Abstract
During a normal course of pregnancy, uterine vascular tone is significantly decreased resulting in a striking increase in uterine blood flow, which is essential for fetal development and fetal growth. Chronic hypoxia during gestation may adversely affect the normal adaptation of uterine vascular tone and increase the risk of preeclampsia and fetal intrauterine growth restriction. In this review, we present evidence that the regulation of K+ channels is an important mechanism in the adaptation of uterine vascular tone to pregnancy and hypoxia. There are four types of K+ channels identified in arterial smooth muscle cells: 1) voltage-dependent K+ (Kv) channels, 2) Ca2+-activated K+ (KCa) channels, 3) inward rectifier K+ (KIR) channels, and 4) ATP-sensitive K+ (KATP) channels. Pregnancy differentially augments the expression and activity of K+ channels via downregulation of protein kinase C signaling in uterine and other vascular beds, leading to decreased uterine vascular tone and increased uterine blood flow. Sex steroid hormones play an important role in the pregnancy-mediated alteration of K+ channels in the uterine vasculature. In addition, chronic hypoxia alters uterine vascular K+ channels expression and activities via modulation of steroid hormones/receptors-mediated signaling, resulting in increased uterine vascular tone during pregnancy.
Keywords: Uterine artery, pregnancy, hypoxia, potassium channels, preeclampsia
1. INTRODUCTION
Pregnancy is associated with decreased uterine vascular tone and a significant increase in uterine blood flow that optimizes the delivery of nutrients and oxygen to the developing fetus. The adaptation of the uterine circulation to pregnancy is complex and is mediated, at least in part by enhanced vasodilation, decreased vascular tone and vascular remodeling. Although the mechanisms contributing to the profound decrease in uterine vascular tone and significant rise in uterine blood flow during pregnancy are not completely understood, increasing evidence suggests that the regulation of K+ channels may play a key role in the adaptations of uterine circulation to pregnancy [1–5]. The K+ channels are the most important ion channels expressed in the plasma membrane of arterial smooth muscle cells and play a key role in the regulation of vascular tone and blood pressure [2, 3]. Stimulation of K+ channels increases K+ efflux in arterial smooth muscle cells, leading to membrane potential hyperpolarization and closure of voltage-dependent Ca2+ channels, which causes vasodilation and decreases in vascular tone. In contrast, inhibition of K+ channels will cause membrane potential depolarization, decrease K+ efflux and enhance voltage-dependent Ca2+ channels activities in arterial smooth muscle cells, resulting in vasoconstriction and increased vascular tone. Therefore, the K+ channels activity is directly linked to contractile tone of vascular smooth muscle, and factors that regulate the activity of K+ channels have major effects on vascular tone and blood flow [2, 6–9].
There are four types of K+ channels that have been identified in arterial smooth muscle: 1) voltage-dependent K+ (Kv) channels, 2) Ca2+-activated K+ (KCa) channels, 3) inward rectifier K+ (KIR) channels, and 4) ATP-sensitive K+ (KATP) channels [2, 10]. In this review, we first present a brief summary of the fundamental physiological role and properties of these four K+ channels in arterial smooth muscle, followed by the discussion of the role and properties of K+ channels in uterine vascular smooth muscle and their adaptations to pregnancy. In addition, since hypoxia is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply and the arterial oxygen concentrations are under the normal physiologic range, short-term (acute) hypoxia or long-term (chronic) hypoxia exposure may result in cardiovascular dysfunction. Indeed, it has been demonstrated that chronic hypoxia during pregnancy is one of the most common insults to the maternal cardiovascular system and fetal development associated with increased uterine vascular tone and heightened risk of preeclampsia [1, 5, 6, 11–16]. Thus, we present the evidence that modulation of K+ channels activity in uterine vasculature may be one of the important mechanisms underlying chronic hypoxia-mediated uterine vascular dysfunction during pregnancy.
2. KATP CHANNELS
2.1. Physiological Roles and Properties of KATP Channels in Vasculature
The ATP-sensitive potassium (KATP) channels were first identified in cardiac muscle [17, 18]. Up to now, they are found to be expressed in most excitable tissues including vasculature [19–21]. KATP channel is composed of at least two subunits: an inwardly rectifying K+ channel six family (Kir6.x) that forms the ion conductive pore and a regulatory sulfonylurea receptor subunit (SUR) that accounts for several pharmacological properties [10, 22, 23]. Different combinations of Kir6.x and SUR.x isoforms/variants produce tissue-specific KATP channel subtypes with different features and distinct functional properties. Two types of KATP channels have been cloned and identified in smooth muscle cell, namely Kir6.2-SUR2B channels [24] and Kir6.1-SUR2B channels [25].
Intracellular ATP acts on Kir6.x to inhibit channel activity, while ADP stimulates channel activity through SUR. Changes in the cytosolic [ATP] to [ADP] ratio thus determine the channel activity. Therefore, it is thought that KATP channels provide a link between cell metabolism and membrane excitability, and play an important role in metabolic regulation of blood flow. In addition, KATP channels may be also active in the resting state and play a role in the maintenance of basal tone in certain vascular beds [26]. Furthermore, KATP channels appear to be the target of a number of vasodilators and vasoconstrictors [27]. Several vasodilators, such as adenosine, prostacyclin, β-agonists enhance KATP channels activities via activating cAMP and protein kinase A (PKA) signaling, resulting in membrane hyperpolarization and vasodilation. In contrast, vasoconstrictors, such as angiotensin II, endothelin-1, serotonin, noradrenaline, α-agonists or neuropeptide decrease the activity of KATP channels via activating protein kinase C (PKC) pathways, causing vascular smooth muscle cell membrane depolarization and contraction [17, 28].
2.2. KATP Channels Blockers
The anti-diabetic sulfonylurea agents, such as glibenclamide, tolbutamide and tolazamide have been developed as KATP channels blockers. Sulfonylurea derivatives have been used since the 1950s for the treatment of noninsulin-dependent diabetes mellitus, in which they stimulate insulin secretion by inhibiting KATP channel activity in pancreatic β cells. Their potency to inhibit KATP channels in smooth muscle cells has been shown subsequently [29, 30]. Based on their property, suifonylureas, in particular the most potent representative agent glibenclamide, have been widely used in many experiments to show the role of KATP channels involved in vascular patho-physiologic functions [29].
2.3. KATP Channels Openers
KATP channels openers are a diverse group of pharmacologic agents that have the ability to increase cellular K+ efflux and induce hyperpolarization of the smooth muscle cell membrane leading to smooth muscle relaxation. The most known representatives of this class of drugs are diazoxide, cromakalim, bimakalim, pinacidil, aprikalim, nicorandil, and minoxidil sulfate [17]. The ability of these compounds to open KATP channels is cell type dependent. However, these compounds have the greatest potency in smooth muscle. Cromakalim is the most widely used type of vasodilators in vascular smooth muscle [29, 30]. Studies in isolated arteries have demonstrated that these compounds directly activate KATP channels and induce vasodilation, which can be blocked by glibenclamide or other KATP channels inhibitors but not by other K+ channels blockers [19, 31]. These studies suggest that KATP channels are the only target for these KATP channels openers. The exact mechanism of action of the KATP channels openers is not fully understood, however, evidence exists suggesting that they act on the KATP channels by decreasing the sensitivity of KATP channels to intracellular ATP [26, 32, 33].
