Abstract
Skeletal muscle is the largest organ in the body and contributes to innumerable aspects of organismal biology. Muscle dysfunction engenders numerous diseases, including diabetes, cachexia, and sarcopenia. At the same time, skeletal muscle is also the main engine of exercise, one of the most efficacious interventions for prevention and treatment of a wide variety of diseases. The transcriptional coactivator PGC-1α has emerged as a key driver of metabolic programming in skeletal muscle, both in health and disease. We review here the many aspects of PGC-1α function in skeletal muscle, with a focus on recent developments.
Keywords: Muscle, Metabolism, PGC-1alpha
Introduction
PGC-1α was originally identified as a PPARγ-binding protein in a search for factors that differentiate brown fat from white fat [1]. Its over-expression conferred on white fat some of the characteristics of brown fat, including mitochondrial biogenesis and thermogenic uncoupling [1, 2]. Since then, it has become apparent that PGC-1α binds to, and coactivates many transcription factors in addition to PPARγ, including most nuclear receptors[3]. Binding to each transcription factor allows PGC-1α to activate individual programs controlled by that factor. For example, activation of PPARα generally leads to induction of genes (e.g. CD36, CPT1) involved in fatty acid import and β-oxidation[4, 5]. PGC-1α has different roles in different tissues, but in nearly every context PGC-1α powerfully drives the transcriptional program of mitochondrial biogenesis [2, 6, 7]. To do so, PGC-1α coactivates NRF1, NRF2 (a tetrameric factor composed of GABPA and GABPB), and ERRα to induce hundreds of nuclear-encoded genes of mitochondrial biology and simultaneously coordinate the induction of mitochondrial replication and transcription of the mitochondrial genome. Recent work has shown that binding of PGC-1α to ERRs likely stabilizes both the ligand-binding domain of ERRs and the activation domain of PGC-1α [8, 9].
In keeping with its powerful role in cellular metabolism, PGC-1α is heavily regulated both transcriptionally and post-transcriptionally, including direct phosphorylation by p38 MAPK, AMPK, PKA, GSK3β, and AKT, acetylation/deacetylation by GCN5/SIRT1 respectively, methylation by PRMT1, glycosylation by the transferase OGT, ubiquitination by SCFCdc4 and others (reviewed in [7]). PGC-1α protein is also intrinsically disordered, rendering it susceptible to proteosomal degradation, with a half-life on the order of 20 minutes. Interestingly, recent work shows that this degradation is inhibited by NQO1 in an NADH-dependent manner, thus providing another regulatory link between redox sensing and PGC-1α [10]. PGC-1β was identified by virtue of its primary homology to PGC-1α (40% of the N-terminal activation domain and 48% of the C-terminal RNA-binding domain [11, 12]) and has many of the same properties as PGC-1α, including the powerful activation of mitochondrial programs, but PGC-1β has been less extensively studied [11–13].
PGC-1α and β are strongly expressed in skeletal muscle, in particular in “red” oxidative muscle. Multiple signals enhance PGC-1α expression in muscle, most notably exercise [14–16] and β-adrenergic signaling[17, 18]. PGC-1β expression appears generally less strongly regulated[19]. Transgenic forced expression of PGC-1α or β in murine skeletal muscle drives mitochondrial biogenesis [20, 21] and engenders mice with increased capacity for endurance exercise [21, 22]. The PGC-1α transgenic mice are protected from denervation atrophy [23], muscle dystrophy [24], aging-associated sarcopenia [25], and other diseases. The PGC-1s, and PGC-1α in particular, have thus received great attention in the context of muscle health and disease. Recent developments in these many studies are outlined below.
Splice variants of mouse PGC-1α
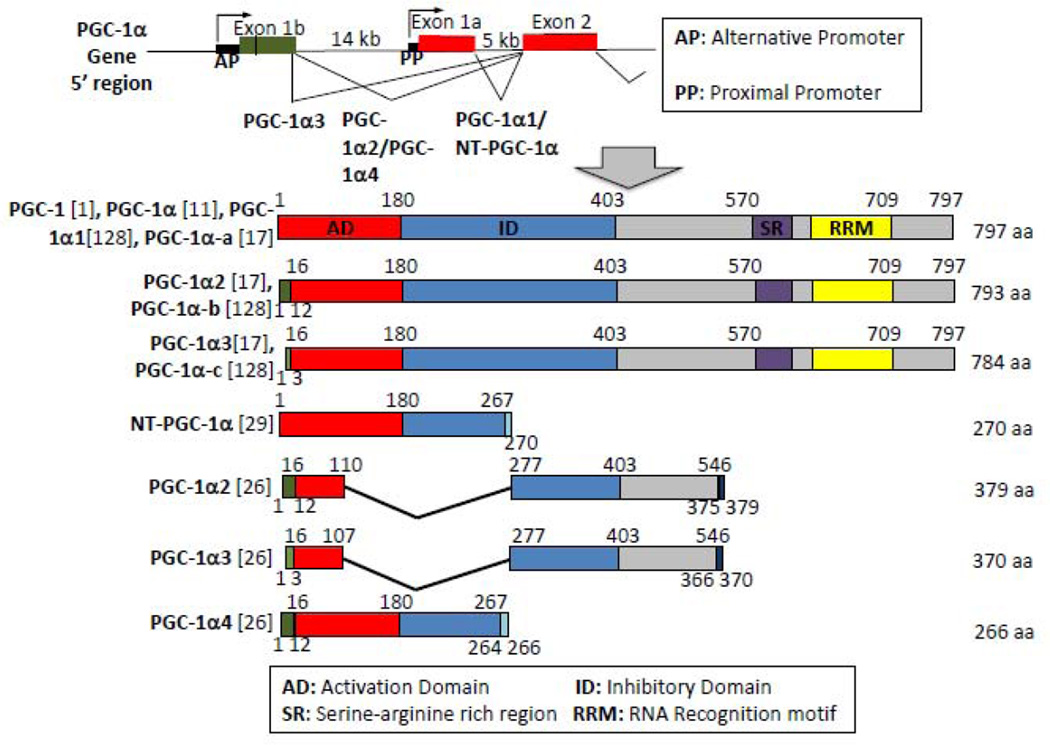
Various isoforms of PGC-1α have been identified since 2008, and have engendered some confusion. The nomenclature for these isoforms is not yet standardized. Figure 1 summarizes the isoforms with the nomenclature used in each study. The full length PGC-1α was first identified in 1998 by Puigserver et al (called PGC-1)[1], which was then changed to PGC-1α with the discovery of family member PGC-1β [11, 12]. The full length PGC-1α has since been called PGC-1α1 [17, 26] or PGC-1α-a [18] to distinguish from other isoforms.
