Abstract
The islet-antigens IA-2 and IA-2β are major autoantigens in type-1 diabetes and transmembrane proteins in dense core vesicles (DCV). Recently we showed that deletion of both IA-2 and IA-2β alters the secretion of hormones and neurotransmitters and impairs behavior and learning. The present study was designed to evaluate the contribution to learning of each of these genes by using single knockout (SKO) and double knockout (DKO) mice in an active avoidance test. After 5 days of training, wild type (WT) mice showed 60–70% active avoidance responses, whereas the DKO mice showed only 10–15% active avoidance responses. The degree of active avoidance responses in the IA-2 SKO mice was similar to that of the DKO mice, but in contrast, the IA-2β SKO mice behaved like WT mice showing 60–70% active avoidance responses. Molecular studies revealed a marked decrease in the phosphorylation of the cAMP Response Element-Binding Protein (CREB) and Ca2+/Calmodulin-Dependent Protein Kinase II (CAMKII) in the striatum and hippocampus of the IA-2 SKO and DKO mice, but not in the IA-2β SKO mice. To evaluate the role of CREB and CAMKII in the SKO and DKO mice, GBR-12909, which selectively blocks the dopamine uptake transporter and increases CREB and CAMKII phosphorylation, was administered. GBR-12909 restored the phosphorylation of CREB and CAMKII and increased active avoidance learning in the DKO and IA-2 SKO to near the normal levels found in the WT and IA-2β SKO mice. We conclude that in the absence of the DCV protein IA-2, active avoidance learning is impaired.
Keywords: autoantigens, type-1 diabetes, dopamine, CREB, CAMKII
The insulinoma-associated proteins IA-2 and IA-2β, also known, respectively, as ICA512 and phogrin, are transmembrane proteins of dense core vesicles (DCV) and are found in neuroendocrine cells throughout the body (Lan et al., 1996, Lu et al., 1996, Takeyama et al., 2009). Based on sequence analysis both are members of the receptorlike protein tyrosine phosphatase (PTP) family, but are enzymatically inactive on standard PTP substrates because of two critical amino acid substitutions in the PTP domain (Lan et al., 1994, Magistrelli et al., 1996). Recently, however, IA-2β was shown to have low phosphatidylinositol phosphatase activity (Caromile et al., 2010). Structurally IA-2 and IA-2β consist of an intracellular, transmembrane and luminal domain and show 74% identity in their intracellular domain, but only 26% identity in their luminal domain. IA-2 is 979 and IA-2β 986 amino acids in length. In humans, the genes for IA-2 and IA-2β are located, respectively, on chromosomes 2q35 and 7q36 and in mice on chromosomes 1 and 12 (Leiter et al., 1997, Saeki et al., 2000).
IA-2 and IA-2β have been of particular interest to the diabetes community because both are major autoantigens in type 1 diabetes (Notkins, 2007, Achenbach et al., 2008). Autoantibodies to these proteins appear years before the onset of clinical disease and in combination with other diabetes-associated autoantibodies have become predictive markers for this disease (Notkins, 2007). Studies on the biological function of these proteins by knockout experiments in mice and knockdown and overexpression experiments in neuroendocrine-secreting cell lines have shown that they affect the half-life and, in turn, the number of DCV (Harashima et al., 2005, Cai et al., 2011).
Alterations in the secretion of hormones (e.g. insulin) and neurotransmitters (e.g. dopamine, serotonin, glutamate) (Nishimura et al., 2009), secondary to the decreased expression of IA-2 and IA-2β, results in a variety of abnormalities including alterations in glucose tolerance, reproduction, behavior, learning and circadian rhythm (Kubosaki et al., 2004, Kubosaki et al., 2006, Kim et al., 2009, Nishimura et al., 2009).
Our initial learning and behavior experiments focused primarily on DKO mice in which both IA-2 and IA-2β were knocked out (Nishimura et al., 2009). The present experiments employing genetic, molecular, pharmacologic and behavioral approaches were initiated to determine the effect of the knockout of the individual IA-2 and IA-2β genes on learning and behavior as evaluated by an active avoidance test. These experiments showed that IA-2, but not IA-2β, is required for normal learning in the active avoidance test.
1. Experimental Procedures
1.1 Mice
Targeted disruption of the C57BL6 mouse IA-2 and IA-2β genes was described previously (Saeki et al., 2002, Kubosaki et al., 2004, Kubosaki et al., 2005). The targeted alleles were backcrossed to the C57BL/6J genetic background for eight (IA-2) and four (IA-2β) generations, and heterozygotes were crossed to give double heterozygotes. Double heterozygotes then were interbred to generate lines of wild-type (WT) (IA-2+/+/IA-2β+/+) mice, two lines of single knockout mice [IA-2-KO (IA-2−/−/IA-2β+/+) and IA-2β-KO (IA-2+/+/IA-2β−/−)], three-allele mutants (IA-2+/−/IA-2β−/−), and DKO mice (IA-2−/−/IA-2β−/−). Mice used in the current study were generated by breeding animals of the same genotype within each line, except that male IA-2−/−/IA-2β−/− mice were bred to three-allele female (IA-2+/−/IA-2β−/−) mice to generate DKO mice because female DKO mice are infertile (Kubosaki et al., 2006). IA-2−/−/IA-2β+/+ mice on the NOD background (IA-2 NOD SKO) were generated as described previously (Kubosaki et al., 2004). Male mice were used in the learning experiments to avoid endocrine changes related to the estrous cycle. All mice were genotyped before use and each mouse was bred, aged and analyzed in the same facility according to National Institutes of Health guidelines. Lights were on from 6:00 AM to 6:00 PM and food and water were available ad libitum. Behavioral experiments were generally conducted between 5:00 AM and 3:00 PM. All experimental procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee.
