Abstract
Introduction
Although rapid response systems (RRS) have been shown to decrease the incidence of cardiac arrest (CA), there are no studies evaluating optimal staffing. We hypothesize that there are no outcome differences between ICU physician and senior resident led events.
Methods
A retrospective study of the RRS database at a single, academic hospital was performed from July 1, 2006 to May 31, 2010. Surgical patients and those in the ICU were excluded. Daytime (D) was defined as 7 am–5 pm Monday through Friday, and weekends were defined as 5 pm on Friday to 6:59 am on Monday. The nurse to patient ratio is constant during all shifts. An ICU physician leads daytime events on weekdays whereas night/weekend (NW) events are led by residents. NW events were compared against D events using chi square or Fischer’s exact test. Significance was defined as p < 0.05.
Results
A total of 1404 events were reviewed with 534 (38%) D and 870 (62%) NW events. Respiratory and staff concerns were more likely during NW compared to D (50% vs. 39% and 46% vs. 34%, p < 0.001, respectively). Following RRS activation, no difference was noted between D and NW periods in the incidence of progression to CA, transfer to ICU, or hospital mortality. Invasive procedures were more common in the NW period.
Conclusion
Resident-led RRS may have similar outcomes to attending intensivist led events. Prospective studies are needed to determine the ideal team composition.
Keywords: Rapid response system, Medical emergency team, Rapid response team
1. Introduction
Rapid response systems (RRS) constitute a proactive system to improve patient safety. The goal of these systems is to provide prompt evaluation and treatment of patients with physiologic deterioration, mainly in non-critical care areas of the hospital. Numerous reports document a significant decrease in cardiac arrest volume following institution of these systems.1–6 Such reports led to the Joint Commission requirement that all hospitals in the United States and Canada have a system to improve recognition and response to changes in a patient’s condition.7
The ideal RRS staffing model has yet to be determined resulting in highly variable resources devoted to RRS implementation. Based on consensus definition, a medical emergency team (MET) includes a clinician who is able to prescribe therapy.8 However, there are no studies comparing outcomes when MET are led by attending physicians, residents or advanced practitioners. There are significant cost and resource implications in mandating attending physician coverage for all activations. It is possible that disruption of an attending physician’s daily work flow may impede care being rendered to established acutely ill patients or may decrease the number of billable encounters scheduled for the day. Thus, demonstration that outcomes are equal between attending and resident led MET may have significant implications in resource allocation.
The purpose of this study was to evaluate differences in outcomes between ICU attending and senior resident physician led MET. We hypothesize that the routine addition of an attending intensivist to all activations does not decrease the incidence of cardiac arrest or ICU transfer following RRS activation.
2. Methods
The Hospital of the University of Pennsylvania implemented a MET in July 2006. When responding to medical inpatients (i.e. patients not on a surgical service as defined below), an ICU attending leads the team from 7 am to 5 pm on weekdays, whereas the team is led by a senior resident (PGY 2–3) at night time and on weekends. An ICU attending is available by telephone as needed during off hours. The team leader (intensivist or senior resident) makes all final decisions regarding the care plan and the need for interventions or ICU transfer. Neither the residents nor the intensivists received specific training in crisis or crew resource management. Other members of the MET, irrespective of time and day, include a nurse–coordinator, respiratory therapist, pharmacist, and the house officer responsible for the patient. The MET members work with the bedside nurses to provide care for the patient. The nurse-to-patient ratio on the wards remains constant at 1:4 throughout all shifts.
To assure uniformity of care, a cadre of 14 nurse coordinators is specifically trained in the RRS protocols for management of various critical events, such as empiric antibiotic therapy for presumed sepsis and mobilization of additional resources (e.g. airway team, cardiac catheterization laboratory), and has oversight over the bed-flow process for the hospital. It should be noted, however, that these nurses act as facilitators to the rapid response process but do not usurp the role of the team leader. The nurse coordinators constitute a group of full time nurses whose sole job is bed management, assessment of patients with risk factors for acute change in physiologic status (e.g. rising oxygen need, hypoglycemic events, significant co-morbidities), and response to MET events. These nurses all have extensive ICU and/or Emergency Department experience and were further specifically trained using the Fundamentals of Critical Support Course provided by the Society of Critical Care Medicine along with regular in-service meetings with the medical directors of the MET. In-service sessions were used to educate nurses on treatment protocols and policies related to the MET.
