Abstract
Objective
Previous studies have shown that inactivation of the group 1B phospholipase A2 (Pla2g1b) suppresses diet-induced obesity, hyperglycemia, insulin resistance, and hyperlipidemia in C57BL/6 mice. A possible influence of Pla2g1b inactivation on atherosclerosis has not been addressed previously. The current study utilized LDL receptor-deficient (Ldlr−/−) mice with plasma lipid levels and distribution similar to hyperlipidemic human subjects as a preclinical animal model to test the effectiveness of Pla2g1b inactivation on atherosclerosis.
Methods and Results
The Pla2g1b+/+Ldlr−/− and Pla2g1b−/−Ldlr−/− mice were fed a low fat chow diet or a hypercaloric diet with 58.5 kcal% fat and 25 kcal% sucrose for 10 weeks. Minimal differences were observed between Pla2g1b+/+Ldlr−/− and Pla2g1b−/−Ldlr−/− mice when the animals were maintained on the low fat chow diet. However, when the animals were maintained on the hypercaloric diet, the Pla2g1+/+Ldlr−/− mice showed the expected body weight gain but the Pla2g1b−/−Ldlr−/− mice were resistant to diet-induced body weight gain. The Pla2g1b−/−Ldlr−/− mice also displayed lower fasting glucose, insulin, and plasma lipid levels compared to the Pla2g1b+/+Ldlr−/− mice, which displayed robust hyperglycemia, hyperinsulinemia, and hyperlipidemia in response to the hypercaloric diet. Importantly, atherosclerotic lesions in the aortic roots were also reduced 7-fold in the Pla2g1b−/−Ldlr−/− mice.
Conclusion
The effectiveness of Pla2g1b inactivation to suppress diet-induced body weight gain and reduces diabetes and atherosclerosis in LDL receptor-deficient mice suggest that pharmacological inhibition of Pla2g1b may be a viable strategy to decrease diet-induced obesity and the risk of diabetes and atherosclerosis in humans.
Keywords: Phospholipase, Atherosclerosis, Glucose tolerance, Lipid and lipoprotein metabolism
1. Introduction
According to the most recent data collected by the World Health Organization, ischemic heart disease and stroke remain the leading cause of death worldwide. The increasing prevalence of obesity and diabetes is also rapidly becoming a major health threat. The risk of these cardiometabolic diseases is dependent on both genetic and lifestyle factors, including the increasing consumption of meals rich in fat and carbohydrates. Reducing consumption of these caloric-rich nutrients or blocking their absorption and endogenous synthesis with drugs such as orlistat, ezetimibe and statins are effective strategies to lower the risk of these cardiometabolic diseases [1]. The development of orlistat and ezetimibe is based on the discovery that lipolysis of the ingested fat by pancreatic lipases and the intestinal cholesterol transporter NPC1L1 are required for optimal fat and cholesterol absorption, respectively [2, 3].
Genome-wide association studies have identified PLA2G1B genetic polymorphism as another risk factor for central obesity in humans [4]. This gene encodes group 1B phospholipase A2 (Pla2g1b), which is predominantly expressed in pancreatic acinar cells with trace amounts of its mRNA found in lung tissues [5]. Unlike other phospholipase As enzymes, Pla2g1b is not found in plasma circulation except under conditions of acute pancreatitis [6, 7]. The Pla2g1b protein synthesized in pancreatic acinar cells is stored in zymogen granules and secreted into the intestinal lumen in response to fatty meal to catalyze phospholipid digestion and lipid nutrient uptake [8]. Interestingly, inactivation of the Pla2g1b gene only has a minor influence on the total amount of lipids absorbed from a single meal, with only tracer amounts of ingested radiolabeled triglyceride found in the feces of Pla2g1b−/− mice [8, 9]. In fact, no significant difference in total fat absorption was observed between Pla2g1b+/+ and Pla2g1b−/− mice measured over a 3-day period [10]. Nevertheless, the absorption of dietary and biliary phospholipids as lysophospholipids was significantly reduced in Pla2g1b−/− mice [11]. Our previous studies showed that Pla2g1b gene inactivation or pharmacological inhibition of Pla2g1b activity in wild type mice protect against diet-induced obesity and hyperglycemia [9], whereas over-expression of Pla2g1b exacerbates diet-induced obesity and insulin resistance [12]. Inactivation of the Pla2g1b gene also suppresses VLDL synthesis and reduces diet-induced hyperlipidemia [13], suggesting that reducing Pla2g1b activity may be a viable option to reduce atherosclerosis. This study was undertaken to test this hypothesis.
