Summary
Developmental timing genes catalyze stem cell progression and animal maturation programs across taxa. C. elegans DRE-1/FBXO11 functions in an SCF E3-ubiquitin ligase complex to regulate the transition to adult programs, but its cognate proteolytic substrates are unknown. Here we identify the conserved Zn-finger transcription factor BLMP-1 as a substrate of the SCFDRE-1/FBXO11 complex. blmp-1 loss-of-function suppressed dre-1 mutant phenotypes and exhibited developmental timing defects opposite to dre-1. blmp-1 also opposed dre-1 for other life history traits including entry into the dauer diapause and longevity. BLMP-1 protein was strikingly elevated upon dre-1 depletion and dysregulated in a stage- and tissue-specific manner. The role of DRE-1 in regulating BLMP-1 stability is evolutionary conserved, as we observed direct protein interaction and degradation function for worm and human counterparts. Taken together, post-translational regulation of BLMP-1/BLIMP-1 by DRE-1/FBXO11 coordinates C. elegans developmental timing and other life history traits, suggesting this two-protein-module mediates metazoan maturation processes.
Introduction
All metazoans develop through successive life stages and mature to reproductive adults. At the cellular level, temporal and positional fates must be precisely regulated to ensure coordination of proliferative, morphogenetic and differentiation events. The nematode C. elegans develops from embryo, through four larval stages, to reach reproductive maturity. Each larval stage is marked by ecdysis and characterized by virtually invariant patterns of stage-specific cellular events (Sulston and Horvitz, 1977).
Temporal selectors called heterochronic genes orchestrate temporal cellular fates during C. elegans larval development across tissues (Ambros and Horvitz, 1984). Mutations in the heterochronic loci result in precocious or retarded expression of stage-specific cellular events, which often manifest as a deletion or repetition of such programs. There are over 30 identified heterochronic loci which encode various transcriptional, translational and post-translational regulators (Resnick et al., 2010). Importantly, most heterochronic genes are highly conserved and have emerged as crucial regulators of proliferation, differentiation and stem cell dynamics in mammals (Rybak et al., 2009; Yu et al., 2007). For example, the RNA binding protein LIN28 has been implicated in precocious puberty in humans (Park et al., 2012), revealing remarkable conservation of function from the molecular to the organismal level.
C. elegans dre-1 was isolated in an enhancer screen for DAF-12/FXR/VDR redundant functions in gonadal heterochrony, as the double mutant gives rise to a synthetic gonadal migration defect in which the gonadal arms fail to turn on schedule (Fielenbach et al., 2007). On their own, dre-1 hypomorphic mutants also display heterochronic phenotypes in epidermal stem cells, called seams, causing precocious seam cell fusion and terminal differentiation a full stage earlier than normal (Fielenbach et al., 2007). dre-1 null mutants arrest after hatching or in the L1 stage often displaying molting defects.
F-box proteins function as the substrate-recognition components of SKP1-CUL1-F-box (SCF) E3-ubiquitin ligase complexes, thus conferring substrate specificity to the ubiquitin-proteasome system (UPS) (Skaar et al., 2013). C. elegans DRE-1 as well as its mammalian homolog FBXO11 are F-box proteins and have previously been shown to work in an SCF complex (Duan et al., 2012; Fielenbach et al., 2007). Interestingly, DRE-1/FBXO11 is one of only 6 F-box proteins highly conserved across all major taxa.
In mice, homozygous mutation of Fbxo11 results in facial clefting and perinatal lethality, as observed in Mutt and Jeff mutant mice, which were identified in ENU mutagenesis screens. Moreover, in mice and humans, Fbxo11 haploinsufficiency is linked to the onset of otitis media, an inflammation of the middle ear affecting children (Hardisty et al., 2003; Hardisty-Hughes et al., 2006; Segade et al., 2006). Two substrates of human FBXO11, BCL6 and CDT2, which promote lymphoma formation and delay cell-cycle exit respectively, have recently been identified (Abbas et al., 2013; Duan et al., 2012; Rossi et al., 2013). However, the substrates mediating developmental timing alterations in dre-1/Fbxo11 mutants have remained largely elusive.