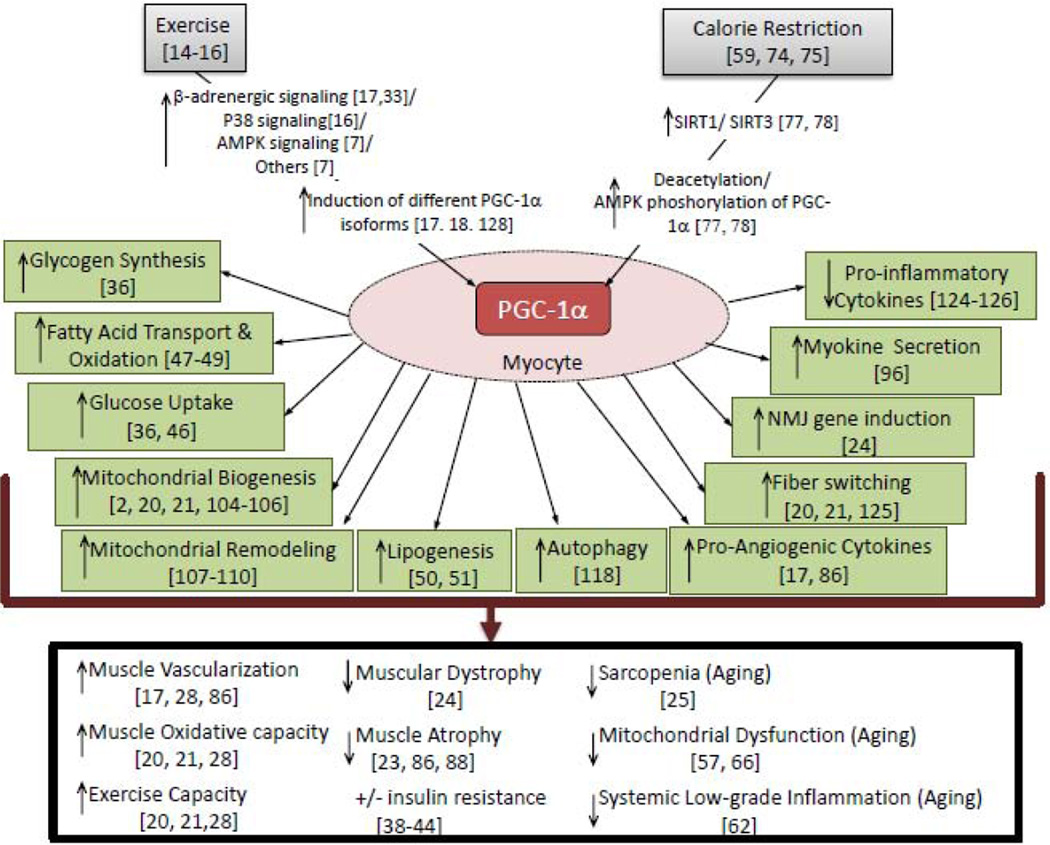
Figure 1. Regulation and roles of PGC-1α in skeletal muscle.
Exercise and calorie restriction induce PGC-1α expression and activation. Induction of PGC-1α affects multiple pathways and processes in the myocyte, as well as systemic physiological effects.
Isoforms of mouse PGC-1α were identified when an alternative promoter was found by two different groups [17, 27]. Transcription from the alternative promoter generates mRNAs that start at two different sites, and that include an alternative exon 1 (Exon 1’) which replaces the canonical exon 1 via alternative splicing. The two new splice variants thus encode different amino acids on the N-terminus (Figure 1), identified as PGC-1α2 and PGC-1α3 by Chinsomboon et al[17], and PGC-1α-b and PGC-1α-c by Miura et al[18, 128]. Splice variants from the alternative promoter are highly expressed in heart, BAT, skeletal muscle and stomach, but not liver[17, 18, 128].
PGC-1α expression is strongly induced by exercise in human and rodent skeletal muscle [14–16]. Recent work has shown that this induction is nearly entirely mediated by dramatic activation of the alternative promoter, with little to no activation of the canonical promoter [17, 18, 128]. β-adrenergic stimulation likely contributes significantly to this induction, activating cAMP signaling and recruitment of the CREB transcription factor to a canonical CRE in the alternative promoter [17].
Characterization of muscle-specific transgenic PGC-1α-b mice revealed that this isoform alone was enough to induce mitochondrial biogenesis, expression of fatty acid transporters, and angiogenesis in muscle [28] . The transgenic mice also displayed increased exercise capacity. Interestingly, despite the use of full length PGC-1α-b cDNA to generate the mice, Tadaishi et al observed increased intensity of multiple different bands on a Western Blot, using an antibody directed to the C-terminus of PGC-1α [28]. This suggests either degradation of the transgene-encoded protein, or more intriguingly, post-translational processes to PGC-1α-b protein.
In 2009, Zhang et al described a 270 amino acid isoform of PGC-1α that lacks amino acid 268–797, and which they named NT-PGC-1α[29]. This isoform results from an alternative splicing event at the exon 7 boundary that leads to an in-frame stop codon shortly into exon 7. NT-PGC-1α is found primarily in brown fat and muscle. In brown fat, NT-PGC-1α behaves much like FL-PGC-1α; expression is increased by fasting or cold exposure, and the isoform binds to both PPARγ and PPARα, leading to the induction of UCP1[29]. However, unlike full length PGC-1α, which is exclusively nuclear, NT-PGC-1α is mostly cytoplasmic. PKA activation increases nuclear localization of NT-PGC-1α, both in BAT and in muscle [29, 30]. Nuclear exclusion of NT-PGC-1α occurs via interaction with the nuclear exportin CRM1, and phosphorylation of NT-PGC-1α by PKA prevents binding to CRM1 [31].
Ruas et al recently described three new isoforms of PGC-1α (figure 2), named PGC-1α2, PGC-1α3, and PGC-1α4 [26]. PGC-1α4 is transcribed from the alternative promoter, but has the same splice variant at exon 7 as NT-PGC-1α [26]. Zhang et al did not describe the N-terminus of NT-PGC-1α [29]. It is thus possible that NT-PGC-1α is the same as PGC-1α-4. Ydfors et al, however, very recently used isoform-specific RT-PCR primers to show that both NT-PGC-1α and PGC-1α4 isoforms are expressed in muscle, and both are induced by exercise [32], suggesting that both forms of PGC-1α exists. PGC-1α4 induces myocyte hypertrophy, a function not described for PGC-1α1[26]. PGC-1α4 in muscle activates Akt and induces the expression of IGF1,a well known inducer of cell growth and proliferation. On the other hand, PGC-1α4 does not induce mitochondrial biogenesis, indicating that the function of this protein is distinct from PGC-1α1[26].
Figure 2. The many isoforms of PGC-1α.
A. Schema of PGC-1α alternative and proximal promoter (top) and of the different PGC-1α isoforms (bottom), along with the names for isoforms used in different publications. Numbers above the isoform are amino acid sequence compared to PGC-1α1/PGC-1α-a isoform. Numbers below the isoforms are amino acid sequence not found on PGC-1α1/PGC-1α-a. References are given in parentheses.
Two other isoforms were described by Ruas et al, PGC-1α2 and PGC-1α3[26]. These isoforms differ from the earlier described PGC-1α2/PGC-1α3 in Chinsomboon et al (Figure 2)[17]. The Ruas isoforms are transcribed from the alternative promoter, but are much shorter, containing only exon 1’,2, 3, 7 and 8, with possible splice variants of exon 3, 7 and 8 compared to PGC-1α1. Resulting proteins are 379 amino acids and 370 amino acids in length respectively. The sequence and size of the previously described PGC-1α2/PGC-1α-b and PGC-1α3/PGC-1α-c (as shown in Fig 1) were confirmed by sequencing of cDNA [28, 33], and Western blotting [17], respectively, indicating that the shorter Ruas isoforms are different from the earlier Miura/Chinsomboon isoforms. Their specific function remains unclear.
In sum, some uncertainties remain over which isoforms are expressed in skeletal muscle, and in response to which stimuli. Working out these details will likely be important, for example for interpreting results from experiments using genetically modified animals. Two murine floxed alleles of PGC-1α have been generated. The first deletes exons 3–4, and thus likely affects all of the isoforms described above [34]. The second leaves exons 1–5 intact, thus likely leaving NT-PGC-1α intact [35]. This difference may in part explain the less pronounced phenotype observed in the latter mice [36–38].
