Abstract
The inositol-1,4,5-trisphosphate receptors (InsP3Rs) are the major intracellular Ca2+-release channels in cells. Activity of InsP3Rs is essential for elementary and global Ca2+ events in the cell. There are three InsP3Rs isoforms that are present in mammalian cells. In this review review we will focus primarily on InsP3R type 1. The InsP3R1 is a predominant isoform in neurons and it is most extensively studied isoform. Combination of biophysical and structural methods revealed key mechanisms of InsP3R function and modulation. Cell biological and biochemical studies lead to identification of a large number of InsP3R-binding proteins. InsP3Rs are involved in the regulation of numerous physiological processes, including learning and memory, proliferation, differentiation, development and cell death. Malfunction of InsP3R1 play a role in a number of neurodegenerative disorders and other disease states. InsP3Rs represent a potentially valuable drug target for treatment of these disorders and for modulating activity of neurons and other cells. Future studies will provide better understanding of physiological functions of InsP3Rs in health and disease.
Keywords: Inositol 1,4,5-trisphosphate receptors; cell nucleus; Ca2+ signalling; neurodegeneration
1. Introduction
The inositol-1,4,5-trisphosphate receptors (InsP3Rs) are the major intracellular Ca2+-release channels in cells. The investigation of mechanisms of inositol-1,4,5-trisphosphate (InsP3)-induced Ca2+-release started in 1980s and InsP3R was first solubilized and purified from the rat cerebellum in 1988 by Snyder’s group (Supattapone et al., 1988). Reconstitution of the purified receptor into lipid vesicles showed that InsP3 and other inositol phosphates stimulate calcium flux (Ferris et al., 1989). Then cDNA of InsP3R was cloned (Furuichi et al., 1989; Mignery et al., 1990) that helps to initiate structure-function studies (Mignery and Sudhof, 1990; Miyawaki et al., 1991). Reconstitution of InsP3R into planar bilayer membranes revealed its single channel permeability, its modulation by Ca2+ and by ATP (Bezprozvanny and Ehrlich, 1993, 1994; Bezprozvanny et al., 1991). Since these initial publications the functional properties of native and recombinant InsP3R have been extensively characterized by Ca2+ flux measurements, planar lipid bilayer or nuclear envelope patch-clamp recordings (Bezprozvanny, 2005; Foskett et al., 2007; Mikoshiba, 2007; Wagner and Yule, 2012). Although some of these studies initially resulted in conflicting data, an agreement has been reached by the field regarding key InsP3R functional properties.
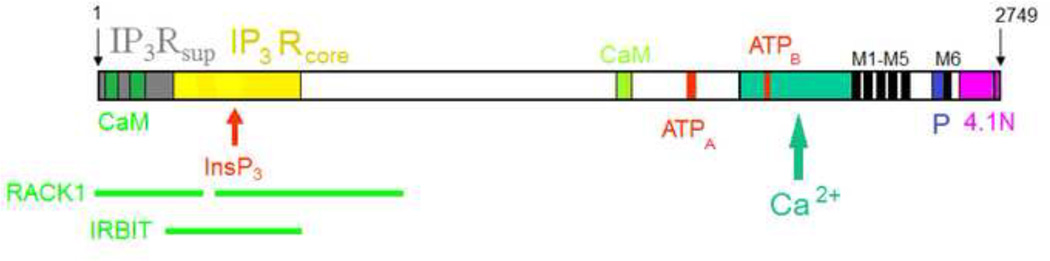
The variety of InsP3-activated Ca2+ channels, including three mammalian InsP3R isoforms (InsP3R type 1 (InsP3R1), InsP3R type 2 (InsP3R2), InsP3R type 3 (InsP3R3), different slicing variants of IP3R1, Drosophila melanogaster InsP3R and Caenorhabditis elegans InsP3R have been discovered and characterised (reviewed in (Bezprozvanny, 2005; Foskett et al., 2007; Mikoshiba, 2007)). The three mammalian InsP3R isoforms are 60–70% identical in sequence (Furuichi et al., 1994) and share a common domain structure (Mignery and Sudhof, 1990; Miyawaki et al., 1991) that consists of an amino-terminal InsP3-binding domain, a carboxyl-terminal Ca2+ channel domain, and a middle coupling domain containing most of the putative regulatory sites and is the most divergent (Fig. 1). InsP3R1 is predominant in the central nervous system, but most other tissues express at least two and often all three InsP3R isoforms at different ratios (Taylor et al., 1999).
Figure 1. Domain structure of InsP3R1.
InsP3Rsup and InsP3Rcore domains, CaM, RACK1, IRBIT and 4.1N binding sites, two ATP (A and B) binding sites, the Ca2+ sensor region, the M1–M6 transmembrane domains and the pore-forming region (P) are shown.
The InsP3R are subjected to multiple levels of regulation (Bezprozvanny, 2005; Foskett et al., 2007; Mikoshiba, 2007; Wagner and Yule, 2012). InsP3Rs are the targets of a number of allosteric regulators, including protein kinases, adenine nucleotides, pH and divalent cations, all of which may play a part in InsP3-induced Ca2+ signaling. Significant effect of phosphorylation on InsP3R is also well documented (Bezprozvanny, 2005; Foskett et al., 2007; Mikoshiba, 2007). Many protein binding with InsP3R have been described, and physiological relevance of these interactions is under intense investigation.
At this moment one can find thousands of papers from different research groups dedicated to various aspects of InsP3R structure, regulation or functional role, but there are still many questions remain to be answered. In this review we focus on InsP3R type 1, which are predominant isoform expressed in mammalian neurons. Here we will briefly review the structure and basic properties of these channels, their role in the cell functions and in several neurodegenerative disorders, such as Hungtington’s disease, spinocerebellar ataxias and Alzheimer’s disease.
2. InsP3Rs in cell functions
A rise in intracellular calcium in neurons in response to InsP3Rs activation is implicated in the control of a numerous cellular functions, including neurotransmission and synaptic plasticity, proliferation, differentiation, development, gene expression, and cell death (Berridge et al., 1998), Evidence at both cellular and behavioral levels implicates InsP3Rs in memory formation, in particular they are required during long-term memory (Baker et al., 2013). It was demonstrated that InsP3R1 is extremely important in embryonic development. InsP3R1 knock-out mice have severe ataxia and tonic or tonic-clonic seizures and die by the weaning period (Matsumoto et al., 1996). Besides, InsP3R1 is a critical regulator of synaptic circuit maintenance in the mature cerebellum; this mechanism may underlie motor coordination and learning in adults (Sugawara et al., 2013). Thus, InsP3R1 are essential for proper brain development and function.
InsP3R1 are highly concentrated in the Purkinje cells of the cerebellum, with lower levels being found in other regions of the brain (Sharp et al., 1993a; Sharp et al., 1993b; Taylor et al., 1999) and in a variety of peripheral tissues (Taylor et al., 1999). Immunohistochemical studies in Purkinje cells, Xenopus oocytes and pancreatic epithelial cells have revealed that at a subcellular level InsP3Rs are localized in the rough and smooth endoplasmic reticulum (ER), Golgi complex and nuclear envelope, but not mitochondria or plasma membranes (Lam and Galione, 2013; Ross et al., 1989; Solovyova and Verkhratsky, 2003). Though, it has been indicated that the plasma membrane in some cell types may also contain InsP3R (Barrera et al., 2004; Dellis et al., 2006; Tanimura et al., 2000), but the role of such localization is rather contradictory.
InsP3 is not the only regulator of InsP3Rs function; Ca2+ plays a critical role in shaping the InsP3R-evoked Ca2+ signals. Low Ca2+ concentrations (<300 nM) activate the channel and increase its open probability, whereas high Ca2+ concentrations inhibit channel opening (Bezprozvanny et al., 1991; Finch et al., 1991; Iino, 1990). These positive and negative feedback cycles are well suited for generating Ca2+ oscillations or waves. It appears that InsP3Rs are activated by simultaneous binding of the two agonists, InsP3 and Ca2+, to the cytoplasmic domain of the molecule that leads to the conformational change in the receptor complex and an increase in the frequency of its Ca2+ channel opening, resulting in Ca2+ release from the intracellular stores. Both InsP3 and Ca2+ are the two main intracellular messengers with their own regulatory pathways (Berridge, 2009, 2012; Decrock et al., 2013). So InsP3R acts as a skilled “analyst” which coordinates two complex streams of signals and forms an integrated response.
