Abstract
Unlimited self renewal capacity and differentiation potential make human pluripotent stem cells (PSC) a promising source for the ex vivo manufacture of red blood cells (RBC) for safe transfusion. Current methods to induce erythropoiesis from PSC suffer from low yields of RBCs, most of which are immature and contain embryonic and fetal rather than adult hemoglobins. We have previously shown that homo-dimerization of the intracellular component of MPL (ic-MPL) induces erythropoiesis from human cord blood progenitors. The goal of the present study was to investigate the potential of ic-MPL dimerization to induce erythropoiesis from human embryonic stem cells (hESC) and to identify the signaling pathways activated by this strategy. We present here evidence that ic-MPL dimerization induces erythropoietin (EPO)-independent erythroid differentiation from hESC by inducing the generation of erythroid progenitors and by promoting more efficient erythroid maturation with increased RBC enucleation as well as increased gamma:epsilon globin ratio and production of beta-globin protein. ic-MPL dimerization is significantly more potent than EPO in inducing erythropoiesis and its effect is additive to EPO. Signaling studies show that dimerization of ic-MPL, unlike stimulation of the wild type MPL receptor, activates AKT in the absence of JAK2/STAT5 signaling. AKT activation upregulates the GATA-1 and FOXO3 transcriptional pathways with resulting inhibition of apoptosis, modulation of cell cycle and enhanced maturation of erythroid cells. These findings open up potential new targets for the generation of therapeutically relevant RBC products from hPSC.
Keywords: human embryonic stem cells, erythropoiesis, erythropoietin, MPL, AKT, GATA-1
INTRODUCTION
Red blood cell transfusion is a widely used cellular therapy with approximately 85 million blood components transfused annually worldwide.[1] Currently, the procedure is exclusively dependent on volunteer donors and is therefore prone to limitations in supply and risk for infectious disease transmission.
Human pluripotent stem cells (hPSC) offer a potentially unlimited source of cells for cellular therapy because they can be propagated and expanded indefinitely, and maintain the ability to differentiate into cell types representative of all three germ layers. Thus hPSC are a potentially ideal source for the ex vivo manufacture of large numbers of red blood cell (RBC) units for safe transfusion. It has been shown that two such hPSC types, human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) can be directed to differentiate into RBCs.[2, 3] However, current methods of hematopoietic differentiation from hPSC, all of which depend on erythropoietin (EPO) stimulation, suffer from low yields of RBCs, most of which are immature and contain predominantly embryonic and fetal rather than adult hemoglobins. Therefore, efficient clinical translation of this strategy is critically dependent on the development of novel methods to enhance the generation of functional mature RBCs from hPSC.
EPO is an essential cytokine for normal erythropoiesis.[4] Our laboratory has demonstrated an EPO-independent approach for the ex vivo expansion and erythroid differentiation of human multilineage hematopoietic progenitors from cord blood.[5] This was achieved by utilizing an inducible system in which a fusion protein (F36V-MPL)[6], consisting of the intracellular domain of the receptor MPL (ic-MPL) and a drug binding domain F36V, is expressed in CD34+ hematopoietic progenitor cells via a lentiviral vector.[5, 7] Signaling through full length MPL is normally accomplished when binding of its natural ligand Thrombopoietin (TPO) to the extracellular portion of the receptor causes homodimerization of the intracellular domain, ultimately leading to the onset of megakaryocytopoiesis.[8] In the F36V-MPL system, only the intracellular component of MPL is expressed and signaling is induced by the addition of a small molecule, AP20187 (CID) that binds to F36V, and homodimerizes ic-MPL in the absence of TPO.[6] The constitutive intracellular expression of F36V-MPL avoids the normal negative feedback from internalization of the cell surface receptor after TPO binding and from down-regulation of MPL transcription during differentiation.[8] Notably, we found that dimerization of F36V-MPL activates a gene expression signature that is distinct from full-length MPL receptor activation.[5]
Based on our previous findings, the goal of the present work was to investigate the potential of ic-MPL dimerization to induce erythropoiesis from hESC and to identify the signaling pathways activated by this strategy. We observed that ic-MPL dimerization during hESC-derived hematopoiesis induces EPO-independent erythroid differentiation through AKT signaling, by both generating erythroid progenitors and promoting output and maturation of RBC from those progenitors. ic-MPL dimerization led to an increase in GATA-1 expression with regulation of its downstream targets associated with cell cycle, apoptosis, and erythroid differentiation; as a functional consequence, ic-MPL increased cell survival and G0/G1 arrest. ic-MPL dimerization was significantly more potent than EPO in inducing erythropoiesis from hESC and was additive when combined with EPO. This is the first demonstration of EPO-independent erythroid differentiation induced in human PSC and reveals the AKT pathway as a novel molecular target through which erythropoiesis can be manipulated.
METHODS
Generation of stably transduced hESC lines
The hESC line H1 (WiCell. Madison, WI) was maintained and expanded on irradiated primary mouse embryonic fibroblasts (Millipore, Billerica, MA). The F36V-MPL plasmid (a generous gift of Dr. C.A. Blau, University of Washington, Seattle, WA) was modified to produce the lentiviral vector, pCCL-c-UbC-F36Vhmpl-IRES-eGFP-WPRE, expressing F36V-MPL and a marker gene (green fluorescent protein) under control of the ubiquitin promoter (UbC) (Supplemental Figure 1A). A lentiviral vector co-expressing only the ligand-binding domain F36V and GFP was used in parallel as a negative control. (See supplementary methods for further details).
Stromal-based co-culture for hematopoietic differentiation
Transduced H1 hESC were plated on confluent layers of OP9-M2 stroma in OP9 medium consisting of MEM-alpha+GlutaMAX (Invitrogen) supplemented with 20% Fetal Bovine Serum (FBS) (Thermo Scientific Hyclone, Logan, Utah) and 1X penicillin/streptomycin (Gemini Bio-Products, West Sacramento, CA). 100nM AP20187 (CID) (Clontech, Mountainview, CA) was added every 3-4 days as indicated in each experiment, from day of plating (day 0) to day 14 (Supplementary Figure 2A). After 14 days of culture, cells were harvested using sequential collagenase IV (Stem Cell Technologies, Vancouver, BC) and Trypsin (Invitrogen) digestion, for FACS analysis or Colony Forming (CFU) assay (Stem Cell Technologies). Megakaryocyte-erythroid progenitors (MEP) were generated as follows to maximize cell output: 10ng/ml BMP-4 (R&D systems, Minneapolis, MN), 10ng/ml bFGF (Invitrogen), and 10ng/ml VEGF (R&D systems) were added to OP9 medium from Day 0-2 for mesoderm induction. Subsequently, half the medium was removed and replaced with OP9 medium containing Stem Cell Factor (SCF, 50 ng/ml), Flt3 Ligand (FL, 25 ng/ml), IL-3 (5 ng/ml), Thrombopoietin (TPO, 25 ng/ml), and Erythropoietin (EPO, 2U/ml) (SFITE medium) (all from R&D Systems) for 11 days for hematopoietic induction (Supplementary Figure 2B). After 14 days of culture, cells were harvested using sequential collagenase IV (Stem Cell Technologies) and Trypsin (Invitrogen) digestion. Cells were then FACS sorted to isolate MEP based on GlyA+CD41a/42a+ and then replated on OP9-M2 with various factors as indicated in each experiment.[10] 10uM LY294002 (Sigma-Aldrich. St. Louis, MO), 200nM Akt inhibitor IV and 25uM AG490 (EMD Millipore. Hayward, CA) were added where indicated.
Erythroblast Induction cultures
The effects of CID vs EPO in inducing the production and maturation of erythroblasts were compared using a combination of stromal co-culture to initiate hematopoiesis from hESC, followed by a two-step protocol in serum-free, suspension culture modified from Qiu et al.[11] (Supplementary Figure 2C). Specifically, transduced H1 hESC were cultured for 14 days on OP9-M2 (without cytokines or CID) as described above. Cells were then harvested from OP9-M2 and transferred to suspension culture in serum-free medium (StemSpan SFEM [Stem Cell Technologies]) for a further 14 days with either CID or EPO. In the first step of this suspension culture (amplification of progenitors), the cultures were supplemented with SCF (100ng/mL), Flt-3L (33.3ng/mL), IL-3 (13.3ng/mL), and BMP4 (13.3ng/mL) with either EPO (2.6U/mL) or CID (100nM) every 2-3 days for 7 days. In the second step (further erythroid differentiation of progenitors), the cells were supplemented with SCF (40ng/mL), IGF-1 (40ng/mL), IL-3 (13.3ng/mL), BMP4 (13.3ng/mL) and continued in either EPO (3.3U/mL) or CID (100nM) for 7 days.
