Abstract
Schistosomiasis is a debilitating parasitic disease of humans, endemic in tropical areas, for which no vaccine is available. Evidence points to glycan antigens as being important in immune responses to infection. Here we describe our studies on the comparative humoral immune responses to defined schistosome-type glycan epitopes in Schistosoma mansoni-infected humans, rhesus monkeys and mice. Rhesus anti-glycan responses over the course of infection were screened on a defined glycan microarray comprising semi-synthetic glycopeptides terminating with schistosome-associated or control mammalian-type glycan epitopes, as well as a defined glycan microarray of mammalian-type glycans representing over 400 glycan structures. Infected rhesus monkeys generated a high immunoglobulin G (IgG) antibody response to the core xylose/core α3 fucose epitope of N-glycans, which peaked at 8–11 weeks post infection, coinciding with maximal ability to kill schistosomula in vitro. By contrast, infected humans generated low antibody levels to this epitope. At 18 months following praziquantel therapy to eliminate the parasite, antibody levels were negligible. Mice chronically infected with S. mansoni generated high levels of anti-fucosylated LacdiNAc (GalNAcβ1, 4(Fucα1, 3)GlcNAc) IgM antibodies, but lacked a robust response to the core xylose/core α3 fucose N-glycan antigens compared with other species studied, and their sera demonstrated an intermediate level of schistosomula killing in vitro. These differential responses to parasite glycan antigens may be related to the ability of rhesus monkeys to self-cure in contrast to the chronic infection seen in humans and mice. Our results validate defined glycan microarrays as a useful technology to evaluate diagnostic and vaccine antigens for schistosomiasis and perhaps other infections.
Keywords: glycan microarrays, glycans, schistosomiasis, schistosomula killing, core xylose-core α3 fucose
Introduction
Schistosomiasis is one of the most prevalent parasitic diseases in the world (The World Health Organization 2010, Tendler et al. 1985; Pearce and MacDonald 2002; Chitsulo et al. 2004). The vasculature-dwelling trematodes of the Schistosoma genus infect humans, non-human primates and other mammals, including domesticated animals such as cattle (Stothard et al. 2012; Zhou et al. 2012). According to the World Health Organization, schistosomiasis is second only to malaria among parasitic diseases in terms of public health impact, with over 200 million people infected, 600 million at risk of exposure and 300,000 deaths per year in Sub-Saharan Africa alone (Chitsulo et al. 2004; Hotez and Ferris 2006; Kupferschmidt 2013). The impact of the disease is manifested in its high morbidity for both children and adults during the chronic phase, which can last for decades (Gryseels et al. 2006; King and Dangerfield-Cha 2008). Mass administration of praziquantel to persons in endemic areas has been instrumental in the management of schistosomiasis (Rollinson et al. 2013). However, re-infections do occur frequently and the emergence of drug-resistant strains is a persistent concern (Cioli and Pica-Mattoccia 2003; Hotez and Ferris 2006; Lamberton et al. 2010; Wang et al. 2012). Thus far, no protective vaccine has been developed against schistosomiasis or any other helminth infection of humans (Hotez et al. 2010).
While most vaccine-related research has focused on development of protein-based vaccines, antibodies in sera from helminth-infected animals and humans also strongly recognize glycan epitopes displayed on the parasite glycoproteins, glycolipids and glycosylphosphatidylinositol-anchored proteins (Nyame et al. 2000, 2004; Eberl et al. 2001; Naus et al. 2003; van Diepen et al. 2012). In order to exploit such glycans as potential vaccine or diagnostic candidates, we need to better understand the basis for their antigenicity, which we address in this manuscript, and immunogenicity, which we explore in our companion manuscript (Prasanphanich et al. 2014).
Anti-glycan antibodies can bind to the helminth surface, have been demonstrated to kill schistosomula in vitro and have protective ability in various in vitro and in vivo models (Dissous et al. 1982; Harn et al. 1984; Grzych et al. 1985, 1987; Ko et al. 1990; Ellis et al. 1994; Nyame et al. 2003; van Stijn et al. 2010). In human studies of naturally acquired immunity to schistosomiasis and resistance to re-infection after cure, elements of both Th1- and Th2-type immune responses have been correlated with protection, notably worm-specific IgE and certain immunoglobulin G (IgG) subtypes (Hagan et al. 1991; Rihet et al. 1991; Dunne et al. 1992; Demeure et al. 1993). Importantly, rhesus macaques (Macaca mulatta), depending on the level of infection with schistosomes, can self-cure (Cheever and Powers 1969). In these animals, egg-laying by adult worms peaks around 8 weeks and then declines (Wilson et al. 2008). The primates are then resistant to secondary infection at 16–20 weeks after primary infection (Smithers and Terry 1967). Whether this natural resistance is due to immunologic or physiologic factors is unclear, but a recent study suggested that IgG binding to crucial proteins such as digestive enzymes might be involved in attenuation of adult worms (Wilson et al. 2008). Given that humoral immunity is important for protection in both of these hosts and that a large portion of their response is anti-glycan, it is feasible that anti-glycan antibodies, as well as those targeting protein antigens, contribute to immune-mediated protection in humans and/or in rhesus macaques.
A handful of studies have examined patterns of anti-glycan antibodies in humans and non-human primate models (Eberl et al. 2001; van Remoortere et al. 2001, 2003; Naus et al. 2003, de Boer et al. 2008; van Diepen et al. 2012). Some of these studies have noted that antibodies to many glycan structures are higher in childhood than in adulthood in endemic persons (van Remoortere et al. 2001; Naus et al. 2003; van Diepen et al. 2012). Chimpanzees partially protected from infection by vaccination with irradiated cercaria appear to make a lower response to certain glycan antigens when compared with un-vaccinated animals (Eberl et al. 2001). To our knowledge, no studies have directly compared the anti-glycan responses of different species, and none have shown direct evidence of the role of anti-glycan antibodies in human infection or in protective models such as rhesus monkeys.
Mice are a well-studied model host for schistosomes and are considered one of the most permissive hosts for Schistosoma mansoni (Warren and Peters 1967). Similar to humans, infected mice develop Th2-type liver granulomas, are unable to clear chronic infections and acquire only partial resistance to super-infection (Pearce and MacDonald 2002; Abdul-Ghani and Hassan 2010). However, because of their small body size, even the lowest infectious dose results in more eggs per gram of tissue and more intense pathology than is usually seen in the heaviest human infections (Cheever 1969). Additionally, resistance to re-infection in mice may be due to physiologic rather than immune factors (McHugh et al. 1987). Thus, we reasoned that comparison of the anti-glycan antibody responses among these three hosts of varying susceptibility to S. mansoni infection would be instructive in elucidating mechanisms that might contribute to protection.
Among the glycans that are highly antigenic in S. mansoni infection are those containing the Lewis X (LeX; Galβ1,4(Fucα1,3)GlcNAc), LacdiNAc (LDN; GalNAcβ1,4-GlcNAc) and fucosylated LacdiNAc (LDNF; GalNAcβ1,4(Fucα1,3)GlcNAc) motifs (Srivatsan et al. 1992a, b; Nyame et al. 1996, 1999, 2000). The LeX epitope is commonly expressed on both parasite and mammalian glycans, whereas expression of LDN and LDNF motifs in mammals appears to be highly restricted to a handful of tissues and/or specific proteins (Dell et al. 1995; Van den Eijnden et al. 1995; Nyame et al. 1996, 1999; Cummings and Nyame 1999; Van den Nieuwenhof et al. 2000). Several other multiply-fucosylated LDN-based motifs which are completely foreign to the host are also major targets of the antibody response to schistosomes (Naus et al. 2003; van Remoortere et al. 2003; de Boer et al. 2008). These motifs can all be expressed as terminal epitopes on a variety of different underlying core structures on schistosome glycoproteins and glycolipids. Additionally, non-mammalian N-glycan core modifications such as core α3 fucose and core β2 xylose are generated by schistosomes and are antigenic in helminth infection (Khoo et al. 1997; van Die et al. 1999; Meevissen et al. 2011; van Diepen et al. 2012). Core α6 fucose, in contrast, is made by both mammals and parasites and is not antigenic. Henceforth, we refer to core α3 fucose as core fucose. The glycans of S. mansoni and other helminths were recently reviewed (Prasanphanich et al. 2013).
Considering the clear need to better understand immune responses to glycan antigens in schistosome-infected humans and animals, we used a chemo-enzymatic approach to generate a “schistosome-type” microarray representing some of the major defined glycan antigens found in S. mansoni. Interrogation of this defined schistosome-type glycan microarray with sera from humans and animals revealed unique and species-dependent anti-glycan responses and may provide a direction to identifying potential glycan-based serodiagnostics and anti-schistosome vaccines.