2. Methods
The Pla2g1b−/− mice in a congenic C57BL/6 background [11, 13] were mated with Ldlr−/− mice on the same background (Jackson laboratories) to obtain Pla2g1b+/−Ldlr+/− offspring, which were then backcrossed and mated to obtain Pla2g1b−/−Ldlr−/− mice for comparison with Pla2g1b+/+Ldlr−/− mice. Age-matched male mice on congenic C57BL/6 background were used for all experiments. Mice were maintained in a specific pathogen-free environment on a 12 hr light/dark cycle and fed either a 5% fat (3.75 kcal/g) standard rodent chow diet (LM485, Harlan Teklad, Madison, WI) or a high caloric diet with 58.5 kcal% fat and 25 kcal% sucrose (D12331, Harlan Teklad) beginning at 10 to 12 weeks of age. Blood samples were collected from fasting mice into EDTA containing tubes. Fasting blood glucose levels were determined with an AccuChek Active Glucometer (Roche Applied Science). Plasma was isolated by centrifugation. Triglyceride and cholesterol levels were measured using colorimetric assay kits (ThermoFisher Scientific). Lipid distribution among various lipoproteins was determined by fast performance liquid chromatography (FPLC) gel filtration on two Superose 6 columns [13]. Plasma insulin levels were measured using an Ultra Sensitive Rat Insulin ELISA kit (Crystal Chem, Chicago). Insulin resistance were estimated by homeostasis model assessment index calculated from fasting glucose and insulin levels [12].
Atherosclerosis lesions were analyzed after 10 weeks of feeding the hypercaloric diets as described previously [14]. Briefly, mice were anesthetized with isofluorane inhalation and the heart and aorta were perfusion fixed with 4% neutral paraformaldehyde solution. Cryosections of 5-µm thickness through the aortic root were prepared for staining with Oil Red O to measure neutral lipid accumulation and counter-stained with hematoxylin. Mean lesion area and total valve area were measured from digitalized images obtained from 5 sections per mouse. Atherosclerotic lesions were analyzed as ratios of lesion area to total valve area from 9 mice in each group. All procedures and animal care were reviewed and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Statistical analysis was performed with SigmaPlot Version 11. Values were expressed as mean ± SD. Multiple comparisons were tested by Student’s t test or ANOVA, with Student Newman-Keuls post-hoc analysis. A difference of P<0.05 was considered statistically significant.