Anyone may activate the team, including non-clinical hospital personnel and families/visitors. Formal activation criteria that are distributed to all hospital staff are listed in Table 1. For the purposes of this study, neurologic triggers for MET activation were defined as: mental status change, loss of consciousness, seizure, suspected stroke, and acute agitation. Cardiovascular triggers were defined as: bradycardia, tachycardia, chest pain, hypotension, and severe hypertension. Respiratory triggers for team activation were defined as: tachypnea, dyspnea, and hypoxemia.
Table 1.
Criteria for rapid response activation.
| Respiratory |
| Rate < 8 or >32 breaths/min |
| Oxygen saturation <85% |
| Acute increase in oxygen need by 50% |
| Dyspnea |
| Cardiac |
| Rate < 40 or >140 beats/min |
| Systolic blood pressure < 80 or >200 mmHg |
| Diastolic blood pressure > 110 mmHg |
| New onset chest pains |
| Neurologic |
| Seizure |
| Acute change in mental status |
| Miscellaneous |
| Uncontrolled bleeding |
| Inability to contact house-officer after 2 pages |
| Nurse concern/discretion |
| Physician concern/discretion |
After obtaining IRB approval, we performed a retrospective analysis of our prospectively maintained RRS database. We analyzed RRS activations from July 1, 2006 to May 31, 2010. RRS activations for visitors, outpatients, ICU patients, and patients on the surgical services (defined as general, cardiothoracic, vascular, otorhinolaryngology, plastics, urology, orthopedic, and neurological surgery and obstetrics/gynecology) were excluded. Surgical service patients were excluded because the team leader during nights and weekends can variably include a surgical ICU fellow or attending. Based on our MET staffing model, night was defined as 5:00 pm to 7:00 am and day was defined as 7:01 am to 4:59 pm, while weekends were defined as between 5 pm on Friday to 6:59 am on Monday morning. For the purpose of analysis, night and weekend (NW) activations were compared as a combined cohort against day (D) RRS activations. To ensure that variables were independent, in patients for whom more than one MET was triggered, all but the final MET were censored from analysis.
The data analyzed included patient demographics, the trigger for RRS activation, intervention(s) rendered by the MET, incidence of cardiac arrest following evaluation by the MET, disposition following activation, and survival to hospital discharge. More than one MET trigger may have been present for any single activation. Chi square or Fisher’s exact test (when fewer than 5 observations were present in any cell) was used to compare groups. Odds ratios (OR) and 95% confidence intervals (CI) were generated using the ‘Risk’ function in SPSS statistical software. Two tailed significance was set at p < 0.05. To assess for the impact of MET trigger and MET timing (D vs. NW) on in-hospital mortality, a multivariable logistic regression model was constructed. All data analyses were performed with SPSS Version 19.0 (IBM SPSS Statistics, Chicago, IL, http://www.spss.com).