1.2 Active Avoidance Learning
Training was conducted using a fully automated shuttle-box located in a ventilated and sound-protected procedure room. The shuttle box (47 × 18 × 26 (h) cm) (Ugo Basile, Comerio Italy) was divided into two compartments with an opening between compartments. A light and sound (670 kHz and 70 dB, respectively) was used as the conditioned stimulus (CS) and preceded by 3 seconds the unconditioned stimulus (UCS) of an electric foot shock of 0.3mA. Animals could avoid the UCS by moving from one compartment into the other within 3 seconds after the onset of the CS. The UCS lasted for a total of 3 seconds. At the end of this period the trial was terminated and after a 30 second inter-trial interval a new trial was initiated. There were two training sessions per day each consisting of 50 trials. Thus, over the 5 days of the experiment each of the animals underwent 500 trials. The daily number of sessions and trials selected for this study was based on published papers using the active avoidance paradigm as an index of learning in mice (Smith et al., 2002, Trigo et al., 2008). The number of trials on which active avoidance responses were made by each animal when avoiding the UCS and the number of times each animal failed to escape the UCS were recorded by a computer attached to the automated shuttle-box. In treatment experiments designed to restore active avoidance learning, the selective dopamine uptake inhibitor GBR-12909 (20.0 mg/kg) or an equal volume of saline was administered intraperitoneally and the animals were returned to their home cages for 30 minutes before initiation of active avoidance testing.
1.3 Western Blotting
Mice were decapitated, their brains were quickly removed, and their hippocampi or striatum were rapidly dissected with the dissecting plate on ice. Samples were immediately homogenized in 10 vol. of ice-cold 80 mM Tris–HCl buffer (pH 7.4) containing 150 mM NaCl, 2 mM EDTA, 0.4 mM DTT and 0.1% SDS. The homogenates were centrifuged at 15,000g at 4 °C for 30-min and supernatants were collected. Protein concentration was determined by the Bradford protein assay. Equal amounts of proteins (50υg) from each group were separated on SDS-PAGE gel and electrophoretically transferred onto PVDF membranes (Millipore, Bedford, MA), blocked, and probed overnight at 4 °C with antibodies. Polyclonal anti-CREB and anti-phospho-CREB antibodies were purchased from Cell Signaling Technology (Beverly, MA). Monoclonal anti-CamKII and polyclonal anti-phospho-CamKII antibodies were purchased from Abcam (Cambridge MA).
1.4 Statistics
The principal dependent variable for the behavioral experiments was the percentage of trials with an active avoidance response during each daily session. The independent variables were genotype (a between-groups variable) and session number (a within-subjects variable). Statistical analysis for Figs. 1 and 2 consisted of two-way analysis of variance (ANOVA) with repeated measures followed by Bonferroni post-hoc analysis to evaluate behavioral data (as shown in Figs. 1 and 2) using GraphPad Prism (version 6; GraphPad Software, San Diego, CA, USA). For the data in Figure 5, in addition to genotype and session number, drug pretreatment (saline versus GBR-12909) was also included as a between-groups independent variable. Data in Figure 5 were analyzed using restricted maximum-likelihood estimation (Proc Mixed, SAS Institute, Cary, NC), and significant interaction effects from this analysis were further analyzed using Holm's multiple-comparison procedure to test for significant differences between GBR-12909 and vehicle groups within each session and genotype. For the phosphorylation analysis (as shown in Figs. 3A & B, and 4A & B), all data are representative of four independent values and presented as means ± SE and analyzed by Student’s t-test. Data shown in the figures are presented as means ± S.E.M. In all cases, a P<0.05 is considered significant.