2.4. Role of KATP Channels in Uterine Vascular Adaptation to Pregnancy
Adaptation of KATP channels in vascular smooth muscle may contribute to the hemodynamic changes associated with normal pregnancy. In a guinea pig animal model, it has been demonstrated that KATP channels play an important role in the regulation of vascular resistance and the activity of KATP channels is vascular beds- and pregnancy-dependent [5]. In this study, it has reported that systemic vascular resistance (SVR) is lower and the SVR response to angiotensin II is diminished in the pregnant compared with the nonpregnant guinea pigs. After treatment with a KATP channels blocker, glibenclamide, the SVR response to angiotensin II was not difference between the pregnant and nonpregnant groups, suggesting that the lower SVR and response to angiotensin II is due to heightened KATP channels activity. Furthermore, pregnancy selectively increased the KATP channels activity in uterine, renal, coronary, and cerebral vascular beds and in the uteroplacental vasculature during angiotensin II infusion [5]. Thus, the pregnancy-induced stimulation of KATP channels activity is likely to be important for the regulation of vascular resistance and maintenance of blood flow in these vascular beds. However, other studies have demonstrated that glibenclamide has no significant effect on phenylphrine-induced contractions in ovine uterine arteries [1], which is in agreement with previous studies that glibenclamide did not affect the basal tone in renal, cerebral and pulmonary arteries [9, 35, 36]. This suggests that the role of KATP channel in regulating baseline vascular tone may be tissues and/or animal species-dependent. Although the baseline vascular tone is not affected by KATP channel, the findings that the KATP channels opener diazoxide caused a significant vasodilation and attenuated phenylphrine-induced contractions suggests that a functional role of KATP channels in the ovine uterine artery [1]. Similar studies have demonstrated that KATP channels are not involved in regulating basal tone in cerebral artery, but KATP channels play a role in vasodialtion caused by changes in metabolic state and by endogenous substances or synthetic KATP channels openers [2, 37]. In consistent with previous studies showing that pregnancy enhances KATP channels activity in guinea pig uterine artery [5], it has been shown that diazoxide-induced relaxations are significantly enhanced in pregnant as compared with that in nonpregnant uterine arteries [1]. These findings further suggest that pregnancy may up-regulate KATP channels activity, resulting in decreased uterine vascular resistance, which may contribute to the increased uterine blood flow during the course of pregnancy.
2.5. Role of KATP Channels in Uterine Vascular Adaptation to Hypoxia
Not only do KATP channels play an important role in regulating vascular contractility in physiologic condition but they also play a key role in vascular dysfunction in various pathophysiological conditions including hypertension, diabetes, ischemia and hypoxia. It is well known that oxygen is a vasoactive substance in the peripheral circulation. Higher oxygen may cause vasoconstriction, whereas hypoxia induces vasodilation. Although the exact mechanisms underlying oxygen-mediated vasoreactivity are not fully understood, increasing evidence suggests that KATP channels may be involved in hypoxia-induced vasodilation [2, 38–40]. Acute hypoxia activates KATP channels either by acting directly on arterial smooth muscle cells or by inducing release of vasodilator metabolites, which in turn activate KATP channels via receptor-coupled signaling [41–43]. KATP channels activities are mainly regulated by intracellular ATP and ADP levels. Thus, hypoxia-mediated vasoreactivity may occur as a consequence of hypoxia-mediated changes of intracellular nucleotide levels in vasculature. Hypoxia can cause a reduction in intracellular ATP or elevation in intracellular ADP leading to vasodilation, which was inhibited by KATP channels blocker glibenclamide in cerebral arteries, coronary, renal and skeletal muscle circulations [2, 38–40]. These observations suggest a key role of KATP channels in hypoxia-mediate vasodilation.
Although the role of KATP channels in hypoxia-mediated vasodilation is well established in many vasculatures including coronary, cerebral, renal, and skeletal muscle circulation, a lack of effect of hypoxia on KATP channels activities has also been reported in rat cremaster arteries [44]. Furthermore, a study has demonstrated that KATP channels are not activated during hypoxia via changes in cell metabolism in rat femoral artery, but that hypoxia-mediated vasodilation is regulated by changes in intracellular Ca2+ concentration [Ca2+]i through modulation of calcium channel activity [45]. The different roles of KATP channels in hypoxia-mediated vasoreactivity may be due to differences in the species, vascular beds, or experimental conditions. In addition, the role of KATP channels is also dependent on the extent and duration of hypoxia. For example, in pulmonary arteries KATP channels are not involved in regulating vascular tone in either normoxia or moderate hypoxia. However, KATP channels play an important role in pulmonary vasoreactivity in sustained and severe pulmonary hypoxia [36]. Furthermore, not only does hypoxia induce vasodilation in many vascular beds, but it also can cause vasoconstriction [46], and KATP channels may also play an important role in hypoxia-mediated vasoconstriction. Indeed, recent studies in high-altitude sheep model have demonstrated that chronic hypoxia selectively enhances uterine vascular tone in pregnant but not nonpregnant sheep [47–49]. Further studies indicated that chronic hypoxia decreased KATP channels opener diazoxide-induced relaxation in pregnant uterine arteries and eliminated pregnancy-mediated response [1]. These observations suggest that chronic hypoxia-mediated enhanced uterine vascular tone may be attributed, in part, to a decrease in KATP channels activities.
2.6. Regulation of KATP Channels by PKC
There is growing body of studies suggest that PKC may play an important role in regulating KATP channels. Inhibition of KATP channels following exposure to vasoconstrictor agonists has been reported in many vascular beds [2, 17]. A modulation of vascular KATP channels by vasoconstrictors is through the activation of PKC signaling [2, 17]. Studies in guinea pig urinary bladder smooth muscle demonstrated a role of PKC in the inhibition of KATP channels by muscarinic receptor agonists [50]. Similar findings have shown that phorbol ester, a PKC activator, inhibits KATP channels currents in mesenteric arteries [51] and insulin secreting cells [52]. Furthermore, many studies suggest that vasoconstrictor hormones and neurotransmitters may inhibit KATP channels function via the activation PKC in vascular smooth muscle [27, 53, 54]. In the recent studies, it has been demonstrated that pregnancy down-regulates the PKC activity associated with an increased KATP channels activity in uterine arteries, whereas chronic hypoxia selectively enhances the PKC activity associated with a decreased KATP channels activity in pregnant but not nonpregnant uterine arteries [1, 47–49]. These observations suggest that pregnancy and chronic hypoxia-mediated changes of KATP channels activities are regulated through PKC-mediated signaling pathway in uterine arteries.
Although the molecular basis for PKC to regulate KATP channels in vasculature is not well established, a number of studies have shown that the KATP channel composed of Kir6.1/SUR2B is inhibited by PKC [28, 54, 55]. In addition, trafficking studies have revealed that PKC initiates internalization of the channel complex leading to the decreased channel activity [56]. Furthermore, PKC-mediated phosphorylation of the channels is also an important mechanism by which the activity of KATP channels can be modulated, which leads to an alteration in channel properties by modifying kinetics and/or the number of channels at the cell membrane [57, 58].
3. BKCA CHANNELS
3.1. Physiological Roles and Properties of BKCa Channels in Vasculature
Large-conductance (200~250 pS), Ca2+-activated K+ channels are activated both by changes in intracellular Ca2+ concentration and membrane depolarization. The channels have a high single-channel conductance, thus it is also called as “big” KCa channels (BKCa channels) [20]. BKCa channels are comprised of a pore formed by four α-subunits and four regulatory β-subunits. The α-subunit has seven transmembrane domains (S0-S6) [20, 21, 59]. In addition, the BKCa channels have four β-subunit isoforms (β1, β2, β3, β4), each with two transmembrane domains. It has been shown that the β1-subunit predominates in vascular smooth muscle [60, 61]. The major role of β1-subunit is to enhance the apparent Ca2+ sensitivity of the channel [62–65]. BKCa channels, a tetramer of α-subunits, associate with auxiliary β-subunits in a tissue-specific manner, modifying the channel’s gating properties.
