Abstract
The dopamine system has been implicated in both substance use disorder (SUD) and schizophrenia. A recent meta- analysis suggests that A1 allele of the DRD2 gene imposes genetic risk for SUD, especially alcoholism and has been implicated in Reward Deficiency Syndrome (RDS). We hypothesize that dopamine D2 receptor (DRD2) gene Taq1 A2 allele is associated with a subtype of non- SUD schizophrenics and as such may act as a putative protective agent against the development of addiction to alcohol or other drugs of abuse. Schizophrenics with SUD may be carriers of the DRD2 Taq1 A1 allele, and/or other RDS reward polymorphisms and have hypodopaminergic reward function. One plausible mechanism for alcohol seeking in schizophrenics with SUD, based on previous research, may be a deficiency of gamma type endorphins that has been linked to schizophrenic type psychosis.. We also propose that alcohol seeking behavior in schizophrenics, may serve as a physiological self-healing process linked to the increased function of the gamma endorphins, thereby reducing abnormal dopaminergic activity at the nucleus accumbens (NAc). These hypotheses warrant further investigation and cautious interpretation. We, therefore, encourage research involving neuroimaging, genome wide association studies (GWAS), and epigenetic investigation into the relationship between neurogenetics and systems biology to unravel the role of dopamine in psychiatric illness and SUD.
Keywords: schizophrenia, substance related disorders, dopaminergic, reward deficiency syndrome (RDS), gamma –endorphins
INTRODUCTION
This hypothesis was developed in consideration of the extensive comorbidity of substance use disorder (SUD) and schizophrenia. The involvement of dopaminergic neurotransmission in the genetic antecedents of schizophrenia, as well as genetic vulnerability to RDS, is discussed. This exploration establishes the circumstances for the hypothesis that a deficiency of gamma type endorphins may trigger self-healing SUD in schizophrenia and that the DRD2 gene Taq1 A2 allele may be protective agent against the development of SUD.
A brief synopsis of the genetic antecedents of schizophrenia
Multiple genes interacting with multiple environmental factors influence many psychiatric conditions (behavioral phenotypes). Evidence suggests that schizophrenia is a complex genetic disorder involving “polygenic” inheritance (1). For example, genetic studies have sought to identify subtypes or endophenotypes for schizophrenia in an effort to improve reliability of diagnosis. A number of chromosomal regions have been shown to have replicated linkage and/or association to schizophrenia susceptibility. Many of the genes that are associated with psychiatric conditions code for the proteins involved in synaptic transmission. Genetic studies are complicated by, fuzzy diagnostic boundaries and the presence of phenocopies, for example, the symptoms produced by schizophrenia that are similar to some symptoms produced by drugs of abuse (2).
Studies have identified a number of candidate genes for schizophrenia. These genes include those that are responsible for development of the messocortical-limbic system. In this regard, genes that control GABA, glutamate and dopamine have shown promising possibilities in animal models of schizophrenia. Moreover, GABA neurons that co-express the calcium binding protein paravalbumin are associated with both glutamatergic metabotropic receptors and dopamine D3 receptors. Other interesting genes include: the gene for catechol -0-methyltransferase (COMT); the gene for neuroregulation that affects the expression and activation of neurotransmitter receptors including glutamate receptors; the gene for dystrobrevin binding protein, with unknown function in the brain; a region of chromosome 13q14-q23 that contains the gene for the serotonin 5-HT-2A receptor; a gene for the alpha 7 nicotinic cholinergic receptor subunit; a breakpoint in chromosome 1 affecting the genes DISC1 and DISC2 linked to both schizophrenia and affective disorders involved in neurite growth (3). The most promising gene regions include but are not limited: 22q12-q13, 8p22-p21, 6p24-p22, 13q14-q32, 5q22-q31, 10p15-p11, 6q21-q22, 15q13-q14, 9q34.3, 4q24-q32, 18 and 1q32-q41. While there is controversy concerning involvement of these gene regions there is emerging evidence of linkage to chromosome 11q and 14p (3). In this regard, the Cannabinoid CB1 receptor gene located at 6q14-q15 may be involved in gene expression during brain development. Hoenicka et al. has shown that the frequency of allele 4 of the cannabinoid receptor 1 (CNR1) gene microsatellite is significantly lower in schizophrenic patients when compared with healthy control individuals. Moreover, no differences have been found with respect to SUD in this schizophrenic population. These results suggest that independent of SUD differences in the cannabinoid system could impart vulnerability to schizophrenia (4).
Interestingly, schizophrenia is also associated with single nucleotide polymorphism of C957T DRD2 gene (chromosome 11q). Specifically, in these patients the C homozygote genotype is overexpressed when compared with controls. It was therefore suggested that variation in the DRD2 gene plays an important role in imparting vulnerability to schizophrenia (5). However, dopamine receptor involvement in hyper functioning of dopaminergic systems in schizophrenia remains controversial. While there are at least five ma in subtypes of dopamine receptors (D1 –D5), traditionally D2 receptors are considered most important. It was shown that clinical efficacy of antipsychotic drugs correlates with their ability to block D2 receptors (6). It was suggested that D2 receptor binding by antipsychotic agents may be “necessary and sufficient” for the anti-psychotic effect (7-16). Since there are few common polymorphisms with the coding region of the DRD2 gene (15) we are not surprised that fewer studies of the DRD2 and antipsychotic drug response have been conducted as compared to the 5-HT system. In recent years D3 and D4 receptors have also been implicated in development of schizophrenic symptoms (7-9).
Comorbidity of substance use disorder (SUD) and schizophrenia
Clinical and epidemiologic studies have found a high frequency of co-occurrence of SUD and psychiatric disorders. Psychiatric comorbidity in drug abusers is associated with greater severity of psychopathology, higher incidence of risky behaviors, higher psychosocial impairment and greater number of violent and criminal behaviors (17).
Since some psychotic symptoms caused by substances of abuse mimic schizophrenia it is difficult to identify phenotypes that are responsible for schizophrenia and not SUD (2,16). The prevalence of SUD is high in schizophrenia and the reason for this comorbidity is unclear. It was suggested that psychiatric patients use substances to cope up with anxiety and cognitive decline (18). Acute self-medication is pursued to ameliorate the symptoms associated with impaired processing of the mesocorticolimbic reward system defined as “Reward Deficiency Syndrome” (RDS) by Blum et al. (19).
Earlier work by van Ree and de Wied (20) provides an interesting hypothesis concerning the putative role of gamma-endorphin in schizophrenia and alternative pathways being involved both schizophrenia and SUD.
There is a high probability that genetic data supports the proposition that, overall schizophrenia vulnerability is distinct from SUD vulnerability. In fact, the truth may be that both co-exist with independent distinct polygenic polymorphisms. The dopamine system, however, has been implicated in both substance use disorder (SUD) and schizophrenia.
HYPOTHESIS
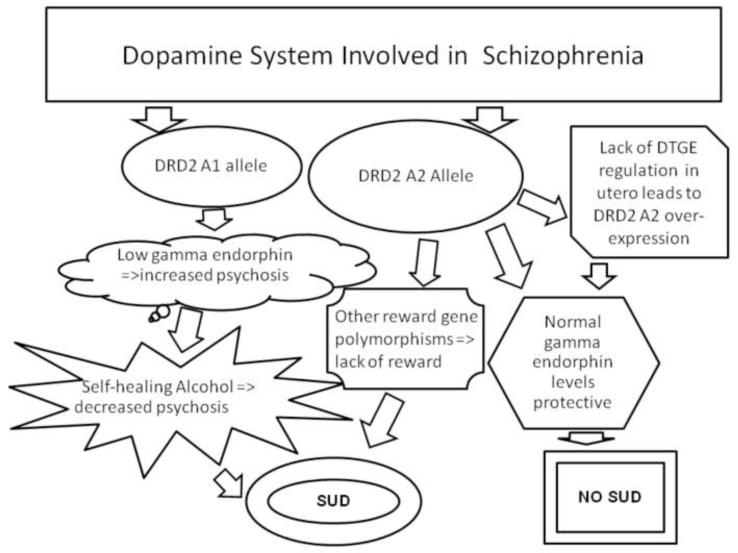
A deficiency of gamma type endorphins may be linked to a sustained increase in dopaminergic activity and consequently hallucinations as observed in Schizophrenia (20). We hypothesize that one plausible mechanism for alcohol seeking in schizophrenics with SUD, may be that it serves as a physiological self-healing process, linked to an increase in gamma endorphins known to reduced hallucination (20)[see figure 1].
Figure 1. Dopamine and opioid peptide interaction in Schizophrenia and alcoholism.
Left side: Carrying the DRD2 A1 increases the “wanting” of alcohol and in carriers with a low gamma endorphin (DTGE) would augment the risk for psychosis leading them to increased self medication by using alcohol. Right side: Carrying the DRD2 A2 may be protective against alcohol drinking in Schizophrenics. Lack of Gamma Endorphin (DTGE) in utero leads to an over-expression of DRD2 A2. Center: However, some Schizophrenics with the DRD2 A2 allele along with unknown reward gene polymorphisms may use substances due to lack of reward.
We hypothesize that DRD2 gene Taq1 A2 allele is associated with a subtype of non- SUD schizophrenics and as such may serve as putative protective agent against the development of addiction to alcohol or other drugs of abuse (21). While schizophrenics with SUD may be carriers of the D2 receptor Taq1 A1 allele, and/or other RDS reward polymorphisms and have hypodopaminergic reward function.
