Abstract
Rabies remains a major neglected global zoonosis. New vaccine strategies are needed for human rabies prophylaxis. A single intramuscular immunization with a moderate dose of an experimental chimpanzee adenovirus (Ad) vector serotype SAd-V24, also termed AdC68, expressing the rabies virus glycoprotein, resulted in sustained titers of rabies virus neutralizing antibodies and protection against a lethal rabies virus challenge infection in a non-human primate model. Taken together, these data demonstrate the safety, immunogenicity, and efficacy of the recombinant Ad-rabies vector for further consideration in human clinical trials.
Keywords: adenoviral vaccine, NHP, rabies virus, VNA
Introduction
Rabies virus causes an acute progressive viral encephalitis, with the highest case fatality of any infectious disease (Jackson, 2009). Although rabies is one of the oldest recognized zoonoses, it remains a neglected disease, resulting in tens of thousands of human fatalities, and tens of millions of annual exposures (http://www.who.int/mediacentre/factsheets/fs099/en/). Rabies virus is most commonly transmitted by dogs in the developing world. Children are at high risk of exposure, and nearly half of rabies-related fatalities affect children below the age of 16. In parts of Africa, Asia, and Latin America, outdated nerve tissue-based vaccines are still used, some unchanged little from the time of Pasteur (Wu, Smith, & Rupprecht, 2011).Modern human vaccines, while highly efficacious, are expensive, and require multiple doses for the elicitation of protective immunity (health.costhelper.com/rabies-vaccine.html ). In general, rabies vaccines are given after a bite, as post-exposure prophylaxis (PEP). In cases of severe exposure, active immunization has to be combined with passive immunization with a rabies virus immune globulin preparation of human (HRIG) or equine origin (ERIG), both of which are costly and habitually in short supply. Development of novel inexpensive, safe and effective rabies biologics for human populations at risk would be a major advantage for rabies prevention in the developing world. Previous studies showed that inclusion of a rabies vaccine into childhood immunizations resulted in the development of memory B cells that rapidly mounted potent rabies virus neutralizing antibody (VNA) recall responses after booster immunization (Malerczyk, Briggs, Dreesen, & Banzhoff, 2007; Suwansrinon et al., 2006). Nevertheless, one study conducted in Thailand concluded that inclusion of rabies vaccines into vaccination programs for children would not be cost effective when compared to standard PEP (Chulasugandha, Khawplod, Havanond, & Wilde, 2006). A less expensive vaccine able to induce sustained rabies VNA titers and durable memory B cell responses in a shortened schedule may render preventative childhood vaccination more cost-effective and thereby reduce the impact of rabies in developing countries, as well as of those deemed at risk of rabies virus exposure in developed countries, such as veterinarians, diagnostic workers, animal handlers, certain travelers, selected members of the military, etc.
The objective of this research was to test the comparative safety, immunogenicity, and efficacy of an adenovirus (Ad) vector expressing the rabies virus glycoprotein in a non-human primate model. Most of these studies were conducted with chimpanzee-derived Ad vectors. Humans only rarely carry neutralizing antibodies to such viruses (Chen et al., 2010; Xiang et al., 2006), which may impair vaccine efficacy. Vectors expressed the full-length glycoprotein of the Evelyn Rokitniki Abelseth (ERA) strain of rabies virus. Immunization of nonhuman primates with the Ad vectors induced high and sustained VNA titers to rabies virus, confirming previous studies performed in mice (Chen et al., 2010; Xiang et al., 2002), which showed that Ad vectors, although most commonly used for induction of cellular immune responses, also elicited potent and durable transgene-specific humoral responses.