BKCa channels play an important physiologic role in regulating vascular smooth muscle contractility and blood pressure [60, 61, 66]. Studies in BK β1-subunit knockout mice have demonstrated that Ca2+ spark-induced BK current is significantly reduced and the mean arterial blood pressure is elevated in the β1-subunit-null mice, leading to left ventricular hypertrophy [67]. In addition, BKCa channels also play a key role in the regulation of myogenic tone. Increased blood pressure induces membrane depolarization and increases [Ca2+]i leading to the activation of BKCa channels [2]. Activation of BKCa channels in turn enhances K+ efflux and counteracts depolarization and constriction-induced by pressure or vasoconstrictors.
3.2. BKCa Channels Blockers and Openers
BKCa channels are very effectively blocked by the scorpion peptide toxin charybdotoxin (ChTX), the related peptide iberiotoxin (IbTX) and slotoxin [68, 69]. These blockers bind to the outer vestibule of the channel to physically occlude the pore and prevent ion conduction. Several tremorgenic indole alkaloids molecules such as paxilline, penitrem A and verruculogen are also potent blockers of BKCa channels. In addition, tetraethylammonium (TEA) is a broad-spectrum K+ channel blocker. However, low concentrations of TEA (≤ 1 mM) can selectively block the BKCa channels.
BKCa channels openers comprise a large series of synthetic benzimidazolone derivatives such as NS004 and NS1619, biaryl amines, biarylureas, pyridyl amines, 3-aryloxindoles, benzopyrans, dihydropyridines, and natural modulators such as dihydrosoyasaponin-1 (DHS-1) and flavonoids. Both NS004 and NS1619 are known as α-subunit-selective BK openers. NS1619 is the only compound without any effects on other ion channels. Other than benzimidazolone derivatives, a wide structural diversity of drugs such as carbonic anhydrase inhibitors has also been shown BK activation properties. In addition, various drugs such as niflumic, flufenamic, and mefenamic acids, as well as 17-β estradiol, can activate BK channels in a nonselective manner [2, 70–74].
3.3. Role of BKCa Channels in Uterine Vascular Adaptation to Pregnancy
Both α and β1-subunits of BKCa channels are expressed exclusively in ovine uterine arterial smooth muscle cells with no evidence of their existence in the endothelium [4, 7, 75]. Recent studies have shown a pregnancy-related modification of BKCa channels gene expression patterns in uterine vasculature [4, 13, 76, 77]. Three α-subunit species were found in uterine arterial smooth muscle of nonpregnant sheep with 83, 100, and 105 kDa. During pregnancy, there was an absence of the 83-kda protein and a marked decrease in the 105-kDa protein, both reappearing ≥30 days after delivery. The 100-kDa α-subunit rises during pregnancy, but it does not appear to equal the fall in the other two species, suggesting that total channel density may actually fall in pregnancy [4]. Other studies showed that the α-subunit of 100 kDa was not significantly different in uterine arteries between nonpregnant and pregnant sheep [6]. One possible reason for this apparent difference may be because of the different sizes of the vessels used [4, 6]. The BKCa β2-subunits are present in ovine uterine arterial smooth muscle cells, but the levels are low and unchanged throughout the reproductive cycle. However, the β1-subunit expression is increased in pregnant uterine arteries as compared with nonpregnant vessels [4, 6]. The increased β1-subunit expression during pregnancy parallels the rise in uterine blood flow [4, 76, 77]. Electrophysiological studies demonstrated a greater whole-cell K+ current density in pregnant, as compared with nonpregnant, uterine arteries. Both of the tetraethylammonium (TEA) and iberiotoxin inhibit K+ currents to the same extent in uterine arterial myocytes. This suggests that the BKCa channel current density is significantly increased in uterine arteries of pregnant animals [6]. Upregulation of β1-subunit expression during pregnancy is likely to enhance the Ca2+ sensitivity of the BKCa channels and facilitate the activation of the channel and the consequent reduction in uterine vascular tone in pregnancy. Indeed, previous studies have demonstrated that intra-arterial infusion of TEA into the uterine artery circulation of late-gestation sheep causes a decrease of basal uterine blood flow from 50% to 80% in the absence of systemic effects [13, 77]. This is consistent with the recent findings that TEA inhibited K+ currents by 53% in pregnant uterine arteries, and TEA significantly increased pressure-dependent vascular tone in ovine pregnant uterine arteries and eliminated the difference of the myogenic response between nonpregnant and pregnant uterine arteries [6]. These observations suggest that the heightened BKCa channels activity is one of important mechanisms in regulating uterine vascular tone and maintaining uteroplacental blood flow in pregnancy.
3.4. Role of BKCa Channels in Uterine Vascular Adaptation to Hypoxia
In many vascular beds, hypoxia causes local vasodilation. This response increases blood flow to the affected organ and thus promotes restoration of tissue oxygenation. Numerous studies suggest that the hypoxia-induced vasodilation and blunted vasoconstriction are associated with an increased BKCa channels expression and/or their activities in the vasculatures [9, 78–81]. In the lung, hypoxia causes local vasoconstriction. Paradoxically, the hypoxia-induced pulmonary hypertension is also associated with an increased expression of BKCa channels [82, 83], which might suggest an adaptive mechanism counteracting pulmonary hypertension since BKCa channels activation serves as a feedback modulator of vascular tone when cytoplasmic calcium becomes elevated [2]. In the uteroplacental circulation, hypoxia-induced fetoplacental vascular constriction has been well demonstrated [84]. The hypoxia-induced fetoplacental vascular constriction is largely mediated by hypoxic inhibition of Kv channels rather than its effect on BKCa channels in smooth muscle of small fetoplacental arteries [85]. In pregnant sheep, chronic hypoxia enhances uterine vascular tone [47]. Although the mechanisms underlying chronic hypoxia-mediated elevation of uterine vascular tone in pregnant animals are not completely understood, the reduction of uterine vascular BKCa channels activities is a possible mechanism, given a key role of BKCa channels in the regulation of uterine vascular tone during normal course of pregnancy [6].
3.5. Regulation of BKCa Channels by Sex Steroid Hormones
Pregnancy is a state with substantially higher levels of estrogen and progesterone as compared with the nonpregnant state. Growing evidence suggests that the increased levels of sex steroid hormones may regulate uterine vascular tone and uterine blood flow via alteration of BKCa channels-mediated signaling [4, 6, 76, 77, 86–88]. In ovariectomized sheep or mice, the estrogen treatment enhanced β1-subunit mRNA and protein expression in uterine arteries and myometrial smooth muscle, which suggests a possible role of the steroid hormone in modulating BKCa channels expression [7, 89]. Indeed, the direct treatment of uterine arteries from nonpregnant animals with estrogen and progesterone for 48 hours ex vivo significantly enhanced β1-subunit protein expression in uterine arterial smooth muscle [6]. The expression of β1 subunit was also found higher in the follicular phase as compared with the luteal phase of the ovarian cycle in nonpregnant sheep, probably because of relatively high estrogen levels that were produced endogenously by the ovaries [75, 90]. Furthermore, TEA had no significant effects on basal uterine vascular resistance and blood flow, but produced a dose-dependent inhibition of the estradiol-17β (E2β)-induced rise in uterine blood flow when infused into the uterine arterial circulation of ovariectomized nonpregnant ewes [91]. In addition, E2β-mediated uterine vasodilation is also associated with BKCa channels activation [4, 76, 77, 88, 91–95]. These observations suggest that the regulation of uterine vascular tone by BKCa channels is modulated by sex steroids.
