Abstract
While a significant component of atherosclerotic plaques has been characterized as amyloid, the specific proteins remain to be fully identified. Probable amyloidogenic proteins are apolipoproteins (Apos), which are vital for the formation and function of lipoproteins. ApoCIII is an abundant protein implicated in atherosclerosis, and we show it forms a ribbonlike looped amyloid, strikingly similar to that previously reported for ApoAI and ApoCII. Triangles and squares with a width of ∼50 nm were also observed, which may be a novel form of amyloid or related to previously reported amyloid rings.
The assembly of proteins into amyloid fibrils is associated with many diseases such as Parkinson’s disease or Alzheimer’s disease as well as functional processes.1 The accumulated study of different amyloid-forming proteins has led to the general observation that amyloids share common features, including cross-β sheet structure and binding by Congo red (CR) and thioflavin T (ThT). Atherosclerotic plaques contain significant amounts of amyloid with a poorly understood composition.2 Several apolipoproteins (Apos), which bind to lipoproteins, form amyloid in vitro and are associated with atherosclerotic plaques.3 ApoAI in particular has known mutations linked to atherosclerosis, is found in atherosclerotic plaques,4 and forms amyloid.5 ApoCII spontaneously forms amyloid and colocalizes with amyloid-containing human atherosclerotic plaque tissue.6
ApoCIII is predominantly found on very-low-density lipoprotein (VLDL), high-density lipoprotein (HDL), and chylomicrons7 at a plasma concentration of ∼14 μM.8 A growing body of evidence demonstrates correlations among ApoCIII, triglycerides, and heart disease.7,9 While ApoCIII has been shown to inhibit lipoprotein lipase10 and inhibit the uptake of lipoprotein particles by isolated rat liver,11 the mechanisms by which ApoCIII levels impact health are not understood. We explored the possibility that ApoCIII is amyloidogenic in vitro, as has been shown for other apolipoproteins, including ApoAI and ApoCII.
A common strategy for promoting amyloid formation is to agitate while monitoring ThT emission, which is expected to increase if amyloid forms. Figure 1A shows ThT intensity increasing as a function of incubation time for three ApoCIII concentrations, where an apparent lag and a subsequent exponential growth phase were observed. Interestingly, lag times did not decrease with increasing protein concentration as is typically found for amyloid formation kinetics. The three native Trp residues of ApoCIII can also be used to probe conformational changes. Trp emission increased, showing kinetic trends similar to those of ThT (Figure 1B and inset). To confirm the presence of amyloid at the end of these experiments, we used CR, another amyloid-specific dye. A clear shift toward 540 nm is observed, indicating binding (Figure 1C) and the presence of amyloid.12 Circular dichroism spectroscopic data also indicate the presence of a β-sheet conformation corroborating the presence of amyloid structure (Figure 1D).
Figure 1.
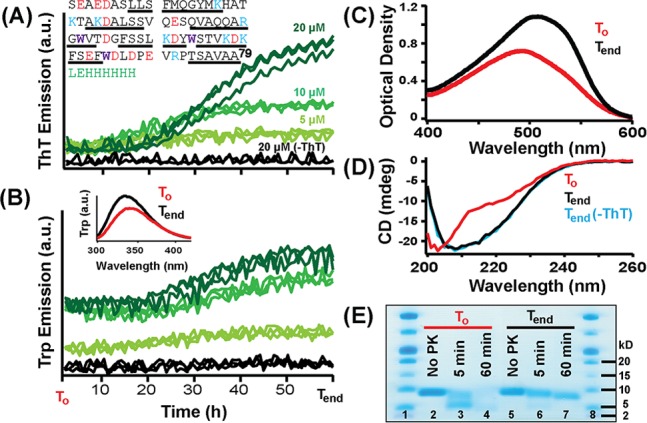
ApoCIII aggregation kinetics simultaneously monitored by ThT (A) and Trp (B) fluorescence. Using a 96-well plate, samples in 10 mM NaPi, 100 mM NaCl, 1 mM EDTA, pH 7.4 buffer, including a 2 mm glass bead, were incubated at 37 °C with orbital shaking (1 mm) using a microplate reader. In the inset of panel A, the recombinant ApoCIII sequence includes a C-terminal hexahistidine tag following two spacer residues (green). Basic, acidic, and Trp residues are colored blue, red, and purple, respectively, and α-helical regions13 are underlined. The inset of panel B shows the ApoCIII Trp fluorescence (20 μM) at the beginning (T0) and end (Tend) of the experiment. (C) CR absorption spectra measured in the presence of ApoCIII (2 μM) at T0 (red) and Tend (black). (D) CD spectra of ApoCIII (20 μM) at T0 (red) and Tend (black) and Tend without ThT (blue). (E) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis results for limited PK digestion at 37 °C for 20 μM ApoCIII at T0 without PK and with PK for 5 and 60 min (lanes 2–4) and at Tend without PK and with PK for 5 and 60 min (lanes 5–7).