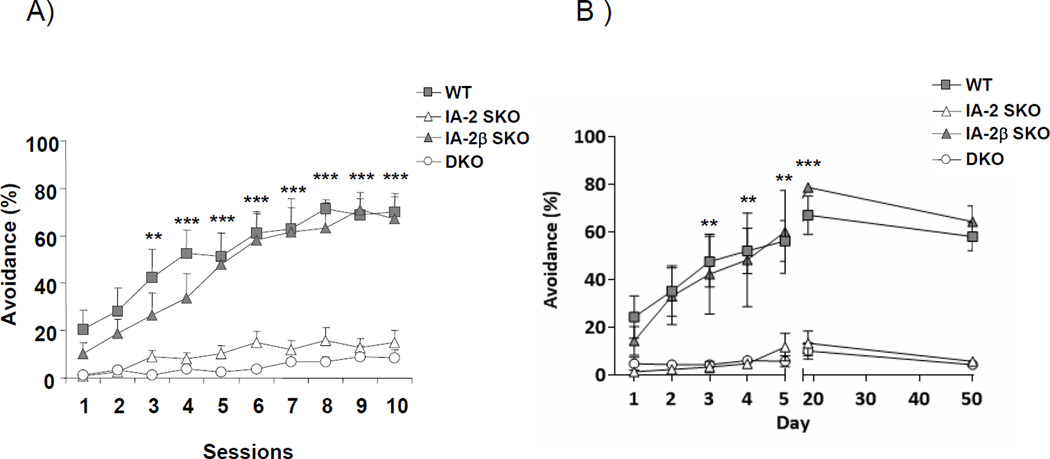
Figure 1. Impaired active avoidance learning in IA-2, but not IA-2β, SKO mice.
(A) Mean percentage of trials on which active avoidance responses was made during each session of 50 trials (2 sessions per day) over 5 days. Both the DKO and IA-2 SKO mice failed to show active avoidance learning in contrast to the WT and IA-2β SKO mice. (B) Duration of active avoidance learning after 5 days of training. Mice then were retested on days 20 and 50. Both WT and IA-2β SKO mice continued to show strong active avoidance. Each group initially consisted of 6 mice. At the start of the experiment mice were 10 ± 2 weeks of age. We were unable to determine if active avoidance responses were maintained across genotypes at day 50 due to the death of several animals resulting in loss of statistical power. Vertical lines represent mean ± SEM. (**) P<0.01, (***) P<0.001.
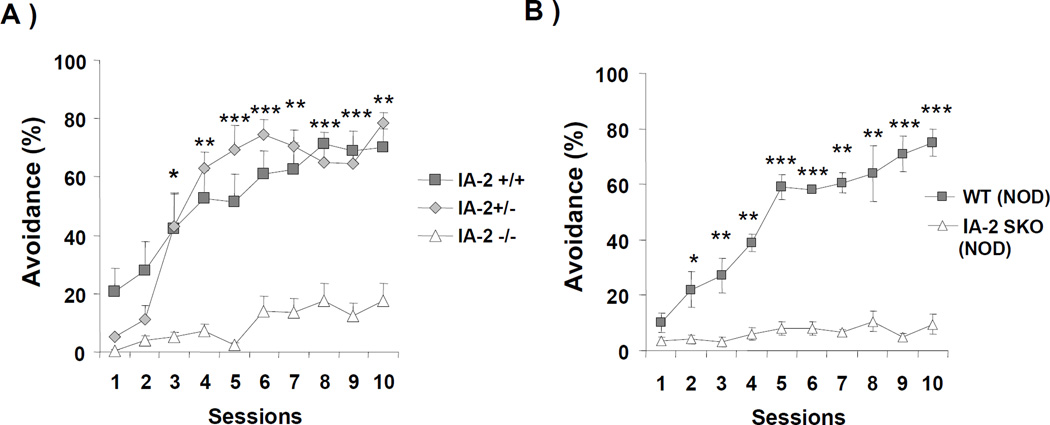
Figure 2. IA-2 genes and genetic background of mice.
(A) Mean percentage of trials on which active avoidance responses was made by IA-2+/+, IA-2+/− and IA-2−/− C57BL/6 mice. (B) Mean percentage of trials on which active avoidance responses was made by IA-2 NOD SKO mice as compared to WT NOD mice. Each group consisted of 4 mice. Data are expressed as the mean ± SEM. (*) P<0.05, (**) P<0.01, (***) P<0.001.