3. Results
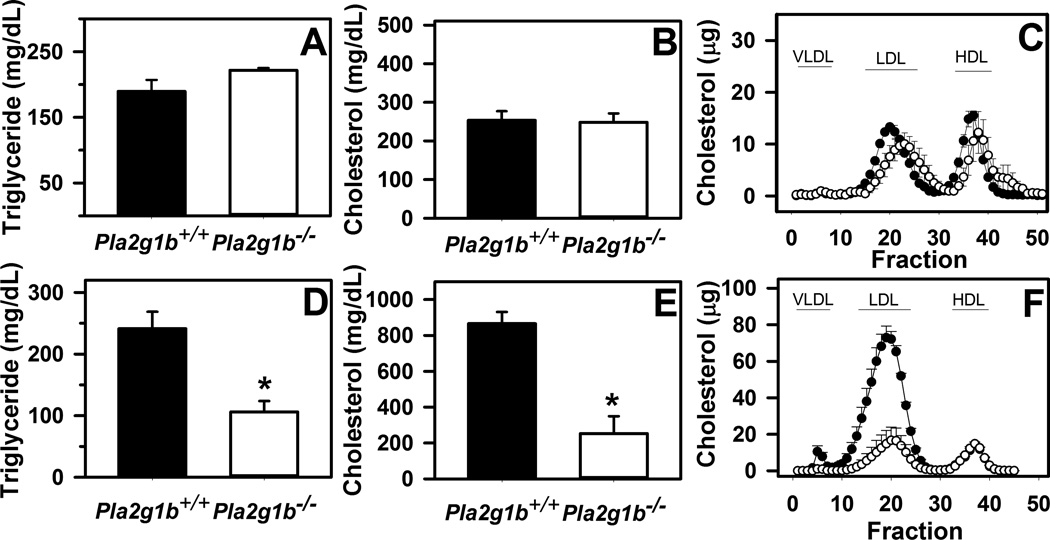
Previous studies have shown that Pla2g1b inactivation suppressed diet-induced obesity, hyperglycemia, and hyperlipidemia in wild type C57BL/6 mice [9, 11, 13]. The current study showed that Pla2g1b gene inactivation had minimal effect on plasma lipid levels in chow-fed LDL receptor-deficient mice but both plasma triglyceride and cholesterol levels were significantly lower in Pla2g1b−/−Ldlr−/− mice compared to Pla2g1b+/+Ldlr−/− mice when the animals were fed a hypercaloric diet (Fig. 1). Lipoprotein profile analysis by FPLC revealed the reduced plasma lipid levels in Pla2g1b−/−Ldlr−/− mice were due to reduction of both VLDL and LDL (Fig. 1).
Figure 1. Fasting plasma lipid levels in Pla2g1b+/+Ldlr−/− and Pla2g1b−/−Ldlr−/− mice.
Male Pla2g1b+/+Ldlr−/− (filled bars and symbols) and Pla2g1b−/−Ldlr−/− mice were fed low fat chow diet (A–C) or hypercaloric diet (D–F) for 10 weeks. Animals were fasted overnight prior to obtaining blood samples to measure plasma triglyceride (A,D) and cholesterol (B,E) levels. An 100 µL of the samples were fractionated on FPLC to determine lipid distribution among the various lipoprotein fractions compared to standards as indicated (C,F). Data represent mean ± SEM from 4–6 mice in each group. * denotes differences from Pla2g1b+/+Ldlr−/− mice at P < 0.01.
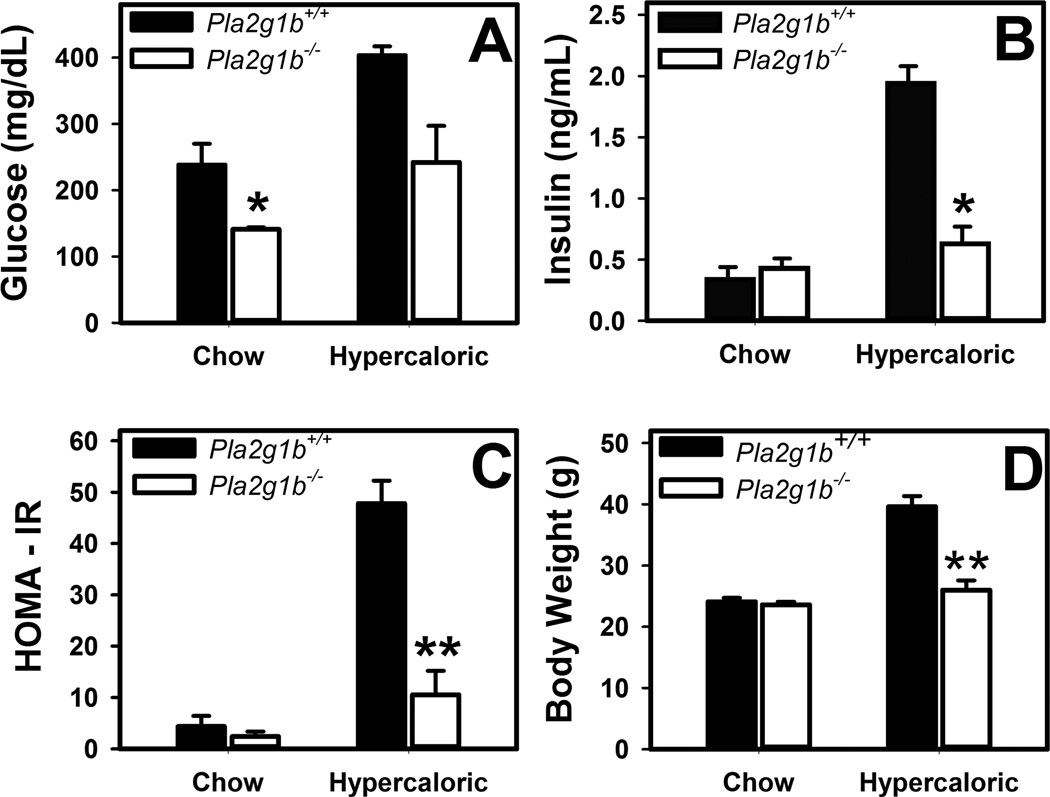
