Abstract
Since 2000, Clostridium difficile isolates of ribotype 027 have been linked to outbreaks in North America and Europe and also an increased rate of colectomy and death among infected individuals. It has been proposed that enhanced sporulation and toxin production were associated with this apparent increase in virulence of 027 isolates. Since only a limited number of isolates have been examined, the relationship of these phenotypes to a specific ribotype, and as well as to clinical disease severity, remains controversial. 106 recent clinical isolates from the University of Michigan Health System were characterized for the ability to sporulate, produce viable spores, grow in rich media, and produce toxins in vitro. Significant variation was observed between isolates for each of these phenotypes. Isolates of ribotype 027 produced higher levels of toxin and exhibited slower growth compared to other ribotypes. Importantly, increased spore production did appear to be relevant to severe C. difficile infection, as determined by available clinical meta-data. These data provide the first significant difference between isolates from severe vs. less severe disease based on an in vitro C. difficile phenotype and suggest that clinical outcome is better predicted by bacterial attributes other than ribotype.
Keywords: Clostridium difficile, Ribotype, NAP1/027, Sporulation
1. Introduction
Clostridium difficile is an anaerobic Gram-positive, spore forming bacterium commonly isolated from soil, humans, and other mammals [1]. Approximately 1e3% of humans are asymptomatic carriers of C. difficile. Clinically manifest C. difficile infection (CDI) of the distal gastrointestinal tract most commonly occurs after exposure to antibiotic treatment. Both the prevalence and clinical severity of CDI have been increasing over the last 12 years [2,3].
Coincident with the recent change in CDI epidemiology, a single pathogenic clone, identified by PCR ribotyping as ribotype 027, emerged and became a prominent pathogen in hospitals worldwide [3]. It appeared that the C. difficile 027 ribotype (hereafter referred to simply as 027) was responsible for disproportionately more severe CDI cases compared to other ribotypes during this time and so it was referred to as “hypervirulent.” As a result, the term “hypervirulent” has become a popular topic in the CDI field and has reached near dogmatic status.
A number of studies have reported potential mechanisms for this apparent increase in virulence. Higher rates of sporulation and greater levels of toxin production have been observed in 027 isolates [4–6]. More recent studies have begun to question whether 027 and related ribotypes truly are associated with increased disease severity and specific in vitro phenotypes. A recent study examining 743 C. difficile isolates from CDI patients was unable to find a significant association between ribotype and severe CDI after adjustment for other clinical and epidemiologic variables [7]. Another study examining sporulation of greater numbers of representative 027 isolates found no significant increase in sporulation between these isolates and other representative ribotypes [8].
To address this controversy, we sought to determine whether in vitro phenotypes were specific to 027 isolates at our institution and to also determine whether these phenotypes were associated with severe human disease, regardless of ribotype. Spore formation, spore viability, isolate-specific growth rates, and levels of toxin production were measured for a collection of 106 isolates.
2. Methods and materials
2.1. Strain isolation and selection
Clinical cases of C. difficile [7,9]were identified by the clinical microbiology laboratory at the University of Michigan as previously described. C. difficile isolates were cultured from patient stool samples using taurocholate-cefoxitin-cycloserine-fructose agar plates using a published protocol [10]. Isolates were selected using three criteria. First, we included a broad selection of isolates to represent the diversity of ribotypes in circulation at our hospital between January 2010 and February 2011. Second, we purposefully enriched the study for the epidemic ribotype 027 (n = 27) and the predominant ribotype in our hospital at the time, 014 (n = 21). Third, isolates from every case of severe CDI identified at our hospital during the sampling time were included in the study (n = 34). Cases exhibiting an infection that either: i) required admission to an ICU, ii) required an interventional surgery (e.g. colectomy), or iii) resulted in death, within 30 days of diagnosis were defined as severe, according to CDC recommendations [11]. These presentations had to be attributable to C. difficile infection in order for the case to be considered severe CDI.
2.2. Bacterial growth conditions and spore stocks
C. difficile isolates were cultured in an anaerobic chamber (Coy Laboratory Products, MI). For in vitro experiments, C. difficile isolates were cultured in BHIS (brain–heart infusion broth supplemented with 0.5% yeast extract and 0.1% cysteine; no glucose or iron were added to this medium) unless otherwise indicated. Spore stocks for all isolates were produced as follows. Freezer stocks of clinical isolates (single passage) were plated on BHIS. An isolated colony was used to inoculate an overnight culture. Four plates were then inoculated with 100 ml of overnight culture and incubated for seven days at 37 °C under anaerobic conditions before being moved to normal oxygen for one day to kill vegetative bacteria. Bacterial lawns were then resuspended in 4 °C water and washed at least four times to remove vegetative cell debris [10]. Spore suspensions were also heated at 65 °C for 30 min to ensure killing any remaining vegetative bacteria Spore stocks were stored in water at 4 °C.
