Abstract
Mesenchymal stem cells (MSCs) have been suggested to participate in immune regulation and airway repair/remodeling. Transforming growth factor β1 (TGFβ1) is critical in the recruitment of stem/progenitor cells for tissue repair, remodeling and cell differentiation. In this study, we sought to investigate the role of TGFβ1 in MSC migration in allergic asthma. We examined nestin expression (a marker for MSCs) and TGFβ1 signaling activation in airways in cockroach allergen (CRE) induced mouse models. Compared with control mice, there were increased nestin+ cells in airways, and higher levels of active TGFβ1 in serum and p-Smad2/3 expression in lungs of CRE-treated mice. Increased activation of TGFβ1 signaling was also found in CRE-treated MSCs. We then assessed MSC migration induced by conditioned medium (ECM) from CRE-challenged human epithelium in air/liquid interface (ALI) culture in Transwell assays. MSC migration was stimulated by ECM, but was significantly inhibited by either TGFβ1 neutralizing antibody or TβR1 inhibitor. Intriguingly, increased migration of MSCs from blood and bone marrow to the airway was also observed after systemic injection of GFP+-MSCs, and from bone marrow of Nes-GFP mice following CRE challenge. Furthermore, TGFβ1 neutralizing antibody inhibited the CRE-induced MSC recruitment, but promoted airway inflammation. Finally, we investigated the role of MSCs in modulating CRE induced T cell response, and found that MSCs significantly inhibited CRE-induced inflammatory cytokine secretion (IL-4, IL13, IL17 and IFN-γ) by CD4+ T cells. These results suggest that TGFβ1 may be a key pro-migratory factor in recruiting MSCs to the airways in mouse models of asthma.
Keywords: asthma, cockroach allergen, TGFβ1, mesenchymal stem cells (MSCs)
Introduction
Asthma is a serious chronic illness that affects an estimated 300 million people and has a severe public health impact both in the U.S. and worldwide (1). The major obstacle in preventing and treating asthma has been our incomplete understanding of its etiology and biological mechanisms. Recent studies have changed our understanding of asthma as a purely inflammatory disease to a disease in which both inflammatory and structural components are equally involved (2). Asthma is often associated with structural remodeling of the airways characterized by airway epithelial damage, wall thickening, and sub-epithelial fibrosis (2). It has been suggested that there is a strong link between allergen exposure and sensitization and development of asthma (3, 4). In particular, exposure to cockroach allergen in early life can lead to allergic airway inflammation and increased risk of developing asthma (5-7). In inner-city populations, 60-80% of children with asthma are sensitized to cockroach allergen (8, 9). However, fundamental questions remain to be fully elucidated regarding the genesis of cockroach allergen induced asthma.
Stem/progenitor cells are known to participate in tissue repair and remodeling (10, 11). MSCs are adult connective tissue progenitor cells with the ability of self-renewal and differentiation into multiple cell types, such as osteoblasts, adipocytes, chondrocytes, and fibroblasts (12). Of interest to us, MSCs can suppress lung inflammation (13) (14) and participate in tissue repair/remodeling by differentiating into a number of mature cell types such as epithelial cells (15-19) and fibroblasts/myofibroblasts (20). In mouse asthmatic models, MSCs are significantly increased in the lungs after allergen sensitization and challenge (13, 21-23), suggesting that MSCs may play a role in the pathogenesis of allergic asthma. The role of MSC recruitment in airway repair/remodeling during asthma seems controversial. Several lines of evidence suggest that the migrated MSCs at early stages repair the damaged airway through cell differentiation and paracrine effects (secretion of growth factors and cytokines at sites of tissue injury and inflammation) (24, 25). However, in chronic stages when there are repetitive allergen challenges and sustained release of local growth factors, MSCs may contribute to progressive fibrosis and pathological remodeling due to their differentiation into myofibroblasts (20). Importantly, MSCs have been shown to modulate the immune responses of various immunocytes like T cells (26, 27), B cells (28), and dendritic cells (DCs) (29), interact with resident alveolar epithelial cells(AECs) (30), and cause mature DCs to promote T cell tolerance and impairment of T cell priming by DCs (31). However, the primary endogenous factor(s) and signaling pathways that control MSC migration to the lung are largely unknown. Further, it is still controversial as to whether MSCs can directly engraft and differentiate into epithelial cells at sites of airway damage. It has been suggested that the mobilization of stem cells from bone marrow/blood circulation is regulated by locally released chemotactic substances (32). Indeed, our recent studies have suggested that activated TGFβ1 released from the injured vessels controls mobilization and recruitment of MSCs to participate in tissue repair/remodeling (33).
TGFβ1 is a mutifunctional cytokine that plays a critical role in cell growth, differentiation, and immune regulation, and has been considered a principal mediator of airway remodeling (34-38). TGFβ1, while in a latent form maintained in a sequestered state in the cell matrix, is considered to be a molecular sensor that releases active TGFβ1 in response to the perturbations of the extracellular matrix at the sites of mechanical stress, wound repair, tissue injury, and inflammation (39, 40). Recent studies have demonstrated that disruption in TGFβ1 signaling imposes a strong predisposition for human allergic diseases (41). Specifically, increased active TGFβ1 has been observed in airways from asthmatic patients (42) and from experimental mice during allergic airway inflammation (43). Further, TGFβ1 has been shown to promote immune responses in the presence of MSCs (44). The stage is thus set to critically evaluate the functional significance of TGFβ1 activation in the migration of MSCs which may be critical in anti-inflammatory responses and tissue repair/remodeling.
In the present study, we have specifically focused on the functional significance of TGFβ1 activation in the migration of MSCs which may be critical in modulating allergen induced airway allergic inflammation. We found increased MSC expression and activated TGFβ1 signaling in airway of cockroach allergen (CRE) induced mouse asthmatic models and in CRE-treated MSCs. We then demonstrated that active TGFβ1 released from CRE-challenged human epithelium is a primary allergen-activated messenger for MSC migration in vitro. Importantly, we observed the increased recruitment of MSCs to the airway from blood and bone marrow in CRE-induced mouse asthmatic model in a TGFβ1-dependent process. Finally, we investigated the effect of TGFβ1on allergen-induced allergic inflammation and the role of MSCs in modulating CRE induced T cell response. These studies provide an important basis for further detailed investigation of the role of TGFβ1signaling in MSC-involved airway inflammation and repairing/remodeling in allergen-induced asthma.
Materials and Methods
Mice
Four- to six-week-old male and female C57BL/6J mice and nestin-GFP-transgenic mice(45) and C57BL/6J-GFP mice(46) were used. All animals were maintained under specific pathogen-free conditions in the animal facility of the Johns Hopkins University School of Medicine. The experimental protocols were reviewed and approved by the Animal Care and Use Committee at Johns Hopkins University School of Medicine.
Cockroach allergen-induced asthma mouse model
To generate a mouse model of cockroach allergen-induced asthma, mice were sensitized intraperitoneally with cockroach extract (CRE) at a concentration of 20μg/mouse with 2mg alum as adjuvant on day 0, 7, and then challenged with the same amount of CRE via intranasal instillation for three successive days (day 21, 22, and 23). Control mice received the same volume of PBS in alum. On day 24, mice were sacrificed, bronchoalveolar lavage (BAL) fluid was collected and lungs were harvested for proposed studies. Serum was harvested for the measurement of cockroach allergen specific IgE.
