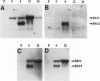
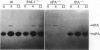
Abstract
Several lines of indirect evidence suggest that plasminogen activation plays a crucial role in degradation of the follicular wall during ovulation. However, single-deficient mice lacking tissue-type plasminogen activator (tPA), urokinase-type plasminogen activator (uPA), or PA inhibitor type 1(PAI-1) gene function were recently found to have normal reproduction, although mice with a combined deficiency of tPA and uPA were significantly less fertile. To investigate whether the reduced fertility of mice lacking PA gene function is due to a reduced ovulation mechanism, we have determined the ovulation efficiency in 25-day-old mice during gonadotropin-induced ovulation. Our results reveal that ovulation efficiency is normal in mice with a single deficiency of tPA or uPA but reduced by 26% in mice lacking both physiological PAs. This result suggests that plasminogen activation plays a role in ovulatory response, although neither tPA nor uPA individually or in combination is obligatory for ovulation. The loss of an individual PA seems to be functionally complemented by the remaining PA but this compensation does not appear to involve any compensatory up-regulation. Our data imply that a functionally redundant mechanism for plasmin formation operates during gonadotropin-induced ovulation and that PAs together with other proteases generate the proteolytic activity required for follicular wall degradation.
Full text
PDF
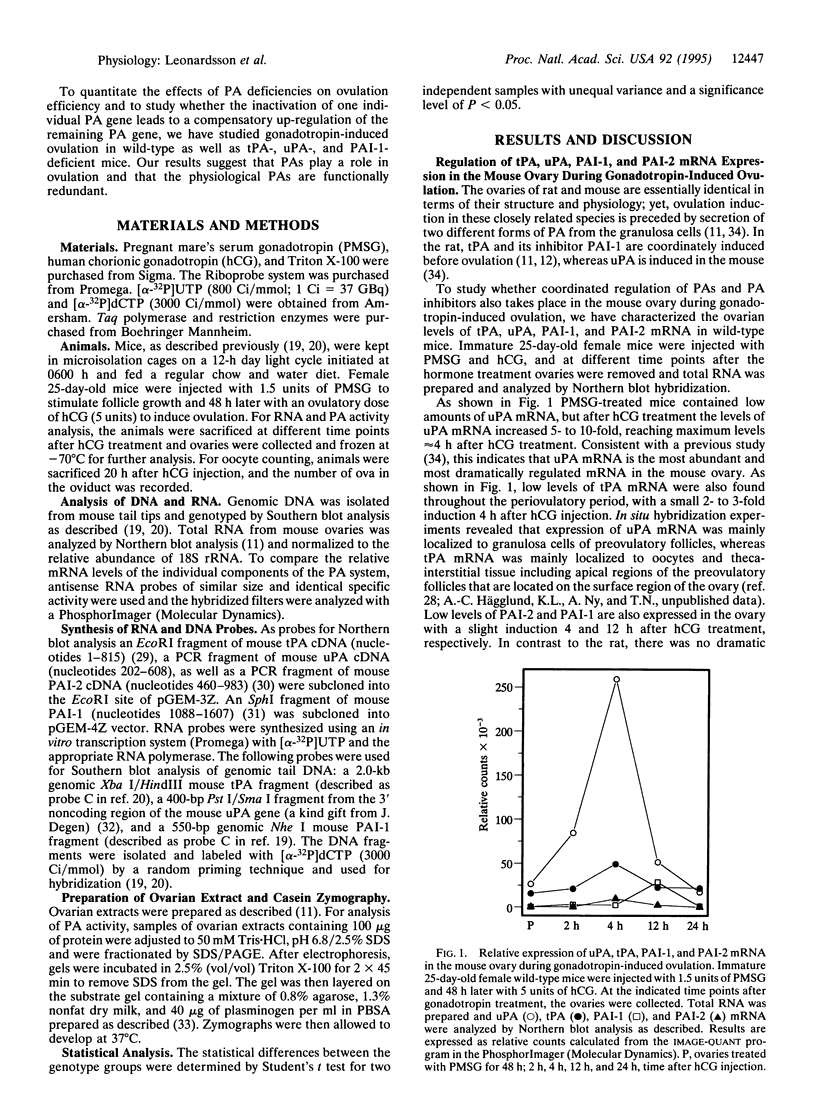

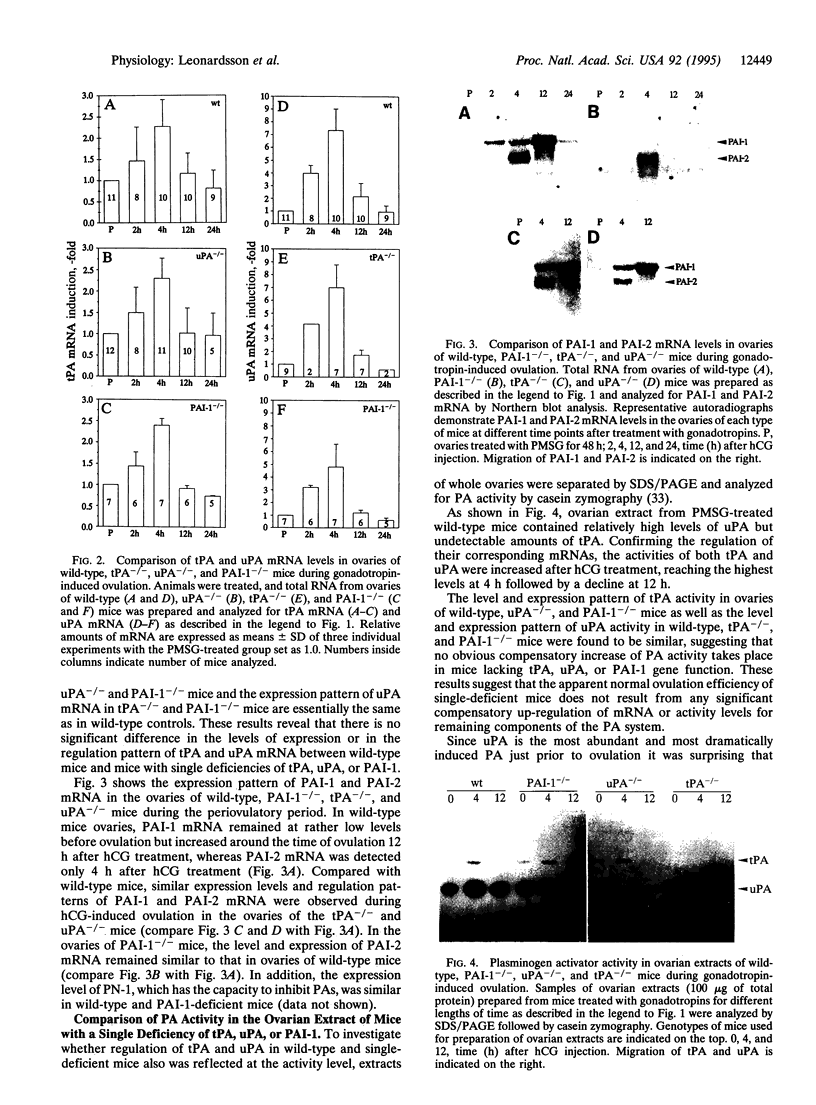

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagavandoss P., Midgley A. R., Jr, Wicha M. Developmental changes in the ovarian follicular basal lamina detected by immunofluorescence and electron microscopy. J Histochem Cytochem. 1983 May;31(5):633–640. doi: 10.1177/31.5.6341456. [DOI] [PubMed] [Google Scholar]
- Beers W. H. Follicular plasminogen and plasminogen activator and the effect of plasmin on ovarian follicle wall. Cell. 1975 Nov;6(3):379–386. doi: 10.1016/0092-8674(75)90187-7. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Belin D., Wohlwend A., Schleuning W. D., Kruithof E. K., Vassalli J. D. Facultative polypeptide translocation allows a single mRNA to encode the secreted and cytosolic forms of plasminogen activators inhibitor 2. EMBO J. 1989 Nov;8(11):3287–3294. doi: 10.1002/j.1460-2075.1989.tb08489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield J. Can genes be truly redundant? Curr Biol. 1992 Oct;2(10):553–554. doi: 10.1016/0960-9822(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Canipari R., O'Connell M. L., Meyer G., Strickland S. Mouse ovarian granulosa cells produce urokinase-type plasminogen activator, whereas the corresponding rat cells produce tissue-type plasminogen activator. J Cell Biol. 1987 Aug;105(2):977–981. doi: 10.1083/jcb.105.