Abstract
Life-long neurogenesis is a characteristic feature of many vertebrate and invertebrate species. In decapod crustaceans, new neurons are added throughout life to two cell clusters containing local (cluster 9) and projection (cluster 10) interneurons in the olfactory pathway. Adult-born neurons in clusters 9 and 10 in crayfish have the anatomical properties and chemistry of mature neurons by 6 months after birth. Here we use 5-bromo-2'-deoxyuridine (BrdU) incorporation to pulse label mitotically active cells in these cell clusters, followed by a survival time of up to 8 months, during which crayfish (Cherax destructor) were sacrificed at intervals and the numbers of BrdU-labeled cells quantified. We find a decrease in the numbers of BrdU-labeled cells in cell cluster 10 between the first and second weeks following BrdU exposure, suggesting a period of cell death shortly after proliferation. Additional delayed cell divisions in both cell clusters are indicated by increases in labeled cells long after the BrdU clearing time. Detection of the first immunoreactivity for the transmitter SIFamide in cluster 10 BrdU-labeled cells was used to define the differentiation time of these cells into neurons, which begins at 4 weeks after BrdU-labeling; the numbers of SIFamide-labeled cells continues to increase over the following month. Experiments testing whether proliferation and survival of Cluster 10 cells are influenced by locomotor activity provided no evidence of a correlation between activity levels and cell proliferation, but suggest a strong influence of locomotor activity on cell survival.
Keywords: adult neurogenesis, neuronal development, neuronal proliferation, SIFamide, allatostatin
INTRODUCTION
The generation of new neurons in the adult brains of many vertebrate and invertebrate species is a well-established phenomenon (Kempermann, 2006). Much of the evidence for adult neurogenesis has come from the incorporation of the synthetic nucleoside BrdU, a thymidine analog, into the DNA of cells during the S phase of the cell cycle. Cells that incorporate BrdU can be visualized following immunocytochemical treatment. Early studies in crustaceans demonstrated that a brief (6 to 12h) single BrdU exposure to living animals was sufficient to label cells in two bilaterally symmetrical clusters of interneurons (clusters 9 and 10; terminology according to Sandeman et al., 1992) located in the midbrain (Harzsch and Dawirs, 1996; Schmidt, 1997; Schmidt and Harzsch, 1998; Harzsch et al., 1999) (Fig. 1A, B). Cell clusters 9 and 10 contain local and projection neurons, respectively, in the olfactory pathway; all cells in cluster 9 innervate both the olfactory and accessory lobes, while cells in cluster 10 innervate either of these synaptic regions and also project to higher-order regions in the lateral protocerebrum. Short-survival time experiments show that the numbers of labeled cells in these clusters vary with the size/age of the animals (Sandeman et al., 1998; Zhang et al., 2009), and in addition are influenced by environmental factors (Sandeman and Sandeman, 2000; Ayub et al., 2011), seasonality (Hansen and Schmidt, 2004) and time of day (Goergen et al., 2002). Endogenous signals such as serotonin and nitric oxide play important roles in regulating the numbers of labeled cells (Beltz et al., 2001; Benton et al., 2007; 2008), indicating that these factors also influence the cell cycle of the neuronal precursors and/or the survival of the newborn cells.
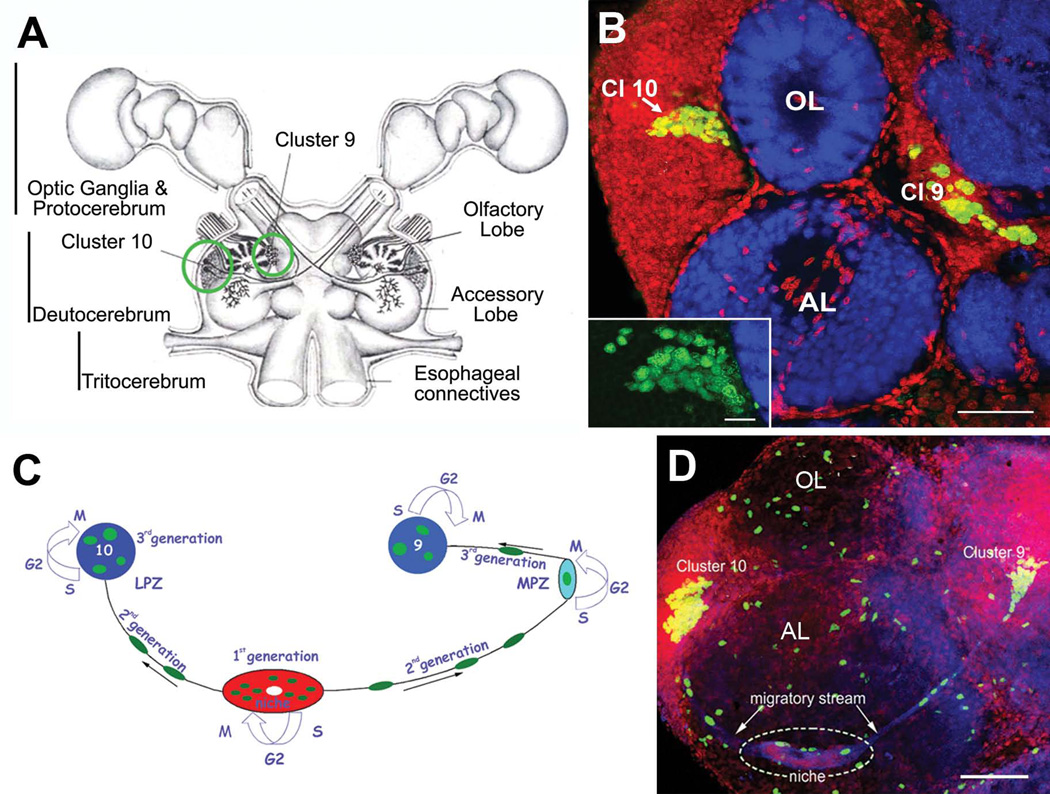
Figure 1.
Neurogenesis in the adult crayfish (C. destructor) brain. (A) Schematic diagram of the crayfish brain. Cell clusters 9 and 10 (circled in green), where adult-born neurons are incorporated, flank the olfactory and accessory lobes of the deutocerebrum. (B) Horizontal section through the olfactory (OL) and accessory lobes (AL) of C. destructor labeled immunocytochemically for BrdU (green) and Drosophila synapsin (blue) and counterstained with propidium iodide (red), a marker of nucleic acids. BrdU-labeled cells are observed within the proliferation zone in soma cluster 10 (Cl 10) (arrow), which lies adjacent to the olfactory lobe and in cluster 9 (Cl 9). The inset shows a higher-magnification view of BrdU-labeled cells within the cluster 10 proliferation zone. (C) A model summarizing our current understanding of events leading to the production of olfactory interneurons in adult crayfish. First generation neuronal precursor cells reside in a neurogenic niche where they divide symmetrically. Their daughters (second-generation precursors) migrate towards the lateral proliferation zone in Cluster 10 (LPZ) or the medial proliferation zone (MPZ) in Cluster 9 along tracts created by the fibers of bipolar niche cells. At least one more division occurs in the LPZ and MPZ before the progeny (third and subsequent generations of precursors) differentiate into neurons. (D) Left side of the brain of Procambarus clarkii labeled immunocytochemically for the S-phase marker BrdU (green). Labeled cells are found in the lateral proliferation zone contiguous with Cluster 10 and in the medial proliferation zone near Cluster 9. The two zones are linked by a chain of cells in the migratory stream, which labels immunocytochemically for glutamine synthetase (GS; blue). These streams originate in the oval region ‘niche’ (dotted circle) containing cells labeled with the nuclear marker propidium iodide (PI, red). The BrdU-labeled cells scattered irregularly throughout the OL and AL (which do not contain neuronal cell bodies) are glial cells. Scale bars: 100 µm in (B); 20 µm in insert in (B); 75µm in (D).
Adult-born neurons in crayfish (Procambarus clarkii) are the progeny of first-generation precursor cells (functionally analogous to neuronal stem cells in vertebrates) that are located in a neurogenic niche on the ventral surface of the brain (Fig. 1C, D) (Sullivan et al., 2007). The daughters of these precursors (second-generation progenitors) migrate along the processes of bipolar niche cells to the medial (MPZ; cluster 9) and lateral proliferation zones (LPZ; cluster 10) (Fig. 1C). Here they divide at least one more time to produce third and subsequent cellular generations (Sullivan et al., 2007; Zhang et al., 2009). One of the intriguing problems related to adult neurogenesis in crayfish is that the first-generation precursors cells are not self-renewing, as both daughter cells migrate away from the niche towards the proliferation zones (Benton et al., 2011; 2013). Nevertheless, the niche is not depleted as the animals grow and age. Based on our latest studies, we have therefore concluded that the niche is not a closed system and that there must be an extrinsic source of first-generation neuronal precursors. Experimental evidence to date indicates that the innate immune (hematopoietic) system may be the source of these neuronal precursor cells (Benton et al., 2011; 2012; 2013; Beltz et al., 2011; Chaves da Silva, 2013).