Fasting glucose levels were statistically lower in chow-fed Pla2g1b−/−Ldlr−/− mice compared to Pla2g1b+/+Ldlr−/− mice but their fasting insulin levels were similar. Homeostasis model assessment of insulin resistance (HOMA-IR) revealed no difference in insulin resistance between the two groups of animals under chow-fed conditions (Fig. 2). In contrast, robust elevation of fasting plasma glucose and insulin levels, leading to ~8-fold increase in HOMA-IR was observed in Pla2g1b+/+Ldlr−/− mice when the animals were fed a hypercaloric diet for 10 weeks. Inactivation of the Pla2g1b gene protected against the hypercaloric diet-induced hyperglycemia, hyperinsulinemia, and insulin resistance in Ldlr−/− mice (Fig. 2). Additionally, consistent with previous results observed in wild type C57BL/6 mice [9], Pla2g1b inactivation also suppressed diet-induced body weight gain in hypercaloric diet-fed Ldlr knockout mice (Fig. 2D).
Figure 2. Blood chemistry and body weight of Pla2g1b+/+Ldlr−/− and Pla2g1b−/−Ldlr−/− mice.
Male Pla2g1b+/+Ldlr−/− (filled bars) and Pla2g1b−/−Ldlr−/− mice (open bars) were fed either a low fat regular mouse chow or a hypercaloric diet containing 58.5 kcal% fat and 25 kcal% sucrose for 10 weeks. The mice were fasted overnight. (A) Blood glucose levels were measured by glucometer. (B) Plasma insulin levels were determined by ELISA. (C) Insulin resistance was estimated based on homeostasis model assessment index of insulin resistance (HOMA-IR) calculated from the glucose and insulin data. (D) Body weight was measured using a Denver 300K scale. The data represent mean ± SEM from 5 mice in each group. * and ** denote differences from Pla2g1b+/+Ldlr−/− mice on the same diet at P = 0.02 and P < 0.001, respectively.