Muscle PGC-1α and diabetes
Skeletal muscle is the main site of glucose disposal after glucose ingestion. Insulin resistance in skeletal muscle is thus the main driver of post-prandial hyperglycemia. Mitochondrial dysfunction and insufficiency has long been noted in muscle biopsies from diabetic patients[39, 40]. Consistent with this observation, PGC-1α and β are mildly repressed in diabetic muscle, as is the PGC-1-dependent mitochondrial program in general [41–43]. These observations have led to the hypothesis that PGC-1 dysfunction, and thus mitochondrial insufficiency, may contribute to insulin resistance in skeletal muscle. Genetic models with PGC-1α have challenged this notion, however. Deletion of PGC-1α specifically in skeletal muscle has little effect on systemic glucose handling and insulin sensitivity [44], and deletion of PGC-1β specifically in skeletal muscle in the context of a whole-body PGC-1α hypomorphic allele (see above) similarly reveals few changes in glucose handling, despite profound defects in mitochondrial function [38]. Conversely, overexpression of PGC-1α in skeletal muscle increases insulin resistance in response to a high fat diet, likely as a result of increasing fatty acid influx in excess to its consumption in myocytes [44]. Interestingly, exercising these same PGC-1α-overexpressing mice not only rescues the insulin resistance, but in fact renders the mice more insulin sensitive than similarly exercised wildtype controls [45]. PGC-1α overexpression in muscle thus enhances the insulin-sensitizing effects of exercise, though how this occurs is not clear. Genotype-environment interactions thus remain incompletely understood, and whether PGC-1 insufficiency ultimately contributes to insulin resistance in muscle remains similarly unclear.
PGC-1α affects glycogen and lipid levels in the muscle
Exercise increases metabolic demand in the muscle. Induction of PGC-1α likely helps to meet this increased demand. PGC-1α fulfills this in a number of ways, such as increasing mitochondrial biogenesis, thus increasing the capacity for generation of ATP, or increasing angiogenesis, thus providing more nutrients to the muscle. Another mechanism is the effect of PGC-1α on fuel intake and storage in the muscle. For example, increased PGC-1α expression in the muscle increases glucose uptake, likely via increase in GLUT4 expression [36, 46]. Peak induction of PGC-1α occurs post-exercise, which has suggested a role in replenishment of glycogen stores depleted during exercise. In support of this hypothesis, transgenic increase in PGC-1α expression in adult skeletal muscle dramatically increases glycogen synthesis and storage, and inhibits breakdown of glycogen[36]. Conversely, PGC-1α KO mice have reduced basal glycogen storage, delayed replenishment of glycogen stores after exercise, and therefore reduced exercise capacity due to insufficient glycogen available during exercise[36].
More recent work has also revealed that PGC-1α also increases lipogenesis in skeletal muscle. The role of PGC-1α in increasing catabolism of lipids in muscle is well established [47–49]. Espinoza et al showed in myocyte cell culture that PGC-1α as well as PGC-1β not only increased catabolism, but also increased anabolism of lipids, including intracellular fatty acids and ceramides [50]. Later studies showed that this was also true in transgenic animals [51]. Fatty acid synthase, FATP4 and de novo lipogenesis was increased in muscle specific PGC-1α transgenics. Higher FAS and lipid levels were also observed in the muscle of athletes after exercise. Overall, these effects on homeostasis strongly support the post-exercise role of PGC-1α in replenishing and increasing the amount of energy stores available for a subsequent exercise session [36, 47]. Interestingly, in the absence of exercise, inappropriately high PGC-1α and thus rate of glycogen and fat anabolism might lead to metabolic imbalance, including diabetes, as discussed above.
PGC-1α, exercise and aging
Aging is associated with progressive decline in the physiological functions of different organs, eventually leading to organ failure and death. Many have hypothesized that aging is in part caused by progressive mitochondrial dysfunction. Aged animals bear accumulated mitochondrial DNA damage, more enlarged or ‘giant’ mitochondria, a decrease in respiratory capacity, an increase in ROS production, a decrease in autophagy of damaged mitochondria, increased opening of the permeability transition pore, and other abnormalities [52–55]. These damages can lead to decreased ATP supply, increased damage to other organelles and mutation of nuclear DNA, as well as increased apoptosis. Mice with accelerated rates of mitochondrial DNA mutation (mtDNA mutator mice), have decreased mitochondrial function, and show accelerated aging, decreased mobility and decreased life span. [56].
Exercise reverses at least some aspects of aging. Recent findings in the mtDNA mutator mice support this hypothesis[57]. Sustained (5 months) endurance exercise in these mice improved mobility in the mice, and increased mitochondrial biogenesis, not only in the skeletal muscle, but also in other organs including the lung and the heart. The morphology of mitochondria also improved markedly. Overall, exercise decreased age-related degeneration of multiple different systems, and life-span increased[57]. This suggests that exercise not only reverses sarcopenia, but also has systemic benefits.
The activity of PGC-1α and PGC-1β in different tissues has been shown to decrease with age [58–61], and could be a contributing factor for mitochondrial dysfunction and development of sarcopenia. Conversely, as noted above, PGC-1α expression increases in muscle with exercise, and may thus confer some of the benefits of exercise in aging. Transgenic increase in PGC-1α expression in the muscle strikingly protects against development of sarcopenia in old (22 month old) mice, with increased respiration in individual mitochondria and in the muscle, decreased inflammation, improved preservation of muscular structure and decreased apoptosis [62]. Conversely, exercising mice that entirely lack PGC-1α failed to protect against age-associated decrease in mitochondrial function and increases in inflammatory markers in skeletal muscle [63, 64]. On the other hand, muscle-specific deletion of PGC-1α does not alter the ability of exercise to stimulate mitochondrial biogenesis in young mice [65], reflecting either a difference between young and old mice, or a non-muscle effect of total-body deletion of PGC-1α.
In a recent report, specific transgenic expression of PGC-1α in the muscle of mitochondrial mutator mice increased mitochondrial biogenesis and function in skeletal muscle, as seen when PGC-1α is expressed in wildtype mice [66]. There were however, no improvements in the accelerated age-associated defects in bone, body weight, fat percentage, fat content, liver function or kidney function in these mice. A mild improvement in heart mitochondrial function and ejection fraction was seen, which may reflect transgene expression of PGC-1α in the heart [66]. Overall, there thus appeared no evidence of delay or inhibition of systemic aging, and there was no increase in life span in the mice. The lack of effect of muscle PGC-1α in this study contrasts with the remarkable effect seen in the previous study (with natural aging)[62], and may reflect an inability to surmount the more dramatic accelerated aging of the mutator mouse, and/or that the mutator mouse is an imperfect model for aging itself. On the other hand, exercise itself almost completely reversed the accelerated aging of the mutator mouse [57]. Therefore, the benefits of exercise, though perhaps in part dependent on induction of PGC-1α, extend beyond PGC-1α alone.
PGC-1α and calorie restriction
Caloric restriction (CR) increases life span and decreases aging in a number of different experimental organisms, although the effect on humans remains uncertain. The effect of CR on mitochondria has been extensively investigated. CR reduces emission of ROS from mitochondria in a number of different cell-types including skeletal muscle [67, 68], increases respiratory capacity of both cells and individual mitochondria [69, 70], increases mitochondrial coupling[70], decreases mitochondrial DNA damage, and reduces sensitivity and rate of opening of PTP [71, 72]. Overall, there is a large body of evidence that CR improves mitochondrial function and reduces mitochondrial dysfunction with age.
Many different studies have shown that PGC-1α is increased with CR. Increase in PGC-1α was first observed in brain, liver, heart and brown adipose tissue with CR [73]. Baker et al soon followed showing significant increase in PGC-1α in gastrocnemius of rats after 40% CR diet beginning at 16 weeks of age[59]. Other studies have shown increased PGC-1α expressing in cell culture models of CR [74] and in mouse skeletal muscle and visceral adipose tissue [75]. Generally, there is good evidence that PGC-1α expression is increased in a number of different organs in different organisms with different CR protocols, although some controversy remains [76]. The mechanisms by which PGC-1α is induced by CR remain incompletely understood, but may in part involve the class III deacetylases SIRT1 and SIRT3. Expression of Sirt1 and 3 decreases with age and increases with caloric restriction, and both Sirt1 and Sirt3 increase PGC-1α biological activity [77, 78]. Sirt1 directly deacetylates PGC-1α [78], and/or acts indirectly by activating AMPK which then phosphorylates PGC1-α[77]. The expression of PGC-1α is also regulated by SIRT3 [77], although the mechanism of this control remains unknown.