Reward deficiency syndrome (RDS) and genetic vulnerability
In 1996, Blum’s laboratory initially described RDS to define the symptoms associated with a common polymorphism of the DRD2 gene (22,23). These symptoms include impulsive, compulsive and addictive behaviors (19). The DRD2 gene has been associated with pleasure, referred to as a ‘reward gene’ (24). Taq1 A1 allele of the DRD2 gene has been widely studied in neuropsychiatric disorders and SUD (25) and associations with antisocial personality disorder (25), high novelty seeking behavior (27), and related traits (28) have been found.
The dopaminergic mesocorticolimbic pathway is important in mediating reinforcement of addiction and may be a feature of addictions and various psychiatric disorders (29-32). Drug-seeking behavior (31-32) is a form of RDS that results when certain genetic variants cause mesocorticolimbic dopamine reward system dysfunctions (19). This breakdown of the reward cascade, (33-41) due to specific genetic and environmental influences, (42) that result in aberrant conduct, is referred to as RDS. It is well known that abuse of psychoactive drugs including; alcohol, (43) as well as most positive reinforcers like sex, food, gambling and aggression activate the release of neuronal dopamine (44-56) which can satisfy abnormal cravings (46) and increase positive feelings (57). A deficiency of D2 receptor then predisposes individuals to multiple addictive, impulsive, and compulsive behaviors (58-60). Although other neuromodulators and neurotransmitters like glutamate, GABA, (61) serotonin (62) and enkephalin, (63) have a role in determining the rewarding and stimulating effects of drugs of addiction, dopamine may be essential for initiating and reinstating drug use after protracted abstinence (63,64-67).
Following the initial findings of a positive association of the Taq1 A1 of the DRD2 gene and severe alcoholism (24) there have been a plethora of studies both positive (26,28,30,33,57,65,67-91) and negative (92-105) [see reviews (25, 58, 78, 95,105-125)]. A number of studies have found that the Taq1 A1 allele is associated with low dopamine D2 density in alcoholics (72,79,125,127). There are conflicting results regarding dopamine transporter (DAT) densities (128-132) among alcoholics, but subtypes were not considered (84,85).
The concept of the dopamine D2 receptor gene as a specific target for alcohol, was appropriately dismissed by, Blum et al. (24), who initially suggested, that they have found a non-specific “reward” gene (133). Moreover, the DRD2 TaqA1 allele is also associated with sensitivity to stress and anxiety (83, 134-135) both symptoms have been related to sensitivity of presynaptic D2 receptors (110). The sensitivity is elevated in high anxiety subjects compared with low anxiety subjects. Other RDS and related neurological and psychiatric disorders are also found to be associated with polymorphisms of the DRD2 gene, and is the subject of another article. However, we do provide a list of PUBMED articles especially SUD (see table 1) a known subset related to a number of psychiatric problems also associated with dopamine gene polymorphisms such as DRD2 gene including borderline personality (4 studies), anxiety (101studies), panic attacks (10 studies), depression (187), conduct disorder (24), anti-social personality (7 studies), obsessive –compulsive disorder (38) amongst others.
Table 1.
The number of Pub Med listed papers that associate various Substance Related Disorders and the DRD2 gene polymorphisms (1-26-14)
| Substances | Pub Med Listed |
|---|---|
| Alcohol | 346 |
| Caffeine | 20 |
| Hallucinogens | 13 |
| Inhalants | 0 |
| Opioids | 51 |
| Sedatives/Hypnotics | 16 |
| Stimulants | 177 |
| Tobacco/Nicotine | 131 |
| Glucose | 35 |
Table 1. The number of Pub Med listed papers that associate various Substance Related Disorders and the DRD2 gene polymorphisms as of January 26th 2014.
A major difficulty with associating the DRD2 TaqA1 allele with alcoholism is that the Taq1 A polymorphism is located more than 10kb downstream from the coding region of the DRD2 gene and a mutation at this site would not be expected to lead to any structural change in the dopamine receptor. The most likely explanation for an association is that the Taq1 A polymorphism is in linkage disequilibrium with an upstream regulatory element, or a 3′ flanking element, or another gene which confers susceptibility to RDS behaviors. Several linkage disequilibrium studies have found strong linkage disequilibrium between the Taq1 A1 allele and the Taq1 B allele and the SSCP 1 allele (53,70,88,134). As we have pointed out, the dopamine D2 receptor has been implicated extensively in relation to alcoholism, nicotine dependence, anxiety, memory, glucose control, pathological aggression, pathological gambling, and certain sexual behaviors -- all of these are RDS behaviors (24,135). The Taq1 A restriction fragment length polymorphism is the most frequently examined polymorphism linked to the DRD2 gene and has been associated with a reduction in D2 receptor density. Neville and associates identified and named the “ankyrin repeat” gene, a kinase gene located in the 10kb downstream region of the Taq1 A1 RFLP, and in a single serine/threonine kinase domain containing 1 (ANKK1), which is expressed at low levels in whole spinal cord RNA and placenta and is a protein one of the many involved in signal transduction pathways (136). Taq1A allele of the DRD2 is a single nucleotide polymorphism (SNP) responsible for an amino acid substitution located within the 11th ankyrin repeat of ANKK1 (p. Glu713lYs), which although it is unlikely to affect structural integrity, may effect substrate-binding specificity. If it does effect substrate-binding, then in ANKK1 activity alterations may provide an alternative explanation for previously described (136) associations between the DRD2 gene and RDS behaviors
Understanding the neural circuitry of rewards may provide a mechanism for understanding how positive reinforces motivate behavior (137). A positive reinforcer is operationally defined as an event that increases the probability of a subsequent response, and drugs of abuse are considered to be stronger positive reinforcers than natural reinforcers (e.g. food and sex) (138-140). The distinction between the primary or natural rewards like satisfaction of physiological drives like hunger and reproduction, and secondary or unnatural rewards is an important one. In fact, learned unnatural rewards like hedonic sensations (141) derived from alcohol and other drugs, as well as from gambling and other risk-taking behaviors are similarly important (138,142-143).
Specifically RDS refers to an insensitivity and inefficiency in the system that controls secondary (or unnatural) reward (19, 25, 58). The acquired need to escape or avoid negative affects created by repeated cycles of alcohol abuse (144) and dependence (145-151) is also encompassed by RDS and result in dopamine release. Dopamine is therefore associated with pleasure, and has been called anti-stress or pleasure molecule (31,32,83,152). The neural circuitry for positive reinforcement involves multiple brain regions and structures (201,153-158).includes the limbic system and the striatum (59). Functions of the limbic system include monitoring of internal homoeostasis, mediating emotional memory and learning, emotional processing (57,159) and processing of aspects of motivational behaviors including sexual behavior.
In disagreement with our initial hypothesis, a number of studies have found that the complex comorbidity of schizophrenia and SUD is also associated with high prevalence of DRD2 A2 allele (17,158-161). If the dopamine receptor gene is not involved in this population of Schizophrenics with SUD then we propose that other multiple pathways that result in hypodopaminergic reward function must be considered as putative inducers of substance seeking behavior. These include polymorphisms in the dopamine D1 and D3 receptors, cannabinoid receptor, tryptophane hydroxylase, serotonin receptors, GABA receptors, opioid receptors, dopamine transporter, dopamine beta hydroxylase receptors, n-acetyltransferase, homer 2 genes, (3,162-175) that are associated with RDS.
Gamma type endorphins deficiency and increased dopaminergic activity
Processing pro-opiomelacortin (POMC) yields alpha, beta and gamma endorphins. Located predominantly in the pituitary, they are also found in neuronal pathways of the brain. Many studies have revealed that Gamma –endorphin has unique pharmacologic properties as compared to other endorphins (20). Certain effects of this compound are independent of the opioid peptide systems and receptors. In fact, removal of the N-terminal group of this substance eliminates opiate-like actions, and the resultant peptide (des-tyr)-gamma-endorphin (DTGE) resembles antipsychotic drugs in a number of tests. However, since this substance did not displace haloperidol from its binding site, it has been suggested that DTGE or a closely related peptide is an endogenous substance with anti-psychotic –like-action (176-177).
The endorphin (DTGE) and other gamma type endorphins function as antagonists of D2 and/or D3 receptors, which are present in the NAc, a terminal area of the mesolimbic dopaminergic pathway (178-179). It has been proposed that endogenous gamma type endorphins exert control over the dopamine system and that a chronic deficiency of these peptides, an RDS phenomenon, may lead to a sustained increase in dopaminergic activity as observed in Schizophrenia (179). Most interestingly, the postulate that, psychosis of the schizophrenic type may result from a deficiency of gamma-type endorphins (180) stimulated research on antipsychotic effects of these peptides (181-182).
Self-healing using alcohol in schizophrenics with SUD
It was reported that alcohol increases these peptides in the brain and may be in part a physiological reason to abuse alcohol by the schizophrenic patients to reduce psychosis. In animal studies Jackson et al. reported an attenuation of behavioral effects of ethanol by desenkephalin –gamma- endorphin (183-184). Alcohol abuse in a subtype of schizophrenics may be explained by this finding.
DRD2 gene taq1 A2 allele may be protective against the development of SUD (alcohol) in schizophrenia
It is plausible that certain sub-populations of schizophrenics carry the DRD2 A2 allele, and this might confer a protection against SUD since the DRD2 A1 allele, and not the DRD2 A2 allele, has been associated with SUD and other addictive behaviors (18,185-193) Possibly, during embryonic development normal regulatory controls of dopaminergic activity are deficient (i.e. lack of DTGE) leading to an increased release of dopamine. Thus, we hypothesize that, there is an overexpression of the DRD2 A2 allele. Noble et al. (72) pointed out that the number of D2 receptors is determined by the DRD2 genotype: A1/A1 = lowest number of D2 receptors; A1/A2 = moderate reduction of D2 receptors (one-third normal) and A2/A2 = highest number of D2 receptors. The overexpression of the DRD2 A2 allele may be an adaptive mechanism necessary to balance hyperactivity of the dopamine system.