MATERIAL AND METHODS
Viral Vector
Briefly, all Ad vectors were generated from recombinant viral molecular clones, as described (Zhou et al., 2010). A number of different Ad vectors derived from human and simian serotypes were tested, specifically vectors based on human serotype 5 (HAd-V5, vectors referred to as AdHu5), and simian serotypes SAd-V23, (vectors called AdC6), SAd-V24, (vectors called AdC7) and SAd-V25 (vectors called AdC68). Of note, SAd-V24 and SAd-V25 are closely related and induce cross-neutralizing antibodies, while neutralizing antibodies to SAd-V23 fail to neutralize SAd-V24 or SAd-V25. The viruses were deleted in the E1 region, and hence were replication-defective. An expression cassette containing the rabies virus glycoprotein gene, under the control of a CMV promoter, was placed into the E1 domain. Upon rescue and expansion on HEK 293 cells, vectors were purified by CsCl gradient centrifugations. Vectors were titrated for content of virus particles [vps] by spectrophotometry at 260 nm using the formula: OD260 x dilution x 1.1 × 1012. Content of infectious virus particles was measured by nested RT-PCR with transgene or Ad (hexon)-specific primers on RNA isolated from HEK 293 cells infected for 5–7 days with serial dilutions of the viral vector. Batches were tested for endotoxin using the Limulus Amebocyte Lysate (LAL) gel-clot method and a commercial kit. Genetic integrity and identity were assessed by isolation of viral DNA. The recombinant DNA, in parallel with the original molecular clones and shuttle plasmids used for generating molecular clones, were digested with a set of restriction enzymes and analyzed by gel electrophoresis. Expression of the transgene product was confirmed by immunoprecipitation of lysates from transfected HeLa cells, and primary vaccine immunogenicity was demonstrated in mice, as described (Xiang et al., 2002). The Ad vectors expressing green fluorescent protein were used to measured antibodies to Ad vectors. These vectors have been described previously.
Nonhuman Primates
Female or male cynomologous monkeys (Macaca fasicularis) or Chinese rhesus macaques (Macaca mulatta) were enrolled into the study. The M. facicularis were 1–2 years of age with weights ranging from 1–6 kg. They were houses in the animal facility of the Centers for Disease Control and Prevention (CDC), Atlanta, GA. The M. mulatta were 2–3 years of age, housed in the Nonhuman Primate Facility of the Division of Medical Genetics of the University of Pennsylvania, Philadelphia, PA. The animals were housed in pairs until the time of rabies virus infection. Prior to handling, animals were sedated with ketamine hydrochloride (10 mg/kg) or Telazol (3–5 mg/kg) IM. Blood samples were collected via venipuncture from a peripheral vein and placed in serum separator tubes. Typically, bleeding occurred once a week to once a month, with ~ 6–7 ml collected per kg per month. Upon challenge with rabies virus, animals were observed multiple times per day. Any alterations in body mass, food consumption, and water intake were monitored closely. Upon the demonstration of compatible clinical signs (e.g., paresis, cranial nerve deficitis, etc.), animals were sedated, and euthanized by intravenous barbiturate overdose. Research was approved by the Institutional Animal Care and Use Committees.
Virus neutralization assays
Serum was separated from clotted blot after low speed centrifugation. Rabies virus neutralizing antibodies (VNAs) were assayed using the rapid fluorescent focus inhibition test, using CVS-11 virus propagated upon MNA cells, as described (Louie et al., 1975). Comparative rabies VNAs were defined arbitrarily using the World Health Organization recommendations, with a level of 0.5 IU/ml considered as a minimum adequate level of acceptable comparable induction as compatible with standard human clinical trial criteria (WHO 2013). Neutralizing antibodies to Ad viruses were measured as described (Xiang et al., 2006). Animals with circulating neutralizing antibody titers ≥1:20 to the vaccine vectors were not enrolled into the study.
Rabies virus challenge
Challenge viruses consisted of street rabies viruses of canine origin, chosen based upon global public health relevance from the New and Old Worlds, and preliminary non-human primate susceptibility data, prepared as previously described (Franka et al., 2009; Rupprecht et al., 2005). Challenge virus stocks were maintained at −80 C, and were diluted using sterile PBS/2% heat-inactivated equine serum or FBS. Typical rabies virus challenge concentrations ranged from ~105.2 – 106.4 mouse intracerebral lethal dose (MICLD)50/ml. Sedated animals were inoculated in the masseter muscles with 0.5 ml of canine rabies virus, with an approximate lethal dose (LD)50 or LD100 dose, based upon prior titrations in naïve animals from prior studies. Brain tissue was removed from euthanized animals. Rabies virus antigens were detected using the direct fluorescent antibody test, as described (Reid, Hall, Smith, & Baer, 1983).