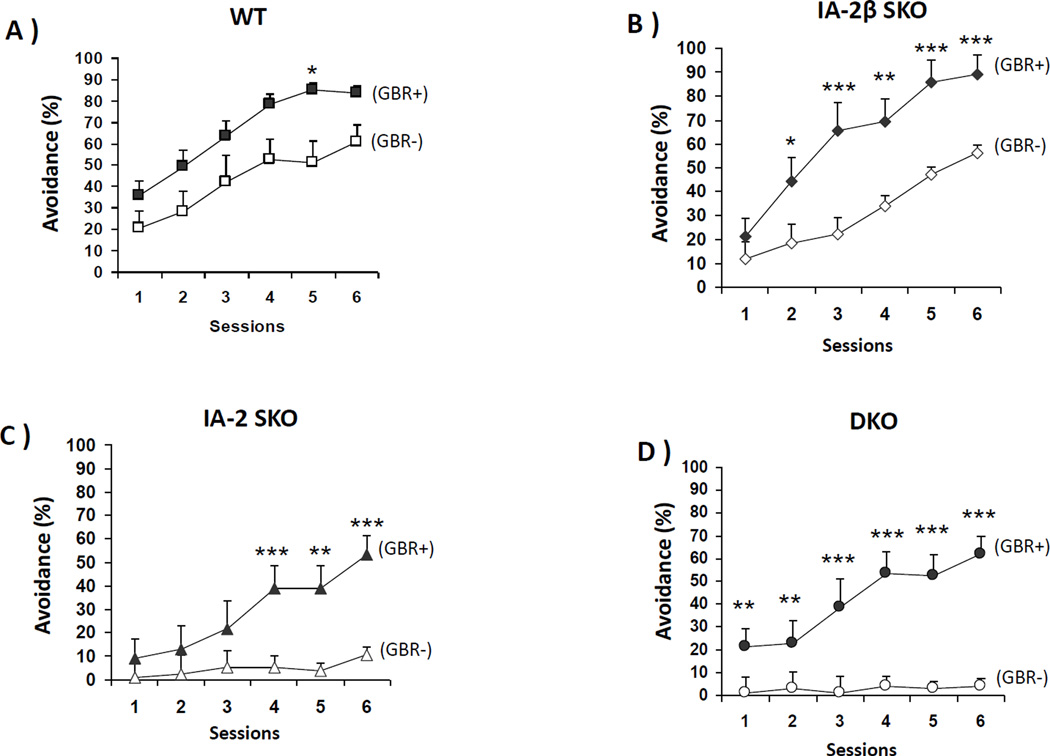
Figure 5. Active avoidance learning is restored by GBR-12909.
GBR-12909 was administered once daily for five days and active avoidance responses were recorded. Each group consisted of 6 mice. (A) WT, (B) IA-2β SKO, (C) IA-2 SKO, (D) DKO. Data are expressed as the mean ± SEM. (*) P<0.05, (**) P<0.01, (***) P<0.001.
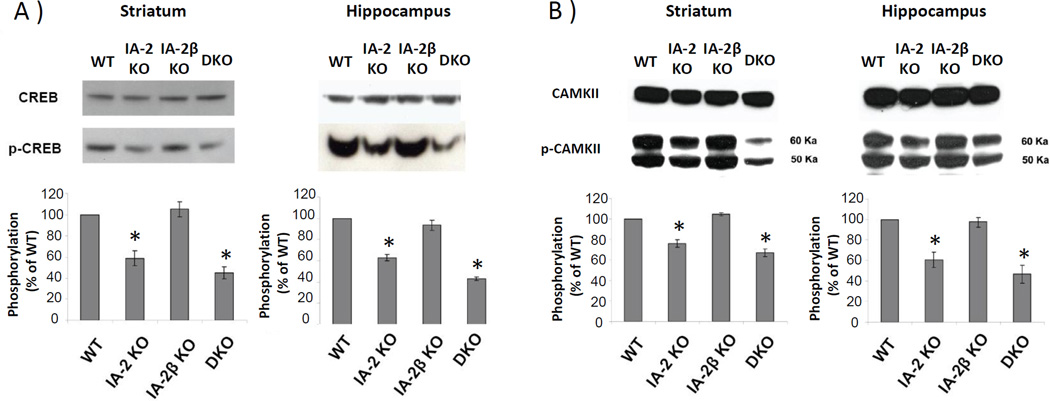
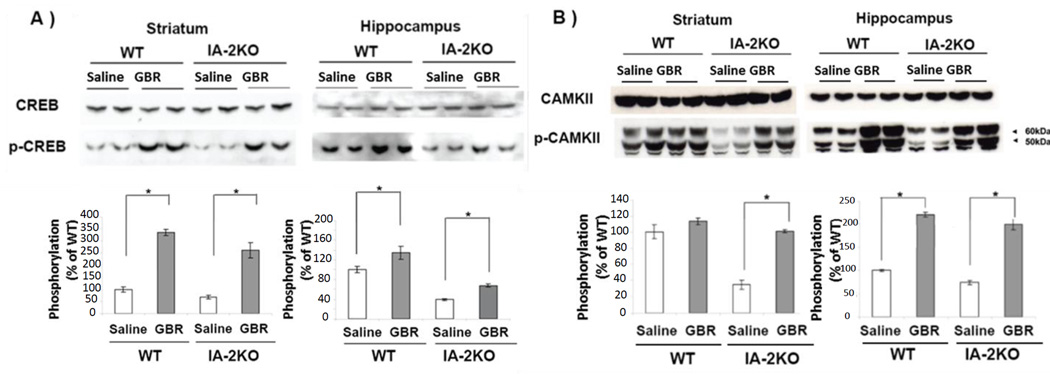
Figure 3. Phosphorylation of CREB and CAMKII is reduced in IA-2, but not in IA-2β, SKO mice.
A) Phosphorylation of CREB and (B) CAMKII in the striatum and hippocampus as determined by Western blot. Phosphorylation ratios were calculated as the ratio of phospho-CREB and phospho-CAMK-II in the KO as compared to the WT mice and then converted to a percentage. Data are expressed as the mean ± SEM. (*) P<0.05.
