Abstract
BACKGROUND
A growing body of research shows that mindfulness meditation can alter neural, behavioral and biochemical processes. However, the mechanisms responsible for such clinically relevant effects remain elusive.
METHODS
Here we explored the impact of a day of intensive practice of mindfulness meditation in experienced subjects (n= 19) on the expression of circadian, chromatin modulatory and inflammatory genes in peripheral blood mononuclear cells (PBMCs). In parallel, we analyzed a control group of subjects with no meditation experience who engaged in leisure activities in the same environment (n= 21). PBMCs from all participants were obtained before (t1) and after (t2) the intervention (t2-t1= 8 hours) and gene expression was analyzed using custom pathway focused quantitative-real time PCR assays. Both groups were also presented with the Trier Social Stress Test (TSST).
RESULTS
Core clock gene expression at baseline (t1) was similar between groups and their rhythmicity was not influenced in meditators by the intensive day of practice. Similarly, we found that all the epigenetic regulatory enzymes and inflammatory genes analyzed exhibited similar basal expression levels in the two groups. In contrast, after the brief intervention we detected reduced expression of histone deacetylase genes (HDAC2, 3 and 9), alterations in global modification of histones (H4ac; H3K4me3) and decreased expression of pro-inflammatory genes (RIPK2 and COX2) in meditators compared with controls. We found that the expression of RIPK2 and HDAC2 genes was associated with a faster cortisol recovery to the TSST in both groups.
CONCLUSIONS
The regulation of HDACs and inflammatory pathways may represent some of the mechanisms underlying the therapeutic potential of mindfulness-based interventions. Our findings set the foundation for future studies to further assess meditation strategies for the treatment of chronic inflammatory conditions.
Keywords: mindfulness, meditation, epigenetics, inflammation, HDAC, stress
INTRODUCTION
Poor stress-coping contributes to the development of chronic diseases and accelerated aging (Epel et al., 2009; Juster et al., 2010; Karatsoreos and McEwen, 2011). Therefore, a growing body of scientific research is devoted to understanding the neurophysiological and cellular responses induced by methods that improve stress management. Among them, mindfulness-based meditation practices, which intentionally cultivate attentional skills, have become an increasingly popular approach, with accumulating experimental evidence of beneficial effects on psychological, neurological, endocrine and immune variables (Kabat-Zinn et al., 1998; Ludwig and Kabat-Zinn, 2008; Lutz et al., 2008; Schmidt et al., 2011; Farb et al., 2012; Rosenkranz et al., 2013). However, our molecular understanding of how they can influence a broad range of biological processes, from brain networks to the immune system, remains limited.
To date, few studies have analyzed the effects of mindfulness techniques at the cellular level. Studies in blood cells have found that the mindfulness-based stress reduction (MBSR) program reduced cytokine secretion, oxidative stress and DNA damage (Carlson et al., 2003), increased natural killer cell activity and decreased interleukin secretion in women recently diagnosed with early stage breast cancer (Witek-Janusek et al., 2008), and increased CD4+ T lymphocyte counts in HIV infected subjects (Creswell et al., 2009). Some reports have also described the molecular impact of other meditation-based interventions using blood cells; for example, RNA microarray studies suggested that the expression of genes involved in cellular metabolism and oxidative stress pathways in blood cells are modulated by body-mind relaxation response training (Dusek et al., 2008; Bhasin et al., 2013). Recent bioinformatic analyses from PBMC genome-wide microarrays have suggested that yogic meditation in family dementia caregivers decreased pro-inflammatory NFk-B signaling and increased the activity of interferon response factors (Black et al., 2013). Increased telomerase activity was detected in response to the same intervention (Lavretsky et al., 2013).
Environmental stimuli influence most body functions, including stress responsiveness and behavior, through extracellular and intracellular pathways that interact with the epigenetic machinery (Graff et al., 2011). In rodents, psychological stress during adulthood induces dynamic epigenetic events such histone acetylation and phosphorylation in the dentate gyrus as soon as 2 h after the start of exposure to a novel environment or forced swimming (Chandramohan et al., 2007; Chandramohan et al., 2008) and in the hippocampus 1 h after training using a fear conditioning paradigm (Chwang et al., 2007). Rapid epigenetic changes in response to environmental exposures such as diet and physical exercise have also been detected in human peripheral tissues (Kaliman et al., 2011; Pham and Lee, 2012). However, no data are currently available regarding the possibility of an epigenetic basis for the effects of mindfulness meditation. Here we show evidence of rapid gene expression changes in chromatin regulatory enzymes, alterations in histone modifications and downregulation of proinflammatory genes after a short intensive session of mindfulness meditation in experienced subjects. In addition, we observe relations between these changes and stress-evoked cortisol responses.
METHODS
Participants
A group of 19 long-term meditators, and a control group of 21 meditation-naïve participants with similar distributions of age, gender, race and body-mass index (S1) were studied before (0800 h) and after (1600 h.) an intensive day of mindfulness meditation or leisure time in the same environment.
Participants provided written informed consent prior to the study procedures, which were approved by the UW-Madison Health Sciences Internal Review Board. They were informed of the study requirements and screened through telephone interviews for exclusion/inclusion criteria.
The mindfulness meditation group was recruited in the US at meditation centers and through related mailing lists, in addition to flyers and advertisements in newspapers. Mindfulness meditation group participants had a historical daily meditation practice spanning a minimum of 3 years, with a minimum of 30 minutes of daily sitting meditation and had to have attended a minimum of 3 intensive retreats lasting 5 or more days. They had an average of 6240 lifetime hours of meditation practice, ranging from 1440 to 14730 total hours. All experienced meditators practiced both standard mindfulness-related meditations (e.g. Vipassana and concentration meditations) and compassion-related meditations (e.g. metta meditation) as taught in the Tibetan and Theravada Buddhist traditions.
The control group comprised individuals with no prior meditation experience who responded to a local advertisement, recruiting participants for research in a non-pharmacological intervention designed to promote well-being. Exclusion criteria included medication with CNS effects, ongoing medical problems, chronic medication, diagnosed sleep disorders, lifetime history of a mental disorder, brain damage, seizures or acute or chronic immune or inflammatory disorders. Women who were pregnant or breast feeding or who had given birth in the last six months were also excluded. Participants were asked to arrive for their first blood draw with a light breakfast that did not include caffeinated beverages.
Interventions
We designed the one-day mindfulness practice period to largely overlap with the contents of the day-long session of the Mindfulness Based Stress Reduction intervention program (MBSR) (Kabat-Zinn, 1982), a meditation program routinely used in North-America hospitals. Mindfulness meditation is a form of attentional control training in which individuals develop the ability to direct and maintain attention towards a chosen object. To this end, mindfulness practice requires skills involved in monitoring the focus of attention and in detecting distraction, disengaging attention from the source of distraction, and flexibly (re)directing and engaging attention to the intended object. In addition to this training of attention, mindfulness meditation cultivates the skill to maintain a non-judgmental, open presence to the present moment. This form of meta-awareness consists of non-reactively monitoring the content of experience from moment to moment without being carried away by thoughts, emotions, or perceptions.