3. Results
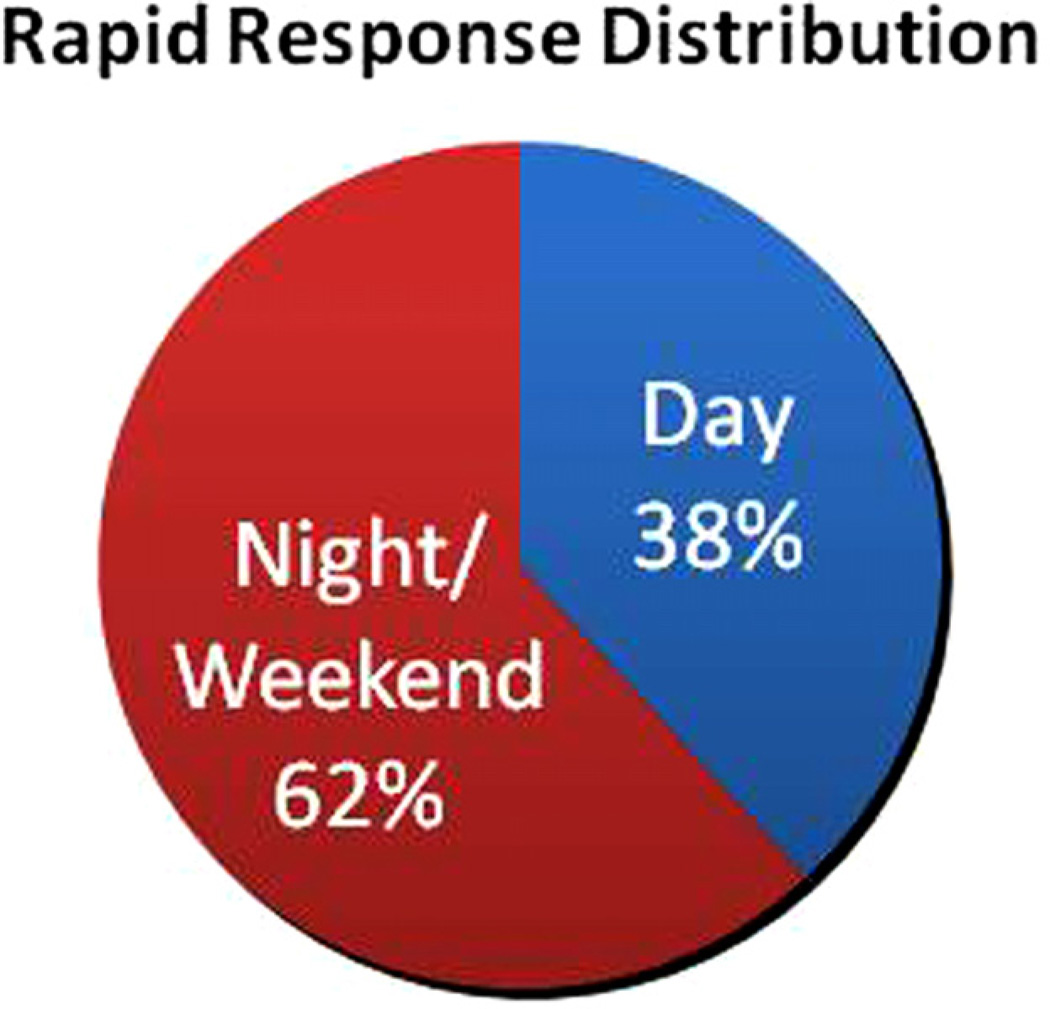
During the study period, there were a total of 139,182 patients admitted to our institution; 80,741 (58%) were medical admissions. There were 1642 RRS activations in 1404 patients and 523 cardiac arrest events during this time period. Thirty eight percent of RRS activations and 43% of cardiac arrest cases occurred during the D period. The distribution of rapid response events throughout a 24 h period is depicted in Fig. 1.
Fig. 1.
Percentage of rapid response calls in each cohort.
Triggers for MET activation varied by time period (Table 2). Respiratory concerns were significantly more likely to trigger RRS activation during the NW period than the D period (51% NW vs. 40% D, p < 0.001), while neurologic concerns were less likely to trigger METs during the NW period (42% NW vs. 48% D, p = 0.04). The incidence of cardiovascular triggers did not differ significantly between the two time periods (56% NW vs. 53% D period, p = 0.38). Beyond specific physiologic triggers, staff concern in the absence of other ‘hard’ triggers was more likely to trigger RRS activation during the NW period (4% NW vs. 2% D, p < 0.07) (Table 3).
Table 2.
