Abstract
All organisms are equipped with systems for detoxification of the metalloids arsenic and antimony. Here, we show that two parallel pathways involving the AP-1–like proteins Yap1p and Yap8p are required for acquisition of metalloid tolerance in the budding yeast S. cerevisiae. Yap8p is demonstrated to reside in the nucleus where it mediates enhanced expression of the arsenic detoxification genes ACR2 and ACR3. Using chromatin immunoprecipitation assays, we show that Yap8p is associated with the ACR3 promoter in untreated as well as arsenic-exposed cells. Like for Yap1p, specific cysteine residues are critical for Yap8p function. We further show that metalloid exposure triggers nuclear accumulation of Yap1p and stimulates expression of antioxidant genes. Yap1p mutants that are unable to accumulate in the nucleus during H2O2 treatment showed nearly normal nuclear retention in response to metalloid exposure. Thus, our data are the first to demonstrate that Yap1p is being regulated by metalloid stress and to indicate that this activation of Yap1p operates in a manner distinct from stress caused by chemical oxidants. We conclude that Yap1p and Yap8p mediate tolerance by controlling separate subsets of detoxification genes and propose that the two AP-1–like proteins respond to metalloids through distinct mechanisms.
INTRODUCTION
Exposure to the toxic metalloids arsenic and antimony is a serious challenge to all organisms. In humans, arsenic compounds are associated with an increased incidence of a variety of diseases, including cancer. Yet, metalloid-containing drugs are used as chemotherapeutic agents to combat infectious diseases caused by pathogenic parasites as well as cancer, including acute promyelocytic leukemia (Murray, 2001; Waxman and Anderson, 2001). The emergence of metalloid tolerance is a considerable threat to effective medical treatment and makes the elucidation of the mechanisms that form the basis of tolerance a high priority (Tamás and Wysocki, 2001)
A number of proteins involved in metalloid transport and tolerance have been described in various organisms. In the eukaryotic model organism Saccharomyces cerevisiae (bakers' yeast) two transport systems contribute to metalloid removal from the cytosol, Acr3p, and Ycf1p. Acr3p is a plasma membrane protein that extrudes As(III) from the cell (Wysocki et al., 1997; Ghosh et al., 1999), whereas the ATP-binding cassette-transporter Ycf1p mediates uptake of glutathione-conjugates of As(III) and Sb(III) into the vacuole (Ghosh et al., 1999). Inactivation of ACR3 sensitizes cells to As(III) and As(V), whereas inactivation of YCF1 causes As(III) and Sb(III) sensitivity (Wysocki et al., 1997; Ghosh et al., 1999; Wysocki et al., 2001). Because arsenic is removed from the cytosol in the form of As(III), yeast cells reduce As(V) to As(III) by the action of the cytosolic arsenate reductase Acr2p (Mukhopadhyay et al., 2000).
To date, little is known about metalloid-specific signal transduction and transcriptional regulation of detoxification genes. As(III) seems to stimulate transcription of various stress-responsive genes in mammals, probably via an AP-1 transcription factor (Del Razo et al., 2001). S. cerevisiae contains eight fungal-specific AP-1–like proteins: Yap1p to Yap8p. These proteins contain a bZIP DNA binding domain as well as conserved cysteine-rich domains (CRD) in their amino and carboxy termini (n-CRD and c-CRD, respectively) (Fernandes et al., 1997; Toone et al., 2001). Yap1p is crucial for oxidative stress tolerance in yeast; YAP1 deletion results in hypersensitivity to peroxide, the thiol oxidant diamide, certain electrophiles, and cadmium (Toone et al., 2001). When cells are exposed to oxidants, Yap1p transiently accumulates in the nucleus (Kuge et al., 1997; Yan et al., 1998; Delaunay et al., 2000; Kuge et al., 2001) and activates transcription of genes coding for proteins that maintain a favorable cellular redox balance as well as for enzymes involved in detoxification of reactive oxygen species (ROS) (Lee et al., 1999; Gasch et al., 2000). In addition, Yap1p mediates cadmium tolerance by controlling YCF1 expression (Wemmie et al., 1994). Interestingly, different oxidants activate Yap1p in distinct ways; activation by H2O2 occurs through formation of an intramolecular disulfide bond between C303 and C598 (Delaunay et al., 2000), whereas diamide activation involves the C-terminal cysteines C598, C620, and C629 (Kuge et al., 2001). It has recently been shown that the glutathione peroxide-like protein Gpx3p is required for Yap1p oxidation by H2O2 (Delaunay et al., 2002). Whether Yap1p oxidation by other stress agents also involves effector proteins has yet to be demonstrated.
Whereas Yap1p has been extensively studied, the physiological functions of the other yeast AP-1–like proteins are currently unknown, and data are largely restricted to overexpression phenotypes (Toone and Jones, 1999; Toone et al., 2001; Toledano et al., 2002). Overexpression of Yap2p confers cellular tolerance to cadmium and the cytotoxic drug 1,10-phenanthroline (Bossier et al., 1993; Wu et al., 1993), and it seems to control transcription of genes involved in protein folding and stabilization in an oxidative environment (Cohen et al., 2002). Yap4p (Cin5p) and Yap6p (Hal7p) are nuclear proteins that confer resistance to the antitumor drug cisplatin when overexpressed (Furuchi et al., 2001). Yap6p (Hal7p) and Yap7p (Hal6p) produce increased sodium and lithium tolerance when present in multiple copies (Mendizabal et al., 1998). Previous studies indicated that Yap8p (Acr1p/Arr1p) is involved in arsenic tolerance, probably by controlling ACR2 and ACR3 expression (Bobrowicz et al., 1997; Bobrowicz and Ulaszewski, 1998; Bouganim et al., 2001). However, the molecular details of this regulation have not been investigated.
The goal of the present study was to explore the function of the S. cerevisiae AP-1–like proteins in metalloid tolerance. We show that Yap1p and Yap8p mediate arsenic and antimony tolerance by activating transcription of separate subsets of defense genes. We also provide evidence that metalloids activate these two proteins through distinct mechanisms.
MATERIALS AND METHODS
Yeast Strains and Growth Conditions
Yeast strains used in this study are described in Table 1. All deletion mutants were constructed according to the method of Güldener et al. (1996) as described previously (Wysocki et al., 2001). Yeast strains were grown on rich YPAD medium (1% yeast extract, 2% peptone, 2% glucose, 0.004% adenine sulfate) or on minimal YNB medium (0.67% yeast nitrogen base) supplemented with auxotrophic requirements and 2% glucose as a carbon source. Metalloid sensitivity assays were carried out as described previously (Wysocki et al., 2001). Yeast transformations were performed by a modified lithium acetate method using PLATE mixture (Kaiser et al., 1994). To create a genomic integration of the Myc-tag in the YAP8 gene the PFA6a-Myc plasmid (kindly provided by J.-Y. Masson, Laval University, Québec, Canada) was used to amplify the fragment (YAP8-Myc) by polymerase chain reaction (PCR) by using the following primers: Yap8-Myc-F1 5′-TAGCCTCAAGCATTTCATTAAGGTCTTTTCGTCAAAATTACGGATCCCCGGGTTAATTAA-3′ and Yap8-Myc-R1 5′-ATAAGAAAGACAATGTTGCGCTGTGCTTACAGGAAGAATAGAATTCGAG C T C G T T T A A A C-3′. The resulting 2.1-kb fragment was integrated in frame at the penultimate codon into wild-type BY4741 strain, and proper integration was verified by PCR.
Table 1.