The control group was engaged in intentional activities such as reading, watching documentaries or playing computer games, and walking. These activities did not include mindfulness practice nor external communication via internet or cell phones.
The one-day intervention aimed to guide meditators to cultivate an open, non-reactive present-moment awareness throughout the day toward their emotions, thoughts and feelings during various activities such as sitting, walking or eating. Both control and mindfulness meditation interventions lasted eight hours and the programs were matched in terms of physical activity. Interventions were held at the Center for Investigating Healthy Minds, University of Wisconsin—Madison simultaneously in small cohorts (total n=4/day), in which both groups were represented with their corresponding intervention (mindfulness meditation for expert meditators or leisure time for meditation-naïve participants). There was no social interaction among participants during the intervention. The intervention rooms were balanced across groups during the study. Food and drink provided during the day was the same for both groups.
The current study was not designed to parse the contribution of group (trained meditators versus naïve controls), from the contribution of the intervention (mindfulness practice versus leisure). Here we chose to combine the effect of meditation expertise with the effect of an intensive day of mindfulness meditation in order to increase our capacity to detect an overall effect and assess the feasibility of more complex studies. For the mindfulness meditation group, the morning audio consisted of a 30-minute inspirational meditation talk, followed by a 40-minute guided mindfulness meditation, and a 10-minute guided walking meditation. After the audio sessions, there was a 20-minute unguided walking meditation, a 40-minute unguided sitting mindfulness meditation and a 30-minute unguided walking meditation. Before lunch there was a 10-minute audio on mindful eating, followed by a 1 hour lunch period. The afternoon audio consisted of a 15-minute inspirational meditation talk, followed by four 40-minute meditation sessions alternating between walking and sitting meditations. The audios were teaching excerpts from Joseph Goldstein, a well-known Vipassana teacher (Goldstein, 1993).
Isolation of peripheral blood mononuclear cells (PBMC)
Blood samples (24 ml) were obtained on arrival (8 am, t1) and at the end of the session (4 pm, t2) from each participant. Immediately after each blood extraction, PBMC were isolated using the Ficoll-Paque-plus method according to the manufacturer’s instructions (Sigma, St Louis, MO). Briefly, blood was diluted with an equal volume of PBS, overlaid on Ficoll-Paque-plus and centrifuged. PBMC were recovered from the interface and contaminant erythrocytes were lysed using ACK lysing buffer (Lonza BioWhittaker) and centrifuged. PBMC counts were performed for each participant and time using the Countess® Automated Cell Counter (Invitrogen) and viability was determined through Trypan blue staining. Cell counts were similar between control and meditation group (meditation group mean: 2.66·107 (t1) and 2.63·107 (t2); control group: 2.28·107 (t1) and 2.95·107 (t2)) as well as the percentage of cell viability (meditation group mean: 96%(t1) and 96% (t2); control group: 95% (t1) and 96% (t2)).
Cell pellets were resuspended in PBS and aliquoted for immediate processing for RNA and protein isolation, before storage at −80°C.
Preparation of protein fractions
Cytosolic and acidic histone fractions from PBMC were obtained as described by Fischer et al. (Fischer et al., 2007). Briefly, cell pellets for immunoblotting were lysed in a buffer containing 50 mM Tris HCl, 150 mM NACl, 2 mM EDTA, protease inhibitor cocktail (Pierce) and 1% Triton-X100 at 4 °C for 15 min and then centrifuged 10 min at 400g. Supernatants containing the cytosolic fractions were immediately frozen at −80°C. The remaining pellets were washed in lysing buffer and dissolved in the same buffer supplemented with 0.2 M HCl, incubated on ice for 30 min and then centrifuged for 10 min at 9,300g. The supernatants containing the histone fractions were immediately frozen at −80°C.
Western blots
Cytosolic proteins (50 μg) or histones (5 μg) were electrophoretically fractionated on 12% bis-Tris polyacrylamide gels and transferred to a 0.45 μm nitrocellulose membrane. Membranes were blocked for 1 h with 5% BSA in PBS and incubated overnight at 4°C with the specific primary antibodies (1:1000, Millipore). Membranes were washed and incubated with peroxidase-labeled secondary antibodies at room temperature for 1 h. Immunoreactive bands were detected by autoradiography. Specific bands from Western blot were quantified by scanning densitometry using Quantity One® 1-D analysis 4.6.3. software (Bio-Rad USA, Life Science Research, Hercules, CA). The same number of cells were lysed in all samples and protease inhibitors were added in the lysing buffer, however, we did not obtain enough protein to perform Western blots in some samples (seven controls and five meditators). Protein levels were corrected by total histone or β-actin (for histone modifications or COX2, respectively) and results were expressed as percentage of basal levels for each group.
Total RNA extraction
Cell pellets for RNA extraction were conserved in RNA Later (Sigma St Louis, MO) overnight at 4°C and thereafter at −80°C until processed. Total RNA was extracted using mirVana™ RNA Isolation Kit (Applied Biosystems) following the manufacturer’s instructions. The yield, purity and quality of RNA were determined spectrophotometrically (NanoDrop, USA) and using the Bioanalyzer 2100 capillary electrophoresis, obtaining RNAs with 260/280 ratios and RIN higher than 1.9 and 7.5 respectively.
Recovery from a laboratory social stressor and correlation with gene expression
Participants completed the Trier Social Stress Test (Kirschbaum et al., 1993) to induce acute psychological stress. Briefly, this standardized laboratory stressor consisted of a 5-minute impromptu speech on a given topic followed by 5 minutes of mental arithmetic, performed standing in front of a microphone before a panel of two (one male, one female) judges and a video camera. Each participant performed the TSST twice, separated by approximately 12 weeks. The spacing between T1 and T2 measures is due to aspects of the study not reported here. Data were collected from the long-term meditators and wait-list controls on the same timeline. The temporal separation between T1 and T2 helps lower the risk of habituation to this challenge. The first TSST (T1) was a baseline measure. The second assessment followed the day of mindfulness practice for the meditation group, but did not follow the day of intervention for the control group. The speech topic and mental arithmetic task were changed at each assessment.
Salivary cortisol was collected using the Salivette device (Sarstedt, Inc., Nümbrecht, Germany) and was used as an index of the magnitude of stress response. Saliva samples were collected after a 20-minute rest period (baseline), immediately after the TSST, and at subsequent 10-minute intervals for 40 minutes — 6 samples per assessment in total. Saliva samples were frozen at −80°C until assayed. Cortisol levels in saliva were quantified using a commercially available luminescence immunoassay (CLIA; IBL-Hamburg, Hamburg, Germany), as previously described (Rohleder and Nater, 2009). In order to assess the temporal dynamics of the stress response, cortisol recovery was defined as the percentage of the baseline value that cortisol levels returned to after the peak, in the 40 minutes of sampling ((cortisol peak − post-peak trough)/(cortisol peak − baseline)). Subsequent values were log-transformed to normalize their distribution. In order to capture changes in the cortisol response after the day of mindfulness practice, a difference score, T2-T1 was calculated, which reflects the post-day of mindfulness response controlling for the response at baseline. In control participants, this metric reflects habituation due to repeated experience with the TSST. Pearson correlations were used to test the association between changes in cortisol recovery with changes in gene expression.
