Abstract
Reorganization of eloquent cortex enables rescue of language functions in patients who sustain brain injury. Individuals with left-sided, early-onset focal epilepsy often show atypical (i.e. bilateral or right-sided) language dominance. Surprisingly, many patients fail to show such interhemispheric shift of language despite having major epileptogenic lesions in close proximity to eloquent cortex. Although a number of epilepsy-related factors may promote interhemispheric plasticity, it has remained unexplored if neuroanatomical asymmetries linked to human language dominance modify the likelihood of atypical lateralization. Here we examined the asymmetry of the planum temporale, one of the most striking asymmetries in the human brain, in relation to language lateralization in children with left-sided focal epilepsy. Language functional magnetic resonance imaging was performed in 51 children with focal epilepsy and left-sided lesions and 36 healthy control subjects. We examined the association of language laterality with a range of potential clinical predictors and the asymmetry of the length of the planum temporale. Using voxel-based methods, we sought to determine the effect of lesion location (in the affected left hemisphere) and grey matter density (in the unaffected right hemisphere) on language laterality. Atypical language lateralization was observed in 19 patients (38%) and in four controls (11%). Language laterality was increasingly right-sided in patients who showed atypical handedness, a left perisylvian ictal electroencephalographic focus, and a lesion in left anterior superior temporal or inferior frontal regions. Most striking was the relationship between rightward asymmetry of the planum temporale and atypical language (R = 0.70, P < 0.0001); patients with a longer planum temporale in the right (unaffected) hemisphere were more likely to have atypical language dominance. Voxel-based regression analysis confirmed that increased grey matter density in the right temporo-parietal junction was correlated with right hemisphere lateralization of language. The length of the planum temporale in the right hemisphere was the main predictor of language lateralization in the epilepsy group, accounting for 48% of variance, with handedness accounting for only a further 5%. There was no correlation between language lateralization and planum temporale asymmetry in the control group. We conclude that asymmetry of the planum temporale may be unrelated to language lateralization in healthy individuals, but the size of the right, contra-lesional planum temporale region may reflect a ‘reserve capacity’ for interhemispheric language reorganization in the presence of a seizure focus and lesions within left perisylvian regions.
Keywords: epilepsy, planum temporale, language lateralization, children
Introduction
One of the remarkable properties of the human brain is the asymmetrical distribution of language function between the two hemispheres (Geschwind, 1970). The scientific account of this phenomenon goes back to the work of Dax, Broca and Wernicke, with earlier descriptions dating even before the 19th century (Meyer, 1974).
Deviation from a typical, i.e. left-hemisphere, dominance for language is often seen in patients with unilateral, left-sided brain pathology, suggesting a major restructuring of the genetically predetermined organization of language (Vargha-Khadem et al., 2000). Focal epilepsy is an important pathological condition for studying the factors that facilitate this reorganization of language. Indeed, the rate of atypical lateralization, i.e. language being represented bilaterally or in right-sided homologues of Broca’s and Wernicke’s regions, increases to ∼30% in patients suffering from left-sided focal epilepsy (Gaillard et al., 2007). Several factors are widely accepted to be associated with atypical language lateralization in patients with epilepsy, including early age at onset of seizures, left-handedness, and a high frequency of interictal epileptiform discharges arising from the left hemisphere (Janszky et al., 2006). Although left perisylvian lesions were identified as a critical factor for language reorganization in early studies from the pre-imaging era (Rasmussen and Milner, 1977), later functional imaging studies identified a significant proportion of patients with left perisylvian injury who did not show evidence of language reorganization (Liegeois et al., 2004; Mbwana et al., 2009; see example cases in Fig. 1). This has remained a surprising finding, with some studies now implicating mesial temporal injury as the major driving force for interhemispheric plasticity (Janszky et al., 2003; Knecht, 2004; Weber et al., 2006).
Figure 1.
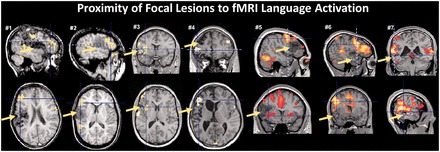
Example cases with lesions in the left inferior frontal region (Cases 1–5) and left temporal cortex (Cases 6 and 7) and prominent functional MRI (fMRI) activation in close proximity to the lesion site (arrows). All cases showed functional MRI lateralization indices compatible with left hemispheric dominance. The orthogonal plane shown in the row below is indicated by a dashed line. Cases 1 and 2 were reported originally by Liegeois et al. (2004).
However, it is not yet known if neuroanatomical factors, beyond lesion location and size, could impede or facilitate language reorganization. There is a long history of anatomical studies reporting on the asymmetrical features of the perisylvian region (Eberstaller, 1884; Flechsig, 1908; Economo and Horn, 1930; Geschwind and Levitsky, 1968; reviewed in Meyer, 1974). Nevertheless, the relationship between functional and structural asymmetries has remained a controversial topic.
The leftward asymmetry of the planum temporale is the most pronounced and consistent asymmetry in the human brain (Toga and Thompson, 2003; van Essen, 2005) and has received considerable attention in relation to language dominance (Geschwind and Levitsky, 1968; Galaburda et al., 1978). The planum temporale is the triangular cortical surface posterior to auditory cortex (Heschl’s gyrus) extending to the end of the Sylvian fissure (Economo and Horn, 1930), which contains higher order auditory and language cortex. The planum temporale is a particularly interesting macro-structural brain asymmetry that can be determined using visual analysis as its length varies on a scale of several centimetres between individuals. It is established early in foetal life (Witelson and Pallie, 1973; Chi et al., 1977) thus predating the onset of epilepsy in most patients. Previous studies investigating a potential link between this striking asymmetry and language dominance have however yielded very mixed results in both healthy populations (Tzourio et al., 1998; Eckert et al., 2006; Keller et al., 2011) and in adult patients with lesional epilepsy (Ratcliff et al., 1980; Foundas et al., 1994; Dorsaint-Pierre et al., 2006). Although earlier studies reported a positive correlation (Ratcliff et al., 1980; Foundas et al., 1994) this could not be confirmed in subsequent studies with adequate sample sizes (Dorsaint-Pierre et al., 2006; Keller et al., 2011). Dorsaint-Pierre et al. (2006) conducted a detailed study of planum temporale anatomy and did not find any association of planum temporale asymmetry with language lateralization in adults with epilepsy. However, left and right-hemisphere epilepsy groups were combined in this study, potentially masking the effects of epilepsy-induced reorganization to the unaffected hemisphere. More recent studies suggest that subtle microstructural asymmetries of white matter language tracts (Powell et al., 2006) and grey matter density (Dorsaint-Pierre et al., 2006; Labudda et al., 2012) could reflect experience-dependent plasticity associated with language reorganization.