Resveratrol, a polyphenol found in red wine, has been shown to have potent antitumor activity (Jang 1997). In addition, feeding of resveratrol to rodents on a high fat diet has been shown to prevent obesity, improve metabolic function, reduce inflammation and prevent diabetes development [79–82]. The gene expression profile of mice on resveratrol resembles that of mice subjected to caloric restriction, including increased mitochondrial pathways, which has led to the suggestion that resveratrol can be used as a caloric restriction mimetic [83]. In 2006, Lagouge et al first reported that resveratrol activates SIRT1, leading to deacetylation and activation of PGC-1α[79]. Price et al recently expanded on these findings with transgenic mice [84]. When SIRT1 expression was suppressed in adult inducible SIRT1 knockout mouse, the resveratrol-induced increase in mitochondrial biogenesis and function was reduced, as was the induction of AMPK activity and increase in NAD+ levels[84], indicating that SIRT1 can act upstream of AMPK. On the other hand, previous studies have suggested that the activation of AMPK was upstream of SIRT1, and that activation of AMPK (by Ca2+ store release from ER) was necessary for SIRT1 activation [85]. Furthermore, AMPK also phosphorylates PGC-1α, which then improves availability of PGC-1α for SIRT1 deacetylation. It is possible therefore that there is feedback between SIRT1 and AMPK activation, and both components must be present for resveratrol induction of mitochondrial biogenesis. Further elucidation will be necessary.
If CR improves mitochondrial function and increases expression of PGC-1α, is there evidence that PGC-1α induction is important for benefits to mitochondria? In particular is induction of PGC-1α and subsequent mitochondrial biogenesis in muscle important for anti-aging effects of CR? Finley et al studied this question using mice with muscle specific deletions of PGC-1α[44]. They reported that CR-induced increases in oxidative mitochondrial biogenesis were lost in these animals. However, CR-induced improvements in whole body metabolic homeostasis were not dependent on muscle PGC-1α[44]. This study therefore shows that PGC-1α induction may be important for reversal of sarcopenia with CR, but other mechanisms mediate systemic metabolic benefits of CR. This is in line with studies that showed that overexpression of PGC-1α by itself is not sufficient to explain overall systemic benefits of exercise [62, 65]. There is currently no report on the effect of CR on whole body PGC-1α KO, nor on other tissue-specific PGC-1α deletions. A putative role of PGC-1α in mediating the benefits of CR, though often assumed to be critical, thus remains uncertain.
PGC-1α and angiogenesis
PGC-1α in skeletal muscle cells potently drives angiogenesis, i.e. the formation of new blood vessels, concomitant with mitochondrial biogenesis [86]. PGC-1α thus coordinates fuel/oxygen consumption in mitochondria with fuel/oxygen delivery via blood vessels. PGC-1α accomplishes this task by inducing the expression and secretion of VEGF and other angiogenic factors from myocytes. Interestingly, the induction of VEGF occurs independently of the hypoxia-inducible factor (HIF) system[86]. Instead, PGC-1α coactivates ERRα on, for example, an enhancer in the first intron of the VEGF gene[86]. Exercise is a potent angiogenic stimulus in skeletal muscle, and deletion of PGC-1α in skeletal muscle of mice abrogates exercise-induced angiogenesis[17, 33]. β-adrenergic signaling appears to be important for this process by potently inducing specific isoforms of PGC-1α (see Isoforms section). Exercise thus induces angiogenesis in skeletal muscle via PGC-1α. PGC-1β also induces angiogenesis, although PGC-1β is not induced by exercise [87]. Muscle specific expression of the exercise-inducible isoform PGC-1α-b is also sufficient to induce angiogenesis in the skeletal muscle[28].
The powerful ability of PGC-1α to activate angiogenesis in skeletal muscle raised the possibility that it may be useful in the treatment of ischemic limb diseases. Implantation of mesenchymal stem cells is a promising but as yet unused treatment for various ischemic diseases including PAD. In a recent study, Lu et al showed that increased expression of PGC-1α in implanted MSCs increased both the engraftment of the cells and the stimulation of angiogenesis in diabetic hindlimb ischemia [88]. Transgenic expression of PGC-1α in skeletal myocytes was also beneficial in the hindlimb ischemia model [86], though this approach is clearly less clinically applicable. These results are promising, but numerous questions remain, including whether PGC-1α in muscle also regulates arterioles and larger arteries, and the optimal mode of delivery.
Recent work has implicated PGC-1s in the regulation of angiogenesis in a number of other organs, including fat [89], retina [90], and heart [91], underscoring the important role of PGC-1α in vascular homeostasis in the muscle and elsewhere.
Myokines and PGC-1α
The mechanism of how induction of PGC-1α by exercise in skeletal muscle can have beneficial effects in other organs remains unknown. One possibility is that exercised muscle releases ‘myokines’, secreted proteins and small molecules that circulate and signal to other cells or other organs[92, 93]. Evidence for the existence and secretion of cytokines can be found in experiments with paralyzed muscle. Electrostimulation of muscles in paralyzed patients was found to induce many of the same factors as exercise in uninjured muscle, including myostatin, IL-6,IG-1 FGF-2 and FSTL [94, 95].
In 2012, Bostrom et al reported the finding of a new myokine, irisin, a protein which they reported to be increased by both forced endurance exercise and in PGC-1α muscle specific transgenic mice[96]. Irisin is the cleaved product of the membrane protein FNDC5. Increase in systemic irisin levels decreases high-fat diet-induced obesity, and increases glucose tolerance in high-fat fed mice. Importantly, irisin induced ‘browning’ of white adipose tissue through induction of UCP1[96]. Therefore, irisin could be therapeutic in combating obesity and diabetes. Since then, a number of reports have supported these findings to various extents. Skeletal muscle FNDC5 expression and circulating irisin was found to be lower in obese patients [97], and there was an increase in serum irisin after acute exercise [98]. Furthermore, Roca-Rivada et al (2012) reported that adipose tissue might also secrete irisin with exercise[99]. Other reports, however, find irisin to be higher in obese (compared to non-obese) rats, and not associated with improvement in insulin sensitivity[100, 101]. Level of irisin in active humans also showed no correlation with diabetes status or BMI [102] or different forms of exercise[103]. In summary, the precise role of irisin in human exercise remains incompletely understood. No matter the role in physiology, however, the potential for irisin as a therapeutic target or agent seems high. It is highly likely that other PGC-1α-regulated myokines also exist.
PGC-1α and Mitochondrial remodeling
The role of PGC-1α as a regulator of mitochondrial biogenesis, respiration, and antioxidant defenses, is well studied [2, 104–106]. Post-natal expression of PGC-1α in the muscle of adult transgenic mice is sufficient to induce robust mitochondrial biogenesis and respiratory capacity [36]. More recent work has focused on the effect of PGC-1α on changing the role and composition of existing mitochondria. There is growing consensus that not only mitochondrial biogenesis, but also mitochondrial adaptation to changing physiological conditions is modulated by expression of PGC-1α. PGC-1α is now for example known to regulate mitochondrial fusion and fission [107, 108]. Isolated mitochondria from muscle specific PGC-1a transgenics (MCK-PGC-1a) also have increased respiration and ROS production from complex I and III [109]. Interestingly, the enhanced respiration requires fatty acids as substrates (palmitoyl co-A with carnitine) [110], while, conversely, mitochondria isolated from PGC-1α knock-out mice have decreased respiration capacity with palmitoyl/carnitine fuel[38]. Multiple reports showed increases in the levels of some proteins in isolated mitochondria from MCK-PGC-1α muscle (subunit V-α of the ETC[109] and ANT1[110]), and no change or even decrease in other mitochondrial proteins (UCP3[110]) compared to WT, again demonstrating mitochondrial remodeling.