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Kieckens L., Schoonjans L., Ream B., van Nuffelen A., Prendergast G., Cole M., Bronson R., Collen D., Mulligan R. C. Plasminogen activator inhibitor-1 gene-deficient mice. I. Generation by homologous recombination and characterization. J Clin Invest. 1993 Dec;92(6):2746–2755. doi: 10.1172/JCI116892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Schoonjans L., Kieckens L., Ream B., Degen J., Bronson R., De Vos R., van den Oord J. J., Collen D., Mulligan R. C. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994 Mar 31;368(6470):419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- Chun S. Y., Popliker M., Reich R., Tsafriri A. Localization of preovulatory expression of plasminogen activator inhibitor type-1 and tissue inhibitor of metalloproteinase type-1 mRNAs in the rat ovary. Biol Reprod. 1992 Aug;47(2):245–253. doi: 10.1095/biolreprod47.2.245. [DOI] [PubMed] [Google Scholar]
- Collen D., Lijnen H. R. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991 Dec 15;78(12):3114–3124. [PubMed] [Google Scholar]
- Curry T. E., Jr, Mann J. S., Huang M. H., Keeble S. C. Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod. 1992 Feb;46(2):256–264. doi: 10.1095/biolreprod46.2.256. [DOI] [PubMed] [Google Scholar]
- Degen S. J., Heckel J. L., Reich E., Degen J. L. The murine urokinase-type plasminogen activator gene. Biochemistry. 1987 Dec 15;26(25):8270–8279. doi: 10.1021/bi00399a038. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Gene knockouts of c-src, transforming growth factor beta 1, and tenascin suggest superfluous, nonfunctional expression of proteins. J Cell Biol. 1993 Mar;120(5):1079–1081. doi: 10.1083/jcb.120.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2632–2636. doi: 10.1073/pnas.86.8.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch B., Leonhardt S., Jarry H., Reich R., Tsafriri A., Wuttke W. In vivo measurement of rat ovarian collagenolytic activities. Endocrinology. 1993 Dec;133(6):2761–2765. doi: 10.1210/endo.133.6.8243302. [DOI] [PubMed] [Google Scholar]