However, the relationship of perisylvian asymmetries with language lateralization has not yet been examined in the context of neurodevelopmental pathology. The aim of this study was to determine if structural asymmetry of the planum temporale region has an impact on language reorganization in the context of epilepsy- and lesion-related factors in a cohort of children with left-sided lesional focal epilepsy. In addition to manual morphometry of the planum temporale, we used user-independent, automated analysis methods to evaluate the role of lesion location in the affected left hemisphere on language lateralization using voxel-based lesion symptom mapping (Bates et al., 2003). Finally, we explored if grey and white matter density differences at a micro-structural level, i.e. varying on a scale of millimetres and less (Draganski and May, 2008), in the unaffected right hemisphere contribute to such association. The use of voxel-based morphometry analysis served (i) to seek convergence with findings from manual sulcal measurements (Dorsaint-Pierre et al., 2006; Eckert et al., 2008); and (ii) to explore microstructural (experience-dependent) changes in the right hemisphere associated with atypical language dominance (Dorsaint-Pierre et al., 2006; Labudda et al., 2012).
Materials and methods
Participants
The group of patients comprised 51 children (Table 1) suffering from drug-resistant left-sided lesional focal epilepsy who were enrolled in the epilepsy surgery programme at Great Ormond Street Hospital NHS Foundation Trust, London, UK. Patients were from an unselected population referred for language functional MRI as part of their routine pre-surgical evaluation. Children with right-sided or bilateral lesions were excluded and those who failed functional MRI scanning, i.e. could not perform the task or moved excessively in the scanner. A group of 36 healthy children [18 females, mean age 13.1 years, range 10–17 years, mean verbal IQ score 109 (SD 19), mean performance IQ score 111 (SD 13)], free of neurological and developmental disorders, were recruited through advertisement from local schools and served as a control group.
Table 1.
Patient characteristics
| Typical language (n = 32) | Atypical language (n = 19) | Statistics |
||
|---|---|---|---|---|
| Demographics and clinical variables | Test | P-value | ||
| Age (years) | 12.9 (2.9) | 13.6 (2.3) | t(49) = 0.90 | 0.372, n.s. |
| Age at onset (years) | 6.0 (4.3) | 5.6 (3.7) | t(44) = 0.35 | 0.725, n.s. |
| Gender (M/F) | 15/17 | 9/10 | χ2(1) = 0.001 | 0.973, n.s. |
| Atypical handedness | 2 (11%) | 12 (63%) | Fisher’s exact | <0.0001 |
| Seizure frequency (number/week) | 13 (19) | 22 (29) | t(49) = 1.36 | 0.178, n.s. |
| Pathology | χ2(3) = 6.50 | 0.090, n.s. | ||
| MTS | 4 (13%) | 1 (5%) | ||
| Lesion | 22 (69%) | 9 (47%) | ||
| Stroke | 2 (6%) | 6 (32%) | ||
| Inflammatory | 4 (13%) | 3 (16%) | ||
| Verbal IQ | 85 (17) | 78 (15) | t(47) = 1.46 | 0.150, n.s. |
| Performance IQ | 88 (14) | 85 (19) | t(47) = 0.67 | 0.545, n.s. |
| Volumetric data | ||||
| Lesion volumea (in cm3) | 11.0 (11.8) | 29.5 (44.6) | F(1,48) = 5.06 | 0.029 |
| Planum temporale - LI | 0.25 (0.19) | −0.11(0.22) | t(44) = 5.83 | P < 0.0001 |
a Covaried for intracranial volume.
MTS = medial temporal sclerosis; n.s. = not significant. Values in brackets are SD = standard deviations, unless shown as %.
Procedures
Clinical work-up
All patients underwent presurgical evaluations, which included neurological and neuropsychological assessments, video EEG telemetry, high-resolution MRI and functional MRI scanning. Seizure semiology was categorized using the Lüders classification (Lüders et al., 1998). Interictal epileptiform discharges and ictal seizure-onset were categorized whether they were detected in the frontal, temporal, parietal, occipital or perisylvian regions.
Neuropsychological evaluation
The age-appropriate Wechsler intelligence scale (WISC-III, WISC-IV, WAIS) was administered to all participants. Handedness was determined during clinical neuropsychological interview and using the Edinburgh Handedness Inventory: in the patient group 37 were right-handed, one ambidextrous, and 13 were left-handed; in the control group, 31 were right-handers and five were left-handers (Table 1).
Magnetic resonance imaging acquisition and analysis
All participants were scanned with a 1.5 T Avanto Siemens scanner. Conventional T2-weighted images were acquired using an axial multi-slice sequence (repetition time = 4920 ms, echo time = 101 ms, field of view = 220 mm, slice thickness = 4 mm, slices = 25, matrix size = 384 × 384). Three-dimensional data sets were acquired using a T1-weighted 3D-FLASH sequence (repetition time = 11 ms, echo time = 4.94 ms, flip angle = 15°, field of view = 256 mm, matrix size = 256 × 256) and a T2-weighted FLAIR sequence (repetition time = 6000 ms, echo time = 353 ms, flip angle = 150°, field of view = 256 mm, matrix = 256 × 256).
Functional MRI was used to investigate hemispheric language lateralization in all participants using a covert semantic retrieval (verb generation) task. This task shows excellent correspondence with invasive tests (Lehericy et al., 2000; Liegeois et al., 2002, 2006). Due to the risk of attenuation of the blood oxygen level-dependent response after severe seizure activity (Jayakar et al., 2002) all epilepsy participants were screened for a recent history of seizure clusters before conducting functional MRI scanning. Lateralization with functional MRI is generally robust in relation to the more chronic abnormalities of cerebral metabolism associated with focal epilepsy (Gaillard et al., 2011). Two sets of functional data were acquired using a whole brain echo-planar pulse sequence (repetition time = 2570 ms, echo time = 50 ms, flip angle = 90°, field of view = 192 × 192, slice thickness = 3 mm, 1 mm interslice gap, slices = 30, matrix size = 64 × 64, voxel size = 3 × 3 × 4 mm3). A block design consisted of 10 active task (covert verb generation) and rest (listening to amplitude-modulated white noise) phases. All participants practiced the task outside the scanner until satisfactory performance was achieved and they were comfortable performing the task inside the scanner.
Functional magnetic resonance imaging language lateralization
Functional images were processed with SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) using a standard procedure that included coregistration and realignment, spatial normalization and computation of the first-level contrast (verb generation versus rest) after covariation with movement regressors. Three expert raters independently performed a quality control by visually evaluating all individual functional MRI data, including evaluation of reproducibility across the two blocks. Calculation of a laterality index for each participant was performed using the LI toolbox (Wilke and Lidzba, 2007). Mean laterality index (LI) values were determined for each individual and region of interest after sampling across 20 different activation thresholds using a boot strapping method (Wilke and Schmithorst, 2006). The Broca’s region of interest included the inferior frontal gyrus, premotor cortex and middle frontal gyrus and additional regions of interest included the temporal lobe and cerebellum. The temporal lobe region of interest included the superior, middle and inferior temporal gyri, fusiform and parahippocampal gyri as well as temporal pole. Laterality indices of +1 indicate complete left-sided lateralization and −1 complete right-sided lateralization. Participants were categorized into typical and atypical lateralization groups according to LI values in Broca’s region as this most closely correlates with results from invasive tests of language dominance (Lehericy et al., 2000; Liegeois et al., 2002). The atypical language lateralization group was comprised of participants with bilateral (LI between +0.2 and −0.2) and right-lateralized-language (LI < −0.2) distribution, consistent with laterality index cut-off values commonly used in language functional MRI-studies (Binder et al., 1996; Pujol et al., 1999; Gaillard et al., 2002, 2007; Seghier, 2008; Labudda et al., 2012). Mean group activation maps were created for typical and atypical lateralization groups using a random-effects model in SPM8.