Real-time quantitative PCR
Random-primed cDNA synthesis was performed at 37 °C starting with 0.3 μg of RNA, using the High Capacity cDNA Archive kit (Applied Biosystems). Quantitative real time (q-RT) PCR custom pathway focused assays were performed using in an ABI Prism 7900HT Real Time PCR system using TaqMan FAM-labeled specific probes (Applied Biosystems). A list of the probes used is presented in S6. Results were normalized to TBPgene expression.
Statistical Methods
Continuous outcome measures for gene expression data (t2-t1) were tested with an analysis of covariance (ANCOVA model), using baseline scores as fixed effect covariates and controlling for potential confounders (age, gender, body mass index and ethnicity). The tables show means and standard deviation (SD) and results are expressed as estimated differences and their 95% Confidence Intervals (95% CI). Tables indicate when arithmetical transformations of data (log transformation, square-root transformation and inverse of the variable) were applied due to skewed distributions. Statistical outliers (≥ two standard deviations from the mean) were removed from the analyses (ranging from 0 to 2 across the different measures). Partial correlations (controlling for group, age, gender, race and BMI) were used to examine the relationship between RIPK2 and HDACs. Basal gene expression levels and Western blot data were evaluated using the Student’s t-test for unpaired data or the non-parametric Mann Whitney U-test, as indicated. Additionally, bootstrap estimations of 95% CI were performed with n=1000 samples to control for multiple comparisons. P values ≤ 0.05 were considered statistically significant. All statistical analyses were performed with SPSS ver. 18.
RESULTS
Expression of circadian rhythm regulatory genes
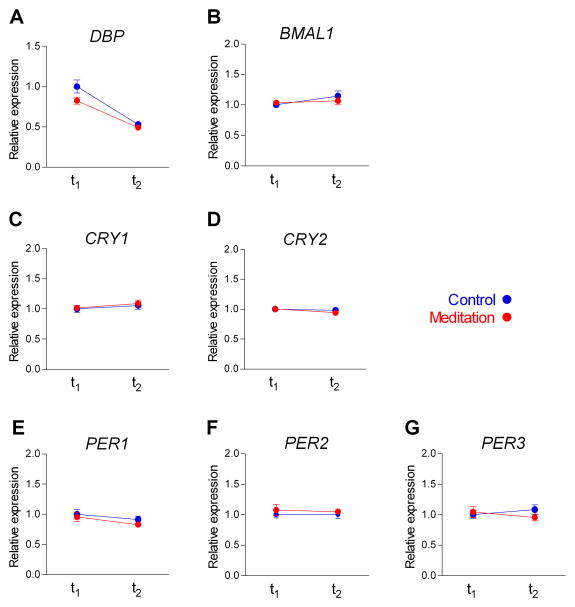
Circadian clock genes synchronize the light-dark phases with physiological functions such as cellular metabolism, hormone secretion, feeding behavior and body temperature (Nakahata et al., 2007; Yan, 2009). Diurnal changes at 4-hour intervals have been detected in the gene expression of circadian regulators (Per2 and Bmal1) and neurohormones (melatonin and cortisol) in human PBMC in response to alterations of light exposure and sleep deprivation (Kavcic et al., 2011). Since eyes closed rest and concurrent abnormal light exposure can occur during the practice of meditation, we explored the expression of core circadian genes before (t1) and after (t2) the intervention in the mindfulness meditation and control groups using a pathway-focused quantitative-real time (q-RT) PCR assay. Both groups exhibited similar gene expression levels at t1 and t2 for all the factors analyzed (Table 1; Fig. 1), which included the bZip-family geneDBP (albumin D-site binding protein) (Fig. 1,A), the core circadian feedback loop regulator BMAL1 (brain and muscle ARNT-like 1, a bHLH transcriptional factor) (Fig. 1, B), and the genes that sustain circadian rhythm in peripheral tissues: thecryptochrome family genes CRY1 and CRY2 (Fig. 1, C–D) and the period family genes PER1, PER2 and PER3, (Fig. 1, E–G). DBP was the only circadian gene that showed a strong rhythmicity between t1 and t2; this oscillation was similar in the two groups. Our data show that the basal expression levels of the main circadian regulators was similar in meditation experts and controls and that their rhythmicity was not influenced by intensive meditation practice.
Table 1.
Statistical analysis of circadian gene expression in MT vs control group.
| Gene | Mean (SD)
|
Student t-test
|
ANCOVA estimators
|
Bootstrapping
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | n | Meditation | n | Control (basal, t1) vs Meditation (basal, t1) | Adj. Diff. | 95% CI | p-value | 95% CI | p-value | ||
|
|
|
|
|
||||||||
| DBP | t1 | 1.09 (0.4) | 20 | 0.89 (0.21) | 19 | t(31)=1.90; p=0.067 | 0.02 | −0.07; 0.10 | 0.722 | −0.61; 0.10 | 0.762 |
| t2 | 0.57 (0.17) | 0.53 (0.16) | |||||||||
|
|
|
|
|
||||||||
| BMAL1 | t1 | 1.03 (0.26) | 21 | 1.12 (0.23) | 19 | t(38)= −1.13; p=0.268 | −0.06 | −0.24; 0.13 | 0.532 | −0.22; 0.11 | 0.507 |
| t2 | 1.21 (0.25) | 1.16 (0.3) | |||||||||
|
|
|
|
|
||||||||
| CRY1 | t1 | 1.03 (0.26) | 21 | 1.04 (0.22) | 19 | t(38)= −0.26; p=0.796 | 0.01 | −0.06; 0.09 | 0.735 | −0.06; 0.09 | 0.752 |
| t2 | 1.08 (0.29) | 1.12 (0.27) | |||||||||
|
|
|
|
|
||||||||
| CRY2 | t1 | 1.01 (0.22) | 21 | 1.02 (0.18) | 19 | t(38)= −0.04; p=0.966 | −0.04 | −0.15; 0.06 | 0.414 | −0.15; 0.05 | 0.395 |
| t2 | 1 (0.23) | 0.95 (0.14) | |||||||||
|
|
|
|
|
||||||||
| PER1 | t1 | 1.01 (0.34) | 19 | 0.96 (0.31) | 19 | t(37)=0.45; p=0.657 | −0.10 | −0.26; 0.05 | 0.192 | −0.26; 0.06 | 0.225 |
| t2 | 0.92 (0.26) | 0.83 (0.21) | |||||||||
|
|
|
|
|
||||||||
| PER2 | t1 | 1.03 (0.28) | 21 | 1.11 (0.42) | 19 | t(31)= −0.69; p=0.498 | 0.03 | −0.14; 0.21 | 0.727 | −0.12; 0.19 | 0.713 |
| t2 | 1.04 (0.32) | 1.08 (0.21) | |||||||||
|
|
|
|
|
||||||||
| PER3 | t1 | 1.04 (0.31) | 21 | 1.09 (0.4) | 19 | t(38)= −0.43; p=0.672 | −0.16 | −0.34; 0.03 | 0.101 | −0.32; 0.03 | 0.1 |
| t2 | 1.13 (0.39) | 1 (0.25) | |||||||||
Student’s independent t-test was conducted to contrast mean value differences between groups (control, meditation) at t1. ANCOVA model was used to compare relative gene expression between groups. Square Root transformation was applied to normalize CRY1 distribution. Bootstrap adjusted difference, 95% confidence interval and two-tailed p values are reported for all variables. p values < 0.05 were considered statistically significant. t1, t2, and SD stand for pre-intervention, post-intervention, and standard deviation, respectively.