Serum cockroach allergen specific IgE measurement
Serum IgG was depleted with Protein G Sepharose 4 Fast Flow beads (GE Healthcare Life Sciences) to increase the sensitivity of IgE measurement according to the reference(47). The IgG depleted serum was analyzed by ELISA according to our previous work(48). Briefly, plates were coated with CRE (10 μg/ml) at 4 °C overnight. Plates were blocked for 1 h with ELISA blocking buffer (eBiosciences). Serum samples was added and incubated overnight at 4 °C. Biotinylated anti-mouse IgE (BioLegend) were added, followed by streptavadin-HRP and TMB substrate. Absorption at 450 nm was measured with Bio-Rad iMark Microplate Absorbance Reader.
Lung histology
Harvested lungs were fixed in 10% neutral buffered formalin overnight, and embedded in paraffin or OCT for frozen section. 5-μM lung sections were cut and then stained with hematoxylin and eosin (H&E) to evaluate general morphology. For immunohistochemical staining, non-specific binding was blocked using 10% blocking serum in PBS for 1 hour, and the tissue samples were then incubated with primary antibodies to nestin (clone 10C2, Abcam), GFP (clone 4B10, Cell Signaling Technology Inc.), MBP (clone MT-14.7, Lee Laboratory, Mayo Clinic), and p-Smad2/3 (Santa Cruz Biotechnology Inc.) overnight at 4°C. A horseradish peroxidase-streptavidin detection system (Dako, Glostrup, Denmark) was used to detect immunoactivity followed by counterstaining with hematoxylin. For immunofluorescent staining, secondary antibodies conjugated with fluorescence were added, and slides were incubated at room temperature for 1 h. Isotype-matched negative control antibodies (R&D Systems) were used under the same conditions. Nuclei were counterstained with 6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Sigma). The sections were mounted with the ProLong Gold Anti-fade Kit (Molecular Probes, Grand Island, NY) and observed under a microscope (Olympus). All histomorphometric parameters in four randomly selected visual fields per specimen, in five specimens per mouse in each group were measured. Similar approach was used for the immunofluorescent staining of p-Smad2/3 in cultured bone marrow-derived MSCs.
Bronchoalveolar lavage fluid and cellular differential count
Harvested BAL fluid was centrifuged; the supernatants were then collected for the measurement of active TGFβ1 by enzyme-linked immunosorbent assay (ELISA) (eBioscience). Total cell number was counted using a hemacytometer (Hausser-Scientific), and cellular differential percentage was determined by means of flow cytometry. Briefly, BALF cells were first blocked with 10μg/ml IgG Fc receptor blocking reagent(2.4G2, BD Biosystems) for 10min, then stained with the mixture of the following antibodies, Anti-CD107b (Mac-3) FITC(M3/84,BD Biosystems); anti-Siglec-F-PE (E-50-2440, BD Biosystems); Anti-Ly-6G (Gr-1) PerCP-Cyanine5.5 (RB6-8C5, eBioscience); anti-CD3ε APC(145-2C11, eBioscience) and anti-CD19 APC(1D3, eBioscience) for 30 min. Cells were analyzed on a FACS Calibur cytometer (BD Biosystems). Lymphocytes were identified as FSClow/SSClow and expressing CD3 or CD19. Granulocytes were recognized as SSChigh Gr-1+cells; Eosinophils were defined as SSChigh SiglecF+Mac-3−cells. Alveolar macrophages cells were identified as SSChigh SiglecF+ Mac-3+ cells.
Western blotting
Cells were washed twice with ice cold PBS and lysed in RIPA buffer containing protease and phosphatase inhibitor cocktails (Sigma). Protein content was measured with BCA reagent (Pierce). Equivalent protein samples were subjected to SDS-PAGE electrophoresis and then transferred to a polyvinylidene difluoride membrane (Millipore). After blocking with 5% nonfat dry milk in TBST, the membrane was incubated with primary anti-p-Smad2/3 and anti-Smad2 (Santa Cruz Biotechnology Inc.). Proteins reactive with primary Abs were visualized with an HRP-conjugated secondary Ab and ECL reagents (Amersham).
Migration assay
To prepare the airway epithelial conditioned medium (ECM), we utilized an Air-Liquid Interface (ALI) culture system for human airway epithelial cells. The isolated epithelial cells from central airway of human subjects (provided by Dr. Allen Myers, n=3), were cultured for 21 days as described previously (49), then cultured with serum-free Dulbecco’s modified Eagle’s medium (DMEM), and treated with cockroach extract (B46, 100μg/ml, GREER Laboratories) at 37°C for 24 hours; the cultured medium was collected and stored at −80°C. Cell migration was performed in 96-well Transwells (Corning, Inc., Acton, MA). A total of 2×104 MSCs (Texas A&M, Institute for Regenerative Medicine) in 50 μl serum-free DMEM were placed in the pre-coated upper chambers with 0.5μg/ml type I collagen (BD Biosciences, San Diego, CA), and 150 μl undiluted ECM was added to the type I collagen coated lower chamber of the transwell. In some experiments, neutralizing antibodies against TGFβ1 (1D11, R&D Systems), or SDF1 (Cell Signaling Technology) or TGFβ type I receptor (TβRI) inhibitor (SB-505124, Sigma-Aldrich) were added to the ECM. After 10 hours of incubation, cells that migrated to the lower side of the filter were fixed with 10% formaldehyde, stained with hematoxylin (Sigma-Aldrich, St. Louis, MO), and then counted under a microscope. Counted cells were expressed as number of migrated cells in five fields (×20).
Isolation of MSCs
Bone-marrow derived MSCs were isolated from mice and characterized using the established approaches in our previous work (50) (51) (52). In brief, the isolated cells from bone marrow were cultured with DMEM and 20% fetal bovine serum (FBS, Atlanta Biologicals) at 37°C in a 5% CO2 humidified incubator. After 72 hours, non-adherent cells were removed and adherent cells were cultured for additional 14 days. The adherent cells were retrieved by 0.25% trypsin digestion containing 0.02% ethylenediaminetetraacetic and sorted by markers Sca-1+, CD29+, CD45− and CD11b− (Bio-Legend). The sorted cells were enriched by further culture. A similar procedure was performed on GFP-labeled MSCs to sort GFP-labeled Sca-1+CD45− CD11b− MSCs(52).
In vivo MSC migration by GFP+MSC injection or in Nes-GFP mice
To examine the MSC migration from blood to the lung, sorted GFP+MSCs (2×106) from C57BL/6J-GFP mice were injected into cockroach allergen sensitized C57BL/6J mice through tail vein right before challenge (day 20). After three day consecutive CRE challenge, mice were sacrificed on day 24, and lung tissues were harvested for the analysis of GFP+ cells. To further examine the migration of endogenous MSCs, we used Nes-GFP mice; a transgenic mouse reporter line expressing GFP under the control of enhancer/promoter of nestin gene(53), to generate CRE induced mouse models of asthma using the same protocol as above. Similarly, lung tissues were harvested for the analysis of GFP+ cells. To see the effect of TGF-β1 on MSC migration, the cockroach allergen sensitized mice were injected intraperitoneally with TGFβ1 neutralizing antibody (1D11) or isotype control antibody (13C4) at a concentration of 0.25 mg/mouse on day 20 (1 day before the initial challenge). The mice were sacrificed at 24 hours after the last challenge (day 24). Recruitment of MSCs to the allergen-challenged airways was analyzed by immunofluorescent staining for GFP.