Lesion identification and voxel-based lesion symptom mapping
All scans were evaluated by an experienced paediatric neuroradiologist. Focal, left-sided lesions were found in all patients (including 10 low-grade tumours, 11 focal cortical dysplasias, four cavernomas, one gliotic lesion, two polymicrogyrias, one CNS melanosis, one meningioangiomatosis, one focal atrophy); eight had a history of a vascular event (three perinatal intracranial bleeds, five ischaemic insults); seven suffered from a suspected Rasmussen’s encephalitis; and five had mesial temporal sclerosis, of which two had a dual pathology. Lesion maps were traced on T1-weighted images using MRIcron software (C. Rorden, http://www.cabiatl.com/mricron/) guided by additional tissue contrast information derived from T2-weighted images.
Voxel-based lesion symptom mapping analysis (Bates et al., 2003) was conducted to evaluate differences in lesions density between individuals with atypical compared to typical lateralization using NPM software (http://www.cabiatl.com/mricron/) and Liebermeister statistic for binomial data (Rorden et al., 2007). Lesion maps were spatially normalized to the MNI template using SPM8 software.
Tissue segmentation and voxel-based morphometry
Volumes of CSF, grey matter and white matter were calculated from 3D-FLASH images using the voxel-based morphometry toolbox (C. Gaser, http://dbm.neuro.uni-jena.de/vbm/) for SPM8 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). In contrast to the default SPM8 algorithm the final tissue probabilities were estimated without a spatial template of tissue distribution (‘priors’), which allows reliable segmentation of grossly abnormal brains (for further details see Northam et al., 2011). This was achieved by using the unified segmentation procedure implemented in SPM8, modified to include a Hidden Markov Field model as an additional spatial constraint (see http://dbm.neuro.uni-jena.de/vbm/markov-random-fields/). For consistency, this method was applied to all brains and tissue segments were visually inspected for accuracy. Grey and white matter segments were normalized (non-linear modulation) to a study-specific template created using the TMP-O-Matic toolbox (Wilke et al., 2008) and smoothed using an 8 mm full-width at half-maximum Gaussian kernel.
A group-level whole brain voxel-wise regression analysis was performed with language functional MRI laterality index value for each participant as the covariate of interest and age and sex as covariates of no interest. This voxel-based morphometry analysis was performed in the right hemisphere only, because a meaningful tissue-specific analysis was not possible in the left hemisphere due to the variability of imaging abnormalities across patients, i.e. some lesions were only visible on T2-weighted imaging. Regions where there was an a priori hypothesis (i.e. in posterior superior temporal and inferior frontal regions) were evaluated at a threshold of P < 0.001, uncorrected for multiple comparisons. The more stringent statistical threshold of P < 0.05, family-wise error-corrected for multiple comparisons, was used for regional differences outside perisylvian language areas where there was no prior hypothesis. The minimum cluster size was 240 as in Labudda et al. (2012).
Planum temporale measurement
We measured the length of the planum temporale on its lateral border; this measure shows a higher inter- and intra-rater reliability than area or volume measures (Shapleske et al., 1999) and is not biased by asymmetries in gyrification (Steinmetz et al., 1990). The anterior border of the planum temporale was defined according to the widely used ‘Pfeiffer’s criterion’ and the posterior border of the planum temporale was defined as the posterior end of the horizontal portion of the Sylvian fissure excluding the planum parietale (Steinmetz, 1990, 1996). See Supplementary material for further details. All anatomical measurements were made blind to clinical and functional MRI laterality information. A first investigator performed all planum temporale measurements twice showing excellent intra-rater reliability in both hemispheres [left planum temporale: single measures intra-class coefficient (ICC) = 0.86; right: ICC = 0.89]. Because measurements were made without being blind to hemispheric side of planum temporale, this potential confound was addressed by a second rater who measured the planum temporale using the identical protocol, but after having about half of the scans randomly flipped in the left to right direction. This showed a similar degree of agreement (left planum temporale: ICC = 0.84; right planum temporale: ICC = 0.88). For comparison with the functional MRI laterality index, a similar planum temporale index was computed using the analogous formula:
Planum temporale length was adjusted for intracranial volume using linear regression analysis, to account for interindividual differences in head size.
Ethics
Ethical approval for the study was obtained from Great Ormond Street Hospital for Children/UCL Institute of Child Health Research Ethics Committee and written informed consent was obtained from all participants or their parents (depending on age at assessment).
Statistical analysis
Group differences in demographic, clinical and cognitive data were tested using independent samples t-tests, analysis of variance and χ2 or Fishers exact tests, where appropriate. Group comparisons of tissue or lesion volumes were made using analysis of covariance, after correcting for intracranial volume. Pearson’s correlations and partial correlations were used in the patient group to demonstrate the relationship between brain measures and clinical factors. Stepwise linear regression analyses were performed to determine predictors of the degree of functional MRI language lateralization (LI value) from amongst those variables that were significant in previous group comparisons. Diagnostic analyses included examination of influential data points, normality of residuals and multi-collinearity. Model selection was performed in a stepwise fashion using the R2 change statistic (Field, 2009).
Results
Language lateralization groups
Language lateralization
As expected, the mean functional MRI laterality index in Broca’s area of 0.29 (SD 0.56) in patients was lower (i.e. less left-lateralized) than in controls [LI = 0.55 (SD 0.32), T = 2.7, P = 0.008]. Lateralization in the temporal lobes was also reduced in patients (LI = 0.03, SD = 0.51) compared with controls [LI = 0.23 (SD = 0.24), T = 2.4, P = 0.019]. Atypical language lateralization was found in 19 (37%) patients (bilateral: five, right-lateralized: 14) compared with four (11%) healthy control subjects (bilateral two, right-lateralized two; χ2 = 7.4, P = 0.006). Group mean activation maps for the atypical lateralization group showed activation in homologous regions of the right hemisphere compared to those in the typical lateralization group (Fig. 2A).
Figure 2.
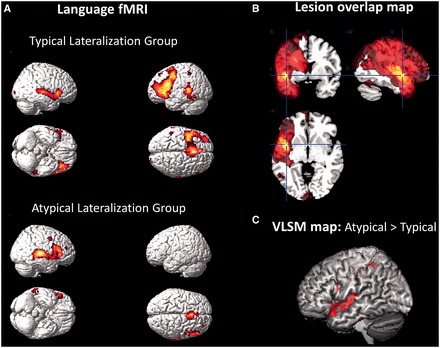
(A) Group functional MRI maps of patients with typical (left-sided, n = 32) and atypical (right-sided and bilateral, n = 19) language activation. Maps are displayed at family-wise error corrected threshold of P = 0.05. (B) Lesion overlap map showing the distribution of left hemisphere lesions in the patient sample (scale ranges from 0–30% overlap). Most lesions clustered around the perisylvian region. (C) Voxel-based lesion-symptom (VLSM) map showing greater likelihood of lesion location in anterior temporal and inferior frontal regions in the atypical compared to typical lateralization group (Liebermeister statistic at false discovery rate at P < 0.05). Smaller clusters are visible in the posterior inferior frontal and inferior parietal regions.