Proteinase K (PK) was used to probe whether ApoCIII samples have proteolytic resistance as would be expected for amyloids (Figure 1E). Clearly, ApoCIII at Tend is more resistant as an apparent protected band, slightly smaller than the original monomer, persists after reaction for 1 h, whereas complete proteolysis was observed for ApoCIII at T0. Mass spectrometry analysis indicates that almost all ApoCIII residues are incorporated into the core of the structure, though unambiguous assignments could not be made (Table S1 of the Supporting Information). Taken together, the spectroscopic and biochemical data support the finding that ApoCIII is amyloidogenic.
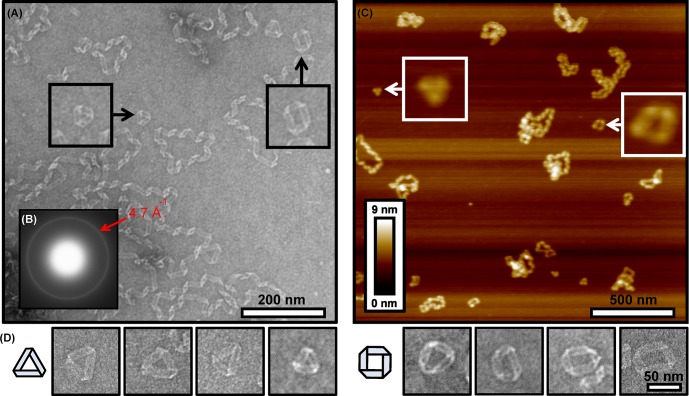
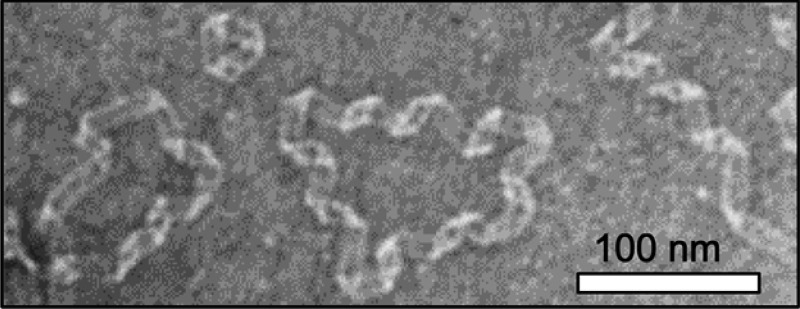
To visualize the ultrastructural features of ApoCIII conformations, transmission electron microscopy (TEM) was employed revealing a ribbonlike morphology with a consistent helical twist (Figure 2A), though the chirality cannot be determined as TEM images are projections. A typical interstrand spacing of (4.7 Å)−1 for the β-sheet conformation was measured by electron diffraction (Figure 2B), demonstrating that these amyloid loops contain well-ordered β-sheet structure.14 This measurement does not distinguish cross-β structure, which would require alignment of the fibrils and X-ray diffraction. Though some free ends were observed, most structures appear to form highly regular loops. An average width of ∼13.5 nm is found (Figure S1 of the Supporting Information), comparable to widths for ApoCII15 and ApoAI.5a The periodicity was ∼77 nm for ApoCIII segments that appeared to have helical repeats without sharp kinks (Figure S2 of the Supporting Information). For comparison, periodicities were somewhat shorter, ∼53 and 25–60 nm for ApoCII15 and ApoAI,5a respectively.
Figure 2.
(A) Representative TEM image at Tend with the indicated scale bar. (B) Electron diffraction ring with a spacing of (4.7 Å)−1 for ApoCIII at Tend. (C) Representative AFM image at Tend with the indicated scale bar. Insets of panels A and C show magnified sections (100 nm) that highlight triangles and squares. (D) Additional examples and possible models for the triangles (left) and squares (right).
These TEM images are notable for their clarity, allowing us to interpret finer features in the ribbonlike morphology and identify novel structures not previously reported. Panels A and D of Figure 2 reveal a triangular conformation in which the ribbon may be joined at the ends in an antiparallel manner as in a Möbius strip and a square conformation in which the ends appear aligned in a parallel manner. Proposed models are shown in Figure 2D, though the direction of the folds cannot be definitively inferred from the TEM images. If the ribbons are continuous, this would suggest that the underlying structure has rotational symmetry.