S. cerevisiae strains used in this study
| Strains: | Genotype | Source |
|---|---|---|
| W303-1A | MATa ura3-1 leu2-3/112 trp1-1 his3-11/15 ade2-1 can1-100 GAL SUC2 mal0 | Thomas and Rothstein (1989) |
| RW104 | W303-1A acr3Δ :: loxP-kanMX-loxP | Wysocki et al. (2001) |
| RW105 | W303-1A acr3Δ :: loxP-kanMX-loxP ycf1Δ :: loxP | Wysocki et al. (2001) |
| RW117 | W303-1A yap8Δ :: loxP | This work |
| RW118 | W303-1A ycf1Δ :: loxP | Wysocki et al. (2001) |
| RW120 | W303-1A yap1Δ :: loxP-kanMX-loxP yap8Δ :: loxP | This work |
| RW124 | W303-1A yap1Δ :: loxP | This work |
| RW127 | W303-1A yap1Δ :: loxP-kanMX-loxP ycf1Δ :: loxP | This work |
| RW128 | W303-1A acr3Δ :: loxP-kanMX-loxP yap1Δ :: loxP | This work |
| RW136 | W303-1A yap8Δ :: loxP acr3Δ :: loxP-kanMX-loxP | This work |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| YAP8-Myc | BY4741 YAP8-Myc HIS3 | This work |
Plasmid Constructs
The plasmids used in this study are listed in Table 2. The ACR3 promoter region from –352 to +34, where +1 represents the start of translation, was obtained by digesting plasmid pRW3 (Wysocki et al., 1997) containing wild-type ACR3, with KpnI and ligating the promoter region into KpnI-digested pALTER-1 (Promega, Madison, WI) to generate the pALTER1-ACR3 template for site-directed mutagenesis. Deletion of the TTAATAA sequence from the ACR2-ACR3 intergenic region was achieved using the Altered Sites II in vitro mutagenesis system (Promega) and the mutagenic oligonucleotide MUT2 (5′-GCTCTTAATTATCTTTTTGTTTGATCAACTTTAGCGGCAACGCTCC-3′). The mutated fragment on plasmid pALTER1-mutACR3 was sequenced to confirm the desired mutation. ACR3-lacZ fusion plasmids were constructed by transferring an EcoRI-BamHI fragment from pALTER1-ACR3 and pALTER1-mutACR3 into EcoRI-BamHI-cleaved pSEYC102 (Emr et al., 1986) to produce pEM19 and pEM20, respectively. The wild-type ACR3 promoter from pRW3 was replaced with the KpnI-KpnI fragment from pALTER1-mutACR3. The resulting pRW33 plasmid contains the ACR3 gene under the control of its endogenous promoter lacking the TTAATAA sequence. The ACR2 promoter region from –323 to +6 was generated by PCR by using primers PE17 (5′-GCCTGCAGGGTTGCATCCTCGTTGGAGGT-3′) and HACR2 (5′-CCCAAGCTTGTACCATTACGCTTGCTGGATTG-3′), and the templates pALTER1-ACR3 and pALTER1-mutACR3 to obtain wild-type and mutated forms of the ACR2 promoter, respectively. The PCR products were cleaved with HindIII-PstI and cloned into YEp357R (Myers et al., 1986), producing plasmids ACR2-lacZ (pEM16) and mutACR2-lacZ (pEM18). The ACR3 promoter fragment (from –354 to + 14) was also cloned into BamHI-PstI-cleaved YEp363 (Myers et al., 1986) to produce the pRW11 plasmid with the LEU2 marker. The GFP-YAP1 fusion plasmid was kindly provided by S.W. Moye-Rowley (University of Iowa, Iowa City, IA) and has been described previously (Coleman et al., 1999). Other GFP-YAP1 plasmids expressing either wild-type Yap1p or the CRD mutants Yap1p-TAT, Yap1p-3Cys, and Yap1p-3Cys C620A were kindly provided by S. Kuge (Tohoku University, Sendai, Japan) and have been described previously (Kuge et al., 2001).
Table 2.
Plasmids used in this study
| Plasmid | Description | Source/Reference |
|---|---|---|
| pUG6 | Vector containing the loxP-kanMX-loxP deletion cassette | Güldener et al. (1996) |
| YEp357R | 2μ vector with lacZ reporter gene, URA3 | Myers et al. (1986) |
| YEp363 | 2μ vector with lacZ reporter gene, LEU2 | Myers et al. (1986) |
| pSEYC102 | CEN vector with lacZ reporter gene, URA3 | Emr et al. (1986) |
| GFP-Yap1p | pRS316 (CEN, URA3), Yap1p fused to GFP | Coleman et al. (1999) |
| pYES2 | 2μ vector with GAL1 promoter, URA3 | Invitrogen |
| pGAL-GFP-Yap8p | GFP fused to N terminus of YAP8 in pYES2 | This work |
| pEM16 | ACR2-lacZ fusion in YEp357R | This work |
| pEM19 | ACR3-lacZ fusion in pSEYC102 | This work |
| pRW11 | ACR3-lacZ fusion in YEp363 | This work |
| pRW3 | ACR3 in pFL39 (CEN, TRP1) | Wysocki et al. (1997) |
| pRW33 | ACR3 behind its own promoter lacking the TTAATAA sequence (mutACR3) on pRW3 | This work |
| pEM18 | mutACR2-lacZ fusion lacking the TTAATAA sequence in YEp357R | This work |
| pEM20 | mutACR3-lacZ fusion lacking the TTAATAA sequence in pSEYC102 | This work |
| GFP-YAP8-C132A | C132A point mutation in pGAL-GFP-Yap8p (pYES2) | This work |
| GFP-YAP8-C274A | C274A point mutation in pGAL-GFP-Yap8p (pYES2) | This work |
| GFP-YAP8-C132A C274A | C132A C274A point mutations in pGAL-GFP-Yap8p (pYES2) | This work |
| pRS cp-GFP HA YAP1 | GFP-fused Yap1p (wt) in pRS314 (CEN, TRP1) | Kuge et al. (1997) |
| pRS cp-GFP HA yap1 cm46A5 | GFP-fused Yap1p-TAT (C598T C620A C629T) in pRS314 | Kuge et al. (1997) |
| pRS cp-GFP HA yap1 3Cys | GFP-fused Yap1p-3Cys (C303T C310T C315T) in pRS314 | Kuge et al. (2001) |
| pRS cp-GFP HA yap1 3Cys, C620A | GFP-fused Yap1p-3Cys C620A (C303T C310T C315T C620A) in pRS314 | Kuge et al. (2001) |
| YEp195-GFP-YAP8 | GFP-YAP8 controlled by the endogenous YAP8 promoter in YEplac195 (2μ, URA3) | This work |
| pJAW96 | YCF1-lacZ fusion in pSEYC102 | Wemmie et al. (1994) |
| pAW14 | GSH1-lacZ fusion in pSEYC102 | Wu and Moye-Rowley (1994) |
| pCM188 | CEN vector, URA3, tetO-CYC1 promoter | Gari et al. (1997) |
| pMiT004 | HIS6-FLAG-tagged YAP8 in pCM188 | This work |
To construct pGAL-GFP-YAP8, the PIR1 gene was excised from plasmid pGAL-GFP-PIR1 (Vongsamphanh et al., 2001) with BamHI-XbaI and replaced by a PCR fragment containing YAP8 by using wild-type genomic DNA as template and primers YAP8-F1-BamHI (5′-–10GACCAAGAGGATCCCAAAACCGCGTGGAAGAAAAGGCGGC+29-3′) and YAP8-R2-XbaI (5′-+1006GGCGAAGGTATCTAGACGTAATAATCTTCACGCC+973-3′) bearing the BamHI and XbaI sites (underlined), respectively. The BamHI-XbaI–cleaved PCR product was subcloned next to the green fluorescent protein (GFP) gene and placed under the control of the GAL1 promoter to produce plasmid pGAL-GFP-YAP8. Cysteine to alanine mutations within Yap8p were created by the QuickChange site-directed mutagenesis kit (Stratagene. La Jolla, CA) by using pGAL-GFP-YAP8 as template. The oligonucleotides used to create the C132A mutation were YAP8-C132A-A (5′-GCAACTGCTCTCAGTGGCTGTTGGAAAGAATTGTACAGTGCCG-3′) and YAP8-C132A-B (5′-CGGCACTGTACAATTCTTTCCAACAGCCACTGAGAGCAGTTGC-3′), bearing the centrally located changes underlined. Similarly, the oligonucleotides YAP8-C274A-A (5′-CTTAAGAAAAGTGCTACGGCTTCTAATTTTGATATTTTAATTAGCC-3′) and YAP8-C274A-B (5′-GGCTAATTAAAATATCAAAATTAGAAGCCGTAGCACTTTTCTTAAG-3′) were used to create the C274A mutation as well as the double mutation C132A C274A. The mutations in the resulting plasmids pYAP8-C132A, pYAP8-C274A, and pYAP8-C132A C274A were verified by DNA sequence analysis. The various GFP-YAP8 fusion proteins (wild-type and cysteine mutants) were also placed under the control of the endogenous YAP8 promoter. GFP-YAP8 was amplified by PCR by using the pGAL-GFP-YAP8 plasmids as templates and primers GFP-1 (5′-GCGGTACCATGAAAGGAGAAGAACTTTTC; KpnI-site underlined) and YAP8-R2-XbaI (see above). The YAP8 promoter was amplified using plasmid pA10 (Bobrowicz et al., 1997) as template with the primers 5′-GGGGAATTCCCTCAAGCTTTATTGTTCCAGC and 5′-GGGGGTACCTATCTTGGTCACTTATCTTCCG, bearing EcoRI and KpnI sites (underlined), respectively. The resulting PCR products were digested with KpnI-XbaI and EcoRI-KpnI, respectively, and cloned into EcoRI-XbaI digested YEplac195.
To construct HIS6 and FLAG-tagged Yap8p, the YAP8 gene was amplified from genomic DNA by PCR by using the primers 5′-CATGCCATGGGTATGGCAAAACCGCGTGGAAGA-3′ and 5′-ACGCGTCGACTTATAATTTTGACGAAAAGAC-3′ bearing NcoI and SalI sites (underlined), respectively. The NcoI-SalI cleaved PCR product was cloned into plasmid pET30f (Ghislain et al., 1996), and the resulting plasmid was sequenced to confirm in-frame ligation of YAP8 with HIS6 and FLAG-tags. HIS6-FLAG-YAP8 was in turn amplified by PCR by using the primers 5′-ATGCATCGATTAATACGACTCACTATAGGG (ClaI site underlined) and 5′-GCTAGTTATTGCTCAGCGG, and the ClaI-NotI–cleaved fragment was cloned into the yeast vector pCM188 (Gari et al., 1997) to generate plasmid pMiT004.