Triggers for rapid response activation.
| Day, N (%) 534 (38) | Night/WE, N (%) 870 (62) | OR | 95% CI | p | |
|---|---|---|---|---|---|
| Respiratory | 215 (40%) | 443 (51%) | 1.54 | 1.25–1.92 | <0.001 |
| Cardiovascular | 284 (53%) | 484 (56%) | 1.10 | 0.89–1.37 | 0.37 |
| Neurologic | 254 (48%) | 366 (42%) | 0.80 | 0.65–0.99 | 0.04 |
| Staff concern alone | 10 (2%) | 31 (4%) | 1.94 | 0.94–3.98 | 0.07 |
Univariate analysis using Chi square. WE: weekend; OR: Odds Ratio; CI: Confidence Interval.
Note: patients may have had more than 1 trigger.
Table 3.
Associations between MET triggers, time of MET, and mortality.
| OR | 95% CI | p | |
|---|---|---|---|
| Respiratory trigger | 1.77 | 1.37–2.29 | 0.01 |
| Cardiac trigger | 1.40 | 1.09–1.81 | 0.01 |
| Neurologic trigger | 1.42 | 1.09–1.84 | 0.01 |
| Staff concern trigger | 1.13 | 0.48–2.70 | 0.78 |
| Night or weekend | 0.87 | 0.68–1.11 | 0.26 |
Multivariable logistic regression model including MET triggers and timing of MET with mortality as the dependent variable. OR: Odds Ratio; 95% CI: 95% Confidence Interval. Trigger predictors OR are referent to MET not having stated trigger; Night and Weekend OR referent to Daytime activation.
To assess for the impact of MET trigger and timing on mortality, we constructed a multivariable logistic regression model. While Respiratory (OR 1.77, 95% CI 1.37–2.29), Cardiac (OR 1.40, 95% CI 1.09–1.81), Neurologic (OR 1.42, 95% CI 1.09–1.84) triggers were all associated with increased in-hospital mortality, Staff-alone triggering (OR 1.13 95% CI 0.48–2.70) and Day vs. NW timing (OR 0.26, 95% CI 0.68–1.11) were not significant.
In terms of RRS interventions (Table 4), patients who experienced a daytime event were more likely to undergo a stat echocardiogram (3% D vs. < 1% NW, p = 0.01) than those who underwent RRS activation at night. RRS activations in the D period were less likely to receive diuretic therapy (5% D vs. 8% NW, p = 0.03), bronchodilators (2% D vs. 7% NW, p < 0.001), supplemental oxygen (62% D vs. 69% NW, p = 0.01), or undergo chest radiography (29% D vs. 40% NW, p < 0.001) compared to those that occurred in the NW period. These findings persisted even after controlling for the increased likelihood of respiratory triggers for RRS activation at night. Invasive procedures were also more common during the NW period, with more patients likely to undergo central line placement (12% D vs. 18% NW, p = 0.002), peripheral line placement (33% D vs. 38% NW, p = 0.07), and indwelling urinary catheter placement (3% D vs. 5% NW, p = 0.05). The percentage of patients undergoing intubation was not different between the two time periods (9% D vs. 11%NW, p = 0.36) nor was the rate of ICU transfer following MET activation (57% D vs. 57% NW, p = 0.82). All other bedside interventions were similar between the two groups.
Table 4.
Interventions during rapid response activation.