In general, it is difficult to follow the contour of the longer structures as there are ambiguous sites regarding the direction of the ribbon fold, resulting from either a seam, a kink in the ribbon, a loss of clarity due to viewing the ribbon edge-on, or damage induced by the staining process. Loop closure could have implications for the mechanism of amyloid formation as it may trap the protein conformational state by blocking fibril end growth.15,16 One unanswered question is whether the loops are continuous β-sheets or connected end to end through different types of interactions.
ApoCIII loops were imaged by atomic force microscopy (AFM) to verify structures are formed in the absence of a stain (Figure 2C). Clearly, triangles, squares, and loops are observed (Figure 2C). A height of ∼2–3 nm is measured (Figure S3 of the Supporting Information), comparable to those of ApoCII loops,15 while the periodicity was ∼35 nm (Figure S4 of the Supporting Information).
Apart from the comparisons to ApoCII17 and oxidized ApoAI amyloid ribbons,5a the triangular and square conformations we observed are remarkably reminiscent of those previously reported for other amyloid-forming proteins. For example, loops formed by 1:1 A53T/WT α-synuclein (α-syn) have a diameter of ∼50 nm, a periodicity of ∼23 nm, and a height of ∼2–4 nm.16 TEM images of α-syn loops formed in the presence of trifluoroethanol have similar dimensions as well as an apparent helical twist.18
ApoAI, ApoCII, and ApoCIII all adopt a helical conformation when they are bound to lipid membranes but there is no obvious relationship that would explain why they form loops while other proteins do not. They do share some general properties of interest that may be irrelevant to loop formation. All three proteins are found on HDL and can remodel lipids into “nanodiscs” that are similar to HDL particles in size and shape.19 ApoAI is directly implicated as an important amyloid component of atherosclerotic plaques,4b while ApoCIII levels have been correlated with atherosclerosis;7 however, whether ApoCIII plays a direct role in the formation of these plaques is unknown. Of note, ApoCII in the presence of lipids can progress from a looped morphology to straight fibrils.20 It has also been shown that the propensity of ApoAI to form fibrils is modulated by mutations21 and oxidation.5a Further studies of ApoCIII should consider the effect of lipids as well as oxidation as they may be critical factors for amyloid formation in atherosclerotic plaques.
In conclusion, we have presented evidence that recombinant ApoCIII forms amyloid, albeit a rather unusual looped structure, which is stable at physiologically relevant concentrations of 5–20 μM and blood pH. Numerous features of amyloids have been characterized: ThT and CR binding, β-sheet structure determined by CD and electron diffraction data, and protease resistance. TEM and AFM demonstrate that ApoCIII forms ribbonlike amyloid loops, and we postulate that they are related to those reported for two other apolipoproteins, ApoCII and ApoAI. Apart from raising the possibility of a biological role for ApoCIII amyloid, we have identified novel loops in the shapes of triangles and squares. Future work is needed to determine how these structures play a role in the mechanism of amyloid assembly and to address whether these conformations are also related to potentially toxic oligomers (intermediates) characterized for other amyloid-forming proteins.
Acknowledgments
TEM, CD, and MS data were collected in the National Heart, Lung and Blood Institute Electron Microscopy, Biophysics, and Biochemistry Cores, respectively. We thank Philippa Talmud (University College London, London, U.K.) for providing the ApoCIII plasmid and Alasdair Steven (National Institute of Arthritis and Musculoskeletal and Skin Diseases) for the use of the Philip CM120 electron microscope for electron diffraction.
Supporting Information Available
Experimental details, Table S1, and Figures S1–S4. This material is available free of charge via the Internet at http://pubs.acs.org.