Flow cytometric analysis
For the analysis of Tregs in lung lymph nodes, the pulmonary hilar lymph nodes were collected and teased apart into a single cell suspension by pressing with the plunger of a 3 ml syringe. The cells were first stained with anti-CD4-FITC(RM4-5, eBioscience, San Diego, CA, USA) and anti-CD25-PE antibodies (PC61.5, eBioscience), followed by intracellular staining with FoxP3-APC(FJK-16s ,eBioscience) or APC-conjugated rat immunoglobulin (Ig) G2a isotype control (eBioscience) using a FoxP3 staining kit (eBioscience). The samples were then analyzed on a FACSCalibur flow cytometer (BD Biosystems). Similar approaches were used for the analysis of TβRI and TβRII (Santa Cruz Biotechnology Inc.) in bone morrow derived MSCs.
Functional effect of MSCs on CRE induced T cell responses
To test if MSCs could inhibit CRE induced T cell response in vitro, mice were sensitized with 50μg CRE plus 2mg alum as adjuvant intraperitoneally on day 0 and 7, and sacrificed on day 21. CD4+ T cells were purified from the spleen and lymph nodes using purification systems from Miltenyi Biotec (Auburn, CA) following the manufacturer’s instructions. Isolated CD4+ T cells (3×105/200ul) were co-cultured with the rest of splenocytes (3×105) that were irradiated with 20Gy as antigen presenting cells in the presence or absence of 50ug/ml CRE in a 96 well plate for 3 days. For MSCs inhibition analysis, 2 × 104 MSCs were added to the culture systems 2h before adding T cells. The culture supernatant was collected 3 days later and the abundance of cytokines was measured by ELISA for IL-4, IL-13, IL-17 and IFN-γ (eBioscience).
Statistical analysis
Data are expressed as the means ± SEM for each group. Statistical significance for normally distributed samples was assessed using an independent two-tailed Student’s t-test or with analysis of Variance by using GraphPad Prism version 5.1 software (GraphPad Software, La Jolla, CA). Differences with P< 0.05 were considered statistically significant.
Results
MSCs are accumulated in lung tissue of CRE-challenged mice
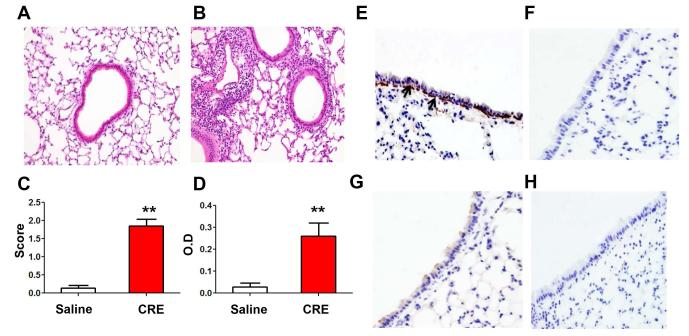
MSCs have been shown to be increased in the lungs after allergen sensitization and challenge (13, 21-23). To investigate whether cockroach allergen can also induce MSC migration to the lungs, we established a cockroach allergen-induced asthmatic mouse model. Compared with control mice (Fig. 1A), cockroach allergen-induced mouse models showed significant recruitment of inflammatory cells to the lung (Fig. 1B) and dense peribronchial infiltrates (Fig. 1C) in the histological examination as well as increased serum levels of cockroach allergen specific IgE (Fig. 1D). We then used this established model to examine whether MSCs migrated to the airway after cockroach allergen sensitization and challenge. It was reported that nestin can serve as a marker for bone marrow MSCs that have both self-renewal and multi-lineage potential in vivo (54). Our histological analysis demonstrated a high number of nestin+ cells in airway epithelial cells and sub-epithelial inflammatory cells from mice after CRE challenge (Fig. 1E), as compared to saline treated mice (Fig. 1G). No positive staining was seen for control IgG (Fig. 1F, H). The results suggest that MSCs are increased in airway after allergen sensitization and challenge.
Figure 1.
Nestin+ cells in lungs of cockroach extract (CRE)-challenged mice. (A-B) Representative H&E stained sections from mice immunized and challenged with saline (A) and CRE (B). (C) Dense peribronchial infiltrates. Score was defined by the number of infiltrates. (D) Serum levels of cockroach allergen specific IgE. (E-H) Nestin+ cells (MSCs, arrows) in airway sections of CRE-(E) and saline-treated mice (G). Control IgG staining in airway sections of CRE-(F) and saline-treated mice (H).**P<0.01. Data are representative of 3 independent experiments (n=4-6 mice/group).
TGFβ1 signaling is activated in lung tissue of allergic asthma and in CRE-treated MSCs
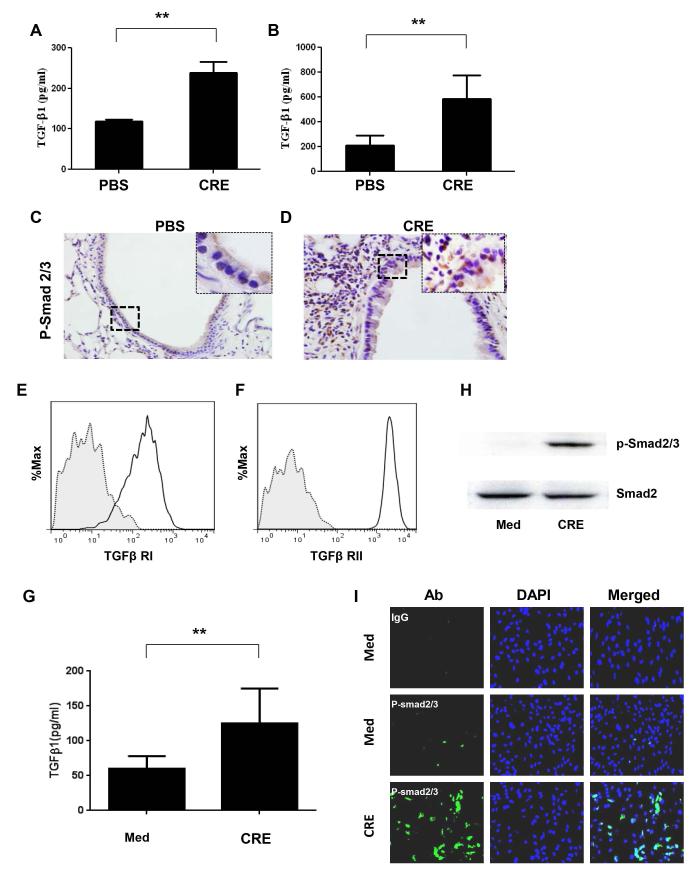
Our recent studies have suggested that activated TGFβ1 released from the injured vessels controls mobilization and recruitment of MSCs to participate in tissue repair/remodeling (33). To investigate whether TGFβ1 signaling is involved in the migration of MSCs to lungs in asthma, we have examined the levels of active TGFβ1 in blood and BALF in CRE-challenged mice. The concentrations of active TGFβ1 were significantly higher in both BALF (P<0.01, Fig. 2A) and peripheral blood (P<0.01, Fig. 2B) in mice after allergen challenge, when compared with control mice. We also detected p-Smad2/3 in the airways of the mice. As compared to those of saline-treated mice (Fig. 2C), much higher numbers of phospho-Smad2/3+ (p-Smad2/3+) epithelial cells and subepithelial inflammatory cells were found in the airways of mice after CRE challenge (Fig. 2D). The results indicate that cockroach allergen induces the activation of TGFβ1 signaling in airways in asthma. To further examine whether cockroach allergen can induce the activation of TGFβ1 signaling in MSCs, we detected the expression of TGFβ receptors (TβRI, TβRII), cockroach allergen induced TGFβ1 secretion as well as p-Smad2/3 expression in MSCs. We found that both TβRI (Fig. 2E) and TβRII (Fig. 2F) were constitutively expressed in MSCs, and MSCs can secrete a large amount of active TGFβ1 in response to cockroach allergen (Fig. 2G). Furthermore, cockroach allergen can induce the increased activation of TGFβ1 signaling in MSCs as determined by western blotting (Fig. 2H) and immunofluorescent staining (Fig. 2I).