FIGURE 1. Effect of mindfulness meditation on circadian gene expression.
Gene expression analysis by real-time RT-PCR from PBMC mRNA using TaqMan FAM-labeled specific probes and expressed relative to TBP (mean ± SE). A, DBP (albumin D-site binding protein). B, BMAL1 (brain and muscle ARNT -like 1). C, D, CRY1 and 2 (Cryptochrome 1 and 2). E–G, PER 1–3 (Period 1–3).
Expression of chromatin modulatory genes and global histone modifications
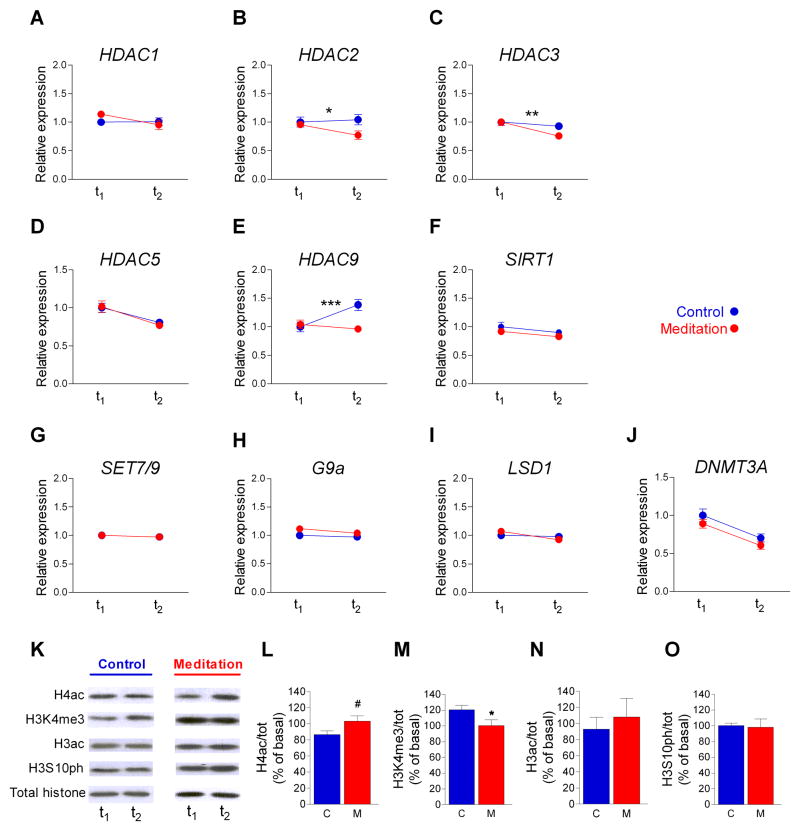
The gene expression levels of key epigenetic chromatin modification enzymes such as histone deacetylases (HDACs), histone methylases and demethylases and DNA methylases was analyzed using custom pathway-focused q-RT PCR assays. We found similar baseline levels for all the epigenetic modulatory genes analyzed in the two groups (Table 2). HDACs remove acetyl groups from different histone or non-histone residues (Akimova et al., 2012) and after the intervention, the meditation group showed a decrease in the gene expression of histone deacetylases HDAC2 (adjusted difference= −0.137, 95%CI= −0.250;−0.025, p<0.05) (Fig. 2 B), HDAC3 (adjusted difference= −0.23, 95%CI= 0.34; 0.09, p< 0.01) (Fig. 2 C) and HDAC9 (adjusted difference= −0.21, 95%CI= −0.30;−0.11, p< 0.001) (Fig. 2 E) compared with controls. Bootstrap estimations of 95% CI with n=1000 samples are shown in Table 2. No difference between groups was observed for HDAC1, HDAC5 and SIRT1, a gene that codes for a sirtuin isoform mainly involved in the deacetylation of non-histone substrates (Fusco et al., 2012) (Fig. 2 A, D and F; Table 2).
Table 2.
Statistical analysis of epigenetic chromatin modification gene expression in MT vs control group.
| Gene | Mean (SD)
|
Student t-test
|
ANCOVA estimators
|
Bootstrapping
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | n | Meditation | n | Control (basal, t1) vs Meditation (basal, t1) | Adj. Diff. | 95% CI | p-value | 95% CI | p-value | ||
|
|
|
|
|
||||||||
| HDAC1 | t1 | 1.05 (0.26) | 21 | 1.20 (0.28) | 19 | t(37)= −1.68; p=0.101 | −0.08 | −0.32; 0.16 | 0.505 | −0.28; 0.13 | 0.430 |
| t2 | 1.06 (0.32) | 1.00 (0.37) | |||||||||
|
|
|
|
|
||||||||
| HDAC2 | t1 | 1.07 (0.43) | 21 | 1.03 (0.2) | 19 | t(38)= −0.09; p=0.931 | −0.14 | −0.25; −0.02 | 0.022 | −0.25; −0.03 | 0.032 |
| t2 | 1.12 (0.45) | 0.83 (0.35) | |||||||||
|
|
|
|
|
||||||||
| HDAC3 | t1 | 1.03 (0.26) | 21 | 1.03 (0.28) | 19 | t(38)=0.02; p=0.982 | −0.23 | −0.38; − 0.08 | 0.003 | 0.09; 0.38 | 0.006 |
| t2 | 0.96 (0.2) | 0.78 (0.13) | |||||||||
|
|
|
|
|
||||||||
| HDAC5 | t1 | 1.03 (0.29) | 21 | 1.05 (0.33) | 19 | t(38)= −0.12; p=0.908 | −0.03 | −0.08; 0.03 | 0.398 | −0.09; 0.03 | 0.433 |
| t2 | 0.83 (0.15) | 0.79 (0.19) | |||||||||
|
|
|
|
|
||||||||
| HDAC9 | t1 | 1.07 (0.42) | 21 | 1.11 (0.36) | 19 | t(38)= −0.45; p=0.656 | −0.21 | −0.30; −0.11 | 0.000 | −0.30; −0.11 | 0.001 |
| t2 | 1.48 (0.48) | 1.03 (0.24) | |||||||||
|
|
|
|
|
||||||||
| SIRT1 | t1 | 1.06 (0.38) | 21 | 0.97 (0.18) | 19 | t(30)=0.51; p=0.612 | −0.03 | −0.09; 0.03 | 0.308 | −0.08; 0.02 | 0.278 |
| t2 | 0.95 (0.22) | 0.88 (0.19) | |||||||||
|
|
|
|
|
||||||||
| SET7 | t1 | 1.04 (0.29) | 21 | 1.04 (0.24) | 19 | t(38)= −0.02; p=0.987 | 0.00 | −0.11; 0.11 | 0.996 | −0.11; 0.11 | 1.000 |
| t2 | 1.01 (0.21) | 1.01 (0.19) | |||||||||
|
|
|
|
|
||||||||
| G9a | t1 | 1.02 (0.23) | 21 | 1.14 (0.21) | 19 | t(38)= −1.68; p=0.101 | 0.06 | −0.08; 0.19 | 0.401 | −0.06; 0.17 | 0.363 |
| t2 | 0.99 (0.19) | 1.06 (0.21) | |||||||||
|
|
|
|
|
||||||||
| LSD1 | t1 | 1.01 (0.17) | 21 | 1.09 (0.2) | 19 | t(38)= −1.25; p=0.219 | −0.08 | −0.16; 0.00 | 0.053 | −0.15; −0.01 | 0.035 |
| t2 | 0.99 (0.15) | 0.94 (0.12) | |||||||||
|
|
|
|
|
||||||||
| DNMT3A | t1 | 1.07 (0.42) | 21 | 0.95 (0.27) | 19 | t(38)=1.06; p=0.298 | −0.07 | −0.22; 0.07 | 0.313 | −0.22; 0.07 | 0.313 |
| t2 | 0.75 (0.25) | 0.65 (0.22) | |||||||||
Student’s independent t-test was conducted to contrast mean value differences between groups (control, meditation) at t1. ANCOVA models were used to compare relative gene expression between groups. HDAC2 and SIRT1 were log transformed to normalize distribution. Square Root and inverse transformation were applied to normalize HDAC9 and HDAC3 distribution respectively. Bootstrap adjusted difference, 95% confidence interval and two-tailed p values are reported for all variables. p values < 0.05 were considered statistically significant. t1, t2, and SD stand for pre-intervention, post-intervention, and standard deviation, respectively.