In vertebrate and invertebrate species, adult-born neurons differentiate and are incorporated into brain circuitry. The survival and incorporation of newborn cells into brain circuits can be explored with long-survival time experiments. Animals are exposed to BrdU for a specific time period, and then left for several months before sacrifice and examination of the brains for the presence of BrdU-labeled cells. The persistence of labeled cells in the brain many months after exposure to BrdU is an indication that the cells may have differentiated and become incorporated into the brain. Differentiation of BrdU-labeled cells can then be assessed by examining the anatomical development of axons and dendrites, acquisition of physiological properties, and expression of transmitters normally found in mature neurons of the same type.
Studies in a number of crustacean species have provided strong evidence that the surviving adult-born neurons are incorporated as new functional units. In adult shore crabs (Carcinus maenas), BrdU labeling in conjunction with biocytin backfills of olfactory projection neurons through the olfactory globular tract demonstrated the anatomical differentiation of proliferating cells after 3 weeks (Schmidt and Demuth, 1998). Further, cells in the spiny lobster, Panulirus argus, express transmitter by 3 months after BrdU exposure (Schmidt, 2001). In the crayfish brain, BrdU-labeled cells display adult characteristics, such as the acquisition of transmitters (i.e., P. clarkii: cluster 10 cells, SIFamide; cluster 9 cells, orcokinin, allatostatin; Sullivan et al., 2007), as well as axonal projections and arborizations in the appropriate synaptic areas (C. destructor, Sullivan and Beltz, 2005), by 6 months after the labeling period. However, intermediate time points were not examined, and thus the period when transmitter differentiation commences or is complete is not known. Therefore, one of the goals of the current study was to define the time when adult-born neurons first express transmitter, as one measure of their differentiated state.
The survival of newborn neurons in mammals is influenced by a number of factors. An enriched environment containing ample space for movement, conspecifics, structures for exploration and exercise wheels has been shown to increase BrdU labeling of hippocampal neurons compared with animals housed alone in simple, small cages (Kempermann et al., 1997; Nilsson et al., 1999; Brown et al., 2003). Further examination has shown that simple activity, such as wheel running, is less effective than when exercise is combined with learning or exploration. It is also well accepted that physical activity promotes both cell proliferation and survival (van Praag et al., 1999; van Praag, 2008, 2009). Studies in crayfish modeled on those performed with rodents have revealed that juvenile animals maintained alone in small containers produce fewer BrdU-labeled olfactory projection neurons (cluster 10 cells) and have fewer long-term surviving neurons, than conspecifics from the same brood living communally in larger containers; this is true even when temperature, lighting, water and feeding conditions are the same (Sandeman and Sandeman, 2000; Ayub et al., 2011). While locomotor activity of the animals in the “enriched” group was presumed to be higher, this was not measured, and therefore the influence of locomotor activity on neurogenesis in crayfish is not known.
The present study extends these earlier studies by exploring stages in the process of neuronal differentiation in crayfish. Animals were exposed to BrdU and were assessed quantitatively to define the rate of initial cell proliferation as well as long-term survival at intervals over a period of 8 months. In addition, the first expression of SIFamide, a transmitter found in mature cluster 10 neurons, was used as a differentiation marker to determine over what time the newborn (BrdU-labeled) cells acquire neuronal characteristics.
We found that the number of BrdU-labeled cells in cluster 10 decreased by about 30% between the first and second weeks after initial BrdU labeling, suggesting that a period of cell death occurs shortly after proliferation. Increases in BrdU-labeled cell counts several weeks later, long after the BrdU clearing time, indicate that some neuronal precursors may undergo a period of quiescence before their final divisions in clusters 9 and 10. Transmitter immunoreactivity was first observed in BrdU-labeled cells at 4 weeks after BrdU administration, and the number of cells expressing transmitter increased over the next 4 weeks. BrdU-labeled cells, like those in the spiny lobster (Schmidt, 2001), move from their initial location in the proliferation zones and along the medial edges of the cell clusters to become distributed over more lateral and peripheral positions in the cell clusters.
In a separate study, we examined the relationship between locomotor activity and BrdU incorporation over a period of 3 weeks. In contrast to findings in mammals, there was no correlation between locomotor activity and the rate of cell proliferation in cluster 10. However, the level of locomotor activity is strongly correlated with the number of cells that survived over the 3-week time period of the study. These experiments suggest that locomotor activity in crayfish influences the rate of survival of newborn neurons, and thus their availability for incorporation into the brain circuitry. It is worth noting that total activity was measured in these experiments, which is not comparable to the wheel running used in prior studies in rodents that examined the effects of locomotion on adult neurogenesis. Our results in crayfish therefore suggest that fewer neurons survive in “couch potatoes” than in physically active individuals, even if this activity does not include aerobic exercise.
The results presented here provide the first quantitative data in support of a period of cell death following cell proliferation in the adult crustacean brain. Further, a timeline for differentiation of the new neurons, beginning at 4 weeks after BrdU labeling, is established. Finally, these studies test the hypothesis that physical activity in crayfish, as in mammalian species, influences the number of cells that survive and differentiate into neurons.
MATERIALS AND METHODS
Proliferation, survival and differentiation studies
Animal Care and Treatment
Freshwater crayfish, Cherax destructor (Malacostraca, Decapoda, Parastacidae; supplied by Yabby Growers and Traders, Bulahdelah, Australia), were bred in the Wellesley Animal Care Facility. Egg-bearing female crayfish were housed at 21± 1°C on a 12:12 light:dark cycle in aquaria supplied with artificial pond water adjusted for mineral balance and pH (ddH2O with NaHCO3, Seachem Equilibrium trace elements from Seachem Laboratories Inc, Coving, GA, pH = 7.6). Eggs from the same mother hatched and developed into adult stage II (ADII) crayfish, which are not yet sexually differentiated (Sandeman and Sandeman, 1991). ADII crayfish in these studies had a carapace length (CL) of 3–4 mm; using siblings from the same brood ensured the least possible genetic variability among the crayfish.
BrdU labeling
Crayfish were incubated in 2 mg/mL BrdU in artificial pond water for 5 days, after which the crayfish were transferred into an aquarium of artificial pond water containing gravel, artificial plants, and pieces of 1" diameter plastic tubing for shelter. Previous research has demonstrated that the clearing time for BrdU in the crayfish brain is 2–3 days (Benton et al., 2011).
Survival and differentiation experiments
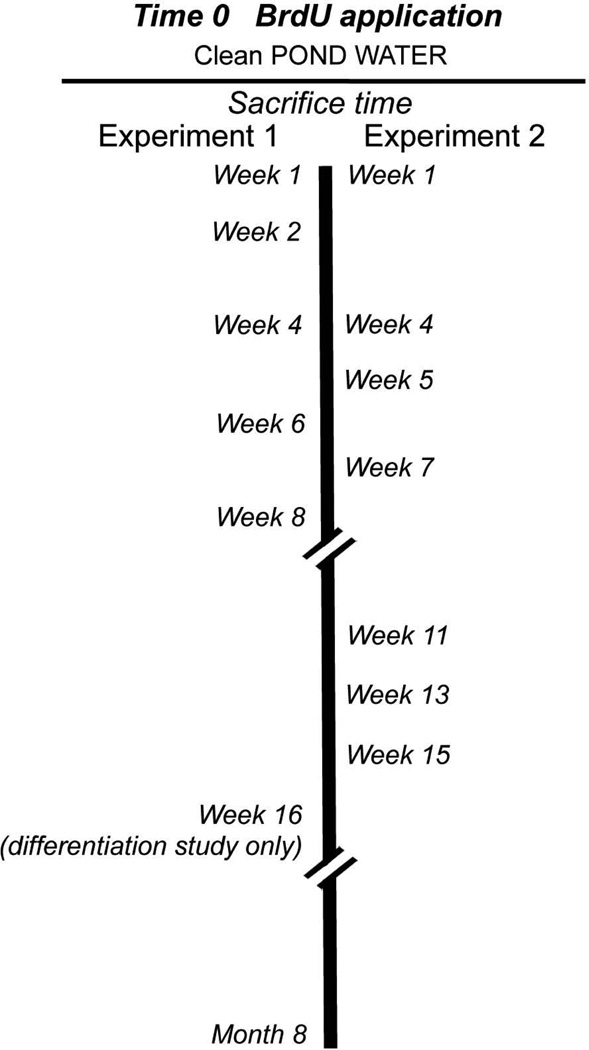
Time zero was defined as the beginning of the BrdU labeling period (see Fig. 2). In the first experiment (Experiment 1), crayfish were killed and their brains dissected and processed immunocytochemically 7 days after the beginning of BrdU exposure (week 1 time point). Subsequently, groups of animals were killed and processed at 2, 4, 6 and 8 weeks, and at 8 months, and BrdU-labeled cells were counted (Fig. 3). These preparations, and in addition brains from the 16-week time point, also were used to determine the timing of transmitter expression in BrdU-labeled cells in clusters 9 and 10, as a measure of cell differentiation (Fig. 2). In a second study (Experiment 2), different time points (weeks 1, 4, 5, 7, 11, 13, 15) were used in order to gather additional information about the survival and differentiation process; however, week 1 data are not reported for cluster 10 because of technical problems processing these brains.