2. Results
2.1 Genetic Approach
Mice deficient in IA-2 exhibit a reduction in active avoidance learning
The effect of the KO of IA-2 and/or IA-2β on learning was evaluated in the active avoidance test. As seen in Fig. 1A, a positive trend in the percentage of trials on which active avoidance responses occurred at the onset of the experiment (sessions 1 and 2) where WT mice showed an active avoidance response on about 20% of the trials in session 1 compared to IA-2 SKO and DKO mice which showed less than 5% of trials with active avoidance responses. The mean percentage of trials on which active avoidance responses was made increased over the 10 sessions for both WT and IA-2β SKO mice. In contrast, the mean percentage of trials on which active avoidance responses was made by the IA-2 SKO mice was markedly lower, showing little evidence of learning across sessions. Even though the IA-2 SKO mice failed to show evidence of avoidance learning, nearly all were able to escape by crossing to the other side of the chamber when the electric shock was initiated, as were the WT and IA-2β SKO mice. Similar to the IA-2 SKO mice, the mean percentage of trials on which active avoidance responses was made by the DKO mice was less than 20%, but these mice often failed to escape the shock, thus suggesting that the learning deficiency was more profound in these animals than in the IA-2 SKO mice. When the data in Fig. 1A were analyzed, there were significant effects of genotype (F3, 20 = 18.86, p<0.0001) and session (F9, 180 = 30.98, p<0.0001). The analysis also showed a significant difference among the genotypes in the percentage of trials on which active avoidance responses occurred beginning in the third session (i.e., the WT and IA-2β SKO mice differ from the IA-2 SKO and DKO mice in sessions 3–10).
A separate experiment was conducted to determine the length of time active avoidance was maintained when mice were trained for 5 days and then tested for retention of active avoidance on days 20 and 50. Figure 1B shows that the DKO and IA-2 SKO mice continued to show little evidence of active avoidance learning across all days tested, including retention days 20 and 50, but the avoidance response was maintained in retention for WT and IA-2 β mice. When the data in Fig. 1B were analyzed, there were significant main effects of genotype (F3, 196 = 512.9, p<0.0001), day (F6, 196 = 50.88, p<0.0001), as well as an interaction (F18, 196 = 10.74, p<0.0001). The analysis also showed the mean percentage of trials on which active avoidance was made by WT and IA-2β SKO mice from days 3–5 were greater than those of the IA-2 SKO and DKO mice (P<0.01), and persisted with WT mice showing ~65% and IA-2β SKO mice showing ~75% active avoidance responses at day 20 (P<0.001). Given that a few mice had died prior to undergoing avoidance testing on day 50, we were unable to determine if active avoidance responses were maintained across genotypes due to a loss of statistical power.
To see whether both copies of the IA-2 gene were needed for active avoidance learning, IA-2+/+ mice were compared with heterozygous IA-2+/− and DKO IA-2−/− mice. As seen in Fig. 2A, the mean percentage of trials on which active avoidance was made by IA-2+/− mice was essentially the same as that of the IA-2+/+ mice from sessions 3 through 10. Thus, a single copy of the IA-2 gene is sufficient for the mice to acquire active avoidance learning. When the data in Fig. 2A were analyzed, there was a main effect of sessions (F9,81=24.56, P<0.0001), as well as a significant interaction between session and genotype (F18,81=4.41, P<0.0001) with post-hoc tests showing significant differences between the groups starting on session 3 (see Figure 2A).
Since the genetic background of an animal can play an important role in learning, we tested the effect of the KO of IA-2 on the NOD genetic background (IA-2 NOD SKO). As seen in Fig. 2B, wild-type NOD mice showed normal avoidance responses, while IA-2 SKO mice on the NOD background were impaired with little evidence of avoidance responses. A two-way ANOVA also showed a significant interaction (F9,100=2.20, P=0.0278), a significant effect of genotype (F1,100=133.7, P<0.0001), as well as a significant effect of session (F9,100=3.06, P<0.0028). Post-hoc analysis showed that from session 2 through 10, WT NOD mice learned the avoidance response significantly better than the IA-2 NOD SKO mice. These findings support the importance of the IA-2 gene in active avoidance learning independent of mouse strain.
2.2 Molecular Approach
Decreased levels of pCREB and pCAMKII and their re-phosphorylation by GBR-12909
A number of molecules are known to play an important role in active avoidance learning including the phosphorylated cAMP Response Element-Binding Protein (CREB), and phosphorylated Ca2+/Calmodulin-Dependent Protein Kinase II (CAMKII). To see whether these proteins were involved in the impairment of active avoidance learning in the IA-2 SKO or DKO mice, the phosphorylation of CREB and CAMKII in the striatum and hippocampus of these mice was examined. Figs. 3A and 3B show that basal levels of CREB and CAMKII proteins were equally expressed in the striatum and hippocampus of the WT and KO mice. However, there was a significant reduction in the phosphorylation of CREB and CAMKII in the IA-2 SKO and DKO mice (P<0.05), but not in the IA-2β SKO mice as compared to the WT mice.