There are other examples of genomic adaptations that protect against alcoholism like. the inactive aldehyde dehydrogenase -2 gene (ALDH2), Individuals with at least one ALDH2*2 allele have little to no ALDH2 activity (194-195). Owing to a high level of blood acetaldehyde after the ingestion of even small doses of alcohol, they exhibit the flushing response (196). This response is unpleasant enough to prevent people with the inactive ALDH2*2 allele form from becoming alcoholic. Although this polymorphic ALDH2*2 allele is found in ~50% of Chinese and Japanese, only 10% of Chinese and Japanese alcoholics possess this allele (197).
It is noteworthy that comparing DRD2 Taq1 A1 allele frequencies; Matsushita et al. (198) found the A1 allele more often in all subjects with ALDH2*2 than in those without it, regardless of whether subjects were alcoholics or healthy controls. Moreover, because alcoholics with inactive ALDH2*2 have overcome severe adverse reactions to develop alcoholism may be due to a genetic drive toward alcoholism. One such genetic trait may be the possession of the DRD2 A1 allele.
Huang et al. (199) examined the relationship between DRD2 gene and alcohol-metabolizing genes, alcohol (ADH1B) and aldehyde (ALDH2) dehydrogenase genes, in a specific subtype of alcoholics. Not only have these genes and associated polymorphisms been considered protective against alcoholism they are both involved in dopamine metabolism (200-201). As expected, the DRD2 A1 allele was associated with anxious –depressive alcoholics (ANX/DEP ALC). Furthermore, the association between the DRD2 A1 allele and ANX/DEP ALC was shown to be under control of both the ADH1B and ALDH2 genotypes.
FUTURE PERSPECTIVES
In the future, we will begin to see many more genetic studies utilizing GWAS, EWAS and neuroimaging experiments that will shed light on the “true” relationship between SUD and other psychiatric conditions. Most interestingly, while there is a significant history of the role of opioid peptides (202) in specific neurons distributed throughout the nervous system (tel-di-mes – rhombencephalon and the spinal cord) little is known about the possible interaction of Gamma – endorphins and SUD. The last study we found on Gamma –endorphin and Schizophrenia was published in 2002 (203). We found a total of 812 articles listed in Pubmed on Gamma – endorphin (9-3-13). As far back as 1982, the neuropeptide des-1-tyrosine-gamma-endorphin (DTGE) was found to have similar behavioral effects in rodents as known drugs acting upon the CNS and it was hypothesized that this effect was due to the accelerating influence of DTGE on tyrosine hydroxylation in the striatal synaptosomes. It was suggested that the effect of DTGE on the brain dopamine biosynthesis might be involved in the development of antipsychotic effects (204). It is noteworthy that Des-tyrosine-gamma-endorphin [beta-endorphin-(2-17); DTG E] lacks direct in-vitro activity at dopaminergic receptors, but does inhibit in vivo [3H] spiperone binding in various rat brain areas an effect similar to beta-endorphin-(6-17) which is now considered a major metabolite of DTGE (205). Additionally, local administration of [Des-Tyr1]-gamma-endorphin (LPH62-77) but not alpha-endorphin (LPH61-76) in either the NAc or the neostriatum mimics the effect of anti-psychotics potentially through ACTH and dopaminergic mechanisms (206).
It is well known that schizophrenic patients are behaviorally supersensitive to dopamine-like drugs (amphetamine, methylphenidate). Accordingly, there is evidence for increased release of dopamine; a slight increase in dopamine D2 receptors and an increase of dopamine D2High receptors in these patients (207) which may explain this supersensitivity. Most interestingly, Seeman (207) pointed out that the elevation in apparent D2High receptors in schizophrenia matches the elevation in D2High receptors, in many animal models of psychosis. This could be due to a number of factors: the rate of phosphorylation and desensitization of D2 receptors by kinases; the attachment of arrestin to D2 receptors; internalization of D2 receptors; rate of receptor de-phosphorylation; formation of D2 receptor dimers; GTP regulation by various GTPases. Importantly and clinically relevant, haloperidol reduces the number of D2high receptors induced by psychostimulants. In terms of SUD, Blum et al. proposed a neurobiological and neurogenetic mechanism involving supersensitivity of DRD2 (208).
The potential role of dopaminergic polymorphisms and psychiatric disease and SUD have been the subject of intense investigation (209-223) since the initial findings of Blum et al. on the DRD2 gene and severe alcoholism (24). We have entered the new genomic era and as such true understanding of the role of gene polymorphisms in SUD and Schizophrenia will be unraveled, as researchers continue to investigate the effect of antipsychotics on brain function (224). One area that has not been evaluated concerning the role of gamma endorphin and vulnerability to schizophrenia is the brain genetics of this important neuropeptide. We are encouraging the scientific community to heretofore initiate genotyping of associated polymorphisms of gamma-endorphin regulatory genes (synthesis, synaptic release; catabolism etc.) to determine schizophrenia susceptibility in large case control studies.
SUMMARY
Both SUD and schizophrenia are complex “polygenic” disorders involving the dopamine system. It was shown that A1 allele of DRD2 gene is a genetic risk for SUD (especially alcoholism) but not for carriers of the DRD2 A2 allele. We hypothesize that a plausible mechanism for alcohol seeking behavior in schizophrenics, carriers of the Taq1 A1 allele, could be a deficiency of gamma type endorphins. This deficiency could be the mechanism of aberrant hyperdopaminergic activity. We further propose that alcohol consumption in schizophrenics, may serve as a physiological self–healing process linked to the increase function of the gamma–type endorphins that consequently, reduced dopaminergic activity at the NAc. We suggested that there may be an overexpression of the DRD2 Taq1 A2 allele, an adaptive mechanism necessary to balance hyperactivity of the dopamine system, due to a lack of DTGE during embryonic development. Further, we hypothesize that in carriers of the DRD2 gene Taq1 A2 allele is associated with a subtype of non-SUD schizophrenics and as such this allele may serve as putative protective agent against the development of addiction to alcohol or other substances. These hypotheses support the proposition that within the dopamine system vulnerability to SUD and schizophrenia may result from two distinct sets of genetic associations that may be studied in sub-populations of schizophrenics and warrant further investigation and cautious interpretation.
We encourage research involving neuroimaging, genome wide association studies (GWAS), and epigenetic investigation into the relationship between neurogenetics and systems biology to unravel the role of dopamine in psychiatric illness and SUD.
Acknowledgements
Marlene Oscar-Berman is the recipient of grants from the National Institutes of Health, NIAAA RO1-AA07112 and K05- AA00219 and the Medical Research Service of the US Department of Veterans Affairs. Dr. Kenneth Blum is a recipients of a grant awarded to PATH Foundation NY from the Life Extension Foundation, Ft Lauderdale, Florida. The work of Dr Badgaiyan was partially supported by the National Institutes of Health grants 1R01NS073884 and 1R21MH073624 and the VA Merit Review Grants CX000479 and CX000780. We appreciate the expert editorial assistance of Margaret A. Madigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest to declare.