Figure 2.
Increased activation of TGFβ1signaling in lung tissue of allergic asthma and CRE-treated SCs. (A-B) Active TGFβ1 levels in BAL (A) and peripheral blood (B) of mice treated with saline and CRE were analyzed by ELISA. (C-D) Histological sections (p-Smad2/3 staining) of mouse airway treated with saline (C) and CRE (D). Data are representative of 3 independent experiments (A-D: n=4-6 mice/group). (E-F) Flow cytometry detected TβRI (E) and TβRII (F) expression in MSCs. (G) Active TGFβ1 levels in supernatants of CRE-treated (50μg/ml) and untreated MSCs for 72 hours. (H) Expression of p-Smad2/3+ in MSCs after exposed to CRE for 24 hours were analyzed by western blotting (H) and immunofluorescence analysis (I). Bars represent mean ± SEM of 3 independent experiments. **P<0.01.
TGFβ1 mediates MSC migration induced by human epithelium conditioned medium
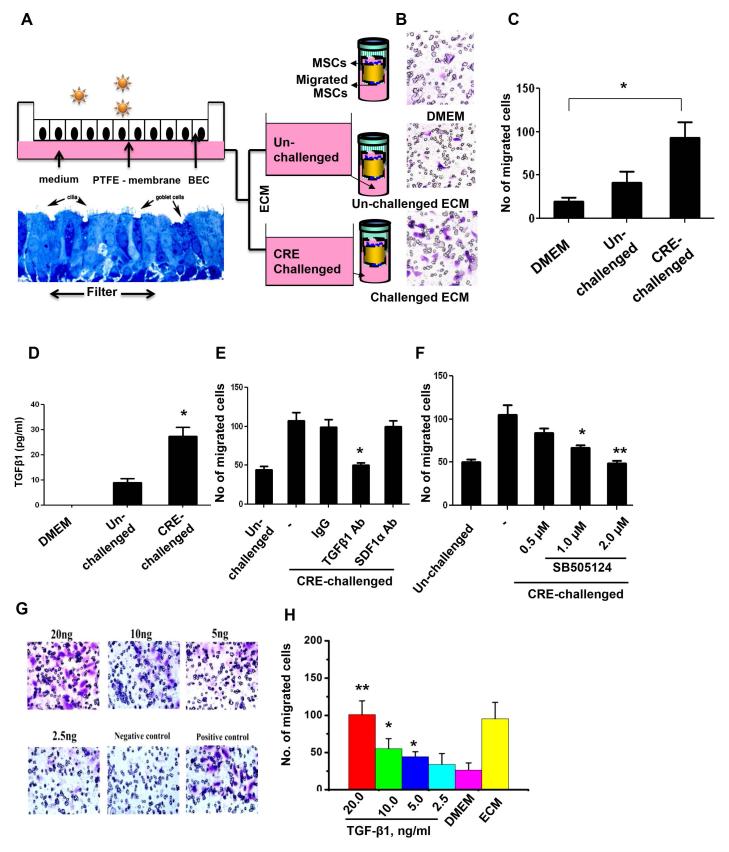
To further examine whether active TGFβ1 released from epithelium in response to environmental allergens can induce MSC migration, we performed a human epithelium conditioned medium (ECM)-based cell migration assay (Fig. 3A), in which ECM collected by incubating human epithelium with or without CRE challenge was placed in the bottom chamber of a transwell and MSCs were placed in the upper chamber of the tranwell. Significantly greater numbers of migrated MSCs were observed in the group with ECM prepared from allergen challenged epithelial cells, compared to those from the resting cells (Fig. 3B, 3C). To examine whether TGFβ1 is one of the major factors in CRE-challenged ECM responsible for the increased migration of MSCs, we firstly measured the levels of active TGFβ1 in ECM. A significantly greater amount of active TGFβ1was observed in ECM prepared by CRE challenged epithelial cells when compared to those from the resting cells (Fig. 3D). To further examine whether TGFβ1 in ECM is essential for MSC migration, we added TGFβ1 neutralizing antibodies in ECM for the migration assay. Interestingly, the migration of MSCs was almost abolished when TGFβ1 neutralizing antibody was used. The migration of MSCs was not affected by adding antibody against SDF-1a/CXCL12, a chemoattractant that can induce the migration of many cell types (Fig. 3E) (55). When different doses of TβR1 kinase-specific inhibitor SB50512 (0.5μM, 1.0μM, and 2.0μM) were added to ECM for the migration assay, the ECM induced migration was significantly inhibited in a concentration-dependent manner (Fig. 3F), suggesting that TGFβ1 signaling is a primary pathway that drives MSC migration. To validate the direct effect of TGFβ1 on MSC migration, we analyzed the migration of MSCs by the addition of the recombinant TGFβ1 in different doses into the DMEM in the lower chamber of transwell. Significant MSC migration was seen at 5, 10, and 20ng/ml TGFβ1 in a dose-dependent manner (Fig. 3G, 3H).
Figure 3.
Epithelium-conditioned medium (ECM) induces MSC migration. (A) Air-liquid interface (ALI) culture system for airway epithelial cells. (B) Transwell assays for migration of MSCs. (C) Number of migrated cells in (B). (D) Levels of active TGF-β1 in ECM from un-challenged and challenged epithelial cells, n=3. (E) Number of migrated cells after ECM was treated with TGF-β1 neutralizing antibody (TGF-β1 Ab) or control antibody (IgG). (F) Number of migrated cells after ECM was treated with different doses of TβR1 inhibitor (SB505124). (G-H) Number of migrated cells after MSCs was directly treated with the addition of different doses of recombinant TGF-β1 into DEME in the lower chamber of Transwell (See B). Numbers of migrated cells were counted in five views (×20). Bars (C, E, and F) represent mean ± SEM of 3 independent experiments. *P<0.05 **P<0.01. BEC: bronchial epithelial cell.