Results
Generation and validation of the defined schistosome-type glycan microarray
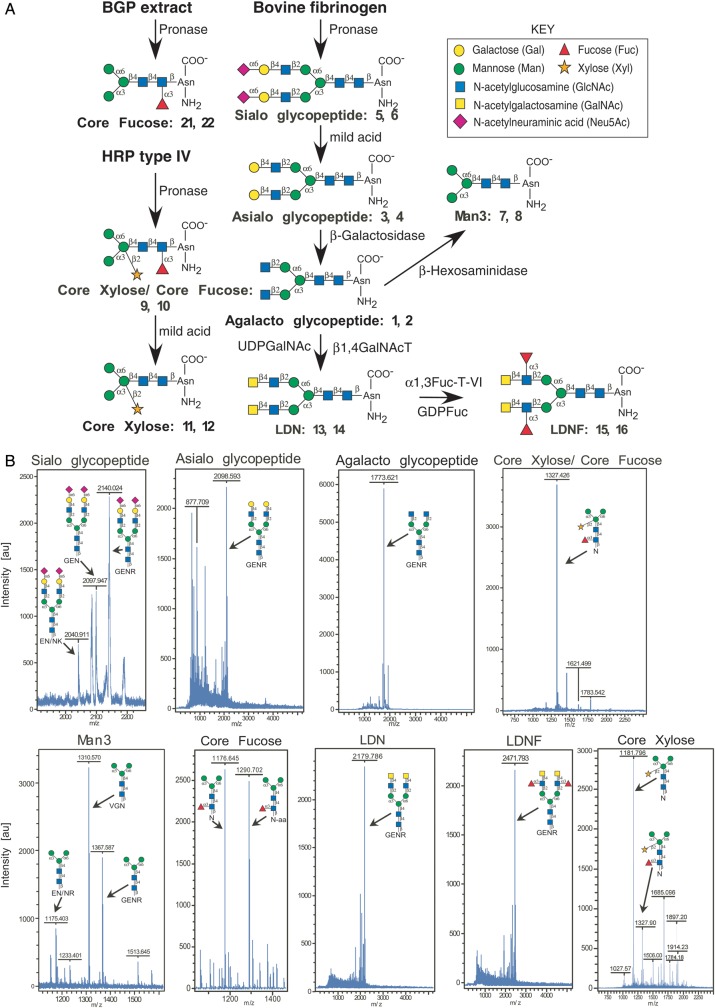
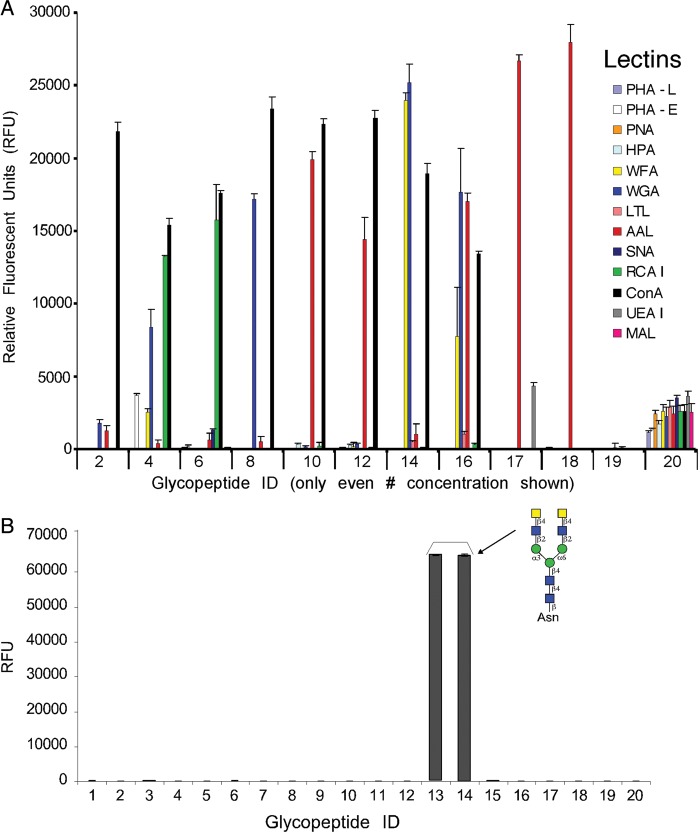
We generated a microarray consisting of defined schistosome glycan epitopes and mammalian-type precursor glycans by using a chemo-enzymatic approach to modify glycopeptides obtained from natural sources (Figure 1A) and printing the glycopeptides on NHS-activated microarray slides. The starting materials were commercial plant or mammalian glycoproteins that were digested with Pronase to generate glycopeptides, many of which contained a single Asn residue. From these starting glycopeptides, glycan modifications were made as described in Figure 1A and the Materials and Methods “Glycopeptide preparation” section to generate the final products. MALDI-TOF analysis was performed on the nine final glycopeptides, including seven glycopeptides containing defined glycan epitopes found in schistosomes (#3–4, 7–16, 21–22), two control glycopeptides (#1–2, 5–6), and several other control glycan structures (#17–20, 23–24). The observed masses of the glycopeptide constructs are shown and closely matched the predicted sizes (Figure 1B). The list of the glycans/glycopeptides that were printed on the defined schistosome microarray, each at 0.2 and 0.4 mg/mL, is given in Table I and Figure 1. To validate the printing, lectins of varying glycan-binding specificities were used in a binding assay. The lectins bound to the different glycopeptides with the expected specificities (Figure 2A). The lectins that bound as expected to appropriate glycans included Con A (concanavalin A), RCAI (Ricinus communis agglutinin I), WGA (wheat germ agglutinin), AAL (Aleuria aurantia lectin) and WFA (Wisteria floribunda agglutinin), which bind to N-glycans, terminal galactose (Gal), terminal N-acetylglucosamine (GlcNAc), fucose (Fuc) and terminal N-acetylgalactosamine (GalNAc), respectively (Cummings 1994; Cummings and Etzler 2009). We noted that AAL, a core fucose-binding lectin (Kochibe and Furukawa 1980), bound somewhat to the defucosylated core xylose-containing glycopeptides #11 and #12. This binding is likely due to incomplete defucosylation of the core xylose/core fucose starting material using mild acid, leaving residual amounts of intact core fucose. Other lectins that showed no or very little binding to schistosome glycans, as expected, but only recognized appropriate control glycans, include MAL (Maackia amurensis lectin), UEA-I (Ulex europaeus I), SNA (Sambucus nigra agglutinin), LTL (Lotus tetragonolobus lectin), HPA (Helix pomatia agglutinin), PNA (peanut agglutinin), PHA-E (Phaseolus vulgaris agglutinin E) and PHA-L (Phaseolus vulgaris agglutinin L), which recognize terminal sialic acid (Neu5Ac) in α2,3-linkage, α1,2-linked Fuc, terminal Neu5Ac in α2,6-linkage, poly-N-acetyllactosamine, terminal α-GalNAc, complex-type bisected biantennary N-glycans and complex-type tri/tetraantennary N-glycans, respectively (Cummings 1994; Cummings and Etzler 2009). Though some minor peaks, not corresponding to any known glycopeptide compositions, could be seen in the MALDI-TOF profiles of printed fractions, lack of binding by the lectins listed above indicates that the printed glycans were sufficiently pure for analysis. For additional validation, a monoclonal antibody to the LDN epitope (Nyame et al. 1999) showed specific binding to LDN-containing glycopeptides #13 and #14 on the microarray (Figure 2B).
Fig. 1.
(A) Synthesis of glycopeptide epitopes. All of the glycopeptides printed on the defined schistosome microarray were synthesized by a combination of chemical and enzymatic methods using natural starting products as shown in the schematic. BGP, Bermuda grass pollen; HRP, horseradish peroxidase. The structures, names, and glycopeptide ID numbers correspond to the microarray list given in Table I. (B) MALDI-TOF profiles of the synthesized glycopeptides and glycans used to generate the defined schistosome microarray. Panels are labeled with the glycan/glycopeptide name, corresponding structure(s), and obtained masses.
Table I.
List of glycopeptides and controls on the defined schistosome microarray
| Glycopeptide ID | Compound name | Printed concentration |
|---|---|---|
| 1 | Agalacto glycopeptide | 0.4 mg/mL |
| 2 | Agalacto glycopeptide | 0.2 mg/mL |
| 3 | Asialo glycopeptide | 0.4 mg/mL |
| 4 | Asialo glycopeptide | 0.2 mg/mL |
| 5 | Sialo glycopeptide | 0.4 mg/mL |
| 6 | Sialo glycopeptide | 0.2 mg/mL |
| 7 | Man3 glycopeptide | 0.4 mg/mL |
| 8 | Man3 glycopeptide | 0.2 mg/mL |
| 9 | Core xylose/core fucose glycopeptide | 0.4 mg/mL |
| 10 | Core xylose/core fucose glycopeptide | 0.2 mg/mL |
| 11 | Core xylose glycopeptide | 0.4 mg/mL |
| 12 | Core xylose glycopeptide | 0.2 mg/mL |
| 13 | LDN glycopeptide | 0.4 mg/mL |
| 14 | LDN glycopeptide | 0.2 mg/mL |
| 15 | LDNF glycopeptide | 0.4 mg/mL |
| 16 | LDNF glycopeptide | 0.2 mg/mL |
| 17 | Lewis a (Lea) glycan | 100 µM |
| 18 | Lewis x (LeX) glycan | 100 µM |
| 19 | PBS | |
| 20 | Biotin | |
| 21 | Core fucose (Fuc-Man3) glycopeptide | 0.4 mg/mL |
| 22 | Core fucose (Fuc-Man3) glycopeptide | 0.2 mg/ml |
| 23 | Asialo glycan-AEAB | 100 μM |
| 24 | Man5 glycan-AEAB | 100 μM |
Structures 1–16 and 21–24 are symmetrical biantennary structures. 21–24 were added on a second iteration of the array. See Figure 1 for the structural details.
Fig. 2.
Lectin and monoclonal antibody binding specificities on the defined schistosome-type glycan array. (A) After incubation with the glycan microarray slides, bound biotinylated lectins (0.1 µg/mL) were detected by cyanine5-streptavidin. Only the lower concentration of each printed glycopeptide (even numbers) are shown. PHA-L, Phaseolus vulgaris agglutinin L; PHA-E, Phaseolus vulgaris agglutinin E; PNA, peanut agglutinin; HPA, Helix pomatia agglutinin; WFA, Wisteria floribunda agglutinin; WGA, wheat germ agglutinin; LTL, Lotus tetragonolobus lectin; AAL, Aleuria aurantia lectin; SNA, Sambucus nigra agglutinin; RCA I, Ricinus communis agglutinin I; ConA, concanavalin A; UEA I, Ulex europaeus I; MAL, Maackia amurensis lectin. (B) Binding of monoclonal antibody to LDN measured by Alexa488-labeled goat anti-mouse IgM secondary antibody. ID numbers on x-axes correspond to glycopeptides listed in Table I. RFU, relative fluorescence units.
Schistosome-infected rhesus monkeys target core xylose/core α3 fucose
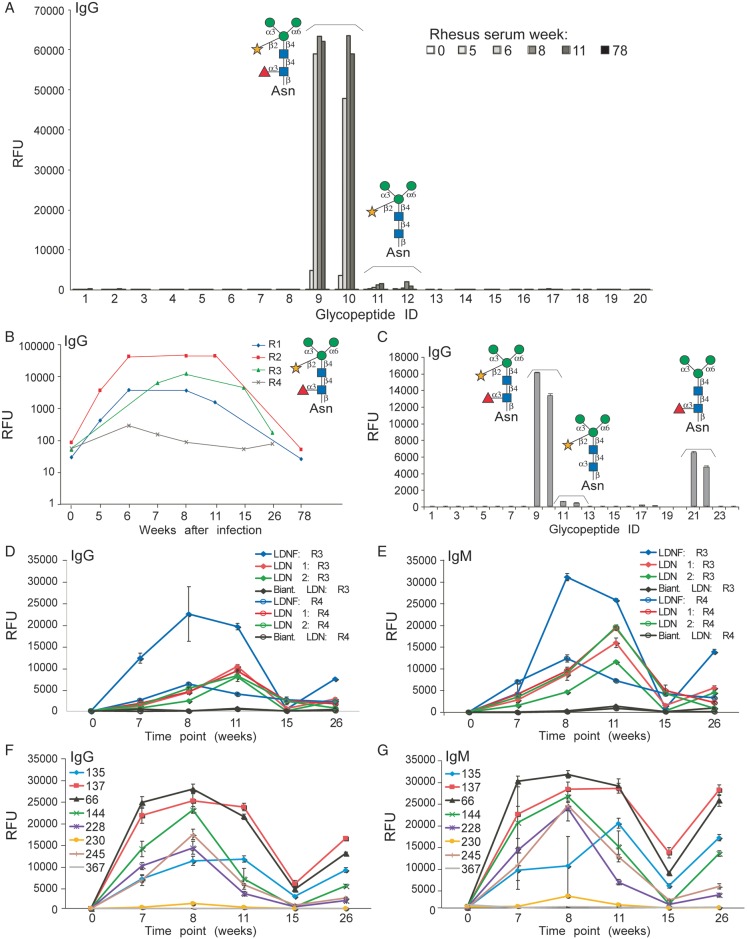
We investigated humoral immune responses to schistosome-specific glycan antigens in four rhesus monkeys infected with S. mansoni. Sera from pre-bled animals lacked detectable IgG to any of the schistosome-related antigens, while sera from infected monkeys at weeks 5, 6, 8 and 11 robustly recognized core xylose/core fucose structures on the defined schistosome microarray at a 1:1000 dilution of sera (Figure 3A, ID 9, 10). In contrast, the anti-IgG response to a version of the glycan that had been defucosylated by mild acid hydrolysis, resulting in the core xylose epitope alone, was substantially lower (Figure 3A, ID 11, 12). Interestingly, these anti-core xylose/core fucose responses were not detectable by 78 weeks post-infection in any of the four monkeys (Figure 3A and B), a time point at which the animals had stopped shedding detectable eggs in the stool. At 6–11 weeks after infection, sera from monkeys showed high levels of anti-core xylose/core fucose IgGs on this microarray (Figure 3B). On a similar version of the microarray generated to also contain the core fucose glycopeptide, which lacks xylose, pooled sera at 1:1000 from two monkeys also showed a response to this epitope (Figure 3C). Although anti-core xylose/core fucose IgM responses were also observed, the reactivity appeared much lower compared with IgG and was not examined further; this trend was observed in all four monkeys throughout the time course of infection and self-cure.