Cell sorting and flow cytometry analysis
FACS analysis was performed on LSRII or LSRFortessa and cell sorting on a FACSAria II (all from Becton Dickinson, San Jose, CA) by direct immuno-fluorescence staining with human specific monoclonal antibodies. Non-specific binding was blocked with intravenous immunoglobulin (0.1%) (IVIG; Cutter, Berkley, CA) prior to staining with fluorochrome conjugated antibodies. Cell acquisition used FACSDivaTM (Becton Dickinson) and analysis was performed using FlowJo (Tree Star, Ashland, OR). The following antibodies were used for staining; CD41a-PE, CD42a-PE, Glycophorin-A-APC, CD43-APC, VE-CAD-PE, CD31-PE, Annexin V-APC, anti-BrdU-APC (all from Becton Dickinson) and mCD29-Alexa 647 (AbD Serotec, Raleigh, NC). mCD29-Alexa 647 was used to gate out murine cells. Unstained cells were used as negative controls. Hoechst 33342 (Invitrogen) was used as a means to distinguish enucleated cells from nucleated cells. FSC/SSC and DAPI (Invitrogen) were used to identify live cells. For analysis of cultured cells, the following gating strategy was used for all experiments: FSC/SSC, GFP+, with/without DAPI neg, with/without mCD29 neg gating. For intracellular staining, the following antibodies were used; p21-PE, GATA1-Rabbit-mAb, BCL-xL-Rabbit-mAb, and Anti-rabbit-Alexa 647 (all from Cell Signaling Technologies, Danvers, MA). Cells were prepared (fixation, permeabilization, and staining) according to manufacturer's instructions.
Cell signaling analysis
Ba/F3 cells were starved overnight (~16hrs) in R10 medium without mIL-3. Cultures were supplemented 10ng/mL mIL-3 for 30min, 10ng/mL TPO, or 100nM CID for 60min prior to fixation using Cytofix Buffer (BD Biosciences). 10uM LY294002 (Sigma-Aldrich), 10uM Wortmannin (Sigma-Aldrich), or 200nM Akt inhibitor IV (EMD Millipore. Hayward, CA) were added for 20min where indicated. Cells were then immuno-fluorescence stained with anti-pAKT(S473)-PE, anti-pSTAT5a(Y694)-PerCP-cy5.5, anti-p-p38-MAPK(pT180/pY182)-PE-Cy7, and anti-pERK1/2(pT202/pY205)-APC (all from Becton Dickinson). Cells were also used for protein extraction and western blot analysis. Cells were pelleted and resuspended in RBC lysis buffer (0.5% NP40 150mM NaCl 50mM Tris pH 8.0) with Roche complete protease inhibitor tablets (EMD Millipore), and 1X Phosphatase Inhibitor Cocktail Set III (EMD Millipore). Protein extracts were resolved by SDS-PAGE and transferred onto Immobilon-FL PVDF membranes. Blots were blocked in LiCor Odyssey buffer (LiCor Biosciences, Lincoln, NE) and incubated with primary antibodies (all from Cell Signaling Technologies): p-JAK2 (3771S); B-Actin (3770S); p-AKT-Ser473 (587F11); p-AKT-Thr308 (2965S); pan-AKT (4685S); p-BAD (5284S). The following secondary fluorescent antibodies were utilized: goat anti-mouse IgG IRDye 800CW, goat anti-rabbit IgG IRDye 680LT LiCor Biosciences). Finished blots were scanned with the LiCor Odyssey Imaging System.
Colony forming assay
Total cells from 14 days of hematopoietic induction as mentioned above (Supplementary Figure 2A) were made into a single cell suspension and plated in MethoCult® GF +H4435 (Stem Cell Technologies) in 35 mm cell culture dishes (Nalgene Nunc, Rochester, NY). 150,000 cells (mixture of OP9 and human cells) were plated per culture dish in duplicate. 100nM CID was also supplemented to the assay where indicated. Hematopoietic colonies were counted on day 14 with an inverted Olympus CKX41 microscope.
HPLC for globin protein analysis
MEP (generated as described above in “Stromal-based coculture for hematopoietic differentiation” Supplementary Figure 2B) were sorted and cultured on OP9 stroma for 14 days in OP9 medium +/− 2U/mL EPO or 100nM CID. GlyA+ cells were then collected using a FACSAria III (Becton Dickinson) and washed twice with PBS, and lysed in water by 3 rapid freeze-thaw cycles. Debris was eliminated by centrifugation at 16,000g and the lysates stored in liquid nitrogen before HPLC analysis. HPLC were performed as previously described.[12]
Quantitative RT-PCR for gene expression analysis
RNA from erythroblasts generated as mentioned above in “Erythroblast induction cultures” (Supplementary Figure 2C) was extracted using a Qiagen Micro Kit (Qiagen, Valencia, CA). An omniscript reverse transcriptase kit was used to make cDNA, which was subjected to quantitative polymerase chain reaction (qPCR) using TaqMan probe-based gene expression analysis assays (Applied Biosystems, Carlsbad, California) for Hs00362216_m1 (HBE), Hs01629437_s1 (HBG), Hs00758889_s1 (HBB), Hs01085823_m1 (GATA1), Hs00355782_m1 (CDKN1A), Hs01078066_m1 (Rb1), Hs00236329_m1 (BCL2L1), Hs00900055_m1 (VEGFA), and Hs01060665_g1 (B-actin). Reactions were loaded onto a MicroAmp Optical 96-Wel Reaction Plate (Applied Biosystems), sealed with MicroAmp Optical Adhesive Film (Applied Biosystems) and amplified in a reaction volume of 20uL on a 7500 Real Time PCR system (Applied Biosystems). The data was analyzed using the comparative C(T) method.[13]
Cell cycle and apoptosis assay
Erythroblasts generated as mentioned above in “Erythroblast induction cultures” (Supplementary Figure 2C) were harvested and stained with Annexin V-APC and Propidium Iodide (PI) (both from Becton Dickinson) for apoptosis analysis and apoptotic cells scored as positive for either Annexin V or PI or both. Unstained cells were used to set negative gates. Erythroblasts were also incubated with bromodeoxyuridine (BrdU) for 30 or 120 minutes before harvest and for cell cycle analysis. Harvested cells were fixed, permeabilized, and stained with 7-AAD and APC conjugated antibody to BrdU. Unstained cells were used to set negative gates and scored according to manufacture's guidelines: apoptotic (BrdUneg7AADneg), G0/G1 (BrdUneg7AADdim), S-phase (BrdUpos7AADdim), and G2/M cells (BrdUneg7AADhi).
Statistical analysis
Prism version 5 (GraphPad Software Inc) was used for statistical analysis. Results are presented as the average value of at least three experimental repeats (indicated accordingly) +/− standard deviation. Unpaired Student's t-test was utilized and P values <0.05 were considered statistically significant.
RESULTS
Dimerization of intracellular MPL induces erythropoiesis from human embryonic stem cells
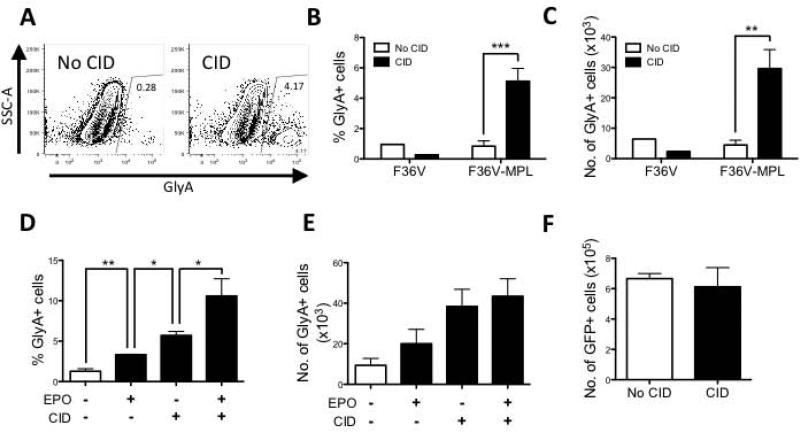
To determine if dimerization of ic-MPL induces erythropoiesis during mesoderm differentiation of human embryonic stem cells (hESC), the H1 cell line was stably transduced to express either the F36V-MPL fusion protein (Supplementary Figure 1A) or a control vector expressing only the ligand binding domain, F36V. Each vector co-expressed the marker gene GFP, allowing selection of transduced hESC (Supplementary Figure 1B,C) and monitoring of vector expression in hematopoietic derivatives. GFP+ hESC were plated onto the murine stromal line OP9 and cultured in serum-containing medium without additional cytokines for 14 days (Supplementary Figure 2A), in the presence or absence of the synthetic ligand AP20187 (aka chemical inducer of dimerization, CID) which binds to F36V and induces homodimerization of the fusion protein.[6] In the absence of CID, glycophorin A+ (GlyA+) erythroid cells were barely detectable in these conditions. However, CID treated cultures consistently generated a population of erythroid cells, producing significant increases in both frequency (P=0.0001; n=5; Figure 1A,B) and cell number (P=0.003; n=5; Figure 1C) of GlyA+ cells. CID also induced erythropoiesis in serum-free medium (supplemented with BMP4, VEGF, and FGF from Day 0-2 and then SCF, Flt-3L, IL-3, TPO +/− EPO and/or CID from Day 3-14) (Supplementary Figure 3). Thus dimerization of ic-MPL through the F36V-MPL fusion protein was sufficient to induce erythropoiesis from hESC in the absence of EPO, a critical regulator of normal erythroid proliferation and differentiation.[4] Notably, erythropoiesis from hESC was significantly greater in the presence of CID than with EPO (P=0.015, n=5, Figure 1D,E) while the addition of CID to EPO further enhanced production of GlyA+ cells (P=0.018; n=5; Figure 1D,E, Supplementary Figure 3B) indicating that ic-MPL dimerization may act through a different pathway to EPO to induce erythropoiesis. The number of total cells generated from hESC was not significantly different between treated and untreated cultures (Figure 1F). CID treatment also had no detectable effect on proliferation or differentiation of undifferentiated hESC.