REFERENCES
- 1.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic –epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 2.Riley BP. Linkage studies of Schizophrenia. Neurotoxicity Res. 2004;6(1):17–34. doi: 10.1007/BF03033293. [DOI] [PubMed] [Google Scholar]
- 3.Fernanade-zRuiz J, Gomez M, Hennandez M, De Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. In: Palomo T, Beninger R, Kostrezewa T Archer, editors. Gene –environment interplay in disease states. Neurotoxcity Res. E.P. Graham Publishing Co.; Mountain Home Tenn. USA: 2004. pp. 389–409. Chapter 25. [Google Scholar]
- 4.Hoenicka J, Ponce G, JiméNez-Arriero MA, et al. Association in alcoholic patients between Psychopathic Traits and the additive effect of allelic forms of theCNR1 and FAAH endocannabinoid genes, and the 3′ Region of the DRD2 Gene. Neurotoxicity Research. 11(1):51–59. doi: 10.1007/BF03033482. [DOI] [PubMed] [Google Scholar]
- 5.Tybura P, Grzywacz A, Samochowiec A, Samochowiec J. Associations between candidate genes with schizophrenia susceptibility and the treatment efficiency. Psychiatr Pol. 2011;45(6):811–23. [PubMed] [Google Scholar]
- 6.Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am. J. Psychiatry. 1976;133:197–203. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- 7.Leriche L, Diaz J, Sokoloff P P. Dopamine and glutamate dysfunctions in schizophrenia : role of dopamine D3 receptor. Neurotoxicity Res. 2004;6:63–72. doi: 10.1007/BF03033298. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz JC, Diaz J, Pilon C, Sokoloff P. Possible implication of the dopamine D3 receptor in schizophrenia and in anti-psychotic drug actions. Brain Res. Rev. 2000;31:277–278. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 9.Lecrubier Y. A partial D3 receptor agonist in schizophrenia. Eur. Neuropsychopharmacol. 2003;13(Suppl. 4):S167–S168. [Google Scholar]
- 10.Malhotra AK. Candidate gene studies of antipsychotic drug efficacy and drug –induced weight gain. Neurotoxcity Res. 2004;6:51–56. doi: 10.1007/BF03033296. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra AK, Buchanan RW, Kim S, Kestler L, Brier A, Pickar D, Goldman D. Allelic variation in the promoter region of the dopamine D2 receptor gene and clozapine response. Schizopr. Res. 1999;36:92–93. (1999) [Google Scholar]
- 12.Shafer M, Rujescu D, Giergling I, Gunterman A, Erfurth A, Bondy B, Moller H. Association of the short –term response to haloperidol treatment with a polymorphism in the dopamine D2 receptor gene. Am. J. Psychiatr. 2001;158:802–804. doi: 10.1176/appi.ajp.158.5.802. [DOI] [PubMed] [Google Scholar]
- 13.Mata I, Arranz MJ, Lai T, et al. The seroternergic system influences individual’s response to risperidone. Am. J. Med. Genet. 2002;114:728. [Google Scholar]
- 14.Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 15.Gejman PV, Ram A, Gelernter J, et al. No structural mutation in the dopamine D2 receptor gene in alcoholism or schizophrenia. Analysis using denaturing gradient gel electrophoresis. JAMA. 1994;271:204–208. doi:10.1001/jama.1994.03510270050038. [PubMed] [Google Scholar]
- 16.Roca M, Martin-Santos R, Saiz J, et al. Diagnostic Interview for Genetic Studies (DIGS): inter-rater and test-retest reliability and validity in a Spanish population. Eur Psychiatry. 2007;22(1):44–48. doi: 10.1016/j.eurpsy.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Mewton L, Teesson M, Slade T, Grove R. The epidemiology of DSM-IV alcohol use disorders amongst young adults in the Australian population. Alcohol Alcohol. 2011;46(2):185–191. doi: 10.1093/alcalc/agq091. [DOI] [PubMed] [Google Scholar]
- 18.Laruelle M, Abi-Dargham A, van Dyck CH, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum K, Cull JG, Braverman ER, Comings DE. Reward Deficiency Syndrome. The American Scientist. 1996;84:132–145. [Google Scholar]
- 20.van Ree JM, de Wied D. Neuropeptides and addiction. In: Blum K, Manzo L, editors. Neurotxicology. Marcel Dekker, Inc.; New York: 1984. pp. 135–161. Chapter 7. [Google Scholar]
- 21.Volkow ND, Wang GJ, Begleiter H, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 22.Grandy DK, Litt M, Allen L, Bunzow JR, et al. The human dopamine D2 receptor gene is located chromosome 11 at q22-q23 identified a TaqI RFLP. Am J Hum Genet. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- 23.Smith M, Wasmuth J, McPherson JD. Cosegregation of an 11q22.3-9p22 translocation with affective disorder: proximity of the dopamine D2 receptor gene relative to the translocation breakpoint. Am J Hum Genet. 1989;45:A220. [Google Scholar]
- 24.Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. Journal of the American Medical Association. 1990;263:2055–2060. [PubMed] [Google Scholar]
- 25.Blum K, Braverman ER. Reward Deficiency Syndrome: A Biogenetic Model for the Diagnosis and Treatment of Impulsive, Addictive and Compulsive Behaviors. Journal Of Psychoactive Drugs. 2000;32:1–112. doi: 10.1080/02791072.2000.10736099. (Supplement) [DOI] [PubMed] [Google Scholar]
- 26.Ponce G, Jimenez –Arriero MA, Rubio G, Hoenicka J, Ampuero I, Ramos JA, Palomo T. The A1 allele of the DRD2 gene (Taq1 A polymorphisms) is associated with antisocial personality in a sample of alcohol –dependent patients. European Psychiatry. 2003;18:356–360. doi: 10.1016/j.eurpsy.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Noble EP, Ozkaragoz TZ, Ritchie T, Zhang X, Bekin TR, Sparkes RS. D2 and D4 dopamine receptor polymorphisms and personality. Am. J. Med. Genet. 1998;81:257–267. [PubMed] [Google Scholar]
- 28.Hill SY, Zezza N, Wipprecht G, Xu J, Neiswanger K. Linkage studies of D2 and D4 receptor genes and alcoholism. Am J Med Genet. 1999;88(6):676–685. doi: 10.1002/(sici)1096-8628(19991215)88:6<676::aid-ajmg18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Lowinson J, Ruiz P, Millman R, Langrod J. Substance abuse: A comprehensive textbook. 4th Edition Lippincott William & Wilkens; Philadelphia USA: 2005. [Google Scholar]
- 30.Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. J.Am.Med.Assn. 1991;266:1793–1800. [PubMed] [Google Scholar]
- 31.Blum K, Braverman ER, Wood RC, et al. Increased prevalence of the Taq1 A1 allele of the dopamine receptor gene in obesity with comorbid substance use disorder. Pharmacogenetics. 1996;6:297–305. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratsma JE, van der Stelt O, Schoffelmeer ANM, Westerveld A, Ginning WB. P3 event-related potential, dopamine D2 A1 allele, and sensation –seeking in adult children of alcoholics. Alcohol. Clin. Exp. Res. 2001;25:960–967. [PubMed] [Google Scholar]
- 34.Romstad A, Dupont E, Krag-Olsen B, Ostergaard K, Guldberg P, Guttler F. Dopa-responsive dystonia and Tourette syndrome in a large Danish family. Arch Neurol. 2003;60(4):618–622. doi: 10.1001/archneur.60.4.618. [DOI] [PubMed] [Google Scholar]
- 35.Singer HS, Szymanski S, Giuliano J, et al. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159(8):1329–1336. doi: 10.1176/appi.ajp.159.8.1329. [DOI] [PubMed] [Google Scholar]
- 36.Smith KM, Daly M, Fischer M, Yiannoutsoss CT, Bauer L, Barkley R, Navia BA. Association of the dopamine beta hydroxylase gene with attention deficit hyperactivity disorder: Genetic analysis of the Milwaukee longitudinal study. Am J Med Genet. 2003;119(1):77–85. doi: 10.1002/ajmg.b.20005. [DOI] [PubMed] [Google Scholar]
- 37.Comings DE, Gade R, MacMurray JP, et al. Genetic variants of the human obesity (OB) gene: association with body mass index in young women psychiatric symptoms, and interaction with the dopamine D2 receptor gene. Molecular Psychiatry. 1996;1:325–335. [PubMed] [Google Scholar]
- 38.Comings DE, Wu S, Chiu C, et al. Polygenic inheritance of Tourette syndrome, stuttering, attention deficit hyperactivity, conduct, and oppositional defiant disorder: The additive and subtractive effect of the three dopaminergic genes DRD2, DBH, and DAT1. Am J Med Genet. 1996;67:264–88. doi: 10.1002/(SICI)1096-8628(19960531)67:3<264::AID-AJMG4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 39.Comings DE, Gade-Andavilu R, Gonzalez N, et al. Multivariate analysis of associations of 42 genes in ADHD, ODD and conduct disorder. Clin Genet. 2000;58:31–40. doi: 10.1034/j.1399-0004.2000.580106.x. [DOI] [PubMed] [Google Scholar]
- 40.Comings D, Johnson P, Dietz G, Muhleman D. Dopamine D2 Receptor Gene (DRD2) Haplotypes and the Defense Style Questionnaire in Substance Abuse, Tourette Syndrome and Controls. Biol.Psychiatry. 1995;37:798–805. doi: 10.1016/0006-3223(94)00222-O. [DOI] [PubMed] [Google Scholar]
- 41.Blum K, Kozlowski GP. Ethanol and neuromodulator interactions: a cascade model of reward. In: Ollat H, Parvez S, Parvez H, editors. Alcohol and Behavior. Utrecht, Netherlands, VSP Press; 1990. pp. 131–149. [Google Scholar]
- 42.Rowe DC Genetic and environmental components of antisocial behavior: A study of 265 twin pairs. Criminology. 1986;24:513–532. [Google Scholar]
- 43.Cools AR, Gingras MA, Nijmegen J. High and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol Biochem Behav. 1998;60:151–159. doi: 10.1016/s0091-3057(97)00586-8. [DOI] [PubMed] [Google Scholar]
- 44.Comings DE, Gade-Andavolu R, Gonzalez N, et al. The additive effect of neurotransmitter genes in pathological gambling. Clin Genet. 2001;60(2):107–116. doi: 10.1034/j.1399-0004.2001.600204.x. [DOI] [PubMed] [Google Scholar]