The mechanisms of mitochondrial remodeling are under active investigation. One intriguing hypothesis is that PGC-1α remodels mitochondria by directly controlling transcription of mitochondrial genes. Aquilano et al reported the presence of PGC-1α and the PGC-1α activating protein SIRT1 in mitochondria of different cells in vivo and in cell culture[111]. They further showed that PGC-1α and SIRT1can be immuno-precipitated in a protein complex with Mitochondrial Transcription Factor A (TFAM) in liver purified mitochondria [111]. This supports the hypothesis that PGC-1α might play a role in transcriptional control on mitochondrial genes, a surprising notion because the transcriptional apparatus in the mitochondrion differs extensively from that in the nucleus. Safdar et al advanced this notion by presenting evidence that acute exercise increases PGC-1α nuclear and mitochondrial subcellular localization in the skeletal muscle of mice[112]. They further showed that the complex with TFAM in skeletal muscle is increased with exercise. Future studies focused on identifying if and how PGC-1α modulates the mitochondrial genome will be of great interest.
PGC-1α and autophagy
Autophagy is the degradation of cytoplasmic constituents within lysosomes [113]. By breaking down damaged or unused cytoplasmic materials, autophagy serves to recycle biological building material and conserve energy in the process of cellular renewal and homeostasis. The level of basal autophagy in the skeletal muscle has been shown to be reduced with age [114]. Decreased or inhibition of autophagy can lead to atrophy of skeletal muscle and muscle loss [115]. However, basal autophagy levels in the muscle can be restored by either caloric restriction or CR with exercise [114]. Exercise results in activation of autophagy and increased autophagy flux in muscle [116]. In order to test if exercise increase in autophagy plays an important role in the beneficial effects of exercise, two different groups used different transgenic mice with normal levels of resting/sedentary autophagy, but with inhibition of exercise induced autophagy[117, 118]. He et al showed that mice with constitutively active anti-autophagic BCL2 gene had decreased exercise endurance, abnormal glucose metabolism after exercise, and exercise in these mice failed to prevent glucose intolerance after high fat feeding [117]. Lira et al, in comparison used the haplodeficient Atg6+/− mice[118]. Atg6 plays an essential role in autophagosome formation. They observed that inhibiting the increase in autophagy after exercise abrogates exercise-induced increases in genes associated with mitochondrial biogenesis. Increase in capillary density and increase in endurance were also lost in the mutant mice[118]. These studies directly show that increased autophagy is necessary for some of the beneficial metabolic effects of exercise. If autophagy plays an important role in exercise muscle homeostasis, does PGC-1α plays a role in controlling autophagy? Lira et al also observed that overexpression of PGC-1α skeletal muscle of the mice induced increase in basal autophagy flux and in some mitophagic proteins including Bnip3[118]. Whether changes in autophagy are due to direct effects of PGC-1α and/or due to increased mitochondrial biogenesis is unknown. Further studies using PGC-1α knockout mice to investigate if PGC-1α is necessary for exercise induced basal autophagy will need to be done.
PGC-1α and inflammation
Aging is accompanied by an increase in persistent, low grade systemic inflammation, characterized by increased pro-inflammatory cytokines, including IL-1β, IL-6 and TNFα [119–121]. Increased low-grade inflammation can contribute to specific inflammatory diseases, including type 2 diabetes with inflammation of white adipose tissue, development of cardiovascular diseases including arteriosclerosis, and neuroinflammation that contributes to progression of Parkinson’s disease. Exercise decreases low-grade inflammation, possibly in part explaining the benefits of exercise on these diseases [64, 122, 123].
Experimental evidence in mice and cells supports the hypothesis that exercise reduction of inflammation is dependent on PGC-1α expression. Fifteen-month old mice with whole-body knockout of PGC-1α have elevated plasma TNFα and IL-6 level [64], but surprisingly no organ specific increase in TNFα either in the adipose, liver or quadriceps. In muscle specific PGC-1α knockout mice (MKO), Handschin et al also observed increased systemic IL-6, with increases in mRNA levels of TNFα, IL-6 and CD68 [124], TNFα expression in muscle and protein in serum was also shown to be significantly induced with exercise in muscle of MKO mice compared to WT controls [125].
In cell culture, increased expression of PGC-1α decreased inflammation and expression of pro-inflammatory cytokines induced by TNF-α, TLR agonists or fatty acids [126]. The mechanism for this is through decrease in p65 phosphorylation and does not affect NFKB binding to promoter or NFKB expression levels. Interestingly, in PGC-1α KO mice, the decrease in TNFα with exercise was abrogated [64]. In a different study, 2-weeks of forced exercise on 8 month old diabetic mice was enough to induce increase in mouse heart expression of PGC-1α mRNA and protein [122], and this corresponded with decreased inflammation in the heart and decreases in circulating inflammatory cytokines.
In summary PGC-1α in muscle appears to block pro-inflammatory pathways, and this may in part explain the anti-inflammatory effects of exercise, though mechanistic details remain to be elucidated.
Conclusions
As outlined above, the PGC-1 coactivators are central to the regulation of metabolism and ancillary programs in skeletal muscle. Much remains to be understood of the molecular mechanisms involved. In addition, the therapeutic potential of targeting PGC-1α in muscle is exciting but remains uncertain and untested. Increasing PGC-1α in muscle to treat diabetes has been proposed, but as noted above, more recent experimental results may no longer justify this approach. Induction of PGC-1α and consequent neovascularization is protective in mouse models of peripheral artery disease , but this has not yet been tested in larger pre-clinical models. Activation of PGC-1α in muscle also prevents denervation-induced atrophy [23] and aging-associated sarcopenia [25], but the mechanisms remain poorly understood, and again the approach has not been tested in larger models. Similarly, transgenic induction of PGC-1α prolongs the maintenance of muscle function in the SOD1 mouse model of Amyotrophic lateral sclerosis (ALS)[127]. PGC-1α expression in muscle also partially prevents muscle degeneration in the mdx mouse model of Duchene Muscle Dystrophy[24], most commonly ascribed to the induction of utrophin as a compensation of loss of dystrophin, though this mechanism has not been proven.
In summary, preclinical findings in rodents suggest that the induction of PGC-1α (and perhaps PGC-1β, though it is much less studied) may have significant benefits on a number of muscle diseases. The challenges ahead will include delineating the mechanisms involved, determining to what extent metabolic effects of PGC-1α are involved, identifying ways to induce PGC-1α expression [86] or activate PGC-1α protein, and testing the approaches in larger animal models. Excitement is high, but the road ahead remains long.
Acknowledgements
MCC is supported by a T32 grant from the NHLBI, and ZA is supported by R01 funding from the NHBLI and by the Ellison Foundation.