2.3. Germination assays
Germination was measured by tracking loss of spore heat resistance over time as previously described [12]. Spores were incubated anaerobically with 0.1% taurocholate (Tc) in BHIS for 10 min. Following incubation, an aliquot was heated at 65 °C for 20 min to kill germinated spores. Remaining spores were enumerated by plating for colony-forming units (CFU) on BHIS plates supplemented with 0.1% Tc. Colonies that grew on these plates were considered to be ungerminated spores and were compared back to spore stocks to quantify the percent germination. Assays were performed in triplicate for each isolate tested.
2.4. Measurement of spore viability
Spore viability was defined as the ratio of total spores to those capable of outgrowth [13]. Total spore particles in each stock were enumerated using a light microscope and a hemocytometer. Numbers of viable spores were quantified by plating spore stocks for CFU onto BHIS agar supplemented with 0.1% Tc. Assays were performed in triplicate for each isolate tested.
2.5. Sporulation assays
In order to quantify spore production from each isolate of C. difficile, each isolate was grown in broth culture for 24 h and examined for the presence of heat resistant CFU. Sporulation of clinical isolates was defined by an increase in heat resistant colonies over time in culture. In order to insure that all cultures were actively growing prior to setting up sporulation assays, overnight cultures were diluted 10-fold into fresh BHIS and incubated for 4 h at 37 °C. Following this incubation, fresh BHIS cultures were inoculated at an OD600 = 0.01 and cultured at 37 °C for 24 h. Following this incubation, samples were incubated at 65 °C for 30 min to kill vegetative bacteria. Total spores in each culture were then enumerated by plating for heat-resistant colony forming units (CFU) on BHIS agar supplemented with 0.1% Tc. Assays were performed in triplicate for each isolate tested.
2.6. Measurement of in vitro growth rates
Growth assays were performed in 96-well plates in a Power-wave HT plate reader (BioTek) inside of an anaerobic chamber (Coy Laboratories). Each culture was inoculated with ~4 viable spores in 200 ml of BHIS supplemented with 0.1% Tc. Growth experiments were run for 48 h with OD600 readings taken every 10 min. Data for each well were normalized to a blank (an un-inoculated well) and used to estimate growth parameters. A model-free, smoothed cubic spline was fit to data for each isolate using the grofit package in R and the maximum growth rate (μ = OD600/hour) parameter was estimated and used from comparative analysis [14,15]. Five independent growth curves were performed for each isolate tested.
2.7. Toxin production assay
Vero cell viability was used to measure toxin production from C. difficile clinical isolates. Triplicate C. difficile cultures were initiated with early log-phase vegetative bacteria at an OD600 0.01 in BHIS + 0.1% Tc and incubated at 37 °C for 18 h. Following incubation, samples were centrifuged to remove bacteria and culture supernatants were frozen at −20 °C. Cell cytotoxicity assays were modified from Lyras et al. [16] and performed as follows: Vero cells were seeded in 96-well plates containing DMEM supplemented with 10% FBS at a concentration of 5 × 103 cells/well and incubated overnight at 37 °C. The following day, culture supernatants were thawed, filtered through a 0.2 μm filter, and diluted serially (2-fold) in culture media before overlaying onto wells containing Vero cells. Dilutions and transfer to plates containing Vero cells were performed using a Biomek FX (Beckman Coulter Inc.). Plates containing the supernatant/Vero cell mixtures were then incubated at 37 °C + 5% CO2 for an additional 48 h. Following incubation, the viability of Vero cells was determined using the Roche cell proliferation I kit (MTT) according to the manufacturer's protocol. Toxin activity in culture supernatants was quantified based on a standard curve, which was generated using known concentrations of puri-fied C. difficile toxin B (List Biological Laboratories, Inc). Assays were performed in triplicate for each isolate tested.
2.8. Statistical analyses
All statistical methods were performed using GraphPad Prism version 5.05 for Windows (GraphPad Software, San Diego California USA). For comparison of two groups, the nonparametric Manne–Whitney test was used. For three group comparisons, the nonparametric Kruskal–Wallis test was used with Dunn's post-test to compare column pairs. Spearman's correlation coefficient and corresponding p-value were used to investigate whether there was significant correlation between growth rate and toxin production.
2.9. Human subjects approval
The University of Michigan Institutional Review Board approved all sample and clinical data collection protocols used in this study. Where applicable, written, informed consent was received from all patients prior to inclusion in this study.
3. Results
3.1. Clinical isolate description
C. difficile isolates were collected from symptomatic patients at the University of Michigan Hospital (Ann Arbor, Michigan, USA) and/or affiliated outpatient clinics between January 2010 and February 2011. Clinical data were reviewed for each patient and severe CDI cases were identified according to CDC recommended guidelines [11]. The University of Michigan Institutional Review Board approved all sample and clinical data collection protocols.