As compared with estrogen, the effect of progesterone in the regulation of BKCa channels is less well established. In contrast to estrogen, progesterone inhibits the BKCa channel current in Xenopus oocytes [96]. The inhibitory effect of progesterone on BKCa channels may partly explain its antagonism against estrogen-mediated vasorelaxation as shown in vitro in porcine coronary arteries [97]. Given the fact that progesterone plays an important role in regulating uterine blood flow during pregnancy [92, 98], whether progesterone-mediated uterine vascular tone is regulated through modulation of BKCa channels needs to be further investigated.
3.6. Regulation of BKCa by PKC
The activation of PKC has been shown an inhibition of BKCa channels in various vascular beds [99, 100]. Studies in porcine coronary artery have demonstrated that PKC activators inhibit the BKCa channels activation by increasing in cytosolic free Ca2+ and phosphorylation of the channel protein [99, 101]. In addition, PKC-induced phsophorylation of the channel protein inhibits BKCa channels activities in smooth muscle, and decreases its sensitivity to be activated by cGMP-dependent protein kinas I or PKA [60, 101]. Recent studies have shown that the activation of PKC by PDBu significantly inhibits the whole-cell K+ current in uterine arterial myocytes. The inhibition of K+ currents by PDBu is significantly greater in the myocytes of pregnanat sheep than that in nonpregnant animals [6]. It has been further demonstrated that the PDBu-induced reduction of K+ currents is predominately mediated by inhibiting the BKCa channels. PKC plays an important role in the regulation of vascular smooth muscle contractility [102, 103]. The finding that the activation of PKC inhibited BKCa channels activity and increased pressure-dependent myogenic tone in pregnant uterine arteries provides a functional link between BKCa channels and PKC-mediated attenuation of myogenic tone of uterine arteries in pregnancy.
4. KV CHANNELS
4.1. Physiological Roles and Properties of Kv Channels in Vasculature
Voltage-dependent K+ (Kv) channels including Kv 1.5 and Kv 1.6 families have been identified in smooth muscle of most vascular beds [2, 104–109]. They have been sub-classified on the basis of their voltage dependence and pharmacology. The basic structure of Kv channels is conserved among different families. Each channel is composed of four α-subunits, themselves composed of six regions of trans-membrane hydrophobic amino acids (S1–S6). The S4 region is considered as the voltage sensor. In addition to these α-subunits, there are β-subunits that may play an important role in modulating the gating properties of the α-subunits [65, 110–112]. Kv channels are voltage dependent. Kv channels are activated in response to membrane depolarization and they are involved in action potential repolarization in electrically excitable muscle such as cardiomyocytes. In addition, the activity of Kv channels contributes to the regulation of resting membrane potential and basal vascular tone [2].
4.2. Pharmacological Blockers and Openers
Because of the ubiquitous expression of multiple classes of Kv channels in vasculatures, there is lack of selective blockers/openers. 4-Aminopyridine (4-AP) is the most selective known blockers of Kv channels in vasculatures [105, 113, 114]. The half-block concentration of 4-AP for Kv channels is in the range of 0.2 ~1.1 mM, which does not inhibit BKCa channels. Thus it has been used to separate Kv currents from BKCa currents that are also activated by membrane depolarization [2, 115–118]. In addition to 4-AP, there are some other agents that may inhibit Kv channels such as agitotoxin-2, phencyclidine, tedisamil and quinidine [2, 105, 113, 114]. At higher concentrations, TEA and glibenclamide may also inhibit Kv channels in vasculatures [2].
Kv channels are opened by membrane depolarization. In addition, these channels can be activated by cAMP-protein kinase A signaling pathway and some other vasodilators such as adenosine, PGI2 and CGRP [119, 120]. On the other hand, vasoconstrictors, including endothelin and angiotensin II, appear to close Kv channels through PKC-mediated signaling [121–123]. Recent studies have demonstrated that Rho kinase-mediated signaling pathway may close Kv channels [122]. Thus, the inhibition of Kv channels activation may contribute to vasoconstrictor-induced depolarization of arteriolar smooth muscle cells.
4.3. Role of Kv Channels in Uterine Vascular Adaptation to Pregnancy
Pressure-induced membrane depolarization is one of the important mechanisms in the development of myogenic tone in pressurized arterial beds and the extent of pressure-induced depolarization is regulated by Kv channels [2, 124, 125]. In rats, it has been demonstrated that pregnancy enhances pressure-induced myogenic tone in small uteroplacental arteries associated with an increased Ca2+ influx and enhanced smooth muscle cell depolarization [126]. Pretreatment with 4-AP in the uterine arteries from nonpregnant rats inhibited the activity of Kv channels and mimicked the effects of pregnancy by increasing pressure-induced depolarization, elevation of [Ca2+]i, and development of myogenic tone. Furthermore, Kv channels currents were also decreased in the uterine arterial myocytes isolated from pregnant rats compared with those of nonpregnant control. These observations suggest that a decrease in Kv channels activity may be a mechanism mediating the pregnancy-induced augmentation of myogenic tone in rat uetroplacental arteries.
4.4. Role of Kv Channels in Vascular Adaptation to Hypoxia
Kv channels may play an important role in hypoxia-mediated pulmonary vasoconstriction [127–129]. Since Kv channels contribute to the membrane potential in pulmonary vascular smooth muscle cells as they do in the systemic arteries, hypoxia induced pulmonary arterial depolarization may be regulated via inhibiting Kv channels. Indeed, acute hypoxia inhibits Kv channels activity in pulmonary artery smooth muscle cells and induces membrane depolarization and a rise in intracellular Ca2+ that triggers vasoconstriction. Prolonged hypoxia decreases the expression of Kv channels and reduces Kv channels currents in the vessel [10, 129]. These observations suggest that the reduction of Kv channels activity in response to acute or chronic hypoxia may contribute to the hypoxia-mediated pulmonary hypertension.
Similar to pulmonary arteries, human fetoplacental vessels also produce vasoconstriction in response to hypoxia. In human fetoplacental artery perfused at a constant flow rate [85], both hypoxia and 4-AP reversibly increased perfusion pressure in non-additive manner, suggesting they act via a common mechanism. Western blotting and RT-PCR analyses showed the expression of Kv channels in the fetoplacental vessels. In addition, patch-clamp experiments demonstrated that hypoxia reversibly inhibited Kv, but not BKCa or ATP-dependent currents, in the fetoplacental arteries smooth muscle cells. These observations suggest that human fetaplcental vessels produce vasoconstriction in response to hypoxia and this response is mainly mediated by hypoxic inhibition of Kv channels in the smooth muscle of small fetoplacental arteries [85].
5. KIR CHANNELS
5.1. Physiological Roles and Properties of KIR Channels in Vasculature
Inward rectifier K+ (KIR) channels have been found in smooth muscle of small-diameter resistance vessels such as small coronary and cerebral arteries [111, 130, 131]. KIR channels conduct K+ ions into cells from negative potentials to more positive potentials, which cause strong inward K+ currents [17]. The inward current is much larger than the outward current. KIR channels are composed of two trans-membrane regions M1 and M2 and a H5 region dipping into the membrane to line the outer part of the pore [10]. There are several KIR gene subfamilies such as KIR 2.0, KIR 2.1–4 and KIR 6.0-2. KIR 2.1 is predominately expressed in smooth muscle of small-diameter resistance vessels, rather than in larger arteries. The activity of KIR channels depends on the membrane potential and the extracellular K+ concentrations ([K+]o) [117, 132]. With a small increase in [K+]o, the channel conducts inward current at membrane potentials negative to the new Ek, but the outward current maintains the same situation [117, 132]. In contrast to Kv and BKCa channels that are activated by membrane depolarization, KIR channels are activated by membrane hyperpolarization and induce vasodilation [2]. KIR channels provide the dominant K+ conductance near the resting membrane potential and may modulate basal vascular tone.