TGFβ1 mediates the recruitment of MSCs to the lungs in asthma
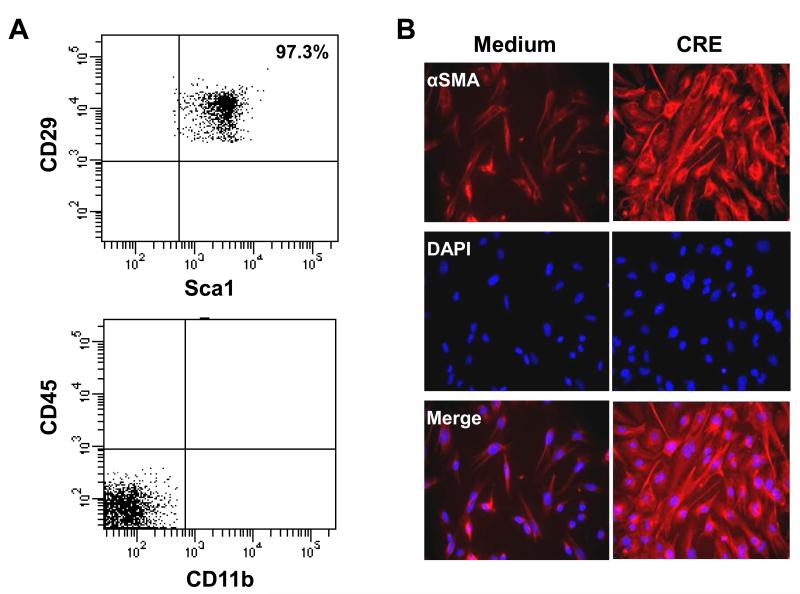
While MSCs were increased in airway after CRE sensitization and challenge, it remains unclear whether these MSCs are bone marrow-derived or local in origin. We thus examined whether there was an increased recruitment of MSCs to the lung from peripheral blood or bone marrow. We first examined whether transplanted MSCs can be recruited to the lungs in CRE-treated mice. These transplanted MSCs were sorted by markers Sca-1+, CD29+, CD45− and CD11b− (Fig. 4A). The ability of MSCs to differentiate into fibroblast/myofibroblast was evaluated by the expression of α-SMA (Abcam) with DAPI for nuclei immune-staining after cells were treated with CRE (50μg/ml) for 72 hours (Fig. 4B). A total of 2×106 sorted MSCs were then injected into CRE-challenged mice through the tail vein right before CRE challenge according to the protocol in Fig. 5A. Significantly greater numbers of GFP+ cells were observed in the lungs from these CRE sensitized and challenged mice, when compared with those in the lungs that received saline alone (Fig. 5B, 5C). To examine whether TGFβ1is required for GFP+ MSC recruitment, we systemically injected TGFβ1neutralizing antibody on the day before MSC injection and CRE challenge. We found that the increased GFP+ MSCs in lungs of CRE-induced mice were significantly inhibited when the mice were pretreated with TGFβ1neutralizing antibody (Fig. 5B, 5C). Next, we examined whether TGFβ1 is also essential for the recruitment of endogenous MSCs to the lungs in asthma using Nes-GFP mice, in which MSCs express GFP under the regulatory elements of the nestin promoter (54). Increased numbers of engrafted GFP+MSCs in the airways (Fig. 5D) and in BAL (Fig. 5E) were observed after the mice were treated with CRE. No nestin+ cells were detected in other tissues including liver, kidney, spleen, heart, adipose tissue, and aorta after allergen sensitization and challenge. Importantly, the increased GFP+MSCs were significantly diminished in mice receiving TGFβ1neutralizing antibody (Fig. 5D, 5E). These findings suggest that TGFβ1 is a key factor that recruits MSCs from bone marrow/peripheral blood to the airway following CRE sensitization and challenge.
Figure 4.
Characterization of mouse GFP+MSCs. (A) Isolated and individually expanded GFP+MSCs were analyzed by fluorescence-activated cell sorting (FACS) using antibodies for Sca-1, CD29, CD45, and CD11b. (B) The ability of MSCs to differentiate into fibroblast/myofibroblast was evaluated by the expression of α-SMA (Abcam) with DAPI for nuclei immune-staining after cells were treated with CRE (50μg/ml) for 72 hours.
Figure 5.
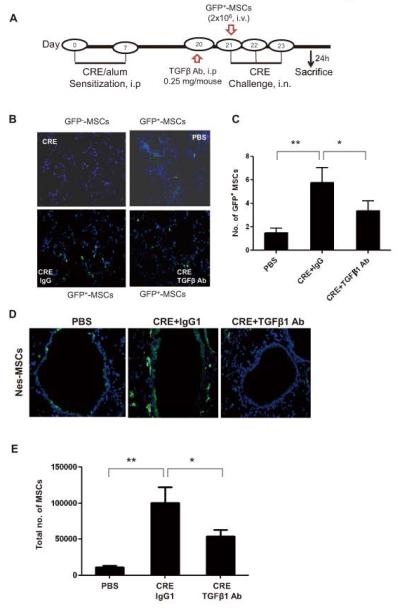
MSCs mobilize to the lungs from peripheral blood through TGFβ1. (A) Schematic of experimental protocol for mouse models of asthma. (B) Immunofluorescence analysis of injected GFP+MSCs in the airways of CRE-challenged or saline-treated mice with or without TGFβ1 Ab. (C) Number of injected GFP+MSCs were counted per field of view (FV, x20 magnification) and analyzed. Bars represent mean ± SEM for 4-6 mice/group. (D) GFP+ cells in the airways of CRE-challenged or saline-treated Nes-GFP mice. (E) Total numbers of MSCs in BAL detected by flow cytometry (anti-GFP). Bars represent mean ± SEM of 3 independent experiments. *P<0.05 **P<0.01.
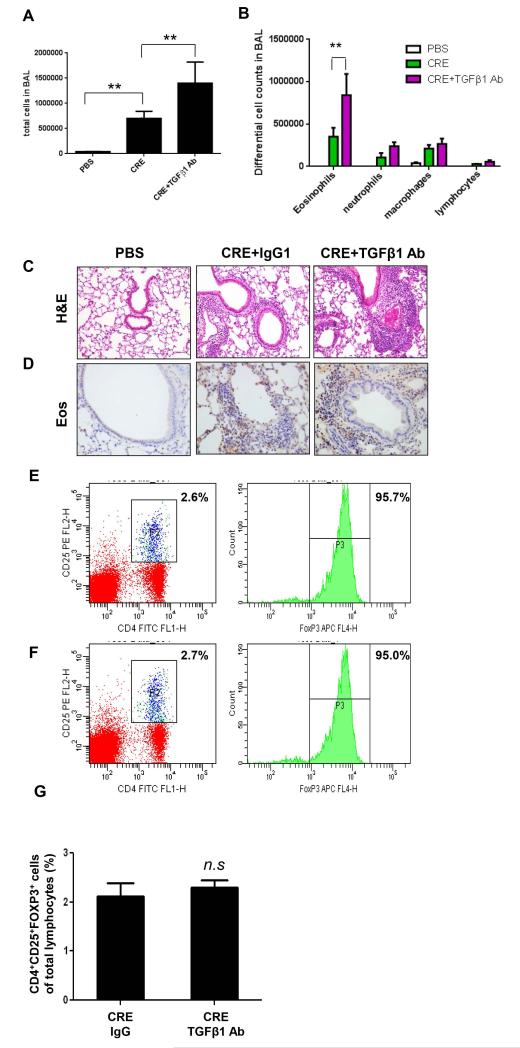
TGFβ1 limits the allergic inflammation
We then examined whether TGFβ1 can suppress cockroach allergen induced allergic inflammation in our established cockroach allergen induced mouse models. Increased recruitment of total inflammatory cells (Fig. 6A) and neutrophils, eosinophils, macrophages, and lymphocytes (Fig. 6B) were detected in BAL of the mice following CRE treatment. Moreover, dense peribronchial infiltrates were also found in the lung tissue by histological examination (Fig. 6C). TGFβ1 Ab treatment further elevated total number of inflammatory cells in BAL, specifically eosinophils (Fig. 6B), and increased the airway inflammation (Fig. 6C) in CRE-induced mice. The increase of eosinphils in the airway was further confirmed by staining for MBP, a marker for eosinophils (Fig. 6D). The increased inflammation in lungs after treatment with TGFβ1 neutralizing antibody was correlated with decreased numbers of MSCs detected in the airway (Fig. 5D) and in BAL (Fig. 5E). In addition, TGF β1 has been shown to be essential for Treg development that is capable of suppressing inflammation in vivo (56). To test whether TGF β1 suppresses inflammation through Treg, we examined the percentage of CD4+CD25+FoxP3+ Treg cells in the lung hilar lymph nodes in TGFβ1 neutralizing antibody treated and untreated mice. As shown in Fig. 6E, 6F, and 6G, no significant difference was observed in the percentage of Treg cells between TGFβ1 neutralizing antibody treated and untreated groups. The results suggest that the effect of TGFβ1 on inflammatory response is not through a direct effect on Treg development.