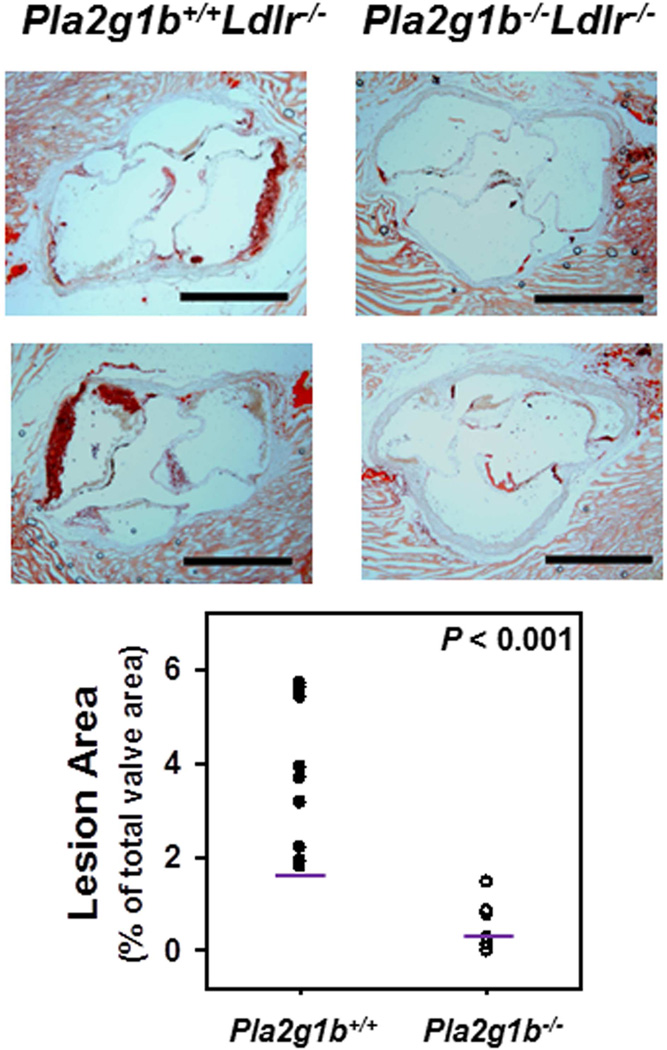
The influence of Pla2g1b on atherosclerosis was assessed by examining the aortic roots of Pla2g1b+/+Ldlr−/− and Pla2g1b−/−Ldlr−/− mice after 10 weeks of feeding the hypercaloric diet. The data revealed an approximate 7-fold reduction in atherosclerotic lesion area in mice without Pla2g1b expression (3.72 ± 0.5% vs. 0.525 ± 0.16% of valve area, P < 0.001) (Fig. 3). These data documented that inactivation of Pla2g1b is effective in suppression of diet-induced atherosclerosis.
Figure 3. Atherosclerotic lesions in the aortic roots of Ldlr−/− mice with or without Pla2g1b expression.
Male Pla2g1b+/+ and Pla2g1b−/−Ldlr−/− mice were fed a hypercaloric diet for 10 weeks. Atherosclerotic lesions were assessed in the aortic roots. The top panels show photomicrographs of aortic roots from 2 different mice in each group stained with oil red O. Scale bar = 500 µm. The bottom panel shows morphometric analysis of lesion areas expressed as ratios of lesion area to total valve area from 9 mice in each group. Each data point represents lesion area in one mouse with the geometric mean indicated with a line.
4. Discussion
Previous studies have shown that genetic inactivation or pharmacologic inhibition of Pla2g1b is effective in suppressing high fat diet-induced obesity, hyperglycemia, insulin resistance, and hyperlipidemia in wild type C57BL/6 mice [9, 11, 13, 15]. The underlying mechanism is related to the reduced absorption of lysophospholipids and the consequential effects of the absorbed lysophospholipids on hepatic fatty acid oxidation and mitochondrial activity [10, 11, 16]. Results of the current study showed that Pla2g1b inactivation is also effective in reducing diet-induced obesity, hyperglycemia, insulin resistance and hyperlipidemia in an animal model with plasma lipid distribution similar to that in human subjects, i.e., the hypercaloric diet-fed Ldlr−/− mice. Importantly, the current study also documented that Pla2g1b inactivation also suppressed atherosclerosis in LDL receptor-deficient mice fed the hypercaloric diet. Since Pla2g1b is present only in the digestive tract [5], where it generates and transport lysophospholipids to the liver resulting in reduced fatty acid oxidation and increased VLDL synthesis [10, 13], the reduced atherosclerosis observed in Pla2g1b−/−Ldlr−/− mice is likely mediated through reduced VLDL production and the consequential benefit of reduced plasma lipid levels on atherosclerosis. Taken together, our data obtained from studies in LDL receptordeficient mice demonstrated proof of principle in a preclinical animal model of human hyperlipidemia and atherosclerosis.