Phosphorylation of CREB and CAMKII can be increased by treatment of animals with GBR-12909, a dopamine reuptake inhibitor that is selective for the dopamine transporter. To see if GBR-12909 was effective in WT and IA-2 SKO mice, GBR-12909 was administered daily for 5 days. As seen in Figs. 4A and 4B, GBR-12909 had little if any effect on the basal level of CREB and CAMKII proteins, but significantly increased the phosphorylation of both of these proteins in the striatum and hippocampus of the IA-2 SKO mice (P<0.05). In contrast, while the administration of GBR-12909 significantly increased the phosphorylation of CREB in both striatum and hippocampus in WT mice (P<0.05), it increased the phosphorylation of CAMKII in the hippocampus (P<0.05), but not in the striatum of WT mice.
Figure 4. Phosphorylation of CREB and CAMKII is increased in IA-2 SKO mice by GBR-12909 treatment.
(A) Phosphorylation of CREB and (B) CAMKII in striatum and hippocampus following treatment with GBR-12909 as determined by Western blot. Phosphorylation ratios of KO mice (n=4) were normalized based on the phosphorylation ratio of WT mice treated with saline. Data are expressed as the mean ± SEM. (*) P<0.05.
2.3 Pharmacologic Approach
Active avoidance learning is restored by GBR-12909
WT, IA-2β SKO, IA-2 SKO and DKO mice were treated with GBR-12909. The data in Fig. 5C and 5D showed that GBR-12909 restored active avoidance responses in the IA-2 SKO and DKO mice. These mice failed to show an increase in active avoidance learning when treated with saline. GBR-12909 treatment also increased active avoidance responses in the WT and IA-2β SKO mice (Fig. 5A and 5B). Whereas the WT mice showed enhanced active avoidance in only one of six sessions, the IA-2β SKO mice showed a significant enhancement in five of the six sessions. When the data in Fig. 5 were analyzed, there were significant main effects of genotype (F3,45=28.92, P<0.0001), GBR-12909 treatment (F1,45=75.14, P<0.0001), and session (F5,45=65.54, P<0.0001), Moreover, the interaction of these three effects was also significant (F1,10=5.438, P=0.0176). This interaction was further explored by performing paired-comparisons (using the Holm procedure to maintain a family-wise significance level of 0.05), comparing GBR-12909 and vehicle-treated mice within genotypes in each session. This analysis showed that the administration of GBR-12909 had a significant enhancing effect in only one of the six active avoidance sessions in the WT controls (Fig. 5A; session 5), but in five of the six sessions of the IA-2β SKO mice (Fig. 5B).
The IA-2 SKO and DKO mice treated with saline showed no evidence of learning (Figs 5C and 5D), and comparisons between the saline-treated groups (collapsed across sessions) indicated that these two genotypes were significantly impaired relative to WT controls. However, GBR-12909 administration dramatically improved avoidance responses in these mice, significantly increasing active avoidance responses in the IA-2 SKO mice (Fig. 5C) during the last three sessions and in the DKO mice during all six sessions (Fig. 5D).
Discussion
Recently, using the dopamine secreting pheochromocytoma cell line PC12, we showed that the knockdown of IA-2 by siRNA decreased the cellular content and secretion of dopamine, whereas the overexpression of IA-2 increased both the cellular content and secretion of dopamine (Nishimura et al., 2010). Additionally, by HPLC we showed that the level of catecholamines in the brain of DKO mice and their secretion from synaptosomes was significantly reduced (Nishimura et al., 2009). In the animal experiments described here we used active avoidance learning, which relies in part on the dopaminergic-signaling pathway (Stark et al., 2004, Darvas et al., 2011), to study the effect of the KO of the individual IA-2 and IA-2β genes on learning. Our experiments showed that the KO of IA-2, but not IA-2β, resulted in marked impairment of active avoidance learning. Impairment of learning also was observed in IA-2 SKO NOD mice, demonstrating that the KO effect was not dependent on the strain of the animal. Moreover, studies on IA-2+/− heterozygous as compared to IA-2+/+ homozygous mice revealed that one copy of the gene was sufficient to maintain active avoidance learning.