Figure 2.
Diagram illustrating the protocol used to determine the time course of survival (Experiment 1, left; and Experiment 2, right) and differentiation (Experiment 1) of adult-born neurons. Time 0 is defined as the beginning of the BrdU labeling period (5 days), after which crayfish were transferred to an aquarium with pond water and later sacrificed at the times indicated. See Materials and Methods for additional details.
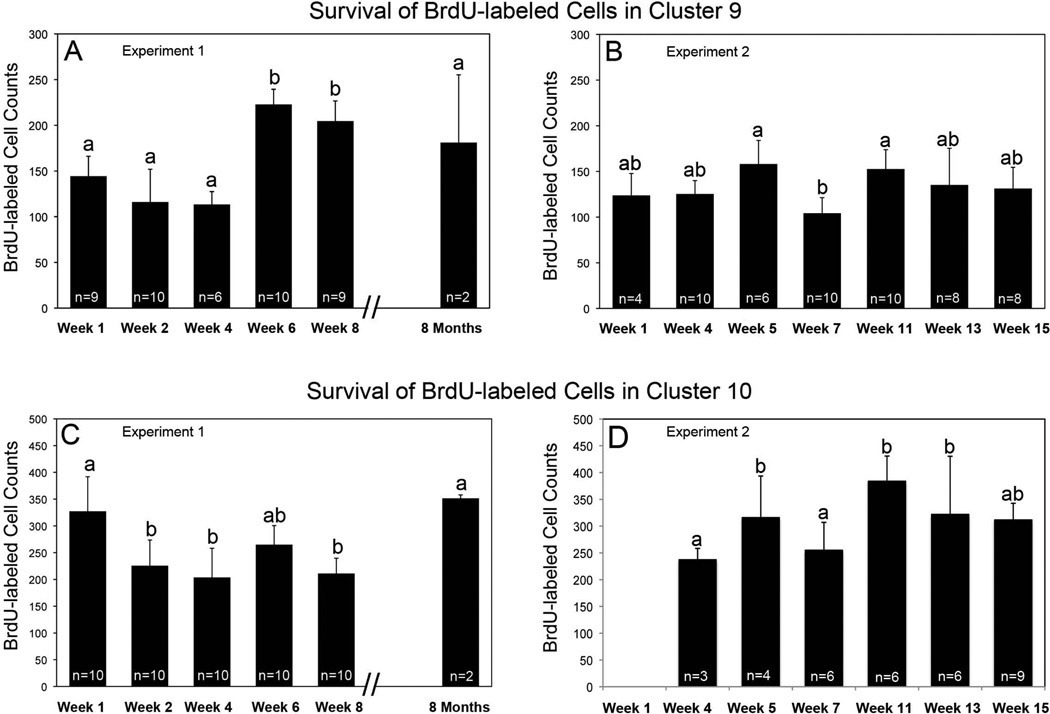
Figure 3.
Histograms of mean numbers of BrdU-labeled cells counted in cluster 9 in Experiment 1 (A: F=28.82, df=5, p=0.0001, One-way ANOVA) and Experiment 2 (B: F=4.75, df=6, p=0.0007), and in cluster 10 in Experiment 1 (C:, F=11.08, df=5, p=0.0001) and 2 (D: F=9.54, df=5, p=0.0001). One-way ANOVA analysis was followed by Tukey multiple comparison test. Statistical similarity is indicated by the same letters. These histograms provide evidence for a period of cell death in cluster 10 between weeks 1 and 2 after BrdU labeling (C), as well as delayed divisions and increased cell numbers at weeks 5–6, and 11 in both clusters 9 (A, B) and 10 (C, D). Cell death following these late divisions is also indicated in B and D between weeks 5 and 7, and in C between weeks 6 and 8. The number of cells clusters (n) counted for each time point is indicated on the histogram.
Immunocytochemical labeling
Brains were dissected and desheathed in cold crayfish saline (205 mM NaCl, 5.4 mM KCl, 34.4 mM CaCl2, 1.2 mM MgCl2, 2.4 mM NaHCO3, pH 7.4), then fixed overnight in 4% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4) at 4°C. Brains were rinsed several times over 2 hours in PB containing 0.3% Triton X-100 (PBTx), incubated in 2N HCl for 20 minutes, and rinsed again in PBTx. Brains were incubated for 20 minutes at room temperature in Image-iT FX signal enhancer (Molecular Probes, Grand Island, NY). After thorough rinsing in PBTx, brains were incubated for 3 hours in mouse anti-BrdU (1:50, BD Biosciences Pharmingen, San Jose, CA). Preparations were rinsed several times in PBTx, incubated overnight at 4°C in polyclonal rabbit anti-SIFamide (1:12,000; a gift from Dr. A. Yasuda), rinsed again, and immersed overnight in goat anti-mouse Alexa 488 and goat anti-rabbit Alexa 594 (1:50, Molecular Probes, Eugene, OR). Preparations were then rinsed in PB and mounted in Gelmount (Biomeda, Foster City, CA).
Data and statistical analysis
Data are presented as means ± standard deviation (S.D.). The number of differentiated (SIFamide-and BrdU-labeled) cells at each time point is presented as a ratio of the number of doubled-labeled cells divided by the total number of BrdU-labeled cells. N values denote the number of cell clusters analyzed for each time point. Comparisons between different time points were done with one-way ANOVA followed by the Tukey multiple comparison test using SPSS 15.0 software.
Locomotor activity, proliferation and survival of adult-born neurons
Animals
Large adult C. destructor (>25mm CL) used for the locomotor studies were obtained from Yabby Growers and Traders, Bulahdelah, Australia.
Activity measurements
Locomotor activity of individual animals was measured with a radio telemetry system designed for small mammals (Series ER-4000 Minimitter; Respironics Company, Bend, OR) and adapted for use with crayfish. Small transponders (15.5×6mm; 0.52g in water) were attached to the dorsal medial surface of the cephalothorax of each crayfish with superglue. Each individual crayfish was placed in a tank (30cm × 60cm) filled to a depth of 20cm with artificial pond water and containing washed gravel and a short length of black plastic pipe as a shelter. The Minimitter transducers did not impede the animals in their movement or from entering and leaving their shelters. The 8 individual monitoring systems were mounted in a rack in which illumination, controlled by a time switch, was provided by wide spectrum fluorescent lamps (Sylvania 40watt GRO-Lux), arranged so that all tanks received the same light intensity (135 lux). The rack was contained in a light- and sound-proof room maintained at 18–20°C. The animals were submitted to a 12:12 light:dark cycle.
The Minimitter transducers provided a continuous, quantitative measure of the animals’ activity, summed into 10-min bins, over the duration of each experiment. The telemetry system does not provide information about positional or directional changes so that no distinction is made, for example, between an animal that moves in a tight circle to one moving at the same speed in a straight line, but will discriminate between these animals if they move at different speeds, and hence cover greater distances per unit time. We found that movements of less than 1.5 mm/s were not recorded. The raw activity data was first displayed as actograms and the data were analyzed by various methods described in the text.
Correlating cell proliferation and cell survival with locomotor activity
To examine the relationship between cell proliferation, cell survival and activity levels, animals were fitted with transponders; 8 were used for the cell proliferation study and 8 for the cell survival experiments. The carapace lengths ranged from 50mm to 58mm. Each animal received a single injection of 0.1 ml BrdU (2 mg/ml). For cell proliferation measurements this injection was given 24 hours before the end of the experimental period. For the survival measurements, the animals were injected with BrdU only on the first day of the three-week experimental period. The animals were killed at the end of the experimental period, their brains were dissected and fixed in 4% paraformaldehyde, desheathed and processed immunocytochemically with mouse anti-BrdU antibody (1:50; BD Biosciences, San Jose, CA) and goat anti-mouse Alexa 488 (1:100; Jackson Immunoresearch, West Grove, PA). After immunocytochemical processing, the brain of each individual animal was mounted with Gel/mount™ (Biomeda Corp., Foster City, CA) and compressed lightly by placing small weights on the coverslip. All 8 animals in the cell proliferation experiment survived the entire experimental period. Two of the eight crayfish used for the cell survival experiment died before the termination of the experiment and therefore no cell counts were obtained for these animals.