Figure 6.
TGFβ1 neutralizing antibody inhibits airway inflammation. (A-B) Total numbers of cells in BAL (A) and differential cell counts in BAL (B) from different treatment groups of mice as indicated. (C-D) H&E (C) and MBP staining (D) of lung tissue section were performed for different treated groups. (E-F) CD4+CD25+FOXP3+ cells isolated from the lung lymph nodes of CRE-challenged mice were treated with IgG (E) and TGFβ1 Ab (F). (G) Percentage of Treg cells among lymphocytes was illustrated. Bars represent mean ± SEM of 3 independent experiments, n=4-6 mice/group. *P<0.05 **P<0.01. n.s., not significant.
MSCs modulate T responses to CRE in vitro
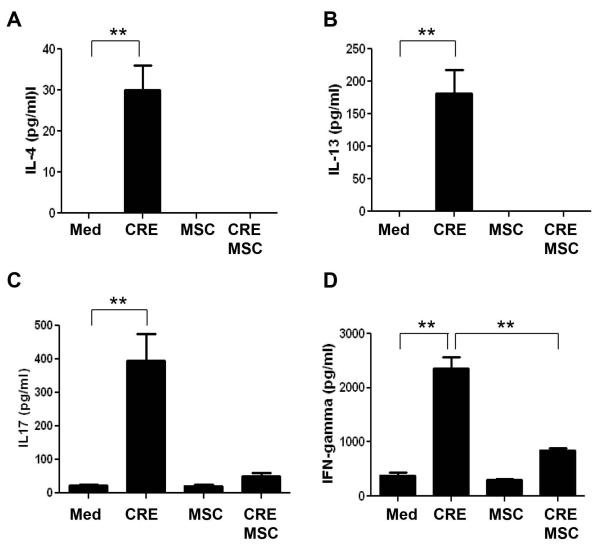
Bone marrow-derived MSCs have been shown to suppress allergic responses in a mouse model of ragweed induced asthma(57), but the mechanism remains unclear. It is possible that TGFβ1 neutralizing antibody may increase the immune response through inhibition of MSC recruitment. To test this possibility, we performed an in vitro analysis to examine whether MSCs can inhibit CRE induced T cell responses. Indeed, when we co-cultured the CD4+ T cells isolated from CRE sensitized mice with irradiated spleen cells and treated the cells with CRE for 3 days, IL-4 (Fig. 7A), IL-13 (Fig. 7B), IL17 (Fig. 7C), and IFN γ (Fig. 7D) production were significantly increased. However, when MSCs were added to the co-cultures, these cytokines were dramatically inhibited.
Figure 7.
Modulatory effects of MSCs on CRE-induced T responses in vitro. MSCs were co-cultured with isolated CD4+ T cells from CRE-sensitized mice. Cytokines including L-4 (A), IL-13 (B), IL17 (C), and IFN γ (D) were measured by ELISA. Bars represent mean ± SEM of 3 independent experiments. **P<0.01. Med: medium.
Discussion
In the current study, we evaluated the functional significance of TGFβ1 activation in the recruitment of MSCs to the airways in asthma. We, for the first time, examined whether MSCs can be recruited to the lungs after cockroach allergen sensitization and challenge, and most importantly, identified the primary pro-migratory factor(s) that controls the MSC migration in this process. We found increased MSCs and TGFβ1 activation in lungs of cockroach allergen induced mouse models and an increased activation of TGFβ1signaling in CRE-treated MSCs. Further, using an epithelium-conditioned medium (ECM)-based transwell assay, we found that active TGFβ1 released from allergen-activated epithelium is a primary allergen-activated messenger for MSC migration. Findings from these studies were further validated by the detection of increased numbers of transplanted GFP+MSCs and endogenous MSCs (GFP+ cells) in the lung tissues in mouse asthma models. Importantly, the neutralizing antibody against TGFβ1 abrogated the migration of MSCs in both ex vivo ECM-based migration assay or in vivo cockroach allergen induced mouse models. Thus, TGFβ1 is a primary pro-migratory factor produced in the CRE-challenged lung tissue to control the recruitment of MSCs in asthma.
MSCs have the ability of self-renewal and differentiation into multiple cell types in vitro and in vivo (12). Several lines of evidence have suggested the involvement of MSCs in asthma (13, 21-23). Our studies have provided further supporting evidence that MSCs are significantly increased after cockroach allergen sensitization and challenge. Histological analysis demonstrated a high number of nestin+ MSCs in airway epithelial and sub-epithelial layers from mice after CRE challenge as compared to saline. Notably, when we detected MSCs in the airway tissues from patients with allergic asthma, we found that those nestin+ MSCs were only found in the airway sub-epithelial region, not airway epithelium (Data not shown). It is likely that MSCs from patients with chronic asthma have resumed their mesenchymal features and are associated with fibrosis/remodeling in the sub-epithelial region. By contrast, MSCs from patients with acute asthma may be recruited into airway epithelial regions and undergo associated differentiation into lung epithelial cells (58). Moreover, we have used nes-GFP transgenic mice to track the endogenous MSC migration to the lung tissues, and found that the recruitment of nes-GFP+ cells was increased after cockroach allergen sensitization and challenge. Nestin, a class VI intermediate filament protein, was originally described as a neuronal stem cell marker expressed during central nervous system development. Specifically, nestin is expressed mainly in migrating and proliferating cells during development; whereas in adult tissues, nestin is mainly expressed in bone marrow MSCs and neuronal stem cells in brain. More than 95% of MSCs express nestin. However, postnatal nestin expression in neuronal stem cells is extremely limited unless there is brain or nerve injury (59) (60). Nestin+ bone marrow cells have also been functionally characterized as MSCs based on their colony-forming-unit fibroblastic activity, ability to be propagated as non-adherent ‘mesenspheres’ that can self-renew and expand in serial transplantations and in vivo contribution to osteochondral lineages under homeostasis(54). While these nes-GFP+ cells in our models mark the MSC population in bone-marrow (61), we could not absolutely exclude the possibilities of the resident lung-derived MSCs or some types of airway epithelial cells types function as progenitors or stem cells that are positive for nestin. Future studies are needed on the clarification of whether bone marrow nestin+ MSCs are the main source of lung nestin+ cells after allergen challenge. We have also examined nestin+ cells in other tissues including liver, kidney, spleen, heart, adipose tissue, and aorta after allergen sensitization and challenge in cockroach allergen induced nes-GFP transgenic mouse models of asthma. We found that no nestin+ cells were detected in these examined other tissues after cockroach allergen sensitization and challenge, suggesting that the damaged lung tissue is the major target site where the nestin+ cells were recruited.