- 45.Reuter J, Raedler R, Rose M, Hand I, Glasher, Buchel C. Pathologucal gambling is linked to reduced activation of the mesolimbic system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 46.Eshleman AJ, Henningsen RA, Neve KA, Janowsky A. Release of dopamine via the human transporter. Mol Pharmacol. 1994;45:312–316. [PubMed] [Google Scholar]
- 47.Carboni E, Silvagni A, Rolando MTP, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gessa Gl., Mutoni F, Coller M, Vargin L, Mercer G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Reseach. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- 49.Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 50.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 51.Di Chiara G, Tanda G, Bassare V, et al. Drug addiction as a disorder of associative learning, Role of nucleus accumbens shell/extended amygdala dopamine. Ann NY Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 52.Di Chiara G, Impereto A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic systems of freely moving rats. Proceedings of the National Academy of Science USA. 1988;84:1413–1416. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch RJ, Sadlack F, Sheffield D. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- 54.Adler CM, Elman I, Weisenfield N, Kestler L, Pickar D, Breier A. Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacolgy. 2000;22(5):545–550. doi: 10.1016/S0893-133X(99)00153-0. [DOI] [PubMed] [Google Scholar]
- 55.Hallbus M, Magnusson T, Magnusson O. Influence of 5-HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: A microdialysis study. Neurosci Lett. 1997;225:57–60. doi: 10.1016/s0304-3940(97)00178-x. [DOI] [PubMed] [Google Scholar]
- 56.Koepp MJ, Gunn RN, Lawrence AD, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 57.Noble EP, Noble RE, Ritchie T, Grandy DK, Sparkes RS. D2 Receptor gene and obesity. Internat. J. Eating Disorders. 1994;15:205–217. doi: 10.1002/1098-108x(199404)15:3<205::aid-eat2260150303>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 58.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 59.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 60.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 61.Dick DM, Edenberg HJ, Xuei X, et al. Association of GABAG3 with alcohol dependence. Alcohol Clin Exp Res. 2004;28(1):4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- 62.Goldman D, Dean M, Brown GL, Bolos AM, Tokola R, Virkkunen M, Linnoila M. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholisms in Finland and the United States. Acta Psychiatr Scand. 1992;86:351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- 63.Comings DE, Dietz G, Johnson JPMJP. Association of the enkephalinase gene with low amplitude P300 waves. NeuroReport. 1999;10:2283–2285. doi: 10.1097/00001756-199908020-00011. (1999) [DOI] [PubMed] [Google Scholar]
- 64.Gardner EL. Brain Reward Mechanisms. In: Lowenson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 1997. pp. 51–58. [Google Scholar]
- 65.Connor JP, Young RM, Lawford BR, Ritchie TL, Noble EP. D2 dopamine receptor (DRD2) polymorphism is associated with severity of alcohol dependence. Eur Psychiatry. 2002;17:17–23. doi: 10.1016/s0924-9338(02)00625-9. [DOI] [PubMed] [Google Scholar]
- 66.Rommelspacher H, Raeder C, Kaulen P, Bruning G. Adaptive changes of dopamine-D2 receptors in rat brain following ethanol withdrawal: a quantitative autoradiographic investigation. Alcohol. 1992;9:355–362. doi: 10.1016/0741-8329(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 67.Blum K, Noble EP, Sheridan PJ, Finley O, et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8(5):409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- 68.Blum K, Sheridan PJ, Noble EP. The A1 allele of the dopamine D2 receptor gene as a genetic marker for risk of severe alcoholism. In: Racagnie G, Brunello N, Fukudo T, editors. Biological Psychiatry vol. 2. Vol. 2. Elsevier Science Publishers B.V.; Amsterdam: 1991. pp. 14–17. [Google Scholar]
- 69.Blum K, Noble EP, Sheridan PJ, et al. Genetic predisposition in alcoholism: Association of the D2 dopamine receptor TaqI B1 RFLP with severe alcoholics. Alcohol. 1993;10:59–67. doi: 10.1016/0741-8329(93)90054-r. [DOI] [PubMed] [Google Scholar]
- 70.O’Hara BF, Smith SS, Bird G, et al. Dopamine D2 receptor RFLPs, haplotypes and their association with substance use in black and Caucasian research volunteers. Hum Hered. 1993;43(4):209–218. doi: 10.1159/000154133. [DOI] [PubMed] [Google Scholar]
- 71.Comings DE, Muhlman D, Ahn C, Gysin R, Flanagan SD. The dopamine D2 receptor gene : a genetic risk factor in substance abuse. Drug Alcohol Depend. 1994;34:175–180. doi: 10.1016/0376-8716(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 72.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan P. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics. Archives of General Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 73.Parsian A, Todd RD, Devor EJ, O’Malley KL, Suarez BK, Reich T, Cloninger CR. Alcoholism and alleles of the human D2 dopamine receptor locus: Studies of Association and linkage. Arch Gen Psychiatry. 1991;48:655–663. doi: 10.1001/archpsyc.1991.01810310073013. [DOI] [PubMed] [Google Scholar]
- 74.Parsian A, Cloninger CR, Zhang ZH. Functional variant in the DRD2 receptor promoter region and subtypes of alcoholism. Am J Med Genet. 2000;96:407–411. doi: 10.1002/1096-8628(20000612)96:3<407::aid-ajmg32>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 75.Noble EP, Syndilko K, Fitch RJ, et al. D2 dopamine receptor Taq1 A alleles in medically ill alcoholic and nonalcoholic patients. Alcohol Alcohol. 1994;29:729–744. [PubMed] [Google Scholar]
- 76.Lawford BR, Young RM, Rowell JA, et al. A1 allele with alcoholism: medical severity of alcoholism and type of controls. Biol Psychiat. 1997;41:386–393. doi: 10.1016/S0006-3223(96)00478-7. [DOI] [PubMed] [Google Scholar]
- 77.Lawford BR, Young RM, Rowell JA, et al. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nat Med. 1995;1(4):337–341. doi: 10.1038/nm0495-337. [DOI] [PubMed] [Google Scholar]
- 78.Neiswanger K, Kaplan BB, Hill SY. What can the DRD2/alcoholism story teach us about association studies in psychiatric genetics? Am J Med Genet. 1995;60(4):272–275. doi: 10.1002/ajmg.1320600403. [DOI] [PubMed] [Google Scholar]
- 79.Hietata J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacol. 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- 80.Hill SY. Alternative strategies for uncovering genes contributing to alcoholism risk: unpredictable findings in a genetic wonderland. Alcohol. 1998;16:53–59. doi: 10.1016/s0741-8329(97)00177-8. [DOI] [PubMed] [Google Scholar]
- 81.Hill SY, Neiswanger K. The value of narrow psychiatric phenotypes and “super” normal controls. In: Blum K, Noble EP, editors. Handbook of Psychiatric Genetics. CRC Press; Boca Raton: 1997. [Google Scholar]
- 82.Hill SY, Zezza N, Wipprecht G, Locke J, Neiswanger K. Personality traits and dopamine receptors (D2 & D4): Linkage studies in families of alcoholics. Am J Med Genet. 1989;88:634–641. doi: 10.1002/(sici)1096-8628(19991215)88:6<634::aid-ajmg11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 83.Bau CHD, Almeida S, Hutz MH. The TaqI A1 allele of the dopamine D2 receptor gene and alcoholism in Brazil: Association and interaction with stress and harm avoidance on severity prediction. Am J Med Genet. 2000;96:302–306. doi: 10.1002/1096-8628(20000612)96:3<302::aid-ajmg13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 84.Laine TPJ, Ahonen A, Torniainen P, et al. Dopamine transporter increase in human brain after alcohol withdrawal. Mol Psychiat. 1999;4:189–191. doi: 10.1038/sj.mp.4000514. [DOI] [PubMed] [Google Scholar]
- 85.Laine TP, Ahonen A, Rasanen P, Tiihonen J. Dopamine transporter availability and depressive symptoms during alcohol withdrawal. Psychiat Res. 1999;90:153–157. doi: 10.1016/s0925-4927(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 86.Arinami T, Itokawa M, Komiyama T, et al. Association between severity of alcoholism and the A1 allele of the dopamine D2 receptor gene TaqI A RFLP in Japanese. Biol Psychiat. 1993;33:108–114. doi: 10.1016/0006-3223(93)90309-2. [DOI] [PubMed] [Google Scholar]
- 87.Kono Y, Yoneda H, Sakai T, et al. Association between early-onset alcoholism and the dopamine D2 receptor gene. Am J Med Genet. 1997;74:179–182. doi: 10.1002/(sici)1096-8628(19970418)74:2<179::aid-ajmg13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 88.Goldman D, Brown GL, Albaugh B, et al. DRD2 dopamine receptor genotype, linkage diseqilibrium and alcoholism. In American Indians and other populations. Alcohol Clin Exp Res. 1993;17:199–204. doi: 10.1111/j.1530-0277.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 89.Goldman D, Dean M, Brown GL, et al. D2 dopamine receptor genotype and cerebrospinal fluid homovanillic acid, 5-hydroxyindoleacetic acid and 3-methoxy-4-hydroxyphenylglycol in alcoholisms in Finland and the United States. Acta Psychiatr Scand. 1992;86:351–357. doi: 10.1111/j.1600-0447.1992.tb03279.x. [DOI] [PubMed] [Google Scholar]
- 90.Goldman D, Urbanek M, Guenther D, Robin R, Long JC. Linkage and association of a functional DRD2 variant [Ser311Cys] and DRD2 markers to alcoholism, substance abuse and schizophrenia in Southwestern American Indians. Am J Med Genet. 1997;74:386–394. doi: 10.1002/(sici)1096-8628(19970725)74:4<386::aid-ajmg9>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 91.Xu K, Lichterman D, Kipsky RH, et al. Association of specific Haplotypes of D2 dopamine Receptor Gene with Vulnerability to heroin dependence in distinct populations. Arch Gen Psychiatry. 2004;61:567–606. doi: 10.1001/archpsyc.61.6.597. [DOI] [PubMed] [Google Scholar]