Fig. 3.
Infected rhesus anti-glycan response on two versions of the defined schistosome-type microarray and the CFG glycan microarray. (A) Pre-bleed (0) and schistosome-infected rhesus monkey sera at various time points (weeks) after infection were used to probe the defined schistosome microarray at a dilution of 1:1000. Data show representative results from one of four monkey sera (R2). (B) Binding of pre-bleed (0) and schistosome-infected rhesus sera at various time points (weeks) from four rhesus monkeys (R1-R4) to the core xylose/core fucose epitope (ID 9) plotted on a log scale to visualize the pattern of binding over time. (C) Binding of 8-week post-infection sera pooled from 2 monkeys on the microarray bearing an additional core fucose epitope (ID 21, 22). ID numbers on the x-axes correspond to glycopeptides listed in Table I. (D–G) The humoral immune responses in rhesus monkeys infected with S. mansoni over a period of 26 weeks were analyzed on the CFG glycan microarray. (D and E) Data shown are from two representative monkeys (R3 and R4), which generated anti-LDN and -LDNF IgG antibodies (D) and IgM antibodies (E). Responses to various LeX antigens for IgG (F) and IgM (G) are from one representative monkey (R3). The legend for F and G correspond to the LeX-containing glycan structures listed in Table II.
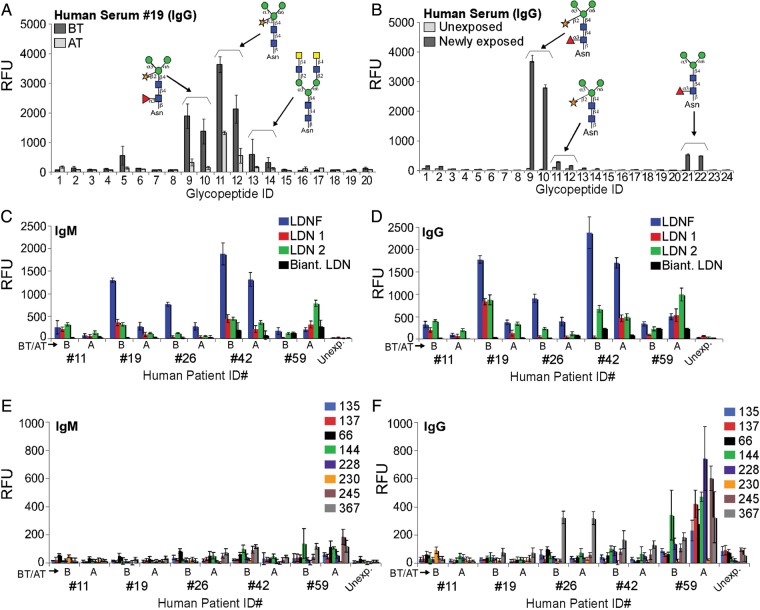
In a parallel study, sera from these four monkeys were used to interrogate anti-glycan responses to various glycan epitopes printed on the mammalian CFG glycan microarray. The CFG array (version 3.2) lacks glycans with core xylose/core fucose epitopes, but it does contain glycans with LDN (the disaccharide on two different linkers, as well as on a biantennary N-glycan structure), LDNF (the trisaccharide) and LeX determinants (several different versions, detailed in Table II), which differ from the biantennary configurations presented on the defined array, as well as hundreds of other mammalian-type glycans. Anti-LDN and -LDNF antibody (IgG and IgM) levels were highest at 8–11 weeks post-infection. These antibody levels decrease significantly after the 11th week. At the 26th week, the anti-LDN and -LDNF responses were similar to those observed at the 7th week post-infection (Figure 3D and E). Levels of anti-LDNF antibodies were generally higher than anti-LDN, and the binding to the straight-chain LDN was much higher than to the biantennary structure. Additionally, during the course of infection, monkeys responded actively to glycan chains terminating with LeX and containing internal and/or poly-LeX antigens (Figure 3F and G and Table II). The anti-LeX responses were highest from 7 to 11 weeks after infection. These anti-LeX responses increased again 15 weeks after infection, with anti-LeX antibody levels at 26 weeks having similar levels as those observed at 11 weeks after infection (Figure 3F and G). This increase in anti-LDN, LDNF and LeX antibodies also coincided with presumed spontaneous cure of the infection. The CFG array data with multiple LeX structures aids in elucidating the anti-LeX antibody specificity in the sera. The LeX derivatives that were most highly bound were poly-LeX with three repeating units of LeX (ID 137 and 66) even in the presence of a terminal fucose residue (LeY). Lower antibody binding was seen to a single LeX epitope (ID 135) and to glycans with sialic acid (ID 228, 230), with no binding to internal LeX on a biantennary chain (ID 367).
Table II.
Selected glycans on the CFG glycan microarray v3.2 that contain the Lewis X (LeX) antigen motif (R-Galβ1-4(Fucα1-3)GlcNAcβ1-R)
| Glycan No. | Glycan structure |
|---|---|
| 135 | Galβ1-4(Fucα1-3)GlcNAcβ-Sp8 |
| 137 | Galβ1-4(Fucα1-3)GlcNAcβ1-4Galβ1-4(Fucα1-3)GlcNAcβ1-4Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 |
| 66 | Fucα1-2Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 |
| 144 | Galβ1-4GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 |
| 228 | Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 |
| 230 | Neu5Acα2-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp8 |
| 245 | Neu5Acα2-6Galβ1-4GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4(Fucα1-3)GlcNAcβ-Sp0 |
| 367 | Galα1-3Galβ1-4(Fucα1-3)GlcNAcβ1-2Manα1-3(Galα1-3Galβ1-4(Fucα1-3)GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAcβ-Sp20 |
LeX epitope is bolded in each structure below.
Trivial names: 135, terminal LeX; 137, terminal and poly-LeX; 66, 144 and 245, internal poly-LeX; 228, sialylated poly-LeX; 230, sialylated LeX; 367, internal LeX on biantennary glycan structure.
Sera from schistosome-infected rhesus monkeys show killing of schistosomula
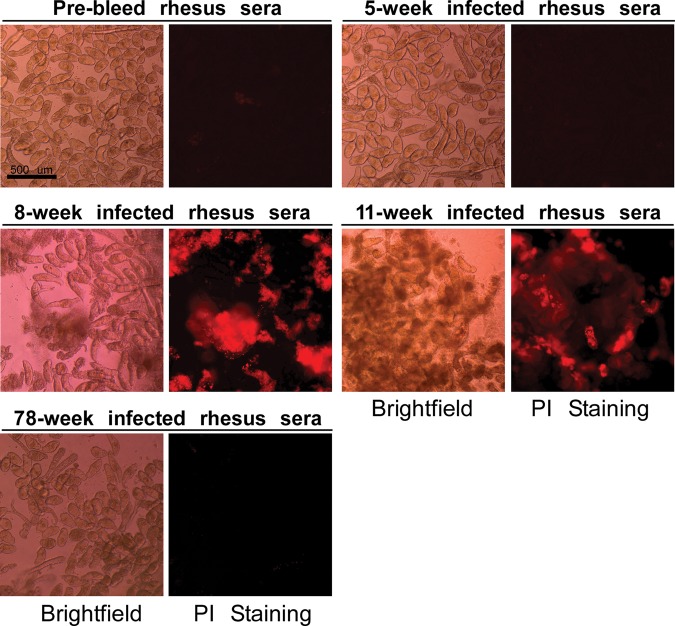
We tested whether the presence of anti-glycan antibodies correlated with the ability of the monkey serum to effectively kill schistosomula. We established an in vitro killing assay in which freshly prepared 3-h-old schistosomula were treated with sera and tested for killing in a follow-up method to our earlier observations using monoclonal antibodies to glycan antigens (Nyame et al. 2003). The schistosomula were visualized by brightfield microscopy and also tested for integrity by staining with propidium iodide (PI), which can stain DNA in permeable cells, but cannot penetrate intact membranes. Pooled sera from these infected monkeys at 1:10 dilution in culture medium was directly lethal to 3-h-old mechanically transformed schistosomula in vitro after 48 h incubation. Due to the limited amount of rhesus sera available, we were not able to test killing at every time point indicated on the microarrays. Sera from 8 and 11 weeks post-infection, which had the highest anti-core xylose/core fucose responses on the defined schistosome microarray and anti-LDN, LDNF and LeX responses on the CFG array, also had the highest schistosomula killing effects compared with other time points during infection (Figure 4 and Table III). Killing was so extensive in 8- to 11-week rhesus serum that PI stain could be seen extruded into the media as well as localized to dying schistosomula. By gross morphology, very few live schistosomula remained at 48 h. Thus, these studies demonstrated that effective antibody-mediated killing correlates with levels of antibodies to the core xylose/core fucose, LDN, LDNF and LeX antigens in the rhesus monkey.
Fig. 4.
Infected rhesus sera are lethal to 3-h-old schistosomula in vitro. Mechanically transformed schistosomula were treated with pooled sera from S. mansoni infected rhesus diluted in culture medium at various time points. Death of schistosomula was assessed by gross anatomy observation (brightfield) and fluorescent propidium iodide (PI) staining. Killing was observed as clumping, rotund body shape, protrusions of acetabular gland, lack of movement, and PI uptake in 8- and 11-week infected sera. Data are an average of three independent fields in one experiment, and are representative of two replicate experiments.
Table III.
In vitro schistosomula lysis
| PI staining |
Gross anatomy observation |
|||||
|---|---|---|---|---|---|---|
| Total no. of somules (48 h) | Total no. of dead somules | % dead | Total no. of somules (48 h) | Total no. of dead somules | % dead | |
| Rhesus sera | ||||||
| Pre-bleed | 150 | 2 | 1.3 | 150 | 1 | 0.7 |
| 5 week infected | 120 | 1 | 0.8 | 120 | 2 | 1.7 |
| 8 week infected | 139 | 130 | 93.5 | 139 | 132 | 95 |
| 11 week infected | 135 | 135 | 100 | 135 | 135 | 100 |
| 78 week infected | 125 | 1 | 0.8 | 125 | 2 | 1.6 |
| Human sera | ||||||
| Normal | 120 | 0 | 0 | 120 | 1 | 0.8 |
| Infected, before treatment | 122 | 3 | 2.5 | 122 | 5 | 4 |
| Infected, after treatment | 125 | 3 | 2.4 | 125 | 4 | 3.2 |
| Pooled infected | 120 | 1 | 0.8 | 120 | 4 | 3.3 |
| Mouse sera | ||||||
| Normal | 159 | 1 | 0.6 | 159 | 2 | 1.3 |
| 8 week infected | 109 | 79 | 72.5 | 109 | 70 | 64.2 |
| Media alone | 150 | 0 | 0 | 150 | 1 | 0.7 |
Data are an average of three independent fields in one experiment, and are representative of two replicate experiments.