Figure 1. Dimerization of ic-MPL induces erythropoiesis from hESC.
H1 hESCs were transduced with F36V-MPL or F36V (control vector) and cultured on murine OP9 stroma for 14 days with or without CID or EPO (no other cytokines added) (schema Supplementary Figure 2A). After 14 days of culture, the cells were counted and analyzed by flow cytometry for generation of erythroid (GlyA+) cells. A) Representative immunophenotype of F36V-MPL transduced cells cultured +/− CID (day 14, gated on GFP+ human [murine CD29-] cells). B-F) Summary of FACS data from Day 14 cultures (n=5 experiments). D-F) FACS data is shown from F36V-MPL expressing cells only. *P<0.05, **P<0.01, ***P<0.001. GlyA+ cell number was calculated by multiplying the total cell count with the total GlyA+ percentage represented in each experiment. F) Total number of DAPI-mCD29-GFP+ cells from Day 14 culture (n=3 experiments).
Dimerization of ic-MPL induces the generation of erythroid progenitors
Erythropoiesis proceeds from the undifferentiated hESC stage by progressing through key transition points of increasingly restrictive lineage specification events, beginning with the onset of mesoderm specification prior to hemato-endothelial commitment and further lineage restriction to a bi-potent megakaryocyte-erythroid progenitor (MEP), then ultimately to the generation of erythroid cells. Further analysis of 14 day cultures generated from hESC plated directly on OP9 stroma (Supplementary Figure 2A) revealed that ic-MPL dimerization increased the frequency of hematopoietic progenitor cells that express CD43 (a marker of hematopoiesis that appears early in culture),[14] but had no significant effect on the generation of endothelial cells (based on VE-cadherin or CD31 expression) (Supplementary Figure 4).
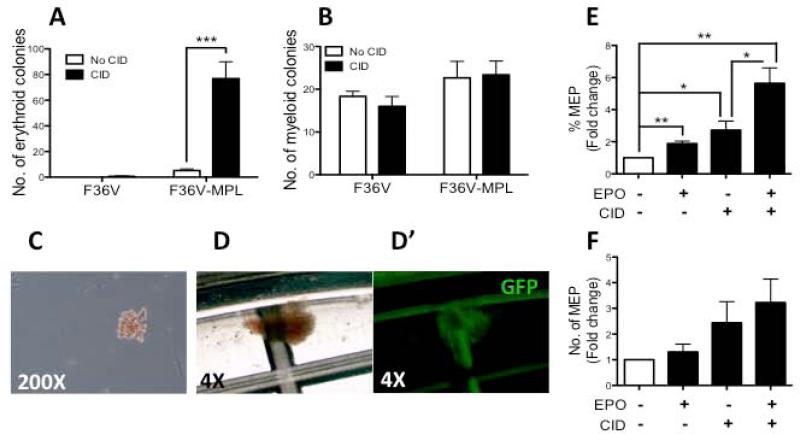
To determine if ic-MPL dimerization during hESC differentiation caused an increase in the production of clonogenic hematopoietic progenitors, cells from day 14 stromal co-cultures (cultured ±CID; Supplementary Figure 2A) were re-plated onto a standard CFU assay (without CID). Cultures that were initially treated with CID prior to CFU plating generated 10-fold more erythroid colonies (BFU-E and CFU-E) compared to untreated controls (P=0.0007; n=15; Figure 2A). In contrast, the addition of CID did not affect the generation of clonogenic myeloid progenitors (Figure 2B), suggesting that the effect of ic-MPL signaling was specific to the erythroid lineage.
Figure 2. Dimerization of ic-MPL induces the generation of erythroid progenitors.
H1 hESCs were transduced with F36V-MPL or F36V (control vector) and cultured on OP9 stroma for 14 days with or without CID and/or EPO. After 14 days of culture, the cells were counted and (A-D’) replated in methylcellulose (with no CID) to measure CFU or (E,F) analyzed by FACS. A) number of erythroid colonies (BFU-E and CFU-E) and B) myeloid colonies (CFU-GM and CFU-M) per 150,000 cells (n=15 experiments;). C) CFU-E and D) BFU-E were both scored as ‘erythroid colonies’ in A. D’) shows GFP expression of BFU-E shown in D. E, F) Summary of FACS data (n=3 experiments), shown as E) frequency of MEP (%GlyA+CD42a/CD42a+) and F) number of MEP; Fold increase is relative to no agent control. *P<0.05, **P<0.01, ***P<0.001.
To further explore the effect of ic-MPL dimerization on progenitor development, Day 14 cultures (Supplementary Figure 2A) were analyzed by FACS for the presence of bipotent megakaryocyte-erythroid progenitors (MEP) previously described as cells that co-express CD41a and GlyA along with CD34 and CD43.[10] Both CID and EPO induced MEP production, and combined treatment further significantly enhanced MEP output (P=0.003; n=4) (Figure 2E,F; Supplementary Figure 5A). The majority of MEP also expressed CD34 (Supplementary Figure 5B-C). These data indicate that ic-MPL dimerization specifically induces the generation of early CD34+ clonogenic progenitors with erythroid potential from hESC.
Dimerization of ic-MPL in MEP induces greater terminal erythroid proliferation and differentiation than EPO
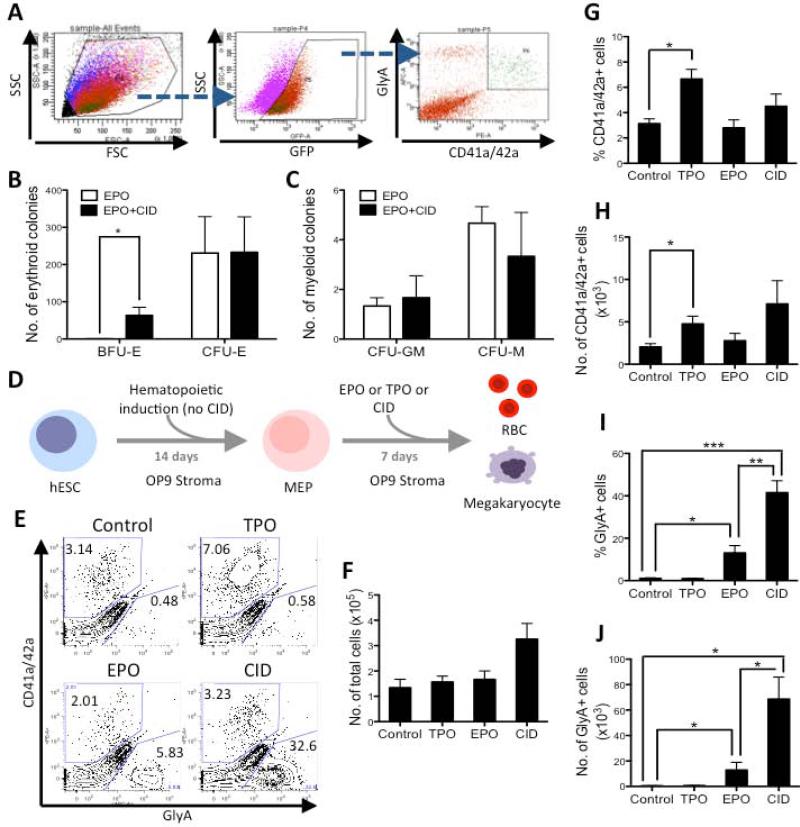
The effect of ic-MPL dimerization on differentiation downstream from the MEP stage was next assessed. All MEP were generated from hESC in the same conditions on OP9 stroma (in the absence of CID) (Supplementary Figure 2B). At Day 14 MEP were isolated by FACS (Figure 3A) and plated into CFU assay containing either EPO or EPO and CID. As expected, MEP generated mostly erythroid colonies in both conditions; however combined EPO and CID stimulation of MEP generated larger colonies (typical of BFU-E), whereas only small CFU-E were generated in the presence of EPO alone (P=0.0462, n=3; Figure 3B). The small number of myeloid colonies (CFU-GM, and CFU-M) generated from MEP was no different between the EPO and CID cultures (Figure 3C) further indicating that ic-MPL induces a strong and specific erythroid effect from the MEP stage.
Figure 3. Dimerization of ic-MPL in MEP induces robust erythroid differentiation.
Hematopoietic differentiation was induced from F36V-MPL transduced hESC on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and then Day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO (schema Supplementary Figure 2B). A) GFP+ MEP were isolated by FACS on day 14 and cultured in B,C) CFU assay containing EPO ± CID for 14 days or D-J) on OP9 stroma for a further 7 days (i.e. total 21 days of culture) in the presence of either TPO, EPO, CID or no agent (Control) and then analyzed by FACS. A) Representative FACS sort gating for isolation of Day 14 MEP (GFP+ MEP are shown as green events in back-gating to FSC/SSC panel). B) Number of erythroid colonies (BFU-E and CFU-E) and C) myeloid colonies per dish per 10,000 cells plated are shown (n=3 experiments; *P<0.05). D) Schema of experimental setup for E-J. E) Representative FACS analysis Day 21. F) Total number of DAPI-GFP+ cells from Day 21 culture (n=3 experiments). G-J) Summary of FACS data at Day 21 shown as: G,H) % and number of megakaryocytes (GlyA-CD41a/CD42a+); I,J) % and number of erythroid (GlyA+CD41a/42a-) cells (n=3); *P<0.05, **P<0.01, ***P<0.001.