FIGURE 2. Effect of mindfulness meditation on gene expression of chromatin modulatory enzymes and histone-tail modifications.
A–H, Gene expression analysis by real-time RT-PCR analyses from PBMC mRNA using TaqMan FAM-labeled specific probes and expressed relative to TBP (mean ± SE; * p<0.05; ** p<0.01; *** p<0,001). A–F, histone deacetylases HDACs 1, 2, 3, 5, 9 and sirtuin 1 (SIRT1). G–H, histone methyltransferases SET7/9and G9a. I, lysine -specific demethylase 1 (LSD1). J, DNA -methyl transferase 3a (DNMT3a).
K–O, PBMC nuclear acidic lysates were probed with antibodies detecting histone-tail modifications. K, representative Western blot images. L–O, quantification of Western blots by scanning densitometry. L, global acetylation of histone H4 (H4ac). M, trimethylation of histone H3 lysine 4 (H3K4me3). N, global acetylation of histone H3 (H3ac). O, phosphorylation of serine 10 in histone H3 (H3S10ph). Histone modifications were corrected by total histone, and data were expressed as t2/t1; #, p<0.05, one tail t-test for unpaired data and p=0,064, two tail t-test; *, p<0.05, 2 tails t-test for unpaired data (mean ± SE; n=10–14/group).
Regarding the histone lysine methylation machinery, no difference between groups was observed for Set7/9, a histone methyltransferase that monomethylates lysine 4 in histone H3 (Del Rizzo and Trievel, 2011) (Fig. 2 G), G9a, a histone methyltransferase that also recruits DNMT3a and DNMT3b to DNA, thus regulating DNA methylation independently of its histone methyltransferase activity (Shinkai and Tachibana, 2011) (Fig. 2 H), or the lysine-specific demethylase LSD1 gene, which mediates gene repression in vivo by maintaining an unmethylated H3K4 status on a set of target promoters (Kooistra and Helin, 2012) (Fig. 2 I). Similarly, no difference between groups was observed for DNMT3a, a gene involved in de novo DNA methylation activity (Jurkowska et al., 2011) (Fig. 2 J). We found a dynamic diurnal DNMT3a gene transcription oscillation that was similar in the two groups (p<0.001 in both groups by two-tailed paired t-test analysis).
One of the most characterized epigenetic mechanisms is the modification of histone C-terminal domains. Some histone modifications are dynamic sensors of stress reactivity (Chandramohan et al., 2007; Chandramohan et al., 2008) and can occur through extremely rapid and reversible processes (Kouzarides, 2007)). After the intervention, the global levels of histone H4 acetylation (H4ac) tended to be higher in the mindfulness meditation group than in controls (Fig. 2 L) (two tailed t-test p=0,064; one-tailed t-test p<0.05; bootstrap resampling n=1000: p=0.067). We also observed a different pattern between groups for histone H3 trimethylation in lysine 4 (H3K4me3), a histone tag involved in gene activation (Vermeulen and Timmers, 2010) (two-tailed t-test p<0.05, bootstrapping n=1000; p<0.05) (Fig. 2 M). The control group presented a diurnal rhythmicity of H3K4me3 levels which is consistent with recent findings for this specific methylation mark (Le Martelot et al., 2012). In contrast, the mindfulness meditation group showed no change in H3K4me3 levels after the intervention. We did not detect any difference between groups in the levels of histone H3 global acetylation (H3ac) or phosphorylation of serine 10 in histone H3 (H3S10ph) (Fig. 2, N–O).
These data show rapid gene expression changes in specific HDACs and alterations in histone modifications in experienced subjects after an intensive day of mindfulness meditation compared with the control group after the leisure intervention.
Expression of inflammatory pathways: correlation with HDACs and social stress recovery
A substantial body of evidence shows that HDACs modulate inflammatory pathways (Halili et al., 2009; Shakespear et al., 2011; Akimova et al., 2012). We therefore examined whether the downregulation of HDAC gene expression in the meditation group was accompanied by reduced expression of proinflammatory genes.