Statistics and correlations
The total activity of individual crayfish during both light and dark phases over three separate weekly periods (week 1, 2 or 3) was extracted from the Minimitter data and the mean weekly activity calculated for each animal. The activity data was then plotted against the cell counts for each animal, obtained at the end of the experimental period. A linear regression line was fitted to the data using a least squares method that provided an estimate of the slope of the regression line. The correlation coefficient (R) was calculated in order to assess the strength of the relationships between cell proliferation and activity, and cell survival and activity.
Confocal microscopy and counts of BrdU-labeled cells
All fluorescently-labeled specimens were viewed with a Leica TCS SP laser-scanning confocal microscope equipped with argon, krypton and helium-neon lasers. Color balance and contrast of the images were adjusted within the Leica TCS SP program. To obtain cell counts for the proliferation and survival studies, as well as for correlations with locomotor activity, serial optical sections were taken at 1µm intervals and saved as two-dimensional stacks. Labeled cells in clusters 9 and 10 were counted by tracing the cell profiles onto a transparent sheet attached to the confocal microscope monitor.
For the differentiation study, sections were examined for the presence of cluster 10 neurons labeled with both BrdU and the neuronal marker SIFamide, from confocal images taken at 5µm intervals throughout cell clusters 9 and 10 on each side of the brain. Neurons were considered double-labeled when a BrdU-labeled nucleus was completely surrounded by a SIFamide-immunoreactive cytoplasm.
RESULTS
1. Cell survival
Experiment 1
In cluster 9, an average of 144 cells incorporated BrdU at the week 1 time point, a number that was maintained at 2 and 4 weeks (Figure 3A); this stability in numbers of labeled cells in cluster 9 during the first month after BrdU exposure was also observed in Experiment 2 (see Fig. 3B). The numbers of BrdU-labeled cells increased between weeks 4 and 6 (p < 0.001) and remained at this heightened level at week 8. By 8 months, the number of BrdU-positive cells decreased (p < 0.001) to the levels seen at week 1.
In cluster 10, an average of 327 cells incorporated BrdU at 1 week (Fig. 3C), while ~30% fewer BrdU-labeled cells were counted at weeks 2 and 4 (p < 0.001). Mean labeled cell counts in animals sacrificed at 6 weeks were intermediate between counts at weeks 1 (p=0.06) and 4 (p=0.06). At week 8, BrdU-labeled cell counts decreased slightly, to levels seen at weeks 2 and 4, and were significantly lower than the initial counts at week 1 (p<0.0001). Eight months after initial BrdU incubation, the number of BrdU-labeled cells in cluster 10 is significantly higher than at week 8 (p= 0.005), and (as observed in cluster 9) did not differ significantly from week 1 (p=0.99) (Figure 3C).
These quantitative assessments indicate that there are both similarities and differences in labeling trends in clusters 9 and 10. Cluster 10 shows a significant decline in BrdU-labeled cell counts at the 2-week time point compared with week 1, while there is no significant decrease in cluster 9 cell labeling. However, both cell groups show unexpected increases in BrdU incorporation at 6 weeks after the beginning of the BrdU labeling period, although this increase was proportionally greater in cluster 9.
Experiment 2
In order to verify these findings, this study was repeated with slightly different sampling times over a 15-week period. This second time course experiment (Fig. 3B, D) confirmed two findings suggested by the initial study. First, BrdU-labeled cell counts are constant in cluster 9 during the first four weeks after BrdU exposure (Fig. 3B), in contrast to the significant drop in BrdU-labeled cells in cluster 10 during the first month following BrdU exposure (Fig. 3C). Second, the delayed peaks in the numbers of BrdU-labeled cells were confirmed, as significant increases in BrdU-positive cell counts were seen at week 5 in cluster 10 (Figs. 3B, D; compare with week 6, Figs. 3A, B). In addition, this experiment also demonstrated a second delayed peak in BrdU labeled cells in cluster 10 at 11 weeks following the initial BrdU labeling period. Cell counts in cluster 9 suggest a trend towards this same pattern of delayed increases in BrdU-labeled cells. Significant decreases in labeled cell counts were observed within two weeks after some of these delayed peaks, suggesting again that a period of cell death may cull the population of recently proliferated cells.
2. Differentiation
In the crayfish P. clarkii, endogenous transmitters have been identified in both cluster 9 and cluster 10 neurons (Yasuda-Kamatani and Yasuda, 2006; Sullivan et al., 2007). The peptide transmitter SIFamide is found in a majority of cells in cluster 10, while orcokinin and allatostatin-like peptide are found in separate, smaller populations of neurons in cluster 9 (Yasuda-Kamatani and Yasuda, 2006).
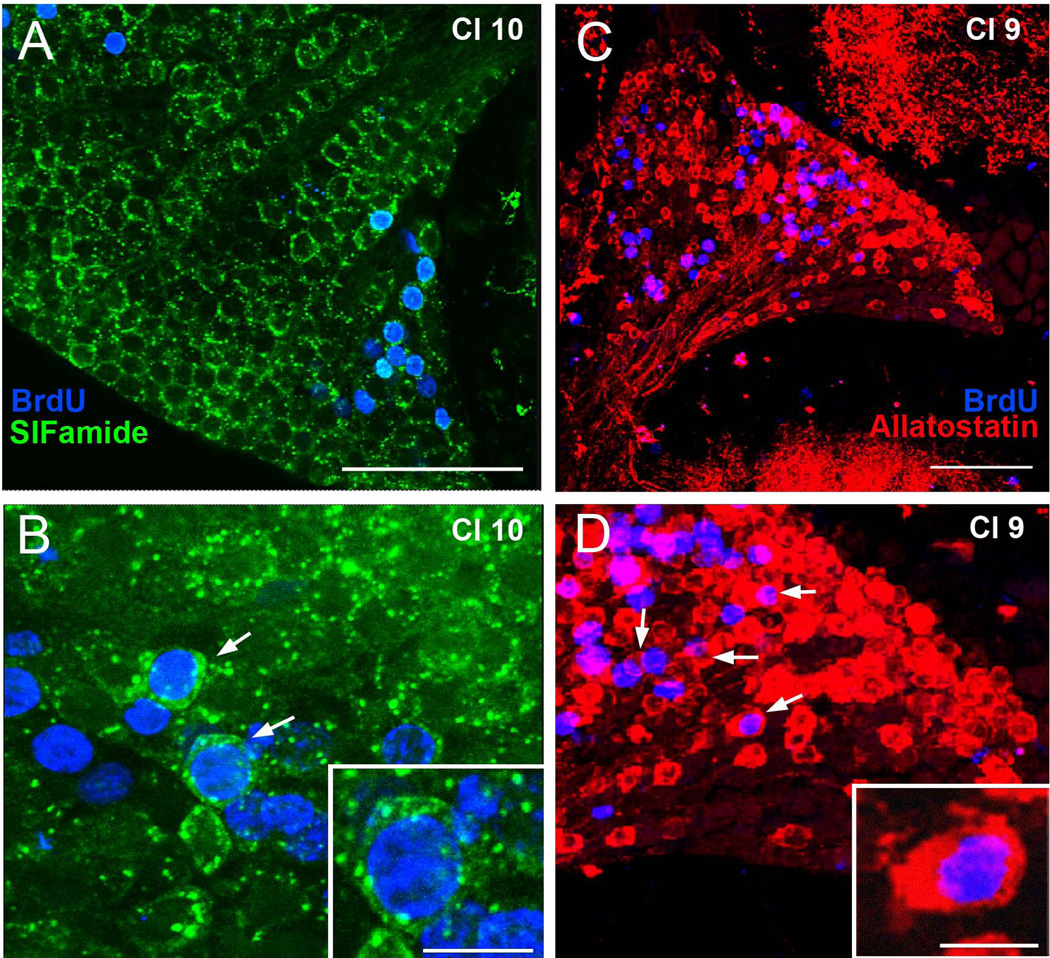
The presence of SIFamide immunoreactivity in cluster 10 neurons, and of allatostatin-like peptide immunoreactivity in cluster 9 cells, was confirmed in C. destructor (Fig. 4). The first appearance of these peptides in BrdU-labeled cells was then used as a marker of neuronal differentiation. For example, cluster 10 cells containing labeling for both BrdU (nuclear labeling) and SIFamide (cytoplasmic labeling) were deemed to have been born during incubation in BrdU and neurochemically differentiated by the time the animals were sacrificed. The nuclear to cytoplasmic ratio in cluster 9 and 10 neurons is large, with allatostatin-like peptide and SIFamide labeling in the cytoplasm appearing as a thin ring around the BrdU-labeled nucleus (Figure 4B, D).
Figure 4.
Differentiation of cluster 9 and cluster 10 BrdU-labeled cells. (A, B) Immunocyto-chemical labeling of cluster 10 cells for BrdU (blue) and SIFamide (green). By week 8, many BrdU-labeled cells are co-labeled with SIFamide (arrows in B), indicating that these have differentiated into neurons. (C, D) Images of BrdU (blue) and allatostatin (red) labeling of cluster 9 neurons (arrows), 8 weeks after the BrdU-labeling period. Scale bars = 100µm in A and B; 15µm in insert in B and D.