All 106 isolates tested were toxigenic based on diagnostic testing (ELISA and/or PCR for the toxin B gene, tcdB) and a Vero cell toxicity assay in the laboratory. The collection contained multiple representatives of 12 ribotypes as well as 27 single representatives of other ribotypes (see Table S1 for specific isolate information). Isolates of ribotype 027 were intentionally over-represented in this data set (n = 27) as were the number of isolates from severe CDI cases (n = 34) in order to specifically address whether they are statistically associated with the assayed phenotypes. Isolates representing ribotype 014 were also over-represented in this data set (n = 21), as this was the most common ribotype observed in patients at the University of Michigan Health System during the collection time period. The availability of clinical meta-data associated with each isolate allowed for the elucidation of any potential relationships between specific in vitro microbiological phenotypes and either isolate ribotype or disease severity. Complete descriptions of each isolate can be found in Table S1.
3.2. Germination and spore viability
It is possible that the differences in clinical severity associated with infection by various C. difficile isolates are related to altered spore dynamics (germination and sporulation). To address this, 36 of the 106 isolates, including 11 representative 027 isolates, were assayed for efficiency of spore germination as scored by loss of heat resistance following exposure to sodium taurocholate, the primary germinant of C. difficile [17], in rich media (BHIS + Tc). Each isolate tested exhibited ≥90% germination within 10 min of exposure to this germinant (data not shown). Since each isolate tested had nearly identical germination efficiencies under these conditions, this assay was not performed for the entire collection.
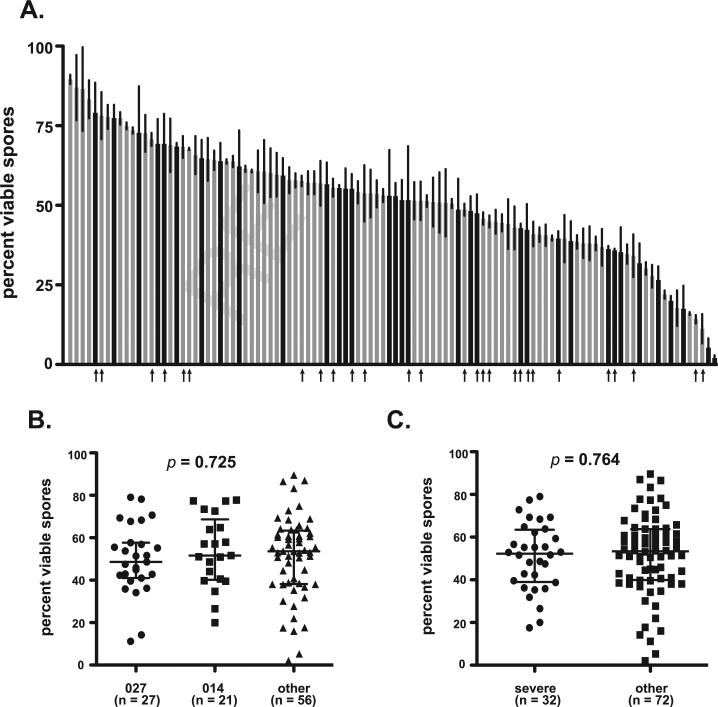
As an alternative, the percent viability of spores produced by each of the clinical isolates was examined. To this end, a colony forming efficiency assay was performed to determine the percentage of total spore particles that were capable of outgrowth [13]. This assay was performed in triplicate for each isolate and the mean percent viabilities are presented in Fig. 1. Significant differences in spore viability were observed across isolates and ranged from 2.0% to 89.5% (Fig. 1A). To determine if spore viability differed between isolate ribotypes, three separate groups were analyzed: 1.) ribotype 027 (previously defined as “hypervirulent”); 2.) ribotype 014, (the most common ribotype isolated at the University of Michigan Hospitals); and 3.) all other ribotypes. All three ribotype groups exhibited a similar range of spore viability and no significant differences were detected between group medians (Fig. 1B, p = 0.725).
Fig. 1.
Viability of spores produced by C. difficile clinical isolates. (A) Viability of spores for each clinical isolate was determined using colony forming efficiency assay. All measurements were performed in triplicate for each isolate and presented as the percent viable spores = ((CFU/ml)/(spore particles/ml)) × 100. Data are presented as % viable spores and represent mean ± SD of triplicate measurements. Black bars represent isolates that caused severe disease, while gray bars represent those that did not. Arrows indicate strains of ribotype 027. A similar figure labeled with labeled with all strain designations is available as supplementary data (Supplementary Figure 1A). (B) Comparison of median values with interquartile range for isolates representing ribotypes 027, 014, or “other.” Groups were compared for statistical significance using KruskaleWallis test (p = 0.725) followed by Dunn's test for column comparisons (no significant differences). (C) Comparison of median values with interquartile range for isolates that caused severe disease were compared to other using unpaired ManneWhitney t-test (p = 0.764).
Next, the relationship between disease severity and spore viability was examined. Isolates that caused severe and non-severe disease had similar spore viability levels (severe median = 53.3%, non-severe median = 53.3). As was observed for ribotype comparisons, isolates from severe CDI cases did not exhibit a statistical difference in spore viability when compared to isolates from non-severe CDI (Fig. 1C, p = 0.764).