Sera from schistosome-infected humans have low reactivity with core xylose/core fucose and other glycan epitopes
We obtained sera from five individuals infected with S. mansoni and analyzed the sera on the defined schistosome microarray. The results showed that all five infected individuals responded predominantly with IgG to both core xylose/core fucose and core xylose epitopes, with four of the five sera responses being higher before (BT) than after treatment (AT) with praziquantel (Figure 5A). Although the time at which these individuals were infected is not known, the trends of their anti-glycan responses were comparable. While the rhesus sera reactivities to core xylose/core fucose were at the threshold of maximal detection, human sera detected with the same secondary antibodies had more than 10-fold lower reactivity. Results from the CFG glycan microarray show that most individuals with schistosome infections had relatively higher levels of anti-LDNF and -LDN antibodies (IgG and IgM) before treatment than after treatment (Figure 5C and D). The levels of anti-LeX antibodies (IgG and IgM) are in most cases not higher than the levels seen in normal human serum, although a few individuals made low-level IgG to internal and/or poly-LeX (Figure 5E and F). Pooled sera from another group of human subjects who were newly infected (1–2 years living in schistosome-endemic regions in Africa) showed anti-glycan responses to core xylose/core fucose, core xylose, and to core fucose, which were predominantly IgG (Figure 5B). Serum from humans with no history of schistosome exposure showed very low or undetectable responses to these glycans (Figure 5B). In contrast to the results with sera from infected rhesus monkeys, pooled sera from infected humans before and after praziquantel treatment lacked killing activity toward schistosomula in vitro as did pooled sera from newly infected humans and normal human sera (Table III). More than 95% of the schistosomula remained alive in all cases. Thus, a less robust anti-glycan antibody response in the human sera compared with rhesus sera correlated with lack of antibody-dependent killing of the schistosomula in vitro.
Fig. 5.
Antibody responses in infected humans. (A) Sera from infected humans before (BT) and after treatment (AT) with praziquantel were used to probe the printed defined schistosome glycan microarray at a dilution of 1:1000. Data show one representative (ID 19) of five human serum samples. (B) Binding of pooled newly infected human sera and normal (unexposed) on the defined schistosome microarray bearing an additional core fucose epitope (ID 21, 22). ID numbers on x-axes correspond to glycopeptides listed in Table I. (C–F) Human sera samples (ID 11, 19, 26, 42, 59) before (B) and after treatment (A) were used to probe a CFG glycan microarray. IgM (C) and IgG (D) responses to LDN and LDNF, and IgM (E) and IgG (F) responses to various LeX antigens are shown. Numbers in the legend for E and F correspond to the LeX-containing glycans listed in Table II. Five human samples (IDs on x-axes) were analyzed plus a normal human serum (Unexp.) control.
Mice generate IgM to LDNF 8 weeks after infection and exhibit complement-dependent killing of schistosomula
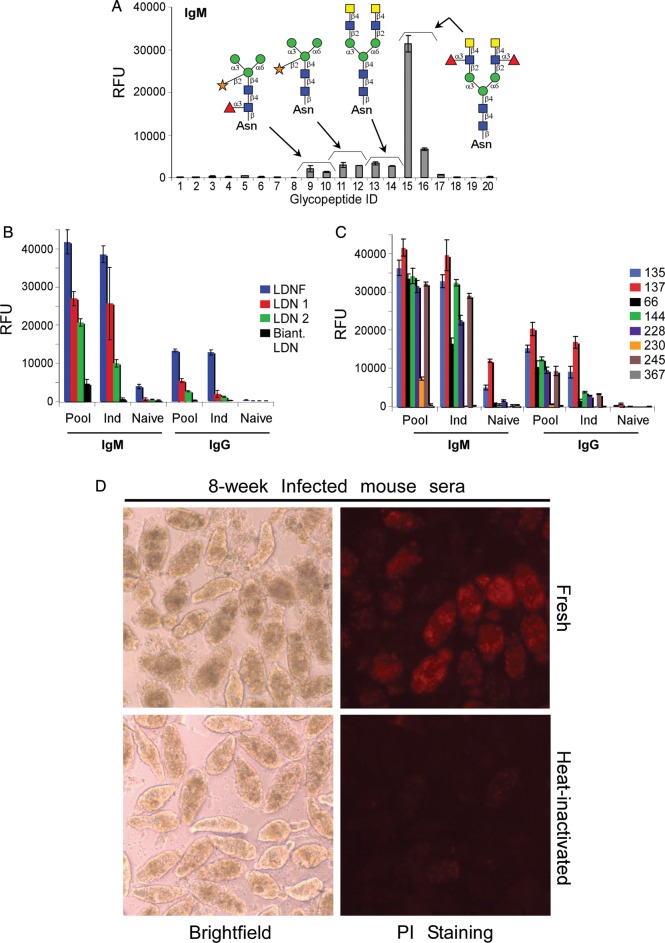
In contrast to the observations in monkeys and humans, S. mansoni-infected outbred Swiss Webster mice generated a predominantly IgM antibody response to the LDNF glycopeptides, and exhibit lower antibody responses to the LDN and core xylose/core fucose antigens (Figure 6A). Similarly, on the CFG glycan microarray, 8 weeks post-infection sera had more robust IgM antibody activity to the LDNF glycan epitope compared with several LDN glycoforms. The anti-LDN IgM responses toward straight chain LDN were many-fold higher than those toward biantennary LDN on the CFG glycan microarray (Figure 6B). Additionally, mice responded to glycans terminating with LeX and poly-LeX antigens, similar to monkeys (Figure 6C). In the in vitro schistosomula killing assays, neither normal mouse sera nor culture media alone demonstrated killing. Sera from 8 weeks post-infection did demonstrate moderate killing, around 70%; however, this was not as effective as the rhesus sera at a similar time point (Table III). To determine whether the schistosomula killing by infected mouse serum was due to complement fixation, we compared fresh and heat-inactivated mouse serum (Figure 6D). Similar to previous assays, the mouse serum killing was 72% with fresh serum but only 2% with heat-inactivated serum, and killing by fresh naïve mouse serum was 16%. These results demonstrated that antibodies from infected mice target LDNF rather than the core xylose/core fucose antigens, and that the parasite-specific antibodies in mouse sera are able to affect moderate complement-dependent killing of schistosomula in vitro at 8 weeks post-infection.
Fig. 6.
Antibody responses and complement-dependent killing activity in infected mice. (A) Sera from 8 week-infected mice were used to probe the defined schistosome glycan microarray for IgM binding (IgG data not shown). (B and C) Sera from infected mice were used to probe a CFG glycan microarray. IgM and IgG (B) responses to LDNF and various LDN structures, and IgM and IgG (C) responses to various LeX epitopes are shown from the following samples: pooled (Pool), individual (Ind) and an individual naïve mouse. Numbers in the legend for C correspond to the glycans listed in Table II. (D) Mechanically transformed schistosomula were treated with pooled sera from 8-week S. mansoni infected mice diluted in culture medium to 1:10. Death of schistosomula was assessed by granularity and loss of membrane integrity (brightfield) and fluorescent propidium iodide (PI) uptake. Data are representative of two replicate experiments. Serum was either fresh (obtained from mice the same day and kept at room temperature) or heat-inactivated to destroy complement at 56°C for 1 h. A section of an image taken at 10× is enlarged to show localization of PI stain to individual dead schistosomula.
Discussion
We have used a defined glycan microarray of glycopeptides presenting schistosome-associated glycan epitopes, as well as a defined glycan microarray from the CFG, to compare the immune responses to glycan antigens in three infected animal species. A few studies have examined the antibody response to helminth glycans using microarrays, but this is the first to make use of a defined schistosome-type glycan microarray and the CFG glycan microarray together in comparing samples from multiple species, in concert with parasite killing assays (Naus et al. 2003; de Boer et al. 2008; Aranzamendi et al. 2011; van Diepen et al. 2012). An advantage of this approach is that only nanogram amounts of glycans are used and sub-microliters of serum samples are used per analysis, and that the sensitivity on these microarrays is considerably higher than that of a standard ELISA (Alvarez and Blixt 2006). The defined arrays also offer a high degree of specificity when similar structures are present, as seen with the IgM monoclonal antibody to LDN in Figure 2B and differentiation of varied LeX-containing structures targeted by sera in Figure 3F. One limitation of this approach is that what we learn using defined glycan arrays is restricted to what helminth glycan structures are known and can be synthesized with available technologies. We and others have developed glycan microarrays from natural parasite sources to complement the defined array approach and we are also working on chemical and enzymatic methods to expand the range of schistosome glycan antigens that can be synthesized (van Diepen et al. 2012; Prasanphanich et al. 2013). Another limitation is the small sample size, owing to the ethical considerations of non-human primate research and constraints on transporting human samples internationally. Our results suggest that microarrays with parasite-specific glycan antigens may be useful for vaccine antigen discovery and identifying improved diagnostic antigens for a variety of parasite infections, and should open doors for conducting more glycan-related research on resistant human populations and protective animal models in the future. This type of work may be especially useful in areas where many parasite infections are co-endemic.
Using this sensitive technology, we compared the anti-glycan humoral immune responses generated during the course of infection with S. mansoni in rhesus monkeys, which naturally self-cure, mice, which are affordable and permissive hosts, and humans. Here, we show that during schistosome infection, monkeys generate anti-glycan IgG antibodies to specific glycans, but after natural resolution of the infection within 78 weeks, the anti-glycan responses diminish. A major novel finding of our studies was that most of the IgG responses in infected monkeys were directed specifically toward the core xylose/core α3 fucose epitope among the glycans tested. Core xylose/core α3 fucose is known to be expressed in S. mansoni (Khoo et al. 2001) and, interestingly, it is also a common plant epitope associated with IgE production in humans and subsequent allergic reactions (Altmann 2007; Kaulfuerst-Soboll et al. 2011). Core xylose and/or core α1,3 fucose have been demonstrated only in glycoproteins from plants, insects, select molluscs and helminths (reviewed in Altmann 2007), and it was demonstrated that these glycans contribute to Th2-biasing in S. mansoni-infected mice and are themselves the target of a Th2-biased antibody response during infection (Faveeuw et al. 2003). To our knowledge, this is the first definitive report of glycan-specific targeting of the core xylose/core fucose epitope in infected rhesus monkeys and, to a lesser degree, in humans.
We observed that sera from infected rhesus monkeys were able in vitro to kill schistosomula, a stage of schistosome life cycle vulnerable to immune lysis (Nyame et al. 1996). This killing could be complement-dependent, similar to 8-week infected mouse serum where virtually all of the killing was complement dependent, or due to neutralization by antibodies. The maximal killing effect was observed with 8- and 11-week post-infection sera, which correlated with maximal anti-glycan antibody levels on both the defined schistosome glycan microarray (to core xylose/core fucose) and to the CFG glycan microarray (toward LDN, LDNF and LeX) (Figure 3D–G). The larval stages of S. mansoni express a complex glycocalyx comprising antigenic glycans, one of them being core xylose and/or core fucose. Faveeuw et al. (2003) demonstrated by western blot and immunofluorescence the expression of core xylose and core α3 fucose epitopes on all life stages of S. mansoni, including on cercaria, the post-acetabular glands of schistosomula, the lining of the adult worm gut, in sexual organs of male worms and in forming eggs of females. Our group has previously demonstrated the presence of LDN, LDNF and LeX on the surface of intramammalian stages of S. mansoni, and that monoclonal IgM antibody to the LDN epitope causes lysis of schistosomula in vitro in a complement-mediated manner (Nyame et al. 2003). Ongoing studies in our lab show that mouse monoclonal antibodies to a variety of parasite glycans, including some of those bound on the schistosome-type array, are cytolytic to schistosomula in vitro (manuscript in preparation). It is thus reasonable to hypothesize that the robust anti-glycan IgM and IgG responses observed in infected rhesus sera, most likely in addition to antibodies targeting many other glycan and protein targets, are associated with its ability to agglutinate and kill schistosomula in vitro.