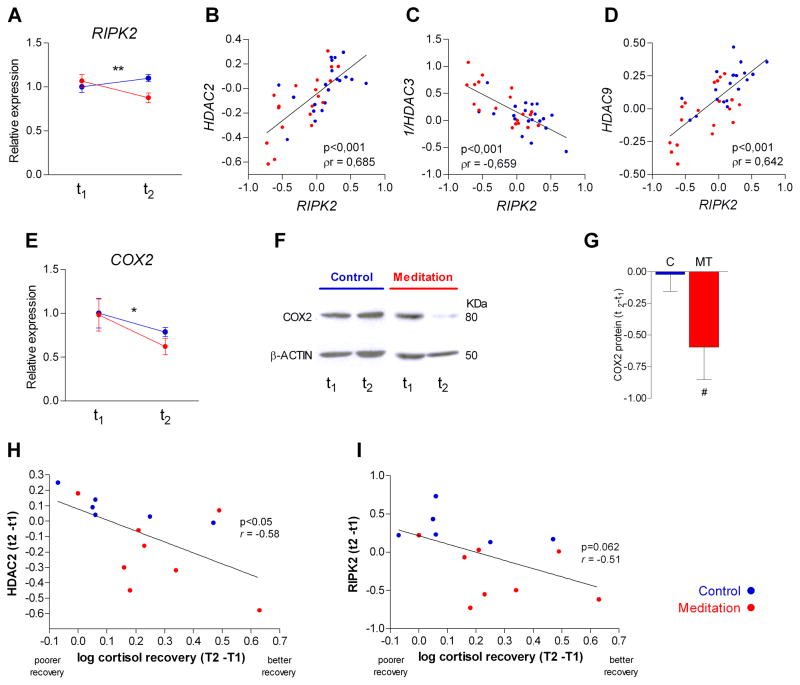
Participants in both groups presented similar baseline (t1) levels for all factors analyzed using custom inflammatory pathway-focused q-RT PCR assays (Table 3). After the intervention, we found a significant downregulation in the mindfulness meditation group of the receptor-interacting serine-threonine kinase 2 (RIPK2) which is a pivotal regulator of inflammatory processes (Yin et al., 2010), (adjusted difference= −0.25, 95% CI= −0.39; −0.11, p< 0.01 by ANCOVA analysis and bootstrap resampling n=1000) (Fig. 3 A and Table 3). A significant correlation was found between the expression of RIPK2 and HDAC2, HDAC3 and HDAC9 (ρr>0.64 and p<0.001 in all cases) (Fig. 3 B–D). These results were confirmed by bootstrap estimations of 95% CI with n=1000 samples (ρr>0.68 and p<0.001 in all cases).
Table 3.
Statistical analysis of proinflammatory genes in MT vs control group.
| Gene | Mean (SD)
|
Student t-test
|
ANCOVA estimators
|
Bootstrapping
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | n | Meditation | n | Control (basal, t1) vs Meditation (basal, t1) | Adj. Diff. | 95% CI | p-value | 95% CI | p-value | ||
| RIPK2 | t1 | 1.04 (0.29) | 21 | 1.11 (0.34) | 18 | t(38)= −0.69; p=0.493 | −0.25 | −0.40; −0.10 | 0.001 | −0.39; −0.11 | 0.002 |
| t2 | 1.14 (0.2) | 0.91 (0.25) | |||||||||
|
|
|
|
|
||||||||
| COX2 | t1 | 1.25 (0.96) | 21 | 1.22 (0.99) | 19 | t(38)=0.11; p=0.913 | −0.15 | −0.26; −0.05 | 0.007 | −0.26; −0.06 | 0.012 |
| t2 | 0.98 (0.31) | 0.77 (0.51) | |||||||||
|
|
|
|
|
||||||||
| CCR7 | t1 | 1.08 (0.45) | 21 | 0.89 (0.27) | 19 | t(38)=1.53; p=0.135 | −0.05 | −0.14; 0.05 | 0.344 | −0.14; 0.04 | 0.361 |
| t2 | 1.23 (0.43) | 1 (0.38) | |||||||||
|
|
|
|
|
||||||||
| CXCR1 | t1 | 1.09 (0.42) | 19 | 1.24 (0.45) | 19 | t(38)= −1.08; p=0.286 | −0.19 | −0.45; 0.06 | 0.132 | −0.42; 0.05 | 0.125 |
| t2 | 1.35 (0.46) | 1.23 (0.34) | |||||||||
|
|
|
|
|
||||||||
| IL-6 | t1 | 1.19 (0.66) | 21 | 1.14 (0.72) | 19 | t(38)=0.23; p=0.821 | 0.02 | −0.26; 0.30 | 0.874 | −0.23; 0.30 | 0.879 |
| t2 | 1.04 (0.42) | 1.04 (0.55) | |||||||||
|
|
|
|
|
||||||||
| TNF-α | t1 | 1.06 (0.4) | 21 | 1.07 (0.43) | 19 | t(38)= −0.05; p=0.957 | −0.11 | −0.36; 0.15 | 0.398 | −0.33; 0.15 | 0.401 |
| t2 | 1.02 (0.31) | 0.92 (0.44) | |||||||||
Student’s independent t-test was conducted to contrast mean value differences between groups (control, meditation) at t1. ANCOVA models were used to compare relative gene expression between groups. COX2 was log transformed to normalize distribution. Square Root transformation was applied to normalize CCR7 and TNF-α distribution. Bootstrap adjusted difference, 95% confidence interval and two-tailed p values are reported for all variables. p values < 0.05 were considered statistically significant. t1, t2, and SD stand for pre-intervention, post-intervention, and standard deviation, respectively.
FIGURE 3. Effect of mindfulness meditation on inflammatory pathways: correlation with HDAC gene expression and social stress recovery.
A, receptor-interacting serine-threonine kinase 2 (RIPK2) gene expression (mean ± SE; ** p<0.01). B–D, partial correlations between RIPK2 and HDAC gene expression. P values (p) and partial correlation coefficients (ρr) are indicated. HDAC3 was normalized using inverse-transformed values. Partial correlations were controlled for group variable (n=40). E, cyclooxygenase 2 (COX2) gene expression (mean ± SE; * p< 0.05). F, PBMC cell lysates were probed with Cox2 antibody, representative Western blot images are shown. G, Quantification of Western blots by scanning densitometry. Cox2 protein was corrected by β-actin, and data were expressed as (t2-t1) (mean ± SE; n=6/group; #, p=0.05, two-tailed Mann Whitney U test). H–I HDAC2 and RIPK2 gene expression correlation with cortisol recovery after acute psychological stress. Participants completed the Trier Social Stress Test twice. T2-T1 was calculated, which reflects the post-intervention response (T1) controlling for the response at baseline, 12 weeks before (T2). Cortisol was determined in saliva samples and recovery was defined as the percentage of the baseline value that cortisol levels returned to after the peak. For HDAC2 and RIPK2, a difference score (t2-t1) was calculated, which reflects the intervention response controlling for baseline levels (t1). Pearson correlations were used to test the association between changes in cortisol recovery with changes in gene expression.
Similarly, after the intervention the mindfulness meditation group showed reduced gene expression of the COX2, (adjusted difference= −0.15, 95%CI= −0.26;−0.06, p<0.05 by ANCOVA and bootstrapping n=1000 analysis) (Fig. 3 E; Table 3). Supporting the gene expression data, COX2 protein levels corrected by β-actin were significantly reduced in cell extracts from the mindfulness meditation group compared with control group (Mann Whitney U-test, p=0.05, Fig. 2F–G).
We did not find statistically significant differences between groups for CCR7, CXCR1, TNF-α and IL6 although with the exception of the latter, they all tended to show lower levels in the mindfulness meditation group after the intervention (Table 3).