The clinical implication of our observations is that Pla2g1b inactivation may be a viable strategy to reduce atherosclerosis and metabolic diseases associated with chronic consumption of hypercaloric diets. Currently, the most effective strategy to reduce hyperlipidemia and atherosclerosis is the use of statins to inhibit endogenous cholesterol biosynthesis and to promote plasma LDL catabolism through LDL receptor induction. However, a recent survey estimated that 1/200 of the general population is heterozygous for familial hypercholesterolemia due to LDL receptor mutation, and a large proportion of these individuals failed to achieve recommended cholesterol levels upon statin therapy [17]. The advent of ezetimibe to block intestinal cholesterol absorption has provided an alternative strategy to reduce plasma cholesterol levels and decrease atherosclerosis risk [18]. The current study showing the effectiveness of Pla2g1b inactivation to reduce plasma cholesterol levels and suppress atherosclerosis in animals with LDL receptor deficiency suggests another novel strategy through Pla2g1b inhibition to enhance hepatic fatty acid oxidation for treatment of atherosclerosis. Since cholesterol absorption is not impaired in Pla2g1b−/− mice [8], this strategy is independent of cholesterol absorption efficiency and may be used in combination with ezetimibe for treatment of a large population of individuals that are resistant to statin therapy. Furthermore, in normal hyperlipidemic and diabetic individuals, inhibition of Pla2g1b may also complement statin and metformin therapies, respectively. Since the predominant mechanism by which Pla2g1b promotes hyperlipidemia and cardiometabolic diseases is through the production and absorption of lysophospholipids in the intestinal lumen [10, 11, 13, 15], and the advantage of minimal side effects of non-systemic drugs that act primarily in the gastrointestinal tract [19], non-absorbable Pla2g1b inhibitors should be considered for development as novel therapeutics for the treatment of hyperlipidemia and cardiometabolic diseases.
Highlights.
Inactivation of the group 1b phospholipase A2 ameliorates diet-induced atherosclerosis.
Group 1b phospholipase A2 inactivation improves insulin sensitivity.
Group 1b phospholipase A2 inactivation suppresses diet-induced body weight gain.
Acknowledgments
This work was supported by grant DK069967 from the National Institutes of Health. N.I. Hollie was a fellowship recipient of grants F31HL110527 from the National Heart, Lung, and Blood Institute, Grant T32GM063483 from the National Institute of General Medical Sciences, and Grant 11PRE7310047 from the American Heart Association during the course of this study.
Non-standard Abbreviations
- Pla2g1b
Group 1B phospholipase A2
- FPLC
fast performance liquid chromatrography
- VLDL
Very low density lipoproteins
- LDL
Low density lipoproteins
- LDLR
LDL receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No conflicts of interest to disclose.