- 92.Bolos AM, Dean M, Lucas-Derse S, Ramsburg M, Brown GL, Goldman D. Population and pedigree studies reveal a lock of association between the dopamine D2 receptor gene and alcoholism. JAMA. 1990;264:3156–3160. [PubMed] [Google Scholar]
- 93.Gelernter J, O’Malley S, Risch N, et al. No association between an allele at the D2 dopamine receptor gene (DRD2) and alcoholism. JAMA. 1991;266:1801–1907. [PubMed] [Google Scholar]
- 94.Gelernter J, Goldman D, Risch N. The A1 allele at the D2 dopamine receptor gene and alcoholism. A reappraisal. JAMA. 1993;269:1673–1677. [PubMed] [Google Scholar]
- 95.Cook BL, Wang ZW, Crowe RR, Hauser R, Freimer M. Alcoholism and the D2 Alcohol Clin Exp Res. 1992;16(4):806–809. doi: 10.1111/j.1530-0277.1992.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 96.Turner E, Ewing J, Shilling P, Smith TL, Irwin M, Schuckit M, Kelsoe JR. Lack of association between an RFLP near the D2 dopamine receptor gene and severe alcoholism. Biol. Psychiat. 1992;3:285–290. doi: 10.1016/0006-3223(92)90052-2. [DOI] [PubMed] [Google Scholar]
- 97.Suarez BK, Parsian A, Hampe CL, Todd RD, Reich T, Cloninger CR. Linkage disequilibria at the D2 Dopamine receptor locus (DRD2) in alcoholics and controls. Genomics. 1994;19:12–20. doi: 10.1006/geno.1994.1005. [DOI] [PubMed] [Google Scholar]
- 98.Chen CH, Chien SH, Hwu HG. Lack of association between TaqI A1 allele of dopamine D2 receptor gene and alcohol-use disorders in Atayal Natives of Taiwan. Am J Med Genet. 1996;67:488–490. doi: 10.1002/(SICI)1096-8628(19960920)67:5<488::AID-AJMG10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 99.Chen WJ, Loh EW, Hsu YP, Cheng AT. Dopamine D2 receptor gene and alcoholism among four aboriginal groups and han in Taiwan. Am J Med Genet. 1997;74:129–136. [PubMed] [Google Scholar]
- 100.Cruz C, Camarena B, Mejia JM, et al. The dopamine D2 receptor gene TaqI A1 polymorphism and alcoholism in a Mexican population. Arch Med Res. 1995;26:421–426. [PubMed] [Google Scholar]
- 101.Gejman PV, Gelernter RJ, Friedman E, et al. No structural mutation in the dopamine D2 receptor gene in alcoholism or schizophrenia. Analysis using denaturing gradient gel electrophoresis. JAMA. 1994;271(3):204–209. [PubMed] [Google Scholar]
- 102.Edenberg HJ, Foroud T, Koller DL, et al. A family-based analysis of the association of the dopamine D2 receptor (DRD2) with alcoholism. Alcohol Clin Exp Res. 1998;22:505–512. [PubMed] [Google Scholar]
- 103.Gebhardt C, Leisch F, Schussler P, et al. Non-association of dopamine D4 and D2 receptor genes with personality in healthy individuals. Psychiatr Genet. 2000;10:131–137. doi: 10.1097/00041444-200010030-00005. [DOI] [PubMed] [Google Scholar]
- 104.Lu RB, Ko HC, Chang FM, Yin SJ, Pakstis AJ, Kidd KK. No association between alcoholism and multiple polymorphism at the dopamine D2 receptor gene (DRD2) in three distinct Taiwanese populations. Biol Psychiatry. 1996;39:419–429. doi: 10.1016/0006-3223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 105.Merikangas KR. The genetic epidemiology of alcoholism. Psychol Med. 1990;20:11–22. doi: 10.1017/s0033291700013192. [DOI] [PubMed] [Google Scholar]
- 106.Gorwood P. Contribution of genetics to the concept of risk status for alcohol dependence. J Soc Biol. 2000;194(1):43–49. [PubMed] [Google Scholar]
- 107.Noble EP. DRD2 gene and alcoholism. Science. 1998;281(5381):1287–1288. doi: 10.1126/science.281.5381.1285h. [DOI] [PubMed] [Google Scholar]
- 108.Noble EP. The D2 dopamine receptor gene: a review of association studies in alcoholism and phenotypes. Alcohol. 1998;16:33–45. doi: 10.1016/s0741-8329(97)00175-4. [DOI] [PubMed] [Google Scholar]
- 109.Noble EP. The DRD2 gene in psychiatric and neurological disorder and its phenotypes. Pharmacogenomics. 2000;1(3):309–333. doi: 10.1517/14622416.1.3.309. [DOI] [PubMed] [Google Scholar]
- 110.Noble EP. D2 Dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet. 2003;116B:103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 111.Bowirrat A. Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am J Med Gen Part B. Neuropsychiatric Genetics. 2005;132B(1):29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 112.Barr CL, Kidd KK. Population frequencies of the A1 allele at the dopamine D2 receptor locus. Biol Psychiat. 1993;34:204–209. doi: 10.1016/0006-3223(93)90073-m. [DOI] [PubMed] [Google Scholar]
- 113.Blum K, Payne J. Alcohol and The Addictive Brain. The Free Press; Simon & Schuster New York NY: 1991. [Google Scholar]
- 114.Bouchard TJ. Genes, environment and personality. Science. 1994;9:415–421. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- 115.Cadoret RJ, Troughton E, Bagford J, Woodworth G. Genetic and environmental factors in adoptee antisocial personality. Eur Arch Psychiatr Neurol Sci. 1990;239:231–240. doi: 10.1007/BF01738577. [DOI] [PubMed] [Google Scholar]
- 116.Carey G. Genetic association study in psychiatry: Analytical evaluation and a recommendation. Am J Med Genet (Neuropsychiatr Genet) 1994;54:311–317. doi: 10.1002/ajmg.1320540407. [DOI] [PubMed] [Google Scholar]
- 117.Crowe RR. Candidate genes in psychiatry: An epidemiological perspective. Am J Med Genet (Neuropsychiatr Genet) 1993;48:74–77. doi: 10.1002/ajmg.1320480203. [DOI] [PubMed] [Google Scholar]
- 118.Grandy DK, Eubanks JH, Evans GA, Civelli O, Litt M. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991;10(3):527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- 119.Greenberg DA. Linkage analysis of “necessary” disease loci versus “susceptibility” loci. Am J Hum Genet. 1993;52:135–143. [PMC free article] [PubMed] [Google Scholar]
- 120.Oakley NR, Hayes AG, Sheehan MJ. Effect of typical and atypical neuroleptics on the behavioural consequences of activation by muscimol of mesolimbic and nigrostriatal dopaminergic pathways in the rat. Neuropharmacol (Berl) 1991;105(2):204–208. doi: 10.1007/BF02244310. [DOI] [PubMed] [Google Scholar]
- 121.Pato CN, Macciardi F, Pato MT, Verga M, Kennedy JL. Review of the putative association of dopamine D2 receptor and alcoholism. Am J Med Genet (Neuropsychiatr Genet) 1993;48:78–82. doi: 10.1002/ajmg.1320480204. [DOI] [PubMed] [Google Scholar]
- 122.Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism. Arch Gen Psychiat. 1991;43:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- 123.Clark WR. We Hardwired? The Role Of Genes In Human Behavior. Oxford University Press; New York: 2000. Grunstein Are. [Google Scholar]
- 124.Hodge SE. What association analysis can and cannot tell us about the genetics of complex disease. Am J Med Genet (Neuropsychiatr Genet) 1994;54:318–323. doi: 10.1002/ajmg.1320540408. [DOI] [PubMed] [Google Scholar]
- 125.Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science. 1994;264:1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- 126.Volkow ND, Wang GJ, Fowler JS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 127.Tiihonen J, Kuikka J, Bergstrom K, Hakola P, Kalni J, Rynanen OP, Fohr J. Altered striatal dopamine re-uptake site densities in habitually violent and non-violent alcoholics. Nature Med. 1995;1:654–657. doi: 10.1038/nm0795-654. [DOI] [PubMed] [Google Scholar]
- 128.Kuikka JT, Baulieu JL, Hiltunen J, et al. Pharmacokinetics and dosimetry of iodine-123 labeled PE2I in humans, a radioligand for dopamine transporter imaging. Eur J Nuc1 Med. 1998;25:531–534. doi: 10.1007/s002590050254. [DOI] [PubMed] [Google Scholar]
- 129.Little KY, McLaughlin DP, Zang L, et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users. Arch Gen Psychiat. 1998;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- 130.Little KY, Zang L. Striatal dopaminergic abnormalities in human cocaine users. Am J. Psychiat. 1999;156:238–245. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- 131.Repo E, Kuikka JT, Bergstrom KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacol. 1999;147:314–318. doi: 10.1007/s002130051173. [DOI] [PubMed] [Google Scholar]
- 132.Kuikka JT, Repo E, Bergstrom KA, Tupala E, Tiihonen J. Specific binding and laterality of human extrastriatal dopamine D2/D3 receptors in the late onset type 1 alcoholic patients. Neurosci Lett. 2000;292(1):57–59. doi: 10.1016/s0304-3940(00)01423-3. [DOI] [PubMed] [Google Scholar]
- 133.Panagis G, Nomikos GG, Miliaressis E, Chergui K, Kastellakis A, Svensson TH, Spyaki C. Ventral pallidum self-stimulation induces stimulus dependent increase in c-fos expression in reward-related brain regions. Neuroscience. 1997;77(1):175–186. doi: 10.1016/s0306-4522(96)00471-x. [DOI] [PubMed] [Google Scholar]
- 134.Jonsson EG, Cichon S, Gustavsson P, et al. Association between a promoter dopamine D2 receptor gene variant and the personality trait detachment. Biol Psychiat. 2003;53:577–584. doi: 10.1016/s0006-3223(02)01732-8. [DOI] [PubMed] [Google Scholar]
- 135.Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1-2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 136.Neville MJ, Johnstone EC, Walton RT. Identification and Characterization of ANKK1: A novel kinase gene closely linked to DRD2 on chromosome band 11q23.1. Human Mutation. 2004;23:540–545. doi: 10.1002/humu.20039. [DOI] [PubMed] [Google Scholar]