Demographic, clinical and cognitive characteristics of laterality groups
Left or ambidextrous handedness was more common in patients with atypical compared to typical language lateralization (Table 1). This atypical handedness was associated with lesions located in posterior frontal regions (13/14 patients, Fisher’s exact: P < 0.0001) and with motor abnormalities of the right hand on neurological examination (10/14 patients, χ2 = 12.5, P = 0.002). Among factors connected with pregnancy, birth history and early development reports of delayed language development were more common in the atypical lateralization group (47%, compared with 12% in the typical group, Fisher’s exact: P = 0.009). There was no difference in other clinical and epilepsy-related factors, with the exception of left perisylvian ictal EEG-onset, which was more common in the atypical lateralization group (92%, compared with 52% in the typical group, Fisher’s exact: P = 0.015).
Given the proximity of epileptogenic lesions near eloquent cortex in this sample (Fig. 2B) it is of interest to determine if language reorganization had incurred a cost in terms of verbal abilities. There were no statistical differences in verbal and performance IQ scores between laterality groups (Table 1), and in a subgroup of 24 children, receptive and expressive language scores from the Clinical Evaluation of Language Fundamentals (CELF-4UK) were also not different (data not shown). These findings were not altered if only patients with lesions in proximity to eloquent cortex in the frontal and temporal lobes were included in the comparisons. Across the patient sample there was nevertheless a weak positive correlation between verbal IQ and functional MRI LI in Broca’s region (R = 0.29, P = 0.043).
Magnetic resonance imaging-derived measures in relation to language lateralization
Planum temporale asymmetry
The mean laterality index of the planum temporale in the patient group (LI = 0.12, SD 0.26) did not differ (T = 0.86, P = 0.395) from control values (LI = 0.17, SD 0.24). Over 70% of participants in both groups showed a leftward planum temporale asymmetry. However, within the patient sample the language laterality groups differed in these scores (Table 1), with the atypical group showing a more right-lateralized planum temporale than the typical lateralization group. There was indeed a strong positive relationship between the laterality index values for functional MRI lateralization and planum temporale asymmetry in patients (R = 0.70, P < 0.0001, Fig. 3) but not in controls (R = 0.03, P = 0.879).
Figure 3.
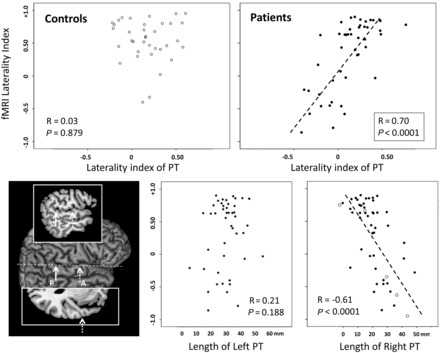
Scatterplots showing distribution of functional MRI laterality indices in Broca’s regions in controls and patients against planum temporale (PT) laterality indices (top). The two graphs below show the functional MRI laterality against the length of the left and right planum temporale (adjusted for intracranial volume), separately, in the patient group. Cases in which the left planum temporale could not be determined due to lesions in the superior temporal lobe are shown with open symbols. The inset on the left shows the definition of the anterior (A) and posterior (P) borders of the planum temporale, shown on the surface rendering of the right side of the brain. Heschl’s sulcus defined the anterior border (shown on the axial slice inset above) and the posterior border was marked by the end of the horizontal portion of the sylvian fissure (shown on the sagittal slice inset below).
In the patient group this correlation was mainly driven by the length of the right planum temporale (R = −0.61, P < 0.0001), rather than the left (R = 0.21, P = 0.188). Interestingly, in five patients the left planum temporale could not be determined due to lesions encroaching on the superior temporal gyrus. Even in these patients (marked separately in Fig. 3) the length of the right planum temporale alone was a good predictor of language lateralization. It is worth noting that the functional MRI laterality index in the temporal lobes was also moderately correlated with planum temporale lateralization (R = 0.36, P = 0.018), an effect also driven by the length of the right planum temporale (R = −0.30, P = 0.044).
Lesion volume and location in the left hemisphere
Patients with atypical compared to typical language lateralization had greater lesion volumes; however, this difference was entirely due to the inclusion of the larger stroke lesions. Voxel-based lesion symptom mapping analysis suggested that lesions were more common in patients with atypical compared to typical language in two regions of the left hemisphere: the anterior superior temporal gyrus and the posterior inferior frontal lobe [Liebermeister statistic at false discovery rate (FDR) P < 0.05, Fig. 2C]. Based on these voxel-based findings, a categorical variable was created (for inclusion in a final regression model, see below) that specified after visual analysis of all scans if lesions encroached the anterior or posterior portions of the inferior frontal and superior temporal regions, in addition to lesion location in any of the other lobes. Using this categorical variable, we confirmed that atypical language was indeed associated with lesions in anterior superior temporal gyrus (χ2 = 4.4, P = 0.036) and the posterior inferior frontal gyrus (χ2 = 6.7, P = 0.010) but none of the other regions examined.
Voxel-based morphometry regression analysis in the patient group
A group-level analysis conducted only in the right hemisphere identified a prominent cluster of negative correlations between language functional MRI laterality index values and grey matter, indicating higher density in right posterior temporo-parietal cortex with increasing right-sided language lateralization (Fig. 4). The peak T-value of 4.41 was located at coordinate [44, −19, 24] in the parietal operculum. The parameter estimates in the planum temporale grey matter cluster (extracted from all patients and averaged across the 1216 voxels of this cluster) correlated with the sulcal length of the right planum temporale (R = 0.40, P = 0.004). At a lower statistical threshold (P = 0.005) this cluster extended inferiorly into the planum temporale, including the medial part of Heschl’s gyrus. Smaller areas of correlation in the posterior and anterior temporal cortex and anterior frontal lobe did not survive the predefined statistical height and extent thresholds. No regions of white matter density correlation were found.
Figure 4.
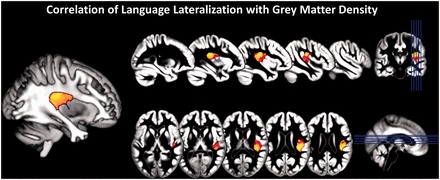
Results of voxel-based morphometry regression analysis indicating a relationship of grey matter density with atypical language lateralization in the patient sample. Statistical thresholds are indicated in yellow (P < 0.001, uncorrected for multiple comparisons) and red (P < 0.005, uncorrected) superimposed on group mean grey matter segments. The analysis was restricted to the right hemisphere only. Cluster extent threshold was 240 as in Labudda et al. (2012). Insets on the right indicated the position of slices displayed. Image on the left shows prominent cluster of correlation within the parieto-temporal junction superimposed on a white matter segment.