Antibodies have also been associated with attenuation of adult worms in rhesus monkeys (Wilson et al. 2008). Wilson et al. observed that over the course of infection, total IgG to adult worm antigen rose steadily from onset to 16–20 weeks, which coincides with resistance to re-infection and maximal lethality to schistosomula in vitro observed in early studies (Clegg and Smithers 1972; Wilson et al. 2008). Though we used different time points in our study, the observed anti-glycan peaks appear to be earlier than the patterns observed in these two studies, occurring at 8–11 weeks. IgG to core xylose/core fucose was declining between 15 and 26 weeks (Figure 3A and B). The anti-core xylose/core fucose response may arise from distinct in vivo events from those responsible for the overall peak in IgG to worm antigen. Whether the same rhesus antibodies that cause lysis of schistosomula in vitro also contribute to the rhesus monkey's spontaneous resolution of schistosome infections at the adult stage is still an open question that warrants further study.
Sera from humans infected with S. mansoni also recognized the core xylose/core fucose epitopes, but less robustly than did sera from infected rhesus monkeys. The IgM responses followed the same trend but their levels were even lower. It should be noted that the times when these individuals were infected and the actual worm burden are not known, and hence their infections may have been either chronic or acute, factors that could also account for the differences seen between human and monkey sera. On the CFG glycan microarray, human IgM and IgG responses to LDNF and LDN epitopes were also less robust compared with rhesus monkeys, with responses to LDNF being the highest in most samples. Similarly to what is seen following self-cure in rhesus, in all but one of the human samples we examined, IgG and IgM levels to core xylose/core fucose, LDN and LDNF declined after treatment with praziquantel. Results from the schistosome-type glycan array and the CFG array were consistent in that both showed extremely low responses to biantennary LDN and to LeX epitopes in humans. These results suggest two important features of glycan array data that could be useful in diagnostics: they can differentiate among antibodies to very slightly different presentations of glycans, which might be advantageous in the setting of co-endemic helminth infections, and they may be able to differentiate between individuals with active infection and those who have recently cleared infection. More studies of individuals in endemic areas need to be done to assess the potential of glycan microarrays in this regard.
Consistent with the low responses in infected people against the glycans we tested, these sera showed minimal schistosomula killing both before and after praziquantel treatment. Pooled sera from newly infected humans also had similar array reactivity, but no schistosomula killing effect. In these infected individuals, although the pattern of anti-glycan IgG responses mirrored those observed in rhesus monkeys, their levels were many-fold lower at the same serum dilution. The differential schistosomula killing ability of sera from these two species could be due to differences in the titer of antibodies to some of the glycan epitopes examined here, differences in the type of antibodies produced, or differences in the titer or specificity of antibodies to glycan or protein epitopes not measured in this study. An important future direction will be to determine whether the anti-glycan antibodies we observed are directly contributing to schistosomula killing, and whether they are responsible for the inter-species variability in serum schistosomula killing. The defined glycan microarray, in combination with more standard ELISA methods, will be helpful to this end, enabling us to confirm addition or removal of antibodies of particular types and specificities and determine which contribute to killing.
In contrast to the results of immune responses in infected rhesus monkeys and humans, the humoral immune response to schistosome glycan epitopes observed in infected mice was predominantly directed toward LDNF, LDN and LeX epitopes. IgM responses were more prominent than in the other two species examined, and there was a less pronounced response to the core xylose/core fucose antigens. Like infected monkeys, but unlike infected humans, infected mice responded strongly to LeX epitopes, especially to structures bearing a terminal and/or polymeric LeX, but largely with IgM. van Roon et al. observed a similarly IgM-biased response to LeX in mice, peaking around 8 weeks post-infection which also preferentially bound multimeric epitopes (Van Roon et al. 2004). In in vitro killing assays, 8-week post-infection mouse sera moderately killed schistosomula, although not as robustly as rhesus serum. This implicates antibodies to LeX as another potential contributor to the in vitro schistosomula killing effect observed with both rhesus and mouse serum. Consistent with this hypothesis are previous studies by our lab in which antibodies to LeX were shown to be cytolytic to myeloid cells (Nyame et al. 1996), and work by another group which characterized a set of protective mouse monoclonal antibodies to the LeX antigen (Harn et al. 1984; Ko et al. 1990). The killing could also be attributed to a combination of many types of anti-schistosome IgGs generated over time in both of these animals, as shown in a previous study of mice (Dean 1983). However, given that mice do not self-cure and can develop only partial immunity to S. mansoni over multiple vaccinations and/or infections, perhaps it is the timing, isotype composition or superior memory component of the rhesus antibody response, and other immune or non-immune mechanisms, which allow the rhesus monkey to generate a highly effective anti-schistosomal response in contrast to mice.
The high anti-glycan antibodies at 8 weeks post-infection in rhesus monkeys and mice observed on both microarrays is not unusual, because in the schistosome life cycle such periods of infection coincide with maximal egg laying by the established worm pairs in host vasculature (Nyame et al. 2000; Wilson et al. 2008). The leaky schistosome eggs likely allow for glycoproteins, glycolipids and other egg products to be more easily processed and presented to the host immune system. Additionally, eggs have high concentrations of glycosyltransferases, including fucosyltransferases, which catalyze the formation of glycans with fucose (Marques et al. 2001; Hokke and Yazdanbakhsh 2005). Thus, it is hard to discern whether the observed peaks in anti-glycan antibody responses are a consequence of maximal antigen availability, are indicative of parasite-destructive forces or both. However, the decline in anti-glycan antibodies observed in the rhesus monkey between 11 and 15 weeks, followed by the steep increase in antibodies to LDNF and LeX between 15 and 26 weeks, is interesting in view of early literature where maximal schistosomula killing ability of rhesus serum had a small peak at 8 weeks and a larger peak from 16 to 20 weeks (Clegg and Smithers 1972). Due to limited amounts of serum at certain time points, killing was not measured during the later time period in our study, but it would be interesting to test whether the second increase in anti-glycan titers could also contribute to in vitro killing.
Another interesting question raised by our work is whether previously infected rhesus monkeys and humans develop memory to glycan antigens, and whether this correlates with their ability to resist re-infection after primary exposure. Unlike rhesus monkeys, most humans are permissive hosts for chronic infection and mount immune responses to the parasites that are ineffective at preventing worms from becoming established in the vasculature. However, infected adult humans in schistosome endemic regions can develop resistance to super-infection with the same schistosome strain. Young children in schistosome endemic areas do not readily acquire protective immunity even after repeated exposure to schistosomes, which may be due to the immaturity of their immune systems (Kabatereine et al. 1999). Some older individuals in these regions continue to become re-infected after multiple rounds of treatment, while others, although under constant exposure, do not become re-infected and hence are “endemic normals” (Correa-Oliveira et al. 1989; Karanja et al. 2002). These individuals probably develop some form of immunity to new schistosome infection episodes, a phenomenon originally termed concomitant immunity (Clegg et al. 1971). Such immunity could be a result of immunologic memory, and might correlate to increasing anti-glycan antibodies generated during subsequent infections, but this remains to be studied. Children appear to mount a somewhat higher response than adults to many of the glycans studied, including core xylose, core fucose, LeX and fucosylated LDN variants, and highly variable anti-glycan patterns were seen among individuals (van Remoortere et al. 2001; Naus et al. 2003; van Diepen et al. 2012). Future studies of anti-glycan memory responses in both rhesus monkeys and resistant humans are warranted to better understand the role of these epitopes.
Some of the antigenic epitopes found in schistosome glycans, such as LDN, LDNF and LeX, are present in glycans on both our defined schistosome glycan microarray and the CFG glycan microarray. Interestingly, we observed that infected human and monkey sera differentially recognized those antigenic determinants between the two arrays. This may result from differences in the underlying structure of the synthetic glycans carrying those antigenic determinants on the two arrays, and likely reflects the fine specificity of the antibody responses. For example, the two LDN- and one LDNF-containing glycans on the CFG glycan microarray are straight-chain glycans, whereas on the schistosome-type array those epitopes are part of biantennary N-glycopeptides, suggesting that the presentation of the antigenic determinant on different glycan backbones may be important for antibody recognition. This is consistent with the observation that LDN is present on a biantennary N-glycan on both microarrays, and the infected rhesus monkey sera fails to recognize it on either, while infected mouse IgM recognizes it at low levels on the defined schistosome array and on the CFG array. Sera from all three species appear to preferentially recognize the straight-chain versions of LDN and LDNF over their respective biantennary presentations. Our group has observed a similar preference using an anti-schistosome monoclonal antibodies to LDNF and to LeX (Mandalasi et al. 2013; Prasanphanich et al. 2014). With the increasing sophistication of glycan microarrays, we can now explore whether this is a general trend seen in IgGs to other glycan epitopes and whether this is a result of how the parasite presents the glycan antigen or the host's tolerization to certain structural presentations.
In summary, our data indicate that high antibody reactivity to multiple glycans, including core xylose/core fucose, LDNF, LDN and LeX in both 8–11 week rhesus monkey serum and, to a lesser degree, 8 week mouse serum coincides with the superior in vitro schistosomula killing abilities of those sera, while human anti-sera which lack robust anti-glycan binding also lack robust in vitro killing ability. Whether such antibodies are making a direct contribution to in vitro killing and/or to in vivo protection warrants further investigation. Our observations suggest that defined glycan microarrays may facilitate the development of diagnostic tools and discovery of vaccine candidates for schistosomiasis.
Materials and methods
Materials
Aspergillus oryzae β-galactosidase, Jack bean β-N-acetylglucosaminidase (β-hexosaminidase), both research-grade quality, bovine fibrinogen and horseradish peroxidase (HRP—type VI) were purchased from Sigma-Aldrich (St. Louis, MO) and were used without further purification. Defatted Bermuda grass pollen (BGP) was obtained from Greer (Lenoir, NC). ConA-Sepharose was obtained from GE Healthcare Bio-Sciences (Uppsala, Sweden). Pronase, a mixture of proteinases isolated from Streptomyces griseus, research-grade quality, was purchased from Calbiochem (San Diego, CA). One thousand molecular weight-cutoff Float-A-lyzer dialysis tubing was purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Graphitized carbon cartridges were purchased from Alltech (Deerfield, IL) while reverse phase (RP) Sep-Pak C18 columns were obtained from Waters (Ireland). N-Hydroxysuccinamide (NHS)-activated slides were purchased from Schott Microarray solutions, Inc. (Louisville, KY). Anti-LDN IgM monoclonal antibody was obtained as described previously (Nyame et al. 1999). All other chemicals were purchased from Sigma-Aldrich and used without further purification.
Source of sera
Sera from S. mansoni-infected adult humans before and after treatment with praziquantel and a newly infected group of adults were obtained from a study that had been conducted in Kisumu, Kenya and had received approval by the ethical review boards of the Kenya Medical Research Institute and the Division of Parasitic Diseases and Malaria at the Centers for Disease Control and Prevention (CDC). Prior to this study, the sera and personal identifiers had been delinked. Normal adult human sera were obtained from a control population that had no history of international travel. In accordance with the “reduce and re-use” premises of animal welfare, sera from adult Chinese rhesus macaques (M. mulatta) at various time points after S. mansoni exposure were obtained from an IACUC-approved study conducted for a different purpose and performed at the Division of Parasitic Diseases and Malaria at the CDC. A limited amount of sera was available and not all of the same time points were available from all four animals. Due to the limited amounts of sera, pooled samples were used when possible. Infected mouse sera were obtained from female adult Swiss Webster mice at pre-infection and 8 weeks post-infection with S. mansoni under an approved IACUC protocol at Emory University.