Expression Analysis
Northern Blot. Sodium arsenite (1 mM) or potassium antimonyl tartrate (1 mM) was added to exponentially growing cells on YNB medium. Total RNA was isolated at the time points indicated in Figure 3 and separated on formaldehyde-agarose gels by using standard methods. Blots were hybridized with 32P-labeled PCR fragments in buffer containing 7% SDS, 0.5 M sodium phosphate buffer, pH 7.0, and 1 mM EDTA. To quantify transcript levels, signal intensity was quantified with a Molecular Dynamics PhosphorImager and normalized to 18S rRNA. At least two independent Northern blot analyses were performed for each growth condition and transcript examined.
Figure 3.
Yap1p and Yap8p control As(III)-induced expression of different subsets of defense genes. (A) Northern blot analysis of total RNA extracted from wild-type, yap1Δ, yap8Δ and yap1Δ yap8Δ cells in the absence (lane 1) or presence of 1 mM As(III) for 60 min (lane 2) and 300 min (lane 3). The filter was hybridized to 32P-labeled DNA fragments recognizing the indicated genes. (B) Metalloid-dependent induction of the ACR3-lacZ reporter in wild-type, yap1Δ, yap8Δ and yap1Δ yap8Δ cells. Cells exposed to metalloids were assayed for β-galactosidase activity as described in MATERIALS AND METHODS. The results are the average of three independent experiments. The error bars represent the SD.
β-Galactosidase Activity Measurements. Cells expressing the various lacZ fusion genes were grown in YNB glucose medium for 20 h in the presence of low concentration of metalloids: wild type [0.1 mM As(III), As(V), Sb(III)], yap8Δ [0.05 mM As(III), As(V); 0.1 mM Sb(III)], yap1Δ [0.05 mM As(III), Sb(III); 0.1 mM As(V)], yap1Δ yap8Δ [0.05 mM As(III), As(V), Sb(III)]. For the last 2 h of incubation, the concentration of metalloids were increased to 1 mM. β-Galactosidase activity assays were performed on permeabilized cells as described previously (Guarente, 1983) at least two times for three independent transformants. The values are given with SD.
Chromatin Immunoprecipitation (ChIP) Assays
Exponentially growing yeast cells expressing FLAG-tagged Yap8p were cross-linked with 1% formaldehyde, incubated with 125 mM glycine, harvested, and washed in phosphate-buffered saline. Cell breakage was performed in lysis buffer (50 mM HEPES, pH 7.5, 140 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 1× complete protease inhibitor cocktail; Roche Diagnostics, Indianapolis, IN) by using glass bead grinding followed by sonication for 90 s to yield an average DNA fragment size of 500 bp. Immunoprecipitation was performed with agarose beads, which were coated with anti-mouse-IgG (Sigma Aldrich, St. Louis, MO) and incubated with anti-FLAG monoclonal antibody (M2; Sigma Aldrich) beforehand. Precipitates were washed and processed for DNA purification as described previously (Kuras and Struhl, 1999). Coprecipitated DNA was used for PCR to amplify promoter fragments of ACR3 and YAP8 by using the following primers: ACR3 (5′-CCTTCCTCTGATTTTCAATTAGGCCC and 5′-GCGATTCACCATATTAACCTTAGAAGGTACCG) and YAP8 (5′-CCTCAAGCTTTATTGTTCCAGC and 5′-TATCTTGGTCACTTATCTTCCG). The PCR products were separated in 2% agarose gel and visualized with ethidium bromide.
Cell Fractionation, Protein Extraction, and Western Analysis
Cells were grown in YPD, either untreated or exposed to 1.0 mM sodium arsenite for 3 h, nuclear extracts and cytoplasmic fractions were prepared and probed by Western analysis as described previously (Vongsamphanh et al., 2001). Anti-myc antibodies (Sc-40; Santa Cruz Biotechnology, Santa Cruz, CA) were kindly provided by J.-Y. Masson (Laval University).
Fluorescence Microscopy
To analyze the distribution of GFP fusion proteins, transformants expressing the proteins of interest were grown in YNB medium lacking the appropriate amino acid to mid-log phase. To visualize DNA, 2 μg/ml 4′,6-diamino-2-phenylindole was added directly to the culture. Cells were washed twice with water or phosphate-buffered saline, and GFP signals were observed in living cells before and 10 min after exposure to 1 mM As(III), 10 mM Sb(III), or 0.5 mM H2O2 by using a Leica DM R fluorescence microscope.
RESULTS
Cells Lacking YAP1 and YAP8 Display Distinct Phenotypes
To investigate the role of the yeast AP-1–like proteins in metalloid tolerance, we first compared growth of the eight single yap mutants (yap1Δ to yap8Δ) to that of wild type (BY4741 background; EUROSCARF strain collection) in the presence of As(III), As(V), and Sb(III). The yap1Δ mutant was sensitive to As(III) and Sb(III) but grew as well as the wild type in the presence of As(V). The yap8Δ mutant displayed hypersensitivity to As(III) and As(V) and a slight sensitivity to Sb(III). Growth of the other yap mutants (yap2Δ to yap7Δ) was unaffected by the metalloids (our unpublished data). We then created single and double yap1Δ and yap8Δ mutants in the W303-1A background to test whether the phenotype is strain specific. The single W303-1A yap1Δ and yap8Δ mutants had the same distinct phenotypes as the corresponding BY4741 mutants (Figure 1A). The yap1Δ yap8Δ double mutant was clearly more sensitive to As(III) and As(V) than the single mutants, whereas the Sb(III) sensitivity of yap1Δ yap8Δ was only slightly higher than that of yap1Δ (Figure 1A). The data indicate a requirement of both Yap1p and Yap8p for As(III) and As(V) tolerance. In addition, Yap1p, and not Yap8p, is required for Sb(III) tolerance. Thus, the two transcriptional activators display both common and distinct metalloid specificity, indicating different biological functions and/or target genes.
Figure 1.
Phenotypes of S. cerevisiae cells lacking AP-1 proteins and proteins involved in metalloid detoxification. (A) Metalloid sensitivity. Cells of W303-1A were grown in liquid medium, and 10-fold serial dilutions of the cultures were spotted on agar plates with or without metalloid salts. Growth was monitored after 3 d at 30°C. (B) Sensitivity of yap8Δ is limited to metalloids, whereas yap1Δ is sensitive to several oxidizing agents. Cells were cultivated as described above, and growth was monitored after 2–3 d at 25°C.
We next compared growth and minimum inhibitory concentrations (MICs) of yap1Δ and yap8Δ mutants to those of ycf1Δ and acr3Δ mutants lacking the transporters mediating metalloid detoxification (Figure 1A and Table 3). The MICs on As(III)-containing medium of yap1Δ (0.75 mM) and yap8Δ (0.20 mM) were very similar to those of ycf1Δ (0.75 mM) and acr3Δ (0.10 mM), respectively. Likewise, the MICs on As(III) of yap1Δ yap8Δ (0.07 mM) and acr3Δ ycf1Δ (0.05 mM) were similar. On As(V), yap8Δ and acr3Δ had identical MICs (both 1.0 mM). The ycf1Δ mutant was more strongly affected by Sb(III) (MIC of 0.25 mM) than yap1Δ (MIC of 2.0 mM). Collectively, these data indicate that Ycf1p plays a more important role in Sb(III) detoxification, whereas Acr3p is more active against As(III). Similarly, Yap8p is critical for As(III) and As(V) tolerance, whereas Yap1p protects cells against As(III) and Sb(III). To test whether Yap1p and Yap8p mediate tolerance through their known targets Ycf1p and Acr3p, respectively, we created yap1Δ ycf1Δ and yap8Δ acr3Δ double mutants and determined their MICs (Table 3). yap8Δ acr3Δ and acr3Δ cells had identical MICs under all conditions tested, suggesting that Yap8p mediates its effect via its target Acr3p. The yap1Δ ycf1Δ mutant was equally sensitive to As(V) and Sb(III) as ycf1Δ, but showed a higher As(III) sensitivity than ycf1Δ. Hence, Yap1p might control expression of several additional genes required for As(III) detoxification (see further).
Table 3.
Sensitivity of yeast strains to arsenic and antimony salts
| MICa (mM)
|
|||
|---|---|---|---|
| Strain | As(III)b | As(V)c | Sb(III)d |
| Wild type | 1.20 | 6.00 | 15.0 |
| yap1Δ | 0.75 | 5.00 | 2.00 |
| yap8Δ | 0.20 | 1.00 | 10.0 |
| yap1Δ yap8Δ | 0.07 | 0.75 | 1.00 |
| acr3Δ | 0.10 | 1.00 | 10.0 |
| ycf1Δ | 0.75 | 5.00 | 0.25 |
| acr3Δ ycf1Δ | 0.05 | 0.40 | 0.15 |
| yap1Δ ycf1Δ | 0.50 | 5.00 | 0.25 |
| yap8Δ acr3Δ | 0.10 | 1.00 | 10.0 |
MIC is the concentration at which no growth was observed on glucose medium. MIC values were determined on the basis of three independent experiments with identical results.