Predictors of atypical language lateralization
To determine independent predictors of functional MRI language lateralization in this cohort from among the clinical and MRI markers identified above, we entered the following variables into a stepwise linear regression model: handedness, lesion volume, lesion location within anterior superior temporal gyrus or posterior inferior frontal gyrus, perisylvian ictal EEG onset and planum temporale length in the right hemisphere. Functional MRI lateralization (Broca’s area LI value) was explained by a model (R2adjusted = 0.52, F = 28.1, P < 0.0001), which included length of the right planum temporale (β = −0.48, P < 0.0001) and handedness (β = 0.39, P = 0.001) as predictors (see Supplementary material for further details).
Discussion
The key finding of this study is that asymmetry of the posterior perisylvian region, which includes the planum temporale, modifies the likelihood of atypical language lateralization in children with early-onset lesional focal epilepsy. We did not find compelling evidence for a relationship between language lateralization and planum temporale asymmetry in healthy control children, in line with other recent studies (Dos Santos et al., 2006; Eckert et al., 2006; Keller et al., 2011). This suggests that sulcal patterns emerging early in foetal life and predating the onset of epilepsy have a lasting impact on the capacity for language reorganization. This finding provides one potential answer to the often surprising degree of resilience of left-hemisphere language cortex to interhemispheric reorganization even in cases with major lesions affecting left perisylvian cortex (Fig. 1; Liegeois et al., 2004; Mbwana et al., 2009). We suggest that the size of the planum temporale region in the right hemisphere may be used as a predictive marker for interhemispheric language reorganization.
Our study confirms that about one-third of patients with left-sided focal epilepsy show atypical language dominance (Binder et al., 1996; Yetkin et al., 1998, Gaillard et al., 2002, 2004, 2007; Adcock et al., 2003; Woermann et al., 2003, Thivard et al., 2005; Weber et al., 2006; Duke et al., 2012). This reorganization involves predominantly right hemisphere homologues of Broca’s and Wernicke’s areas, including associated regions in the middle frontal gyrus and angular gyrus (Mbwana et al., 2009). Intrahemispheric reorganization involves areas adjacent to classical language cortex (Liegeois et al., 2004; Rosenberger et al., 2009). Our study also replicates the influence of a number of factors driving interhemispheric language plasticity in this population, such as atypical handedness, lesion- and seizure-related factors.
The role of clinical and lesion-related factors for language reorganization
In line with previous studies atypical handedness was associated with atypical language reflecting the impact of underlying pathology (Satz et al., 1985; Sveller et al., 2006; Gaillard et al., 2007), which in the majority of cases was located in the posterior frontal lobe and was associated with mild to moderate motor impairment of the right hand (Isaacs et al., 1996). This pathology-induced association is therefore in distinction to the genetic association observed in healthy populations (Anneken et al., 2004; Corballis, 2009).
Among epilepsy-related factors, only perisylvian ictal EEG onset was associated with atypical language, which is in keeping with previous studies indicating that epileptiform activity emanating from perisylvian regions may alter language lateralization (Janszky et al., 2003; Lillywhite et al., 2009; Monjauze et al., 2011).
We were able to replicate findings from classical and more recent studies (Rasmussen and Milner, 1977; Korman et al., 2010; Duke et al., 2012) regarding the importance of lesion localization for language lateralization, although our study did not have a uniform distribution of lesion density across the brain to address this issue comprehensively. Among aetiological groups we observed that stroke lesions had the greatest impact on language laterality, in agreement with the findings of Gaillard et al. (2007). Using a liberal statistical threshold, we observed that lesions within posterior inferior frontal and anterior superior temporal cortex were more frequently associated with atypical language. We speculate that these cortical regions (Fig. 2C) are compatible with the connectivity of what has been termed the ‘ventral language pathway’ (Scott et al., 2000; Saur et al., 2008). This pathway includes the projections from the anterior temporal lobe to the inferior frontal cortex (through the uncinate fasciculus and extreme capsule fibre system) and is assumed to be functionally distinct from a ‘dorsal pathway’ through the arcuate fasciculus. One reason for such a selective effect could be the earlier structural and functional maturation of the ventral compared with the dorsal pathway (Brauer et al., 2013). This would make the ventral system more important to preserve during language development (<7 years) and therefore be driving interhemispheric reorganization more effectively than injury to the less mature dorsal pathway. We did not find any evidence to implicate the cortical projections of the dorsal pathway in the posterior superior temporal lobe. This explanation might also account for the frequently observed atypical language dominance in patients with mesial temporal epilepsy (Janszky et al., 2003; Liegeois et al., 2004; Weber et al., 2006; Duke et al., 2012) in whom seizure activity impacts on anterior temporal regions and could spread to frontal regions through the uncinate fasciculus.
We observed no apparent or minimal cost to verbal abilities in patients with atypical compared to typical language lateralization. We interpret this finding as indicating overall compensatory plasticity, in particular in patients with lesions in proximity to eloquent cortex. The evidence for or against the compensatory nature of interhemispheric language shifts is still contradictory and the causal nature of this relationship is difficult to establish, as other mediating aetiological factors cannot be ruled out (reviewed by Vlooswijk et al., 2010). For example the laterality groups differed in lesion size and location as well as seizure frequency, which by themselves impact on neuropsychological performance. Nevertheless, the weak correlation between verbal IQ scores and functional MRI lateralization observed in our patients is in line with findings in other cohorts with and without brain injury (Whitehouse and Bishop, 2008; Myers et al., 2010; Northam et al., 2012), accounting for ∼10% of verbal abilities. Of interest, in this context, are the parental reports of delayed speech acquisition in our patients with atypical lateralization. This retrospective observation is in agreement with the finding of atypical receptive functional MRI language lateralization in young children with idiopathic speech delay (Bernal and Altman, 2003). It is also important to stress that reorganization to the right hemisphere is only one possible mechanism with additional compensation possible through interhemispheric commissures and through the ventral language pathway in the left hemisphere (Northam et al., 2012; Dick et al., 2013).
The relationship between structural and functional language asymmetries
In agreement with earlier studies (Geschwind and Levitsky, 1968; Witelson and Pallie, 1973; Rubens et al., 1976) we observed a clear leftward asymmetry of the planum temporale in the majority of patients and controls and no overall difference between groups. The laterality index is in line with literature values (Steinmetz, 1996; Shapleske et al., 1999), given appropriate conversions are made. Similarly, there was also no difference in planum temporale length or lateralization according to age of epilepsy onset, pathology type or estimated age at injury (data not shown). Thus, the morphological asymmetry of the planum temporale, which is set by the last trimester (Chi et al., 1977) with no change during later post-natal life (Preis et al., 1999; van Essen, 2005), seems unaffected by the presence or type of developmental pathology. We argue here that this innate asymmetry, or more precisely the size of the planum temporale region in the right hemisphere, may either facilitate (in cases with a large right-sided planum temporale) or impede (in cases with a small right-sided planum temporale) the shift of language representation under pressure from pathological factors affecting left perisylvian cortex, such as epileptic discharges or destructive lesion. However, only longitudinal studies can rule out the alternative, albeit currently implausible, hypothesis that functional language reorganization can have a profound effect on macrostructure of sulcal morphology of the planum temporale, i.e. inducing changes in the order of several centimetres.