To elucidate the mechanism of post-translational control of developmental timing by DRE-1/FBXO11, we sought to identify proteolytic targets by performing genetic suppressor screens in C. elegans. We discovered that the highly conserved Zn-finger transcriptional repressor BLMP-1/BLIMP-1 is regulated by the SCFDRE-1/FBXO11 complex via proteasome-dependent degradation. Our studies reveal that blmp-1 is a heterochronic gene, whose misregulation accounts for most developmental alterations observed in dre-1 mutants and demonstrate that DRE-1-dependent BLMP-1 regulation is conserved across taxa.
Results
blmp-1 and nhr-25 Suppress dre-1 Epidermal Heterochrony
Because DRE-1/FBXO11 acts as the substrate-recognition component of an SCF E3-ubiquitin ligase complex, we reasoned that dre-1 loss-of-function would lead to aberrant accumulation of client substrates. If so, then knockdown of such substrates should suppress the developmental timing defects seen in dre-1 mutants. We therefore performed an RNAi-based suppressor screen looking for amelioration of developmental timing defects in partial loss-of-function dre-1(dh99) mutants. We focused our screen initially on transcription factors as well as known heterochronic loci as candidates. We identified the Zn-finger transcription factor blmp-1 as a potent suppressor of dre-1 heterochronic phenotypes. The predicted C. elegans BLMP-1 protein harbors an N-terminal SET domain, a proline rich domain, and 5 paired-domain Zn-fingers at the C-terminus. An existing deletion allele tm548 lacks part of exon 3 and results in a frameshift that disrupts the SET domain and all subsequent Zn-fingers, suggesting it is a null allele. Mammalian BLIMP-1 is the major B-lymphocyte maturation factor, but also governs various cell fate decisions including primordial germ cell specification and skin differentiation, generally working as a transcriptional repressor (Horsley et al., 2006; Ohinata et al., 2005; Turner et al., 1994). Importantly, C. elegans BLMP-1 and mammalian BLIMP-1 show high domain conservation, with 43% similarity in the SET domain and 70% similarity in the Zn-fingers (Tunyaplin et al., 2000).
blmp-1 loss-of-function suppressed dre-1 phenotypes in multiple tissues, including epidermis and gonad. During normal terminal differentiation of the hypodermis, epidermal seam cells exit the cell cycle early in the L4 larval stage, fuse, and then synthesize a continuous ridged cuticular structure called adult alae (Figure 1A). In dre-1 partial loss-of-function mutants (e.g. dre-1(dh99)) seam cells undergo precocious terminal differentiation in L3, a full stage ahead of schedule (Fielenbach et al., 2007) (Figures 1A-C). Using the adherens junction marker ajm-1:gfp to mark seam cell boundaries, we observed that blmp-1 knockdown by RNAi reduced dre-1(dh99) precocious seam cell fusion from 90% to 15% of the animals (Figures 1B and C). Similarly only 7.5% of dre-1;blmp-1(tm548) double mutant animals showed seam cell fusion in L3 (Figure 1C). Notably, blmp-1 mutants themselves displayed heterochronic phenotypes: Although blmp-1 mutants had normal timing for seam cell division and fusion (Table S1), they exhibited incomplete adult alae synthesis, a phenotype typical of weak retarded mutants (Figures 1D and E). Incomplete alae formation was rescued by introducing a fosmid-based blmp-1::gfp translational fusion construct (Figure 1E), demonstrating that the phenotype arises from lesions in blmp-1. Moreover, in dre-1;blmp-1 double mutants, the incomplete alae formation phenotype of blmp-1 prevailed, placing blmp-1 genetically downstream (Figures 1A and E). Thus blmp-1 and dre-1 have opposite phenotypes in the seams, and dre-1 phenotypes depend on functional blmp-1(+).