Many regions of the brain, including the accessory and olfactory lobes that receive projections from the cluster 10 neurons, as well as the median protocerebrum, are immunoreactive for SIFamide. In cluster 10, SIFamide labeling is primarily concentrated in cells towards the lateral edge of the cluster, and is absent from the medial areas of the cluster where new BrdU-labeled cells emerge in the proliferation zones. All double-labeled cells (BrdU- and SIFamide- immunoreactive) were located laterally in the cluster, outside the proliferation zone.
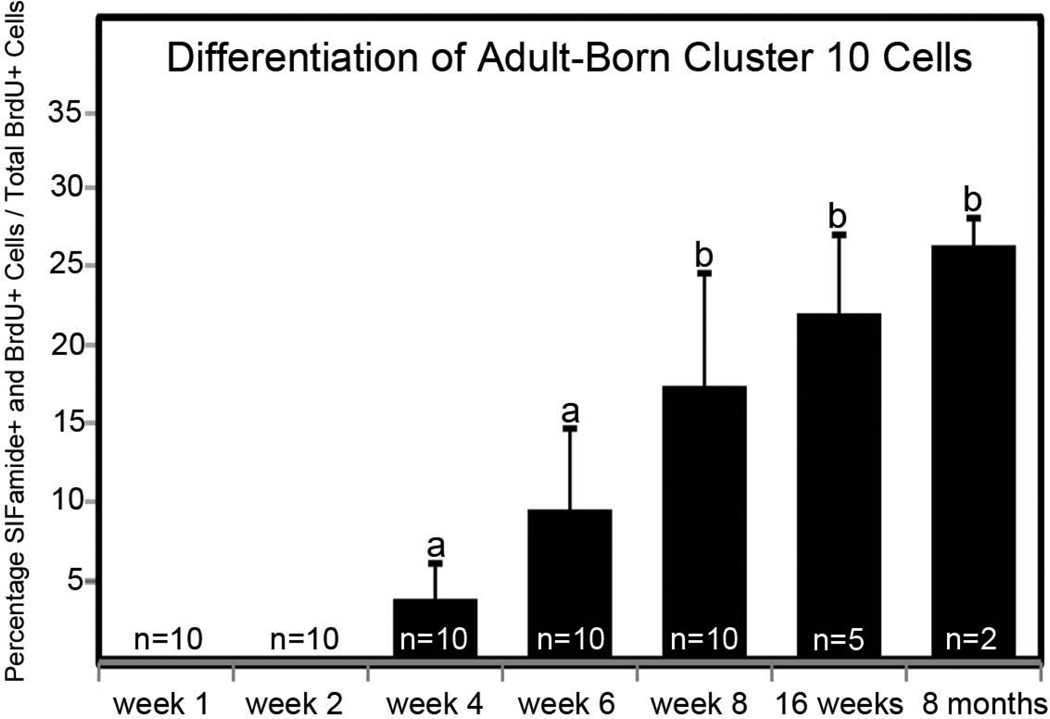
Double-labeled cells were counted for each time point and data are presented as a percentage of the total BrdU-labeled population (Figure 5). In cluster 9, fewer than 3% of BrdU-labeled cells were immunoreactive for allatostatin-like peptide by the 15-week time point and the variability between brains of different crayfish was high; these data could not be treated statistically and are not presented. However, SIFamide labeling in cluster 10 neurons was much more robust, with 20–25% of BrdU-labeled cells expressing transmitter by 16 weeks. Neurons labeled for both BrdU and SIFamide were first observed within cluster 10 four weeks after the initial BrdU exposure (Figure 5). The percentage of the total BrdU population that labels with SIFamide increased until week 8, after which the ratio of differentiated double-labeled neurons reached a plateau at about 25%, even at the 8-month time point.
Figure 5.
Differentiation of adult-born cluster 10 neurons. Mean percentages (± standard deviations) of SIFamide and BrdU double-labeled neurons to total BrdU-labeled neurons in cluster 10 analyzed over the time course of Experiment 1. The “n” represents the number of cell clusters assessed for each time point. One-way ANOVA analysis (F = 17.920, df = 4, p < 0.001) followed by Tukey multiple comparison test revealed significant differences between groups a and b (p < 0.001).
3. Localization of BrdU-labeled cells
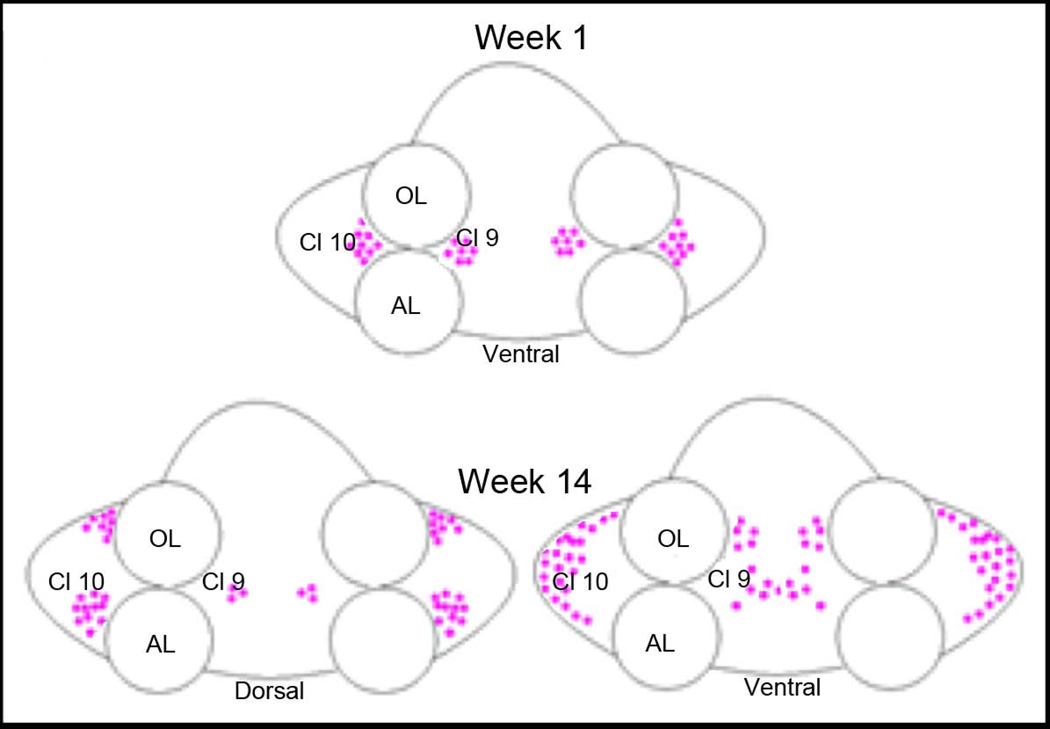
The distribution of BrdU-labeled cells within clusters 9 and 10 changes over time (Fig. 6). At week 1, BrdU-labeled cells were nestled ventrally at the medial and lateral margins of the OL and AL (see Fig. 6, top, and Fig. 1B, D), where the migratory streams empty into the proliferation zones associated with clusters 9 and 10. Over time, some BrdU-labeled cells in cluster 10 were found more laterally and, by the later sampling times, near the rostral and caudal edges of the cluster. By 4 months after BrdU administration, BrdU-labeled cells were found throughout cluster 10 from ventral to dorsal surfaces (Figure 6, bottom). In cluster 9 at week 1, BrdU-labeled cells were found in the migratory streams associated with cluster 9 and in the medial proliferation zone (MPZ). As in cluster 10, BrdU-labeled cells in cluster 9 were gradually displaced from the MPZ, and were distributed throughout the cell cluster by 4 months after BrdU labeling.
Figure 6.
Schema of the changing distribution of newborn neurons over time. At week 1, BrdU-labeled cells are nestled on the medial and lateral sides of the olfactory (OL) and accessory (AL) lobes, near the proliferation zones of cell clusters 9 and 10. By week 14, BrdU-labeled cells are found throughout the dorsal-ventral extent of clusters 9 and 10, and cells have dispersed from the sites where they proliferated. Dorsally, cluster 9 cells occupy a small region, while more ventrally these are distributed throughout the cluster. BrdU-labeled cells occupy positions at the rostral and caudal ends of cluster 10 dorsally, while ventrally the labeled cells are found in the lateral margins of the soma cluster.
Two spatially separated groups of BrdU-labeled cells were often present in both clusters 9 and 10 at the later time points. One group of cells expanded to the edges of these clusters, as described above, while a second group of BrdU-labeled cells, some of which were intensely labeled, were often (but not always) observed within the proliferation zones at the entrances of the clusters. The cells just lateral to the proliferation zone in cluster 10 did not label for SIFamide, suggesting that in this cluster the regions where proliferation and differentiation occur are distinct and spatially separated.