To further delineate the lineage specific differentiation induced by ic-MPL dimerization, MEP were again generated on OP9 stroma in the absence of CID (Supplementary Figure 2B), isolated at Day 14 by FACS and differentiated in the presence or absence of either Thrombopoietin (TPO), EPO, or CID (Figure 3D). Total cell number tended to be higher in CID than either EPO or TPO (Figure 3F). As expected, TPO generated significantly more megakarocytes (P=0.014; n=3; Figure 3E,G,H) whereas EPO induced more erythroid cells (P=0.026; n=3; Figure 3E,I,J) from MEP than non-treated controls, consistent with the lineage specificity of these cytokines and demonstrating the bipotency of the MEP population. Notably, CID induced a ~40-fold increase in erythroid differentiation from MEP compared to non-treated controls (P=0.0004; n=3; Figure 3I). Megakaryocyte frequency and number also tended to be greater with CID, although the increase was not statistically significant (Figure 3G,H). Thus dimerization of ic-MPL produced qualitatively different effects than stimulation of full-length MPL with its natural ligand TPO, primarily inducing erythroid rather than megakaryocytic differentiation.
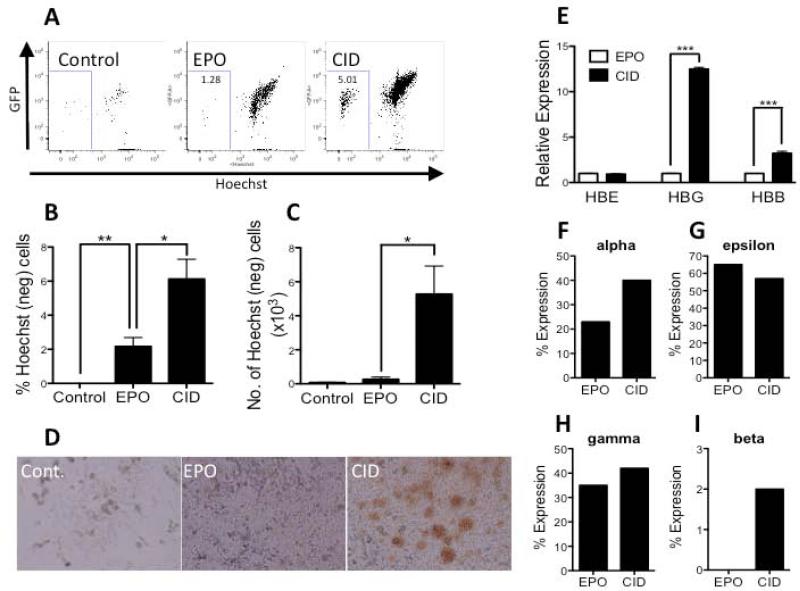
Next, the ability of CID and EPO to drive terminal erythroid differentiation from MEP was compared (Supplementary Figure 2B). Although the overall number of enucleated cells remained low in both conditions, CID treatment generated a 4-fold greater number of enucleated RBCs than EPO (n=4; Figure 4A-C, Supplementary Figure 6) and also produced more visually prominent hemoglobinization (Figure 4D). Additional experiments performed in the absence of serum confirmed that erythroid differentiation from MEP induced by ic-MPL dimerization was EPO-independent (Supplementary Figure 7).
Figure 4. Dimerization of ic-MPL induces more efficient erythroid maturation than erythropoietin.
A-C) Erythroid enucleation, D) hemoglobinization, and E-I) Globin expression were analyzed after differentiation of isolated MEP in either EPO, CID or neither (Control). MEP were initially generated after 14 days without CID and then cultured on OP9 stroma in the three conditions shown for a further 7 days (see schema Fig 3D). A) Representative FACS analysis of Hoechst and GFP expression to assess enucleation of erythroid cells (gated from GlyA+ gated cells). B,C) Summary of FACS data measuring enucleated erythroid cells (% Hoechstneg cells within GlyA+ gate) (n=4). D) Light microscopy of Day 21 stromal co-culture showing large, hemoglobinized clones only in CID culture. E) Relative mRNA of globins HBE (embryonic), HBG (fetal), and HBB (adult) by q-PCR. β-actin was used as a housekeeping gene (n=3). HPLC results shown as F) % alpha-globin expression relative to total globin (i.e. beta, alpha, gamma, and epsilon) and G-I) % expression from beta-locus (beta, gamma, epsilon). *P<0.05, **P<0.01, ***P<0.001
RBCs generated in the presence of CID also gave higher expression of both gamma (fetal) and beta (adult) globin at the transcriptional level than those generated with EPO (Figure 4E). Globin protein analysis by HPLC[11] demonstrated an increase in alpha-globin expression with CID treatment compared to EPO (Figure 4F). Also, although no detectable beta-globin expression was observed with EPO, CID treatment induced the expression of low levels of beta-globin and increased the gamma:epsilon globin ratio, suggesting a maturational shift in erythropoiesis (Figure 4G-I). Thus, MPL dimerization is more potent than EPO in the production of erythroid cells and in inducing terminal erythroid differentiation.
Dimerization of ic-MPL requires AKT signaling for erythroid induction
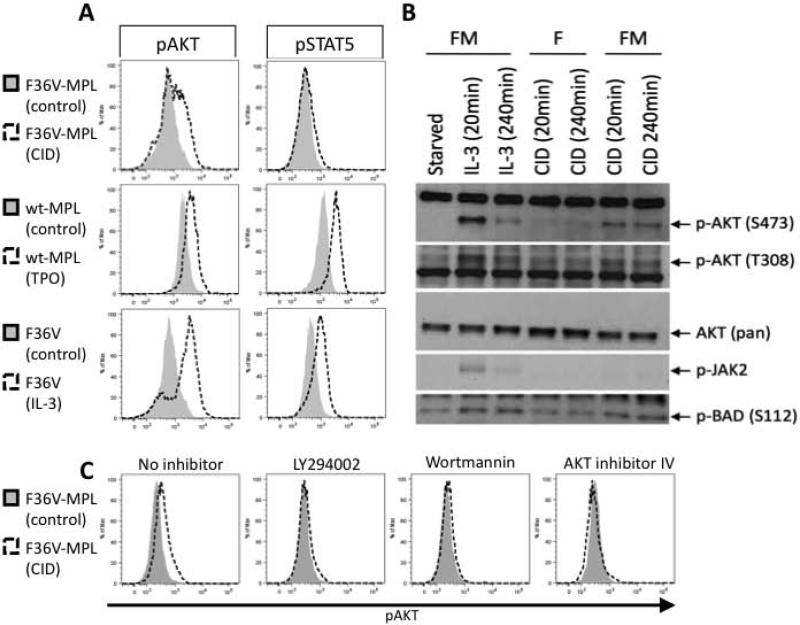
To compare the signaling mechanism(s) activated by ic-MPL dimerization with those in which TPO stimulates wildtype full length MPL, phosphoflow and western blot analysis was performed on Ba/F3 cells, an IL-3 dependent leukemia cell line, transduced with either F36V (control vector), F36V-MPL or wild type full length MPL. IL-3 independent growth/survival was achieved with ic-MPL dimerization in cells expressing F36V-MPL but not the control F36V (data not shown) indicating the ligand specificity and functionality of the system. As expected, TPO stimulation of Ba/F3 cells that expressed wild type MPL induced phosphorylation of both AKT and STAT5 (Figure 5A), as well as p38, and ERK-MAPK (Supplementary Figure 8).[8] Interestingly, unlike the full-length MPL receptor, ic-MPL dimerization with CID activated only the AKT pathway; no phosphorylation of STAT5, JAK2, (Figure 5A,B), ERK1/2 or p38 (Supplementary Figure 8) was detected. ic-MPL dimerization induced phosphorylation of BAD (Figure 5B; Supplementary Figure 9), a pro-apoptotic protein which is a downstream target of AKT signaling [15, 16]. In the presence of CID, AKT phosphorylation was blocked with PI3K inhibitors (LY294002 and Wortmannin) and an AKT specific inhibitor (AKT inhibitor IV) (Figure 5C).
Figure 5. Dimerization of ic-MPL activates AKT but not JAK2/STAT5 signaling.
Signal transduction analysis of Ba/F3 cells expressing F36V-MPL (FM), wild-type (full length) human MPL (wt-MPL), or F36V (F) transgenes. Cells were cytokine-starved overnight prior to stimulation with IL-3, TPO, CID or no agent (control), after which the cells were processed for A,C) phosphoflow and B) western blot analysis. A) Representative FACS analysis of pAKT (S473) and pSTAT5. The time point of maximal activation is shown for each agent: CID 60min, TPO 60min, and IL-3 30min. B) Representative Western Blot analysis of pAKT (S473 and T308 residues), pan-AKT (control), pJAK2, and pBAD (S112). F36V-MPL (FM); F36V (F). C) Cells were stimulated +/− CID for 60min with or without inhibitors as shown. Representative FACS analysis for pAKT (S473) is shown.