Active TGFβ1 has been observed to be increased in human asthmatic airways (42, 62), sputum supernatants(63), and airway in mouse models during allergic reactions (43). Over-expression of TGFβ1 in primary airway fibroblasts has been associated with the regulation of granulocyte activation and trafficking(63) and progression of airway remodeling (64). Our studies provided further evidence for the increased activation of TGFβ1 signaling in mouse models of asthma by showing increased levels of active TGFβ1 in BAL and peripheral blood, and p-Smad2/3+ in airway of mouse models during allergic reactions. Although active TGFβ1 can be released from different cellular sources, such as bronchial epithelium (65), eosinophils (66), macrophages (67) and fibroblasts (64), our study suggests that MSCs may also be one of the major cell types releasing active TGFβ1 and have an increased activation of TGFβ1 signaling in response to cockroach allergen treatment. In this study, we found that MSCs constitutively express both TβRI and TβRII and have increased p-Smad2/3+ when expose to cockroach allergen.
We observed increased levels of active TGFβ1 released from cockroach allergen treated primary epithelial cells under the ALI culture, which includes the isolation of airway epithelial cells from central airway, culturing of these isolated cells at ALI and exposure to experimental allergen (49, 68). The ALI culture model allows the culture of airway epithelia in response to allergen exposure that more closely resembles their physiological setting than ordinary liquid culture system (69). We postulated that active TGFβ1 released from damaged airway epithelial cells after repeated exposure to allergens may be a major resource for promoting MSC recruitment to the lung tissues. Indeed, we found that the migration of MSCs was significantly inhibited when either TGFβ1 neutralizing antibody or TβRI inhibitor was added into ECM that was used for migration assays. Furthermore, we examined the role of TGFβ1 signaling in the recruitment of MSCs to the lung tissue in mouse asthma models. We found that TGF-β1 neutralizing antibody can significantly inhibit MSC migration from blood circulation to the lungs. Interestingly, the total number of inflammatory cells, especially eosinophils, was enhanced by TGF-β1 neutralizing antibody. In addition, we found a high number of phospho-Smad2/3+ (p-Smad2/3+) cells in the lungs from mice after CRE challenge, and the increased p-Smad2/3+ cells were abrogated by TGFβ1 neutralizing antibody. This was consistent with the previous report indicating that anti-TGFβ1 can regulate active TGFβ1 signaling in situ with a reduction of p-Smad 2 in lung sections.
Very recent studies by Dr. Guerrerio et al. demonstrated that disruption in TGFβ1 signaling imposes a strong predisposition for human allergic diseases, and suggested that inhibition of TGFβ1 signaling could be a potential basis for beneficial therapeutic strategies (41). However, our studies demonstrated that TGFβ1 plays an anti-inflammatory role in an allergen induced mouse model of asthma. In particular, the blocking of TGFβ1 can promote allergic inflammation as determined by increased total numbers of inflammatory cells in BAL and peribronchial infiltrates. In particular, significantly increased eosinophils were observed in BAL and lung tissue from anti-TGFβ1 treated mice. This is consistent with the previous report that TGFβ1 can suppress eosinophilic lung disease(70), and mutations in the receptor for TGFβ1 are associated with a strong predisposition towards eosinophilic gastrointestinal disease (41). In contrast, MSCs in BAL and lung tissue were significantly reduced, providing evidence for the possibility that the enhanced allergen-induced allergic inflammation by anti-TGFβ may be due to the reduction of migrated MSCs. Indeed, injected BMSCs have been shown to suppress Th2-driven allergic responses in a mouse model of ragweed induced asthma through TGFβ1 (57). However, the detailed underlying mechanism concerning the suppressive role of MSCs in inflammation remains unclear. To test the possibility that TGF β1 suppressed inflammation through Treg, we examined the percentage of CD4+CD25+FoxP3+ Treg cells in the lung hilar lymph nodes from TGFβ1 neutralizing antibody treated and untreated mice. However, no significant difference was observed for the percentage of Treg cells, suggesting that TGFβ1 neutralizing antibody may not directly affect Treg development. Thus, it is likely that these migrated MSCs may be the major factor in suppressing inflammation by TGFβ1. In fact, MSCs have been suggested to be involved in modulating the immune responses through paracrine effects and interaction with different immunocytes (26-30). In this study, we found that MSCs significantly inhibited CRE-induced inflammatory cytokine secretion by CD4+ T cells (IL-4, IL13, IL17 and IFN-γ). The findings provide further evidence for the recruited MSCs in modulating immune and inflammatory responses in asthma. However, we failed to find clear differences in the percentage of Th1 and Th2 cells among CD4+ T cells isolated from these lymph nodes of CRE-treated mice between TGFβ1 neutralizing antibody treated and untreated mice (data not shown). This suggests the complexity of the underlying mechanisms regarding TGFβ1 suppressed inflammation.
The role of TGFβ1 in modulation of inflammation is controversial. The model presented in this study represents an acute type immunological response where their data clearly demonstrates a role of the recruited MSCs in modulating immune and inflammatory responses in asthma. We believe, in early stage, active TGFβ1 released from damaged/repairing epithelium in response to allergens suppress immune response either directly by inhibiting the function of immune cells, such as T cells, B cells NK cells, and macrophages, or indirectly by recruiting MSCs into the major damaged lung tissue to suppress inflammation by the secretion of cytokines and growth factors at sites of tissue inflammation and repair the damaged epithelium. While in the late stage, these sustained active TGF β1 released locally due to repetitive allergen challenge may cause aberrant excessive recruitment of MSCs and promote MSC differentiation into myofibroblasts. This will lead to a progressive fibrosis and pathological remodeling in asthma.
Taken together, our studies have demonstrated that TGFβ1 released from allergen-activated epithelium is a primary pro-migratory factor controlling the recruitment of MSCs to the lungs in asthma. Moreover, we demonstrated that MSCs can inhibit CRE induced inflammatory cytokine secretion by CD4+ T cells. These findings suggest that TGFβ1 may play a role in MSC migration which could be critical in allergen induced airway allergic inflammation. Importantly, these studies provide a basis for a further investigation into the role of TGFβ1 in regulating MSC-involved airway inflammation and repairing/remodeling in allergen-induced asthma.
ACKNOWLEDGEMENTS
We thank Dr. Allen C Myers for supplying human lung tissue for an Air-Liquid Interface (ALI) culture and technical assistance. We also thank Drs. Zhang Cui, Bin Yu, Kai Jiao, and Wenying He for excellent technical assistance.
Funding: This research was supported by National Institutes of Health (NIH) grants 1R21AI088406 (to PG), RO1ES021739 (to PG), and American Heart Association (AHA) grant 13GRNT17050013 (to M.W.)