4. Locomotor activity, cell proliferation and cell survival in cluster 10
1. Activity and cell proliferation
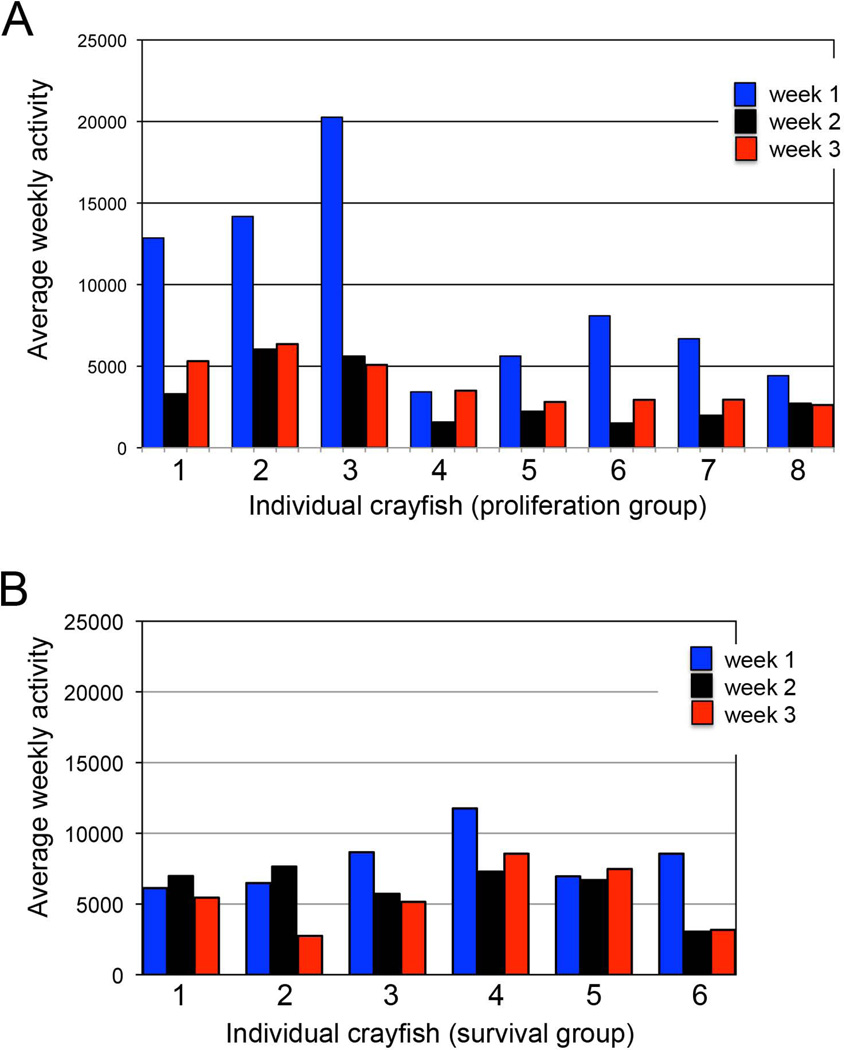
In the large adult animals used in these experiments, locomotor activity tended to be higher in the first week of confinement in individual aquaria than in subsequent weeks (Fig. 7A). This is a common phenomenon exhibited by crayfish placed in “novel” surroundings (Basil and Sandeman, 2000). The range of activity exhibited varied considerably between animals (from 3422 to 20261 in week 1, 1500 to 6029 in week 2, 2622 to 6357 in week 3; see Methods for explanation of quantification). However, variation for each individual animal was relatively low, with a tendency for each animal to maintain a relatively constant level over the course of the experiment.
Figure 7.
Average activity of individual crayfish for each of the 3 experimental weeks. (A) Crayfish 1–8 in the cell proliferation study; (B) Crayfish 1–6 in the cell survival study. Animals tend to exhibit increased activity in the first week after placement in the aquaria, which we attribute to their exploration of the novel environment. Their activity then decreases to a relatively constant level, which is maintained at over the next 2 weeks. Activity levels in weeks two and three were relatively consistent for individual crayfish, but varied between crayfish.
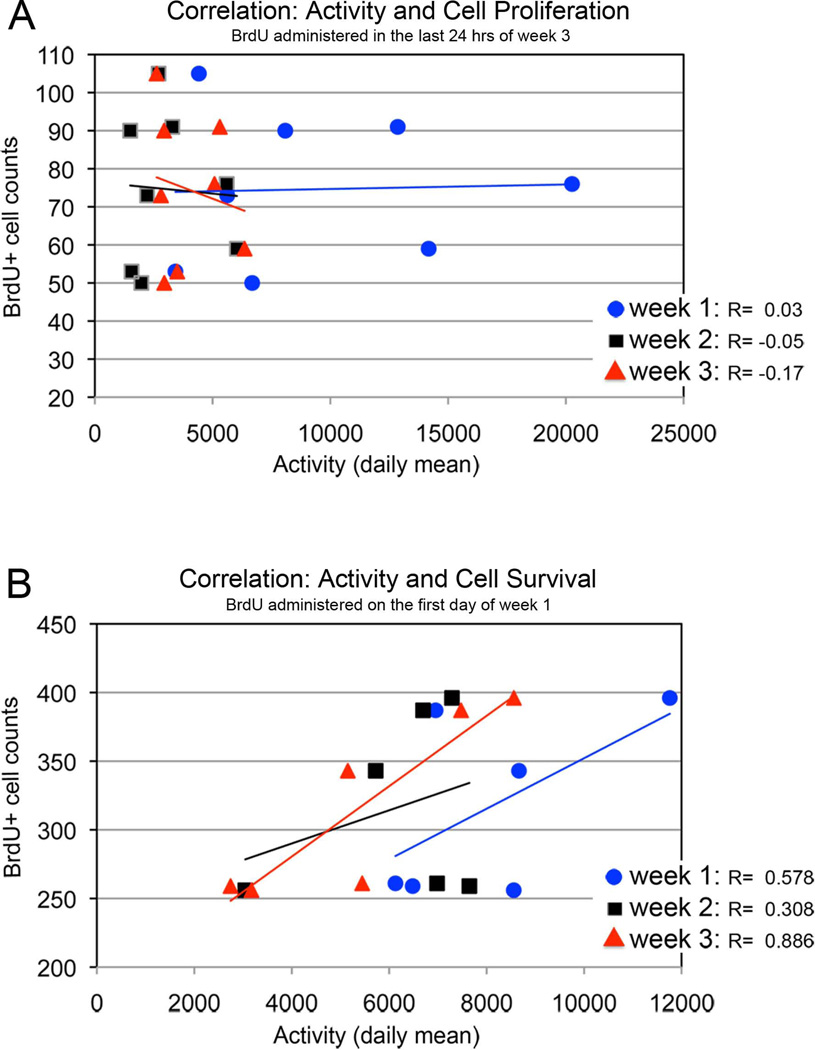
To reveal cells that were proliferating at the end of the 3-week experimental period, animals were injected with BrdU only once, 24 hours prior to sacrifice. The resulting BrdU-labeled cell counts varied between animals, ranging from 50 to 105 per cluster 10. Despite the differences in activity and BrdU-labeled cell counts, regression lines fitted through scatter plots of the weekly data are almost flat for each of the three weeks (Fig. 8A). The Pearson’s correlation coefficient (R) indicates that cell proliferation over the last 24 hours of the experimental period is not correlated with (and therefore is not influenced by) the levels of locomotor activity over the 3 weeks prior to sacrifice.
Figure 8.
(A) Correlations between the number of BrdU-labeled cells in cluster 10 of animals that were injected with BrdU one day before sacrifice, and the activity during weeks 1–3 of the experiment. The graph indicates that there is no correlation between cell proliferation and activity levels. (B) Graph showing positive correlations between the number of cells that survive in the cluster 10 over the period of the experiment and the activity during weeks 1–3. Crayfish were injected with BrdU on the first day of the 3-week experimental period. These correlations suggest a moderate interaction between cell survival and activity during weeks 1 and 2, and a strong interaction between cell survival and activity during week 3.
2. Activity and cell survival
Of the six crayfish that survived the three weeks of this experiment, some (but not all) exhibited higher activity levels in the first week compared with the following period (Fig. 7B). Again there was a tendency for active and less active animals to maintain their individual levels over the experimental period, and for there to be considerable variation in the extent of activity between individuals. Crayfish were injected with BrdU only once at the beginning of the experimental period and sacrificed three weeks later, in order to examine the influence of activity on the survival of newborn cells.
Counts of BrdU-labeled cells in cluster 10 represent cells that survived for the entire experimental period (3 weeks). The correlations between activity levels in weeks 1, 2 and 3 and cell survival are positive (R= 0.58, week 1; 0.31, week 2; 0.89, week 3), indicating a moderate influence of locomotor activity in weeks 1 and 2, and a strong interaction between locomotor activity during week 3, on the number of surviving cells at sacrifice (Fig. 8B). Week 3 activity levels show the strongest correlation with BrdU-labeled cell counts, indicating a strong interaction between locomotor activity over this period and the number of surviving cells.