Glycopeptide preparation
Glycopeptides were generated from commonly available natural sources. Sialylated, asialo-, agalacto-, LDN, LDNF and Man3 N-linked biantennary glycopeptides were prepared from bovine fibrinogen, which exclusively contains biantennary N-glycans lacking fucose and containing one or two sialic acid residues (Debeire et al. 1985). To prepare these sialylated glycopeptide precursors, bovine fibrinogen (1 g) was treated with 100 mg of Pronase in 200 mL of 1× Pronase buffer (10×, 1 M Tris–HCl, pH 8, 10 mM MgCl2, 10 mM CaCl2 and 1% NaN3) overnight at room temperature. To remove undigested protein, the resultant digest was passed over an RP C18 Sep-Pak column and the flow-through collected. To purify the glycopeptide product, the sample was applied to a 100 mL column of ConA-Sepharose. ConA lectin (Canavalia ensiformis lectin) binds oligomannose-type and complex-type biantennary N-glycans (Baenziger and Fiete 1979; Brenckle and Kornfeld 1980; Brewer and Bhattacharyya 1986). The column was washed three times with 1× Pronase buffer and bound glycopeptides were eluted using 1.5 L of 100 mM α-methyl mannoside. The collected glycopeptides were desalted over a graphitized carbon cartridge, to which the glycopeptides bound. After washing the cartridge several times with deionized water, the bound glycopeptides were eluted with three column volumes of 30% acetonitrile with 0.1% trifluoroacetic acid (TFA) and dried in a speed-vac. The dry glycopeptides were reconstituted with deionized water and their concentration estimated by the standard phenol-sulfuric acid method, using glucose as a standard, and their weights determined by drying and weighing (Dubois et al. 1956). To generate the asialo-glycopeptide, the reconstituted sialo-glycopeptides were desialylated by treatment with 100 mM HCl at 80°C for 30 min. To isolate the asialo-glycopeptides, the sample was neutralized with 100 mM NaOH, desalted over graphitized carbon cartridge and the desired glycopeptide was eluted with three column volumes of 30% acetonitrile with 0.1% TFA. Complete desialylation was confirmed by matrix-assisted laser-desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) profiling.
For MALDI-TOF MS analysis, 0.5 µL of serially diluted glycopeptides in water was mixed with 0.5 µL of 2,5-dihydroxybenzoic acid matrix, air-dried and analyzed in a MALDI-TOF mass spectrometer (Bruker Daltonics, Billerica, MA). Agalacto-glycopeptides were generated by digesting 24 mg of asialo-glycopeptides in 10 mL of sodium acetate (NaOAc) buffer pH 5.2 with 20 mg of β-galactosidase at 37°C for 12 h. β-Galactosidase was previously dialyzed twice against 2 L of 30 mM NaOAc, pH 5.2, for 12 h at 4°C. The digested sample was passed sequentially over an RP C18 column and a graphitized carbon cartridge before elution with 30% acetonitrile with 0.1% TFA and dried in a speed-vac to generate degalactosylated (agalacto)-glycopeptides. Complete degalactosylation was confirmed by MALDI-TOF MS profiling. From these agalacto-glycopeptides, the LDN-glycopeptides were generated using recombinant β1,4-N-acetylgalactosaminyltransferase (B4GALNT) from Caenorhabditis elegans, based on previous methods (Kawar et al. 2002). Briefly, 50 mL medium containing HPC4-tagged B4GALNT was centrifuged at 1500 × g for 5 min to remove cellular debris and incubated with HPC4-UltraLink beads (5 mg of anti-HPC4 antibody/mL of beads; 0.1 µL of beads/mL of medium) for 1 h at room temperature on a rotating platform. The beads were collected by centrifugation at 600 × g for 3 min and washed three times with 10 mL of 100 mM sodium cacodylate buffer, pH 7.0 with 2 mM CaCl2. The beads were resuspended in the same buffer with the addition of 20 mM MnCl2 and used as the enzyme source. Ten mg of agalacto-glycopeptide was incubated with the beads in 100 mM sodium cacodylate buffer, pH 7.0 containing 2 mM of UDP-GalNAc, 20 mM MnCl2 and 3 units of calf intestinal alkaline phosphatase to degrade the generated UDP in a total volume of 10 mL. The mixture was incubated for 48 h at room temperature with slow rotation. After incubation, supernatant was recovered by centrifugation at 600 × g for 3 min and passed sequentially over an RP C18 column and a graphitized carbon cartridge as described above and dried in a speed-vac to generate LDN-glycopeptide.
LDNF glycopeptide was generated from LDN glycopeptide by the addition of fucose using GDP-fucose as sugar donor and recombinant human α1,3 fucosyltransferase 6 (FUT6) as the enzyme based on a previous method (De Vries et al. 1997). The preparation of FUT6 from medium containing the enzyme and its subsequent immobilization on beads followed the procedure used to prepare B4GALNT, except that no MnCl2 was added. LDN-glycopeptides (10 mg) were incubated with the beads in 100 mM sodium cacodylate buffer, pH 7.0, containing 50 mM of GDP-fucose, and 3 units of calf intestinal alkaline phosphatase. The mixture was incubated for 48 h at room temperature with slow rotation. Every 12 h, fresh 50 mM GDP-fucose was added and incubation continued. After incubation, the LDNF glycopeptide was purified from the supernatant in a similar way as LDN-glycopeptides. To generate Man3-glycopeptides, 15 mg of agalacto-glycopeptides were treated with 150 µL (10 units) β-hexosaminidase in 5 mL of 30 mM NaOAc, pH 5.2. The digest was transferred into 500 molecular-weight-cutoff Float-A-lyzer dialysis tubing and digestion continued for 12 h while dialyzing against 30 mM NaOAc buffer, pH 5.2, at room temperature. The Man3-glycopepetide product was purified in the same way as the other glycopeptides above. Core xylose/core fucose N-linked biantennary glycopeptides Manα1,6(Manα1,3)(Xylβ1,2)Manβ1,4GlcNAcβ1,4(Fucα1,3)GlcNAcβ-R were prepared from HRP type VI. HRP exclusively contains biantennary N-glycans with an α1,3 core fucose and a β1,2 xylose (van Remoortere et al. 2003; Wuhrer et al. 2005). Briefly, 100 mg of Pronase was used to digest 1 g HRP, type VI, in 100 mL of 1× Pronase buffer. Glycopeptides from the digest were then sequentially purified in the same way as the sialylated or asialo-glycopeptides above.
Core xylose-glycopeptides were chemically derived from core xylose/core fucose glycopeptides by incubation with 25 mM sulfuric acid at 80°C for 30 min. The sample was neutralized with 50 mM NaOH, desalted over graphitized carbon cartridge as described above and dried in a speed-vac. Core fucose (fucosylated Man3) was prepared from BGP which contains IgE-binding proteins carrying N-linked glycans with α1,3 core fucose (Ohsuga et al. 1996). Briefly, crude extract was prepared by extracting 5 g defatted pollen with 1× PBS for 16 h at 4°C with constant stirring. The extract was filtered through a Whatman No. 1 filter and the eluent centrifuged at 800 × g for 45 min at 4°C. The supernatant was dialyzed at 4°C against 1× Pronase buffer. The dialyzed sample was lyophilized, dissolved in 100 mL of 1× Pronase buffer, and digested with 100 mg of Pronase overnight at room temperature. Core fucosylated glycopeptides were sequentially purified in the same way as the sialylated or asialo-glycopeptides above.
Preparation and analysis of a defined schistosome glycan microarray
The printing of glycopeptides to generate a defined schistosome glycan microarray on NHS-activated slides (Schott, Elmsford, NY) was carried out at two different glycopeptide concentrations, 0.4 and 0.2 mg/mL, using a Piezorray printer from Perkin Elmer following a previously described procedure (de Boer et al. 2007). Briefly, all samples were printed in phosphate buffer (300 mM sodium phosphate, pH 8.5). The average spot volume was within 10% variation of 1/3 nL. After printing, the slides were boxed loosely and placed in a high moisture chamber at 55°C and incubated for 1 h, washed and blocked with 50 mM ethanolamine in 50 mM sodium borate (pH 8.5) for 1 h. The slides were rinsed with water and dried by centrifugation and used immediately or stored desiccated at −20°C. Before using, the slides were rehydrated for 5 min in TSM buffer (20 mM Tris–HCl, 150 mM NaCl, 0.2 mM CaCl2, 0.2 mM MgCl2, 0.05% Tween). To validate the printing, biotinylated lectins were used in a binding assay as detailed by Heimburg-Molinaro et al. (2011). For multi-panel experiments on a single slide, the microarray layout was designed using Piezorray software according to the dimension of a standard 16-chamber adaptor. The adaptor was applied to the slide to separate a single slide into multiple chambers each containing a complete array sealed from each other during the assay. Detection of bound biotinylated lectins was carried out by incubation with cyanine5-labeled streptavidin. To investigate specific humoral responses, all sera were analyzed at 1:100 and/or 1:1000 dilutions. Detection of bound mouse antibodies from serum was carried out by incubation with Alexa568-labeled goat anti-mouse IgG and Alexa488-labeled goat anti-mouse IgM (Invitrogen). Detection of bound human antibodies and rhesus monkey antibodies from serum was carried out by incubation with Alexa488- or Alexa555-labeled goat anti-human IgG and Alexa488-labeled goat anti-human IgM (Invitrogen). Anti-human secondary antibodies were used to detect rhesus and human sera due to high homology in the Fc region (Scinicariello et al. 2004). The slides were scanned with a Perkin Elmer ProScanarray microarray scanner equipped with four lasers covering an excitation range from 488 to 647 nm. The scanned images were analyzed with ScanArray Express software.
CFG glycan microarray preparation and analysis
Glycan microarrays (Version 3.2) available from the Consortium for Functional Glycomics (CFG) (http://www.functionalglycomics.org/), termed the CFG glycan microarray, were obtained on request from the CFG (Blixt et al. 2004). Briefly, aminoalkyl glycosides were covalently coupled to N-succinimidyl-activated glass slides (1 × 3 inch) in a sodium phosphate buffer, pH 8.5, 0.005% Tween 20. Slides were immersed in 50 mM ethanolamine, 50 mM sodium borate (pH 9.0), for 1 h, rinsed with water, dried under a stream of microfiltered air and stored in a desiccator until use. For sera recognition of glycans on the printed glycan microarray, dilutions of 1:100 for mice and 1:1000 for monkeys and humans were performed in TSM buffer containing 1% BSA for 1 h at room temperature in a dark humid chamber. The slide was washed by successive immersion in TSM buffer (four times), TSM without Tween (four times), and then incubated with fluorescently-labeled appropriate secondary antibody (anti-mouse IgG or IgM, or anti-human IgG or IgM). Anti-human secondary antibodies were used to detect rhesus and human sera due to high homology in the Fc region (Scinicariello et al. 2004). After 1 h at room temperature in a dark humid chamber, the slide was washed by successive immersion in TSM (four times), TSM without Tween (four times) and water (four times). The slide was dried by microcentrifugation. An image of bound fluorescence was obtained using a microarray scanner (ScanArray Express, Perkin Elmer Life Sciences). The integrated spot intensities were determined using Imagene software (BioDiscovery).