Sodium arsenite.
Sodium arsenate.
Potassium antimonyl tartrate.
We expanded the growth analysis by including a variety of oxidative stress-generating agents in the growth medium (Figure 1B). Although yap1Δ was sensitive to a range of chemical oxidants, including diamide, menadione, methyl viologen (paraquat) and tert-butylhydroperoxide (t-BOOH), yap8Δ displayed parental resistance to these drugs. Moreover, the sensitivity of yap1Δ yap8Δ was identical to that of yap1Δ cells (Figure 1B). We conclude that unlike Yap1p, Yap8p is not involved in the general oxidative stress response.
Yap1p and Yap8p Have Different Subcellular Localizations
Yap1p has previously been shown to undergo cellular redistribution in response to oxidative stress (Kuge et al., 1997; Yan et al., 1998). We therefore tested whether metalloids could also alter Yap1p cellular localization. To do this, we introduced a centromeric plasmid containing Yap1p fused to GFP (Coleman et al., 1999) into yeast and followed the distribution of the fusion protein in living cells (Figure 2A). In the absence of metalloids, GFP-Yap1p was evenly distributed throughout the cell. GFP-Yap1p showed a predominant nuclear signal (colocalization with 4′,6-diamino-2-phenylindole; our unpublished data) within 5 min of As(III) exposure (1.0 mM). The timing and the extent of GFP-Yap1p nuclear accumulation was similar in cells exposed to As(III) and to H2O2, which was used here as a positive control for oxidative stress (Figure 2A). Sb(III) exposure (10 mM) resulted in a somewhat weaker effect; GFP-Yap1p was present both in the cytoplasm and the nucleus and the nuclear signal was evident only after 10 min. This is somewhat surprising because Yap1p seems to play a more active role in detoxification of Sb(III) than of As(III) (Figure 1A). Nonetheless, the data clearly demonstrate that agents other than oxidants are able to trigger Yap1p activation and nuclear retention.
Figure 2.
Cellular localization of Yap1p and Yap8p. (A) Plasmids carrying GFP fusions of Yap1p and Yap8p were transformed into yap1Δ and yap8Δ cells, respectively. Transformants were cultivated in selective medium with either 2% glucose (GFP-Yap1p) or 2% raffinose +0.5% galactose followed by induction with 2% galactose for 1–2 h (GFP-Yap8p). Living cells were analyzed by fluorescence microscope for Yap1p and Yap8p localization (GFP) before and 10 min after exposure to As(III) (1 mM), Sb(III) (10 mM), and H2O2 (0.5 mM). (B) Localization of GFP-Yap8p in yap8Δ cells transsformed with a plasmid expressing the fusion protein from the endogenous YAP8 promoter before and after 10 min of As(III) exposure. (C) Cells with a genomic copy of Yap8p fused to the Myc-tag were grown in YPD, either untreated or exposed to 1.0 mM As(III) for 3 h, and nuclear extracts and cytoplasmic fractions were prepared and probed by anti-myc antibodies. Cells lacking Yap8p-Myc was used as a control.
We also conducted parallel experiments to examine Yap8p cellular distribution in response to metalloid exposure. Because the YAP8 promoter did not produce sufficient amounts of the GFP-Yap8p fusion protein to allow detection when integrated into the genome, we placed GFP-Yap8p under the control of the galactose-inducible GAL1 promoter. To avoid massive overproduction of Yap8p that could lead to localization artifacts, cells were grown on raffinose and low galactose (0.5%). GFP-Yap8p was functionally active because it complemented the As(III) sensitivity of the yap8Δ mutant (Figure 6C). Direct fluorescence analysis revealed that GFP-Yap8p, in contrast to Yap1p, was already present in the nucleus of untreated cells (Figure 2A) and remained nuclear in cells exposed to As(III), Sb(III), or H2O2 (Figure 2A). We repeated the experiments by introducing a multi-copy plasmid where the GFP-Yap8p was controlled by the endogenous YAP8 promoter into yeast. Also in this case, GFP-Yap8p was functional and showed a distinct nuclear localization under all conditions tested (Figure 2B; our unpublished data). To confirm nuclear residence of Yap8p, we created a genomic integration of a Myc-tag at the C-terminal end of the YAP8 gene. Nuclear and cytoplasmic extracts were prepared from untreated and As(III)-exposed cells expressing functional Yap8p-Myc and probed by Western blotting (Figure 2C). Yap8p-Myc was present in nuclear extracts both in untreated as well as As(III)-exposed cells, supporting the notion that Yap8p is a nuclear protein. We note, however, that a minor portion of Yap8p is present in the cytoplasm as observed both by fluorescence microscopy and by immunoblotting. Together, the data indicate that Yap1p and Yap8p have distinct cellular localizations under normal growth conditions and that they may be regulated in different ways to mediate metalloid stress-specific transcriptional activation.
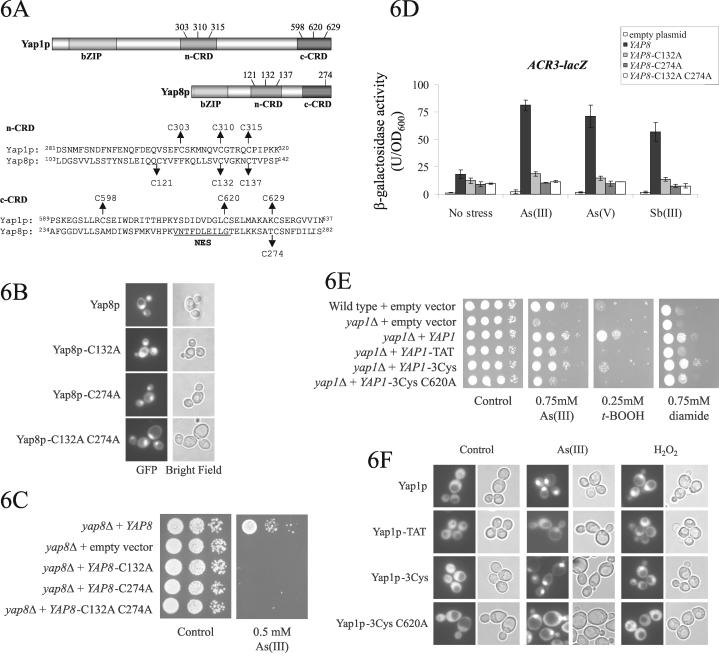
Figure 6.
Functional analysis of Yap1p and Yap8p mutants bearing substitutions of conserved CRD cysteines. (A) Domain organization of Yap1p and Yap8p and position of conserved cysteine residues involved in redox regulation and metalloid tolerance. NES, nuclear export sequence. (B) Subcellular localization of Yap8p cysteine mutants. Plasmids carrying GFP fusions of Yap8p and the Yap8p-C132A, Yap8p-C274A, and Yap8p-C132A C274A mutants were transformed into yap8Δ cells, and the proteins were visualized by fluorescence microscopy. For simplicity, the GFP-Yap8p mutants are labeled in abbreviated form. (C) Yap8p cysteines C132 and C274 are both required for Yap8p function. yap8Δ cells were transformed with plasmids expressing either native GFP-Yap8p or the indicated GFP-Yap8p mutants. The yap8Δ mutant was also transformed with an empty control plasmid. Transformed cells were grown in liquid medium containing 2% raffinose and 0.5% galactose followed by induction with 2% galactose for 1–2 h. Serial 10-fold dilutions of the cultures were spotted on agar plates (with glucose as a carbon source) with or without As(III). (D) GFP-Yap8p cysteine mutants are unable to induce ACR3-lacZ expression. yap8Δ cells were transformed with empty control plasmid and with plasmids expressing either native GFP-Yap8p or the indicated GFP-Yap8p mutants. β-Galactosidase activity was measured as described in Figure 3. (E) Yap1p cysteines are important for As(III) and oxidative stress tolerance. yap1Δ cells were transformed with plasmids expressing either native GFP-Yap1p or the indicated GFP-Yap1p mutants. Wild-type and yap1Δ cells were also transformed with an empty control plasmid. Ten-fold serial dilutions of the cultures were spotted on agar plates and incubated at 30°C (As(III)) or 25°C (t-BOOH and diamide) for 2–3 d. For simplicity, the GFP-Yap1p mutants are labeled in abbreviated form. (F) Subcellular localization of Yap1p CRD mutants. Plasmids carrying GFP fusions of Yap1p and the indicated Yap1p mutants were transformed into yap1Δ cells, and the proteins were visualized before and 10 min after exposure to As(III) (1.0 mM) or H2O2 (0.5 mM) by fluorescence microscopy.