DISCUSSION
The goals of the current experiments were to examine adult neurogenesis over an extended time frame in order to understand the temporal relationships between cell birth, survival and differentiation in the crayfish brain, and to determine if levels of locomotor activity influence proliferation or survival of the newborn cells.
Survival of BrdU-labeled cells
Between the first and second weeks after BrdU exposure, there was a decrease in the number of BrdU-labeled cells in cluster 10, and this low level of BrdU-positive cell counts was maintained through the week 4 time point. Such a reduction in BrdU labeling was not observed in cluster 9 following the initial labeling period, in either time-course study. However, significant decreases in the number of labeled cells was observed after the delayed peaks at 5 weeks in both clusters 9 and 10. These decreases in BrdU-labeled cells are most likely explained by a period of cell death following cell proliferation, a well-known phenomenon during both embryonic and adult neurogenesis (Oppenheim, 1981; Sanes et al., 2006).
Previous studies in decapod crustaceans have provided differing opinions on whether cell death plays a role in adult neurogenesis. In Carcinus maenas, it was reported that adult-born neurons in cluster 10 do not undergo programmed cell death (Schmidt and Demuth, 1998). This conclusion was based on comparisons of BrdU-labeled cell counts of short (4 hr–5 days) versus long (1 month) survival time experiments, which demonstrated roughly a doubling in total BrdU-labeled cell counts in cluster 10 between these times. Because of the lack of data from intervening time points, it is not clear whether cell death is absent, representing a mechanistic difference between C. maenas and C. destructor, or whether a higher rate of cell proliferation may have masked an underlying cell death component. The long-term survival of adult-born neurons also has been investigated in Panulirus argus, where BrdU-labeled cells in cluster 9 and 10 were quantified at 14 hours, 14 days, 3 months and 11 months after BrdU exposure (Schmidt, 2001). Based on the locations of pyknotic cells in the proliferation zones in clusters 9 and 10, this paper suggests that some of the newborn cells may be eliminated by apoptosis shortly after birth. However, quantitative data from the same study showed no indication of cell loss, with gradually increasing BrdU-labeled cell counts over the various sampling times. The present study therefore is the first to provide quantitative data in support of cell death following adult neurogenesis in crustacean species. The contrasting data gathered from other species may be explained by species-specific differences in the proportion of cell birth and death in the adult brain, by differences in the time course of these mechanisms and/or by the times sampled in the various studies. It is clear from the present data in the crayfish C. destructor, that a significant cell loss (~30%) occurs within the first week after BrdU incorporation in cluster 10. In contrast, if cell loss occurs in cluster 9, it is far less pronounced and is only supported by a significant quantitative decrease following a late peak in BrdU labeling (Fig. 3B, see weeks 5 and 7). In cluster 9, therefore, if apoptosis is occurring, there must be a balance between cell birth and death so that overall reductions in cell numbers are not generally observed.
Cell death is an important characteristic of adult neurogenesis in the dentate gyrus and olfactory bulb, two regions where new neurons are added in the adult mammalian brain (Biebl et al., 2000; Medrano and Scrable, 2005; Lepousez and Lledo, 2011). It has been estimated that 50% of the thousands of cells produced by the subventricular zone, which are destined to become local interneurons in the olfactory bulb, are eliminated by apoptosis (Winner et al., 2002). The degree of both neurogenesis and apoptosis is an order of magnitude lower in the dentate gyrus. In rodents, dying cells have been documented in all regions where neurogenic activity occurs in the adult brain: the subventricular and subgranular zones, rostral migratory stream, olfactory bulb and dentate gyrus (Biebl et al., 2000). However, the majority of cell death in the olfactory system occurs as the newborn cells reach their target area in the olfactory bulb. This contrasts with cell death in the hippocampus, where most newly generated cells undergo apoptosis locally in the subgranular zone, before they begin to migrate or differentiate into granule cells. In both cases, however, mechanisms of cell death play an important role in regulating the number of newly generated neurons in the mammalian forebrain. In the olfactory pathway this turnover appears to be important in optimizing olfactory performance (Mouret et al., 2009).
Whether the cell death suggested by our time course studies contributes to a turnover of cells in the olfactory pathway, or whether apoptosis is primarily occurring locally in the proliferation zone in cluster 10 to cull the newborn population of cells, has been debated (Schmidt, 2001). Apoptosis can be observed using the TUNEL (Terminal transferase UTP Nick End Labeling) method, which marks exposed ends of DNA fragments with labeled nucleotides. TUNEL profiles and toluidine blue staining suggest the presence of dying cells in the proliferation zone of cluster 10 in the lobster Homarus americanus (Harzsch et al., 1999), as do histological observations of pyknotic nuclei in P. argus (Schmidt, 2001). These data correlate well with our findings of cell death within 1–2 weeks after BrdU incubation. However, additional evidence from TUNEL assays demonstrates that labeled cells are also scattered throughout cluster 10, suggesting a turnover of cells (Sandeman et al., 1998; Harzsch et al., 1999). Further, it is difficult to imagine that cluster 10 continually increases in cell number and size over the long lifetimes of many crustaceans, such as the American lobster, which has been proposed to live for a century or more (Klapper et al., 1998). Therefore, it seems logical to propose that some turnover of olfactory interneurons must occur in the crustacean brain, as in the mammalian olfactory bulb; likewise, the maintenance of an optimal number of neurons, as well as a balance between newborn and older, differentiated neurons, is likely to be critical for adult brain function (Mouret et al., 2009).
Secondary and tertiary peaks in BrdU-labeled cell numbers
In addition to the initial peak of BrdU incorporation at week 1 in clusters 9 and 10, our data also indicate secondary (5–6 weeks) and tertiary (11 weeks) peaks in BrdU-labeled cell counts (see Fig. 3). The BrdU clearing time in the closely related crayfish P. clarkii (Benton et al., 2011) is between 2 and 3 days. Therefore, all labeling observed at extended time points must be generated by BrdU incorporation during the initial labeling period and the subsequent divisions of these cells. Thus, the delayed peaks must be due to synchronized divisions of cells that were labeled 5/6 weeks and 11 weeks earlier. Many of the cells composing these peaks are intensely labeled, further suggesting that some cells in clusters 9 and 10 were arrested after BrdU incorporation, and did not immediately continue to divide after taking up the label. A period of cell death may also be associated with these late proliferative periods, as decreases in BrdU-labeled cell counts are observed in both clusters 9 and 10 during the weeks immediately following some of these secondary and tertiary peaks (Fig. 3B, C, D). Delayed increases in the number of BrdU-labeled cells have also been reported in P. argus (Schmidt, 2011), suggesting that delayed, synchronous divisions of neuronal precursors may be a general property of adult neurogenesis in crustacean species.
Differentiation into neuropeptide-producing neurons
The neuropeptide SIFamide, which plays an integral role in olfaction in crustaceans, is localized in adult crayfish in cluster 10 cell bodies, in terminals within the olfactory and accessory lobes, and in the olfactory globular tract (Yasuda-Kamatani and Yasuda, 2006; Polanska et al., 2007; Verleyen et al., 2008). The presence of SIFamide in BrdU-labeled cells in cluster 10 in our preparations may therefore be taken as evidence that these cells have differentiated into neurons and become integrated into the brain as functional units.
In cluster 10, cells that were double labeled with both BrdU and SIFamide were first observed at week 4, and their numbers increase at later time points, reaching a plateau by 8 weeks (see Fig. 5). This timeline is similar to what has been reported for differentiation of adult-born neurons in the mouse brain (van Praag et al., 2002). In the present study, only ~25% of BrdU-labeled cluster 10 neurons double-label with SIFamide by 8 months after BrdU incubation, indicating that the other BrdU-labeled cells either have not differentiated, or have differentiated into non-SIFamide-producing neurons that use a different transmitter.
The location of the BrdU/SIFamide-labeled neurons in cluster 10 provides an indication of the timing of the differentiation process and where this takes place. Cells within the proliferation zone of cluster 10 contain intensely labeled BrdU-containing cells that do not label for SIFamide at the week 1 time point. Later, after the newly born cells migrate away from the proliferation zone, BrdU labeled cells are found that also express the SIFamide transmitter, indicating chemical differentiation. This suggests the existence of a “differentiation zone" that is distinct from the proliferation zone, and a process of maturation and differentiation that corresponds with the migration of the cells away from the proliferation zones. Such a relationship has also been found in P. argus (Schmidt, 2001). Similarly, pulse-chase experiments in 7-year-old adult H. americanus found that by six weeks after BrdU-injection, labeled cells had dispersed in cluster 10 and obtained shapes and sizes similar to adult interneurons in the surrounding cluster, suggesting their differentiation (Harzsch et al., 1999).