Glossary
Abbreviations
- PPAR
peroxisomal proliferator activator receptor
- PGC-1
PPARgamma-coactivator 1
- CD36
Cluster of differentiation 36
- CPT1
Carnitine palmitoyl transferase
- NRF
Nuclear respiratory factor
- ERR
Estrogen related receptor
- MAPK
Microtubule associated protein kinase
- AMPK
Adenosine monophosphate kinase
- PKA
Protein kinase A
- GSK
Glycogen synthase kinase
- PRMT1
Protein arginine methyl transferase 1
- GABPA
GA binding protein transcription factor A
- GABPB
GA binding protein transcription factor B
- SIRT1
sirtuin-1
- cAMP
cyclic AMP
- CRE
cAMP responsive element
- CREB
CRE-binding protein
- BAT
Brown adipose tissue
- GLUT4
Glucose transporter 4
- FATP4
Fatty acid transporter protein 4
- PTP
Permeability transition pore
- CR
Calorie restriction
- IGF
Insulin-like growth factor
- IL6
Interleukin 6
- FGF
Fibroblast growth factor
- FNDC5
fibronectin type III domain containing 5
- MCK
Muscle creatine kinase
- ETC
Electron transport chain
- UCP
Uncoupling protein
- TNF
Tumor necrosis factor
- TFAM
Mitochondrial Transcription Factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest/financial disclosure statement: none
Author contributions
MCC and ZA wrote the manuscript together.
References
- 1.Puigserver P, Wu Z, Park CW, et al. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, Puigserver P, Ansersson, et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1α. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm D, Eriksson O, Mäkelä J, et al. PGC-1α: a master gene that is hard to master. Cell. Mol. Life Sci. 69(15):2465–2468. doi: 10.1007/s00018-012-1043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vega RB, Huss JM, Kelly DP. The Coactivator PGC-1 Cooperates with Peroxisome Proliferator-Activated Receptor α in Transcriptional Control of Nuclear Genes Encoding Mitochondrial Fatty Acid Oxidation Enzymes. Mol. Cell. Biol. 2000;20(5):1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan JG. Peroxisome Proliferator Activated Receptor-α(PPARα) and PPARγCoactivator-1α (PGC-1α) Regulation of Cardiac Metabolism in Diabetes. Pediatr Cardiol. 32(3):323–328. doi: 10.1007/s00246-011-9889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochemical Pharmacology. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. American Journal of Clinical Nutrition. 2011;93(4):884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarakonda S, Gupta K, Chalmers MJ, et al. Disorder-to-order transition underlies the structural basis for the assembly of a transcriptionally active PGC-1α/ERRγcomplex. Proc. Natl. Acad. Sci. U.S.A. 2011;108(46):18678–18683. doi: 10.1073/pnas.1113813108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takacs M, Petoukhov MV, Atkinson RA, et al. The Asymmetric Binding of PGC-1α to the ERRα and ERRγ Nuclear Receptor Homodimers Involves a Similar Recognition Mechanism. PLoS ONE. 2013;8(7):e67810. doi: 10.1371/journal.pone.0067810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamovich Y, Shlomai A, Tsvetkov P, et al. The Protein Level of PGC-1α, a Key Metabolic Regulator, Is Controlled by NADH-NQO1. Mol. Cell. Biol. 2013;33(13):2603–2613. doi: 10.1128/MCB.01672-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J, Puigserver P, Donovan J, Spiegelman BM. Peroxisome Proliferator-activated Receptor γ Coactivator 1β (PGC-1β ), A Novel PGC-1-related Transcription Coactivator Associated with Host Cell Factor. Journal of Biological Chemistry. 2001;277(3):1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 12.Kressler D, Schreiber S, Knutti D, Kralli A. The PGC-1-related Protein PERC Is a Selective Coactivator of Estrogen Receptor α. Journal of Biological Chemistry. 2002;277(16):13918–13925. doi: 10.1074/jbc.M201134200. [DOI] [PubMed] [Google Scholar]
- 13.Lelliott CJ, Vidal-Puig A. PGC-1β: A Co-activator That Sets the Tone for Both Basal and Stress-Stimulated Mitochondrial Activity. Adv Exp Med Biol. 2009;646:133–139. doi: 10.1007/978-1-4020-9173-5_15. [DOI] [PubMed] [Google Scholar]
- 14.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1αgene in human skeletal muscle. The Journal of Physiology. 2003;546(3):851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1αprotein expression in rat epitrochlearis and soleus muscle. American Journal of Physiology. Endocrinology and metabolism. 2003;286(2):208E–216E. doi: 10.1152/ajpendo.00051.2003. [DOI] [PubMed] [Google Scholar]
- 16.Akimoto T, Pohnert SC, Li P, et al. Exercise Stimulates Pgc-1α Transcription in Skeletal Muscle through Activation of the p38 MAPK Pathway. Journal of Biological Chemistry. 2005;280(20):19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 17.Chinsomboon J, Ruas J, Gupta RK, et al. The transcriptional coactivator PGC-1αmediates exercise-induced angiogenesis in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 2009;106(50):21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, Kawanaka K, Kai Y, et al. An Increase in Murine Skeletal Muscle Peroxisome Proliferator-Activated Receptor-γCoactivator-1α(PGC-1α) mRNA in Response to Exercise Is Mediated by β-Adrenergic Receptor Activation. Endocrinology. 2007;148(7):3441–3448. doi: 10.1210/en.2006-1646. [DOI] [PubMed] [Google Scholar]
- 19.Rowe GC, Jang C, Patten IS, Arany Z. PGC-1β regulates angiogenesis in skeletal muscle. American Journal of Physiology. Endocrinology and metabolism. 2011;301(1):E155–E163. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nat Cell Biol. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 21.Arany Z, Lebrasseur N, Morris C, et al. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metabolism. 2007;5(1):35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. Journal of Applied Physiology. 2008;104:1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 23.Sandri M, Lin J, Handschin C, et al. PGC-1αprotects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. U.S.A. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handschin C, Kobayashi YM, Chin S, et al. PGC-1αregulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes & Development. 2007;21(7):770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenz T, Rossi SG, Rotundo RL, et al. Increased muscle PGC-1αexpression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U.S.A. 2009;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Ruas JL, White JP, Rao RR, et al. A PGC-1α Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka T, Inagaki K, Noguchi T, et al. Identification and characterization of an alternative promoter of the human PGC-1α gene. Biochemical and Biophysical Research Communications. 2009;381(4):537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 28.Tadaishi M, Miura S, Kai Y, et al. Skeletal Muscle-Specific Expression of PGC- 1α-b, an Exercise-Responsive Isoform, Increases Exercise Capacity and Peak Oxygen Uptake. PLoS ONE. 2011;6(12):e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Huypens P, Adamson AW, et al. Alternative mRNA Splicing Produces a Novel Biologically Active Short Isoform of PGC-1. Journal of Biological Chemistry. 2009;284(47):32813–32826. doi: 10.1074/jbc.M109.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen T, Liu Y, Schneider MF. Localization and Regulation of the N Terminal Splice Variant of PGC-1α in Adult Skeletal Muscle Fibers. Journal of Biomedicine and Biotechnology. 2012;2012(2):1–8. doi: 10.1155/2012/989263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang JS, Huypens P, Zhang Y, et al. Regulation of NT-PGC-1αSubcellular Localization and Function by Protein Kinase A-dependent Modulation of Nuclear Export by CRM1. Journal of Biological Chemistry. 2010;285(23):18039–18050. doi: 10.1074/jbc.M109.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ydfors M, Fischer H, Mascher H, et al. The truncated splice variants, NT- PGC- 1α and PGC- 1α4, increase with both endurance and resistance exercise in human skeletal muscle. J. Cell. Physiol. 2013;1(6) doi: 10.1002/phy2.140. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadaishi M, Miura S, Kai Y, et al. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1 mRNA: a role of 2-adrenergic receptor activation. American Journal of Physiology. Endocrinology and metabolism. 2011;300(2):E341–E349. doi: 10.1152/ajpendo.00400.2010. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Wu P-H, Tarr PT, et al. Defects in Adaptive Energy Metabolism with CNS-Linked Hyperactivity in PGC-1α Null Mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Leone TC, Lehman JJ, Finck BN, et al. PGC-1α Deficiency Causes Multi-System Energy Metabolic Derangements: Muscle Dysfunction, Abnormal Weight Control and Hepatic Steatosis. PLoS Biol. 2005;3(4):e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wende AR, Schaeffer PJ, Parker GJ, et al. A Role for the Transcriptional Coactivator PGC-1αin Muscle Refueling. Journal of Biological Chemistry. 