- 137.Robbins TW, Everitt BJ. Neurobiobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 138.Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with “reward. J Neurochem. 2002;82:721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- 139.Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- 140.Cooper M, Frone M, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Personality Social Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- 141.Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- 142.Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20(9):1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 143.Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA (A) receptors in specific limbic brain regions. Psychopharmacol (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- 144.Koehnke MD, Schich S, Lutz U, Willecke M, Koehnke AM, Kolb W, Gaertner I. Severity of alcohol withdrawal symptoms and the T1128C polymorphism of the neuropeptide Y gene. J Neural Transm. 2002;109(11):1423–1429. doi: 10.1007/s00702-002-0752-1. [DOI] [PubMed] [Google Scholar]
- 145.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol. 1994;5:383–404. doi: 10.1097/00008877-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 147.Gordon AS, Yao L, Jiang Z, Fish burn CS, Fuchs S, Diamond I. Ethanol acts synergistically with a D2 dopamine agonist to cause translocation of protein kinase C. Mol Pharmacol. 2001;59:153–160. doi: 10.1124/mol.59.1.153. [DOI] [PubMed] [Google Scholar]
- 148.Lobos EA, Todd RD. Association analysis in an evolutionary context: Cladistic analysis of the DRD2 locus to test for association with alcoholism. Am J Med Genet. 1998;81:411–419. [PubMed] [Google Scholar]
- 149.Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychol. 2002;21(2):139–146. [PubMed] [Google Scholar]
- 150.Myers RD, Robinson DE. Mu and D2 receptor antisense oligonucleotides injected in nucleus accumbens suppress high alcohol intake in genetic drinking HEP rats. Alcohol. 1999;18(2-3):225–233. doi: 10.1016/s0741-8329(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 151.Mash DC, Staley JK, Doepel FM, Young SN, Ervin FR, Palmour RM. Altered dopamine transporter densities in alcohol-preferring vervet monkeys. Neuro Report. 1996;7:457–462. doi: 10.1097/00001756-199601310-00020. [DOI] [PubMed] [Google Scholar]
- 152.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: A study and replication. Biol Psychiat. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 153.Kotter R, Stephan KE. Useless or helpful? The “limbic system” concept. Rev Neurosci. 1997;8:139–145. doi: 10.1515/revneuro.1997.8.2.139. [DOI] [PubMed] [Google Scholar]
- 154.Oscar-Berman M. Neuropsychological vulnerabilities in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA’s neuroscience and behavioral research portfolio. National Institute on Alcohol Abuse and Alcoholism Research Monograph-34. The Institute; Bethesda, MD: 2000. pp. 149–158. [Google Scholar]
- 155.Oscar-Berman M, Hutner N, Beaumont JG, Kenealy PM, Rogers MJC, editors. The Blackwell dictionary of neuropsychology. Blackwell Publishers; Cambridge, MA: 1996. Alcoholism; pp. 33–38. [Google Scholar]
- 156.Oscar-Berman M, McNamara P, Freedman M. Delayed-response tasks: Parallels between experimental ablation studies and finding in patients with frontal lesions. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal lobe function and injury. Oxford University Press; NY: 1991. pp. 231–255. [Google Scholar]
- 157.Koob GF. Neurobiology of addiction: Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 158.Arinami T, Itokawa M, Aoki J, Shibuya H, Ookubo Y, Iwawaki A, Ota K, Shimizu H, Hamaguchi H, Toru M. Further association study on dopamine D2 receptor S311C in schizophrenia and affective disorders. Am J Med Genet. 1996;67(2):133–138. doi: 10.1002/(SICI)1096-8628(19960409)67:2<133::AID-AJMG2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 159.Comings DE, Comings BG, Muhleman D, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- 160.Golimbet VE, Aksenova MG, Nosikov VV, Orlova VA, Kaleda VG. Analysis of the linkage of the Taq 1A and Taq 1B loci of the dopamine D2 receptor gene with schizophrenia in patients and their siblings. Neurosci Behav Physiol. 2003;33(3):223–225. doi: 10.1023/a:1022191012698. [DOI] [PubMed] [Google Scholar]
- 161.Schindler KM, Pato MT, Dourado A, et al. Association and linkage disequilibrium between a functional polymorphism of the dopamine-2 receptor gene and schizophrenia in a genetically homogeneous Portuguese population. Mol Psychiat. 2002;7(9):1002–1005. doi: 10.1038/sj.mp.4001126. [DOI] [PubMed] [Google Scholar]
- 162.Noble EP, Zhang X, Ritchie T, et al. D2 dopamine receptor and GABA (A) receptor beta3 subunit gene alcoholism. Psychiat Res. 1998;81:133–147. doi: 10.1016/s0165-1781(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 163.Parsons LH, Weiss F, Koob GF. Serotonin 1b receptor stimulation enhances dopamine-mediated reinforcement. Psychopharmacol. 1996;128:150–160. doi: 10.1007/s002130050120. [DOI] [PubMed] [Google Scholar]
- 164.Santiago M, Machado A, Cano J. Regulation of the prefrontal cortical dopamine release by GABAA and GABAB receptor agonists and antagonists. Brain Res. 1993;630:28–31. doi: 10.1016/0006-8993(93)90638-4. [DOI] [PubMed] [Google Scholar]
- 165.Tupala E, Hall H, Bergstrom K, et al. Dopamine D(2)/D(3)-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and type 2 alcoholics. Mol Psychiat. 2001;6(3):261–267. doi: 10.1038/sj.mp.4000859. [DOI] [PubMed] [Google Scholar]
- 166.Tupala E, Kuikka JT, Hall H, et al. Measurement of the striatal dopamine transporter density and heterogeneity in type 1 alcoholics using human whole hemisphere autoradiography. Neuroimage. 2001;1:87–94. doi: 10.1006/nimg.2001.0793. [DOI] [PubMed] [Google Scholar]
- 167.Pilla M, Perachon S, Sautel F, et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400(6742):371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 168.Comings DE, Wu S, Chiu C, et al. Polygenic inhertance of Tourette Syndrome, stuttering, attention-deficit-hyperactivity, conduct and oppositional defiant disorder. The addictive and subtractive effect of the three dopaminergic genes-DRD2, DBH and DAT1. Am J Med Gen (Neuropsychiatric Genetics) 1996;67:264–288. doi: 10.1002/(SICI)1096-8628(19960531)67:3<264::AID-AJMG4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 169.Tupala E, Hall H, Bergstrom K, et al. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20(2):91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacol. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 171.Comings DE, Blake H, Dietz G, et al. The preenkephalin gene(PENK) and opioid dependence. Neuroreport. 10:1133–135. doi: 10.1097/00001756-199904060-00042. [DOI] [PubMed] [Google Scholar]
- 172.Comings DE, Muhleman D, Wu S, McMurray JP. Association of the N-alpha- acetyltransferase gene (NAT1) with mild and sever substance abuse. Neuroreport. 2000;11(6):1227–1230. doi: 10.1097/00001756-200004270-00017. [DOI] [PubMed] [Google Scholar]
- 173.Ary AW, Lominac KD, Wroten MG, et al. Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci. 2013;33(19):8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Comings DE, Gade R, Wu S, Chiu C, et al. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Molecular Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- 175.Comings DE, Gade R, Muhlman D, et al. Exon and intron variants in the human tryptophan 2,3 dioxygenase gene: Potential association with Tourette Syndrome, substance abuse and other disorders. Pharmacogenetics. 1996;6:307–318. doi: 10.1097/00008571-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 176.Versteeg DH, Van Heuven-Nolsen D, De Wied D. Pre-decapitation state of arousal of rats predetermines the effect of des-Tyr1-gamma-endorphin on dopamine release from nucleus accumbens slices in vitro. Life Sci. 1984;34(16):1549–1554. doi: 10.1016/0024-3205(84)90609-x. [DOI] [PubMed] [Google Scholar]
- 177.De Wied D, Kovacs GL, Bohus B, Van Ree JM, Greven HM. Neuroleptic activity of the neuropeptide Beta- LPH - ( [Des- 1]– 62 77 Tyr gamma-endorphin; DT gamma E) Eur. J. Pharmacol. 1978;49:427–436. doi: 10.1016/0014-2999(78)90317-5. [DOI] [PubMed] [Google Scholar]
- 178.van Ree JM, Witter A, Leysen JE. Interaction of des-tyrosine-gamma-endorphin with neuroleptic binding sites in various areas of rat brain. Eur. J. Pharmacol. 1978;52:411–413. doi: 10.1016/0014-2999(78)90300-x. [DOI] [PubMed] [Google Scholar]
- 179.van Ree JM, Caffe AM, Wolterink G. Non-opiate Beta- endorphin fragments and dopamine. III. Gamma –type endorphins and various neuroleptics counteract the hyperactivity elicited by injection of apomorphine into the nucleus accumbens. Neuropharmacology. 1982;21:1111–1117. doi: 10.1016/0028-3908(82)90168-x. [DOI] [PubMed] [Google Scholar]
- 180.van Ree JM, De Wied D. Neuroleptic –like profile of gamma type endorphins as related to schizophrenia. Trends Pharmacol Sci. 1982;3:358–361. [Google Scholar]
- 181.De Wied D. Psychopathology as a neuropeptide dysfunction. In: vanRee JM, Terenius L, editors. Characteristics and Function of Opioids. Elsevier/North –Holland; Amsterdam: 1978. pp. 113–122. [Google Scholar]
- 182.van Ree JM, Verhoeven WMA, De Wied D, Van Praag HM. The use of the synthetic peptides gamma-type endorphins in mentally ill patients. Ann. N.Y. Acad. Sci. 1982;398:478–495. doi: 10.1111/j.1749-6632.1982.tb39519.x. [DOI] [PubMed] [Google Scholar]