Abbreviations
- MSCs
Mesenchymal stem cells
- TGFβ
Transforming growth factor β
- ALI
Air liquid interface
- ECM
Epithelial conditioned medium
- CRE
Cockroach extract
- TGFβ1 Ab
TGFβ1 neutralizing antibody
REFERENCES
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–462. doi: 10.1016/j.jaci.2011.04.047. quiz 463-454. [DOI] [PubMed] [Google Scholar]
- 3.Phipps S, Benyahia F, Ou TT, Barkans J, Robinson DS, Kay AB. Acute allergen-induced airway remodeling in atopic asthma. Am J Respir Cell Mol Biol. 2004;31:626–632. doi: 10.1165/rcmb.2004-0193OC. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd CM, Robinson DS. Allergen-induced airway remodelling. Eur Respir J. 2007;29:1020–1032. doi: 10.1183/09031936.00150305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 6.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, Eggleston PA. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112:87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura KE, T Demoor,, Rauch M, Faruqi AA, Jang S, C CJ, Boushey HA, Zoratti E, Ownby D, Lukacs NW, Lynch SV. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, Mortimer KM, Mitchell H, Ownby D, Slavin R, Malveaux F. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 9.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 11.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 12.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011;236:1461–1467. doi: 10.1258/ebm.2011.011221. [DOI] [PubMed] [Google Scholar]
- 14.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc. 2008;5:323–327. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr., Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Sun Z, Qiu X, Li Y, Qin J, Han X. Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2009;390:1309–1314. doi: 10.1016/j.bbrc.2009.10.143. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Wang Y, Gong X, Su H, Han X. Secretion of rat tracheal epithelial cells induces mesenchymal stem cells to differentiate into epithelial cells. Cell Biol Int. 2012;36:169–175. doi: 10.1042/CBI20110121. [DOI] [PubMed] [Google Scholar]
- 20.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–671. doi: 10.1016/j.biocel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Bentley JK, Popova AP, Bozyk PD, Linn MJ, Baek AE, Lei J, Goldsmith AM, Hershenson MB. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respir Res. 2010;11:127. doi: 10.1186/1465-9921-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–1148. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 26.Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolome ST, Sambuceti G, Traggiai E, Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, Hodges MG, Jelinek I, Madala S, Karpati S, Mezey E. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010;107:5652–5657. doi: 10.1073/pnas.0910720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Sun B, Wang D, Ji Y, Kong Q, Wang G, Wang J, Zhao W, Jin L, Li H. Murine bone marrow mesenchymal stem cells cause mature dendritic cells to promote T-cell tolerance. Scand J Immunol. 2008;68:607–615. doi: 10.1111/j.1365-3083.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 30.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol. 2011;45:809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YS, Jeong JA, Lim DS. Mesenchymal stem cell-mediated immature dendritic cells induce regulatory T cell-based immunosuppressive effect. Immunol Invest. 2012;41:214–229. doi: 10.3109/08820139.2011.619022. [DOI] [PubMed] [Google Scholar]
- 32.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 33.Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen YF, Jan De Beur S, Yu B, Cao X. Injury-activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells. 2012;30:2498–2511. doi: 10.1002/stem.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 35.Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG. Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease. Am J Respir Crit Care Med. 2007;176:974–982. doi: 10.1164/rccm.200702-334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-beta in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 37.Peng X, Mathai SK, Murray LA, Russell T, Reilkoff R, Chen Q, Gulati M, Elias JA, Bucala R, Gan Y, Herzog EL. Local apoptosis promotes collagen production by monocyte-derived cells in transforming growth factor beta1-induced lung fibrosis. Fibrogenesis Tissue Repair. 2011;4:12. doi: 10.1186/1755-1536-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, Wrana JL. Switch Enhancers Interpret TGF-beta and Hippo Signaling to Control Cell Fate in Human Embryonic Stem Cells. Cell reports. 2013 doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1-an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Tenney RM, Discher DE. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol. 2009;21:630–635. doi: 10.1016/j.ceb.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, Oliva-Hemker M, Wood RA, Dietz HC. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5:195ra194. doi: 10.1126/scitranslmed.3006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–313. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosendahl A, Checchin D, Fehniger TE, ten Dijke P, Heldin CH, Sideras P. Activation of the TGF-beta/activin-Smad2 pathway during allergic airway inflammation. Am J Respir Cell Mol Biol. 2001;25:60–68. doi: 10.1165/ajrcmb.25.1.4396. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, Yu P, Han X, Du L, Gan J, Wang Y, Shi Y. TGF-beta promotes immune responses in the presence of mesenchymal stem cells. Journal of immunology. 2014;192:103–109. doi: 10.4049/jimmunol.1302164. [DOI] [PubMed] [Google Scholar]
- 45.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. The Journal of comparative neurology. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 46.Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 47.Lehrer SB, Reish R, Fernandes J, Gaudry P, Dai G, Reese G. Enhancement of murine IgE antibody detection by IgG removal. Journal of immunological methods. 2004;284:1–6. doi: 10.1016/j.jim.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Y, Kawasaki H, Hsu SC, Lee RT, Yao X, Plunkett B, Fu J, Yang K, Lee YC, Huang SK. Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med. 2010;16:1128–1133. doi: 10.1038/nm.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 50.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nature medicine. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nature medicine. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, Wan M, Cao X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2012;27:2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 54.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 56.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med (Berl) 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35:1466–1475. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Yamada M, Burke C, Colditz P, Johnson DW, Gobe GC. Erythropoietin protects against apoptosis and increases expression of non-neuronal cell markers in the hypoxia-injured developing brain. The Journal of pathology. 2011;224:101–109. doi: 10.1002/path.2862. [DOI] [PubMed] [Google Scholar]
- 60.Kutlu O, Ross AE, Schaeffer EM, Gratzke C, Stief CG, Strong TD, Burnett AL, Hedlund P, Bivalacqua TJ. Increased expression of nestin in the major pelvic ganglion following cavernous nerve injury. International journal of impotence research. 2012;24:84–90. doi: 10.1038/ijir.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mendez-Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)- and beta(3)-adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139–144. doi: 10.1111/j.1749-6632.2010.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang K, Chen HB, Wang Y, Lin JH, Hu Y, Fang YR. [Changes in IL-17 and TGF-beta1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma] Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:604–608. [PubMed] [Google Scholar]
- 63.Gagliardo R, Chanez P, Gjomarkaj M, La Grutta S, Bonanno A, Montalbano AM, Di Sano C, Albano GD, Gras D, Anzalone G, Riccobono L, Profita M. The role of transforming growth factor-beta1 in airway inflammation of childhood asthma. Int J Immunopathol Pharmacol. 2013;26:725–738. doi: 10.1177/039463201302600316. [DOI] [PubMed] [Google Scholar]
- 64.Mirzamani MS, Nourani MR, Imani Fooladi AA, Zare S, Ebrahimi M, Yazdani S, Ghanei M, Karimfar MH. Increased Expression of Transforming Growth Factor-beta and Receptors in Primary Human Airway Fibroblasts from Chemical Inhalation Patients. Iran J Allergy Asthma Immunol. 2013;12:144–152. [PubMed] [Google Scholar]
- 65.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004;34:567–575. doi: 10.1111/j.1365-2222.2004.1917.x. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol. 2004;31:62–68. doi: 10.1165/rcmb.2003-0305OC. [DOI] [PubMed] [Google Scholar]
- 67.Doherty TA, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, Norris PS, Choi H, Scheu S, Pfeffer K, Zuraw BL, Ware CF, Broide DH, Croft M. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nat Med. 2011;17:596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lam HC, Choi AM, Ryter SW. Isolation of mouse respiratory epithelial cells and exposure to experimental cigarette smoke at air liquid interface. J Vis Exp. 2011 doi: 10.3791/2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeh TH, Tsai CH, Chen YS, Hsu WC, Cheng CH, Hsu CJ, Lee SY. Increased communication among nasal epithelial cells in air-liquid interface culture. Laryngoscope. 2007;117:1439–1444. doi: 10.1097/MLG.0b013e318063e84f. [DOI] [PubMed] [Google Scholar]
- 70.Williams AE, Humphreys IR, Cornere M, Edwards L, Rae A, Hussell T. TGF-beta prevents eosinophilic lung disease but impairs pathogen clearance. Microbes Infect. 2005;7:365–374. doi: 10.1016/j.micinf.2004.11.012. [DOI] [PubMed] [Google Scholar]