Because of complex Ca2+-mediated feedback on InsP3R activity, Ca2+ signals evoked by the receptor activation are complex, restricted in space and time, and this spatiotemporal organization determines physiological effect of the signal (Berridge, 1997; Bootman et al., 2001; Konieczny et al., 2012). Intracellular InsP3-activated Ca2+ signals are organized at three levels, each of them provides different signaling functions and serves as a building block for Ca2+ signals at the next level (Berridge, 1996, 1997; Bootman et al., 2001). At the first, so called “fundamental”, level signals result from openings of a single InsP3R channel. At the resting conditions the cytosolic concentration of Ca2+ is low and InsP3Rs are in a conformation with low affinity for InsP3, but the activating stimuli trigger the rise in the intracellular Ca2+ concentration and the production of InsP3 from the plasma membrane. These low concentrations of the two agonists activate one InsP3R leading to a rapid localized Ca2+flux called “blip” (Parker and Yao, 1996). At the next, “elementary”, level Ca2+ signals, so called “puffs”, arise from the concerted opening of multiple InsP3R channels. It has been demonstrated that InsP3Rs are initially randomly distributed in the membranes, but low concentrations of InsP3 cause them to aggregate rapidly and reversibly into clusters (Yao et al., 1995). There is no agreement about the number of InsP3Rs in a cluster, it was proposed that from four (Rahman, 2012; Rahman and Taylor, 2009) to 25 or 35 InsP3Rs can open simultaneously during puffs (Shuai et al., 2006; Shuai and Jung, 2003; Solovey and Dawson, 2010). At resting cytosolic Ca2+ concentrations clustered InsP3Rs open independently, but at increasing of Ca2+ they are more likely to open and close together (Dickinson et al., 2012; Taylor et al., 2009; Yamasaki-Mann et al., 2013). Ca2+release from one channel acts as an activating ligand to stimulate nearby channels through Ca2+-induced Ca2+release (CICR). So, the spatial organization of InsP3R channels within clusters and the distribution of clusters, together with the positive regulation by InsP3 and Ca2+, enable local and long-range Ca2+signals to be constructed from the activities of single InsP3R. At intermediate InsP3 concentrations activate groups of InsP3Rs which release Ca2+ to form puffs, but at high concentrations of InsP3 all the receptors are excitable and puffs then act as initiation sites to spawn a regenerative wave that spreads through the cell by CICR (Berridge, 2009). It is the third level of the intracellular Ca2+ hierarchy, which is associated with stronger extra-cellular agonist stimulation. Ca2+ released at one cluster can trigger Ca2+ release at adjacent clusters by CICR, leading to the generation of Ca2+ waves that propagate in a saltatory manner in the whole cell.
The nuclear envelope is the Ca2+ store with InsP3Rs in its inner membrane (Fedorenko OA, 2008; Marchenko et al., 2005). It was indicated that the cell nucleus contains the whole set of enzymes required for the InsR3 synthesis from diphosphoinositolphosphate of the nuclear membrane, so the regulation of nuclear InsP3Rs cannot depend on cytoplasmic processes (Gomes et al., 2006; Klein and Malviya, 2008; Rodrigues et al., 2009). Of course, in small cells InsR3 can freely penetrate into the nucleus by diffusion through the nuclear pores, but in large cells the distance from the plasma membrane to the nucleus is large enough so it is unlikely that InsP3Rs in the nuclear membrane are activated by cytosolic InsP3R. This point is proved by the fact that that numerous InsP3-activated channels were recorded from the inner nuclear membrane of Purkinje and CA1 pyramidal neurons, which are the largest cells in the brain. No InsP3Rs were found in the nuclear membrane of granule neurons of the cerebellum (Marchenko et al., 2005) and dentate gyrus (Fedorenko OA, 2007). Therefore the mechanism of regulation of nuclear Ca2+ may vary in different cells. There is a growing body of evidence that nuclear Ca2+ can affect gene transcription (Bading, 2000; Bengtson and Bading, 2012; Greer and Greenberg, 2008; Parekh and Muallem, 2011; Wiegert and Bading, 2011) through activation of nuclear Ca2+-sensitive kinases and phosphatases, or through direct interaction with Ca2+-dependent transcription factors, such as CREB and DREAM.. However, many issues needed to be clarified, in particular the regulation of Ca2+ signals between the cytoplasm and the nucleus and the mechanisms of the intranuclear Ca2+ signaling.
3. Biophysical properties of InsP3R1
Biophysical properties of InsP3R1 have been studied following their isolation and reconstitution into artificial lipid planar bilayer membranes (Bezprozvanny and Ehrlich, 1993, 1994; Bezprozvanny et al., 1991; Tu et al., 2005b) and using patch-clamp recordings from nuclei isolated from a variety of cells, including mammalian neurons (Fedorenko OA, 2008; Mak and Foskett, 1997; Marchenko et al., 2005; Wagner and Yule, 2012).
The InsP3R channel is a Ca2+-selective channel, which is also permeable to other cations such as K+ and Ba2+with permeability ratios PBa/PK = 5–6 and PCa/PK = 4–5 in symmetrical 140–150 mM K+ solutions, with relatively little selectivity among different divalent cations (Bezprozvanny and Ehrlich, 1994; Boehning et al., 2001; Marchenko et al., 2005). The InsP3R are channels with a large single-channel monovalent ion conductance. InsP3Rs recorded in the inner nuclear membrane of rat cerebellar Purkinje cell have a slope conductance of 355 pS (Marchenko et al., 2005) in symmetric solutions with 150 mM K+ and the absence of Mg2+. Similar conductance in the same conditions was recorded for expressed recombinant rat InsP3R1 present in COS-7 cell nuclei (Boehning et al., 2001). Single-channel conductance of InsP3Rs with Ba2+ as the current carrier is 121 pS based on nuclear patch measurements (Marchenko et al., 2005). Conductance and selectivity of InsP3Rs from the nuclear membranes are similar to cerebellar InsP3Rs and recombinant InsP3R1 incorporated into artificial lipid bilayers (Bezprozvanny and Ehrlich, 1994; Tu et al., 2005b)
At a membrane potential 60 mV in symmetrical 150 mm KCl solution open probability (Po) for InsP3R recorded from the nuclear membranes of Purkinje neurons is about 0.036; with Ba2+ as a current carrier under the same conditions Po increases to 0.32 (Marchenko et al., 2005). The presence of Ca2+ in solutions in concentrations ≥10 mM strongly decreased the Po to 0.0023 and that apparently results from the inhibitory effect of high Ca2+ concentrations in the vicinity of the receptor produced by Ca2+ current through the channel rather than an effect of the presence of Ca2+ at the luminal surface of the membrane, because the Po of single inward K+ currents remained practically unchanged (Bezprozvanny and Ehrlich, 1994).
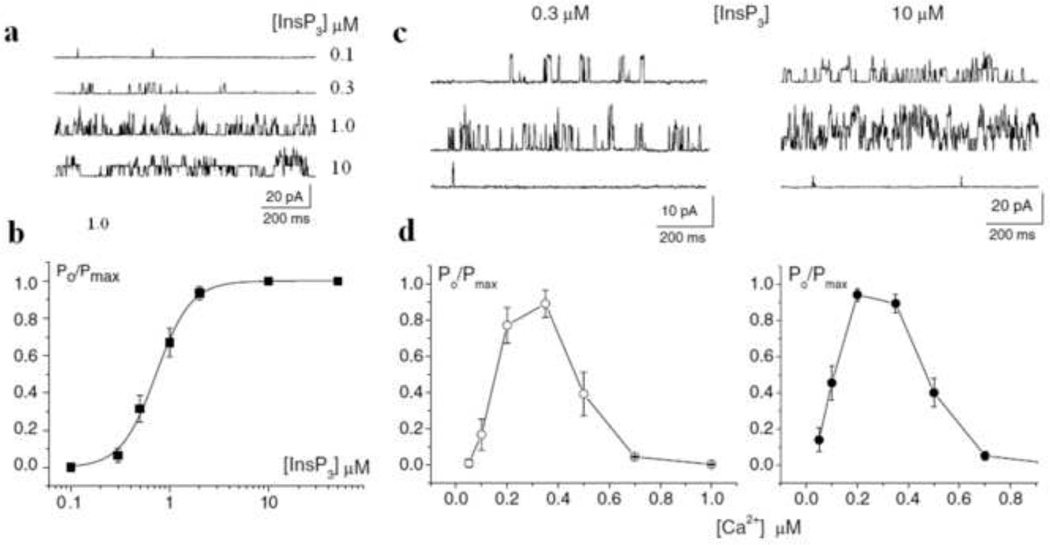
InsP3Rs in the inner nuclear membrane of CA1 pyramidal neurons are activated by IP3 in concentrations ≥50 nM and saturated (Po ≥ 95% of Po at 10 µM IP3) in concentrations of ≥2 µM in the presence of 250 nm free Ca2+ (Fig. 2A, 2B) (Marchenko et al., 2005). InsP3, and other ligands such as ATP, regulate channel activity mainly by modifying the sensitivity of the channels to Ca2+ regulation, though in the absence of InsP3, Ca2+ alone (20 nm–50 mm) is unable to activate the channels. The regulation of the receptors by Ca2+ evokes much controversy (Mak et al., 1998). It has been reported that at steady-state conditions and saturating concentration of InsP3 cerebellar InsP3Rs incorporated into artificial lipid bilayers were activated by Ca2+ at low concentrations and inhibited by higher Ca2+ concentration with the peak of activation renged from 200 to 400 nm (Bezprozvanny et al., 1991). In nuclear patch-clamp recordings Ca2+ inhibited InsP3Rs with the same efficiency both at low (0.3 mm) and saturated (10 mm) InsP3 concentrations (Fig. 2C, 2D). Therefore the inhibitory effect of Ca2+ on InsP3Rs does not depend on the InsP3 concentration (Marchenko et al., 2005). Experiments with flash photolysis of caged InsP3 in Purkinje neurons support these data. It has been shown that Ca2+ entry through plasmalemmal Ca2+ channels strongly suppressed Ca2+ release from stores induced by high (25 mm) InsP3 concentrations (Khodakhah and Ogden, 1995). Recombinant InsP3Rs1 expressed in insect cells also demonstrated a bell-shaped Ca2+ dependence at saturated InsP3 concentrations (Tu et al., 2005a). Thus, most recent results support “narrow” Ca2+ dependence of InsP3R1 within physiological range of Ca2+ concentrations. Besides being activated by InsP3 and suitable concentrations of Ca2+, InsP3R channel activity is also allosterically potentiated by ATP, (Bezprozvanny and Ehrlich, 1993; Mak et al., 1999; Wagner and Yule, 2012). It has been reported that other nucleotides, such ADP (Iino, 1991), AMP (Ferris et al., 1990), and GTP (Bezprozvanny and Ehrlich, 1993; Mak et al., 1999) can also potentiate InsP3R1 channel activity.