The active avoidance test of learning requires an intact hearing process as well as intact locomotor activity for the animal to move from one compartment into the other to avoid the electric foot shock after presentation of the CS (light+tone). In a previous experiment, hearing function was assessed using an animal training clicker (held ~30 cm above the mouse) that delivered soundbursts between 70–90 dB. The presence or absence of an ear flick response (Preyer reflex) was recorded. All genotypes responded similarly with greater than 80% (26/32) of the animals responding with a full Preyer reflex (e.g., complete ear flick), suggesting that insensitivity to the conditioned stimulus (i.e., tone) was not responsible for the learning dysfunction (unpublished data). Our earlier experiments (Kim et al., 2009), in which locomotion was evaluated by telemetry, showed that the spontaneous locomotor activity of the IA-2 SKO mice equaled that of the WT mice. In contrast, the spontaneous motor activity of the IA-2β SKO mice was mildly decreased, whereas that of the DKO mice was moderately decreased. The decreased locomotor activity of the DKO mice was attributed to anxiety/depression since no difference was observed in the gait or walking speed of the DKO mice (Nishimura et al., 2009). Moreover, when put under stress, the motor activity of the DKO mice nearly equaled that of the WT mice (Kim et al., 2009). These experiments, together with our current experiments, show that the failure of the IA-2 SKO mice to learn in the active avoidance test is not due to a decrease in locomotion, nor does the previously observed mild decrease in locomotion in the IA-2β SKO mice prevent them from learning in the active avoidance test. Based on these findings with SKO mice, we conclude that the decreased locomotor activity of the DKO mice is not responsible for impaired active avoidance, but rather is a consequence of the loss of IA-2, which does not affect locomotion. Moreover, all four genotypes responded similarly to painful stimuli based on hot plate and tail flick assays (unpublished data), suggesting that insensitivity to the unconditioned stimulus (i.e., electric shock) was not responsible for the learning dysfunction.
Insight into the molecular basis underlying impaired active avoidance learning comes from the studies on CREB and CAMKII, the phosphorylation of which is known to be important in the dopamine-dependent learning pathway (Silva et al., 1992, Bourtchuladze et al., 1994, Lledo et al., 1995, Berke and Hyman, 2000). Our studies showed a marked decrease in the phosphorylation of CREB and CAMKII in IA-2 SKO and DKO mice as compared to IA-2β SKO and WT mice. Of particular interest, administration GBR-12909 restored phosphorylation and this was associated with an increase in active avoidance learning. This does not mean, however, that the decrease in the phosphorylation of CREB and CAMKII in the IA-2 SKO mice is solely due to impaired dopamine secretion. Instead, the decrease in CREB and CAMKII phosphorylation could be secondary to a decrease in the secretion of one or more of the many other non-dopaminergic neurotransmitters that might be affected as a result of the KO of IA-2. IA-2 SKO mice, thus, may prove to be a useful animal model for screening drugs that enhance the phosphorylation of CREB and CAMKII and improve learning.
Because DCV are present in neuroendocrine cells throughout the body, the KO of IA-2 and IA-2β has a broad effect on the secretion of a number of different hormones and neurotransmitters. Little is known, however, about the relative density of IA-2 and IA-2β on DCV, the distribution or number of these vesicles in different neuroendocrine cells and the effect of the knockout of IA-2 and/or IA-2β on the storage and secretion of neurotransmitters in various parts of the brain. Each of the tests that are commonly used to study behavior and learning involve complex and often interconnecting neuronal pathways encompassing a variety of different neurotransmitters. This may explain why the knockout of IA-2 has a profound effect, as shown here, on the active avoidance paradigm, but little or no effect on circadian rhythm as determined by telemetry and wheel-running activity (Kim et al., 2009). These tests may involve different pathways and different neurotransmitters (Yamamoto, 2007, Mobbs et al., 2009). In this context, it is of particular interest that the knockout of IA-2β did not affect active avoidance even though both IA-2 and IA-2β are transmembrane proteins of DCV and IA-2β also is a transmembrane synaptic vesicle protein (Nishimura et al., 2009). Despite the fact that these two proteins belong to the same family and are closely related, differences in their biological properties and function exist. This could explain why the deletion of IA-2, but not IA-2β, affects active avoidance learning, but why in some behavior and learning paradigms the deletion of both genes produces a stronger phenotype than the deletion of either gene alone.
Highlights.
First demonstration that deletion of a DCV protein profoundly impairs active avoidance learning.
Deletion of both copies of the IA-2 gene is required to impair active avoidance learning.
The deletion of IA-2 results in decreased phosphorylation of CREB and CAMKII in the brain.
GBR12909 increases the phosphorylation of CREB and CAMKII and restores active avoidance learning.
We conclude that the IA-2 gene is required for normal active avoidance learning.