Figure 1. blmp-1 Promotes Terminal Differentiation in the Hypodermis.
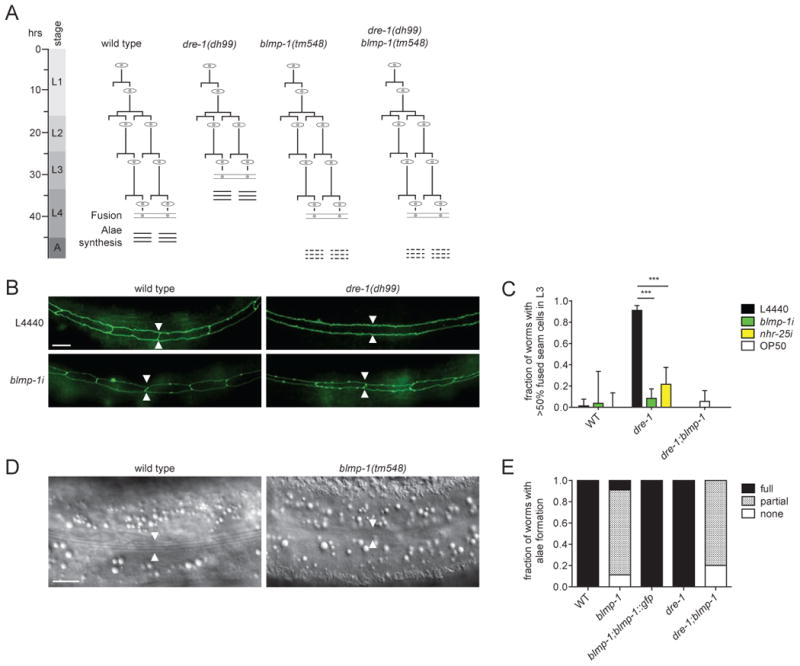
(A) Seam cell lineages from WT worms and various mutants as inferred from inspection. Terminal differentiation is indicated by fusion of the seams (eye-shaped cells) and adult alae formation (three horizontal bars). dre-1 mutants exhibit precocious seam cell terminal differentiation, whereas blmp-1 and the double mutants show incomplete formation of adult alae (three dashed horizontal bars). (B) Representative images of seam cell adherence junctions as visualized by ajm-1::gfp in WT and dre-1 L3 larvae grown on L4440 empty vector control or blmp-1 RNAi, respectively. Arrowheads indicate seam-seam boundaries. (C) blmp-1 and nhr-25 RNAi suppress dre-1 precocious seam cell fusion in L3 larvae. ***p<0.001 (Fisher's exact). Mean+95%CI (n>30). (D) Representative DIC images of young adult WT and blmp-1 mutant worms. Arrowheads indicate seam cell positions, the origin of adult alae synthesis. (E) blmp-1 and dre-1;blmp-1 mutants show poor formation of adult alae. The blmp-1 mutant phenotype is rescued by a blmp-1::gfp transgene. (n>20).
Scale bars = 10 μm. See also Table S1.
Another transcription factor identified in our screen was the homolog of mammalian steroidogenic-factor-1, called nhr-25, a nuclear hormone receptor implicated in C. elegans molting and epidermal developmental timing (Gissendanner and Sluder, 2000; Hada et al., 2010; Silhánková et al., 2005). Notably, nhr-25 depletion reportedly induces retarded seam cell fusion and prevents precocious adult alae formation (Hada et al., 2010). We observed that nhr-25 depletion also significantly suppressed dre-1 precocious seam cell fusion from 90% to 21.5% of the animals (Figure 1C). Thus, complete terminal differentiation of C. elegans epidermal stem cells depends on both blmp-1 and nhr-25.