Figure 2. Modulation of InsP3R1 by Ca2+ and InsP3.
InsP3R1 activity was recorded by patching cerebellar Purkinje cell nuclear membrane (Marchenko et al., 2005)
a, InsP3R channel activity at different InsP3 concentrations. b, dependence of the normalised open probability of InsP3R channels on InsP3 concentration. Data points represent mean±SEM of five experiments. The solid curve is the Hill equation fit with EC50=0.68 µM and Hill coefficient =2.5. The channel activity (c) and normalised open probability of InsP3R channels (d) at different Ca2+ concentrations in the presence of low (0.3 µM, left; n=5) and saturated (10 µM, right; n=7) InsP3 concentrations. At both InsP3 concentrations 1 µM of Ca2+ almost completely inhibited the channel activity. Patch pipettes were filled with BaCl2 solution, bath contained standard KCl solution with [Ca2+]i =250 nM. Holding potential was 40 mV.
InsP3Rs have several phosphorylation sites (Bezprozvanny, 2005). InsP3Rs are regulated by numerous kinases, including cAMP-dependent protein kinase (PKA) (DeSouza et al., 2002; Tang et al., 2003b; Vanderheyden et al., 2009; Wagner et al., 2004; Wagner et al., 2003), protein kinase C (Ferris et al., 1991), cGMP-dependent protein kinase (Haug et al., 1999; Komalavilas and Lincoln, 1994) and others. It has been reported, for example, that phosphorylation by PKA of recombinant type 1 InsP3Rs expressed in insect cells increased the Po of the channels incorporated into artificial lipid bilayers more than 10-fold (from <2–3% to 30–40%) and increased their sensitivity to InsP3 about 4-fold (Tang et al., 2003b).
There are about 40 proteins or even more that can bind InsP3R (Fig. 1), among them as calmodulin (Kasri et al., 2004a; Michikawa et al., 1999; Yamada et al., 1995), RACK1 (Woodard et al., 2010), protein 4.1N (Maximov et al., 2003), IRBIT (Mikoshiba, 2012), Bcl-2 (Chen et al., 2004), AKAP9 (Tu et al., 2004) and may others. Therefore, InsP3Rs in cells act as a critical “signaling hub” that mediate cross-talk between Ca2+ signaling, kinases, phosphatases and protein-protein interaction mechanisms. Not surprisingly, abnormality in modulation or activity of InsP3R1 is connected with variety of neurological disorders (see below).
4. Structural studies of InsP3Rs
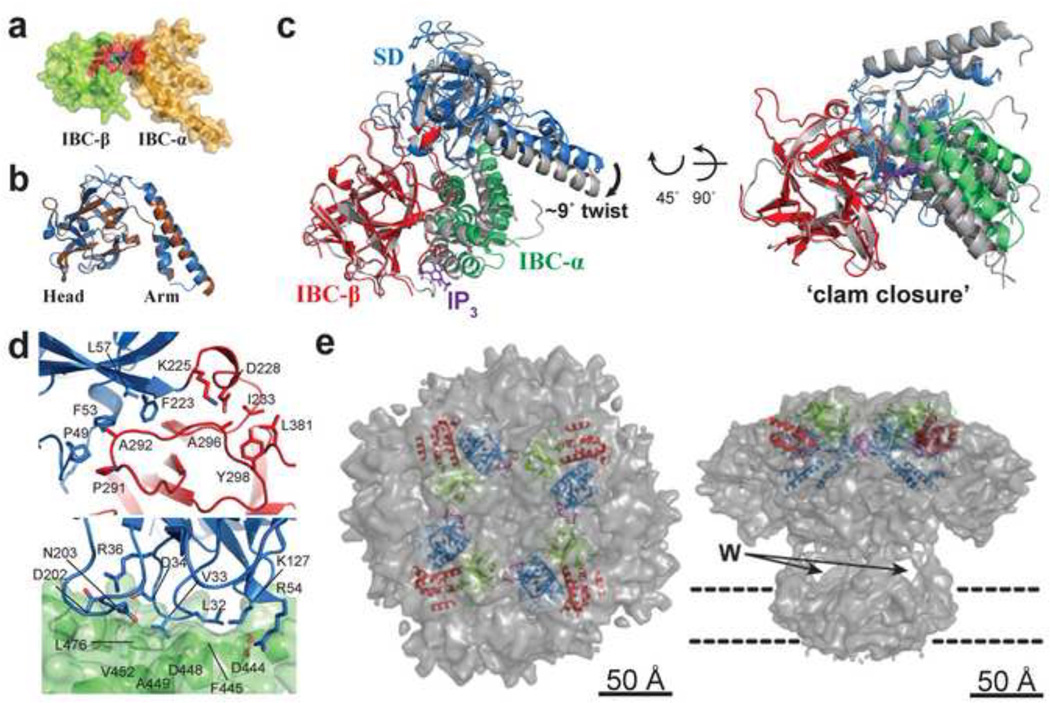
In the past decade, significant advancements have been made in determining atomic-resolution structures of InsP3Rs. We now have a good understanding of the molecular mechanism underlying receptor recognition of the InsP3 molecule and how this binding is transformed into a protein conformational change at the NH2-terminus, essential for the initial step of channel activation. The first high-resolution structure determined by X-ray crystallography was the NH2-terminal InsP3-binding core (IBC) of InsP3R1 (residues 224–604) in complex with InsP3 (Bosanac et al., 2002). The structure of the IBC contains two structurally distinct domains: the β-domain (IBC-β) and α-domain (IBC-α). The IBC-β (residues 224 – 436) adopts a β-trefoil fold comprising 12 β-strands and two single turn helices, whereas the IBC-α (residues 437– 604) adopts an armadillo repeat fold consisting of 8 α-helices (Fig. 3A). The IBC forms an L-shaped structure with the two domains oriented approximately perpendicular to each other; several basic amino acids cluster in a cleft formed by both domains, comprising the InsP3 binding site (Bosanac et al., 2002). The crystal structures of the NH2-terminal suppressor domain (SD) have been determined for InsP3R1 (residues 1–223) (Bosanac et al., 2005) and InsP3R3 (residues 1–224) (Chan et al., 2010); moreover, the two structures are nearly identical showing a backbone root mean square deviation (rmsd) of ~1.3 Å (Fig. 3B). The SD folds into a hammer-like structure with a 12 β-stranded “head” domain and a helix-turn-helix “arm” domain. Furthermore, the head domain of the SD adopts a similar β-trefoil fold as found in the IBC. The InsP3 binding affinity of the entire NH2-terminal region (NT; residues 1– 604 of InsP3R1) is reduced by more than one order of magnitude compared with that of the IBC alone, implying that the SD inhibits or “suppresses” InsP3 binding (Yoshikawa et al., 1996). Evidence suggests that not only is the SD required for suppression of InsP3 binding, but it is also needed for InsP3-induced allosteric channel gating. For example, InsP3R1 lacking the SD shows no measureable InsP3-evoked Ca2+ release (Uchida et al., 2003), and remarkably, a single Tyr167Ala mutation in the SD completely abolishes InsP3-evoked Ca2+ release (Yamazaki et al., 2010).