References
- 1.Agouridis AP, Filippatos TD, Tsimihodimos V, Elisaf MS. Combinations of ezetimibe with nonstatin drug regimens affecting lipid metabolism. Expert Rev Cardiovasc Ther. 2011;9:355–366. doi: 10.1586/erc.11.4. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RH, Miles JM. The use of orlistat in the treatment of obesity, dyslipidaemia and Type 2 diabetes. Expert Opin Pharmacother. 2005;6:2483–2491. doi: 10.1517/14656566.6.14.2483. [DOI] [PubMed] [Google Scholar]
- 3.Altmann SW, Davis HR, Zhu L-J, Yao X, Hoos LM, Tetzloff G, Lyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SG, Adam G, Langdown M, Reneland R, Braun A, Andrew T, Surdulescu GL, Norberg M, Dudbridge F, Reed PW, Sambrook PN, Kleyn PW, Spector TD. Linkage and potential association of obesity-related phenotypes with two genes on chromosome 12q24 in a female dizygous twin cohort. Eur J Hum Genet. 2006;14:340–348. doi: 10.1038/sj.ejhg.5201551. [DOI] [PubMed] [Google Scholar]
- 5.Richmond BL, Hui DY. Molecular structure and tissue-specific expression of the mouse pancreatic phospholipase A2 gene. Gene. 2000;244:65–72. doi: 10.1016/s0378-1119(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 6.Nevalainen TJ, Gronroos JM, Kortesuo PT. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993;34:1133–1136. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aufenanger J, Samman M, Qunitel M, Fassbender K, Zimmer W, Bertsch T. Pancreatic phospholipase A2 activity in acute pancreatitis: a prognostic marker for early identification of patients at risk. Clin Chem Lab Med. 2002;40:293–297. doi: 10.1515/CCLM.2002.046. [DOI] [PubMed] [Google Scholar]
- 8.Richmond BL, Boileau AC, Zheng S, Huggins KW, Granholm NA, Tso P, Hui DY. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A(2) deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- 9.Huggins KW, Boileau AC, Hui DY. Protection against diet-induced obesity and obesity-related insulin resistance in Group 1B PLA2-deficient mice. Am. J. Physiol. 2002;283:E994–E1001. doi: 10.1152/ajpendo.00110.2002. [DOI] [PubMed] [Google Scholar]
- 10.Labonte ED, Pfluger PT, Cash JG, Kuhel DG, Roja JC, Magness DP, Jandacek RJ, Tschop MH, Hui DY. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: the molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010;24:2516–2524. doi: 10.1096/fj.09-144436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labonte ED, Kirby RJ, Schildmeyer NM, Cannon AM, Huggins KW, Hui DY. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes. 2006;55:935–941. doi: 10.2337/diabetes.55.04.06.db05-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cash JG, Kuhel DG, Goodin C, Hui DY. Pancreatic acinar cell-specific overexpression of group 1B phospholipase A2 exacerbates diet-induced obesity and insulin resistance in mice. Int J Obes. 2011;35:877–881. doi: 10.1038/ijo.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollie NI, Hui DY. Group 1B phospholipase A2 deficiency protects against diet-induced hyperlipidemia in mice. J Lipid Res. 2011;52:2005–2011. doi: 10.1194/jlr.M019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingrel JB, Pilcher-Roberts R, Basford JE, Manoharan P, Neumann J, Konaniah ES, Srinivasan R, Bogdanov VY, Hui DY. Myeloid-specific Kruppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ Res. 2012;110:1294–1302. doi: 10.1161/CIRCRESAHA.112.267310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui DY, Cope MJ, Labonte ED, Chang H-T, Shao J, Goka E, Abousalham A, Charmot D, Buysse J. The Phospholipase A2 inhibitor methyl indoxam suppresses diet-induced obesity and glucose intolerance in mice. Br J Pharmacol. 2009;157:1263–1269. doi: 10.1111/j.1476-5381.2009.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollie NI, Cash JG, Matlib MA, Wortman M, Basford JE, Abplanalp W, Hui DY. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbalip.2013.11.013. ePub ahead of print (pii: S1388-1981(1313)00264-00263. doi:00210.01016/j.bbalip.02013.00211.00013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordestgaard BG, Chapman MJ, Humphries Steve E, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed MH, Byrne CD. Potential therapeutic uses for ezetimibe beyond lowering LDL-c to decrease cardiovascular events. Diabetes Obes Metab. 2010;12:958–966. doi: 10.1111/j.1463-1326.2010.01261.x. [DOI] [PubMed] [Google Scholar]
- 19.Chambot D. Non-systemic drugs: A critical review. Curr Pharm Des. 2012;18:1434–1445. doi: 10.2174/138161212799504858. [DOI] [PMC free article] [PubMed] [Google Scholar]