5.2. Pharmacological Blockers and Openers
Ba2+ is a relatively selective inhibitor of KIR channels in vascular smooth muscle cells, with a dissociation constant (Kd) in the micromolar ranges (~2 μM) [117, 133]. This blockade is voltage dependent and the extent of inhibition is greater at more negative membrane potentials. Although Ba2+ can block other K+ channels, it is much less effective at blocking the function of other K+ channels expressed in vasculature. For example, the Kd for the KATP channel at −60 mV is 200 μM, and the Kd values for the BKCa and Kv channels are in the millimolar range. Thus Ba2+ is a useful tool to distinguish the KIR channels from other K+ channels [2, 133]. Both Ca2+ and Mg2+ also block KIR channels activity in vasculature at physiological concentrations. External Ca2+ and Mg2+ (5 mM) reduce the KIR channels currents by 47% and 41%, respectively, at −60 mV, in a largely voltage-independent manner. In addition, external Cs+ also blocks KIR channels in vascular smooth muscle cells with a Kd of 2.9 mM at -60 mV. The inhibition of KIR channels currents in vascular smooth muscle cells is highly voltage dependent with rapid kinetics [17, 133]. Taken together, it suggests that multiple ions can modulate the KIR channels activity. In addition, there are several agents that can activate KIR channels. For example, C-type natriuretic peptide, EDHF, adenosine, and bradykinin, which activate PKA and protein kinase G, may open vascular KIR channels [134–135].
5.3. Regulation of Vascular KIR Channels by PKC and PKA
Vascular KIR channels can be down-regulated by vasoconstrictors [53, 136]. Among other K+ channels, the regulation of KIR channels by vasoconstrictors such as ET-1 and Angiotensin II is closely related to PKC activation. Several PKC isoforms have been identified in vascular smooth muscle cells. Previous studies have shown that ET-1 and angiotensin II activate Ca+-independent PKCε to inhibit KATP and Kv currents. However, the inhibitory effects of ET-1 and angiotensin II on KIR channels activity are mediated by the PKCα activation. These observations suggest that PKC isoforms may contribute differentially to the vasoconstrictors-mediated regulations of different K+ channels activities [53, 136]. In contrast to PKC, PKA may play an important role in the activation of KIR channels in the vasculature. PKA-coupled vasodilators such as adenosine and bradykinin induce vasodilation through the activation of KIR channels in vascular smooth muscle via cAMP-PKA signaling pathway [27].
5.4. Role of KIR Channels in Vascular Adaptation to Pregnancy and Hypoxia
The KIR 6.1 channel mRNA has been readily detected by RT-PCR in human fetoplacental arteries and veins, and a protein expression band at ~ 55 kDa in the vessels has been detected by Western blot analysis [137]. This finding opens a door to further study of the functional role of KIR channels in the regulation of uteroplacental vascular tone in pregnancy. In addition, recent studies have demonstrated that functional KIR 6.1 and KIR 6.2 channels are expressed in human pregnant myometrium smooth muscle cells and the downregulation of KIR 6.1 and KIR 6.2 channels expression in the myometrium may contribute to the enhanced uterine contractility associated with the onset of labor [138–140].
Vasodilations in response to hypoxia in certain peripheral vasculatures such as cerebral and small coronary arteries are likely a protective response to increase local blood flow. One of the mechanisms underlying hypoxia-induced vasodilation is via the activation of KIR channels by hypoxia. Park et al. [53] have examined the effects of acute hypoxia on Ba2+-sensitive inward rectifier K+ (KIR) current in rabbit coronary arterial smooth muscle cells. They have demonstrated that the density of KIR current is greater in cells isolated from small-diameter coronary arteries than in cells from larger arteries. Hypoxia induces an increase in KIR current in small coronary artery smooth muscle cells. The hypoxia-induced increased in KIR currents is attenuated by the inhibition of adenylyl cyclase and PKA. In Langendorff-perfused rabbit hearts, hypoxia-induced increase in coronary blood flow is inhibited by Ba2+. These findings suggest that the hypoxia-induced coronary vasodilation, at least partly, regulated by the activation of KIR channels via cAMP- and PKA-dependent signaling pathways. In contrast to hypoxic vasodilation, hypoxia may induce vasoconstriction in certain vessels including pulmonary and fetoplacental arteries. However, there is no evidence at present to suggest a role of KIR channels in hypoxia-induced vasoconstriction. Given the finding that chronic hypoxia enhanced uterine vascular tone in pregnant sheep associated with an increase in PKC activity in uterine arteries [47], it is plausible to propose that hypoxia may inhibit KIR channels activation via PKC-dependent mechanism and the attenuation of KIR channel activation may play a role in hypoxia-mediated enhanced uterine vascular tone during pregnancy.
6. CONCLUDING REMARKS
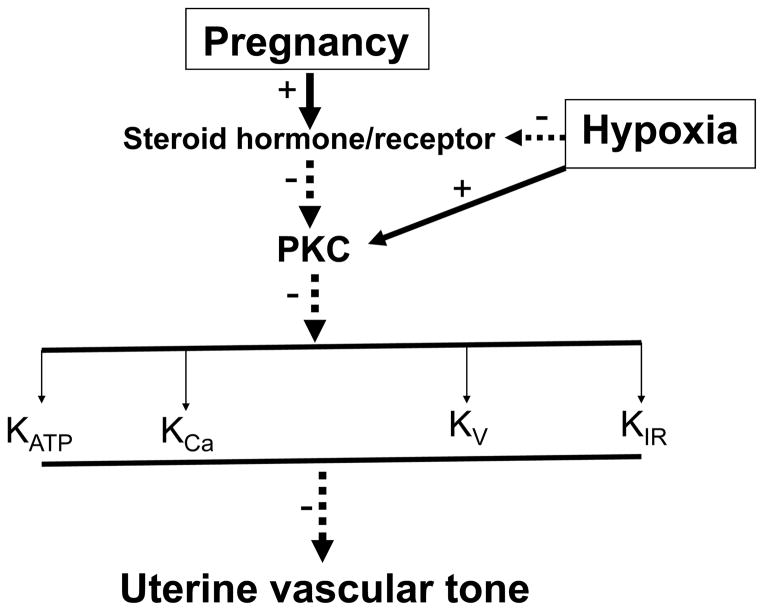
It is evident that K+ channels play an important role in the regulation of vascular tone. In general, the activation of K+ in arterial smooth muscle causes a decrease in vascular tone and an increase in blood flow via vasodilation. In contrast, the inhibition of K+ channels results in vasoconstriction. Pregnancy is associated with increased sex steroids hormones/receptors levels in uterine vasculature (Fig. 1). The increased steroid hormones/receptors differentially attenuate PKC-mediated signaling in uterine arterial smooth muscle cells [15, 142], leading to differential upregulation of K+ channels expression and/or their activities, which are likely to contribute to the decreased uterine vascular tone and increased uterine blood flow in pregnancy. Exposed to hypoxia during pregnancy attenuates the effects of sex steroid hormones/receptors, leading to enhanced PKC activation in pregnant uterine arteries. The selective inhibition of K+ channels activities by the increased PKC activation is likely to contribute significantly to the maladaptation of uterine vascular hemodynamics in pregnancy complicated by preeclampsia and fetal intrauterine growth restriction in response to hypoxia. As the knowledge of structures and properties of each K+ channels and their physiological and pathological roles in uterine vasculature continues to grow, it should become possible to develop pharmacologic therapeutic strategies targeting on K+ channels to prevent or treat uterine vascular dysfunction in pregnancy complications such as diabetes and hypertension in gestation, preeclampsia and fetal intrauterine growth restriction.