Figure 3. Structure of InsP3R1-NT relative to the full-length tetrameric receptor.
a, Crystal structure of the InsP3R1-IBC in complex with InsP3 at 2.2 Å resolution. Ribbon and surface representations of the IBC-β (light green) and IBC-α (orange) are shown. InsP3-coordinating regions are colored red. b, Superimposed structures of the InsP3R1-SD (blue) and InsP3R3-SD (brown). c, Superimposed apo (SD, blue; IBC-β, red; IBC-α, green) and InsP3-bound (i.e. holo) InsP3R1-NT (grey) structures. The structures were aligned by overlaying the IBC-β. d, The two interfaces between the SD and IBC (colored as in c). The short β-interface (top) consists predominantly of hydrophobic interactions between Pro49, Phe53, and Phe223 from the SD, and Pro291 and Ala292 from the IBC-β, and is supported by a salt bridge between Lys225 and Asp228. The longer α-interface (bottom) is stabilized by hydrophobic interactions between Val33 in the SD and a pocket formed by Val452, Phe445, Ala449, and Leu476 within IBC-α. Electrostatic interactions between Arg54 and Lys127 from the SD and Asp444 from IBC-α are also involved in forming the α-interface. e, Cryo-EM structures of full-length InsP3R1 in the closed state are shown from top (left) and side (right) views. The docked crystal structure of InsP3R1-NT is shown as a ribbon representation with SD in blue, IBC-β in red and IBC-α in green. The HS-loop is colored magenta. The windows (W) of InsP3R1 are indicated with arrows. The membrane bilayer boundaries are depicted with broken lines. The figures in a and b are reproduced from (Stathopulos et al., 2012) and in c–e from (Seo et al., 2012).
Recently, four atomic-resolution NT structures of InsP3R1 have been determined. Lin et al., solved two NT structures of rat InsP3R1 at 3.8 Å resolution; moreover, they derived one structure in an InsP3-free state (i.e., apo) and a second structure in an InsP3-bound state (i.e., holo) from a single crystal grown in the presence of InsP3 (Lin et al., 2011). Subsequently, apo and holo NT structures of rat InsP3R1 at higher resolution were separately determined from crystals grown in the absence (3.0 Å) and presence (3.6 Å) of InsP3, respectively (Seo et al., 2012) (Fig. 3C). The individual structures of the three domains comprising NT of InsP3R1 (i.e., SD, IBC-β, and IBC-α) are highly similar to the separately determined SD (Bosanac et al., 2005) and IBC (Bosanac et al., 2002). Nonetheless, these NT structures represent a significant advancement in the understanding of InsP3R function by revealing the arrangement of the SD and IBC domains with respect to one another and providing important clues on the bases for tetrameric channel formation. The three domains in NT form a triangular architecture, and the SD is located on the opposite face of the InsP3-binding site, suggesting that the SD suppresses InsP3 binding by an allosteric mechanism. The SD interacts with both the IBC-β and IBC-α, forming two interfaces (i.e., β-interface and α-interface, respectively) (Fig. 3D). The functional importance of residues associated with the α-interface is demonstrated by the Val33Lys mutation, which almost completely abrogates the effects of the SD on InsP3 binding and attenuates the maximal open probability of the full-length channel (Bosanac et al., 2005; Rossi et al., 2009). The most marked conformational change caused by InsP3 binding is the significant decrease in the domain orientation angle between IBC-β and IBC-α (Fig.3C). This ligand binding-induced structural change in IBC occurs with the hinge region between IBC-β and IBC-α set as the pivot point, resulting in a narrowing of the InsP3-binding cleft and a clam-like closure. Consequently, the SD rotates (~9°) towards the IBC, accompanied by a swing that is approximately perpendicular to the IBC ‘clam closure’ (Fig. 3C).
Recently, Ludtke et al. determined the cryo-EM structure of InsP3R isolated from the rat cerebellum at 9.5 Å resolution (Ludtke et al., 2011; Murray et al., 2013), dramatically improving our view of the tetrameric full-length InsP3R channel compared to earlier studies (da Fonseca et al., 2003; Hamada et al., 2002; Jiang et al., 2002; Sato et al., 2004; Serysheva et al., 2003). The tetrameric structure of InsP3R1 revealed a mushroom-shaped overall architecture with a fourfold symmetry axis along a central plug (Fig. 3D). The cytoplasmic region is located apical to the transmembrane domain with several large openings or “windows” between the two regions (Fig. 3E) and consists of a relatively rigid exterior surface with a more structurally variable and probably flexible interior (i.e. exhibiting higher statistical variability in the calculated density map), possibly due to multiple conformations of InsP3R1 in the closed state. Docking the high resolution crystal structures of InsP3R1-NT into this full-length cryo-EM structure shows that InsP3R1-NT forms a tetrameric ring around the fourfold symmetry axis, with the hot spot loop (HS-loop; so-called due to the high structural homology with a loop where a cluster of disease-associated mutations in ryanodine receptor domain A have been mapped) of InsP3R1 (residues 165–180) involved in intersubunit interactions (Fig. 3E). Importantly, Li et al. produced covalently linked tetrameric InsP3R1-NT through site-specific cysteine insertions in the intersubunit interface that was modeled in the docking studies and demonstrated that InsP3 inhibits cross-linked tetrameric InsP3R1-NT in a concentration-dependent manner, suggesting that InsP3 binding closes the clam-like IBC, disrupting these intersubunit interactions and allowing the channel to open (Fig. 4C) (Li et al., 2013). Taken together, these observations indicate that the InsP3R1-NT plays a critical role in the allosteric modulation of ion channel conductance through a modification of quaternary arrangement.
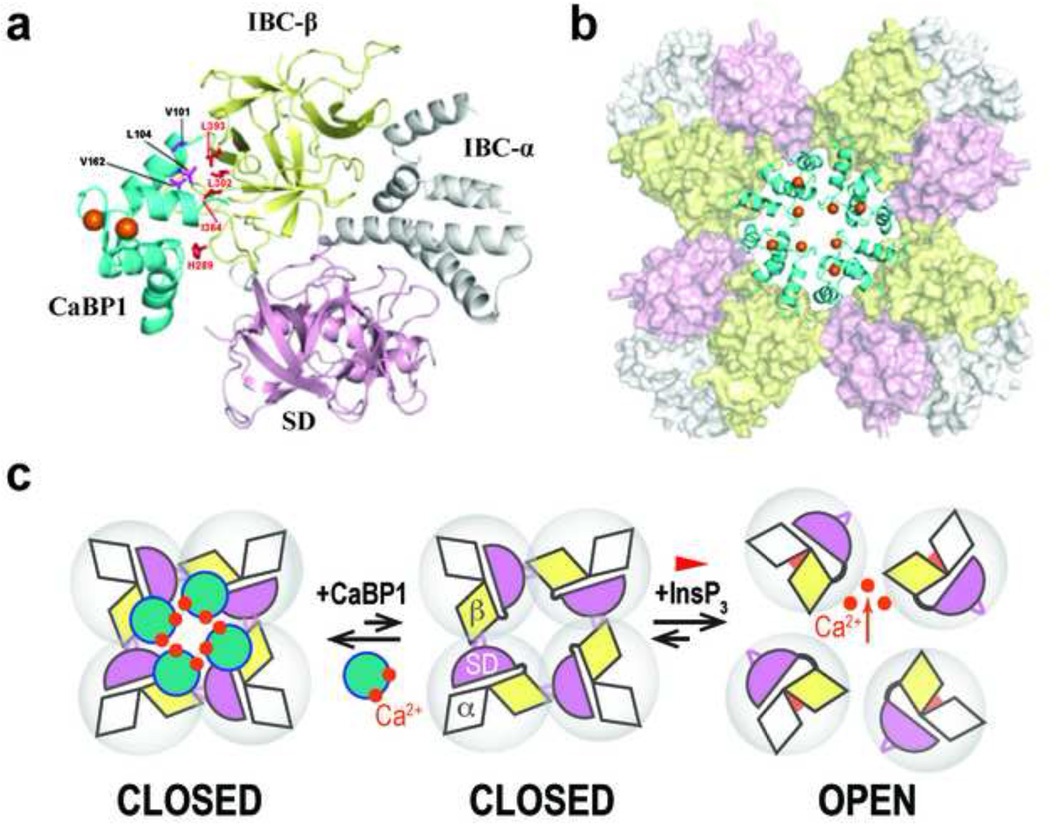
Figure 4. Structure of the InsP3R1-NT/CaBP1 complex and opposing effects of InsP3 and CaBP1 on intersubunit interactions.
a, Structure of the C-lobe of CaBP1 (CaBP1-C) bound to InsP3R1-NT in a 1:1 complex. NMR structural restraints were used to define contacts between InsP3R1-NT (SD, pink; IBC-β, yellow; IBC-α, grey) and the CaBP1-C (cyan, with Ca2+ atoms colored orange). Key residues at the binding interface are highlighted in magenta (CaBP1) and red (InsP3R1). b, Model for the tetrameric InsP3R1-NT/CaBP1-C complex (colored as in f) generated by superimposing the InsP3R1-NT crystal structure (Seo et al., 2012) onto the cryo-EM electron density map of InsP3R1 (Ludtke et al., 2011). c, Interactions between adjacent InsP3R1-NTs that are mediated by IBC-β (yellow) and the HS-loop of the SD (magenta) hold the tetrameric InsP3R1 in a closed state. InsP3 binding closes the clam-like IBC, disrupting these intersubunit interactions, and allowing the channel to open. CaBP1 clamps the intersubunit interactions associated with the closed-state, thereby inhibiting channel opening. The figures are reproduced from (Li et al., 2013).