3.3. Sporulation
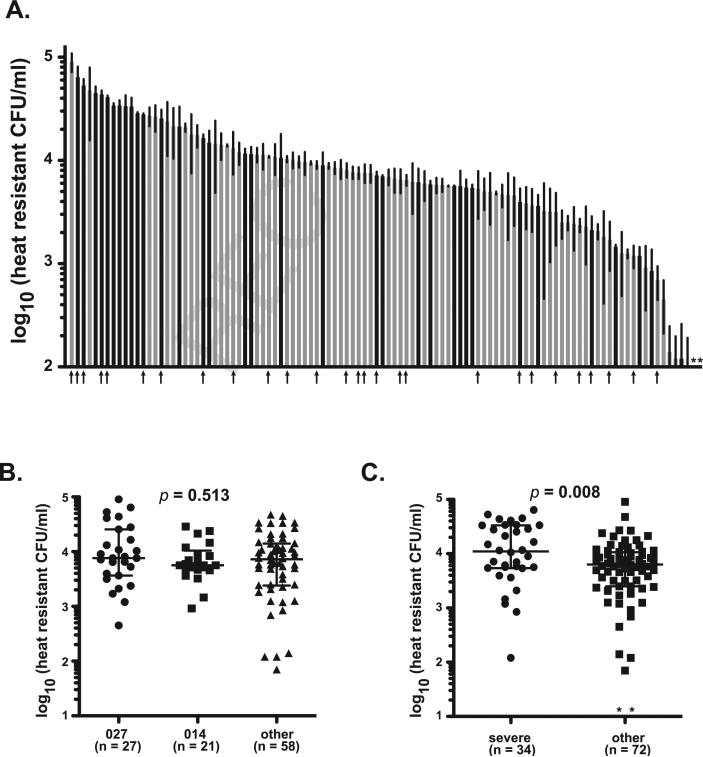
To better understand differences in sporulation characteristics among these clinical isolates, the number of heat-resistant colony forming units (CFU) present following 24 h of growth in culture media was quantified (Fig. 2). Isolates exhibited a wide range of sporulation over this time, ranging from those that produced no detectible spores to 9 × 104 spores/ml (Fig. 2A). The same ribotype groups used in the spore viability assay above were examined for differences in sporulation levels. All three groups exhibited similar median sporulation levels after 24 h and no significant differences were observed (Fig. 1B, p = 0.513).
Fig. 2.
Sporulation characteristic of C. difficile clinical isolates. (A) Total spore production following 24 h of in vitro growth was determined for each clinical isolate by measuring heat-resistant CFU. Data are presented as heat resistant CFU/ml (limit of detection ~100 spores/ml) and represent mean ± SD of three independent cultures. * = No spores were detected in any replicates for these isolates. Black bars represent isolates that caused severe disease, while gray bars represent those that did not. Arrows indicate strains of ribotype 027. A similar figure labeled with all strain designations is available as supplementary data (Supplementary Figure 1B). (B) Comparison of median values with interquartile range for isolates representing ribotypes 027, 014, or “other.” Groups were compared for statistical significance using Kruskal–Wallis test (p = 0.513) followed by Dunn's test for column comparisons (no significant differences). (C) Comparison of median values with interquartile range for isolates that caused severe disease were compared to other using unpaired Mann–Whitney t-test (p = 0.008).
Importantly, isolates that caused severe disease appeared to cluster at the upper end of the sporulation range (Fig. 2A, black bars). Although these isolates exhibited a similar range in values as the entire data set, isolates from severe cases of disease produced nearly twice as many spores compared to isolates from non-severe cases (Fig. 2C, median values: severe = 1.1 × 104 spores/ml; non-severe = 6.3 × 103 spores/ml) and this difference was statistically significant (p = 0.008).
3.4. Growth rates
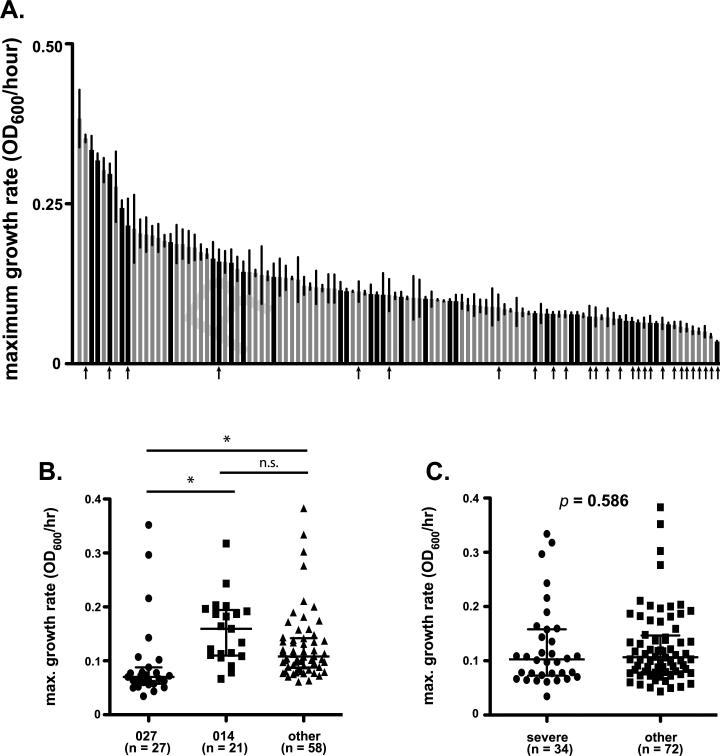
The maximum increase in OD600 per hour was quantified for each isolate and was considered to be an estimate of growth rate (μ). Isolates in this collection exhibited a 10-fold difference in overall growth rates (Fig. 3A; range: 0.38e0.03 OD600/hour; equivalent doubling times = 1.8–22.0 h, median 6.5 h). Interestingly, when growth rates were compared based on isolate ribotype groups, a clear pattern was evident. Although the overall range in growth rates for 027 isolates was similar to other isolates, 20 (74%) of the 027 isolates were in the bottom third of the data range (Fig. 3A, arrows). Median growth rates differed between ribotype groups (Fig. 3B, p < 0.0001), with 027 isolates exhibiting slower growth than the other ribotype groups. No difference was observed between the 014 and other ribotype groups.