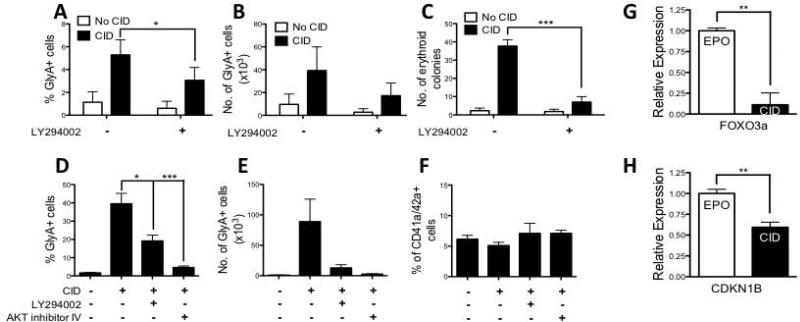
The relevance of AKT signaling was next explored in hESC-derived erythropoiesis. Treatment with the PI3K inhibitor LY294002, during 14 days of CID stimulation on OP9 stroma (Supplementary Figure 2A) significantly reduced the frequency and number of GlyA+ cells (P=0.02; n=5; Figure 6A,B) and clonogenic erythroid progenitors in CFU assay (P<0.0001; n=6; Figure 6C) produced from hESC. PI3K inhibition also significantly reduced the number of GlyA+ cells generated from isolated MEP (P=0.021; n=4; Figure 6D,E) as did treatment using AKT inhibitor IV (P=0.0009; n=4; Figure 6D,E). In the same conditions, the frequency of CD41a/CD42a+ megakaryocytic cells did not change, indicating that AKT inhibition specifically inhibited erythropoiesis (Figure 6F). As further evidence that JAK2/STAT5 signaling is not required for ic-MPL induced erythropoiesis, treatment with the JAK2 inhibitor, AG490, reduced megakaryocyte output in the presence of TPO, but had no effect on GlyA+ output in the presence of CID or EPO (Supplementary Figure 10). Furthermore, treatment with CID caused a significant decrease in expression of FOXO3a, a member of the forkhead family of transcription factors, which is known to be deactivated and downregulated by AKT signaling at the protein level and RNA level, and which induces a pro-apoptotic gene network (Figure 6G).[17, 18] CDKN1B, a downstream target that is upregulated by FOXO3a, was also decreased upon treatment with CID (Figure 6H).[17, 18] These results indicate that AKT activation is required for the erythropoiesis induced in hESC by ic-MPL dimerization while JAK2/STAT5 is not.
Figure 6. Induction of erythropoiesis from hESC by ic-MPL dimerization requires PI3K/AKT activation.
A-C) F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium from Day 0-14 with or without CID (no cytokines added). From Day 7-14, cells were also treated ± PI3K inhibitor (LY294002). Day 14 cells were processed for A,B) FACS analysis and C) CFU assay. A,B) %GlyA+ and number GlyA+ cells, respectively (n=5). C) Total number of erythroid colonies per 150,000 cells plated (n=6). D-F) Day 14 MEP generated from F36V-MPL transduced hESC were isolated by FACS for a further 7 days of culture (i.e. total 21 days of culture) in the presence of SCF, Flt-3L, IL-3, TPO, ± CID along with either LY294002 or AKT inhibitor IV and then analyzed at Day 21 by FACS (n=4). D) % and E) number of erythroid cells (GlyA+CD41a/CD42a-); F) % megakaryocytes (GlyA-CD41a/42a+). G,H) F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium Day 0-14 without morphogens or cytokines, and then in liquid expansion culture Day 14-28 (see Supplementary Figure 2C). Relative gene expression in total Day 28 cultures shown by q-PCR in CID normalized to EPO. β-actin was used as a housekeeping gene. *P<0.05, **P<0.01. ***P<0.001.
ic-MPL induced erythropoiesis is mediated through modulation of cell cycle and survival
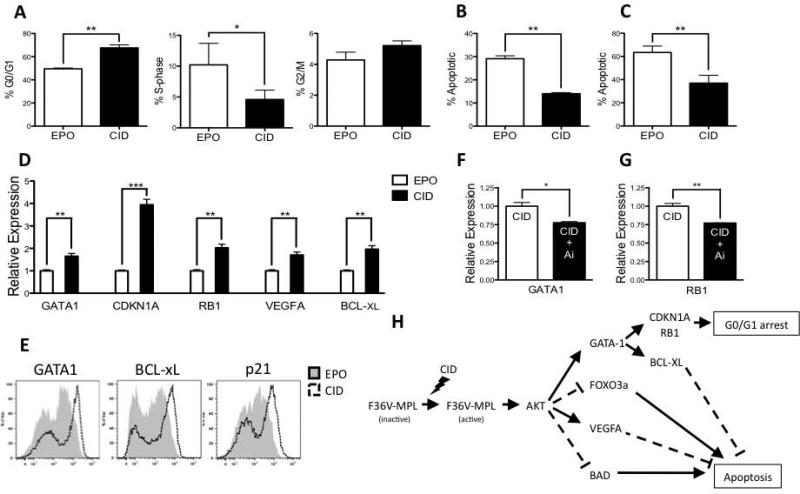
AKT is a key downstream effector of PI3K and plays a major role in mediating cell cycle, survival, and differentiation.[19] To assess the effect of ic-MPL dimerization on apoptosis and cell cycle of erythroid cells, GlyA+CD71+ erythroblasts were generated from hESC for analysis using a combined stroma and suspension 2 step protocol (See Methods and Supplementary Figure 2C).[11] A significantly higher frequency of CID treated erythroblasts were in G0/G1 (P=0.007, n=4) and correspondingly fewer cells were in S-phase (P=0.017, n=4) compared to EPO treatment (Figure 7A; Supplementary Figure 11). The expression of the cell cycle regulators, CDKN1A (both mRNA and its protein p21) [20] and RB1,[21, 22] was also upregulated with CID treatment compared to EPO (Figure 7D,E).
Figure 7. ic-MPL induced erythropoiesis is mediated through modulation of cell cycle and survival.
A-E) F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium Day 0-14 without morphogens or cytokines, and then in liquid expansion culture Day 14-28 (see Supplementary Figure 2C), and analyzed at Day 28. A) Cell cycle analysis: G0/G1 (BrdUneg7AADdim), S-phase (BrdUpos7AADdim), and G2/M cells (BrdUneg7AADhi). B) % Apoptosis defined as BrdUneg7AADneg. C) % Apoptosis based on Annexin V and PI (n=4). D) Relative expression of genes shown by q-PCR in CID normalized to EPO. β-actin was used as a housekeeping gene. E) Representative intracellular flow analysis of GATA1, BCL-xL, and p21 (CDKN1A) expression in EPO (gray) or CID (open). F,G) Day 28 cells were further cultured in the same medium (as in Day 14-28 culture) for 7 days with or without the addition of AKT inhibitor IV prior to q-PCR analysis. H) Proposed model of ic-MPL induced erythropoiesis mediated through AKT signaling. *P<0.05, **P<0.01 ***P<0.001.
Apoptosis in CID treated erythroblasts was significantly reduced (P=0.005, n=4, Figure 7B; P=0.003, n=4, Figure 7C) compared to EPO treatment. Consistent with reduced apoptosis, CID stimulation phosphorylated BAD[15] (Figure 5B; Supplementary Figure 9) and upregulated expression of BCL-xL (P=0.004, n=3) and VEGFA (P=0.008, n=3) (Figure 7D), both of which have been shown to inhibit apoptosis.[23-26] BCL-xL protein levels were also higher in CID treatment compared to EPO (Figure 7E).
CID treatment also upregulated GATA1 expression to significantly higher levels than EPO treatment (Figure 7D,E), and addition of AKT Inhibitor IV caused a reduction in CID induced GATA1 and RB1 expression (Figure 7F,G). GATA1, which is a key regulator of erythroid development essential for the survival of erythroid precursors and their terminal differentiation, is targeted by PI3K/AKT for phosphorylation and activation.[19] Taken together, these results strongly suggest that ic-MPL regulates cell cycle and survival during hESC-derived erythropoiesis through multiple pathways that are dependent on AKT activation (Figure 7H).
DISCUSSION
In these studies we have shown that ic-MPL dimerization during hESC differentiation induces the generation of MEP and their erythroid progeny in an EPO-independent manner. Unlike either TPO or EPO stimulation, ic-MPL dimerization induces activation of the AKT signaling pathway, without concurrent activation of JAK2/STAT5 signaling. Furthermore, we show that ic-MPL dimerization activates a network of genes associated with the regulation of cell cycle, apoptosis, and erythroid differentiation, with a corresponding reduction in both apoptosis and cell cycle progression. The consequence of these combined events is to promote RBC production and maturation from hESC significantly more efficiently than EPO.
EPO induced erythropoiesis from hESC is believed to resemble primitive (embryonic yolk sac) rather than definitive (fetal and adult) erythropoiesis, with predominant expression of embryonic globins in most conditions. The majority of erythroid colonies produced by EPO comprise small CFU-E rather than the larger BFU-E and consist of few if any mature enucleated RBCs.[10, 27] In our studies, MEPs stimulated by ic-MPL dimerization generated large numbers of BFU-Es, and increased the production of enucleated RBCs relative to EPO stimulation, suggesting that ic-MPL induces a shift toward a more mature, definitive state of erythropoiesis. This hypothesis is further strengthened by an increase in fetal (gamma) globin production relative to embryonic (epsilon) globin production, and the detection of adult beta-globin exclusively in CID stimulated cells. The ability of ic-MPL to increase both output and maturation of erythroid cells provides a promising step toward the ultimate goal of generating therapeutically relevant RBCs for transfusion.