Very recently, the structure of InsP3R1-NT in complex with calcium binding protein 1 (CaBP1) was determined using nuclear magnetic resonance (NMR) spectroscopy-based chemical shift perturbation mapping and paramagnetic relaxation enhancement (PRE) data (Fig. 4A) (Li et al., 2013). The structure showed that exposed hydrophobic residues in Ca2+-bound CaBP1 (i.e. Val101, Leu104, and Val162) interact with clustered hydrophobic residues (i.e. Leu302, Ile364, and Leu393) in the IBC-β domain of InsP3R1 (Fig. 4A). Superimposing the structure of the InsP3R1-NT/CaBP1 complex with the tetrameric InsP3R1-NT shown in Fig. 4B revealed that the four molecules of CaBP1 form a ring-like structure around the central cytosolic vestibule. Further, chemical cross-linking experiments showed that CaBP1 enhances the production of cross-linked tetrameric InsP3R1-NT (Li et al., 2013). In aggregate with data demonstrating that Ca2+-CaBP1 inhibits InsP3-evoked Ca2+ release (Haynes et al., 2004; Kasri et al., 2004b; Li et al., 2013) a novel regulatory molecular mechanism has been proposed where CaBP1 clamps the intersubunit interactions and thereby inhibits channel opening (Fig. 4C) (Li et al., 2013).
5. The role of InsP3Rs in neurodegenerative diseases
The role of InsP3R1 has been shown for several neurodegenerative diseases such as Huntington’s disease, spinocerebellar ataxias (SCAs) and Alzheimer disease (AD) (Bezprozvanny, 2009, 2011; Bezprozvanny and Mattson, 2008; Kasumu and Bezprozvanny, 2012).
HD is an autosomal-dominant neurodegenerative disorder and it is caused by polyglutamine (polyQ) expansion in the amino-terminal region of a protein huntingtin (Htt). Huntington disease (HD) is an autosomal-dominant neurodegenerative disorder with the age of onset between 35 and 50 years and inexorable progression to death 15–20 years after onset. The symptoms include motor abnormalities including chorea and psychiatric disturbance with gradual dementia (Vonsattel and DiFiglia, 1998). Neuropathological analysis reveals selective and progressive neuronal loss in the striatum (caudate nucleus, putamen and globus pallidus) (Vonsattel and DiFiglia, 1998; Vonsattel et al., 1985). GABAergic medium spiny striatal neurons (MSN) are the most sensitive to neuronal degeneration in HD (Vonsattel and DiFiglia, 1998; Vonsattel et al., 1985). It is widely accepted that mutant Htt (mHtt) protein acquires a “toxic gain of function” (Tobin and Signer, 2000). A a number of potential “toxic functions” of mutant Htt have been suggested. Our laboratory is focused on connection between mutant Htt and deregulated neuronal Ca2+ signaling. We first discovered that mHtt binds directly and specifically to the C-terminal region of the type 1 IP3 receptor (IP3R1) (Tang et al., 2003a). Recently, unbiased high-throughput screening assays confirmed mHtt binding to IP3R1 (Kaltenbach et al., 2007). Interestingly the affinity of mHtt to IP3R1 increases when mHtt is associated with HAP1A (Tang et al., 2003a). Moreover, mHtt, but not normal Htt, augmented IP3R1 activity in planar lipid bilayers (Tang et al., 2003a). Similarly, application of subthreshold concentrations of DHPG, an mGluR1/5 agonist, sensitized Ca2+ release in YAC128 primary MSN cultures (Tang et al., 2003a). This is consistent with the fact that glutamate-induced apoptosis of MSNs from YAC128 mice is mediated by mGluR1/5 and NR2B receptors (Tang et al., 2005). In fact, specific blockade of IP3R1 with 2-APB and Enoxaparin is neuroprotective in the same model (Tang et al., 2005). In more recent experiments, we demonstrated that viral delivery of a peptide that disrupts mHtt association with IP3R1 protected YAC128 MSNs in vitro and in vivo (Tang et al., 2009). We also reported that Ca2+ stabilizing agent dantrolene exerts beneficial effects in mouse model of HD (Chen et al., 2011). We also demonstrated that continuous overactivation of InsP3R1 pathway causes compensatory upregulation of neuronal store-operated Ca2+ entry in HD neurons, which contributes to Ca2+ overload and pathology (Wu et al., 2011). The alterations in ER enzymes that have been observed in HD postmortem brains (Kerner et al., 1997; Mao and Wang, 2001, 2002; Tallaksen-Greene et al., 1998; Testa et al., 1995) are consistent with malfunction of ER Ca2+ handling in HD MSN neurons. In recent studies it was discovered that InsP3R1 association of ER stress chaperone protein GRP78 is impaired in HD R6/2 model mice, resulting in misregulation of InsP3R1 gating (Higo et al., 2010). These results provide additional support for the role of InsP3R1 in HD pathology.
Many SCAs are caused by polyQ-expansion in ataxin proteins and typically affect cerebellar Purkinje cells. SCAs are generally characterized by cerebellar atrophy and a progressive incoordination of movement known as ataxia (Filla et al., 1999; Lastres-Becker et al., 2008; Schols et al., 2004). Although there is a phenotypic overlap with cerebellar atrophy and ataxia in all SCA patients, other brain regions may be affected in different type of disease. The pathogenesis of SCAs is not fully understood, however, several different pathogenic mechanisms have been studied in SCAs such as dysregulation of transcription and gene expression, alterations in calcium homeostasis and synaptic neurotransmission, mitochondrial stress and apoptosis (reviewed in (Bezprozvanny and Klockgether, 2010; Carlson et al., 2009; Kasumu and Bezprozvanny, 2012; Matilla-Duenas et al., 2009)).
Studies in our laboratory primarily focused on the role of InsP3Rs in pathogenesis of SCA type 2 (SCA2). The SCA2 is caused by an expansion and translation of unstable glutamine (Q) repeats in the gene encoding ataxin-2 from the normal 22 to more than 31 extra glutamine repeats (Imbert et al., 1996; Pulst et al., 1996; Sanpei et al., 1996). PolyQ-expanded ataxin-2 (ATXN2exp) protein, similar to wildtype ataxin-2 (ATXNwt), is widely expressed in the body tissues. Similar to wildtype ataxin-2, polyglutamine-expanded ataxin-2 protein is ubiquitously expressed in cells without severe aggregation and formation of inclusion bodies (Huynh et al., 2000). Cerebellar PCs in SCA2 patients are mostly affected with a loss of over 75% (Schols et al., 2004). The role of calcium signaling in the pathogenesis of SCA2 is supported by the genetic association between polymorphisms in the CACNA1A gene and the age of disease onset in patients diagnosed with SCA2 (Pulst et al., 2005). The role of aberrant neuronal calcium signaling in SCA2 pathogenesis was strengthened further by a finding in our lab that ATX2exp but not wildtype ATX2 (ATX2wt) specifically binds InsP3R1 (Liu et al., 2009). In a lipid bilayer reconstitution experiment, we found the presence of ATX2exp substantially sensitized IP3R 1 to activation by InsP3 (Liu et al., 2009). Consistent with involvement of excitotoxic mechanism, we discovered that PC cells in SCA2 mouse undergo dark cell degeneration (DCD) mode of cell death (Kasumu and Bezprozvanny, 2012). Long-term feeding of SCA2-58Q mice with a calcium stabilizer dantrolene alleviated the age-dependent motor coordination deficits and PC cell loss in these mice (Liu et al., 2009). More recently we performed partial suppression of InsP3-induced Ca2+ release in PC cells of SCA2-58Q mice by using inositol 5-phosphatase viral construct (Kasumu et al., 2012). We discovered that this approach prevented the onset of PC dysfunction, alleviated motor incoordination, and reduced age-dependent PC degeneration in SCA2-58Q mice (Kasumu et al., 2012). These results indicate that partial suppression of InsP3-induced Ca2+ release is a viable therapeutic strategy for treatment of SCA2 and possibly other SCAs (Kasumu et al., 2012). Remarkably, insufficient InsP3R-mediated Ca2+ signaling also leads to the ataxic phenotype and PC degeneration, such as observed in SCA15/16 patients haploinsufficient for the InsP3R1 gene (Hara et al., 2008; Iwaki et al., 2008; van de Leemput et al., 2007). An ataxic phenotype is also observed in opt mice with reduced levels of InsP3R1 protein (Street et al., 1997) and a severe ataxia is observed in InsP3R1 knockout mice (Matsumoto et al., 1996). These findings indicate that the reduced Ca2+ release via InsP3R1 also leads to PC dysfunction and ataxic phenotype and that there is a relatively narrow range of optimal InsP3-mediated Ca2+ signaling that is compatible with proper function and long-term survival of PCs. Deviation from this optimal range in either direction of InsP3-mediated Ca2+ signaling results in PC dysfunction and an ataxic phenotype (Kasumu et al., 2012).