RESULTS
Immunogenicity of Ad vectors used in a high dose prime-boost regimen
A pilot experiment was conducted in two Chinese rhesus macaques to test if Ad vectors expressing the rabies virus glycoprotein induced rabies VNAs and if such responses could be enhanced by booster immunizations. To this end, two monkeys that had no detectable antibody titers to rabies virus or the Ad vectors were immunized on day 0 with 1012 vp of the AdC7rab.gp vector given intramuscularly. Eight months later, they were boosted with the same dose of the AdC6rab.gp vector. Five months later, they were boosted with 1012 vp of the AdHu5rab.gp vector. Blood was collected at several time points after vaccination. Rabies VNA titers were determined from heat-inactivated plasma. Plasma from naïve monkeys, and monkeys immunized with vectors expressing an unrelated transgene were included, but none of the latter developed detectable rabies VNAs (< 0.2 IU, data not shown). The experimental animals developed VNA titers of approximately 10 IU after the first immunization (Figure 1). Although values fluctuated, titers were sustained for at least 8 months. After the boost with AdC6rab.gp, rabies VNA titers virus increased in both animals ~ 10 fold. In one animal, rabies VNA titers then contracted to levels obtained after priming, while the other animal showed more sustained increases after the first boost. A second boost with an AdHu5rab.gp vector again increased rabies VNA titers by more than 10 fold. Overall, these preliminary results showed that Ad vector immunization induced a potent and sustained rabies VNA response. In addition, vectors induced memory B cells that readily differentiate into antibody-secreting cells upon booster immunizations.
Figure 1. Induction of rabies VNA upon sequential immunizations with Ad vectors.
Two rhesus macaques, shown individually in circles or cross-filled squares, were primed with AdC7rab.gp, and then boosted with AdC6rab.gp, with AdHu5rab.gp administered at the time points indicated by the arrows. The rabies VNA titers were measured from sera at various time points. Titers are shown as international units (IU) according to a reference serum tested in parallel. Control animals immunized with the same vectors, but expressing an antigen of HIV-1, were tested as well, and their sera showed titers consistently below 0.2 IU (not shown).
Vaccine efficacy in a dose escalation study
The vector dose used in the above pilot study exceeded the dose that would be expected to cause significant toxicity in humans. Although the AdC vectors we tested have not yet undergone testing in humans, other E1-deleted Ad vectors of human or simian serotypes typically cause dose-limiting adverse events at 1011 vp (Harro et al., 2009). Therefore, we next tested the AdC68rab.gp vector in a dose escalation experiment at 109 to 1011 vp in small groups of two cynomologous monkeys per group. An AdC vector control was used at 1011 vp. A commercially available human diploid cell rabies vaccine (HDCV), used intramuscularly at the recommended dose for pre-exposure vaccination, was included. The AdC vector vaccine was only given once, and the HDCV was given 3 times on days −41, −27 and −20 in relation to the AdC vaccine. Animals were challenged at 18 weeks after the last vaccine dose with ~ 1 lethal dose (LD)100 of a virulent street strain of canine rabies virus. The two animals that received the control vector developed signs of rabies. All other vaccinated animals had detectable rabies VNA by day 7 post-immunization, and remained disease-free (Figure 2A).
Figure 2. Vaccine Efficacy in Pre-exposure and Post-exposure Regimens.

[A] Dose Escalation: Cynomologous monkeys were vaccinated once with various doses (109–1011 vp) of the AdC68rab.gp vector, a control AdC68 vector given at 1011 vp, or three doses of commercial human raies vaccine, HDCV. Additional control animals received PBS only. Animals were challenged with canine rabies vius at 18 weeks after AdC68 vaccination. [B] Effect of pre-existing HAd-V5 neutralizing antibodies: Groups of monkeys were injected with 1012 vp of an AdHu5 vector expressing a reporter protein, or they were left untreated.. Four weeks later, pre-treated, as well as untreated animals, were vaccinated with 1010 vp of AdC68rab.gp. A positive control group received commercial human rabies vaccine, HDCV given 3 times, a negative control group received PBS. Animals were challenged with canine rabies virus 18 weeks after AdC vaccination and survival was recorded. [C] Post-exposure prophylaxis: Nonhuman primates were infected with canine rabies virus. Six hours later, one group was vaccinated with 1010 vp of AdC68rab.gp and received commercial HRIG infiltrated at the rabies virus inoculation site. An additional group was vaccinated with AdC68rab.gp, but without HRIG. A positive control group was treated with the traditional vaccine regimen, i.e., HRIG combined with 4 doses of commercial PCEC human rabies vaccine, the latter given on days 1, 8, 15 and 29 relative to challenge. Additional control groups only received HRIG, a control AdC68 vector or PBS. Survival was recorded. [NB: need to change the vaccine in @C from HDCV to PCEC.