2007;282(50):36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 37.Lai Y, Thomas GD, Yue Y, et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 2009;119(3):624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zechner C, Lai L, Zechner JF, et al. Total Skeletal Muscle PGC-1αDeficiency Uncouples Mitochondrial Derangements from Fiber Type Determination and Insulin Sensitivity. Cell Metabolism. 2010;12(6):633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowell BB, Shulman GI. Mitochondrial Dysfunction and Type 2 Diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 40.Liesa M, Shirihai OS. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metabolism. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mootha VK, Lindgren CM, Eriksson K-F, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Cell Biol. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 42.Mootha VK, Handschin C, Arlow D, et al. Err and Gabpa/b specify PGC-1αdependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl. Acad. Sci. U.S.A. 2004;101(17):6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. U.S.A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finley LWS, Lee J, Souza A, et al. Skeletal muscle transcriptional coactivator PGC-1αmediates mitochondrial, but not metabolic, changes during calorie restriction. Proc. Natl. Acad. Sci. U.S.A. 2012;109(8):2931–2936. doi: 10.1073/pnas.1115813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summermatter S, Shui G, Maag D, et al. PGC-1α Improves Glucose Homeostasis in Skeletal Muscle in an Activity-Dependent Manner. Diabetes. 2013;52(62):85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michael LF, WU Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1α. Proc. Natl. Acad. Sci. U.S.A. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Summermatter S, Troxler H, Santos G, Handschin C. Coordinated balancing of muscle oxidative metabolism through PGC-1α increases metabolic flexibility and preserves insulin sensitivity. Biochemical and Biophysical Research Communications. 2011;408(1):180–185. doi: 10.1016/j.bbrc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26(7):1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonen A. PGC-1α-induced improvements in skeletal muscle metabolism and insulin sensitivity. Applied Physiology, Nutrition and Metabolism. 2009;34:307–314. doi: 10.1139/H09-008. [DOI] [PubMed] [Google Scholar]
- 50.Espinoza DO, Boros LG, Crunkhorn S, et al. Dual modulation of both lipid oxidation and synthesis by peroxisome proliferator-activated receptor- γ coactivator-1α and-1β in cultured myotubes. The FASEB Journal. 2010;24(4):1003–1014. doi: 10.1096/fj.09-133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Summermatter S, Baum O, Santos G, et al. Peroxisome Proliferator-activated Receptor Coactivator 1α(PGC-1α) Promotes Skeletal Muscle Lipid Refueling in Vivo by Activating de Novo Lipogenesis and the Pentose Phosphate Pathway. Journal of Biological Chemistry. 2010;285(43):32793–32800. doi: 10.1074/jbc.M110.145995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper JM, Mann VM, Schapira AHV. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: Effect of ageing. Cell Metabolism. 1992;113(1):91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 53.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U.S.A. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Cell Metabolism. 2013;24(5):247–256. doi: 10.1016/j.tem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safdar A, Hamadeh MJ, Kaczor JJ, et al. Aberrant Mitochondrial Homeostasis in the Skeletal Muscle of Sedentary Older Adults. PLoS ONE. 2010;5(5):e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nat Cell Biol. 2004;429(6990):417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 57.Safdar A, Bourgeois JM, Ogborn DI, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108(10):4135–4140. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Arnold A-S, Egger A, Handschin C. PGC-1α and Myokines in the Aging Muscle -A Mini-Review. Gerontology. 2011;57(1):37–43. doi: 10.1159/000281883. [DOI] [PubMed] [Google Scholar]
- 59.Baker DJ, Betik AC, Krause DJ, Hepple RT. No Decline in Skeletal Muscle Oxidative Capacity With Aging in Long-Term Calorically Restricted Rats: Effects Are Independent of Mitochondrial DNA Integrity. Journal of Gerontology A Biol Sci Med Sci. 2006;61(7):675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- 60.Hepple RT, Baker DJ, McConkey M, et al. Caloric Restriction Protects Mitochondrial Function with Aging in Skeletal and Cardiac Muscles. Rejuvenation Research. 2006;9(2):219–222. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 61.Finley LWS, Haigis MC. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Research Reviews. 2009;8(3):173–188. doi: 10.1016/j.arr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenz T, Rossi SG, Rotundo RL, et al. Increased muscle PGC-1αexpression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U.S.A. 2009;106(48):20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Leick L, Lyngby SS, Wojtasewski JF, Pilegaard H. PGC-1α is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Biochemical Pharmacology. 2010;45(5):336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Olesen J, Ringholm S, Nielsen MM, et al. Role of PGC-1α in exercise training-and resveratrol-induced prevention of age-associated inflammation. Biochemical Pharmacology. 2013;48(11):1274–1284. doi: 10.1016/j.exger.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowe GC, Patten IS, Zsengeller ZK, et al. Disconnecting Mitochondrial Content from Respiratory Chain Capacity in PGC-1α-Deficient Skeletal Muscle. Cell Reports. 2013;3(5):1449–1456. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dillon LM, Williams SL, Hida A, et al. Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse. Human Molecular Genetics. 2012;21(10):2288–2297. doi: 10.1093/hmg/dds049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bevilacqua L, Ramsey JJ, Hagopian K, et al. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. American Journal of PHysiology. Endocrinology and metabolism. 2004;286(5):E852–E861. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- 68.Bevilacqua L, Ramsey JJ, Hagopian K, et al. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. American Journal of PHysiology. Endocrinology and metabolism. 2005;289(3):E429–E438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 69.Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. The FASEB Journal. 2005;19(10):1320–1322. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- 70.Lanza IR, Zabielski P, Klaus KA, et al. Chronic Caloric Restriction Preserves Mitochondrial Function in Senescence without Increasing Mitochondrial Biogenesis. Cell Metabolism. 2012;16(6):777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofer T, Servais S, Seo AY, et al. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: Effects of aging and lifelong calorie restriction. Mechanisms of Ageing and Development. 2009;130(5):297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shinmura K, Tamaki K, Sano M, et al. Impact of long-term caloric restriction on cardiac senescence: Caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. Journal of Molecular and Cellular Cardiology. 2011;50(1):117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Nisoli E. Calorie Restriction Promotes Mitochondrial Biogenesis by Inducing the Expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U.S.A. 2006;103(6):1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cerqueira FM, Laurindo FRM, Kowaltowski AJ. Mild Mitochondrial Uncoupling and Calorie Restriction Increase Fasting eNOS, Akt and Mitochondrial Biogenesis. PLoS ONE. 2011;6(3):e18433. doi: 10.1371/journal.pone.0018433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gouspillou G, Hepple RT. Facts and controversies in our understanding of how caloric restriction impacts the mitochondrion. Biochemical Pharmacology. 2013;48(10):1075–1084. doi: 10.1016/j.exger.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Palacios OM, Carmona JJ, Michan S, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging Journal. 2009;1(9):771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nemoto S, MM F, T F. SIRT1 Functionally Interacts with the Metabolic Regulator and Transcriptional Coactivator PGC-1α. Journal of Biological Chemistry. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 79.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 80.Barger JL, Kayo T, Pugh TD, et al. Short-term consumption of a resveratrolcontaining nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Cell Metabolism. 2008;43(9):859–866. doi: 10.1016/j.exger.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 81.