Alzheimer disease (AD) is another neurodegenerative disease in whose pathogenesis abnormal InsP3R signaling is likely to be involved. AD progresses slowly and affects neurons mainly in the cortex and hippocampus. The exact mechanism of AD pathogenesis is not known. One to two percent of AD cases constitute early onset familial Alzheimer disease cases. FAD is caused by mutations in genes coding presenilin 1 (PS1), presenilin 2 (PS2) or amyloid precursor proteins (APP). The first observation of exaggerated InsP3R-mediated Ca2+ release from ER has been detected in fibroblasts from AD patients (Ito et al., 1994). Later similar results have been obtained in AD mouse models as well as in Xenopus oocytes expressing human PS1 and PS2 mutant constructs (Bezprozvanny and Mattson, 2008; Stutzmann, 2007). The mechanism of abnormal InsP3R signaling in AD is not fully understood. Our laboratory discovered that presenilns function as ER Ca2+ leak channels, and FAD mutations in presenilins result in elevated ER Ca2+ levels (Nelson et al., 2007; Tu et al., 2006; Zhang et al., 2010). Increased ER Ca2+ levels are in turn result in enhanced InsP3R1-mediated Ca2+ release. The role of presenilins as ER Ca2+ leak channels was recently confirmed in unbiased screen for modulators of calcium homeostasis (Bandara et al., 2013). An alternative hypothesis is that mutant presenilins enhance InsP3R1 gating via direct protein-protein interaction (Cheung et al., 2010; Cheung et al., 2008). Future studies will be need to understand the mechanism of InsP3R1 disregulation in AD and its importance for pathology.
6. Conclusions
A remarkable progress has been made since original discovery of InsP3R family 25 years ago. Using electrophysiological and molecular methods we dissected major functional properties of all 3 mammalian InsP3R isoforms. Thanks to application of structural biology methods we start to understand function of InsP3R as a “molecular machine” in atomic details. Identification of a large number of InsP3R-binding partners highlighted a role of InsP3R as a key “signaling hub” in cells. We discovered that dysfunction of InsP3R is linked to a variety of neurodegenerative disorders, and demonstarted that InsP3R is a potential target for therapeutic interference in these and other disorders. There is no doubt that further research of InsP3R family in healthy and disease states will continue to provide additional valuable insights into basic biology and medicine.
Acknowlegments
IB is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer’s Disease Research. MI holds the Canada Research Chair in Cancer Structural Biology. This work was supported by the Dynasty Foundation grant DP–B-11/13 (EP), Welch Foundation grant I-1754 (IB), NIH grants R01NS080152 (IB), R01NS074376 (IB), R01NS056224 (IB), by the contract with the Russian Ministry of Science 11.G34.31.0056 (IB), by the State Fund for Fundamental Researches in Ukraine DFFD F 46.2/001 (O.F), the Heart and Stroke Foundation of Canada (MI), the Natural Sciences and Engineering Research Council of Canada (MI), and the Canadian Institutes of Health Research (MI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bading H. Transcription-dependent neuronal plasticity the nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. doi: 10.1046/j.1432-1327.2000.01565.x. [DOI] [PubMed] [Google Scholar]
- Baker KD, Edwards TM, Rickard NS. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev. 2013;37:1211–1239. doi: 10.1016/j.neubiorev.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Bandara S, Malmersjo S, Meyer T. Regulators of Calcium Homeostasis Identified by Inference of Kinetic Model Parameters from Live Single Cells Perturbed by siRNA. Science signaling. 2013;6:ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera NP, Morales B, Villalon M. Plasma and intracellular membrane inositol 1,4,5-trisphosphate receptors mediate the Ca(2+) increase associated with the ATP-induced increase in ciliary beat frequency. Am J Physiol Cell Physiol. 2004;287:C1114–C1124. doi: 10.1152/ajpcell.00343.2003. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Microdomains and elemental events in calcium signalling. Cell Calcium. 1996;20:95–96. doi: 10.1016/s0143-4160(96)90098-6. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Exp Biol. 1997;200:315–319. doi: 10.1242/jeb.200.2.315. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. Role of inositol 1,4,5-trisphosphate receptors in pathogenesis of Huntington's disease and spinocerebellar ataxias. Neurochem Res. 2011;36:1186–1197. doi: 10.1007/s11064-010-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. ATP modulates the function of inositol 1,4,5-trisphosphategated channels at two sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Klockgether T. Therapeutic prospects for spinocerebellar ataxia type 2 and 3. Drugs of the Future. 2010;34:991–999. doi: 10.1358/dof.2009.034.12.1443434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Boehning D, Joseph SK, Mak DO, Foskett JK. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys J. 2001;81:117–124. doi: 10.1016/s0006-3495(01)75685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Lipp P, Berridge MJ. The organisation and functions of local Ca(2+) signals. J Cell Sci. 2001;114:2213–2222. doi: 10.1242/jcs.114.12.2213. [DOI] [PubMed] [Google Scholar]
- Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. doi: 10.1038/nature01268. [DOI] [PubMed] [Google Scholar]
- Bosanac I, Yamazaki H, Matsu-Ura T, Michikawa T, Mikoshiba K, Ikura M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Molecular cell. 2005;17:193–203. doi: 10.1016/j.molcel.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Carlson KM, Andresen JM, Orr HT. Emerging pathogenic pathways in the spinocerebellar ataxias. Curr Opin Genet Dev. 2009;19:247–253. doi: 10.1016/j.gde.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Yamazaki H, Ishiyama N, Seo MD, Mal TK, Michikawa T, Mikoshiba K, Ikura M. Structural studies of inositol 1,4,5-trisphosphate receptor: coupling ligand binding to channel gating. J Biol Chem. 2010;285:36092–36099. doi: 10.1074/jbc.M110.140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wu J, Lvovskaya S, Herndon E, Supnet C, Bezprozvanny I. Dantrolene is neuroprotective in Huntington's disease transgenic mouse model. Mol Neurodegener. 2011;6:81. doi: 10.1186/1750-1326-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Mei L, Mak DO, Hayashi I, Iwatsubo T, Kang DE, Foskett JK. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Sci Signal. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca PC, Morris SA, Nerou EP, Taylor CW, Morris EP. Domain organization of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis. Proc Natl Acad Sci U S A. 2003;100:3936–3941. doi: 10.1073/pnas.0536251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decrock E, De Bock M, Wang N, Gadicherla AK, Bol M, Delvaeye T, Vandenabeele P, Vinken M, Bultynck G, Krysko DV, Leybaert L. IP3, a small molecule with a powerful message. Biochim Biophys Acta. 2013;1833:1772–1786. doi: 10.1016/j.bbamcr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein Kinase A and Two Phosphatases Are Components of the Inositol 1,4,5-Trisphosphate Receptor Macromolecular Signaling Complex. J Biol Chem. 2002;277:39397–39400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- Dickinson GD, Swaminathan D, Parker I. The probability of triggering calcium puffs is linearly related to the number of inositol trisphosphate receptors in a cluster. Biophys J. 2012;102:1826–1836. doi: 10.1016/j.bpj.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko OA DD, Marchenko SM. Nuclear ionic channels of the granule cells from the dentate gyrus. Fiziol Zh. 2007;53:9–15. [PubMed] [Google Scholar]
- Fedorenko OA DD, Marchenko SM. Calcium channels in the nuclear envelope of pyramidal neurons of the hippocampus. Neurophysiology. 2008;40:238–242. [Google Scholar]
- Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. Inositol trisphosphate receptor - phosphorylation by protein kinase-C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc. Natl. Acad. Sci. USA. 1991;88:2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Snyder SH. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc. Natl. Acad. Sci. USA. 1990;87:2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Supattapone S, Snyder SH. Purified inositol 1,4,5- trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989;342:87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Filla A, De Michele G, Santoro L, Calabrese O, Castaldo I, Giuffrida S, Restivo D, Serlenga L, Condorelli DF, Bonuccelli U, Scala R, Coppola G, Caruso G, Cocozza S. Spinocerebellar ataxia type 2 in southern Italy: a clinical and molecular study of 30 families. J Neurol. 1999;246:467–471. doi: 10.1007/s004150050385. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO. Inositol Trisphosphate Receptor Ca2+ Release Channels. Physiological reviews. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T, Kohda K, Miyawaki A, Mikoshiba K. Intracellular channels. Current Opinion Neurobiol. 1994;4:294–303. doi: 10.1016/0959-4388(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gomes DA, Leite MF, Bennett AM, Nathanson MH. Calcium signaling in the nucleus. Can J Physiol Pharmacol. 2006;84:325–332. doi: 10.1139/y05-117. [DOI] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hamada K, Miyata T, Mayanagi K, Hirota J, Mikoshiba K. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J Biol Chem. 2002;277:21115–21118. doi: 10.1074/jbc.C200244200. [DOI] [PubMed] [Google Scholar]