Acknowledgment
This work was supported by the intramural research program of the National Institute of Dental and Craniofacial Research, NIH. The authors thank Drs. Irwin Kopin (Scientist Emeritus/NIH/NINDS), Carl Lupica and Alex Hoffman (NIH/NIDA, Baltimore, MD, USA) for helpful discussions and Mr. Talmo Pereira for assisting in genotyping the mice and running the experiments. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- DCV
dense core vesicles
- SKO
single knockout mice
- DKO
double knockout mice
- WT
wild type mice
- NOD
non-obese diabetic mice
- CREB
cAMP Response Element-Binding Protein
- CAMKII
Ca2+/Calmodulin-Dependent Protein Kinase II
- PTP
protein tyrosine phosphatase
- CS
conditioned stimulus
- UCS
unconditioned stimulus
- PVDF
polyvinylidene fluoride
- GBR-12909
a selective dopamine uptake inhibitor
- IA-2 and IA-2β
Insulinoma associated proteins
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ. Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia. 2008;51:488–492. doi: 10.1007/s00125-007-0912-9. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Cai T, Hirai H, Zhang G, Zhang M, Takahashi N, Kasai H, Satin LS, Leapman RD, Notkins AL. Deletion of Ia-2 and/or Ia-2beta in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia. 2011;54:2347–2357. doi: 10.1007/s00125-011-2221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caromile LA, Oganesian A, Coats SA, Seifert RA, Bowen-Pope DF. The neurosecretory vesicle protein phogrin functions as a phosphatidylinositol phosphatase to regulate insulin secretion. The Journal of biological chemistry. 2010;285:10487–10496. doi: 10.1074/jbc.M109.066563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Learning & memory. Vol. 18. NY: Cold Spring Harbor; 2011. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response; pp. 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harashima S, Clark A, Christie MR, Notkins AL. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8704–8709. doi: 10.1073/pnas.0408887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Power A, Brown TM, Constance CM, Coon SL, Nishimura T, Hirai H, Cai T, Eisner C, Weaver DR, Piggins HD, Klein DC, Schnermann J, Notkins AL. Deletion of the secretory vesicle proteins IA-2 and IA-2beta disrupts circadian rhythms of cardiovascular and physical activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:3226–3232. doi: 10.1096/fj.09-132019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubosaki A, Gross S, Miura J, Saeki K, Zhu M, Nakamura S, Hendriks W, Notkins AL. Targeted disruption of the IA-2beta gene causes glucose intolerance and impairs insulin secretion but does not prevent the development of diabetes in NOD mice. Diabetes. 2004;53:1684–1691. doi: 10.2337/diabetes.53.7.1684. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2beta causes female infertility. Endocrinology. 2006;147:811–815. doi: 10.1210/en.2005-0638. [DOI] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Notkins AL. Dense core vesicle proteins IA-2 and IA-2beta: metabolic alterations in double knockout mice. Diabetes. 2005;54(Suppl 2):S46–S51. doi: 10.2337/diabetes.54.suppl_2.s46. [DOI] [PubMed] [Google Scholar]
- Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA and cell biology. 1994;13:505–514. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6367–6370. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter EH, Tsumura H, Serreze DV, Chapman HD, Rabin DU, Lan MS, Notkins AL. Mapping to chromosomes 1 and 12 of mouse homologs of human protein tyrosine phosphatase, receptor-type, related genes encoding pancreatic beta cell autoantigens. Mammalian genome : official journal of the International Mammalian Genome Society. 1997;8:949–950. doi: 10.1007/s003359900619. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2307–2311. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistrelli G, Toma S, Isacchi A. Substitution of two variant residues in the protein tyrosine phosphatase-like PTP35/IA-2 sequence reconstitutes catalytic activity. Biochemical and biophysical research communications. 1996;227:581–588. doi: 10.1006/bbrc.1996.1549. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Harashima S, Yafang H, Notkins AL. IA-2 modulates dopamine secretion in PC12 cells. Molecular and cellular endocrinology. 2010;315:81–86. doi: 10.1016/j.mce.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kubosaki A, Ito Y, Notkins AL. Disturbances in the secretion of neurotransmitters in IA-2/IA-2beta null mice: changes in behavior, learning and lifespan. Neuroscience. 2009;159:427–437. doi: 10.1016/j.neuroscience.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins AL. New predictors of disease. Molecules called predictive autoantibodies appear in the blood years before people show symptoms of various disorders. Tests that detected these molecules could warn of the need to take preventive action. Scientific American. 2007;296:72–79. [PubMed] [Google Scholar]
- Saeki K, Xie J, Notkins AL. Genomic structure of mouse IA-2: comparison with its human homologue. Diabetologia. 2000;43:1429–1434. doi: 10.1007/s001250051550. [DOI] [PubMed] [Google Scholar]
- Saeki K, Zhu M, Kubosaki A, Xie J, Lan MS, Notkins AL. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–1850. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science (New York, NY) 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Smith JW, Fetsko LA, Xu R, Wang Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience. 2002;113:755–765. doi: 10.1016/s0306-4522(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Stark H, Rothe T, Wagner T, Scheich H. Learning a new behavioral strategy in the shuttle-box increases prefrontal dopamine. Neuroscience. 2004;126:21–29. doi: 10.1016/j.neuroscience.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Takeyama N, Ano Y, Wu G, Kubota N, Saeki K, Sakudo A, Momotani E, Sugiura K, Yukawa M, Onodera T. Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life sciences. 2009;84:678–687. doi: 10.1016/j.lfs.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Trigo JM, Cabrero-Castel A, Berrendero F, Maldonado R, Robledo P. MDMA modifies active avoidance learning and recall in mice. Psychopharmacology. 2008;197:391–400. doi: 10.1007/s00213-007-1045-z. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Brain regions responsible for the expression of conditioned taste aversion in rats. Chemical senses. 2007;32:105–109. doi: 10.1093/chemse/bjj045. [DOI] [PubMed] [Google Scholar]