Fig. 1. The potential role of pregnancy and hypoxia in regulation of K+ channels in uterine vasculatures.
Activation of protein kinase C (PKC) results in inhibition of K+ channels activity. The increased sex steroid hormones/their receptors during pregnancy down-regulate PKC gene expression and/or activity in uterine artery smooth muscle cells, which leads to a selectively increased K+ channels expressions and activities in uterine vasculatures during pregnancy. However, chronic hypoxia during pregnancy enhances PKC activity via down-regulation of steroid hormone-mediated signaling, resulting in decreased K+ channels activities and increased uterine vascular tone.
Acknowledgments
The studies from the authors’ laboratory cited in this review were supported in part by National Institutes of Health Grants HL054094 (LZ), HL057787 (LZ), HL089012 (LZ), HL110125 (LZ), HD031226 (LZ), and DA032510 (DX). We apologize to those authors whose excellent studies covered by the scope of this review were unable to be cited due to space restriction.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
Send Orders for Reprints to reprints@benthamscience.net
References
- 1.Xiao D, Longo LD, Zhang L. Role of KATP and L-type Ca2+ channel activities in regulation of ovine uterine vascular contractility: effect of pregnancy and chronic hypoxia. Am J Obstet Gynecol. 2010;203:596, e6–12. doi: 10.1016/j.ajog.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 3.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–27. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1878–87. doi: 10.1152/ajpheart.01185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyes L, Rodman DM, Curran-Everett D, Morris K, Moore LG. Effect of K+ATP channel inhibition on total and regional vascular resistance in guinea pig pregnancy. Am J Physiol. 1998;275:H680–8. doi: 10.1152/ajpheart.1998.275.2.H680. [DOI] [PubMed] [Google Scholar]
- 6.Hu XQ, Xiao D, Zhu R, et al. Pregnancy upregulates large-conductance Ca(2+)-activated K(+) channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–9. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagar D, Liu XT, Rosenfeld CR. Estrogen regulates {beta}1-subunit expression in Ca(2+)-activated K(+) channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–27. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 8.Chang K, Lubo Z. Review article: steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci. 2008;15:336–48. doi: 10.1177/1933719108317975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long W, Zhang L, Longo LD. Fetal and adult cerebral artery K(ATP) and K(Ca) channel responses to long-term hypoxia. J Appl Physiol. 2002;92:1692–701. doi: 10.1152/japplphysiol.01110.2001. [DOI] [PubMed] [Google Scholar]
- 10.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164:549–57. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 11.Zamudio S, Palmer SK, Dahms TE, et al. Blood volume expansion, preeclampsia, and infant birth weight at high altitude. J Appl Physiol. 1993;75:1566–73. doi: 10.1152/jappl.1993.75.4.1566. [DOI] [PubMed] [Google Scholar]
- 12.Belouchi NE, Roux E, Savineau JP, Marthan R. Effect of chronic hypoxia on calcium signalling in airway smooth muscle cells. Eur Respir J. 1999;14:74–9. doi: 10.1034/j.1399-3003.1999.14a13.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld CR, Cornfield DN, Roy T. Ca(2+)-activated K(+) channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281:H422–31. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 14.Meyer MC, Brayden JE, McLaughlin MK. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol. 1993;169:1510–6. doi: 10.1016/0002-9378(93)90427-k. [DOI] [PubMed] [Google Scholar]
- 15.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290:H2337–43. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- 16.White MM, Zhang L. Effects of chronic hypoxia on maternal vasodilation and vascular reactivity in guinea pig and ovine pregnancy. High Alt Med Biol. 2003;4:157–69. doi: 10.1089/152702903322022776. [DOI] [PubMed] [Google Scholar]
- 17.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 18.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 19.Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- 20.Nelson MT. Ca(2+)-activated potassium channels and ATP-sensitive potassium channels as modulators of vascular tone. Trends Cardiovasc Med. 1993;3:54–60. doi: 10.1016/1050-1738(93)90037-7. [DOI] [PubMed] [Google Scholar]
- 21.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–3. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- 22.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6. X, KATP channels. Annu Rev Physiol. 1998;60:667–87. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617–24. doi: 10.1113/jphysiol.2006.105973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isomoto S, Kondo C, Yamada M, et al. A novel sulfonylurea receptor forms with BIR (Kir6. 2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–4. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 25.Yamada M, Isomoto S, Matsumoto S, et al. Sulphonylurea receptor 2B and Kir6. 1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–20. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamura Y, Tomoike H, Narishige T, Takahashi T, Kasuya H, Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol. 1992;263:H399–404. doi: 10.1152/ajpheart.1992.263.2.H399. [DOI] [PubMed] [Google Scholar]
- 27.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 28.Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–7. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- 29.Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–80. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 30.Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149–56. [PubMed] [Google Scholar]
- 31.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol. 1996;270:H1649–54. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- 33.Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol. 1992;262:C1220–7. doi: 10.1152/ajpcell.1992.262.5.C1220. [DOI] [PubMed] [Google Scholar]
- 34.Cadorette C, Sicotte B, Brochu M, St-Louis J. Effects of potassium channel modulators on myotropic responses of aortic rings of pregnant rats. Am J Physiol Heart Circ Physiol. 2000;278:H567–76. doi: 10.1152/ajpheart.2000.278.2.H567. [DOI] [PubMed] [Google Scholar]
- 35.Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993;265:H581–5. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- 36.Wiener CM, Dunn A, Sylvester JT. ATP-dependent K+ channels modulate vasoconstrictor responses to severe hypoxia in isolated ferret lungs. J Clin Invest. 1991;88:500–4. doi: 10.1172/JCI115331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475:9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990;247:1341–4. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- 39.Loutzenhiser RD, Parker MJ. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994;74:861–9. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- 40.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74:1005–8. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 41.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–86. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daut J, Standen NB, Nelson MT. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. J Cardiovasc Electrophysiol. 1994;5:154–81. doi: 10.1111/j.1540-8167.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 43.Sabouni MH, Hargittai PT, Lieberman EM, Mustafa SJ. Evidence for adenosine receptor-mediated hyperpolarization in coronary smooth muscle. Am J Physiol. 1989;257:H1750–2. doi: 10.1152/ajpheart.1989.257.5.H1750. [DOI] [PubMed] [Google Scholar]
- 44.Jackson WF. Hypoxia does not activate ATP-sensitive K+ channels in arteriolar muscle cells. Microcirculation. 2000;7:137–45. [PMC free article] [PubMed] [Google Scholar]
- 45.Quayle JM, Turner MR, Burrell HE, Kamishima T. Effects of hypoxia, anoxia, and metabolic inhibitors on KATP channels in rat femoral artery myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H71–80. doi: 10.1152/ajpheart.01107.2005. [DOI] [PubMed] [Google Scholar]