- Hara K, Shiga A, Nozaki H, Mitsui J, Takahashi Y, Ishiguro H, Yomono H, Kurisaki H, Goto J, Ikeuchi T, Tsuji S, Nishizawa M, Onodera O. Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology. 2008;71:547–551. doi: 10.1212/01.wnl.0000311277.71046.a0. [DOI] [PubMed] [Google Scholar]
- Haug LS, Jensen V, Hvalby O, Walaas SI, Ostvold AC. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic nucleotide-dependent kinases in vitro and in rat cerebellar slices in situ. J Biol Chem. 1999;274:7467–7473. doi: 10.1074/jbc.274.11.7467. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem. 2004;279:547–555. doi: 10.1074/jbc.M309617200. [DOI] [PubMed] [Google Scholar]
- Higo T, Hamada K, Hisatsune C, Nukina N, Hashikawa T, Hattori M, Nakamura T, Mikoshiba K. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron. 2010;68:865–878. doi: 10.1016/j.neuron.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26:44–50. doi: 10.1038/79162. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Effects of adenine nucleotides on inositol 1,4,5-trisphosphate-induced calcium release in vascular smooth muscle cells. J. Gen.Physiol. 1991;98:681–698. doi: 10.1085/jgp.98.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, Weber C, Mandel JL, Cancel G, Abbas N, Durr A, Didierjean O, Stevanin G, Agid Y, Brice A. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A, Kawano Y, Miura S, Shibata H, Matsuse D, Li W, Furuya H, Ohyagi Y, Taniwaki T, Kira J, Fukumaki Y. Heterozygous deletion of ITPR1, but not SUMF1, in spinocerebellar ataxia type 16. J Med Genet. 2008;45:32–35. doi: 10.1136/jmg.2007.053942. [DOI] [PubMed] [Google Scholar]
- Jiang QX, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 A resolution. The EMBO journal. 2002;21:3575–3581. doi: 10.1093/emboj/cdf380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, Cha GH, Ukani L, Chepanoske CL, Zhen Y, Sahasrabudhe S, Olson J, Kurschner C, Ellerby LM, Peltier JM, Botas J, Hughes RE. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasri NN, Bultynck G, Smyth J, Szlufcik K, Parys JB, Callewaert G, Missiaen L, Fissore RA, Mikoshiba K, de Smedt H. The N-terminal Ca2+-independent calmodulin-binding site on the inositol 1,4,5-trisphosphate receptor is responsible for calmodulin inhibition, even though this inhibition requires Ca2+ Mol Pharmacol. 2004a;66:276–284. doi: 10.1124/mol.66.2.276. [DOI] [PubMed] [Google Scholar]
- Kasri NN, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, De Smedt H, Conway SJ, Holmes AB, Berridge MJ, Roderick HL. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 2004b;23:312–321. doi: 10.1038/sj.emboj.7600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumu A, Bezprozvanny I. Deranged Calcium Signaling in Purkinje Cells and Pathogenesis in Spinocerebellar Ataxia 2 (SCA2) and Other Ataxias. Cerebellum (London, England) 2012;11:630–639. doi: 10.1007/s12311-010-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasumu AW, Liang X, Egorova P, Vorontsova D, Bezprozvanny I. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32:12786–12796. doi: 10.1523/JNEUROSCI.1643-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerner JA, Standaert DG, Penney JB, Jr, Young AB, Landwehrmeyer GB. Expression of group one metabotropic glutamate receptor subunit mRNAs in neurochemically identified neurons in the rat neostriatum, neocortex, and hippocampus. Brain Res Mol Brain Res. 1997;48:259–269. doi: 10.1016/s0169-328x(97)00102-2. [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Ogden D. Fast activation and inactivation of inositol trisphosphate-evoked Ca2+ release in rat cerebellar Purkinje neurones. J Physiol (Lond) 1995;487:343–358. doi: 10.1113/jphysiol.1995.sp020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Malviya AN. Mechanism of nuclear calcium signaling by inositol 1,4,5-trisphosphate produced in the nucleus, nuclear located protein kinase C and cyclic AMP-dependent protein kinase. Front Biosci. 2008;13:1206–1226. doi: 10.2741/2756. [DOI] [PubMed] [Google Scholar]
- Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994;269:8701–8707. [PubMed] [Google Scholar]
- Konieczny V, Keebler MV, Taylor CW. Spatial organization of intracellular Ca2+ signals. Semin Cell Dev Biol. 2012;23:172–180. doi: 10.1016/j.semcdb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Lam AK, Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim Biophys Acta. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Rub U, Auburger G. Spinocerebellar ataxia 2 (SCA2) Cerebellum. 2008;7:115–124. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- Li C, Enomoto M, Rossi AM, Seo MD, Rahman T, Stathopulos PB, Taylor CW, Ikura M, Ames JB. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc Natl Acad Sci U S A. 2013;110:8507–8512. doi: 10.1073/pnas.1220847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Baek K, Lu Z. Apo and InsP(3)-bound crystal structures of the ligand-binding domain of an InsP(3) receptor. Nat Struct Mol Biol. 2011;18:1172–1174. doi: 10.1038/nsmb.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP, Pulst SM, Bezprozvanny I. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Tran TP, Ngo QT, Moiseenkova-Bell VY, Chiu W, Serysheva II. Flexible architecture of IP3R1 by Cryo-EM. Structure. 2011;19:1192–1199. doi: 10.1016/j.str.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Foskett JK. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J Gen Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. U S A. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Foskett JK. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca(2+) activation. J Biol Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Upregulation of preprodynorphin and preproenkephalin mRNA expression by selective activation of group I metabotropic glutamate receptors in characterized primary cultures of rat striatal neurons. Brain Res Mol Brain Res. 2001;86:125–137. doi: 10.1016/s0169-328x(00)00276-x. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Glutamate cascade to cAMP response element-binding protein phosphorylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors. Mol Pharmacol. 2002;62:473–484. doi: 10.1124/mol.62.3.473. [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Yarotskyy VV, Kovalenko TN, Kostyuk PG, Thomas RC. Spontaniously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurons. J Physiol. 2005;563:897–910. doi: 10.1113/jphysiol.2004.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla-Duenas A, Sanchez I, Corral-Juan M, Davalos A, Alvarez R, Latorre P. Cellular and Molecular Pathways Triggering Neurodegeneration in the Spinocerebellar Ataxias. Cerebellum. 2009 doi: 10.1007/s12311-009-0144-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nakagawa T, Inoue T, Nagata E, Tanaka K, Takano H, Minowa O, Kuno J, Sakakibara S, Yamada M, Yoneshima H, Miyawaki A, Fukuuchi Y, Furuichi T, Okano H, Mikoshiba K, Noda T. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379:168–171. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang T-S, Bezprozvanny I. Association of the type 1 inositol (1,4,5)-trisphosphate receptor with 4.1N protein in neurons. Molecular Cellular Neuroscience. 2003;22:271–283. doi: 10.1016/s1044-7431(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- Mignery GA, Newton CL, Archer BT, Sudhof TC. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- Mignery GA, Sudhof TC. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990;9:3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. The discovery and structural investigation of the IP(3) receptor and the associated IRBIT protein. Adv Exp Med Biol. 2012;740:281–304. doi: 10.1007/978-94-007-2888-2_12. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Furuichi T, Ryou Y, Yoshikawa S, Nakagawa T, Saitoh T, Mikoshiba K. Structure-function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc Natl Acad Sci U S A. 1991;88:4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SC, Flanagan J, Popova OB, Chiu W, Ludtke SJ, Serysheva II. Validation of cryo-EM structure of IP(3)R1 channel. Structure. 2013;21:900–909. doi: 10.1016/j.str.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Tu H, Lei T, Bentahir M, de Strooper B, Bezprozvanny I. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Muallem S. Ca(2+) signalling and gene regulation. Cell Calcium. 2011;49:279. doi: 10.1016/j.ceca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Parker I, Yao Y. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J Physiol (Lond) 1996;491:663–668. doi: 10.1113/jphysiol.1996.sp021247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, Pearlman S, Starkman S, Orozco-Diaz G, Lunkes A, DeJong P, Rouleau GA, Auburger G, Korenberg JR, Figueroa C, Sahba S. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- Pulst SM, Santos N, Wang D, Yang H, Huynh D, Velazquez L, Figueroa KP. Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain. 2005;128:2297–2303. doi: 10.1093/brain/awh586. [DOI] [PubMed] [Google Scholar]
