADT on post-treatment erectile function was not apparent in the brachytherapy group (34% versus 35%, respectively; P=0.91).
Comment
Our results demonstrate that, while excellent tumor control rates can be achieved with high-dose conformal EBRT, brachytherapy was associated with superior long-term biochemical outcomes for favorable risk prostate cancer. We believe these improved biochemical tumor-control outcomes are related to the higher biological doses that can be delivered safely to the tumor with brachytherapy compared with EBRT. While prescription doses are limited to 144 Gy for patients treated with brachytherapy alone, it is unavoidable for significant portions of the prostate to receive doses in excess of 250 Gy. Pieters et al13 have observed that the biologic dose in at least 50% of the prostate volume is significantly higher with brachytherapy (and in particular with high-dose-rate brachytherapy) than with what is routinely achieved with IMRT alone. In the current report we note that brachytherapy is associated with lower post-treatment PSA nadir values compared with EBRT and this likely reflects its greater ability to more effectively ablate the prostatic epithelium compared with EBRT-based approaches.
With the increased dose levels delivered via brachytherapy-based interventions, it is not surprising that a higher incidence of grade 2 urinary symptoms are observed in our experience compared with patients treated with EBRT. These findings are likely related to the higher dose exposure delivered to the urethra and bladder neck with brachytherapy. Yet the incidence of grade 2 urinary symptoms was only noted in 15.6% of our brachytherapy-treated patients compared with 4.3% of the IMRT group. These symptoms generally included frequent urination requiring alpha-blocker medications, which gradually improved with time; urinary incontinence was unusual in these patients. In addition, we did not observe a higher incidence of urinary-related grade 3 toxicities in the brachytherapy group, which we report as less than 2.5% and not significantly different than what was noted for IMRT-treated patients. We also observed a higher incidence of grade 2 rectal bleeding in the brachytherapy patients compared with IMRT (5.1% vs 1.4%). The use of IMRT has significantly improved the tolerance of high-dose EBRT for prostate cancer, and indeed the higher incidence of rectal effects we observed with non-IMRT treatment in our prior report are no longer observed with the availability of more sophisticated treatment-delivery techniques.
Several reports have made comparisons of various treatment modalities in terms of tumor-control rates, toxicity, and health-related quality-of-life outcomes.14-21 In addition to the nonrandomized, retrospective nature of these comparisons, one of the significant limitations of those reports was the suboptimal quality of one or the other therapy being compared that was not considered necessarily state of the art delivery based on current practice. In order to fairly compare tumor-control outcomes and rates of morbidity between EBRT and brachytherapy, treatment cohorts that need to be included are a high dose (>75 Gy) conformal IMRT and high-quality image-guided brachytherapy group. In one report from Wong et al,21 patients treated with high-dose treatment interventions achieved superior biochemical control rates at 5 years compared to low-dose 70 Gy EBRT. In that study, with a follow-up of 58 months, no differences were noted in those patients treated with IMRT (n=314; median dose 75 Gy) compared with brachytherapy and EBRT combined with brachytherapy (n=269). The authors also noted higher rates of urinary-related toxicities among the brachytherapy-treated patients compared with EBRT, yet the 18% incidence of grade 3 urinary toxicity reported in that series is significantly higher than in other reported series including our current series. The incidence of grade 3 toxicity in our series was only 2%, which was related to urethral strictures and corrected with urethral dilation or transurethral resection of the prostate. In another report, from investigators at the Fox Chase Cancer Center,22 comparable outcomes were noted among low-risk patients treated with IMRT or I-125 implantation, yet the study cohort was small and the overall follow-up was less than 4-years. We nevertheless recognize that these reports and the current study have inherent limitations given their retrospective nature. Selection bias and other potential clinical and pathologic variables can confound outcome differences between treatment groups, making it difficult to claim definitively the superiority of one treatment intervention compared with another.
Based on the current report, our treatment preference for low risk prostate cancer patients is to favor brachytherapy due to the improved tumor-control outcomes. However, in a setting where brachytherapy would be associated with increased functional impairment or treatment-related morbidity, such as for those patients with significant obstructive symptoms, large prostate glands, or other medical co-morbidities, external EBRT would be the preferred nonsurgical treatment intervention. Clearly, quality-of-life considerations are very important in the decision-making process, and issues regarding the importance of incontinence, sexual function, and psychological preferences weigh heavily for the patient in ultimately choosing between a surgical and a nonsurgical approach. We do caution patients not to select their therapy based on presumed advantages of one therapy over another for improved sexual dysfunction. In both treatment groups we noted a relatively high incidence of erectile dysfunction. We observed a similar incidence of impotence among the patients treated with brachytherapy and EBRT (35% and 44%, respectively). Given the abundance of variables impacting upon potency, including baseline functional status, medical condition of the patient, and the use of medications, it is difficult to state with certainty whether brachytherapy represents a significant advantage for the patient in terms of potency preservation.