Although activation of full length MPL by its ligand TPO has been clearly demonstrated to induce megakaryocytopoiesis, several studies have suggested that TPO also enhances erythropoiesis.[28-32] However, in all but one of these studies,[32] hematopoietic progenitors were stimulated with TPO in the presence of EPO and/or serum (which contains EPO). We and others have found that TPO alone does not stimulate human erythropoiesis in conditions that lack EPO [5, 33] whereas ic-MPL dimerization with CID alone is sufficient for erythropoietic induction. Consistent with this dichotomy, we find in this current study that CID induced ic-MPL dimerization does not activate the same set of signaling pathways normally activated by TPO signaling; instead of activating MAPK, JAK/STAT, and AKT signaling, only AKT signaling was detectable with F36V-MPL dimerization. F36V-MPL dimerization in CD34+ cord blood progenitors also induced gene expression profiles distinct from stimulation with TPO, activating genes associated with erythropoiesis.[5] These observations further highlight the distinctness and novelty of ic-MPL signaling.
A fusion protein consisting of the intracellular and transmembrane domain of MPL and the extracellular domain of the Prolactin receptor (PRL-R) has been shown to induce hematopoiesis from murine embryonic stem cells (mESC).[31] In contrast to our studies, the effect of the PRL-R fusion protein was not erythroid specific, as output of both myeloid and erythroid progenitors was increased; the previous study did not report any effect on erythroid maturation[31]. A notable difference between the PRL-R and ic-MPL systems is in the method by which MPL is activated, i.e. induction of homodimerization through ligand binding to the extracellular PRL-R in contrast to direct homodimerization of the intracellular domain of MPL.
TPO and EPO receptors are both composed of extracellular, transmembrane, and intracellular domains, each component contributing to the ultimate conformational arrangement of the receptor.[34] For example, upon activation and dimerization of the EPO receptor (EPO-R), the two extracellular domains of EPO-R induce conformational changes that are transmitted through the transmembrane domain to the intracellular domain which then mediates various signaling pathways.[35] Alterations in the transmembrane or extracellular conformation of EPO-R or replacement with a synthetic extracellular domain has been shown to confer differences in downstream signaling pathway activation.[35, 36] Similarly, changes in tryptophan 515 in the intracellular juxtamembrane boundary of the MPL receptor was shown to influence the angle and dimerization of the transmembrane helix ultimately affecting receptor activation and signal transduction in different ways depending on the amino acid substitution.[37] We postulate that direct dimerization of the intracellular fragment of MPL through the binding of CID to F36V-MPL (which lacks the extracellular and transmembrane domain) results in a conformational arrangement of the signaling domain that is distinct from that induced when activation of the transmembrane and intracellular domains of MPL occurs after ligand binding to an extracellular receptor. These conformational changes still allow AKT activation, but in the context of absent or severely reduced JAK2/STAT5 activation produce a unique pattern of gene expression[5] and cellular function.
JAK2/STAT5 signaling is activated by both EPO and TPO stimulation of their respective receptors[38, 39] and is essential for TPO mediated megakaryocytopoiesis.[40] The lack of JAK2/STAT5 in F36V-MPL signaling provides a likely explanation as to why CID induced MPL activation had little effect on megakarocyte differentiation in our studies. Although CID did induce a moderate increase in total megakaryocyte output from MEP, this was primarily due to an overall increase in cell numbers rather than a specific megakaryoctye specific effect by ic-MPL dimerization. Notably, EPO mediated JAK2/STAT5 activity appears to be required primarily for stress induced erythropoiesis as erythroid differentiation was seen in STAT5a−/−5b−/− mice during normal homeostasis.[39, 41, 42] Contrary to JAK2/STAT5, the PI3K/AKT pathway is critical for erythropoiesis[43, 44], mediating erythroid-cell maturation of JAK2-deficient murine fetal liver progenitor cells.[45]
ic-MPL dimerization resulted in upregulation of GATA-1, a key regulator of erythroid development which is essential for terminal differentiation, including globin gene expression[46]. PI3K/AKT stimulates the phosphorylation and activation of GATA1[19], and activated GATA-1 upregulates its own gene expression through positive feedback regulation[47]. Thus erythroid maturation produced by ic-MPL induced AKT signaling is likely to at least be partly mediated by both GATA-1 activation and up-regulation. GATA1 has also been shown to upregulate the expression of CDKN1A and RB1 which are cell cycle inhibitors inducing G0/G1 arrest.[20] RB1 is required for erythroid differentiation in a cell intrinsic manner and forms a complex with GATA-1 to stall cell proliferation and steer erythroid precursors towards terminal differentiation,[21, 22] highlighting the importance of cell cycle regulation in erythroid differentiation. Along with regulation of cell cycle during erythropoiesis, GATA1 has also been shown to be critical for the survival of erythroid precursors, primarily by the upregulation of the anti-apoptotic factor BCL-xL.[23] In our studies, expression of the genes associated with the AKT/GATA-1 dependent cell cycle/survival network was significantly greater upon ic-MPL dimerization than with EPO. Notably, VEGF was also upregulated by ic-MPL in both hESC in the current studies and our previous report using cord blood progenitors.[5] VEGF is also upregulated by AKT signaling and has also been implicated as an anti-apoptotic factor for erythroid progenitors.[25, 26] Along with the upregulation of anti-apoptotic factors, the pro-apoptotic factor, FOXO3a,[17, 18] was observed to be decreased with ic-MPL dimerization. FOXO3a is inhibited at the protein level by AKT signaling, and has been shown to regulate its own expression through a positive feed-back mechanism further indicating an anti-apoptotic role for ic-MPL through AKT signaling.[17, 18] Thus, the upregulation of these multiple AKT-dependent pathways, along with a corresponding reduction in cell cycle and apoptosis, demonstrates a central role for AKT regulated cell cycle/survival mechanisms in the erythropoiesis induced from hESC by ic-MPL dimerization.
SUMMARY
In summary, we have identified a novel EPO-independent approach that is not only more efficient at erythropoiesis but is also able to augment EPO induced erythropoiesis. The key role for AKT signaling in the production and maturation of erythroid cells highlights the importance of these pathways for further study as targets to enhance erythropoiesis from hESC.
Supplementary Material
Supplementary Figure 1. Generation of stably transduced H1 hESC line expressing F36V-MPL. H1 hESCs were transduced with F36V-MPL and isolated by FACS based on FSC/SSC, SSEA-4+, GFP+, mCD29-, DAPI- gating and subsequently cultured to establish a stably expressing line maintaining ~90% GFP+ expression. A) Schematic of F36V-MPL vector expressing transgenes with the ubiquitin (UbC) promoter. B) Representative immunophenotype analysis of GFP and mCD29 expression to assess expression of vector in human mCD29-cells through continual cell passaging (25 passages from point of transduction). C) Light microscope picture of a stably F36V-MPL transduced hESC colony expressing GFP.
Supplementary Figure 2. General schematic of culture conditions utilized. A) H1 hESCs were transduced with F36V-MPL or F36V (control vector) and cultured on murine OP9 stroma for 14 days with or without CID or EPO (no other cytokines added) prior to analysis. B) To optimize production of MEP, hematopoietic differentiation was induced from F36V-MPL transduced hESC on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and then Day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS on day 14 for use in further analysis. C) To induce erythroblast differentiation, F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium Day 0-14, in serum but without morphogens or cytokines, and then in two step serum-free liquid expansion culture Day 14-28 in EPO or CID. Day 28 cells were used for further analysis.
Supplementary Figure 3. ic-MPL induced erythropoiesis from undifferentiated hESC is serum independent. Hematopoietic differentiation was induced from F36V-MPL transduced hESC by culturing on OP9 stroma Day 0-2 in STEMSPAN (serum-free) supplemented with BMP4, VEGF, and FGF and then from Day 3-14 in SCF, Flt-3L, IL-3, and TPO +/− EPO and/or CID. A) Schematic of culture conditions utilized. B) Immunophenotype analysis of GlyA expression.
Supplementary Figure 4. Dimerization of ic-MPL increases generation of early hematopoietic cells but not endothelial cells. H1 hESCs transduced with F36V-MPL were cultured on OP9 stroma for 14 days with or without CID (no cytokines added), and then counted and analyzed by FACS. Summary of Immunophenotype data shown as: A) % and number of CD43+ cells (n=3); B) % and number of VE-cadherin (VE-CAD)+ cells (n=5); C) % and number of CD31+ cells (n=3). % for each represents the frequency from all GFP+ cells. *P<0.05.
Supplementary Figure 5. ic-MPL dimerization induces the generation of CD34+ MEP. A) F36V-MPL transduced hESC were cultured on OP9 stroma for 14 days with or without EPO or CID (no cytokines). After 14 days of culture, the cells were counted and analyzed by FACS. A) Representative FACS analysis of CD41a/42a and GlyA expression performed on F36V-MPL transduced cells. B-C) F36V-MPL transduced hESC cultured on OP9 stroma were cultured in BMP4, VEGF, and FGF (Day 0-2) and SCF, Flt-3L, IL-3, TPO, and EPO (Day 3-14) prior to flow cytometry analysis. B) Immunophenotype analysis of GlyA and CD41a/42a expression performed on F36V-MPL transduced cells. C) Immunophenotype analysis for CD34 expression of cells in MEP and Eryth gates shown in B.