- 46.Rubanyi G, Paul RJ. Two distinct effects of oxygen on vascular tone in isolated porcine coronary arteries. Circ Res. 1985;56:1–10. doi: 10.1161/01.res.56.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1840–9. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao D, Bird IM, Magness RR, Longo LD, Zhang L. Upregulation of eNOS in pregnant ovine uterine arteries by chronic hypoxia. Am J Physiol Heart Circ Physiol. 2001;280:H812–20. doi: 10.1152/ajpheart.2001.280.2.H812. [DOI] [PubMed] [Google Scholar]
- 49.Xiao D, Zhang L. Calcium homeostasis and contraction of the uterine artery: effect of pregnancy and chronic hypoxia. Biol Reprod. 2004;70:1171–7. doi: 10.1095/biolreprod.103.024943. [DOI] [PubMed] [Google Scholar]
- 50.Bonev AD, Nelson MT. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am J Physiol. 1993;265:C1723–8. doi: 10.1152/ajpcell.1993.265.6.C1723. [DOI] [PubMed] [Google Scholar]
- 51.Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–23. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura J, Suda T, Ogawa Y, Takeo T, Suga S, Wakui M. Protein kinase C-dependent and -independent inhibition of Ca(2+) influx by phorbol ester in rat pancreatic beta-cells. Cell Signal. 2001;13:199–205. doi: 10.1016/s0898-6568(01)00136-x. [DOI] [PubMed] [Google Scholar]
- 53.Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H2461–7. doi: 10.1152/ajpheart.00331.2005. [DOI] [PubMed] [Google Scholar]
- 54.Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6. 1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem. 2008;283:2488–94. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorneloe KS, Maruyama Y, Malcolm AT, Light PE, Walsh MP, Cole WC. Protein kinase C modulation of recombinant ATP-sensitive K(+) channels composed of Kir6.1 and/or Kir6. 2 expressed with SUR2B. J Physiol. 2002;541:65–80. doi: 10.1113/jphysiol.2002.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manna PT, Smith AJ, Taneja TK, Howell GJ, Lippiat JD, Sivaprasadarao A. Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J Biol Chem. 2010;285:5963–73. doi: 10.1074/jbc.M109.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- 58.Light P. Regulation of ATP-sensitive potassium channels by phosphorylation. Biochim Biophys Acta. 1996;1286:65–73. doi: 10.1016/0304-4157(96)00004-4. [DOI] [PubMed] [Google Scholar]
- 59.Wallner M, Meera P, Toro L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca(2+)-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc Natl Acad Sci USA. 1996;93:14922–7. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka Y, Koike K, Toro L. MaxiK channel roles in blood vessel relaxations induced by endothelium-derived relaxing factors and their molecular mechanisms. J Smooth Muscle Res. 2004;40:125–53. doi: 10.1540/jsmr.40.125. [DOI] [PubMed] [Google Scholar]
- 62.Waldron GJ, Cole WC. Activation of vascular smooth muscle K+ channels by endothelium-derived relaxing factors. Clin Exp Pharmacol Physiol. 1999;26:180–4. doi: 10.1046/j.1440-1681.1999.03006.x. [DOI] [PubMed] [Google Scholar]
- 63.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv, cabeta) of maxi K channels. FEBS Lett. 1996;385:127–8. doi: 10.1016/0014-5793(96)83884-1. [DOI] [PubMed] [Google Scholar]
- 64.Clapp LH, Jabr RI. The BK channel: protective or detrimental in genetic hypertension? Circ Res. 2003;93:893–5. doi: 10.1161/01.RES.0000103309.55054.3E. [DOI] [PubMed] [Google Scholar]
- 65.Cox RH, Rusch NJ. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–57. doi: 10.1038/sj.mn.7800140. [DOI] [PubMed] [Google Scholar]
- 66.Korovkina VP, England SK. Detection and implications of potassium channel alterations. Vascul Pharmacol. 2002;38:3–12. doi: 10.1016/s1537-1891(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 67.Brenner R, Perez GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–6. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 68.Wallner M, Meera P, Ottolia M, et al. Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Recept Channels. 1995;3:185–99. [PubMed] [Google Scholar]
- 69.Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–8. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- 70.Hu S, Kim HS. On the mechanism of the differential effects of NS004 and NS1608 in smooth muscle cells from guinea pig bladder. Eur J Pharmacol. 1996;318:461–8. doi: 10.1016/s0014-2999(96)00776-5. [DOI] [PubMed] [Google Scholar]
- 71.Gelband GH, McCullough JR. Modulation of rabbit aortic Ca(2+)-activated K+ channels by pinacidil, cromakalim, and glibenclamide. Am J Physiol. 1993;264:C1119–27. doi: 10.1152/ajpcell.1993.264.5.C1119. [DOI] [PubMed] [Google Scholar]
- 72.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–91. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 74.Jackson WF. Potassium channels and proliferation of vascular smooth muscle cells. Circ Res. 2005;97:1211–2. doi: 10.1161/01.RES.0000196742.65848.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan LH, Rosenfeld CR, Liu XT, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab. 2010;298:E222–8. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenfeld CR. Consideration of the uteroplacental circulation in intrauterine growth. Semin Perinatol. 1984;8:42–51. [PubMed] [Google Scholar]
- 77.Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12:402–8. doi: 10.1016/j.jsgi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Gebremedhin D, Bonnet P, Greene AS, et al. Hypoxia increases the activity of Ca(2+)-sensitive K+ channels in cat cerebral arterial muscle cell membranes. Pflugers Arch. 1994;428:621–30. doi: 10.1007/BF00374586. [DOI] [PubMed] [Google Scholar]
- 79.Naik JS, Walker BR. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am J Physiol Heart Circ Physiol. 2003;285:H220–8. doi: 10.1152/ajpheart.01131.2002. [DOI] [PubMed] [Google Scholar]
- 80.Earley S, Pastuszyn A, Walker BR. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2003;285:H127–36. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- 81.Naik JS, Walker BR. Role of vascular heme oxygenase in reduced myogenic reactivity following chronic hypoxia. Microcirculation. 2006;13:81–8. doi: 10.1080/10739680500466301. [DOI] [PubMed] [Google Scholar]
- 82.Resnik E, Herron J, Fu R, Ivy DD, Cornfield DN. Oxygen tension modulates the expression of pulmonary vascular BKCa channel alpha- and beta-subunits. Am J Physiol Lung Cell Mol Physiol. 2006;290:L761–L8. doi: 10.1152/ajplung.00283.2005. [DOI] [PubMed] [Google Scholar]
- 83.Ahn YT, Kim YM, Adams E, Lyu SC, Alvira CM, Cornfield DN. Hypoxia-inducible factor-1alpha regulates KCNMB1 expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;302:L352–9. doi: 10.1152/ajplung.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hampl V, Jakoubek V. Regulation of fetoplacental vascular bed by hypoxia. Physiol Res. 2009;58:S87–93. doi: 10.33549/physiolres.931922. [DOI] [PubMed] [Google Scholar]
- 85.Hampl V, Bibova J, Stranak Z, et al. Hypoxic fetoplacental vasoconstriction in humans is mediated by potassium channel inhibition. Am J Physiol Heart Circ Physiol. 2002;283:H2440–9. doi: 10.1152/ajpheart.01033.2001. [DOI] [PubMed] [Google Scholar]
- 86.Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol. 1998;275:H731–43. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- 87.Rosenfeld CR, Morriss FH, Jr, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17beta on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol. 1976;124:618–29. doi: 10.1016/0002-9378(76)90064-8. [DOI] [PubMed] [Google Scholar]
- 88.Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989;256:E536–42. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- 89.Benkusky NA, Korovkina VP, Brainard AM, England SK. Myometrial maxi-K channel beta1 subunit modulation during pregnancy and after 17beta-estradiol stimulation. FEBS Lett. 2002;524:97–102. doi: 10.1016/s0014-5793(02)03011-9. [DOI] [PubMed] [Google Scholar]