Therefore, the location of labeled neurons in cluster 10 is indicative of cellular age: older cells are located near the edges, while cells born more recently are located more medially, closer to the proliferation zone. This progression has been described in H. americanus (Harzsch et al., 1999) and quantified in P. argus, where it has been demonstrated that 14 hour-old BrdU-labeled cells are located close to the proliferation zones, while 6 month-old BrdU-labeled cells are located 50–100 µm (in cluster 10) and 30–50µm (in cluster 9) away from the proliferative zones (Schmidt, 2001).
Influence of locomotor activity on cell proliferation and survival
Our data suggest that levels of locomotor activity do not influence neuronal proliferation in cluster 10, but are a strong determinant of cell survival. In rodents, wheel running can result in a 3–4-fold or greater increase in the generation and survival of newborn neurons in the hippocampus (van Praag, 2008), but neither physical activity nor enriched environment alter olfactory bulb neurogenesis (Brown et al., 2003). While the present data appear to contrast with results related to the olfactory system in mammals, it is important to emphasize that distinct groups of projection neurons in cluster 10 innervate the olfactory lobe (the primary olfactory processing area, analagous to the olfactory bulb) and the accessory lobe (a higher order processing area that may subserve functions related to those that are localized in the hippocampus of mammals) (Sandeman et al., 1995). Further, both of these cell types are produced during adult neurogenesis (Sullivan and Beltz, 1995). Therefore, it may be that the specific neuronal type that is influenced by locomotor activity may be the cluster 10 neurons that specifically target the accessory lobe.
Conclusions
These studies provide the first quantitative evidence for a period of cell death following cell proliferation during adult neurogenesis in the crustacean brain. In addition, the chemical differentiation of the new neurons is first documented at 4 weeks after BrdU exposure, and continues during the next month. Finally, these findings support the hypothesis that levels of physical activity in crayfish, as in mammalian species, strongly influence the number of cells that survive and differentiate into neurons.
Acknowledgements
The authors thank P. Carey and V. LePage for care of the animals used in these studies, and Elizabeth Bless, Emily Cockey, Kristina Costa, Emmy Li and Jody Platto for their critical comments and discussion of the manuscript. This work was supported by NIH R01 MH67157, NSF IOS 0818259, and NSF IOS 1121345.
References
- Ayub N, Benton JL, Zhang Y, Beltz BS. Environmental enrichment influences neuronal stem cells in the adult crayfish brain. Dev Neurobiol. 2011;71:351–361. doi: 10.1002/dneu.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basil J, Sandeman D. Crayfish (Cherax destructor) use tactile cues to detect and learn topographical changes in their environment. Ethology. 2000;106:247–259. [Google Scholar]
- Beltz BS, Benton JL, Sullivan JM. Transient uptake of serotonin by newborn olfactory projection neurons. Proceedings of the Natlonal Academy of Sciences U S A. 2001;98:12730–12735. doi: 10.1073/pnas.231471298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz BS, Zhang Y, Benton JL, Sandeman DC. Adult neurogenesis in the decapod crustacean brain: a hematopoietic connection? European Journal of Neuroscience. 2011;34:870–883. doi: 10.1111/j.1460-9568.2011.07802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Chaves da Silva PGS, Sandeman DC, Beltz BS. First-generation neuronal precursors in the crayfish brain are not self-renewing. International Journal of Developmental Neuroscience. 2013 doi: 10.1016/j.ijdevneu.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Goergen EM, Rogan SC, Beltz BS. Hormonal and synaptic influences of serotonin on adult neurogenesis. General and Comparative Endocrinology. 2008;158:183–190. doi: 10.1016/j.ygcen.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Sandeman DC, Beltz BS. Nitric oxide in the crustacean brain: regulation of neurogenesis and morphogenesis in the developing olfactory pathway. Developmental Dynamics. 2007;236:3047–3060. doi: 10.1002/dvdy.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Kirkhart CR, Sandeman DC, Beltz BS. Primary neuronal precursors in adult crayfish brain: replenishment from a non-neuronal source. BMC Neuroscience. 2011;12:53. doi: 10.1186/1471-2202-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton JL, Zhang Y, Soderhall I, Beltz BS. The crustacean cytokine astakine-1: A link between adult neurogenesis and hematopoiesis? Society for Neuroscience. 2012 Abstracts 427.13/A59. [Google Scholar]
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Chaves da Silva PG, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis in the crayfish brain: the hematopoietic anterior proliferation center has direct access to the brain and stem cell niche. Stem Cells and Development. 2013;22:1027–1041. doi: 10.1089/scd.2012.0583. [DOI] [PubMed] [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. Journal of Neurobiology. 2002;53:90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Hansen A, Schmidt M. Influence of season and environment on adult neurogenesis in the central olfactory pathway of the shore crab, Carcinus maenas. Brain Res. 2004;1025:85–97. doi: 10.1016/j.brainres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Dawirs RR. Neurogenesis in the developing crab brain: postembryonic generation of neurons persists beyond metamorphosis. J Neurobiol. 1996;29:384–398. doi: 10.1002/(SICI)1097-4695(199603)29:3<384::AID-NEU9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Harzsch S, Miller J, Benton J, Beltz B. From embryo to adult: persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. Journal Neuroscience. 1999;19:3472–3485. doi: 10.1523/JNEUROSCI.19-09-03472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Adult Neurogenesis. Cold Spring Harbor Monograph Series. 2006;52 [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Klapper W, Kuhne K, Singh KK, Heidorn K, Parwaresch R, Krupp G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 1998;439:143–146. doi: 10.1016/s0014-5793(98)01357-x. [DOI] [PubMed] [Google Scholar]
- Lepousez G, Lledo PM. Life and death decision in adult neurogenesis: in praise of napping. Neuron. 2011;71:768–771. doi: 10.1016/j.neuron.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Medrano S, Scrable H. Maintaining appearances--the role of p53 in adult neurogenesis. Biochem Biophys Res Commun. 2005;331:828–833. doi: 10.1016/j.bbrc.2005.03.194. [DOI] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. V. Evidence on the role of cell death and neuromuscular function in the formation of specific peripheral connections. J Neurosci. 1981;1:141–151. doi: 10.1523/JNEUROSCI.01-02-00141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska MA, Yasuda A, Harzsch S. Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res. 2007;330:331–344. doi: 10.1007/s00441-007-0473-8. [DOI] [PubMed] [Google Scholar]
- Sandeman D, Sandeman R, Derby C, Schmidt M. Morphology of the brain of crayfish, crabs, and spiny lobsters: A common nomenclature for homologous structures. The Biological Bulletin. 1992;183:340–346. doi: 10.2307/1542217. [DOI] [PubMed] [Google Scholar]
- Sandeman DC, Benton JL, Beltz BS. An identified serotonergic neuron regulates adult neurogenesis in the crustacean brain. Developmental Neurobiology. 2009;69:530–545. doi: 10.1002/dneu.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Clarke D, Sandeman D, Manly M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. Journal of Neuroscience. 1998;18:6195–6206. doi: 10.1523/JNEUROSCI.18-16-06195.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. Stages in development of the embryo of the freshwater Cherax destructor. Roux Archives of Developmental Biology. 1991:27–37. doi: 10.1007/BF02457638. [DOI] [PubMed] [Google Scholar]
- Sandeman R, Sandeman D. "Impoverished" and "enriched" living conditions influence the proliferation and survival of neurons in crayfish brain. J Neurobiol. 2000;45:215–226. [PubMed] [Google Scholar]
- Sandeman RE, Watson AH, Sandeman DC. Ultrastructure of the synaptic terminals of the dorsal giant serotonin-IR neuron and deutocerebral commissure interneurons in the accessory and olfactory lobes of the crayfish. J Comp Neurol. 1995;361:617–632. doi: 10.1002/cne.903610406. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Reh TA, Harris WA. Development of the Nervous System. Oxford, UK: Elsevier Academic Press; 2006. [Google Scholar]
- Schmidt M, Harzch S. Comparative Analysis of Neurogenesis in the Central Olfactory Pathway of Adult Decapod Crustaceans by In Vivo BrdU Labeling. Biological Bulletin. 1999;196:127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Continuous neurogenesis in the olfactory brain of adult shore crabs, Carcinus maenas. Brain Research. 1997;762:131–143. doi: 10.1016/s0006-8993(97)00376-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Neuronal differentiation and long-term survival of newly generated cells in the olfactory midbrain of the adult spiny lobster, Panulirus argus. J Neurobiol. 2001;48:181–203. doi: 10.1002/neu.1050. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Demuth S. Neurogenesis in the central olfactory pathway of adult decapod crustaceans. Ann N Y Acad Sci. 1998;855:277–280. doi: 10.1111/j.1749-6632.1998.tb10583.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Beltz BS. Newborn cells in the adult crayfish brain differentiate into distinct neuronal types. Journal of Neurobiology. 2005;65:157–170. doi: 10.1002/neu.20195. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: a common strategy across diverse species. Journal of Comparative Neurology. 2007;500:574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]