There is variable agreement with our findings among previous investigations into the link between planum temporale lateralization and language dominance in epilepsy populations (see Supplementary material for further details). Although early reports by Foundas et al. (1994) and Ratcliff et al. (1980) are broadly compatible with our data, they did not specify type and side of pathology in their patient samples. In contrast, Dorsaint-Pierre et al. (2006) did not confirm a correlation of planum temporale morphology with Wada language dominance, which, however, can be attributed to their failure to consider pathology-induced reorganization. Their group of patients differed widely in age at epilepsy onset and side of seizure focus and most appeared to have mesial temporal sclerosis, which is frequently associated with atypical language dominance (Weber et al., 2006). Two recent reports nevertheless show some agreement with our findings.
Oh and Koh (2009) reported in adults with temporal lobe epilepsy that among 10 patients with a left-sided focus and right hemisphere language, seven showed rightward planum temporale asymmetry, whereas leftward planum temporale asymmetry was found in most patients with left language dominance. Surprisingly, the authors reported a different distribution of planum temporale lateralization according to side of seizure onset: left patients with temporal lobe epilepsy showed a predominantly bilateral planum temporale distribution whereas patients with right temporal lobe epilepsy had mostly left-lateralized planum temporale. Apart from a possible measurement or recruitment bias, this remains unexplained as it suggests that mesial temporal pathology could influence planum temporale sulcal development despite late seizure onset. In contrast, we did not find evidence for alterations of planum temporale length or asymmetry with even more proximal injury to this region.
Furthermore, a recent voxel-based morphometry study in adults with left-sided temporal lobe epilepsy reported increased grey matter density within right temporo-lateral cortex in patients with atypical language lateralization on functional MRI (Labudda et al., 2012). Although planum temporale morphology was not quantified, the authors also computed correlations of grey matter density with functional MRI lateralization. Agreement with our study is seen in the extension of a voxel-based morphometry correlation cluster into right Heschl’s gyrus. In addition, Labudda et al. (2012) observed correlations within the medial frontal gyrus and superior temporal gyrus, which the present study failed to find. This is likely to be due to the different study populations, older ages of epilepsy onsets and longer duration of seizures. The failure of our study to find robust differences in other regions than the planum temporale may also relate to the possibility that some structural asymmetries may parallel the ontogenetic development of functional asymmetries (experience-dependent plasticity; Josse et al., 2009), and hence might be more difficult to detect in childhood than adulthood.
Overall, the evidence from healthy populations points towards the lack of a major relationship of planum temporale asymmetry to language dominance, which we also confirmed here (Supplementary material).
Development and functional role of perisylvian cortical asymmetries
Asymmetries in the fronto-parietal operculum and superior temporal sulcus are the most consistent and pronounced human cerebral asymmetries (Toga and Thompson, 2003; van Essen, 2005), which emerge from the 23rd gestational week (Habas et al., 2012). They are present in both term-born infants and adults to a similar degree (Hill et al., 2010) and there are no differences in asymmetry between childhood, adolescence and adulthood (Preis et al., 1999; Eckert et al., 2008).
The planum temporale on the dorsal surface of the temporal lobe consists of auditory association cortex (Economo and Horn, 1930) and is engaged in higher order audition (Griffiths and Warren, 2002). This region shows distinct cytoarchitectonic features, thought to enable the processing of complex spectro-temporal patterns of human speech (Galuske et al., 2000). The debate on the functional role of the posterior temporal regions in speech and language is still ongoing, with evidence for its role in semantic and syntactic processing as well as covert articulation (Wise et al., 2001; Price, 2010, 2012). Imaging studies consider it as a functional unit together with the ventral supramarginal gyrus [‘pPT/vSMg’ according to Price (2010), or ‘Spt—Sylvian parieto-temporal’ according to Hickok et al. (2009)].
It is of note that the correlation of planum temporale asymmetry with Broca’s area lateralization also extended to functional MRI lateralization in the temporal lobes, albeit less strikingly. Although it is possible that the verb generation task used here is not optimal for activating posterior temporal language regions, recent studies report greater planum temporale activation during productive (including covert) tasks than purely receptive language tasks (reviewed in Zheng, 2009; Buchsbaum et al., 2011). In addition, receptive language functions are generally more bilaterally represented in the brain than language production (Hickok and Poeppel, 2007). Planum temporale activation was also found in the present study with group level activation extending from lateral superior temporal cortex into the planum temporale. Overall, this is in keeping with the putative role of in the posterior planum temporale region, and specifically of area ‘Spt’, in auditory-to-speech-motor integration (Wise et al., 2001; Hickok and Poeppel, 2007; Buchsbaum et al., 2011).
It remains to be investigated which of its functional and connectivity properties are ultimately critical for the role that the present study attributes to the planum temporale region in constraining interhemispheric language plasticity. When considering which element of the planum temporale asymmetry is most salient for the observed relationship, it is interesting to mention those patients in which the left planum temporale could not be identified due to lesions affecting the superior temporal lobe. In these patients the length of the right planum temporale alone predicted language lateralization, confirming the observation across the cohort (Fig. 3) that it is the not the length of the left planum temporale or planum temporale asymmetry per se, but the size of the contra-lesional planum temporale that is important, akin to a ‘reserve capacity’ for interhemispheric reorganization.
Methodological considerations
It is important to acknowledge potential limitations of this study. The prevalence of atypical language lateralization could be over-represented in our sample because the process of referral for functional MRI investigation includes a consideration by referring physicians of the potential risk from surgery to language function. Nevertheless, the high percentage of atypical language in our cohort is in accordance with that of other centres (Gaillard et al., 2007) and allowed us to robustly estimate the contribution of anatomical factors in language plasticity. Furthermore, the large spectrum of brain pathology included in this study prevented us from comparing pathology groups systematically. Existing evidence does not indicate a critical role for pathology types in influencing language reorganization (Briellmann et al., 2006; Korman et al., 2010), with the exception of stroke lesions (Gaillard et al., 2007). The present study presents one of the largest paediatric cohorts reported to date, showing that the findings extend across the spectrum of brain pathology, representative for young patients with epilepsy coming for neurosurgical work-up (Harvey et al., 2008).
It is important to note that there is overlap between the macro-structural measure of planum temporale asymmetry and voxel-based morphometry grey matter density in the planum temporale region (Eckert et al., 2008), which was also found in this cohort. Eckert et al. (2008) estimated that planum temporale grey matter density accounted for ∼20% of variance in sulcal planum temporale asymmetry, which compares well with a value of 16% in our study. Despite the limitations of voxel-based morphometry in capturing sulcal morphology (Molko et al., 2003) it is reassuring to see the convergence between both methods.