- Rahman T. Dynamic clustering of IP3 receptors by IP3. Biochem Soc Trans. 2012;40:325–330. doi: 10.1042/BST20110772. [DOI] [PubMed] [Google Scholar]
- Rahman T, Taylor CW. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels (Austin) 2009;3:226–232. doi: 10.4161/chan.3.4.9247. [DOI] [PubMed] [Google Scholar]
- Rodrigues MA, Gomes DA, Nathanson MH, Leite MF. Nuclear calcium signaling: a cell within a cell. Braz J Med Biol Res. 2009;42:17–20. doi: 10.1590/s0100-879x2008005000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Meldolesi J, Milner TA, Satoh T, Supattapone S, Snyder SH. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989;339:468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Rossi AM, Riley AM, Tovey SC, Rahman T, Dellis O, Taylor EJ, Veresov VG, Potter BV, Taylor CW. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol. 2009;5:631–639. doi: 10.1038/nchembio.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, Wakisaka A, Tashiro K, Ishida Y, Ikeuchi T, Koide R, Saito M, Sato A, Tanaka T, Hanyu S, Takiyama Y, Nishizawa M, Shimizu N, Nomura Y, Segawa M, Iwabuchi K, Eguchi I, Tanaka H, Takahashi H, Tsuji S. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14:277–284. doi: 10.1038/ng1196-277. [DOI] [PubMed] [Google Scholar]
- Sato C, Hamada K, Ogura T, Miyazawa A, Iwasaki K, Hiroaki Y, Tani K, Terauchi A, Fujiyoshi Y, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J Mol Biol. 2004;336:155–164. doi: 10.1016/j.jmb.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Schols L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, Taylor CW. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serysheva II, Bare DJ, Ludtke SJ, Kettlun CS, Chiu W, Mignery GA. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by electron cryomicroscopy. J Biol Chem. 2003;278:21319–21322. doi: 10.1074/jbc.C300148200. [DOI] [PubMed] [Google Scholar]
- Sharp AH, Dawson TM, Ross CA, Fotuhi M, Mourey RJ, Snyder SH. Inositol 1,4,5-trisphosphate receptors: immunohistochemical localization to discrete areas of rat central nervous system. Neuroscience. 1993a;53:927–942. doi: 10.1016/0306-4522(93)90478-x. [DOI] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP, Snyder SH. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodinesensitive Ca2+ release channels in rat brain. J Neurosci. 1993b;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai J, Rose HJ, Parker I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys J. 2006;91:4033–4044. doi: 10.1529/biophysj.106.088880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai JW, Jung P. Optimal ion channel clustering for intracellular calcium signaling. Proc Natl Acad Sci U S A. 2003;100:506–510. doi: 10.1073/pnas.0236032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey G, Dawson SP. Intra-cluster percolation of calcium signals. PLoS One. 2010;5:e8997. doi: 10.1371/journal.pone.0008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyova N, Verkhratsky A. Neuronal endoplasmic reticulum acts as a single functional Ca2+ store shared by ryanodine and inositol-1,4,5-trisphosphate receptors as revealed by intra-ER [Ca2+] recordings in single rat sensory neurones. Pflugers Arch. 2003;446:447–454. doi: 10.1007/s00424-003-1094-z. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Seo MD, Enomoto M, Amador FJ, Ishiyama N, Ikura M. Themes and variations in ER/SR calcium release channels: structure and function. Physiology (Bethesda) 2012;27:331–342. doi: 10.1152/physiol.00013.2012. [DOI] [PubMed] [Google Scholar]
- Street VA, Bosma MM, Demas VP, Regan MR, Lin DD, Robinson LC, Agnew WS, Tempel BL. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17:635–645. doi: 10.1523/JNEUROSCI.17-02-00635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE. The pathogenesis of Alzheimers disease is it a lifelong "calciumopathy"? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Hisatsune C, Le TD, Hashikawa T, Hirono M, Hattori M, Nagao S, Mikoshiba K. Type 1 inositol trisphosphate receptor regulates cerebellar circuits by maintaining the spine morphology of purkinje cells in adult mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12186–12196. doi: 10.1523/JNEUROSCI.0545-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S, Worley PF, Baraban JM, Snyder SH. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J. Biol. Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Tang TS, Guo C, Wang H, Chen X, Bezprozvanny I. Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington's disease mouse model. J Neurosci. 2009;29:1257–1266. doi: 10.1523/JNEUROSCI.4411-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington's disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003a;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase 1alpha. J Neurosci. 2003b;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Tojyo Y, Turner RJ. Evidence that type I, II, and III inositol 1,4,5- trisphosphate receptors can occur as integral plasma membrane proteins. J Biol Chem. 2000;275:27488–27493. doi: 10.1074/jbc.M004495200. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Taufiq Ur R, Pantazaka E. Targeting and clustering of IP3 receptors: key determinants of spatially organized Ca2+ signals. Chaos. 2009;19:037102. doi: 10.1063/1.3127593. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–252. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- Tobin AJ, Signer ER. Huntington's disease: the challenge for cell biologists. Trends Cell Biol. 2000;10:531–536. doi: 10.1016/s0962-8924(00)01853-5. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee S-F, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer's disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Tang TS, Wang Z, Bezprozvanny I. Association of type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J Biol Chem. 2004;279:19375–19382. doi: 10.1074/jbc.M313476200. [DOI] [PubMed] [Google Scholar]
- Tu H, Wang Z, Bezprozvanny I. Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys J. 2005a;88:1056–1069. doi: 10.1529/biophysj.104.049601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J. 2005b;88:1046–1055. doi: 10.1529/biophysj.104.049593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- van de Leemput J, Chandran J, Knight MA, Holtzclaw LA, Scholz S, Cookson MR, Houlden H, Gwinn-Hardy K, Fung HC, Lin X, Hernandez D, Simon-Sanchez J, Wood NW, Giunti P, Rafferty I, Hardy J, Storey E, Gardner RJ, Forrest SM, Fisher EM, Russell JT, Cai H, Singleton AB. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. Journal of neuropathology and experimental neurology. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington's disease. Journal of neuropathology and experimental neurology. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Li WH, Joseph SK, Yule DI. Functional consequences of phosphomimetic mutations at key cAMP-dependent protein kinase phosphorylation sites in the type 1 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2004;279:46242–46252. doi: 10.1074/jbc.M405849200. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Li WH, Yule DI. Phosphorylation of type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases: a mutational analysis of the functionally important sites in the S2+ and S2- splice variants. J Biol Chem. 2003;278:45811–45817. doi: 10.1074/jbc.M306270200. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Yule DI. Differential regulation of the InsP(3) receptor type-1 and -2 single channel properties by InsP(3), Ca(2)(+) and ATP. J Physiol. 2012;590:3245–3259. doi: 10.1113/jphysiol.2012.228320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49:296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Woodard GE, Lopez JJ, Jardin I, Salido GM, Rosado JA. TRPC3 regulates agoniststimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J Biol Chem. 2010;285:8045–8053. doi: 10.1074/jbc.M109.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Shih HP, Vigont V, Hrdlicka L, Diggins L, Singh C, Mahoney M, Chesworth R, Shapiro G, Zimina O, Chen X, Wu Q, Glushankova L, Ahlijanian M, Koenig G, Mozhayeva GN, Kaznacheyeva E, Bezprozvanny I. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington's disease treatment. Chemistry & biology. 2011;18:777–793. doi: 10.1016/j.chembiol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Miyawaki A, Saito K, Nakajima T, Yamamoto-Hino M, Ryo Y, Furuichi T, Mikoshiba K. The calmodulin-binding domain in the mouse type 1 inositol 1,4,5-trisphosphate receptor. Biochem J. 1995;308:83–88. doi: 10.1042/bj3080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki-Mann M, Demuro A, Parker I. Cytosolic [Ca2+] regulation of InsP3-evoked puffs. Biochem J. 2013;449:167–173. doi: 10.1042/BJ20121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Chan J, Ikura M, Michikawa T, Mikoshiba K. Tyr-167/Trp-168 in type 1/3 inositol 1,4,5-trisphosphate receptor mediates functional coupling between ligand binding and channel opening. J Biol Chem. 2010;285:36081–36091. doi: 10.1074/jbc.M110.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol (Lond) 1995;482:533–553. doi: 10.1113/jphysiol.1995.sp020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:18277–18284. doi: 10.1074/jbc.271.30.18277. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I. Role of presenilins in neuronal calcium homeostasis. J Neurosci. 2010;30:8566–8580. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]