| Day, N (%) 534 (38) | Night/WE, N (%) 870 (62) | OR | 95% C.I. | p | |
|---|---|---|---|---|---|
| Echocardiogram | 11 (2) | 4 (<1) | 0.22 | 0.07–0.69 | 0.01 |
| Diuretics | 26 (5) | 69 (8) | 1.68 | 1.06–2.68 | 0.03 |
| Bronchodilators | 13 (2) | 63 (7) | 3.13 | 1.71–5.74 | <0.001 |
| Supplemental O2 | 331 (62) | 599 (69) | 1.34 | 1.07–1.68 | 0.01 |
| Chest radiography | 154 (29) | 344 (40) | 1.61 | 1.27–2.02 | <0.001 |
| Central venous line placement | 64 (12) | 160 (18) | 1.65 | 1.21–2.25 | 0.002 |
| Peripheral IV placement | 176 (33) | 330 (38) | 1.24 | 0.99–1.55 | 0.07 |
| Urinary catheter placement | 16 (3) | 45 (5) | 1.76 | 0.98–3.15 | 0.05 |
| Intubation | 50 (9) | 95 (11) | 1.18 | 0.82–1.70 | 0.36 |
| Transfer to ICU | 306 (57) | 494 (57) | 0.96 | 0.78–1.21 | 0.82 |
WE: weekend; OR: Odds ratio; IV: intravenous catheter; ICU: intensive care unit.
Progression to cardiopulmonary arrest following RRS activation was not significantly different between the two periods (1.8% D vs. 2.4% NW, p = 0.4). Additionally, death during a RRS event was distinctly rare and did not differ significantly between the two groups (0.2% D vs. 0.8% NW, p = 0.81). Beyond the immediate results of the RRS activation, we found that unadjusted in-hospital mortality did not differ between D and NW periods (27% D vs. 26% NW, p = 0.64).
4. Discussion
Rapid response systems were introduced in the mid-90s after reports that cardiac arrest in hospitalized patients is preceded by slowly progressing signs of worsening physiologic status and that the incidence of in-hospital cardiac arrest may decrease if these signs are identified and rapidly intervened upon.9–11 Since then, groups including the Institute for Healthcare Improvement and the Joint Commission have led efforts that culminated in the wide adoption of RRS across the United States, Canada, and some European countries. However, despite this wide adoption, RRS have not been shown to impact on overall mortality. Thus, debate still continues regarding proper resource allocation for and the utility of these teams in different settings.12
Our system lends itself to study because the MET physician configuration changes at fixed time points whereas the nurse-to-patient ratio and non-physician staffing of the MET remains constant. This provides an opportunity to assess the impact of routine intensivist presence relative to a senior resident on outcomes following RRS activation. Because many centers, including ours, utilize an attending physician to respond to RRS events, use of this resource for all activations may have collateral detriment – delaying evaluation and management by of established critically ill patients by faculty.
Our data clearly demonstrate similar outcomes for daytime events, led by attending-level intensivists, and night/weekend events, led by residents. The incidence of progression to cardiac arrest following RRS activation is too small to reliably assess for this endpoint given the current sample size, but the rates of intubation, ICU transfer, and in-hospital mortality were similar between the two arms. This suggests that attending physicians may be able to assume a more advisory role with residents leading the team in most instances. If so, this may permit an escalation model in which intensivists are consulted to specifically render critical care or negotiate triage for selected populations. However, this finding warrants prospective validation. Furthermore, this finding may be due to the select group of nurse coordinators who are specifically trained in detecting and responding to deteriorating patients on the wards, are familiar with resuscitation protocols, and know how to quickly mobilize more resources as needed. Our study could not assess how often a resident independently led the team versus how often the resident solicited advice from the nurse coordinator.
Our study also reveals interesting information regarding the profile and management of the deteriorating patient stratified by day and time. Respiratory triggers and staff concerns were more common during nights and weekends. This may be related to the effect of various sedatives and/or pain medications on respiratory status, particularly in those with impaired pulmonary function at baseline. Our study was not designed to better assess reasons underlying this finding. Daytime use of urgent echocardiography was substantially higher than night/weekend; likely due to the relative ease of obtaining this study during regular working hours. The logistical barriers to obtaining this study at night may have resulted in much less frequent use despite having an overall larger raw number of cardiac triggered events after-hours. The finding that the daytime patients were less likely to receive diuretics, bronchodilators, or supplemental oxygen likely stems from the preponderance of respiratory events at night; however, additional potential explanations may exist. Residents may be more likely to attempt urgent therapeutic interventions when presented with acute deteriorations. The finding that invasive procedures were also more common in the NW period supports this contention. This possibility may have implications regarding the necessity of many procedures and therapies prescribed after-hours, and future studies should examine the impact of specific interventions on ultimate outcome.