Conclusions and implications
Our study is the first to conclude that neurodevelopmental factors, which shape the early emergence of interindividual differences in planum temporale asymmetry, exert a powerful influence over interhemispheric language plasticity in the presence of multiple injury-related factors promoting a shift. The finding reported here provides a novel account for the apparent resilience to reorganization in cases with lesions in or near eloquent cortex (Fig. 1). The clinical implications derive from the necessity to estimate the risk of neurosurgical treatment or acute injury for language compromise. We introduce an anatomical notion of ‘reserve capacity’ for language reorganization by analogy to reserve capacity for memory outcome after temporal lobe resection. We suggest that individual sulcal/gyral morphology of the planum temporale, unlike voxel-based morphometry group statistics, could prove a useful predictor of degree of reorganization and functional recovery in patients who sustained such injury to language regions. The most informative cases are those who undergo resections in the language-dominant hemisphere for the treatment of severe epilepsy syndromes, tumours or vascular malformations. Based on preoperative language performance, postoperative outcome is often difficult to predict and we expect that innate planum temporale asymmetry may contribute to some of the considerable interindividual differences in linguistic competence after surgery (Liegeois et al., 2008). It also remains to be determined if our findings extend to patients with older ages at insult. For example, in patients with aphasia due to left-hemisphere stroke planum temporale asymmetry could modify right hemisphere auditory functional connectivity and its contribution to the recovery of speech comprehension (Teki et al., 2013).
In addition, our study is an important step towards solving a major puzzle of the structure-function relationships in the human brain. The strong left-hemisphere functional dominance for language has been contrasted with relatively subtle anatomical asymmetries (Dorsaint-Pierre et al., 2006). The finding that a single anatomical measure accounts for a large proportion of variability in hemispheric lateralization calls for further research into the intrinsic properties and connectivity patterns of the planum temporale region. For example, it will be of interest to examine perisylvian pathways (Petrides and Pandya, 1988; Schmahmann et al., 2007) using diffusion-weighted tractography in relation to posterior Sylvian asymmetries. Our observation that anatomical features of this region relate to fronto-temporal language lateralization in a developmental cohort suggest a critical role in language acquisition, most likely acting as an interface for the integration of sensory and vocal tract representations, important for articulation and phonological memory.
Finally, we propose that elucidating the molecular pathways (Sun and Walsh, 2006) and cellular properties (Bianco et al., 2008), which shape the lateralized patterning of cortical language networks might offer new insights into mechanisms of recovery of function after neurological injury.
Supplementary Material
Acknowledgements
We would like to thanks Tina Banks for MRI scanning and Josselyn Hellriegel for help with data analysis.
Glossary
Abbreviation
- LI
laterality index
- fMRI
functional MRI
Funding
This work was supported by a fellowship (G.P.) from the Austrian Chapter of the International League Against Epilepsy, and further funding from Epilepsy Research UK, Action Medical Research UK, and the Great Ormond Street Hospital Children’s Charity. This work was undertaken at GOSH/UCL Institute of Child Health who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Supplementary material
Supplementary material is available at Brain online.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Anneken K, Konrad C, Drager B, Breitenstein C, Kennerknecht I, Ringelstein EB, et al. Familial aggregation of strong hemispheric language lateralization. Neurology. 2004;63:2433–5. doi: 10.1212/01.wnl.0000147265.71911.65. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bernal B, Altman NR. Speech delay in children: a functional MR imaging study. Radiology. 2003;229:651–8. doi: 10.1148/radiol.2293021746. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Carl M, Russell C, Clarke JD, Wilson SW. Brain asymmetry is encoded at the level of axon terminal morphology. Neural Dev. 2008;3:9. doi: 10.1186/1749-8104-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–84. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Perani D, Friederici AD. Dorsal and ventral pathways inlanguage development. Brain Lang. 2013 doi: 10.1016/j.bandl.2013.03.001. pii: S0093-934X(13)00070-9. Advance Access published on May 1, 2013, doi:10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Briellmann RS, Labate A, Harvey AS, Harvey AS, Saling MM, Sveller C, et al. Is language lateralization in temporal lobe epilepsy patients related to the nature of the epileptogenic lesion? Epilepsia. 2006;47:916–20. doi: 10.1111/j.1528-1167.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Baldo J, Okada K, Berman KF, Dronkers N, D’Esposito M, et al. Conduction aphasia, sensory-motor integration, and phonological short-term memory—an aggregate analysis of lesion and fMRI data. Brain Lang. 2011;119:119–28. doi: 10.1016/j.bandl.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–8. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The evolution and genetics of cerebral asymmetry. Philos Trans R Soc Lond B Biol Sci. 2009;364:867–79. doi: 10.1098/rstb.2008.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Raja Beharelle A, Solodkin A, Small SL. Interhemispheric functional connectivity following prenatal or perinatal brain injury predicts receptive language outcome. J Neurosci. 2013;33:5612–25. doi: 10.1523/JNEUROSCI.2851-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins KE, Neelin P, Lerch JP, Bouffard M, et al. Asymmetries of the planum temporale and Heschl’s gyrus: relationship to language lateralization. Brain. 2006;129:1164–76. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dos Santos SS, Woerner W, Walter C, Kreuder F, Lueken U, Westerhausen R, et al. Handedness, dichotic-listening ear advantage, and gender effects on planum temporale asymmetry—a volumetric investigation using structural magnetic resonance imaging. Neuropsychologia. 2006;44:622–36. doi: 10.1016/j.neuropsychologia.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–42. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Duke ES, Tesfaye M, Berl MM, Walker JE, Ritzl EK, Fasano RE, et al. The effect of seizure focus on regional language processing areas. Epilepsia. 2012;53:1044–50. doi: 10.1111/j.1528-1167.2012.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberstaller O. Zur Oberflächen-Anatomie der Grosshirn-Hemisphären. Wien. Med. Blätter. 1884;7:644–6. [Google Scholar]
- Eckert MA, Leonard CM, Possing ET, Binder JR. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang. 2006;98:102–111. doi: 10.1016/j.bandl.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, Walczak AR, Bonihla L, Leonard CM, Binder JR. Manual and automated measures of superior temporal gyrus asymmetry: concordant structural predictors of verbal ability in children. Neuroimage. 2008;41:813–22. doi: 10.1016/j.neuroimage.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economo C, Horn L. Ueber Windungsrelief, Maße und Rindenarchitektonik der Supratemporalfläche, ihre individuellen und ihre Seitenunterschiede. Zeitschr Neurol Psychiatr. 1930;130:678–757. [Google Scholar]
- Field A. Discovering statistics using SPSS. London: Sage Publications Limited; 2009. [Google Scholar]
- Flechsig P. Bemerkungen über die Hörsphäre des menschlichen Gehirns. Neurologisches Zentralblatt. 1908;27:2–7. [Google Scholar]
- Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–31. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–65. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–71. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Duke ES, Ritzl E, Miranda S, Liew C, et al. fMRI language dominance and FDG-PET hypometabolism. Neurology. 2011;76:1322–9. doi: 10.1212/WNL.0b013e31821527b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetrics in the brain. Science. 1978;199:852–6. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- Galuske RA, Schlote W, Bratzke H, Singer W. Interhemispheric asymmetries of the modular structure in human temporal cortex. Science. 2000;289:1946–9. doi: 10.1126/science.289.5486.1946. [DOI] [PubMed] [Google Scholar]