Graphs A–C show percent of animals that survived without developing signs of rabies. Numbers of animals per group are shown within each graph above the bars.
Effect of pre-existing immunity to a human serotype Ad vector on the efficacy of AdC68rab.gp
Humans are exposed to Ad viruses and in response develop neutralizing antibodies, which are serotype-specific, as well as binding antibodies and T cells, which cross-react between different human and simian serotypes (Hutnick et al., 2010; Xiang et al., 2002). Numerous studies have shown that pre-existing neutralizing antibodies to Ad vectors impact their immunogenicity (McCoy et al., 2007; McElrath et al., 2008; Roberts et al., 2006). Neutralizing antibodies to AdC68 virus are only found rarely in humans (Xiang et al., 2002). Nevertheless, pre-existing binding antibodies or T cells may also affect vector-induced immune responses. Therefore, in the next experiment, we assessed the effect of pre-exposure to AdHu5 virus on the efficacy of the AdC68rab.gp vaccine. To this end, groups of 4 animals were either pre-exposed with 1012 vp of an AdHu5 vector expressing a reporter protein, or they were left untreated. Four weeks later, the pre-treated as well as the untreated animals were vaccinated with 1010 vp of AdC68rab.gp. A third positive control group of 2 animals received HDCV, given 3 times on days −41, −27 and −20 in relation to the AdC vaccination (given on day 0). Four control animals received PBS. All vaccinated animals developed detectable rabies VNA within 14 days of immunization. Animals were challenged with ~ 1 LD50 of street rabies virus of canine origin 18 weeks after AdC vaccination. As shown in Figure 2B, all of the AdC68rab.gp and HDCV vaccinated animals survived the challenge, while 2 of 4 control animals developed signs of rabies, indicating that pre-existing immunity to human serotype Ad vectors did not reduce the efficacy of the AdC68rab.gp vector.
Efficacy in post-exposure prophylaxis
In general, prevention of rabies occurs after exposure has occurred. Prophylaxis after severe exposures, which should commence as soon as possible, consists of careful wound cleaning, active immunization given repeatedly over a course of ~ 2–48 weeks, and a RIG preparation injected once into the site of exposure. To assess if AdC68rab.gp would induce protective immunity in a post-exposure model, a total of 18 nonhuman primates were infected with ~1 LD100 of a virulent street rabies virus of canine origin. Six hours later, 5 animals were vaccinated with 1010 vp of AdC68rab.gp. In addition, these animals received commercial HRIG injected close to the rabies virus challenge site, at a dose of 20 IU/kg. An additional 5 animals were vaccinated with AdC68rab.gp, without HRIG. Two animals were administered a traditional PEP regimen, i.e., HRIG combined with 4 doses of a commercial purified chick embryo cell (PCEC) human rabies vaccine, the latter given on days 1, 8, 15 and 29 relative to challenge. Two animals received HRIG only, two animals received a control AdC68 vector, and two animals were treated with PBS only. Thereafter, both animals given PBS only, and 1 of 2 animals administered the AdC68 vector, displayed clinical signs of rabies, were sedated, euthanized, and diagnosed rabid. The combination of PCEC vaccine and HRIG provided complete protection, as did HRIG infiltration, without addition of vaccine. The AdC68rab.gp given with, or without HRIG, only provided partial protection in 4 or 2 of 5 animals, respectively. All surviving animals developed rabies VNA > 0.5 IU/ml within 7 days of PEP initiation, and all had titers > 2.8 IU/ml by conclusion of the study at 4 months. These data, while based on small groups of animals, suggest that AdC68rab.gp, although highly efficacious in a preventative setting, may not reliably protect if given after exposure to rabies virus (Figure 2C).