Supported by the Intramural Research Program at the National Institutes of Health, the National Heart, Lung and Blood Institute, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
The authors declare no competing financial interests.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Shewmaker F.; McGlinchey R. P.; Wickner R. B. (2011) J. Biol. Chem. 286, 16533–16540. [DOI] [PMC free article] [PubMed] [Google Scholar]; b McGlinchey R. P.; Yap T. L.; Lee J. C. (2011) Phys. Chem. Chem. Phys. 13, 20066–20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Walley V. M.; Kisilevsky R.; Young I. D. (1995) Cardiovasc. Pathol. 4, 79–102. [DOI] [PubMed] [Google Scholar]; b Kholová I.; Niessen H. W. M. (2005) J. Clin. Pathol. 58, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bagnato C.; Thumar J.; Mayya V.; Hwang S.-I.; Zebroski H.; Claffey K. P.; Haudenschild C.; Eng J. K.; Lundgren D. H.; Han D. K. (2007) Mol. Cell. Proteomics 6, 1088–1102. [DOI] [PubMed] [Google Scholar]
- Teoh C.; Griffin M. W.; Howlett G. (2011) Protein Cell 2, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Obici L.; Franceschini G.; Calabresi L.; Giorgetti S.; Stoppini M.; Merlini G.; Bellotti V. (2006) Amyloid 13, 191–205. [DOI] [PubMed] [Google Scholar]; b Mucchiano G. I.; Jonasson L.; Häggqvist B.; Einarsson E.; Westermark P. (2001) Am. J. Clin. Pathol. 115, 298–303. [DOI] [PubMed] [Google Scholar]; c Westermark P.; Mucchiano G.; Marthin T.; Johnson K. H.; Sletten K. (1995) Am. J. Pathol. 147, 1186–1192. [PMC free article] [PubMed] [Google Scholar]
- a Wong Y. Q.; Binger K. J.; Howlett G. J.; Griffin M. D. W. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Di Gaetano S.; Guglielmi F.; Arciello A.; Mangione P.; Monti M.; Pagnozzi D.; Raimondi S.; Giorgetti S.; Orru S.; Canale C.; Pucci P.; Dobson C. M.; Bellotti V.; Piccoli R. (2006) Biochem. Biophys. Res. Commun. 351, 223–228. [DOI] [PubMed] [Google Scholar]
- Medeiros L. A.; Khan T.; El Khoury J. B.; Pham C. L. L.; Hatters D. M.; Howlett G. J.; Lopez R.; O’Brien K. D.; Moore K. J. (2004) J. Biol. Chem. 279, 10643–10648. [DOI] [PubMed] [Google Scholar]
- Ooi E. M. M.; Barrett P. H. R.; Chan D. C.; Watts G. F. (2008) Clin. Sci. 114, 611–624. [DOI] [PubMed] [Google Scholar]
- Jong M. C.; Hofker M. H.; Havekes L. M. (1999) Arterioscler., Thromb., Vasc. Biol. 19, 472–484. [DOI] [PubMed] [Google Scholar]
- Pollin T. I.; Damcott C. M.; Shen H.; Ott S. H.; Shelton J.; Horenstein R. B.; Post W.; McLenithan J. C.; Bielak L. F.; Peyser P. A.; Mitchell B. D.; Miller M.; O’Connell J. R.; Shuldiner A. R. (2008) Science 322, 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. V.; Baginsky M. L. (1972) Biochem. Biophys. Res. Commun. 46, 375–381. [DOI] [PubMed] [Google Scholar]
- Windler E.; Havel R. J. (1985) J. Lipid Res. 26, 556–565. [PubMed] [Google Scholar]
- Nilsson M. R. (2004) Methods 34, 151–160. [DOI] [PubMed] [Google Scholar]
- Gangabadage C. S.; Zdunek J.; Tessari M.; Nilsson S.; Olivecrona G.; Wijmenga S. S. (2008) J. Biol. Chem. 283, 17416–17427. [DOI] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366. [DOI] [PubMed] [Google Scholar]
- Hatters D. M.; MacRaild C. A.; Daniels R.; Gosal W. S.; Thomson N. H.; Jones J. A.; Davis J. J.; MacPhee C. E.; Dobson C. M.; Howlett G. J. (2003) Biophys. J. 85, 3979–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K. A.; Lee S.-J.; Rochet J.-C.; Ding T. T.; Williamson R. E.; Lansbury P. T. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters D. M.; MacPhee C. E.; Lawrence L. J.; Sawyer W. H.; Howlett G. J. (2000) Biochemistry 39, 8276–8283. [DOI] [PubMed] [Google Scholar]
- Anderson V. L.; Ramlall T. F.; Rospigliosi C. C.; Webb W. W.; Eliezer D. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 18850–18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricarello D. A.; Smilowitz J. T.; Zivkovic A. M.; German J. B.; Parikh A. N. (2011) ACS Nano 5, 42–57. [DOI] [PubMed] [Google Scholar]
- Griffin M. D. W.; Mok M. L. Y.; Wilson L. M.; Pham C. L. L.; Waddington L. J.; Perugini M. A.; Howlett G. J. (2008) J. Mol. Biol. 375, 240–256. [DOI] [PubMed] [Google Scholar]
- a Petrlova J.; Duong T.; Cochran M. C.; Axelsson A.; Mörgelin M.; Roberts L. M.; Lagerstedt J. O. (2012) J. Lipid Res. 53, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Adachi E.; Nakajima H.; Mizuguchi C.; Dhanasekaran P.; Kawashima H.; Nagao K.; Akaji K.; Lund-Katz S.; Phillips M. C.; Saito H. (2013) J. Biol. Chem. 288, 2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.