- Huarte J., Belin D., Bosco D., Sappino A. P., Vassalli J. D. Plasminogen activator and mouse spermatozoa: urokinase synthesis in the male genital tract and binding of the enzyme to the sperm cell surface. J Cell Biol. 1987 May;104(5):1281–1289. doi: 10.1083/jcb.104.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte J., Belin D., Vassalli J. D. Plasminogen activator in mouse and rat oocytes: induction during meiotic maturation. Cell. 1985 Dec;43(2 Pt 1):551–558. doi: 10.1016/0092-8674(85)90184-9. [DOI] [PubMed] [Google Scholar]
- Huarte J., Vassalli J. D., Belin D., Sakkas D. Involvement of the plasminogen activator/plasmin proteolytic cascade in fertilization. Dev Biol. 1993 Jun;157(2):539–546. doi: 10.1006/dbio.1993.1156. [DOI] [PubMed] [Google Scholar]
- Hummler E., Cole T. J., Blendy J. A., Ganss R., Aguzzi A., Schmid W., Beermann F., Schütz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. X., NY T., Sarkar D., Loskutoff D., Hsueh A. J. Identification and regulation of tissue plasminogen activator activity in rat cumulus-oocyte complexes. Endocrinology. 1986 Oct;119(4):1578–1587. doi: 10.1210/endo-119-4-1578. [DOI] [PubMed] [Google Scholar]
- Liu Y. X., Peng X. R., Ny T. Tissue-specific and time-coordinated hormone regulation of plasminogen-activator-inhibitor type I and tissue-type plasminogen activator in the rat ovary during gonadotropin-induced ovulation. Eur J Biochem. 1991 Jan 30;195(2):549–555. doi: 10.1111/j.1432-1033.1991.tb15736.x. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Ny T., Peng X. R., Ohlsson M. Hormonal regulation of the fibrinolytic components in the ovary. Thromb Res. 1993 Jul 1;71(1):1–45. doi: 10.1016/0049-3848(93)90203-z. [DOI] [PubMed] [Google Scholar]
- Palotie A., Peltonen L., Foidart J. M., Rajaniemi H. Immunohistochemical localization of basement membrane components and interstitial collagen types in preovulatory rat ovarian follicles. Coll Relat Res. 1984 Aug;4(4):279–287. doi: 10.1016/s0174-173x(84)80035-7. [DOI] [PubMed] [Google Scholar]
- Peng X. R., Hsueh A. J., Ny T. Transient and cell-specific expression of tissue-type plasminogen activator and plasminogen-activator-inhibitor type 1 results in controlled and directed proteolysis during gonadotropin-induced ovulation. Eur J Biochem. 1993 May 15;214(1):147–156. doi: 10.1111/j.1432-1033.1993.tb17907.x. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Diamond L. E., Dahl D., Cole M. D. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol Cell Biol. 1990 Mar;10(3):1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich R., Daphna-Iken D., Chun S. Y., Popliker M., Slager R., Adelmann-Grill B. C., Tsafriri A. Preovulatory changes in ovarian expression of collagenases and tissue metalloproteinase inhibitor messenger ribonucleic acid: role of eicosanoids. Endocrinology. 1991 Oct;129(4):1869–1875. doi: 10.1210/endo-129-4-1869. [DOI] [PubMed] [Google Scholar]