Longevity of AdC68rab.gp induced immune responses
Lastly, a longer term pre-exposure vaccination experiment was conducted based upon the above preliminary results. On day 0, six animals were vaccinated IM with 1 ml containing 109 vps of the AdC68rab.gp vector. All vaccinated animals developed rabies VNA within 30 days of immunization (Figure 3A). Titers were remarkably stable for at least 18 months. By 21 months, after vaccination, 4 animals still had titers above 10 IU, and the other two animals had titers ~ 1 IU. The AdC68rab.gp-immunized animals, together with a group of 5 control animals, were challenged with ~1 LD100 of a virulent street rabies virus of canine origin at 22 months after vaccination. All control animals displayed signs of rabies, but all vaccinated animals survived. No rabies virus antigens were detected in the brains of the vaccinated animals at the conclusion of the study, in contrast to control animals (Figure 3B). Vaccinated animals demonstrated a very robust anamnestic response within 7 days post-challenge that at its peak showed on average 240-fold increases in VNA titers compared to those measured pre-challenge. Although rabies VNA responses contracted rapidly after challenge, they remained elevated (~ 8 fold) for the duration of the study (Figure 3C).
Figure 3. Vaccine immunogenicity and efficacy in a long-term study.
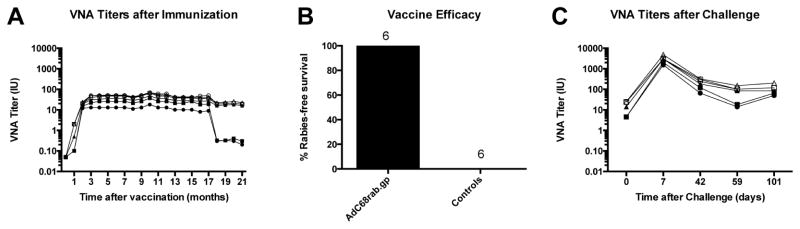
[A] VNA titers after immunization: Groups of 6 animals were vaccinated with 109 vp of AdC68rab.gp. The rabies VNA titers were tested periodically from sera for a period of 21 months. The graph shows rabies VNA titers of individual animals. [B] Vaccine efficacy: The vaccinated animals shown in [A] were challenged with canine rabies virus at 22 months after immunization. An additional 5 unvaccinated animals were challenged as well. Survival was recorded. [C] VNA titers after challenge. Sera from the vaccinated animals in [A] were tested the day of challenge and then at the indicated time points after challenge for VNA titers to rabies virus. The graph shows rabies VNA titers of individual animals. [NB: need to change the number of controls in B from 6 to 5.
Discussion
Globally, more than three quarters of the human population are assumed to be at risk for rabies virus exposure (WHO, 2013). The five billion people living in Asia and Africa are at high risk of exposure to rabid dogs, which accounts for the majority of all human rabies-related fatalities (Knobel et al., 2005). More than 15 million humans receive PEP each year, with an estimated prevention of more than 300,000 deaths (WHO 2013). In South East Asia, approximately 20 million people are bitten each year by dogs. Of those bitten, more than 1.5 million receive PEP. Significantly, PEP is given to ~40% of children by the time they reach 16 years of age in Asia and Africa (Meslin, Briggs, 2013). Based upon such alarming statistics, rabies vaccine may be considered preventatively as part of childhood immunization programs in countries with a high incidence of rabies, and given as a pre-exposure series (Ravish et al., 2013). In case of subsequent exposure, follow-up with wound cleaning and a booster immunization would be recommended (). Immunological memory provided by modern rabies vaccines is long lasting (Malerczyk et al., 2007; Suwansrinon et al., 2006). Recall responses develop rapidly, so that RIG is unnecessary.
As an example, a cost analysis was conducted for different regimens on preventative vaccination vs. traditional PEP in Thai children (CHULASUGANDHA et al., 2006). Using the least expensive vaccine regimens (intradermal vaccination + ERIG) it was estimated that the cost for preventative vaccination followed by booster immunizations would equal the cost of PEP with a dog bite prevalence of 7%. Although the actual dog bite rate is not known for Thailand, about 30% of children have been bitten by a dog by age 15, suggesting an annual incidence rate of ~ 2.3 bites per 100,000. The authors concluded that preventative childhood vaccination was not cost-effective for Thai children (Chulasugandha et al., 2006). One may assume that this type of cost analyses based on available vaccines would be similar for other countries with a high incidence of rabies.