Yap1p and Yap8p Activate Expression of Distinct Subsets of Defense Genes
We next followed the mRNA levels of various stress defense genes in metalloid-exposed cells. As(III) exposure strongly enhanced TRX2 (thioredoxin) and TRR1 (thioredoxin reductase) expression (5- and 2.5-fold, respectively), whereas induction of GSH1 (γ-glutamylcysteine synthetase) and GLR1 (glutathione reductase) was more moderate (both approximately twofold; Figure 3A). These induction levels are comparable with those observed upon cadmium (Dormer et al., 2000; Fauchon et al., 2002), selenite (Pinson et al., 2000), or mercury (Westwater et al., 2002) exposure. Expression of other stress-responsive genes, including CTT1 (cytoplasmic catalase), HSP12 (putative LEA-protein), and GRE2 (similar to plant dihydroflavonol-4-reductases) was strongly stimulated by both As(III) and Sb(III), whereas only As(III) induced HSP104 (chaperone) expression (our unpublished data). Expression of the arsenic detoxification genes ACR2 and ACR3 was also enhanced, although these mRNAs generated weak signals that could not reliably be quantified (see below).
Consistent with the growth data, we found that Yap1p and Yap8p indeed control transcription of distinct subsets of defense genes. As(III)-induction of the oxidative stress-responsive genes TRX2, TRR1, GSH1, GLR1, and GRE2 was Yap1p dependent; their mRNA level was significantly reduced in the yap1Δ mutant compared with the wild type (Figure 3A). Expression of these genes was not significantly affected by YAP8 deletion. On the other hand, Yap8p was required for ACR2 and ACR3 expression; no expression of these genes was detected in the yap8Δ mutant (see below). Finally, induction of CTT1 and HSP12 was somewhat reduced in yap1Δ yap8Δ cells, whereas HSP104 expression was basically unaltered, suggesting that other transcription factors may be more important for the control of these genes (our unpublished data). Similar Yap1p- and Yap8p-dependent control of gene expression was obtained when cells were exposed to Sb(III) (our unpublished data).
To provide further evidence for the role of Yap8p in transcriptional control of ACR2 and ACR3, we fused their promoters to the lacZ gene (ACR2-lacZ and ACR3-lacZ) and measured β-galactosidase activity in the same strains as above. ACR3-lacZ expression was induced 15-fold by As(III), 10-fold by As(V), and approximately fourfold by Sb(III) in wild-type cells transformed with the ACR3-lacZ fusion on a centromeric vector (PEM19) (Figure 3B). Deletion of YAP8 resulted in a complete loss of ACR3-lacZ induction, whereas YAP1 deletion did not significantly affect induction (Figure 3B). Similar results were also obtained using the ACR2-lacZ construct (our unpublished data). Collectively, the expression data clearly demonstrate a role of Yap1p in metalloid-dependent activation of various oxidative stress-responsive genes, whereas the main function of Yap8p seems to be linked to the control of ACR2 and ACR3 expression.
Yap1p Is Hyperactivated in Metalloid-exposed Cells Lacking YAP8 or ACR3
We next examined control of YCF1 expression by using a YCF1 promoter-lacZ fusion construct (YCF1-lacZ) (Wemmie et al., 1994) (Table 4). YCF1-lacZ expression in wild-type cells was not affected by up to 20 h of metalloid exposure. Remarkably, YAP1 deletion did not affect YCF1-lacZ expression, whereas YAP8 deletion caused a nearly threefold increase in the presence of As(III) and As(V), but not Sb(III). This increase was Yap1p-dependent because YCF1-lacZ expression was reduced to basal level in yap1Δ yap8Δ cells. The reason for this observation might be that Yap8p impedes on Yap1p function, i.e., hinders the binding of Yap1p to the YCF1 promoter. Alternatively, Yap1p might be hyperactive in metalloid-exposed cells that lack Yap8p. To test the latter hypothesis, we monitored YCF1-lacZ expression in acr3Δ cells (Table 4). As(III) indeed stimulated YCF1-lacZ expression in the acr3Δ mutant (approximately twofold) albeit not as strongly as in yap8Δ (threefold). YCF1-lacZ expression was similar in yap8Δ acr3Δ and yap8Δ. Because yap8Δ and acr3Δ are equally sensitive to As(III), both possibilities might occur; hyperactivation of Yap1p in yap8Δ and acr3Δ mutants as well as an absence of Yap8p on the Yap1p-binding site of the YCF1 promoter in yap8Δ.
Table 4.
Control of YCF1-lacZ and GSH1-lacZ expression in metalloid-exposed cells
|
YCF1-lacZ/pSEYC102 (U/OD600)a
|
|||||||
| Strain | No stress | As(III)b | As(V)b | Sb(III)b | |||
| Wild-type | 5.4 ± 0.8 | 5.6 ± 0.3 | 5.2 ± 0.8 | 5.4 ± 0.2 | |||
| yap1Δ | 5.2 ± 0.1 | 4.1 ± 0.3 | 4.2 ± 0.3 | 3.6 ± 0.7 | |||
| yap8Δ | 6.1 ± 0.4 | 16.7 ± 0.9 | 13.9 ± 0.4 | 5.0 ± 0.6 | |||
| yap1Δ yap8Δ | 4.6 ± 0.5 | 4.8 ± 1.0 | 5.9 ± 0.4 | 3.5 ± 0.1 | |||
| acr3Δ | 5.1 ± 0.6 | 10.7 ± 0.4 | 8.6 ± 0.3 | 6.3 ± 1.1 | |||
| yap8Δ acr3Δ | 5.0 ± 0.15 | 15.9 ± 0.2 | 13.5 ± 0.45 | 5.9 ± 0.2 | |||
| yap1Δ acr3Δ | 4.0 ± 0.5 | 5.6 ± 1.0 | 6.1 ± 0.7 | 4.35 ± 0.4 | |||
|
GSH1-lacZ/pSEYC102 (U/OD600)a
|
|||||||
| Strain | No stress | As(III)b | As(V)b | Sb(III)b | |||
| WT | 10.3 ± 0.6 | 15.0 ± 0.9 | 14.5 ± 1.3 | 18.3 ± 1.1 | |||
| yap1Δ | 7.0 ± 1.4 | 8.4 ± 1.5 | 9.4 ± 1.5 | 10.0 ± 1.1 | |||
| yap8Δ | 11.3 ± 0.7 | 32.8 ± 3.1 | 29.1 ± 2.1 | 20.2 ± 0.9 | |||
| yap1Δ yap8Δ | 7.6 ± 1.0 | 15.3 ± 1.7 | 13.5 ± 2.9 | 12.1 ± 1.5 | |||
| acr3Δ | 11.3 ± 1.2 | 32.9 ± 3.1 | 28.9 ± 3.4 | 21.0 ± 1.8 | |||
| yap8Δ acr3Δ | 11.9 ± 0.7 | 34.9 ± 2.9 | 29.5 ± 4.3 | 20.8 ± 1.9 | |||
| yap1Δ acr3Δ | 6.7 ± 0.7 | 13.3 ± 1.4 | 11.7 ± 1.9 | 10.7 ± 0.9 | |||
β-Galactosidase activities are the average of three independent experiments, including standard deviation.
YCF1-lacZ and GSH1-lacZ expression from the pSEYC102 centromeric plasmid after 20 h of metalloid preincubation (0.1 mM) followed by 2 h of induction with 1 mM respective metalloid.
Similar observations were made using a GSH1 promoter-lacZ fusion construct (GSH1-lacZ) (Wu and Moye-Rowley, 1994) (Table 4). GSH1-lacZ expression in the wild type was moderately stimulated by As(III) (1.5-fold), As(V) (1.5-fold), and Sb(III) (twofold) in a Yap1p-dependent way (Table 4). In analogy with YCF1-lacZ, deletion of either YAP8 or ACR3 resulted in an approximately threefold higher GSH1-lacZ expression during metalloid exposure. Again, this stimulation was Yap1p-dependent because expression was reduced to the basal level when YAP1 was deleted in combination with either YAP8 or ACR3. In addition, the basal level of TRR1 mRNA was also increased in the yap8Δ mutant (Figure 3A). Hence, the consequences of an apparently hyperactive Yap1p can be seen on different promoters.
Activation of ACR2 and ACR3 Expression Requires a Putative AP-1 Binding Site
Although several studies have documented the promoter sequence recognized by Yap1p, the Yap8p recognition element has not previously been established (Toone and Jones, 1999; Toone et al., 2001). The promoter region between the divergently expressed ACR2 and ACR3 genes contains a putative AP-1 binding site with the sequence TTAATAA (Figure 4A). This sequence differs from the preferential Yap1p binding site TTACTAA on one position (underlined). The promoter of YAP8 itself contains such a TTAATAA sequence. To study the importance of this sequence for Acr3p-mediated As(III) tolerance, we deleted the TTAATAA sequence from the ACR3 promoter in plasmid pRW3, which contains the entire ACR3 gene, and transformed the resulting plasmid (mutACR3) into the acr3Δ strain. Cells containing the mutACR3 plasmid failed to grow in the presence of 0.5 mM As(III), whereas cells containing the ACR3 gene behind its native promoter grew as well as wild-type cells (Figure 4B). The fact that cells with the mutACR3 plasmid are unable to grow in the presence of As(III) is likely to be caused by a lack of ACR3 induction.