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramadori G, Gautron L, Fujikawa T, et al. Central Administration of Resveratrol Improves Diet-Induced Diabetes. Endocrinology. 2009;150(12):5326–5333. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metabolism. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Price NL, Gomes AP, Ling AJY, et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metabolism. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park S-J, Ahmad F, Philp A, et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arany Z, Foo S, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 87.Rowe GC, Jang C, Patten IS, Arany Z. PGC-1βregulates angiogenesis in skeletal muscle. American Journal of Physiology. Endocrinology and metabolism. 2011;301(1):E155–E163. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu D, Zhang L, Wang H, et al. PGC-1 : The Missing Ingredient for Mesenchymal Stem Cell-Mediated Angiogenesis. Diabetes. 2012;61(5):979–980. doi: 10.2337/db12-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pino E, Wang H, McDonald ME, et al. Roles for Peroxisome Proliferatoractivated Receptor γ(PPAR ) and PPAR Coactivators 1αand 1βin Regulating Response of White and Brown Adipocytes to Hypoxia. Journal of Biological Chemistry. 2012;287(22):18351–18358. doi: 10.1074/jbc.M112.350918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saint-Geniez M, Jiang A, Abend S, et al. PGC-1α Regulates Normal and Pathological Angiogenesis in the Retina. Biochemical Pharmacology. 2013;182(1):255–265. doi: 10.1016/j.ajpath.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pedersen BK. Muscle as a Secretory Organ. Compr Physiol. 2013 Jul;3(3):1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 93.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 94.Magnusson SP, Simonsen EB, Dyhre-Poulsen P, et al. Viscoelastic stress relaxation during static stretch in human skeletal muscle in the absence of EMG activity. Scand J Med Sci Sports. 1996;6(6):323–328. doi: 10.1111/j.1600-0838.1996.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 95.Mohr T, JL A, FB B-S, et al. Long term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1–16. doi: 10.1038/sj.sc.3100343. [DOI] [PubMed] [Google Scholar]
- 96.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. Journal of Clinical Endocrinology & Metabolism. 2013;98(4):E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- 98.Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Cell Metabolism. 2012;61(12):1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roca-Rivada A, Al-Massadi O, Castelao C, et al. Muscle tissue as an endocrine organ: Comparative secretome profiling of slow-oxidative and fast-glycolytic rat muscle explants and its variation with exercise. J Proteomics. 2012;75(17):5414–5425. doi: 10.1016/j.jprot.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 100.Sharma N, Castorena CM, Cartee GD. Greater insulin sensitivity in calorie restricted rats occurs with unaltered circulating levels of several important myokines and cytokines. Nutrition Metabolism. 2012 Oct 15;9(1):90. doi: 10.1186/1743-7075-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roberts MD, Bayless DS, Company JM, et al. Elevated skeletal muscle irisin precursor FNDC5 mRNA in obese OLETF rats. Metabolism. 2013;62(8):1052–1056. doi: 10.1016/j.metabol.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488(7413):E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 103.Pekkala S, Wiklund P, Hulmi JJ, et al. Are Skeletal Muscle FNDC5 Gene Expression and Irisin Release Regulated by Exercise and Related to Health? The Journal of Physiology. 2013;(1113):263707. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovascular Research. 2008;79(2):208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 105.Uldry M, Yang W, St-Pierre J, et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metabolism. 2006;3(5):333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 106.St-Pierre J, Drori S, Uldry M, et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1αTranscriptional Coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Soriano FX, Liesa M, Bach D, et al. Evidence for a Mitochondrial Regulatory Pathway Defined by Peroxisome Proliferator-Activated Receptor-γCoactivator-1α, Estrogen-Related Receptor-α, and Mitofusin 2. Diabetes. 2006;55(6):1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 108.Garnier A, Fortin D, Zolll J, et al. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. The FASEB Journal. 2005;19(1):43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 109.Austin S, Klimcakova E, St-Pierre J. Impact of PGC-1α on the topology and rate of superoxide production by the mitochondrial electron transport chain. Biochemical Pharmacology. 2011;51(12):2243–2248. doi: 10.1016/j.freeradbiomed.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 110.Hoeks J, Arany Z, Phielix E, et al. Enhanced lipid-but not carbohydratesupported mitochondrial respiration in skeletal muscle of PGC-1α overexpressing mice. Clinical and Experimental Pharmacology and Physiology. 2011;227(3):1026–1033. doi: 10.1002/jcp.22812. [DOI] [PubMed] [Google Scholar]
- 111.Aquilano K, Vigilanza P, Baldelli S, et al. Peroxisome Proliferator-activated Receptor Co-activator 1α (PGC-1α) and Sirtuin 1 (SIRT1) Reside in Mitochondria: Possible Direct Function in mitochondrial biogenesis. Journal of Biological Chemistry. 2010;285(28):21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Safdar A, Little JP, Stokl AJ, et al. Exercise Increases Mitochondrial PGC- 1αContent and Promotes Nuclear-Mitochondrial Cross-talk to Coordinate Mitochondrial Biogenesis. Journal of Biological Chemistry. 2011;286(12):10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Mizushima N, Komatsu M. Autophagy: Renovation of Cells and Tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 114.Wohlgemuth SE, Seo AY, Marzetti E, et al. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Biochemical Pharmacology. 2010;45(2):138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masiero E, Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy. 6(2):307–309. doi: 10.4161/auto.6.2.11137. [DOI] [PubMed] [Google Scholar]
- 116.Grumati P, Coletto L, Schiavinato A, et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VIdeficient muscles. Autophagy. 2011;7(12):1415–1423. doi: 10.4161/auto.7.12.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He C, Bassik MC, Moresi V, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lira VA, Okutsu M, Zhang M, et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. The FASEB Journal. 2013;27(10):4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm. res. 49(11):561–570. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- 120.Degens H. Age-related skeletal muscle dysfunction: causes and mechanisms. J Musculoskelet Neuronal Interact. 2007;7(3):246–252. [PubMed] [Google Scholar]
- 121.Franceschi C, Capri M, Monti D, et al. Inflammation and anti-inflammation: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Ageing and Development. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 122.Botta A, Laher I, Beam J, et al. Short Term Exercise Induces PGC-1α, Ameliorates Inflammation and Increases Mitochondrial Membrane Proteins but Fails to Increase Respiratory Enzymes in Aging Diabetic Hearts. PLoS ONE. 2013;8(8):e70248. doi: 10.1371/journal.pone.0070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Febbraio MA. Exercise and inflammation. Journal of Applied Physiology. 2007;103:376–377. doi: 10.1152/japplphysiol.00414.2007. [DOI] [PubMed] [Google Scholar]
- 124.Handschin C, CS C, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle–specific PGC-1α knockout mice reveals skeletal muscle–pancreatic β cell crosstalk. J. Clin. Invest. 2007;117(11):3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Handschin C, Chin S, Li P, et al. Skeletal Muscle Fiber-type Switching, Exercise Intolerance, and Myopathy in PGC-1 Muscle-specific Knock-out Animals. Journal of Biological Chemistry. 2007;282(41):30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 126.Eisele PS, Salatino S, Sobek J, et al. The Peroxisome Proliferator-activated Receptor Coactivator 1α/β(PGC-1) Coactivators Repress the Transcriptional Activity of NF- B in Skeletal Muscle Cells. Journal of Biological Chemistry. 2013;288(4):2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Da Cruz S, Parone PA, Lopes VS, et al. Elevated PGC-α Activity Sustains Mitochondrial Biogenesis and Muscle Function without Extending Survival in a Mouse Model of Inherited ALS. Cell Metabolism. 2012;15(5):778–786. doi: 10.1016/j.cmet.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miura S, Kai Y, Kamei Y, Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to β2-adrenergic receptor activation and exercise. Endocrinology. 2008;149(9):4527–4533. doi: 10.1210/en.2008-0466. [DOI] [PubMed] [Google Scholar]