This study has several important limitations. First, surgical patients were excluded and therefore the findings may not apply to this cohort. Second, this is a retrospective analysis and has the limitations inherent in this study design. Patient cases unfold over time and drawing distinct time thresholds does not necessarily capture the impact of RRS behavior on patient outcomes. The lack of difference may be related to sufficient training on the part of the residents to address these patients’ needs, the protocol-driven care rendered by the nurse-coordinators to address most critical events, small sample size, or an amalgam of these factors. In addition, the database does not capture the time from the decision to transfer a patient to the ICU and actual arrival or subsequent complications other than recurrent need for RRS activation or death. Thus, there may be differences in outcomes subsequent to the response, such as changes in goals of care, hospital acquired infection, organ dysfunction, and ICU or hospital length of stay that were not captured. Moreover, there was no way to reliably adjust for confounders that may have differed between the groups, and it is possible that the two groups differ in acuity of illness or some other unmeasured aspects that may have impacted the need for intervention or final outcome. Finally, the possibility of a type II error exists, as the rate of mortality during a rapid response activation was low for both groups.
5. Conclusion
While rapid response systems have been shown to improve the rates of serious adverse events for hospitalized patients, the ideal composition of the responding team remains to be identified. Our study found similar outcomes for teams led by attending intensivists and senior residents when a uniform cadre of nurse coordinators is included in the responding team. A resident-led MET in such a model may enable more efficient use of attending physician time and resources. A prospective study is needed to determine optimal staffing and resource allocation of RRS.
Supplementary Material
Footnotes
A Spanish translated version of the summary of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2012.05.020.
Conflict of interest statement
The authors do not have any financial, personal, or other conflicts of interest with any materials related to this work.
References
- 1.Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV. Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ. 2002;324:387–390. doi: 10.1136/bmj.324.7334.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL. Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care. 2004;13:251–254. doi: 10.1136/qshc.2003.006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galhotra S, DeVita MA, Simmons RL, Dew MA. Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Qual Saf Health Care. 2007;16:260–265. doi: 10.1136/qshc.2007.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D, Bellomo R, Bates S, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9:R808–R815. doi: 10.1186/cc3906. [Epub 2005 November 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Offner PJ, Heit J, Roberts R. Implementation of a rapid response team decreases cardiac arrest outside of the intensive care unit. J Trauma. 2007;62:1223–1227. doi: 10.1097/TA.0b013e31804d4968. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 6.Sarani B, Palilonis E, Sonnad S, et al. Clinical emergencies and outcomes in patients admitted to a surgical versus medical service. Resuscitation. 2011;82:415–418. doi: 10.1016/j.resuscitation.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Commission J. National Patient Safety Goals. 2008 [Google Scholar]
- 8.Devita MA, Bellomo R, Hillman K, et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006;34:2463–2478. doi: 10.1097/01.CCM.0000235743.38172.6E. [DOI] [PubMed] [Google Scholar]
- 9.Schein RM, Hazday N, Pena M, Ruben BH, Sprung CL. Clinical antecedents to in-hospital cardiopulmonary arrest. Chest. 1990;98:1388–1392. doi: 10.1378/chest.98.6.1388. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Bishop G, Hillman KM, Daffurn K. The medical emergency team. Anaesth Intensive Care. 1995;23:183–186. doi: 10.1177/0310057X9502300210. [DOI] [PubMed] [Google Scholar]
- 11.Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care. A pilot study in a tertiary-care hospital. Med J Aust. 1999;171:22–25. doi: 10.5694/j.1326-5377.1999.tb123492.x. [DOI] [PubMed] [Google Scholar]
- 12.Winters BD, Pham J, Pronovost PJ. Rapid response teams—walk, don’t run. JAMA. 2006;296:1645–1647. doi: 10.1001/jama.296.13.1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.