Supplementary Figure 6. Dimerization of ic-MPL induces hemoglobinized nucleated and enucleated RBC. Hematopoietic differentiation was induced from F36V-MPL transduced hESC on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and then Day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS on day 14 and cultured on OP9 stroma for a further 7 days (i.e. total 21 days of culture) in the presence of CID. After 21 days of culture, cells were sorted based on GFP and GlyA expression and stained with Wright-Giemsa and 3’3-diaminobenzidine. Shown are 4 different slides of GFP+GyA+ sorted cells all generated from MEP in the presence of CID (all 40x magnification). White arrows mark enucleated hemoglobinized erythrocytes.
Supplementary Figure 7. ic-MPL induced erythroid maturation from MEP is serum and EPO independent. Hematopoietic differentiation was induced from F36V-MPL transduced hESC by culturing on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS sorting on day 14 (see Figure 3) and re-cultured on retronectin with STEMSPAN (serum-free) medium +/− EPO or CID for 10 more days prior to analysis. A) Light microscope picture of EPO vs CID after 10 days of culture on retronectin with STEMSPAN. B) Immunophenotype analysis of GlyA and CD41a/42a expression. C) Immunophenotype analysis for Hoechst staining of cells in GlyA+ gate.
Supplementary Figure 8. Dimerization of intracellular cMPL does not activate p38 and ERK-MAPK. Ba/F3 cells were transduced with F36V-MPL (FM) or control vector expressing wild-type (full length) human MPL (Mpl) and isolated based on GFP expression. Cells were then cytokine starved overnight prior to stimulating with TPO or CID after which the cells were processed for flow analysis. Representative FACS analysis of pERK1/2 and p-p38.
Supplementary Figure 9. Quantification of Western Blot Analysis for BAD phosphorylation. Western blot analysis of BAD phosphorylation from Figure 5B was quantified by densitometry using ImageJ software. pan-AKT was used as the loading control. F36V-MPL (FM); F36V (F).
Supplementary Figure 10. Induction of erythropoiesis from hESC by ic-MPL dimerization does not require JAK2 activity. A-C) Day 14 MEP generated from F36V-MPL transduced hESC were isolated by FACS sorting for a further 7 days of culture (i.e. total 21 days of culture) in the presence of SCF, Flt-3L, IL-3, TPO, ± CID along with the JAK2 inhibitor AG490 and then analyzed by FACS. A) Summary of Day 21 FACS data shown as: B) % of erythroid cells (GlyA+CD41a/CD42a-); C) % megakaryocytes (GlyA-CD41a/42a+).
Supplementary Figure 11. FACS plot for BrdU/7-AAD staining. F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium for 14 days without that addition of any morphogens or cytokines. The cells were then dissociated and replated in liquid culture in the presence of Flt-3L, SCF, BMP-4, IL-3 and EPO or CID for 7 days and then transferred to IGF-1, SCF, BMP-4, IL-3 and EPO or CID for another 7 days prior to analysis. Representative FACS analysis of BrdU and 7-AAD staining to assess cell cycle. Apoptotic (BrdUneg7AADneg), G0/G1 (BrdUneg7AADdim), S-phase (BrdUpos7AADdim), and G2/M cells (BrdUneg7AADhi). Summary of this data is shown in Figure 7A, B
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (PO1-HL073104) (GMC and DBK), the California Institute for Regenerative Medicine (CIRM) (RC1-00108) (GMC) and the Broad Stem Cell Research Center (BSCRC) at UCLA. W.S.K. was the recipient of a CIRM Training Grant (TG2-01169), BSCRC Fellowship in Stem Cell Science, and the National Institutes of Health Training in Developmental Hematology Award (T32 HL086345). We thank Felicia Codrea and Jessica Scholes (UCLA BSCRC Flow Cytometry core) for assistance with flow cytometry, Shantha Seenadheera for assistance with viral vectors, and the UCLA Jonsson Comprehensive Cancer Center Shared Translational Pathology Core Laboratory.
We thank ARIAD Pharmaceutical, Cambridge, MA, for providing CID. This work was supported by the National Institutes of Health (PO1-HL073104) (GMC and DBK), the California Institute for Regenerative Medicine (CIRM) (RC1-00108) (GMC) and the Broad Stem Cell Research Center (BSCRC) at UCLA. W.S.K. is the recipient of a CIRM Training Grant (TG2-01169), BSCRC Fellowship in Stem Cell Science, and the National Institutes of Health Training in Developmental Hematology Award (T32 HL086345).
Footnotes
Authorship Contributions
William Kim: Conception and design, collection and assembly of data (performance of experiments), data analysis and interpretations, manuscript writing
Yuhua Zhu: Collection and assembly of data (assisted in performance of cell culture experiments)
Qiming Deng: Collection and assembly of data (western blot)
Chi Jia Chen: Collection and assembly of data (assisted in performance of cell culture experiments)
Chong Bin He: Collection and assembly of data (assisted in performance of cell culture experiments)
Amanda Grieco: Collection and assembly of data (Globin HPLC)
Gautam Dravid: Conception and design
Chintan Parekh: Conception and design, manuscript writing
Roger Hollis: Produced viral vectors
Timothy Lane: Collection and assembly of data (western blot)
Eric Bouhassira: Collection and assembly of data (Globin HPLC), manuscript writing
Donald Kohn: Produced viral vectors, manuscript writing
Gay Crooks: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Carson J, Grossman B, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB. Annals of Internal Medicine. 2012;157:49–U95. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 2.Olivier E, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Experimental Hematology. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica-the Hematology Journal. 2010;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendall RG. Erythropoietin. Clin Lab Haematol. 2001;23:71–80. doi: 10.1046/j.1365-2257.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- 5.Parekh C, Sahaghian A, Kim W, et al. Novel Pathways to Erythropoiesis Induced by Dimerization of Intracellular C-Mpl in Human Hematopoietic Progenitors. Stem Cells. 2012;30:697–708. doi: 10.1002/stem.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard RE, Wood B, Zeng H, et al. Expansion of genetically modified primary human hemopoietic cells using chemical inducers of dimerization. Blood. 2000;95:430–436. [PubMed] [Google Scholar]
- 7.Abdel-Azim H, Zhu Y, Hollis R, et al. Expansion of multipotent and lymphoid-committed human progenitors through intraceflular dimerization of Mpl. Blood. 2008;111:4064–4074. doi: 10.1182/blood-2007-08-107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 10.Klimchenko O, Mori M, Distefano A, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabry ME, Bouhassira EE, Suzuka SM, et al. Transgenic mice and hemoglobinopathies. Methods Mol Med. 2003;82:213–241. doi: 10.1385/1-59259-373-9:213. [DOI] [PubMed] [Google Scholar]
- 13.Schmittgen T, Livak K. Analyzing real-time PCR data by the comparative C-T method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 14.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 16.del Peso L, González-García M, Page C, et al. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 17.Essaghir A, Dif N, Marbehant CY, et al. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284:10334–10342. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Tang N, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Kitidis C, Fleming MD, et al. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rylski M, Welch JJ, Chen YY, et al. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol. 2003;23:5031–5042. doi: 10.1128/MCB.23.14.5031-5042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark AJ, Doyle KM, Humbert PO. Cell-intrinsic requirement for pRb in erythropoiesis. Blood. 2004;104:1324–1326. doi: 10.1182/blood-2004-02-0618. [DOI] [PubMed] [Google Scholar]
- 22.Kadri Z, Shimizu R, Ohneda O, et al. Direct binding of pRb/E2F-2 to GATA-1 regulates maturation and terminal cell division during erythropoiesis. PLoS Biol. 2009;7:e1000123. doi: 10.1371/journal.pbio.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory T, Yu C, Ma A, et al. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- 24.Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med (Berl) 2003;81:20–31. doi: 10.1007/s00109-002-0397-4. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Sawai H, Ochi N, et al. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161–171. doi: 10.1007/s11010-009-0154-x. [DOI] [PubMed] [Google Scholar]
- 26.Martin R, Lahlil R, Damert A, et al. SCL interacts with VEGF to suppress apoptosis at the onset of hematopoiesis. Development. 2004;131:693–702. doi: 10.1242/dev.00968. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M, Laver JH, Kato T, et al. Recombinant human thrombopoietin (Mpl ligand) enhances proliferation of erythroid progenitors. Blood. 1995;86:2494–2499. [PubMed] [Google Scholar]
- 29.Kaushansky K, Broudy VC, Grossmann A, et al. Thrombopoietin expands erythroid progenitors, increases red cell production, and enhances erythroid recovery after myelosuppressive therapy. J Clin Invest. 1995;96:1683–1687. doi: 10.1172/JCI118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratajczak MZ, Ratajczak J, Marlicz W, et al. Recombinant human thrombopoietin (TPO) stimulates erythropoiesis by inhibiting erythroid progenitor cell apoptosis. Br J Haematol. 1997;98:8–17. doi: 10.1046/j.1365-2141.1997.1802997.x. [DOI] [PubMed] [Google Scholar]