Fig. 3.
Growth rate differences among C. difficile clinical isolates. (A) Maximal growth rates in BHIS broth were determined for each clinical isolate. Data are presented at maximum increase in OD600 per hour and represent mean ± SD of five independent curves for each isolate. Black bars represent isolates that caused severe disease, while gray bars represent those that did not. Arrows indicate strains of ribotype 027. A similar figure labeled with labeled with all strain designations is available as supplementary data (Supplementary Figure 1C). (B) Comparison of median values with interquartile range for isolates representing ribotypes 027, 014, or “other.” Groups were compared for statistical significance using Kruskal–Wallis test (p < 0.0001) followed by Dunn's test for column comparisons (* = p < 0.05). (C) Comparison of median values with interquartile range for isolates that caused severe disease were compared to other using unpaired Mann–Whitney t-test (p = 0.586).
It was hypothesized that isolates that can rapidly expand would cause more severe disease, due to increased bacterial load and concomitant virulence factor production. On the contrary, isolates from severe CDI cases did not appear to grow more rapidly than others. In fact, these isolates exhibited a similar range of growth rates as those from less severe cases and there was not a significant difference observed between these isolate groups (Fig. 3C, p = 0.586).
3.5. Toxin production
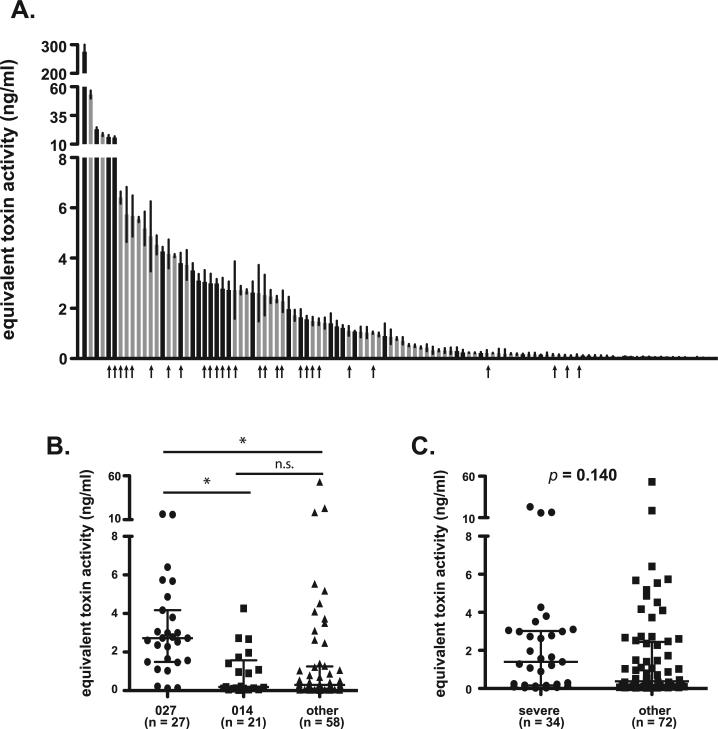
Previous reports have indicated increased levels of toxin production from representative 027 isolates [5,6,18]. To examine this phenotype within the current isolate set, total toxin production from each isolate was measured following 18 h of vegetative growth in culture. The clinical isolates tested exhibited significant differences in in vitro toxin production, with total detectable toxin ranging from 0.028 to 53.2 ng/ml (Fig. 4A). It should be noted that one isolate produced over 250 ng/mL of toxin, but was excluded from statistical analyses as an outlier. Isolates of ribotype 027 exhibited higher levels of toxin production than those of other ribotypes. Toxin production of these isolates ranged from 0.12 to 16.3 ng/mL (median = 2.7 ng/ml) with 18 of 27 isolates falling in the top third of the data set (Fig. 4A, arrows). The increase in toxin production observed from 027 isolates was statistically significant compared to isolates of both the 014 group and the “other ribotypes” group (Fig. 4B, p < 0.0001). No statistical difference was observed between the 014 isolates and the isolates in the “other” ribotype group (Fig. 4B).
Fig. 4.