In conclusion, in our experience brachytherapy is associated with improved PFRS outcomes compared with high-dose IMRT for low risk patients. Severe toxicity is extremely low for both treatment groups yet the incidence of grade 2 late toxicities were higher for the brachytherapy group. To date, the biochemical control improvement has not translated into an enhancement in DMFS outcomes. Patients should be counseled that, with well-delivered brachytherapy, biochemical control rates can be 5%-10% better than what is achieved with high-dose IMRT. Newer EBRT approaches employing image-guided IMRT (IGRT) may further improve accuracy of dose delivery in these patients, yet there is no evidence to date of improved outcomes for IGRT given the relatively short follow-up of treated patients. The apparent biochemical control advantage for brachytherapy may need to be considered in the presence of comorbidities or factors that could predict greater potential toxicities with this modality that could in turn impact quality-of-life outcomes. The patient needs to weigh these issues carefully when ultimately making his treatment selection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002 Aug 1;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005 Sep 14;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006 Oct;176(4 Pt 1):1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Kupelian PA, Ciezki J, Reddy CA, Klein EA, Mahadevan A. Effect of increasing radiation doses on local and distant failures in patients with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008 May 1;71(1):16–22. doi: 10.1016/j.ijrobp.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Yamada Y, Bhatia S, Zaider M, et al. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy. 2006 Jul-Sep;5(3):157–164. doi: 10.1016/j.brachy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007 Feb 1;67(2):327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 7.Morris WJ, Keyes M, Palma D, et al. Population-based study of biochemical and survival outcomes after permanent 125I brachytherapy for low- and intermediate-risk prostate cancer. Urology. 2009 Apr;73(4):860–865. doi: 10.1016/j.urology.2008.07.064. discussion 865-867. [DOI] [PubMed] [Google Scholar]
- 8.Hinnen KA, Jan JB, van Roermund JG, et al. Long-Term Biochemical and Survival Outcome of 921 Patients Treated with I-125 Permanent Prostate Brachytherapy. Int J Radiat Oncol Biol Phys. 2009 Jun 17; doi: 10.1016/j.ijrobp.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Stone NN, Stock RG, Unger P. Intermediate term biochemical-free progression and local control following 125iodine brachytherapy for prostate cancer. J Urol. 2005 Mar;173(3):803–807. doi: 10.1097/01.ju.0000152558.63996.29. [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Wallner KE, Ling CC, et al. Comparison of the 5-year outcome and morbidity of three-dimensional conformal radiotherapy versus transperineal permanent iodine-125 implantation for early-stage prostatic cancer. J Clin Oncol. 1999 Feb;17(2):517–522. doi: 10.1200/JCO.1999.17.2.517. [DOI] [PubMed] [Google Scholar]
- 11.Zelefsky MJ, Yamada Y, Cohen GN, et al. Five-year outcome of intraoperative conformal permanent I-125 interstitial implantation for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007 Jan 1;67(1):65–70. doi: 10.1016/j.ijrobp.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Thames H, Kuban D, Levy L, et al. Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys. 2003;57:929–943. doi: 10.1016/s0360-3016(03)00631-x. [DOI] [PubMed] [Google Scholar]
- 13.Pieters BR, van de Kamer JB, van Herten YR, et al. Comparison of biologically equivalent dose-volume parameters for the treatment of prostate cancer with concomitant boost IMRT versus IMRT combined with brachytherapy. Radiother Oncol. 2008 Jul;88(1):46–52. doi: 10.1016/j.radonc.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007 Jun 1;109(11):2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer M, Suarez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008 Oct 1;72(2):421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Klein EA, Ciezki J, Kupelian PA, Mahadevan A. Outcomes for intermediate risk prostate cancer: are there advantages for surgery, external radiation, or brachytherapy? Urol Oncol. 2009 Jan-Feb;27(1):67–71. doi: 10.1016/j.urolonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Wyler SF, Engeler DS, Seelentag W, Ries G, Schmid HP. Health-related quality of life after radical prostatectomy and low-dose-rate brachytherapy for localized prostate cancer. Urol Int. 2009;82(1):17–23. doi: 10.1159/000176019. [DOI] [PubMed] [Google Scholar]
- 18.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005 Apr 20;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 19.Tward JD, Lee CM, Pappas LM, Szabo A, Gaffney DK, Shrieve DC. Survival of men with clinically localized prostate cancer treated with prostatectomy, brachytherapy, or no definitive treatment: impact of age at diagnosis. Cancer. 2006 Nov 15;107(10):2392–2400. doi: 10.1002/cncr.22261. [DOI] [PubMed] [Google Scholar]
- 20.Fang FM, Wang YM, Wang CJ, Huang HY, Chiang PH. Comparison of the outcome and morbidity for localized or locally advanced prostate cancer treated by high-dose-rate brachytherapy plus external beam radiotherapy (EBRT) versus EBRT alone. Jpn J Clin Oncol. 2008 Jul;38(7):474–479. doi: 10.1093/jjco/hyn056. [DOI] [PubMed] [Google Scholar]
- 21.Wong WW, Vora SA, Schild SE, et al. Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009 Aug 10; doi: 10.1002/cncr.24558. [DOI] [PubMed] [Google Scholar]
- 22.Eade TN, Horwitz EM, Ruth K, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys. 2008;71:338–345. doi: 10.1016/j.ijrobp.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