In vitro killing of schistosomula
Snails were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID, NIH: Schistosoma mansoni, Strain NMRI-exposed Biomphalaria glabrata, Strain NMRI, NR-21962. Schistosoma mansoni (Puerto Rican strain) cercariae were prepared according to published protocol with a slight alteration (Lazdins et al. 1982). Briefly, schistosome-infected Biomphalaria glabrata snails were kept in the dark for 2 days, and then exposed to light for 2 h to induce shedding of cercariae. The free swimming cercariae were filtered through a 100 μm sieve to remove snail and aquarium debris, chilled on ice for 30 min and centrifuged at 500 × g (swinging bucket) for 10 min at 4°C. The cercarial pellet was suspended in DMEM buffer containing penicillin and streptomycin. Transformation to schistosomula was carried out by vortexing the suspension at top speed to effect the dislocation of cercarial tails. Schistosomula bodies were separated from cercarial tails by centrifugation on a 70% Percoll in DMEM gradient at 1100 × g for 10 min at 4°C. Schistosomula were recovered from the pellet fraction and washed twice with DMEM containing penicillin and streptomycin. The schistosomula were subsequently cultured in flat-bottomed microtiter plates (200–250 schistosomula/well) in 90 μL DMEM containing 10% fetal bovine serum, penicillin and streptomycin for 3 h at 37°C in a humidified atmosphere with 5% CO2. To these 3 h transformed schistosomula, 10 μL of rhesus monkey, human or mouse serum was added and the plates re-incubated under the same conditions. After 48 h of incubation, schistosomula killing was assessed by two independent methods. First, gross anatomy observation of the schistosomula bodies including rotund appearance, outer membrane blebbing and protrusion of the acetabular gland, and lack of movement were used as indicators of death. Second, uptake of PI at 10 mg/mL by schistosomula with compromised tegument was used to confirm death of schistosomula. In instances where killing was extensive and individual schistosomula were difficult to distinguish and/or PI staining of schistosomula DNA in the media made counting individual dead parasites impossible, the remaining live schistosomula were counted. For complement dependent-killing assays, schistosomula were treated with pooled sera from 8-week S. mansoni infected mice diluted in culture medium to 1:10. Serum was either fresh (obtained from mice the same day and kept at room temperature) or heat-inactivated to destroy complement at 56°C for 1 h. Viability was assessed at 72 h.
Funding
This work was supported by NIH Grant R01AI101982 to R.D.C. and by a grant from the Georgia Research Alliance (GRA G6397930) to R.D.C.
Conflict of interest
None declared.
Abbreviations
AAL, Aleuria aurantia lectin; BGP, Bermuda grass pollen; BSA, bovine serum albumin; CDC, Centers for Disease Control and Prevention; CFG, Consortium for Functional Glycomics; Con A, concanavalin A; Galβ1,4(Fucα1,3)GlcNAc; HPA, Helix pomatia agglutinin; HRP, horseradish peroxidase; IgG, immunoglobulin G; LDN, LacdiNAc, GalNAcβ1,4GlcNAc; LDNF, fucosylated LacdiNAc, GalNAcβ1,4(Fucα1,3)GlcNAc; LeX, Lewis X; LTL, Lotus tetragonolobus lectin; MAL, Maackia amurensis lectin; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; NaOAc, sodium acetate; NHS, N-hydroxysuccinamide; PBS, phosphate-buffered saline; PHA-E, Phaseolus vulgaris agglutinin E; PHA-L, Phaseolus vulgaris agglutinin L; PI, propidium iodide; PNA, peanut agglutinin; RCAI, Ricinus communis agglutinin I; RP, reverse phase; SNA, Sambucus nigra agglutinin; TFA, trifluoroacetic acid; UEA-I, Ulex europaeus I; WFA, Wisteria floribunda agglutinin; WGA, wheat germ agglutinin.
Acknowledgements
The authors would like to acknowledge The Consortium for Functional Glycomics (CFG) funded by the NIGMS GM62116 and GM98791 for services provided by the Glycan Array Synthesis Core (The Scripps Research Institute, La Jolla, CA) that produced the mammalian glycan microarray and the Protein-Glycan Interaction Core (Emory University School of Medicine, Atlanta, GA) that performed analysis of samples on the array.
References
- Abdul-Ghani RA, Hassan AA. Murine schistosomiasis as a model for human schistosomiasis mansoni: Similarities and discrepancies. Parasitol Res. 2010;107:1–8. doi: 10.1007/s00436-010-1855-5. [DOI] [PubMed] [Google Scholar]
- Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- Alvarez RA, Blixt O. Identification of ligand specificities for glycan-binding proteins using glycan arrays. Methods Enzymol. 2006;415:292–310. doi: 10.1016/S0076-6879(06)15018-1. [DOI] [PubMed] [Google Scholar]
- Aranzamendi C, Tefsen B, Jansen M, Chiumiento L, Bruschi F, Kortbeek T, Smith DF, Cummings RD, Pinelli E, Van Die I. Glycan microarray profiling of parasite infection sera identifies the LDNF glycan as a potential antigen for serodiagnosis of trichinellosis. Exp Parasitol. 2011;129:221–226. doi: 10.1016/j.exppara.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger JU, Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979;254:2400–2407. [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer AR, Hokke CH, Deelder AM, Wuhrer M. General microarray technique for immobilization and screening of natural glycans. Anal Chem. 2007;79:8107–8113. doi: 10.1021/ac071187g. [DOI] [PubMed] [Google Scholar]
- de Boer AR, Hokke CH, Deelder AM, Wuhrer M. Serum antibody screening by surface plasmon resonance using a natural glycan microarray. Glycoconj J. 2008;25:75–84. doi: 10.1007/s10719-007-9100-x. [DOI] [PubMed] [Google Scholar]
- Brenckle R, Kornfeld R. Structure of the oligosaccharides of mouse immunoglobulin M secreted by the MOPC 104E plasmacytoma. Arch Biochem Biophys. 1980;201:160–173. doi: 10.1016/0003-9861(80)90499-3. [DOI] [PubMed] [Google Scholar]
- Brewer CF, Bhattacharyya L. Specificity of concanavalin A binding to asparagine-linked glycopeptides. A nuclear magnetic relaxation dispersion study. J Biol Chem. 1986;261:7306–7310. [PubMed] [Google Scholar]
- Cheever AW. Quantitative comparison of the intensity of Schistosoma manosni infections and man and experimental animals. Trans R Soc Trop Med Hyg. 1969;63:781–795. doi: 10.1016/0035-9203(69)90122-9. [DOI] [PubMed] [Google Scholar]
- Cheever AW, Powers KG. Schistosoma mansoni infection in rhesus monkeys: Changes in egg production and egg distribution in prolonged infections in intact and splenectomized monkeys. Ann Trop Med Parasitol. 1969;63:83–93. doi: 10.1080/00034983.1969.11686603. [DOI] [PubMed] [Google Scholar]
- Chitsulo L, Loverde P, Engels D. Schistosomiasis. Nat Rev Microbiol. 2004;2:12–13. doi: 10.1038/nrmicro801. [DOI] [PubMed] [Google Scholar]
- Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003;90((Supp 1)):S3–S9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- Clegg JA, Smithers SR. The effects of immune rhesus monkey serum on schistosomula of Schistosoma manosni during cultivation in vitro. Int J Parasitol. 1972;2:79–98. doi: 10.1016/0020-7519(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Clegg JA, Smithers SR, Terry RJ. Concomitant immunity and host antigens associated with schistosomiasis. Int J Parasitol. 1971;1:43–49. doi: 10.1016/0020-7519(71)90045-2. [DOI] [PubMed] [Google Scholar]
- Correa-Oliveira R, Pearce EJ, Oliveira GC, Golgher DB, Katz N, Bahia LG, Carvalho OS, Gazzinelli G, Sher A. The human immune response to defined immunogens of Schistosoma mansoni: Elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans R Soc Trop Med Hyg. 1989;83:798–804. doi: 10.1016/0035-9203(89)90334-9. [DOI] [PubMed] [Google Scholar]
- Cummings RD. Use of lectins in analysis of glycoconjugates. Methods Enzymol. 1994;230:66–86. doi: 10.1016/0076-6879(94)30008-9. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Etzler ME. Antibodies and lectins in glycan analysis. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Boston: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- Cummings RD, Nyame AK. Schistosome glycoconjugates. Biochim Biophys Acta. 1999;1455:363–374. doi: 10.1016/s0925-4439(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Dean DA. Schistosoma and related genera: Acquired resistance in mice. Exp Parasitol. 1983;55:1–104. doi: 10.1016/0014-4894(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Debeire P, Montreuil J, Moczar E, van Halbeek H, Vliegenthart JF. Primary structure of two major glycans of bovine fibrinogen. Eur J Biochem. 1985;151:607–611. doi: 10.1111/j.1432-1033.1985.tb09147.x. [DOI] [PubMed] [Google Scholar]
- Dell A, Morris HR, Easton RL, Panico M, Patankar M, Oehniger S, Koistinen R, Koistinen H, Seppala M, Clark GF. Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J Biol Chem. 1995;270:24116–24126. doi: 10.1074/jbc.270.41.24116. [DOI] [PubMed] [Google Scholar]
- Demeure CE, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein AJ. Resistance to Schistosoma mansoni in humans: Influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993;168:1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- De Vries T, Palcic MP, Schoenmakers PS, Van Den Eijnden DH, Joziasse DH. Acceptor specificity of GDP-Fuc:Gal beta 1–>4GlcNAc-R alpha 3-fucosyltransferase VI (FucT VI) expressed in insect cells as soluble, secreted enzyme. Glycobiology. 1997;7:921–927. doi: 10.1093/glycob/7.7.921. [DOI] [PubMed] [Google Scholar]
- van Die I, Gomord V, Kooyman FNJ, Berg TKVD, Cummings RD, Vervelde L. Core alpha1,3-fucose is a common modification of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- van Diepen A, Smit CH, van Egmond L, Kabatereine NB, Pinot de Moira A, Dunne DW, Hokke CH. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Neglect Trop Dis. 2012;6:e1922. doi: 10.1371/journal.pntd.0001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissous C, Grzych JM, Capron A. Schistosoma mansoni surface antigen define by a rat monoclonal IgGa. J Immunol. 1982;129:2232–2234. [PubMed] [Google Scholar]
- Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1956;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of human schistosomiasis: Association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22:1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW, Coulson PS, Wilson RA. Antibodies to glycans dominate the host response to schistosome larvae and eggs: Is their role protective or subversive? J Infect Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- Ellis LA, Reason AJ, Morris HR, Dell A, Iglesias R, Ubeira FM, Appleton JA. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Mallevaey T, Paschinger K, Wilson IB, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, et al. Schistosome N-glycans containing core alpha 3-fucose and core beta 2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Grzych JM, Capron M, Lambert PH, Dissous C, Torres S, Capron A. An anti-idiotype vaccine against experimental schistosomiasis. Nature. 1985;316:74–76. doi: 10.1038/316074a0. [DOI] [PubMed] [Google Scholar]