By comparison, the AdC68 described in this manuscript appears as immunogenic compared to licensed rabies vaccines by reliably inducing high titers of VNA after a single immunization without the addition of adjuvant. The vaccine also induces sustained responses, which as shown here in nonhuman primates, remain at adequate levels for over a year. Equally important, AdC68rab.gp induces memory B cells, which upon rabies virus challenge mount a rapid and potent recall response within a few days. This was achieved with a very modest dose of 109 vp of vector, which is well below the dose that is typically used for Ad vectors undergoing clinical testing as vaccines for other infectious diseases, such as human immunodeficiency virus (HIV-1). A single dose regimen, compared to a three-dose regimen which is administered over a 3–4 week period, would be more convenient, produce better patient compliance, and most likely also more cost-effective.
Currently, Ad vectors are being explored as vaccine vectors for other infectious diseases to which protection is linked to induction of cellular responses, such as those caused by HIV-1 (Baden et al., 2012; McElrath et al., 2008), hepatitis C virus (Barnes et al., 2012), Plasmodium falciparum (Sheehy et al., 2012) or Mycobacterium tuberculosis (Hoft et al., 2012). A surrogate of adequate immunization against rabies virus has been well defined (Johnson, Cunningham, & Fooks, 2010). Rabies VNAs at titers at or above 0.5IU are deemed adequate as evidence of a proper immunological response to prevent a productive infection. Presumably, CD4+ T cells are needed to promote B cell responses, but otherwise cell-mediated immunity does not contribute prominently to the prevention of virus acquisition. Results shown here demonstrate that Ad vectors induce very potent and sustained antibody responses and are thus suited as vaccine carriers for pathogens that are best prevented by humoral responses. These data confirm previous studies in laboratory rodents, where we compared the AdHu5rab.gp vector to a DNA vaccine and a vaccinia-rabies glycoprotein (VRG) recombinant vaccine, both expressing the same transgene product (Xiang, Pasquini, & Ertl, 1999; Xiang, Yang, Wilson, & Ertl, 1996). The VRG vaccine is licensed for wildlife immunization, and as such has shown efficacy in a number of species such as raccoons, coyotes, and foxes (Brochier et al., 1996). By comparison, Ad-based vectors have showed superior induction of rabies VNAs, as compared to the other two vaccine platforms.
When used during PEP, the AdC68rab.gp vaccine did not induce reliable protection. All non-lyssavirus recombinant vaccines, including Ad vectors, V-RG, etc., first have to synthesize the transgene product before an immune response is elicited. In case of PEP, rapid induction of an immune response is essential and the delayed induction of responses may be detrimental. In our mind, such recombinant vaccines, including AdC68rb.gp, therefore may not be suitable in exploration for PEP applications.
Taken together, these data demonstrate the safety, immunogenicity, and efficacy of the AdC68rab.gp vaccine in a non-human primate animal model. Such encouraging results should be supportive towards the near-term consideration of human clinical trials, particularly considering the low, single-dose delivery, without the need for adjuvants. Together with improved local public education, enhanced de-centralized laboratory-based surveillance, mass canine rabies vaccination, and improved human prophylaxis, such modern biologics will have a substantive role to play in global rabies prevention and control strategies (Lembo et al., 2012).
Acknowledgments
The authors thank staff in the CDC Rabies Program and Animal Resources Branch for their assistance.
The views expressed in this communication are those of the authors and do not necessarily reflect the opinion of their host institutions. Use of commercial product names is for comparison purposes only and does not constitute official endorsement.
This work was supported in part by grants from NIH/NIAID 1R01AI055018 and 5R01AI037166.