Figure 4.
ACR3 induction requires the TTAATAA promoter element. (A) Promoters of ACR2, ACR3, and YAP8 contain putative AP-1 binding sites with the sequence TTAATAA. (B) Presence of the TTAATAA sequence in the ACR3 promoter is required for Acr3p-mediated As(III) tolerance. Cells were grown in liquid medium and 10-fold serial dilutions of the cultures were spotted on agar plates with or without As(III). (C) Induction of ACR3-lacZ expression is dependent on the TTAATAA sequence. β-Galactosidase activity was measured as described in Figure 3.
To confirm this notion, we deleted the TTAATAA sequence from the ACR2-lacZ and ACR3-lacZ constructs and measured β-galactosidase activity after metalloid exposure. Induction of ACR2-lacZ and ACR3-lacZ expression was completely lost in the promoter mutants (Figure 4C; our unpublished data). Hence, induction of ACR2 and ACR3 expression by the metalloids is dependent on both Yap8p and the TTAATAA sequence.
Yap8p Interacts with the ACR3 Promoter In Vivo
The most straightforward explanation for the data mentioned above is that Yap8p controls ACR2 and ACR3 expression by direct binding to the promoter separating these genes (Figure 4A). To examine whether Yap8p is present on the ACR3 promoter in vivo, we performed chromatin immunoprecipitation assays by using two different strains: a strain expressing no FLAG-tagged Yap8p and a strain expressing FLAG-Yap8p. ChIP analysis revealed that FLAG-Yap8p was cross-linked with the ACR3 promoter (Figure 5). A comparison of untreated and As(III)-exposed cells revealed that FLAG-Yap8p was present on the ACR3 promoter already in the absence of As(III)-stress and remained bound to this promoter in As(III)-exposed cells.
Figure 5.
Binding of FLAG-Yap8p to the ACR3 promoter as detected by ChIP. PCR was performed on chromatin fragments isolated after immunoprecipitation (Anti-FLAG-IP) in cells expressing FLAG-Yap8p or cells without tagged protein (no tag) with primers that specifically amplify the ACR3 and YAP8 promoters. Cells were either untreated or exposed to 1 mM As(III) for 3 h. PCR was also performed on extracts that were not immunoprecipitated (input).
Because the YAP8 promoter also contains a TTAATAA sequence, we also investigated whether FLAG-Yap8p would bind to its own promoter. However, in contrast to the ACR3 promoter, FLAG-Yap8p was not associated with its own promoter, neither in the absence nor in the presence of As(III) (Figure 5). Hence, despite the fact that both promoters contain the TTAATAA sequence, Yap8p was only present on the ACR3 promoter, suggesting that other factors influence DNA-binding of Yap8p.
Identification of Yap1p and Yap8p Cysteines That Are Critical for Metalloid Tolerance
Yap8p has three cysteines in the n-CRD located at C121, C132, and C137. The position of C132 and C137 is conserved in both Yap1p and Yap8p (Figure 6A). Yap8p has one cysteine in a putative c-CRD (C274), which is at the same position as Yap1p-C629 (Figure 6A). We chose to create alanine substitutions in Yap8p at positions C132 and C274 by site-directed mutagenesis within the plasmid expressing GFP-Yap8p. These residues were chosen as they are conserved in the CRDs of both Yap1p and Yap8p as well as in several fungal AP-1–like proteins, including Schizosaccharomyces pombe Pap1 and Candida albicans Cap1 (Toone et al., 2001). Three mutants were created: GFP-Yap8p-C132A, GFP-Yap8p-C274A, and GFP-Yap8p-C132A C274A. The three Yap8p mutants had the same nuclear localization as the wild-type protein both in the absence (Figure 6B) and presence of metalloids (our unpublished data). Importantly, none of the mutants were able to complement the As(III) sensitivity of the yap8Δ mutant (Figure 6C). Moreover, neither of the single mutants GFP-Yap8p-C132A and GFP-Yap8p-C274A nor the double GFP-Yap8p-C132A C274A mutant was capable of inducing ACR3-lacZ expression upon metalloid exposure, whereas the native GFP-Yap8p protein (Yap8p) proved to be a strong activator of ACR3-lacZ expression in the presence of all metalloids tested (Figure 6D). It is noteworthy that the basal level of ACR3-lacZ was elevated due to higher amounts of GFP-Yap8p expression from the GAL1 promoter. From the above-mentioned data, we conclude that the cysteine residues at position C132 and C274 are essential for proper Yap8p function.
We expanded the analysis to include Yap1p mutants carrying alterations in the n- and c-CRDs; Yap1p-TAT (C598T C620A C629T), Yap1p-3Cys (C303T C310T C315T), and Yap1p-3Cys C620A (C303T C310T C315T C620A). These mutant proteins exhibit reduced nuclear residence in the presence of H2O2 or diamide and consequently a diminished expression of TRX2 (Kuge et al., 2001). We transformed the plasmids containing these GFP-tagged Yap1p mutants into yap1Δ cells and analyzed their capability to complement the yap1Δ phenotype. Importantly, the Yap1p-mutants complemented the As(III) sensitivity of yap1Δ to different degrees: Yap1p-3Cys produced wild-type growth, whereas Yap1p-TAT and Yap1p-3Cys C620A caused partial complementation. The latter two transformants were also sensitive to t-BOOH and diamide, whereas Yap1p-3Cys had a weaker phenotype (Figure 6E). All three alleles produced wild-type growth on Sb(III) medium (our unpublished data).
In agreement with the degree of complementation, Yap1p-3Cys showed strong nuclear retention upon As(III) exposure similar to that of wild-type Yap1p (Figure 6F). Yap1p-3Cys C620A showed an intermediate response; in the presence of As(III), the signal was obvious both in the nucleus and the cytoplasm, and the nuclear signal was more short-lived than that of wild-type Yap1p. In contrast, Yap1p-TAT showed a weak nuclear localization upon As(III) exposure, although most of the protein was in the cytoplasm. Moreover, no additional nuclear accumulation was detectable with Yap1p-TAT after prolonged exposure. It is possible that enough Yap1p-TAT enters the nucleus to provide partial As(III) tolerance (Figure 6E). We also noted that certain Yap1p mutants behaved differently under H2O2 and As(III) exposure (Figure 6F). Yap1p-TAT was mainly cytoplasmic in the presence of As(III), whereas H2O2 triggered a transient nuclear accumulation. Conversely, Yap1p-3Cys C620A was partially nuclear in As(III)-exposed cells, whereas H2O2 was unable to induce nuclear retention of the protein. Hence, despite the fact that the n- and c-CRDs seem to play a role in As(III)-dependent activation of Yap1p, the mechanism by which As(III) activates the protein is clearly different from those involved in H2O2 and diamide-dependent activation.
DISCUSSION
Our data provide important insight into the S. cerevisiae response to metalloid exposure and the involvement of the AP-1–like proteins Yap1p and Yap8p in tolerance acquisition. We show that yeast cells mount an oxidative stress response when challenged with metalloids; the mRNAs coding for proteins that maintain the cellular redox status (TRX2, TRR1), control cellular glutathione levels (GSH1, GLR1), or provide protection against oxidative damage (CTT1) were increased. Expression of other oxidative stress-responsive genes (HSP12, GRE2) was also stimulated. Indeed, As(III) has been shown to provoke increased ROS production in mammalian cells (Liu et al., 2001), and this may also be the case in yeast (our unpublished data). However, the mechanisms responsible for arsenic-induced ROS generation are poorly understood. Several possible mechanisms have been proposed, e.g., production of H2O2 as a result of As(III) oxidation or formation of hydroxyl radicals during the release of iron from ferritin triggered by arsenicals (Del Razo et al., 2001). It has also been postulated that arsenic trioxide induces apoptosis in acute promyelocytic leukemia cells by increasing the cellular H2O2 level possibly by inhibiting glutathione peroxidase (Jing et al., 1999). Hence, in addition to act directly upon the metalloids themselves, cells also increase their ability to deal with damages caused by oxidative stress.
Transcriptional activation of antioxidant genes was rapid; after exposure to metalloids, increased mRNA levels were detected within 15 min and peaked after 30–60 min. This is in contrast to the response of the arsenic detoxification genes ACR2 and ACR3 whose mRNAs were detected after 45–60 min and continued to increase up to 5 h. These results indicate that (expression of) these subsets of metalloid-responsive genes are controlled by different mechanisms and/or that their products may be involved at different stages during the adaptation process leading to tolerance. We did not find any evidence for enhanced YCF1 expression during metalloid exposure, although its deletion clearly caused sensitivity. It is possible that an increase in the expression of YCF1 requires prolonged exposure because two-fold higher YCF1 expression was evident only after 24 h of exposure to cadmium (Li et al., 1997). Alternatively, the basal level of Ycf1p in the vacuolar membranes could be sufficient to mediate tolerance. Thus, conjugation of As(III) and Sb(III) to GSH may represent the rate-limiting step in tolerance acquisition. In that case, increased GSH synthesis might be sufficient to promote increased vacuolar sequestration of GSH-conjugated metalloids by the action of Ycf1p. A similar mechanism of arsenic tolerance was proposed in Leishmania, where increased synthesis of trypanothione, the major source of reduced thiols in trypanosomatidae, is required to produce resistance as a result of formation and extrusion of metalloid–thiol complexes (Mukhopadhyay et al., 1996).