- Reich R., Miskin R., Tsafriri A. Follicular plasminogen activator: involvement in ovulation. Endocrinology. 1985 Feb;116(2):516–521. doi: 10.1210/endo-116-2-516. [DOI] [PubMed] [Google Scholar]
- Reich R., Miskin R., Tsafriri A. Intrafollicular distribution of plasminogen activators and their hormonal regulation in vitro. Endocrinology. 1986 Oct;119(4):1588–1593. doi: 10.1210/endo-119-4-1588. [DOI] [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988 Jan 25;263(3):1563–1569. [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Huarte J., Belin D., Vassalli J. D. Plasminogen activators in tissue remodeling and invasion: mRNA localization in mouse ovaries and implanting embryos. J Cell Biol. 1989 Nov;109(5):2471–2479. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994 Jul 7;370(6484):61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Sherman M. I. Studies on the temporal correlation between secretion of plasminogen activator and stages of early mouse embryogenesis. Oncodev Biol Med. 1980 Aug;1(1):7–16. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Strickland S., Beers W. H. Studies on the role of plasminogen activator in ovulation. In vitro response of granulosa cells to gonadotropins, cyclic nucleotides, and prostaglandins. J Biol Chem. 1976 Sep 25;251(18):5694–5702. [PubMed] [Google Scholar]
- Strickland S., Reich E., Sherman M. I. Plasminogen activator in early embryogenesis: enzyme production by trophoblast and parietal endoderm. Cell. 1976 Oct;9(2):231–240. doi: 10.1016/0092-8674(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Tsafriri A., Bicsak T. A., Cajander S. B., Ny T., Hsueh A. J. Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and alpha 2-antiplasmin. Endocrinology. 1989 Jan;124(1):415–421. doi: 10.1210/endo-124-1-415. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Huarte J., Bosco D., Sappino A. P., Sappino N., Velardi A., Wohlwend A., Ernø H., Monard D., Belin D. Protease-nexin I as an androgen-dependent secretory product of the murine seminal vesicle. EMBO J. 1993 May;12(5):1871–1878. doi: 10.1002/j.1460-2075.1993.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Santulli R., Atlas S. J., Fujii S., Wallach E. E. The effects of proteolytic enzymes on in vitro ovulation in the rabbit. Am J Obstet Gynecol. 1987 Aug;157(2):468–475. doi: 10.1016/s0002-9378(87)80197-7. [DOI] [PubMed] [Google Scholar]