References
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Adenovirus Serotype 26 HIV-1 Env Vaccine (IPCAVD 001) J Infect Dis. 2012;207(2):240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel Adenovirus-Based Vaccines Induce Broad and Sustained T Cell Responses to HCV in Man. Sci Transl Med. 2012;4(115):115ra1–115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, et al. Adenovirus-Based Vaccines: Comparison of Vectors from Three Species of Adenoviridae. J Virol. 2010;84(20):10522–10532. doi: 10.1128/JVI.00450-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulasugandha P, Khawplod P, Havanond P, Wilde H. Cost comparison of rabies pre-exposure vaccination with post-exposure treatment in Thai children. Vaccine. 2006;24(9):1478–1482. doi: 10.1016/j.vaccine.2005.03.059. [DOI] [PubMed] [Google Scholar]
- Harro CD, Robertson MN, Lally MA, O’Neill LD, Edupuganti S, Goepfert PA, et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res Hum Retroviruses. 2009;25(1):103–114. doi: 10.1089/aid.2008.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft DF, Blazevic A, Stanley J, Landry B, Sizemore D, Kpamegan E, et al. A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine. 2012;30(12):2098–2108. doi: 10.1016/j.vaccine.2012.01.048. [DOI] [PubMed] [Google Scholar]
- Hutnick NA, Carnathan D, Demers K, Makedonas G, Ertl HCJ, Betts MR. Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine. 2010;28(8):1932–1941. doi: 10.1016/j.vaccine.2009.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC. Therapy of rabies encephalitis. Biomédica : revista del Instituto Nacional de Salud. 2009;29(2):169–176. [PubMed] [Google Scholar]
- Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. 2010;28(23):3896–3901. doi: 10.1016/j.vaccine.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Louie RE, Dobkin MB, Meyer P, Chin B, Roby RE, Hammar AH, VJC Measurement of rabies antibody: comparison of the mouse neutralization test (MNT) with the rapid fluorescent focus inhibition test (RFFIT) J Biol Stand. 1975;3(4):365–373. doi: 10.1016/0092-1157(75)90061-x. [DOI] [PubMed] [Google Scholar]
- Malerczyk C, Briggs DJ, Dreesen DW, Banzhoff A. Duration of Immunity: An Anamnestic Response 14 Years After Rabies Vaccination With Purified Chick Embryo Cell Rabies Vaccine. J Travel Med. 2007;14(1) doi: 10.1111/j.1708-8305.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- McCoy K, Tatsis N, Korioth-Schmitz B, Lásaro MO, Hensley SE, Lin SW, et al. Effect of Preexisting Immunity to Adenovirus Human Serotype 5 Antigens on the Immune Responses of Nonhuman Primates to Vaccine Regimens Based on Human- or Chimpanzee-Derived Adenovirus Vectors. J Virol. 2007;81(12):6594–6604. doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case–cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid FL, Hall NH, Smith JS, Baer GM. Increased immunofluorescent staining of rabies-infected, formalin-fixed brain tissue after pepsin and trypsin digestion. J Clin Microbiol. 1983;18(4):968–971. doi: 10.1128/jcm.18.4.968-971.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJE, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441(7090):239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, Choudhary P, Biswas S, Halstead FD, et al. ChAd63-MVA–vectored Blood-stage Malaria Vaccines Targeting MSP1 and AMA1: Assessment of Efficacy Against Mosquito Bite Challenge in Humans. Mol Ther. 2012;20(12):2355–2368. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwansrinon K, Wilde H, Benjavongkulchai M, Banjongkasaena U, Lertjarutorn S, Boonchang S, et al. Survival of neutralizing antibody in previously rabies vaccinated subjects: A prospective study showing long lasting immunity. Vaccine. 2006;24(18):3878–3880. doi: 10.1016/j.vaccine.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Wu X, Smith TG, Rupprecht CE. From brain passage to cell adaptation: the road of human rabies vaccine development. Exp Rev Vaccines. 2011;10(11):1597–1608. doi: 10.1586/erv.11.140. [DOI] [PubMed] [Google Scholar]
- Xiang ZQ, Pasquini S, Ertl HC. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J Immunol. 1999;162(11):6716–6723. [PubMed] [Google Scholar]
- Xiang ZQ, Yang Y, Wilson JM, Ertl HC. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219(1):220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, et al. Novel, Chimpanzee Serotype 68-Based Adenoviral Vaccine Carrier for Induction of Antibodies to a Transgene Product. J Virol. 2002;76(6):2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12(10):1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhou X, Bian A, Li H, Chen H, Small JC, et al. An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector. Nature Protoc. 2010;5(11):1775–1785. doi: 10.1038/nprot.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]