- 183.Verhoeven WMA, van Praag HM, van Ree JM, De Wied D. Improvement of schizophrenic patients treated with [ Des-tyr1] – gamma-endorphin (DTgamma E) Arch Gen Psychiatry. 1979;36:294–298. doi: 10.1001/archpsyc.1979.01780030060005. [DOI] [PubMed] [Google Scholar]
- 184.Jackson HC, Ramsay E. Nutt DJ Attenuation of the behavioral effects of ethanol in mice by Desenkephalin-Gamma – Endorphin (ORG 5878) Alcohol & Alcoholism. 1992;27:373–379. [PubMed] [Google Scholar]
- 185.Chen TJH, Blum K, Mathews D, et al. Preliminary Association of both the Taq1 A1 Allele of the Dopamine D2 Receptor Gene and the Dopamine Transporter (DAT1) 480 bp Allele with Pathological Aggression in Adolescents. Med Hypotheses. 2005;65(4):703–707. doi: 10.1016/j.mehy.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 186.Chen CK, Chen SL, Mill J, et al. The dopamine transporter gene is associated with attention deficit hyperactivity disorder in a Taiwanese sample. Mol Psychiat. 2003;8(4):393–396. doi: 10.1038/sj.mp.4001238. [DOI] [PubMed] [Google Scholar]
- 187.Comings DE, Rosenthal RJ, Lesieur HR, et al. A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenet. 1996;6(3):223–234. doi: 10.1097/00008571-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 188.Uhl G, Blum K, Noble E, Smith S. Substance abuse vulnerability and D2 receptor genes. Trends Neurosci. 1993;16(3):83–88. doi: 10.1016/0166-2236(93)90128-9. [DOI] [PubMed] [Google Scholar]
- 189.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiat. 2001;158:377–382. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 190.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 191.Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 192.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- 194.Yoshida A, Hsu L, Yasumami M. Genetics of human alcohol-metabolizing enzymes. Progress in Nucleic Acid Research and Molecular Biology. 1991;40:255–287. doi: 10.1016/s0079-6603(08)60844-2. [DOI] [PubMed] [Google Scholar]
- 195.Crabb DW, Edenberg HJ, Bosron WF, Li TK. Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity: the inactive ALDH2*2 is dominant. J. of Clin. Invest. 1989;83:314–316. doi: 10.1172/JCI113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamamoto K, Ueno Y, Mizoi Y, Tatsuno Y. Genetic polymorphism of alcohol and aldehyde dehydrogenase and the effects on alcohol metabolism. Japan J of Alc. Drug Dep. 1993;28:13–25. [PubMed] [Google Scholar]
- 197.Higuchi S, Matsushita T, Imazeki H, Kinoshta T, Tikagi S, Kono H. Aldehyde dehdrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–742. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- 198.Matsushita S, Muramatsu T, Murayama M, Nakane J, Higuchi S. Alcoholism. ALDH2*2 allele and the A1 allele of the dopamine D2 receptor gene: an association study. Psychiatry Research. 2001;104:19–26. doi: 10.1016/s0165-1781(01)00290-6. [DOI] [PubMed] [Google Scholar]
- 199.Huang S-Y, Lin WW, Ko H-C, et al. Possible Interaction of Alcohol Dehydrogenase and Aldehyde Dehydrogenase Genes With the Dopamine D2 receptor Gene in Anxiety-Depressive Alcohol Dependence. Alcohol Clin Exp Resb. 2004;374:384. doi: 10.1097/01.alc.0000117832.62901.61. [DOI] [PubMed] [Google Scholar]
- 200.Lamensdorf I, Eisenhofer G, Harvey–White J, Nechustan A, Kirk K, Kopin IJ. 3,4 – Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]
- 201.Comings DE, Blum K. International Meeting On Implications Of Comorbidity For Etiology and Treatment Of Neuropsychiatric Disorders. Mazagon (Huelva) Spain; Oct 19-23, 2005. Reward Deficiency Syndrome: Genetic Aspects of Behavioral Disorders and Validation. [Google Scholar]
- 202.Hökfelt T, Elde R, Johansson O, Terenius L, Stein L. The distribution of enkephalin-immunoreactive cell bodies in the rat central nervous system. Neurosci Lett. 1977;5:25–31. doi: 10.1016/0304-3940(77)90160-4. [DOI] [PubMed] [Google Scholar]
- 203.De Wied D, Sigling HO. Neuropeptides involved in the pathophysiology of schizophrenia and major depression. Neurotox Res. 2002;4:453–468. doi: 10.1080/10298420290031432. [DOI] [PubMed] [Google Scholar]
- 204.Raevskiǐ KS, Shemanov AIu, Kudrin VS. Des-1-tyrosine-gamma-endorphin: an assessment of its neuroleptic properties and effect on dopamine synthesis. Farmakol Toksikol. 1982;45:5–8. [PubMed] [Google Scholar]
- 205.Schoemaker H, Davis TP, Pedigo NW, Chen A, Berens ES, Ragan P, Ling NC, Yamamura HI. Identification of beta-endorphin-6(16-17) as the principal metabolite of des-tyrosin-gamma-endorphin (DTgammaE) in vitro and assessment of its activity in neurotransmitter receptor binding assays. Eur J Pharmacol. 1982;81:459–468. doi: 10.1016/0014-2999(82)90111-x. [DOI] [PubMed] [Google Scholar]
- 206.Gispen WH, Ormond D, ten Haaf J, De Wied D. Modulation of ACTH-induced grooming by [Des-Tyr1]-gamma-endorphin and haloperidol. Eur J Pharmacol. 1980;63:203–207. doi: 10.1016/0014-2999(80)90447-1. [DOI] [PubMed] [Google Scholar]
- 207.Seeman P. Are dopamine D2 receptors out of control in psychosis? Prog Neuropsychopharmacol Biol Psychiatry. 2013;46C:146–152. doi: 10.1016/j.pnpbp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 208.Blum K, Chen TJ, Downs BW, Bowirrat A, et al. Neurogenetics of dopaminergic receptor supersensitivity in activation of brain reward circuitry and relapse: proposing “deprivation-amplification relapse therapy” (DART) Postgrad Med. 2009;121(6):176–196. doi: 10.3810/pgm.2009.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xiao L, Shen T, Peng DH, Shu C, Jiang KD, Wang GH. Functional -141C Ins/Del polymorphism in the dopamine D2 receptor genepromoter and schizophrenia in a Chinese Han population. J Int Med Res. 2013;41:1171–1178. doi: 10.1177/0300060513483415. [DOI] [PubMed] [Google Scholar]
- 210.Borroto-Escuela DO, Ravani A, Tarakanov AO, et al. Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem Biophys Res Commun. 2013;435:140–146. doi: 10.1016/j.bbrc.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 211.Pinacho R, Villalmanzo N, Roca M, et al. Analysis of Sp transcription factors in the postmortem brain of chronic schizophrenia: a pilot study of relationship to negative symptoms. J Psychiatr Res. 2013;47(7):926–934. doi: 10.1016/j.jpsychires.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 212.Glass MJ, Robinson DC, Waters E, Pickel VM. Deletion of the NMDA-NR1 receptor subunit gene in the mouse nucleus accumbens attenuates apomorphine-induced dopamine D1 receptor trafficking and acoustic startle behavior. Synapse. 2013;67:265–279. doi: 10.1002/syn.21637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang Y, Sasaoka T, Dang MT. A molecular genetic approach to uncovering the differential functions of dopamine D2 receptor isoforms. Methods Mol Biol. 2013;964:181–200. doi: 10.1007/978-1-62703-251-3_11. [DOI] [PubMed] [Google Scholar]
- 214.Novak G, Fan T, O’Dowd BF, George SR. Striatal development involves a switch in gene expression networks, followed by a myelination event: implications for neuropsychiatric disease. Synapse. 2013;67:179–188. doi: 10.1002/syn.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Giegling I, Balzarro B, Porcelli S, et al. Influence of ANKK1 and DRD2 polymorphisms in response to haloperidol. Eur Arch Psychiatry Clin Neurosci. 2013;263:65–74. doi: 10.1007/s00406-012-0348-1. [DOI] [PubMed] [Google Scholar]
- 216.Sáiz PA, García-Portilla MP, Arango C, et al. Genetic polymorphisms in the dopamine-2 receptor (DRD2), dopamine-3 receptor (DRD3), and dopamine transporter (SLC6A3) genes in schizophrenia: Data from an association study. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):26–31. doi: 10.1016/j.pnpbp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 217.Urigüen L, García-Fuster MJ, Callado LF, et al. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206:313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 218.Ponce G, Pérez-González R, Aragüés M, et al. The ANKK1 kinase gene and psychiatric disorders. Neurotox Res. 2009;16:50–59. doi: 10.1007/s12640-009-9046-9. [DOI] [PubMed] [Google Scholar]
- 219.Lafuente A, Bernardo M, Mas S, et al. Polymorphism of dopamine D2 receptor (TaqIA, TaqIB, and-141C Ins/Del) and dopamine degradation enzyme (COMT G158A, A-278G) genes and extrapyramidal symptoms in patients with schizophrenia and bipolar disorders. Psychiatry Res. 2008;161:131–41. doi: 10.1016/j.psychres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 220.Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- 221.Moriguchi S, Bies RR, Remington G, et al. Estimated Dopamine D2 Receptor Occupancy and Remission in Schizophrenia: Analysis of the CATIE Data. J Clin Psychopharmacol. 2013;33(5):682–685. doi: 10.1097/JCP.0b013e3182979a0a. doi: 10.1097/JCP.0b013e3182979a0a. [DOI] [PubMed] [Google Scholar]
- 222.Hoenicka J, Aragüés M, Rodríguez-Jiménez R, et al. Psychosis and Addictions Research Group (PARG). C957T DRD2 polymorphism is associated with schizophrenia in Spanish patients. Acta Psychiatr Scand. 2006;114:435–438. doi: 10.1111/j.1600-0447.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 223.Virgos C, Martorell L, Valero J, et al. Association study of schizophrenia with polymorphisms at six candidate genes. Schizophr Res. 2001;49:65–71. doi: 10.1016/s0920-9964(00)00106-7. [DOI] [PubMed] [Google Scholar]
- 224.Sweatt JD. The emerging field of neuroepigenetics. Neuron. 2013;80:624–632. doi: 10.1016/j.neuron.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]