Collectively, it seems that the first line of defense during metalloid exposure is an increase in GSH synthesis coupled to Ycf1p activity as well as an oxidative stress response. The fact that gsh1Δ cells (when cultivated on minimal medium supplemented with cysteine to ensure growth) display sensitivity to As(III), As(V), and Sb(III) (our unpublished data) further corroborates this notion. When exposure persists, expression of the detoxification system ACR3 is stimulated to ensure continuous metalloid removal from the cytosol.
It is firmly established that various oxidants activate Yap1p and that the protein is required for oxidative stress tolerance as well as for resistance to heavy metals and cytotoxic agents (Toone et al., 2001). Here, we show that metalloids also activate Yap1p (Figure 7A); the protein is detected in the nucleus within a few minutes of As(III) or Sb(III) exposure and transcription of Yap1p-dependent genes peaks at ∼30–60 min. Moreover, this activation could be coupled with Yap1p-dependent expression of GSH1, TRX2, TRR1, GLR1, and GRE2 under metalloid exposure; the induction of these genes is largely absent in yap1Δ cells. Hence, the metalloid sensitivity of yap1Δ can be attributed to, at least in part, a lack of transcriptional activation of oxidative stress defense genes.
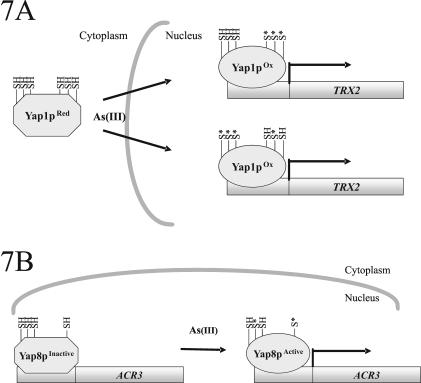
Figure 7.
Model for As(III)-activation of Yap1p and Yap8p. (A) As(III) triggers nuclear accumulation of Yap1p and transcriptional activation of its target genes, including TRX2 (thioredoxin). Mutations within the CRDs that affect Yap1p function under As(III) exposure (Yap1p-TAT and Yap1p-3Cys C620A) are indicated with an asterisk (*). Whether As(III) activates Yap1p through oxidative modification of critical cysteines or through another mechanism is presently unknown. (B) Yap8p is bound to the promoter of ACR3 (arsenic efflux protein) in an apparently inactive form in the absence of stress, whereas As(III) exposure activates Yap8p through an as yet unknown mechanism. Yap8p activation requires specific cysteine residues. Cysteine mutations (C132A or C274A) that affect Yap8p function are marked with an *.
The mechanism of Yap1p activation is clearly complex and may involve disulfide bond formation between distinct cysteines in an oxidant-specific manner. Yap1p mutants with modified cysteines in the c-CRD (Yap1p-TAT) or n-CRD in combination with C620A (Yap1p-3Cys C620A) displayed perturbed nuclear accumulation and were unable to produce wild-type As(III) tolerance. In view of the fact that these Yap1p mutants responded differently to H2O2 and As(III), it is likely that these agents activate Yap1p through distinct mechanisms. Because yap1Δ cells are sensitive to a wide range of oxidants, it is tempting to speculate that Yap1p is regulated by metalloid-induced oxidative modifications of critical cysteines, either directly or via an auxiliary protein as is the case during H2O2 activation (Delaunay et al., 2002). However, a more direct activation mechanism, e.g., binding of As(III) or Sb(III) to Yap1p, cannot be excluded at present.
Unlike Yap1p, Yap8p is a nuclear protein that does not exhibit stress-dependent changes in localization. Instead, ChIP analysis demonstrated that Yap8p is constitutively bound to the ACR3 promoter (Figure 7B). Interestingly, the Yap8p-Acr3p system seems to operate under prolonged As(III) exposure; induced ACR3 expression was only apparent after 45–60 min (mRNA) or after 20 h (β-galactosidase assay). Hence, although Yap1p controls the first-line defense, Yap8p is required for the cellular response during long-term exposure. Our data indicates that the control of Yap8p is neither exerted at the level of localization nor at the level of As(III)-stimulated binding to the ACR3 promoter. Hence, Yap8p activation may involve a novel mechanism.
Activation of Yap8p requires critical cysteine residues that are conserved in several fungal AP-1–like proteins. The Yap8p-C132A or Yap8p-C274A mutants were unable to induce ACR3 expression and, as a consequence, they could not produce As(III) tolerance. In contrast to yap1Δ, yap8Δ cells were not sensitive to chemical oxidants. Moreover, overexpression of Yap8p did not restore H2O2 tolerance of yap1Δ cells (our unpublished data). Although Yap8p activation by metalloid-induced oxidative modifications cannot be excluded solely based on these data, such an activation mechanism may seem less likely. Instead, it is conceivable that these Yap8p cysteines directly bind to As(III) inducing a conformational change such that the modified Yap8p can trigger gene expression.
Our data revealed that Yap8p-dependent activation of ACR2 and ACR3 expression requires a DNA sequence (TTAATAA) that is related to the DNA binding site of Yap1p (TTACTAA). Although these data suggest that Yap8p binds to the TTAATAA sequence, ChIP analysis did not show the presence of Yap8p on the YAP8 promoter, which also contains TTAATAA. It has recently been shown that mutations of nucleotides flanking the Yap1p and Yap2p DNA binding sites decreased expression of their target genes (Cohen et al., 2002). In analogy, the nucleotides flanking the TTAATAA of the two promoters are different; TTGATTAATAATCAA in the ACR3 promoter and TTCTTAATAAATT in the YAP8 promoter, and this difference might affect DNA binding and expression of target genes. Although the exact sequence of the Yap8p DNA binding-site remains to be determined, it is clear that the yeast AP-1–like proteins activate transcription through slightly different sequences. Yap1p to Yap4p preferentially interact with TTACTAA, however, the strongest activation through this site is observed for Yap1p and much weaker for Yap2p and Yap3p (Fernandes et al., 1997). The promoter of the yeast major facilitator encoding FLR1 gene contains three functional but nonequivalent Yap responsive elements: YRE3 (TTACTAA), YRE2 (TGACTAA), and YRE1 (TTAGTCA). Yap1p binds to the YREs with different affinities (YRE3 > YRE2 > YRE1), and mutation of YRE3 caused the largest decrease in FLR1 activation (Nguyen et al., 2001). Finally, recent microarray analysis has shown that expression of one cluster of genes that is specifically induced by Yap1p (but not by Yap2p) in response to H2O2 is significantly increased in the absence of Yap2p (Cohen et al., 2002). Similarly, our data indicated higher expression of the Yap1p-dependent genes YCF1 and GSH1 in the absence of Yap8p. Hence, the yeast AP-1–like proteins may constitute a transcriptional network controlling expression of genes encoding protective functions under various adverse conditions. However, the precise nature of such a network remains to be elucidated.
We conclude that Yap1p and Yap8p mediate metalloid tolerance by activating transcription of distinct defense genes. However, the precise molecular mechanism(s) involved in metalloid-activation of Yap1p and Yap8p have yet to be revealed. Unveiling the tolerance mechanisms in yeast may prove of value for identifying similar mechanisms in other organisms and have important implications for the use of arsenic and antimony in medical therapy.
Acknowledgments
We thank W.S. Moye-Rowley (University of Iowa), S. Kuge (Tohoku University), and M. Ghislain (Université Catholique de Louvain) for providing plasmids; J.-Y. Masson (Laval University) for providing antibodies; and S. Hohmann and T. Nyström (Göteborg University) for critical comments on the manuscript. This work was supported by a fellowship from the Foundation for Polish Science to R.W., by the National Cancer Institute of Canada with funds from the Canadian Cancer Society to D.R., and by the Swedish Research Council (VR) to M.J.T. D.R. was supported by a career scientist award from National Cancer Institute of Canada, and M.J.T. gratefully acknowledges support from the Human Frontier Science Program (grant RG0021/2000-M to Stefan Hohmann).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–04–0236. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–04–0236.