Differences in toxin production by C. difficile clinical isolates. (A) Toxin production in vitro was measured for each clinical isolate using Vero cell cytotoxicity as measured by MTT assay. Toxin concentrations were estimated using a standard curve generated by treating Vero cells with purified C. difficile toxin B. Data are presented at equivalent toxin activity in ng/ml and represent mean ± SD of three independent cultures. Black bars represent isolates that caused severe disease, while gray bars represent those that did not. Arrows indicate strains of ribotype 027. A similar figure labeled with all strain designations is available as supplementary data (Supplementary Figure 1D). (B) Comparison of median values with interquartile range for isolates representing ribotypes 027, 014, or “other.” Groups were compared for statistical significance using Kruskal–Wallis test (p < 0.0001) followed by Dunn's test for column comparisons (* = p < 0.05). (C) Comparison of median values with interquartile range for isolates that caused severe disease were compared to other using unpaired ManneWhitney t-test (p = 0.140).
Since these exotoxins are the primary virulence factors of C. difficile [16,19], it was hypothesized that increased in vitro toxin production would predict severe disease. However, as observed for isolate growth rates, no statistical difference was observed in toxin production in vitro between isolates from severe and non-severe cases. Isolates that caused severe disease exhibited a range of toxin production in vitro (0.024–23.0 ng/ml) similar to those that caused less severe disease (0.024–53 ng/ml) and no statistically significant difference was observed between these groups (Fig. 4C, p = 0.140).
3.6. Relationship between toxin production and growth rate
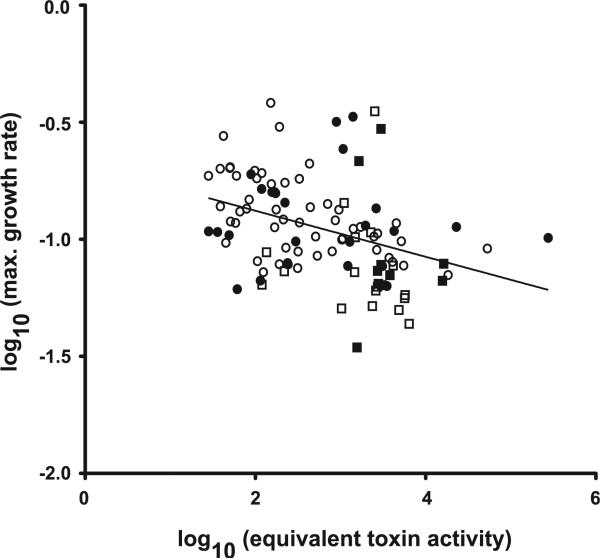
The data presented above show statistical associations between 027 isolates and both increased toxin production and decreased in vitro growth rate. Since toxin production is thought to be growth phase and nutrient dependent [20], the relationship between growth rates and toxin production was examined further. A negative correlation was observed between these two phenotypes (Fig. 5, Spearman r = −0.4591, p < 0.0001). In other words, isolates that produced higher levels of toxin also grew more slowly. This correlation was not dependent on 027 isolates, as the statistical significance remained when these data were removed (p = 0.0004).
Fig. 5.
Negative correlation between isolate toxin production and growth rate. Scatterplot of log transformed growth rates vs. log transformed toxin levels. Closed squares = 027 – severe disease; open squares = 027 – non-severe disease; closed circles = other ribotypes e severe disease; open circles = other ribotypes – non-severe disease. Spearman correlation r = 0.4591; p > 0.0001.
4. Discussion
CDI has become a major healthcare problem throughout the world. This study is the first we know of to report that C. difficile isolates that caused severe CDI, regardless of ribotype, produce more spores than isolates from less severe cases. We also showed that isolates of ribotype 027 expressed higher levels of toxin and grew more slowly than isolates of other ribotypes, but that these in vitro phenotypes were not associated with a severe clinical outcome. The observed correlation between growth rates and toxin production is interesting in terms of cellular regulation of virulence factors and warrants further study.
The increased incidence of CDI over the last decade at healthcare facilities around the globe has raised public awareness and brought about many new clinical and microbiologic questions. Beginning in 2005, a number of studies have focused on the molecular epidemiology and pathogenesis of certain C. difficile genotypes that were referred to as “hypervirulent” [21]. These ribotypes appeared to cause more severe disease compared to other isolates and to also have increased toxin production and sporulation levels in vitro. These findings led some to suggest that such phenotypes were the underlying mechanisms of the observed increase in virulence [4,5]. Recent studies, however, that considered increased numbers of representative isolates have brought some of these findings into question. For example, Burns et al. examined the sporulation potential of 53 isolates, including 28 representative 027 isolates, and concluded that 027 isolates did not exhibit higher levels of sporulation than those of other ribotypes tested [8]. Therefore, the original hypothesis that 027 isolates cause more cases of severe disease because they produce more spores remains controversial. In support of these findings, we also observed that 027 isolates do not inherently produce more spores than isolates of other ribotypes (Fig. 2).