- 31.Challier C, Cocault L, Berthier R, et al. The cytoplasmic domain of Mpl receptor transduces exclusive signals in embryonic and fetal hematopoietic cells. Blood. 2002;100:2063–2070. [PubMed] [Google Scholar]
- 32.Kieran MW, Perkins AC, Orkin SH, et al. Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc Natl Acad Sci U S A. 1996;93:9126–9131. doi: 10.1073/pnas.93.17.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Wang M, Tang DC, et al. Thrombopoietin has a differentiative effect on late-stage human erythropoiesis. Br J Haematol. 1999;105:459–469. [PubMed] [Google Scholar]
- 34.Kaushansky K. Molecular mechanisms of thrombopoietin signaling. J Thromb Haemost. 2009;7(Suppl 1):235–238. doi: 10.1111/j.1538-7836.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- 35.Kubatzky KF, Liu W, Goldgraben K, et al. Structural requirements of the extracellular to transmembrane domain junction for erythropoietin receptor function. J Biol Chem. 2005;280:14844–14854. doi: 10.1074/jbc.M411251200. [DOI] [PubMed] [Google Scholar]
- 36.Constantinescu SN, Huang LJ, Nam H, et al. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol Cell. 2001;7:377–385. doi: 10.1016/s1097-2765(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 37.Defour JP, Itaya M, Gryshkova V, et al. Tryptophan at the transmembranecytosolic junction modulates thrombopoietin receptor dimerization and activation. Proc Natl Acad Sci U S A. 2013;110:2540–2545. doi: 10.1073/pnas.1211560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darnell JE. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 39.Watowich SS. The erythropoietin receptor: molecular structure and hematopoietic signaling pathways. J Investig Med. 2011;59:1067–1072. doi: 10.231/JIM.0b013e31820fb28c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. 1999;274:13480–13484. doi: 10.1074/jbc.274.19.13480. [DOI] [PubMed] [Google Scholar]
- 41.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 42.Socolovsky M, Nam H, Fleming MD, et al. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 43.Myklebust JH, Blomhoff HK, Rusten LS, et al. Activation of phosphatidylinositol 3-kinase is important for erythropoietin-induced erythropoiesis from CD34(+) hematopoietic progenitor cells. Exp Hematol. 2002;30:990–1000. doi: 10.1016/s0301-472x(02)00868-8. [DOI] [PubMed] [Google Scholar]
- 44.Bouscary D, Pene F, Claessens YE, et al. Critical role for PI 3-kinase in the control of erythropoietin-induced erythroid progenitor proliferation. Blood. 2003;101:3436–3443. doi: 10.1182/blood-2002-07-2332. [DOI] [PubMed] [Google Scholar]
- 45.Ghaffari S, Kitidis C, Zhao W, et al. AKT induces erythroid-cell maturation of JAK2-deficient fetal liver progenitor cells and is required for Epo regulation of erythroid-cell differentiation. Blood. 2006;107:1888–1891. doi: 10.1182/blood-2005-06-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferreira R, Ohneda K, Yamamoto M, et al. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi M, Yamamoto M. Regulation of GATA1 gene expression. J Biochem. 2007;142:1–10. doi: 10.1093/jb/mvm122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Generation of stably transduced H1 hESC line expressing F36V-MPL. H1 hESCs were transduced with F36V-MPL and isolated by FACS based on FSC/SSC, SSEA-4+, GFP+, mCD29-, DAPI- gating and subsequently cultured to establish a stably expressing line maintaining ~90% GFP+ expression. A) Schematic of F36V-MPL vector expressing transgenes with the ubiquitin (UbC) promoter. B) Representative immunophenotype analysis of GFP and mCD29 expression to assess expression of vector in human mCD29-cells through continual cell passaging (25 passages from point of transduction). C) Light microscope picture of a stably F36V-MPL transduced hESC colony expressing GFP.
Supplementary Figure 2. General schematic of culture conditions utilized. A) H1 hESCs were transduced with F36V-MPL or F36V (control vector) and cultured on murine OP9 stroma for 14 days with or without CID or EPO (no other cytokines added) prior to analysis. B) To optimize production of MEP, hematopoietic differentiation was induced from F36V-MPL transduced hESC on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and then Day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS on day 14 for use in further analysis. C) To induce erythroblast differentiation, F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium Day 0-14, in serum but without morphogens or cytokines, and then in two step serum-free liquid expansion culture Day 14-28 in EPO or CID. Day 28 cells were used for further analysis.
Supplementary Figure 3. ic-MPL induced erythropoiesis from undifferentiated hESC is serum independent. Hematopoietic differentiation was induced from F36V-MPL transduced hESC by culturing on OP9 stroma Day 0-2 in STEMSPAN (serum-free) supplemented with BMP4, VEGF, and FGF and then from Day 3-14 in SCF, Flt-3L, IL-3, and TPO +/− EPO and/or CID. A) Schematic of culture conditions utilized. B) Immunophenotype analysis of GlyA expression.
Supplementary Figure 4. Dimerization of ic-MPL increases generation of early hematopoietic cells but not endothelial cells. H1 hESCs transduced with F36V-MPL were cultured on OP9 stroma for 14 days with or without CID (no cytokines added), and then counted and analyzed by FACS. Summary of Immunophenotype data shown as: A) % and number of CD43+ cells (n=3); B) % and number of VE-cadherin (VE-CAD)+ cells (n=5); C) % and number of CD31+ cells (n=3). % for each represents the frequency from all GFP+ cells. *P<0.05.
Supplementary Figure 5. ic-MPL dimerization induces the generation of CD34+ MEP. A) F36V-MPL transduced hESC were cultured on OP9 stroma for 14 days with or without EPO or CID (no cytokines). After 14 days of culture, the cells were counted and analyzed by FACS. A) Representative FACS analysis of CD41a/42a and GlyA expression performed on F36V-MPL transduced cells. B-C) F36V-MPL transduced hESC cultured on OP9 stroma were cultured in BMP4, VEGF, and FGF (Day 0-2) and SCF, Flt-3L, IL-3, TPO, and EPO (Day 3-14) prior to flow cytometry analysis. B) Immunophenotype analysis of GlyA and CD41a/42a expression performed on F36V-MPL transduced cells. C) Immunophenotype analysis for CD34 expression of cells in MEP and Eryth gates shown in B.
Supplementary Figure 6. Dimerization of ic-MPL induces hemoglobinized nucleated and enucleated RBC. Hematopoietic differentiation was induced from F36V-MPL transduced hESC on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and then Day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS on day 14 and cultured on OP9 stroma for a further 7 days (i.e. total 21 days of culture) in the presence of CID. After 21 days of culture, cells were sorted based on GFP and GlyA expression and stained with Wright-Giemsa and 3’3-diaminobenzidine. Shown are 4 different slides of GFP+GyA+ sorted cells all generated from MEP in the presence of CID (all 40x magnification). White arrows mark enucleated hemoglobinized erythrocytes.
Supplementary Figure 7. ic-MPL induced erythroid maturation from MEP is serum and EPO independent. Hematopoietic differentiation was induced from F36V-MPL transduced hESC by culturing on OP9 stroma Day 0-2 in BMP4, VEGF, and FGF and day 3-14 in SCF, Flt-3L, IL-3, TPO, and EPO. GFP+ MEP were isolated by FACS sorting on day 14 (see Figure 3) and re-cultured on retronectin with STEMSPAN (serum-free) medium +/− EPO or CID for 10 more days prior to analysis. A) Light microscope picture of EPO vs CID after 10 days of culture on retronectin with STEMSPAN. B) Immunophenotype analysis of GlyA and CD41a/42a expression. C) Immunophenotype analysis for Hoechst staining of cells in GlyA+ gate.
Supplementary Figure 8. Dimerization of intracellular cMPL does not activate p38 and ERK-MAPK. Ba/F3 cells were transduced with F36V-MPL (FM) or control vector expressing wild-type (full length) human MPL (Mpl) and isolated based on GFP expression. Cells were then cytokine starved overnight prior to stimulating with TPO or CID after which the cells were processed for flow analysis. Representative FACS analysis of pERK1/2 and p-p38.
Supplementary Figure 9. Quantification of Western Blot Analysis for BAD phosphorylation. Western blot analysis of BAD phosphorylation from Figure 5B was quantified by densitometry using ImageJ software. pan-AKT was used as the loading control. F36V-MPL (FM); F36V (F).
Supplementary Figure 10. Induction of erythropoiesis from hESC by ic-MPL dimerization does not require JAK2 activity. A-C) Day 14 MEP generated from F36V-MPL transduced hESC were isolated by FACS sorting for a further 7 days of culture (i.e. total 21 days of culture) in the presence of SCF, Flt-3L, IL-3, TPO, ± CID along with the JAK2 inhibitor AG490 and then analyzed by FACS. A) Summary of Day 21 FACS data shown as: B) % of erythroid cells (GlyA+CD41a/CD42a-); C) % megakaryocytes (GlyA-CD41a/42a+).
Supplementary Figure 11. FACS plot for BrdU/7-AAD staining. F36V-MPL transduced hESC were cultured on OP9 stroma in hematopoietic induction medium for 14 days without that addition of any morphogens or cytokines. The cells were then dissociated and replated in liquid culture in the presence of Flt-3L, SCF, BMP-4, IL-3 and EPO or CID for 7 days and then transferred to IGF-1, SCF, BMP-4, IL-3 and EPO or CID for another 7 days prior to analysis. Representative FACS analysis of BrdU and 7-AAD staining to assess cell cycle. Apoptotic (BrdUneg7AADneg), G0/G1 (BrdUneg7AADdim), S-phase (BrdUpos7AADdim), and G2/M cells (BrdUneg7AADhi). Summary of this data is shown in Figure 7A, B