- Geschwind N. The organization of language and the brain. Science. 1970;170:940–4. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–7. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–53. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, et al. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 2012;22:13–25. doi: 10.1093/cercor/bhr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AS, Cross JH, Shinnar S, Mathern BW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–55. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- Hickok G, Okada K, Serences JT. Area Spt in the human planum temporale supports sensory-motor integration for speech processing. J Neurophysiol. 2009;101:2725–32. doi: 10.1152/jn.91099.2008. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hill J, Dierker D, Neil J, Inder T, Knutsen A, Harwell J, et al. A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J Neurosci. 2010;30:2268–76. doi: 10.1523/JNEUROSCI.4682-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs E, Christie D, Vargha-Khadem F, Mishkin M. Effects of hemispheric side of injury, age at injury, and presence of seizure disorder on functional ear and hand asymmetries in hemiplegic children. Neuropsychologia. 1996;34:127–37. doi: 10.1016/0028-3932(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–7. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Jayakar P, Bernal B, Santiago Medina L, Altman N. False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology. 2002;58:490–2. doi: 10.1212/wnl.58.3.490. [DOI] [PubMed] [Google Scholar]
- Josse G, Kherif F, Flandin G, Seghier ML, Price CJ. Predicting language lateralization from gray matter. J Neurosci. 2009;29:13516–23. doi: 10.1523/JNEUROSCI.1680-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SS, Roberts N, Garcia-Finana M, Mohammadi S, Ringelstein EB, Knecht S, et al. Can the language-dominant hemisphere be predicted by brain anatomy? J Cogn Neurosci. 2011;23:2013–29. doi: 10.1162/jocn.2010.21563. [DOI] [PubMed] [Google Scholar]
- Knecht S. Does language lateralization depend on the hippocampus? Brain. 2004;127:1217–8. doi: 10.1093/brain/awh202. [DOI] [PubMed] [Google Scholar]
- Korman B, Bernal B, Duchowny M, Jayakar P, Altman N, Garaycoa G, et al. Atypical propositional language organization in prenatal and early-acquired temporal lobe lesions. J Child Neurol. 2010;25:985–93. doi: 10.1177/0883073809357242. [DOI] [PubMed] [Google Scholar]
- Labudda K, Mertens M, Janszky J, Bien CG, Woermann FG. Atypical language lateralisation associated with right fronto-temporal grey matter increases—a combined fMRI and VBM study in left-sided mesial temporal lobe epilepsy patients. Neuroimage. 2012;59:728–37. doi: 10.1016/j.neuroimage.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–33. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Baldeweg T, Vargha-Khadem F. Speaking with a single cerebral hemisphere: fMRI language organization after hemispherectomy in childhood. Brain Lang. 2008;106:195–203. doi: 10.1016/j.bandl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–36. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17:1861–7. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Cross JH, Gadian DG, Connelly A. Role of fMRI in the decision-making process: epilepsy surgery for children. J Magn Reson Imaging. 2006;23:933–40. doi: 10.1002/jmri.20586. [DOI] [PubMed] [Google Scholar]
- Lillywhite LM, Saling MM, Harvey AS, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009;50:2276–84. doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- Lüders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, et al. Semiological seizure classification. Epilepsia. 1998;39:1006–13. doi: 10.1111/j.1528-1157.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132(Pt 2):7–56. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. The frontal lobe syndrome, the aphasias and related conditions. A contribution to the history of cortical localization. Brain. 1974;97:565–600. doi: 10.1093/brain/97.1.565. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, et al. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–58. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Monjauze C, Broadbent H, Boyd SG, Neville BG, Baldeweg T. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia. 2011;52:e79–83. doi: 10.1111/j.1528-1167.2011.03105.x. [DOI] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51:1445–52. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Chong WK, Wyatt JS, Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann Neurol. 2011;69:702–11. doi: 10.1002/ana.22263. [DOI] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Tournier JD, Croft LJ, Johns PN, Chong WK, et al. Interhemispheric temporal lobe connectivity predicts language impairment in adolescents born preterm. Brain. 2012;135:3781–98. doi: 10.1093/brain/aws276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YM, Koh EJ. Language lateralization in patients with temporal lobe epilepsy: a comparison between volumetric analysis and the Wada test. J Korean Neurosurg Soc. 2009;45:329–35. doi: 10.3340/jkns.2009.45.6.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Preis S, Jancke L, Schmitz-Hillebrecht J, Steinmetz H. Child age and planum temporale asymmetry. Brain Cogn. 1999;40:441–52. doi: 10.1006/brcg.1998.1072. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–47. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–43. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Ratcliff G, Dila C, Taylor L, Milner B. The morphological asymmetry of the hemispheres and cerebral dominance for speech: a possible relationship. Brain Lang. 1980;11:87–98. doi: 10.1016/0093-934x(80)90112-1. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, et al. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72:1830–6. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens AB, Mahowald MW, Hutton JT. Asymmetry of the lateral (sylvian) fissures in man. Neurology. 1976;26:620–4. doi: 10.1212/wnl.26.7.620. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain Cogn. 1985;4:27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Rademacher J, Jaencke L, Huang Y, Thron A, Zilles K. Total surface of temporoparietal intrasylvian cortex: diverging left-right asymmetries. Brain Lang. 1990;39:357–72. doi: 10.1016/0093-934x(90)90145-7. [DOI] [PubMed] [Google Scholar]
- Steinmetz H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci Biobehav Rev. 1996;20:587–591. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev Neurosci. 2006;7:655–62. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Sveller C, Briellmann RS, Saling MM, Lillywhite L, Abbott DF, Masterton RA, et al. Relationship between language lateralization and handedness in left-hemispheric partial epilepsy. Neurology. 2006;67:1813–7. doi: 10.1212/01.wnl.0000244465.74707.42. [DOI] [PubMed] [Google Scholar]
- Teki S, Barnes GR, Penny WD, Iverson P, Woodhead ZVJ, Griffiths TD, et al. The right hemisphere supports but does not replace left hemisphere auditory function in patients with persisting aphasia. Brain. 2013;136(Pt 6):1901–12. doi: 10.1093/brain/awt087. doi:10.1093/brain/awt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage. 2005;24:841–51. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Nkanga-Ngila B, Mazoyer B. Left planum temporale surface correlates with functional dominance during story listening. Neuroreport. 1998;9:829–33. doi: 10.1097/00001756-199803300-00012. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Isaacs E, Watkins K, Mishkin M. Ontogenetic specialisation of hemispheric function. In: Oxbury JM, Polkey CE, Duchowny MS, editors. Intactable focal epilepsy. London: W.B Suanders; 2000. [Google Scholar]
- Vlooswijk MC, Jansen JF, de Krom MC, Majoie HM, Hofman PA, Backes WH, et al. Functional MRI in chronic epilepsy: associations with cognitive impairment. Lancet Neurol. 2010;9:1018–27. doi: 10.1016/S1474-4422(10)70180-0. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, et al. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–51. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV. Cerebral dominance for language function in adults with specific language impairment or autism. Brain. 2008;131:3193–200. doi: 10.1093/brain/awn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–30. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke’s area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–6. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19:1095–8. [PMC free article] [PubMed] [Google Scholar]
- Zheng ZZ. The functional specialization of the planum temporale. J Neurophysiol. 2009;102:3079–81. doi: 10.1152/jn.00434.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