The most significant finding of the present study was that isolates from severe CDI cases had greater sporulation levels after 24 h in culture than isolates from less severe cases. As with other spore-forming, pathogens, the C. difficile spore is believed to be the infectious particle, or the means by which disease is transmitted from person to person. A model supported by our results is that isolates with greater sporulation potential transmit more spores to susceptible individuals, leading to a greater infective dose. This increased inoculum may, in turn, lead to increased vegetative cell numbers and greater levels of toxin production in the antibiotic-perturbed GI tract and ultimately facilitate virulence.
In contrast, neither germination rates, nor spore viability, were significantly greater among 027 isolates or among isolates from cases of severe disease. However, several important observations were made. Unlike the other phenotypes tested, all tested isolates exhibited nearly complete germination within only 10 min (data not shown). This high level of conservation across isolates likely indicates the universal importance of this process in nature and is intriguing, particularly considering the wide range of values observed in each of the other phenotypes tested. For example, spore viability was much more heterogeneous across all isolates, with some producing spores that were nearly all viable and others producing very few viable spore particles. It is interesting to note that low percentages of viable spores did not appear to be dependent on the number of overall particles with several of the least viable spore preparations having large numbers of phase-bright spore particles. This observation indicates two distinct possibilities. Either the process of sporulation is relatively inefficient or some of these isolates are able to germinate in response to a currently undefined/untested germinant that was not present in the assay medium used. The presence of another germinant in vivo seems likely since other pathogenic spore formers, such as Bacillus anthracis, sporulate robustly in vitro, typically producing only viable spores with few-to-no additional spore-like particles that are unable to germinate [13].
Higher toxin production was evident among 027 isolates compared to isolates of other ribotypes. These data support previous studies that considered toxin production of other 027 isolates [5,6]. Although there was an apparent difference in the median values for toxin production between severe and non-severe cases (1.48 ng/ml and 0.38 ng/ml, respectively), this difference was not determined to be statistically significant. While it is possible that these data would reach significance once greater numbers of severe cases are examined, the current results suggest that in vitro toxin production may not be a useful predictor of disease severity. In support of this hypothesis, a large epidemiologic investigation at our institution found that isolation of a hypervirulent ribotype was not predictive of severe CDI [7]. While this study considered both 027 and 078 isolates as “hypervirulent” the same result was obtained if 027 isolates were considered independently. Together these results indicate that in vitro toxin production, while greater for 027 isolates, appears to be a poor measure for determining the potential of a given isolate to cause severe disease.
Along with increased toxin production, 027 isolates also had significantly slower growth rates. These two phenotypes were negatively correlated among all isolates, suggesting a coordinated regulation in all C. difficile isolates. Importantly, this correlation was independent of ribotype, as the significance was retained even when removing 027 isolates from the analysis. The regulatory mechanism responsible for the negative correlation remains to be elucidated, but it is possible that a shift in overall metabolism allows isolates to better commit resources to the production of toxin. Much research has gone into understanding the regulation of toxin and the role of nutrient availability in this process. Toxin genes are induced during states of starvation and repressed when the cell has readily available nutrients [22–26]. These factors, along with specific toxin regulatory genes present in the pathogenicity locus, indicate a complex mechanism that has evolved to regulate toxin production. Alternatively, it is possible that 027 isolates have a decreased ability to acquire nutrients. This could result in a more constitutive state of starvation, leading to increased toxin production. Given the relationship between growth rate and toxin production presented here, a deeper understanding of the metabolic differences between these isolates may increase our knowledge of toxin regulation by this pathogen.
Our results, and those of other recently published studies, do not support the hypothesis that hypervirulence is a ribotype-specific trait. Similarly, reports that “hypervirulent” ribotypes exhibit increased sporulation compared to other ribotypes were not supported by two independent collections of clinical isolates (this study and [8]). Although the data presented here do confirm previous studies showing increased toxin production from isolates of these ribotypes, a correlation between high toxin production and severity of disease was not observed. Collectively, these results provide evidence that ribotyping may not provide clinically relevant information. It remains possible that some C. difficile isolates are hypervirulent, however, other methods are needed for identifying these isolates in the hospital setting.
It is important to note two potential caveats of this study. First, all bacterial phenotypes were examined under in vitro conditions, which may not mimic host environments/conditions. Although the only phenotype significantly associated with disease severity was increased sporulation, this does not exclude all other factors involved. Indeed, the differences in the microenvironments encountered within hosts, or the physical state of the hosts themselves, may also play a role in the severity of a given case of CDI. The second caveat is that the observed differences between isolates that cause more severe disease are dependent on the definition of “severe disease”. Clinical outcomes were used to define severe cases of disease and a more broad definition of clinical severity could, potentially, alter the conclusions. The fact that a strong correlative phenotype was identified even in light of these facts speaks to the strength and importance of this finding. Further studies are currently underway to understand the various host factors that may also play a role in disease, including alterations in immune response and any underlying physiologic patterns, including disease states.