- Grzych J-M, Dissous C, Capron M, Torres S, Lambert P-H, Capron A. Schistosoma mansoni shares a protective carbohydrate epitope with keyhole limpet hemocyanin. J Exp Med. 1987;165:865–878. doi: 10.1084/jem.165.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–245. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- Harn DA, Mitsuyama M, David JR. Schistosoma manosoni anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984;159:1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. Preparation and analysis of glycan microarrays. Curr Prot Protein Sci. 2011:1–33. doi: 10.1002/0471140864.ps1210s64. Chapter 12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite Immunol. 2005;27:257–264. doi: 10.1111/j.1365-3024.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Ferris MT. The antipoverty vaccines. Vaccine. 2006;24:5787–5799. doi: 10.1016/j.vaccine.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, Dunne DW, Fulford AJ. Adult resistance to schistosomiasis mansoni: Age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118(Pt 1):101–105. doi: 10.1017/s0031182098003576. [DOI] [PubMed] [Google Scholar]
- Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: A longitudinal study. Lancet. 2002;360:592–596. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- Kaulfuerst-Soboll H, Rips S, Koiwa H, Kajiura H, Fujiyama K, von Schaewen A. Reduced immunogenicity of Arabidopsis hybrid glycosylation1 (hgl1) N-glycans due to altered accessibility of xylose and core fucose epitopes. J Biol Chem. 2011;286:22955–22964. doi: 10.1074/jbc.M110.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawar ZS, Van Die I, Cummings RD. Molecular cloning and enzymatic characterization of a UDP-GalNAc:GlcNAc(beta)-R beta1,4-N-acetylgalactosaminyltransferase from Caenorhabditis elegans. J Biol Chem. 2002;277:34924–34932. doi: 10.1074/jbc.M206112200. [DOI] [PubMed] [Google Scholar]
- Khoo K-h, Chatteriee D, Caulfield JP, Morris HR, Dell A. Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: Identification of novel core structures and terminal sequences. Glycobiology. 1997;7:663–677. doi: 10.1093/glycob/7.5.663. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Huang HH, Lee KM. Characteristic structural features of schistosome cercarial N-glycans: Expression of Lewis X and core xylosylation. Glycobiology. 2001;11:149–163. doi: 10.1093/glycob/11.2.149. [DOI] [PubMed] [Google Scholar]
- King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- Ko AI, Dräger UC, Harn DA. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc Natl Acad Sci USA. 1990;87:4159–4163. doi: 10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochibe N, Furukawa K. Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry. 1980;19:2841–2846. doi: 10.1021/bi00554a004. [DOI] [PubMed] [Google Scholar]
- Kupferschmidt K. Coming at a snail's pace. Science. 2013;339:502–503. doi: 10.1126/science.339.6119.502. [DOI] [PubMed] [Google Scholar]
- Lamberton PH, Hogan SC, Kabatereine NB, Fenwick A, Webster JP. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am J Trop Med Hyg. 2010;83:1340–1347. doi: 10.4269/ajtmh.2010.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins JK, Stein MJ, David JR, Sher A. Schistosoma mansoni: Rapid isolation and purification of schistosomula of different developmental stages by centrifugation on discontinuous density gradients of Percoll. Exp Parasitol. 1982;53:39–44. doi: 10.1016/0014-4894(82)90090-x. [DOI] [PubMed] [Google Scholar]
- Mandalasi M, Dorabawila N, Smith DF, Heimburg-molinaro J, Nyame AK, Cummings RD. Development and characterization of a specific IgG monoclonal antibody toward the Lewis x antigen using splenocytes of Schistosoma mansoni infected mice. Glycobiology. 2013;23:877–892. doi: 10.1093/glycob/cwt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques ET, Jr, Ichikawa Y, Strand M, August JT, Hart GW, Schnaar RL. Fucosyltransferases in Schistosoma mansoni development. Glycobiology. 2001;11:249–259. doi: 10.1093/glycob/11.3.249. [DOI] [PubMed] [Google Scholar]
- McHugh SM, Coulson PS, Wilson RA. Pathologically induced alterations in the dimensions of the hepatic portal vasculature of mice infected with Schistosoma mansoni. Parasitology. 1987;94(Pt 1):69–80. doi: 10.1017/s0031182000053464. [DOI] [PubMed] [Google Scholar]
- Meevissen MHJ, Balog CIA, Koeleman CAM, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Wuhrer M, Hokke CH. Targeted glycoproteomic analysis reveals that kappa-5 is a major, uniquely glycosylated component of Schistosoma mansoni egg antigens. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.005710. M110.005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus CW, van Remoortere A, Ouma JH, Kimani G, Dunne DW, Kamerling JP, Deelder AM, Hokke CH. Specific antibody responses to three schistosome-related carbohydrate structures in recently exposed immigrants and established residents in an area of Schistosoma mansoni endemicity. Infect Immun. 2003;71:5676–5681. doi: 10.1128/IAI.71.10.5676-5681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyame AK, Kawar ZS, Cummings RD. Antigenic glycans in parasitic infections: Implications for vaccines and diagnostics. Arch Biochem Biophys. 2004;426:182–200. doi: 10.1016/j.abb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Leppanen AM, Bogitsh BJ, Cummings RD. Antibody responses to the fucosylated LacdiNAc glycan antigen in Schistosoma mansoni-infected mice and expression of the glycan among schistosomes. Exp Parasitol. 2000;96:202–212. doi: 10.1006/expr.2000.4573. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Leppanen AM, DeBose-Boyd R, Cummings RD. Mice infected with Schistosoma mansoni generate antibodies to LacdiNAc (GalNAc beta 1–>4GlcNAc) determinants. Glycobiology. 1999;9:1029–1035. doi: 10.1093/glycob/9.10.1029. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. Immunity to schistosomiasis: Glycans are potential antigenic targets for immune intervention. Exp Parasitol. 2003;104:1–13. doi: 10.1016/s0014-4894(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Pilcher JB, Tsang VC, Cummings RD. Schistosoma mansoni infection in humans and primates induces cytolytic antibodies to surface Le(x) determinants on myeloid cells. Exp Parasitol. 1996;82:191–200. doi: 10.1006/expr.1996.0024. [DOI] [PubMed] [Google Scholar]
- Ohsuga H, Su SN, Takahashi N, Yang SY, Nakagawa H, Shimada I, Arata Y, Lee YC. The carbohydrate moiety of the bermuda grass antigen BG60. New oligosaccharides of plant origin. J Biol Chem. 1996;271:26653–26658. doi: 10.1074/jbc.271.43.26653. [DOI] [PubMed] [Google Scholar]
- Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Prasanphanich NS, Luyai A, Song X, Heimburg-Molinaro J, Mandalasi M, Mickum M, Smith DF, Nyame AK, Cummings RD. Immunization with recombinantly expressed glycan antigens from Schistosoma mansoni induces glycan-specific antibodies against the parasite. Glycobiology. 2014;24:619–637. doi: 10.1093/glycob/cwu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host-helminth interactions. Front Immunol. 2013;4(240):1–22. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Remoortere A, Bank CM, Nyame AK, Cummings RD, Deelder AM, van Die I. Schistosoma mansoni-infected mice produce antibodies that cross-react with plant, insect, and mammalian glycoproteins and recognize the truncated biantennary N-glycan Man3GlcNAc2-R. Glycobiology. 2003;13:217–225. doi: 10.1093/glycob/cwg025. [DOI] [PubMed] [Google Scholar]
- van Remoortere A, van Dam GJ, Hokke CH, van den Eijnden DH, van Die I, Deelder AM. Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect Immun. 2001;69:2396–2401. doi: 10.1128/IAI.69.4.2396-2401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol. 1991;21:2679–2686. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuente LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. Rhesus macaque antibody molecules: Sequences and heterogeneity of alpha and gamma constant regions. Immunology. 2004;111:66–74. doi: 10.1111/j.1365-2567.2003.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers SR, Terry RJ. Resistance to experimental infection with Schistosoma mansoni in rhesus monkeys induced by the transfer of adult worms. Trans R Soc Trop Med Hyg. 1967;61:517–533. doi: 10.1016/0035-9203(67)90102-2. [DOI] [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewis X antigen. The Journal of Biological Chemistry. 1992a;267:20196–20203. [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. Schistosoma mansoni synthesizes novel biantennary Asn-linked oligosaccharides containing terminal B-linked N-acetylgalactosamine. Glycobiology. 1992b;2:445–452. doi: 10.1093/glycob/2.5.445. [DOI] [PubMed] [Google Scholar]
- van Stijn CMW, van den Broek M, Vervelde L, Alvarez Ra, Cummings RD, Tefsen B, van Die I. Vaccination-induced IgG response to Galalpha1-3GalNAc glycan epitopes in lambs protected against Haemonchus contortus challenge infection. Int J Parasitol. 2010;40:215–222. doi: 10.1016/j.ijpara.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard JR, Mugisha L, Standley CJ. Stopping schistosomes from ‘monkeying-around’ in chimpanzees. Trends Parasitol. 2012;28:320–326. doi: 10.1016/j.pt.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Tendler M, Pinto RM, Cortes M, Gebara G. Schistosoma mansoni: Comparative evaluation of different routes of experimental infection. Rev Inst Med Trop Sao Paulo. 1985;27:111–114. doi: 10.1590/s0036-46651985000300001. [DOI] [PubMed] [Google Scholar]
- The World Health Organization. 2010. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. p. 1–184. Available: http://www.who.int/neglected_diseases/2010report/en/ . [Google Scholar]
- Van den Eijnden DH, Neeleman AP, Van der Knaap WP, Bakker H, Agterberg M, Van Die I. Novel glycosylation routes for glycoproteins: The lacdiNAc pathway. Biochem Soc Trans. 1995;23:175–179. doi: 10.1042/bst0230175. [DOI] [PubMed] [Google Scholar]
- Van den Nieuwenhof IM, Koistinen H, Easton RL, Koistinen R, Kamarainen M, Morris HR, Van Die I, Seppala M, Dell A, Van den Eijnden DH. Recombinant glycodelin carrying the same type of glycan structures as contraceptive glycodelin-A can be produced in human kidney 293 cells but not in Chinese hamster ovary cells. Eur J Biochem. 2000;267:4753–4762. doi: 10.1046/j.1432-1327.2000.01528.x. [DOI] [PubMed] [Google Scholar]
- Van Roon AM, Van de Vijver KK, Jacobs W, Van Marck EA, Van Dam GJ, Hokke CH, Deelder AM. Discrimination between the anti-monomeric and the anti-multimeric Lewis X response in murine schistosomiasis. Microbes Infect. 2004;6:1125–1132. doi: 10.1016/j.micinf.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang L, Liang Y-S. Susceptibility or resistance of praziquantel in human schistosomiasis: A review. Parasitol Res. 2012;111:1871–1877. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- Warren KS, Peters PA. Comparison of penetration and maturation of Schistosoma mansoni in the hamster, mouse, guinea pig, rabbit, and rat. Am J Trop Med Hygiene. 1967;16:718–722. [PubMed] [Google Scholar]
- Wilson RA, Langermans JA, van Dam GJ, Vervenne RA, Hall SL, Borges WC, Dillon GP, Thomas AW, Coulson PS. Elimination of Schistosoma mansoni adult worms by rhesus macaques: basis for a therapeutic vaccine? PLoS Negl Trop Dis. 2008;2:e290. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Balog CI, Koeleman CA, Deelder AM, Hokke CH. New features of site-specific horseradish peroxidase (HRP) glycosylation uncovered by nano-LC-MS with repeated ion-isolation/fragmentation cycles. Biochim Biophys Acta. 2005;1723:229–239. doi: 10.1016/j.bbagen.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Zhou Y-B, Liang S, Jiang Q-W. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasites Vectors. 2012;5:275. doi: 10.1186/1756-3305-5-275. [DOI] [PMC free article] [PubMed] [Google Scholar]