In sum, these findings show that the basal expression levels of the inflammatory genes analyzed were similar in meditation experts and controls, however in experienced subjects, an intensive day of mindfulness meditation seem to trigger an anti-inflammatory response that is not observed after the same period of time in controls.
RIPK2 and HDAC2 gene expression is inversely associated with a faster cortisol recovery from the Trier psychosocial stress test (TSST)
Activation of proinflammatory signaling in PBMC has been described as an effector for the neuroendocrine response to psychosocial stress (Bierhaus et al., 2003). We therefore analysed the recovery of cortisol levels following the TSST in both groups of participants at study entry (T1) and approximately 12 weeks later (T2) and its correlation with HDACs, RIPK2 and COX2 gene expression. For the meditation group, T2 corresponds to the day following mindfulness practice.
In both groups of participants, a better cortisol recovery was associated with lower post-intervention levels of RIPK2 (r=0.51; p=0.065) and HDAC2 (r=0.58; p=0.029) (Figure 3, I and J), but not with other HDACs or COX2.
DISCUSSION
Here we show that expert meditators display rapid peripheral changes in the expression of histone deacetylase genes (HDAC 2, 3 and 9), global histone modifications (H4ac; H3K4me3) and pro-inflammatory genes (RIPK2 and COX2) at the end of an intensive day of mindfulness practice. Notably, lower levels of HDAC2 and RIPK2 predicted better cortisol recovery in a social stress test.
The current study was not designed to disentangle the effect of acute mindfulness practice from the enduring impact of long-term meditation training. Rather, we intentionally combined these factors to determine if there was a trait-like group difference at baseline (there was not) and to increase our capacity to detect an overall effect in the expert meditators. In the future, it will be important to determine if a one-day intervention in otherwise naïve participants would produce any of the effects we observed in the current study.
To our knowledge this is the first study to explore the influence of meditation in factors that synchronize gene expression: our data indicate that, compared with subjects that have never meditated, the basal expression levels of the main circadian regulators are not affected in PBMC from long-term meditators and that their rhythmicity is not influenced by a day long practice. Similarly, we found that all the epigenetic regulatory enzymes analyzed exhibited similar basal (t1) gene expression levels in the two groups of participants, but the expression of HDACs 2, 3 and 9 was lower in the mindfulness meditation group compared with controls after the intervention (t2). HDACs are enzymes that remove acetyl groups from the amino acid lysine on histones and they regulate transcription through direct effects on histones and non-histone chromatin regulatory factors, as well as alternative substrates. Hence, we cannot definitely conclude from our data that the downregulation of HDAC gene expression had an impact on epigenetic mechanisms. Moreover, due to biological sample limitations, in this study we could not corroborate that the changes in gene expression were associated with altered HDAC protein expression, activity or localization. However, the fact that global acetylation of histone H4 (H4ac) was increased after the meditation practice suggests that HDAC gene downregulation at least in part reduced HDAC enzymatic activity. No change was observed in the global acetylation levels of histone 3. The different dynamics in global acetylation of histones H3 and H4 that we detected may be due to distinct turnover rates for these epigenetic tags. Indeed, it has been recently shown that fast turnover acetylated histones exhibit half-lives between 1 and 2 hours (Zheng et al., 2013).
Consistent with the emerging anti-inflammatory role of HDAC inhibitors (HDACi) (Halili et al., 2009; Shakespear et al., 2011; Akimova et al., 2012), the decrease in HDAC gene expression in the mindfulness meditation group was concomitant with a significant downregulation of proinflammatory RIPK2 and COX2 genes. Notably, HDACs 2, 3 and 9 have been proposed as targets for HDACi to reduce inflammatory markers in blood cell models (Kim et al., 2007; Li et al., 2007; Tao et al., 2007; de Zoeten et al., 2010; Yamaguchi et al., 2010) and both RIPK2 and COX2 gene expression have been reported to be regulated by HDACi in diverse cell systems (Tong et al., 2004; Wu and Guo, 2007; Roger et al., 2011). Moreover, we found a strong positive correlation between HDACs and RIPK2 gene expression in the meditation group. On the other hand, we found that the general inhibitor of HDAC activity trichostatin A (TSA) rapidly inhibited COX2 gene expression levels in a human lymphocyte-derived cell line (MT2) (50% reduction after 30 min of TSA treatment, S2). The overall pattern of our findings indicate that the decreased HDAC mRNA levels may have contributed to lower COX2 expression in the mindfulness meditation group, although the impact of HDAC gene downregulation on HDAC enzymatic activity needs to be confirmed. Finally, we show that a faster recovery of cortisol levels after an acute psychological stress test (Trier Social Stress Test) was associated with lower gene expression levels of RIPK2 and HDAC2. Notably RIPK2 regulates the inflammatory response through the NF-kappaB pathway (Yin et al., 2010), which is an effector of the neuroendocrine response to psychosocial stress in PBMCs (Bierhaus et al., 2003). The shift toward quicker recovery from stress, as well as the regulation of HDAC and inflammatory pathways may be a beneficial combination that helps to explain mechanisms underlying the therapeutic potential of mindfulness meditation in stress-related disorders. Further study is warranted to explore a mechanistic link between HPA axis activity and the peripheral HDAC regulation of inflammation in response to meditation.
The practice of meditation is increasingly associated with pain attenuation (Brown and Jones, 2010; Perlman et al., 2010; Grant et al., 2011; Zeidan et al., 2012; Lutz et al., 2013) and thermal pain reduction was one of few specific outcomes detected in response to an MBSR program versus a carefully matched active control (MacCoon et al., 2012). RIPK2 is now attributed a pivotal role in the regulation of inflammation through both the NF-kappaB and the JAK-STAT pathways and is therefore an interesting new pharmacological target for the inhibition of inflammatory diseases (Yin et al., 2010). COX2 is the target of analgesic and antiinflammatory drugs based on non-specific or selective COX inhibition (e.g. aspirin and ibuprofen) (Ramalho et al., 2009). It has been proposed that central mechanisms such as enhanced emotional and cognitive control mediate the perception of pain in meditators. Our findings suggest that peripheral anti-inflammatory and analgesic molecular mediators may also play a role in the regulation of pain initiation and maintenance in response to meditation. Moreover, our data support the notion that the inhibition of the pro-inflammatory NF-kappaB pathway is a reproducible molecular outcome in blood cells in response to meditation-based practices such as the elicitation of the relaxation response in long-term practitioners (Dusek et al., 2008; Bhasin et al., 2013), mindfulness-based stress reduction training in older adults (Creswell et al., 2012) and yogic meditation in family dementia caregivers (Black et al., 2013).