In summary, we have shown that clinical isolates of C. difficile exhibit wide variation in basic microbiology, with significant differences observed in most of the phenotypes tested. It is perhaps surprising that such a large range of phenotypic results was observed from isolates that are considered currently to be very similar at the genetic level [27]. These differences can add to the difficulty in assigning general characteristics, such as “hypervirulence,” to isolate group by ribotype. Importantly, the data presented here clearly identify the first microbiological correlate of disease severity in C. difficile. Specifically, our work shows that isolates that exhibit increased sporulation tend to cause more severe disease. Specific features of the 027 ribotype group were also identified and could provide a useful tool for examining the specific role of these phenotypes and also for studying the regulation of toxin production.
Supplementary Material
Acknowledgments
This work was supported Clostridium difficile Cooperative Research Center (U19AI09087). PEC was also supported by grant number UL1RR024986 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRRR or the National Institutes of Health. The authors would like to thank Susan Foltin for technical assistance.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.anaerobe.2013.04.003.
References
- 1.Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clinical Microbiology and Infection. 2006;12(Suppl. 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 2.Dubberke E. Clostridium difficile infection: the scope of the problem. Journal of Hospital Medicine. 2012;7(Suppl. 3):S1–4. doi: 10.1002/jhm.1916. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009;136:1913–24. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 4.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, Wullt M, et al. Increased sporulation rate of epidemic Clostridium difficile Type 027/NAP1. Journal of Clinical Microbiology. 2008;46:1530–3. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan VK, Johnson S, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. Journal of Bacteriology. 2010;192:4904–11. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 7.Walk ST, Micic D, Jain R, Lo ES, Trivedi I, Liu EW, et al. Clostridium difficile ribotype does not predict severe infection. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2012;55:1661–8. doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns DA, Heeg D, Cartman ST, Minton NP. Reconsidering the sporulation characteristics of hypervirulent Clostridium difficile BI/NAP1/027. PLoS ONE. 2011;6:e24894. doi: 10.1371/journal.pone.0024894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao K, Walk ST, Micic D, Chenoweth E, Deng L, Galecki AT, et al. Procalcitonin levels associate with severity of Clostridium difficile infection. PLoS ONE. 2013;8:e58265. doi: 10.1371/journal.pone.0058265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorg JA, Dineen SS. Laboratory maintenance of Clostridium difficile. Current Protocols in Microbiology. 2009 doi: 10.1002/9780471729259.mc09a01s12. Chapter 9:Unit9A 1. [DOI] [PubMed] [Google Scholar]
- 11.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infection Control and Hospital Epidemiology. 2007;28:140–5. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 12.Carr KA, Lybarger SR, Anderson EC, Janes BK, Hanna PC. The role of Bacillus anthracis germinant receptors in germination and virulence. Molecular Microbiology. 2010;75:365–75. doi: 10.1111/j.1365-2958.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- 13.Giebel JD, Carr KA, Anderson EC, Hanna PC. The germination-specific lytic enzymes SleB, CwlJ1, and CwlJ2 each contribute to Bacillus anthracis spore germination and virulence. Journal of Bacteriology. 2009;191:5569–76. doi: 10.1128/JB.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahm M, Hasenbrink G, Lichtenberg-Frate H, Ludwig J, Kschischo M. Grofit: fitting biological Growth Curves with R. Journal of Statistical Software. 2010;33:1–21. [Google Scholar]
- 15.R. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. [Google Scholar]
- 16.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–9. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson KH, Kennedy MJ, Fekety FR. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. Journal of Clinical Microbiology. 1982;15:443–6. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vohra P, Poxton I. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology. 2011 doi: 10.1099/mic.0.046243-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuehne SA, Cartman ST, Minton NP. Both, toxin A and toxin B, are important in Clostridium difficile infection. Gut Microbes. 2011;2:252–5. doi: 10.4161/gmic.2.4.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vohra P, Poxton IR. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology. 2011;157:1343–53. doi: 10.1099/mic.0.046243-0. [DOI] [PubMed] [Google Scholar]
- 21.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. The New England Journal of Medicine. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 22.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. Repression of Clostridium difficile toxin gene expression by CodY. Molecular Microbiology. 2007;66:206–19. doi: 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda D, Karasawa T, Yamakawa K, Tanaka R, Namiki M, Nakamura S. Effect of isoleucine on toxin production by Clostridium difficile in a defined medium. Zentralbl Bakteriol. 1998;287:375–86. doi: 10.1016/s0934-8840(98)80174-6. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson S, Burman LG, Akerlund T. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology. 1999;145(Pt 7):1683–93. doi: 10.1099/13500872-145-7-1683. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson S, Burman LG, Akerlund T. Induction of toxins in Clostridium difficile is associated with dramatic changes of its metabolism. Microbiology. 2008;154:3430–6. doi: 10.1099/mic.0.2008/019778-0. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infection and Immunity. 2000;68:5881–8. doi: 10.1128/iai.68.10.5881-5888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Didelot X, Eyre DW, Cule M, Ip CL, Ansari MA, Griffiths D, et al. Microevolutionary analysis of Clostridium difficile genomes to investigate transmission. Genome Biology. 2012;13:R118. doi: 10.1186/gb-2012-13-12-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.