References
- Bobrowicz, P., and Ulaszewski, S. (1998). Arsenical-induced transcriptional activation of the yeast Saccharomyces cerevisiae ACR2 and ACR3 genes requires the presence of the ACR1 gene product. Cell Mol. Biol. Lett. 3, 13–20. [Google Scholar]
- Bobrowicz, P., Wysocki, R., Owsianik, G., Goffeau, A., and Ulaszewski, S. (1997). Isolation of three contiguous genes, ACR1, ACR2 and ACR3, involved in resistance to arsenic compounds in the yeast Saccharomyces cerevisiae. Yeast 13, 819–828. [DOI] [PubMed] [Google Scholar]
- Bossier, P., Fernandes, L., Rocha, D., and Rodrigues-Pousada, C. (1993). Overexpression of YAP2, coding for a new yAP protein, and YAP1 in Saccharomyces cerevisiae alleviates growth inhibition caused by 1,10-phenanthroline. J. Biol. Chem. 268, 23640–23645. [PubMed] [Google Scholar]
- Bouganim, N., David, J., Wysocki, R., and Ramotar, D. (2001). Yap1 overproduction restores arsenite resistance to the ABC-transporter deficient mutant ycf1 by activating ACR3 expression. Biochem. Cell Biol. 79, 441–448. [PubMed] [Google Scholar]
- Cohen, B.A., Pilpel, Y., Mitra, R.D., and Church, G.M. (2002). Discrimination between paralogs using microarray analysis: application to the Yap1p and Yap2p transcriptional networks. Mol. Biol. Cell 13, 1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, S.T., Epping, E.A., Steggerda, S.M., and Moye-Rowley, W.S. (1999). Yap1p activates gene transcription in an oxidant-specific fashion. Mol. Cell. Biol. 19, 8302–8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo, L.M., Quintanilla-Vega, B., Brambila-Colombres, E., Calderon-Aranda, E.S., Manno, M., and Albores, A. (2001). Stress proteins induced by arsenic. Toxicol. Appl. Pharmacol. 177, 132–148. [DOI] [PubMed] [Google Scholar]
- Delaunay, A., Isnard, A.D., and Toledano, M.B. (2000). H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 19, 5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay, A., Pflieger, D., Barrault, M.B., Vinh, J., and Toledano, M.B. (2002). A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481. [DOI] [PubMed] [Google Scholar]
- Dormer, U.H., Westwater, J., McLaren, N.F., Kent, N.A., Mellor, J., and Jamieson, D.J. (2000). Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J. Biol. Chem. 275, 32611–32616. [DOI] [PubMed] [Google Scholar]
- Emr, S.D., Vassarotti, A., Garrett, J., Geller, B.L., Takeda, M., and Douglas, M.G. (1986). The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J. Cell Biol. 102, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchon, M., Lagniel, G., Aude, J.C., Lombardia, L., Soularue, P., Petat, C., Marguerie, G., Sentenac, A., Werner, M., and Labarre, J. (2002). Sulfur sparing in the yeast proteome in response to sulfur demand. Mol. Cell 9, 713–723. [DOI] [PubMed] [Google Scholar]
- Fernandes, L., Rodrigues-Pousada, C., and Struhl, K. (1997). Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Mol. Cell. Biol. 17, 6982–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuchi, T., Ishikawa, H., Miura, N., Ishizuka, M., Kajiya, K., Kuge, S., and Naganuma, A. (2001). Two nuclear proteins, Cin5 and Ydr259c, confer resistance to cisplatin in Saccharomyces cerevisiae. Mol. Pharmacol. 59, 470–474. [DOI] [PubMed] [Google Scholar]
- Gari, E., Piedrafita, L., Aldea, M., and Herrero, E. (1997). A set of vectors with a tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast 13, 837–848. [DOI] [PubMed] [Google Scholar]
- Gasch, A.P., Spellman, P.T., Kao, C.M., Carmel-Harel, O., Eisen, M.B., Storz, G., Botstein, D., and Brown, P.O. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain, M., Dohmen, R.J., Levy, F., and Varshavsky, A. (1996). Cdc48p interacts with Ufd3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 15, 4884–4899. [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M., Shen, J., and Rosen, B.P. (1999). Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 96, 5001–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente, L. (1983). Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101, 181–191. [DOI] [PubMed] [Google Scholar]
- Güldener, U., Heck, S., Fielder, T., Beinhauer, J., and Hegemann, J.H. (1996). A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24, 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, Y., Dai, J., Chalmers-Redman, R.M., Tatton, W.G., and Waxman, S. (1999). Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94, 2102–2111. [PubMed] [Google Scholar]
- Kaiser, C., Michaelis, S., and Mitchel, A. (1994). Methods in Yeast Genetics. A Cold Spring Harbor Laboratory Course Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Kuge, S., Arita, M., Murayama, A., Maeta, K., Izawa, S., Inoue, Y., and Nomoto, A. (2001). Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol. Cell. Biol. 21, 6139–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge, S., Jones, N., and Nomoto, A. (1997). Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 16, 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras, L., and Struhl, K. (1999). Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399, 609–613. [DOI] [PubMed] [Google Scholar]
- Lee, J., Godon, C., Lagniel, G., Spector, D., Garin, J., Labarre, J., and Toledano, M.B. (1999). Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 274, 16040–16046. [DOI] [PubMed] [Google Scholar]
- Li, Z.S., Lu, Y.P., Zhen, R.G., Szczypka, M., Thiele, D.J., and Rea, P.A. (1997). A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. USA 94, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.X., Athar, M., Lippai, I., Waldren, C., and Hei, T.K. (2001). Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc. Natl. Acad. Sci. USA 98, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal, I., Rios, G., Mulet, J.M., Serrano, R., and de Larrinoa, I.F. (1998). Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 425, 323–328. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, R., Dey, S., Xu, N., Gage, D., Lightbody, J., Ouellette, M., and Rosen, B.P. (1996). Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc. Natl. Acad. Sci. USA 93, 10383–10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, R., Shi, J., and Rosen, B.P. (2000). Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 275, 21149–21157. [DOI] [PubMed] [Google Scholar]
- Murray, H.W. (2001). Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 45, 2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, A.M., Tzagoloff, A., Kinney, D.M., and Lusty, C.J. (1986). Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene 45, 299–310. [DOI] [PubMed] [Google Scholar]
- Nguyen, D.T., Alarco, A.M., and Raymond, M. (2001). Multiple Yap1p-binding sites mediate induction of the yeast major facilitator FLR1 gene in response to drugs, oxidants, and alkylating agents. J. Biol. Chem. 276, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Pinson, B., Sagot, I., and Daignan-Fornier, B. (2000). Identification of genes affecting selenite toxicity and resistance in Saccharomyces cerevisiae. Mol. Microbiol. 36, 679–687. [DOI] [PubMed] [Google Scholar]
- Tamás, M.J., and Wysocki, R. (2001). Mechanisms involved in metalloid transport and tolerance acquisition. Curr. Genet. 40, 2–12. [DOI] [PubMed] [Google Scholar]
- Thomas, B.J., and Rothstein, R. (1989). Elevated recombination rates in transcriptionally active DNA. Cell 56, 619–630. [DOI] [PubMed] [Google Scholar]
- Toledano, M.B., Delaunay, A., Biteau, B., Spector, D., and Azevedo, D. (2002). Oxidative stress responses in yeast. In: Yeast Stress Responses, vol. 1, ed. S. Hohmann and W.H. Mager, Heidelberg: Springer, 241–303. [Google Scholar]
- Toone, W.M., and Jones, N. (1999). AP-1 transcription factors in yeast. Curr. Opin. Genet. Dev. 9, 55–61. [DOI] [PubMed] [Google Scholar]
- Toone, W.M., Morgan, B.A., and Jones, N. (2001). Redox control of AP-1-like factors in yeast and beyond. Oncogene 20, 2336–2346. [DOI] [PubMed] [Google Scholar]
- Waxman, S., and Anderson, K.C. (2001). History of the development of arsenic derivatives in cancer therapy. Oncologist 6 (suppl 2), 3–10. [DOI] [PubMed] [Google Scholar]
- Wemmie, J.A., Szczypka, M.S., Thiele, D.J., and Moye-Rowley, W.S. (1994). Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J. Biol. Chem. 269, 32592–32597. [PubMed] [Google Scholar]
- Westwater, J., McLaren, N.F., Dormer, U.H., and Jamieson, D.J. (2002). The adaptive response of Saccharomyces cerevisiae to mercury exposure. Yeast 19, 233–239. [DOI] [PubMed] [Google Scholar]
- Vongsamphanh, R., Fortier, P.K., and Ramotar, D. (2001). Pir1p mediates translocation of the yeast Apn1p endonuclease into the mitochondria to maintain genomic stability. Mol. Cell. Biol. 21, 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, A., Wemmie, J.A., Edgington, N.P., Goebl, M., Guevara, J.L., and Moye-Rowley, W.S. (1993). Yeast bZip proteins mediate pleiotropic drug and metal resistance. J. Biol. Chem. 268, 18850–18858. [PubMed] [Google Scholar]
- Wu, A.L., and Moye-Rowley, W.S. (1994). GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol. Cell. Biol. 14, 5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki, R., Bobrowicz, P., and Ulaszewski, S. (1997). The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 272, 30061–30066. [DOI] [PubMed] [Google Scholar]
- Wysocki, R., Chéry, C.C., Wawrzycka, D., Van Hulle, M., Cornelis, R., Thevelein, J.M., and Tamás, M.J. (2001). The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 40, 1391–1401. [DOI] [PubMed] [Google Scholar]
- Yan, C., Lee, L.H., and Davis, L.I. (1998). Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 17, 7416–7429. [DOI] [PMC free article] [PubMed] [Google Scholar]