Chronic low-grade inflammation is associated with the most common health problems in the modern world including cardiovascular and metabolic disease, cancer and neuropsychiatric disorders. Our findings suggest that mindfulness-based behavioral interventions may produce beneficial effects in subjects with chronic diseases in whom inflammation is a significant correlate. Data presented here suggest that mindfulness meditation practice influences mechanisms similar to those targeted by different anti-inflammatory drugs such as HDACi or cyclooxygenase inhibitors. These findings set the foundation for future studies to further assess the mechanisms through which different forms of meditation modulate inflammation.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Center for Complementary and Alternative Medicine of NIH to RJD and AL (P01-AT004952) and by grants to RJD from the Fetzer Institute, the John Templeton Foundation and an anonymous donor. We would like to thank David Bachhuber for his excellent work with the planning and logistics of data collection and José Ríos MSc Biostatistics and Data Management Platform, IDIBAPS, for assistance in the statistical analysis.
Role of funding sources
None.
Footnotes
Author contribution
Designed research: PK, AL, RD
Performed research: PK, MJA-L, MC-T, MAR
Contributed new reagents/analytic tools PK, RD, AL
Analyzed data PK, MJA-L, MC-T MAR, RD, AL
Wrote the paper PK, AL, RD
Conflict of interest statement
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimova T, Beier UH, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases and T-cell immune responses. Blood. 2012;119(11):2443–2451. doi: 10.1182/blood-2011-10-292003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin MK, Dusek JA, Chang BH, Joseph MG, Denninger JW, Fricchione GL, Benson H, Libermann TA. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS One. 2013;8(5):e62817. doi: 10.1371/journal.pone.0062817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, Nazarian N, Khalsa DS, Lavretsky H. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38(3):348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Jones AK. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150(3):428–438. doi: 10.1016/j.pain.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65(4):571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: A small randomized controlled trial. Brain Behav Immun. 2012;26(7):1095–1101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain Behav Immun. 2009;23(2):184–188. doi: 10.1016/j.bbi.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27(10):2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101(3):815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27(46):12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138(2):583–594. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011;6(9):1059–1067. doi: 10.4161/epi.6.9.16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, Benson H, Libermann TA. Genomic counter-stress changes induced by the relaxation response. PLoS One. 2008;3(7):e2576. doi: 10.1371/journal.pone.0002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry. 2012;57(2):70–77. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fusco S, Maulucci G, Pani G. Sirt1: def-eating senescence? Cell Cycle. 2012;11(22):4135–4146. doi: 10.4161/cc.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. The practice of freedom. Boston: Shambala Publications; 1993. Insight Meditation. [Google Scholar]
- Graff J, Kim D, Dobbin MM, Tsai LH. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev. 2011;91(2):603–649. doi: 10.1152/physrev.00012.2010. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152(1):150–156. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem. 2009;9(3):309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- Jurkowska RZ, Jurkowski TP, Jeltsch A. Structure and function of mammalian DNA methyltransferases. Chembiochem. 2011;12(2):206–222. doi: 10.1002/cbic.201000195. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Wheeler E, Light T, Skillings A, Scharf MJ, Cropley TG, Hosmer D, Bernhard JD. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosom Med. 1998;60(5):625–632. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Parrizas M, Lalanza JF, Camins A, Escorihuela RM, Pallas M. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Res Rev. 2011;10(4):475–486. doi: 10.1016/j.arr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15(12):576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Kavcic P, Rojc B, Dolenc-Groselj L, Claustrat B, Fujs K, Poljak M. The impact of sleep deprivation and nighttime light exposure on clock gene expression in humans. Croat Med J. 2011;52(5):594–603. doi: 10.3325/cmj.2011.52.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Jeong JW, Park JA, Lee JW, Seo JH, Jung BK, Bae MK, Kim KW. Regulation of the HIF-1alpha stability by histone deacetylases. Oncol Rep. 2007;17(3):647–651. [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol. 2012;13(5):297–311. doi: 10.1038/nrm3327. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS, Lin J, Blackburn E, Irwin MR. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Martelot G, Canella D, Symul L, Migliavacca E, Gilardi F, Liechti R, Martin O, Harshman K, Delorenzi M, Desvergne B, Herr W, Deplancke B, Schibler U, Rougemont J, Guex N, Hernandez N, Naef F. Genome-Wide RNA Polymerase II Profiles and RNA Accumulation Reveal Kinetics of Transcription and Associated Epigenetic Changes During Diurnal Cycles. PLoS Biol. 2012;10(11):e1001442. doi: 10.1371/journal.pbio.1001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, Saouaf SJ, Greene MI. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104(11):4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300(11):1350–1352. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Bonus KA, Stoney CM, Salomons TV, Davidson RJ, Lutz A. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behav Res Ther. 2012;50(1):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P. Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol. 2007;19(2):230–237. doi: 10.1016/j.ceb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion. 2010;10(1):65–71. doi: 10.1037/a0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TX, Lee J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients. 2012;4(12):1868–1886. doi: 10.3390/nu4121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho TC, Rocha MV, da Cunha EF, Freitas MP. The search for new COX-2 inhibitors: a review of 2002 – 2008 patents. Expert Opin Ther Pat. 2009;19(9):1193–1228. doi: 10.1517/13543770903059125. [DOI] [PubMed] [Google Scholar]
- Roger T, Lugrin J, Le Roy D, Goy G, Mombelli M, Koessler T, Ding XC, Chanson AL, Reymond MK, Miconnet I, Schrenzel J, Francois P, Calandra T. Histone deacetylase inhibitors impair innate immune responses to Toll-like receptor agonists and to infection. Blood. 2011;117(4):1205–1217. doi: 10.1182/blood-2010-05-284711. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun. 2013;27(1):174–184. doi: 10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152(2):361–369. doi: 10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Shakespear MR, Halili MA, Irvine KM, Fairlie DP, Sweet MJ. Histone deacetylases as regulators of inflammation and immunity. Trends Immunol. 2011;32(7):335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25(8):781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317(2):463–471. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Timmers HT. Grasping trimethylation of histone H3 at lysine 4. Epigenomics. 2010;2(3):395–406. doi: 10.2217/epi.10.11. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22(6):969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Guo SW. Suppression of IL-1beta-induced COX-2 expression by trichostatin A (TSA) in human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2007;135(1):88–93. doi: 10.1016/j.ejogrb.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010;24(5):455–469. doi: 10.1101/gad.552310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L. Expression of clock genes in the suprachiasmatic nucleus: effect of environmental lighting conditions. Rev Endocr Metab Disord. 2009;10(4):301–310. doi: 10.1007/s11154-009-9121-9. [DOI] [PubMed] [Google Scholar]
- Yin X, Krikorian P, Logan T, Csizmadia V. Induction of RIP-2 kinase by proinflammatory cytokines is mediated via NF-kappaB signaling pathways and involves a novel feed-forward regulatory mechanism. Mol Cell Biochem. 2010;333(1–2):251–259. doi: 10.1007/s11010-009-0226-y. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett. 2012;520(2):165–173. doi: 10.1016/j.neulet.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Thomas PM, Kelleher NL. Measurement of acetylation turnover at distinct lysines in human histones identifies long-lived acetylation sites. Nat Commun. 2013;4:2203. doi: 10.1038/ncomms3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.