- 90.Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001;280:H1699–705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- 91.Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279:H319–28. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- 92.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–30. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 94.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol. 1997;272:H2765–73. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 95.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–42. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 96.Wong CM, Tsang SY, Yao X, Chan FL, Huang Y. Differential effects of estrogen and progesterone on potassium channels expressed in Xenopus oocytes. Steroids. 2008;73:272–9. doi: 10.1016/j.steroids.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 97.Teoh H, Man RY. Progesterone modulates estradiol actions: acute effects at physiological concentrations. Eur J Pharmacol. 1999;378:57–62. doi: 10.1016/s0014-2999(99)00438-0. [DOI] [PubMed] [Google Scholar]
- 98.Perrot-Applanat M, Groyer-Picard MT, Garcia E, Lorenzo F, Milgrom E. Immunocytochemical demonstration of estrogen and progesterone receptors in muscle cells of uterine arteries in rabbits and humans. Endocrinology. 1988;123:1511–9. doi: 10.1210/endo-123-3-1511. [DOI] [PubMed] [Google Scholar]
- 99.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–52. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 100.Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190:263–9. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- 101.Crozatier B. Central role of PKCs in vascular smooth muscle cell ion channel regulation. J Mol Cell Cardiol. 2006;41:952–5. doi: 10.1016/j.yjmcc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Baraban JM, Gould RJ, Peroutka SJ, Snyder SH. Phorbol ester effects on neurotransmission: interaction with neurotransmitters and calcium in smooth muscle. Proc Natl Acad Sci USA. 1985;82:604–7. doi: 10.1073/pnas.82.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singer HA, Baker KM. Calcium dependence of phorbol 12,13-dibutyrate-induced force and myosin light chain phosphorylation in arterial smooth muscle. J Pharmacol Exp Ther. 1987;243:814–21. [PubMed] [Google Scholar]
- 104.Al-Uzri A, Stablein DM, RAC Posttransplant diabetes mellitus in pediatric renal transplant recipients: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Transplantation. 2001;72:1020–4. doi: 10.1097/00007890-200109270-00007. [DOI] [PubMed] [Google Scholar]
- 105.Cheong A, Dedman AM, Xu SZ, Beech DJ. K(V)alpha1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281:H1057–65. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- 106.Gelband CH, Hume JR. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res. 1992;71:745–58. doi: 10.1161/01.res.71.4.745. [DOI] [PubMed] [Google Scholar]
- 107.Bonnet P, Rusch NJ, Harder DR. Characterization of an outward K+ current in freshly dispersed cerebral arterial muscle cells. Pflugers Arch. 1991;418:292–6. doi: 10.1007/BF00370529. [DOI] [PubMed] [Google Scholar]
- 108.Ishikawa T, Hume JR, Keef KD. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993;468:379–400. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clapp LH, Gurney AM. Outward currents in rabbit pulmonary artery cells dissociated with a new technique. Exp Physiol. 1991;76:677–93. doi: 10.1113/expphysiol.1991.sp003535. [DOI] [PubMed] [Google Scholar]
- 110.Thorneloe KS, Chen TT, Kerr PM, et al. Molecular composition of 4-aminopyridine-sensitive voltage-gated K(+) channels of vascular smooth muscle. Circ Res. 2001;89:1030–7. doi: 10.1161/hh2301.100817. [DOI] [PubMed] [Google Scholar]
- 111.Korovkina VP, England SK. Molecular diversity of vascular potassium channel isoforms. Clin Exp Pharmacol Physiol. 2002;29:317–23. doi: 10.1046/j.1440-1681.2002.03651.x. [DOI] [PubMed] [Google Scholar]
- 112.Bahring R, Milligan CJ, Vardanyan V, et al. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvbeta subunits. J Biol Chem. 2001;276:22923–9. doi: 10.1074/jbc.M100483200. [DOI] [PubMed] [Google Scholar]
- 113.Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534:691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 115.Okabe K, Kitamura K, Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch. 1987;409:561–8. doi: 10.1007/BF00584654. [DOI] [PubMed] [Google Scholar]
- 116.Smirnov SV, Aaronson PI. Ca(2+)-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. J Physiol. 1992;457:431–54. doi: 10.1113/jphysiol.1992.sp019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quayle JM, McCarron JG, Brayden JE, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol. 1993;265:C1363–70. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- 118.Beech DJ, Bolton TB. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989;98:851–64. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–34. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- 120.Cole WC, Clement-Chomienne O, Aiello EA. Regulation of 4-aminopyridine-sensitive, delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem Cell Biol. 1996;74:439–47. doi: 10.1139/o96-048. [DOI] [PubMed] [Google Scholar]
- 121.Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1996;271:H109–19. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- 122.Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286:H1088–100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- 123.Clement-Chomienne O, Walsh MP, Cole WC. Angiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 1996;495:689–700. doi: 10.1113/jphysiol.1996.sp021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–5. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 125.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- 126.Telezhkin V, Goecks T, Bonev AD, Osol G, Gokina NI. Decreased function of voltage-gated potassium channels contributes to augmented myogenic tone of uterine arteries in late pregnancy. Am J Physiol Heart Circ Physiol. 2008;294:H272–84. doi: 10.1152/ajpheart.00216.2007. [DOI] [PubMed] [Google Scholar]
- 127.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–90. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 128.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol. 1993;264:L107–15. doi: 10.1152/ajplung.1993.264.2.L107. [DOI] [PubMed] [Google Scholar]
- 129.Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9. 3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–25. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol. 1996;494:715–26. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun Park W, Kyoung Son Y, Kim N, et al. The protein kinase A inhibitor, H-89, directly inhibits KATP and Kir channels in rabbit coronary arterial smooth muscle cells. Biochem Biophys Res Commun. 2006;340:1104–10. doi: 10.1016/j.bbrc.2005.12.116. [DOI] [PubMed] [Google Scholar]
- 132.Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988;404:455–66. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robertson BE, Bonev AD, Nelson MT. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+ Am J Physiol. 1996;271:H696–705. doi: 10.1152/ajpheart.1996.271.2.H696. [DOI] [PubMed] [Google Scholar]
- 134.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–31. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rivers RJ, Hein TW, Zhang C, Kuo L. Activation of barium-sensitive inward rectifier potassium channels mediates remote dilation of coronary arterioles. Circulation. 2001;104:1749–53. doi: 10.1161/hc4001.098053. [DOI] [PubMed] [Google Scholar]
- 136.Park WS, Kim N, Youm JB, et al. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341:728–35. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 137.Wareing M, Bai X, Seghier F, et al. Expression and function of potassium channels in the human placental vasculature. Am J Physiol Regul Integr Comp Physiol. 2006;291:R437–46. doi: 10.1152/ajpregu.00040.2006. [DOI] [PubMed] [Google Scholar]
- 138.Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007;18:332–9. doi: 10.1016/j.semcdb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu C, You X, Gao L, et al. Expression of ATP-sensitive potassium channels in human pregnant myometrium. Reprod Biol Endocrinol. 2011;9:35. doi: 10.1186/1477-7827-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang H, Xiao D, Longo LD, Zhang L. Regulation of α1-adrenoceptor-mediated contractions of uterine arteries by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol. 2006;291:H2282–H2289. doi: 10.1152/ajpheart.00321.2006. [DOI] [PubMed] [Google Scholar]