blmp-1 Suppresses dre-1 Gonadal Heterochrony
We next asked whether blmp-1 and nhr-25 also influenced dre-1 developmental timing phenotypes in the gonad. In hermaphrodites, gonadal morphogenesis is led by two distal tip cells (dtcs), which migrate from midbody in opposite directions towards head and tail during L2. In L3, dtcs reflex dorsally and centripetally back towards the midbody to form two U-shaped gonadal arms in adult animals (Figure 2A). Whereas dre-1 mutants on their own show only a weak retarded gonadal migration phenotype, combinations with other heterochronic loci, such as daf-12/FXR/VDR or the lin-29/EGR null mutants, unveil a strong synthetic retarded phenotype in which both gonadal arms fail to reflex (Figures 2A-C) (Fielenbach et al., 2007). The unreflexed gonadal phenotype is interpreted as a reiteration of L2-programs, with concomitant prevention of L3-specific gonadal events.
Figure 2. blmp-1 Impedes Dorsal Migration of the Gonad.
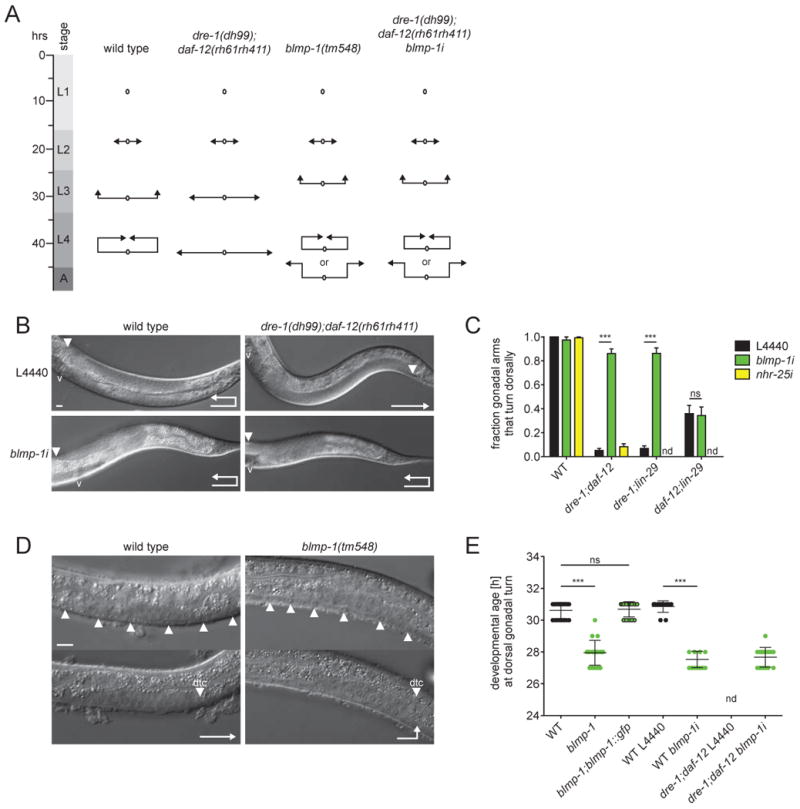
(A) Schematic representation of gonadal migration programs of WT worms and various mutants as inferred from inspection. Arrowheads indicate positions of the dtcs. (B) Representative DIC images of WT and dre-1;daf-12 young adult worms grown on L4440 empty vector control or blmp-1 RNAi, respectively, showing one gonadal arm. Arrowheads indicate the position of the dtc. v = vulva. (C) blmp-1 depletion specifically suppresses gonadal migration defects of dre-1;daf-12 and dre-1;lin-29(n546) mutants. ***p<0.001 (ANOVA). Mean+SEM (n=3, 40 gonadal arms each). (D) Representative DIC images of 27 h post-hatching WT and blmp-1 mutant larvae staged by the vulval precursor cells (arrowheads, upper panel). Dtcs migrate precociously in the blmp-1 mutant (arrowheads, lower panel). (E) In blmp-1 mutants, as well as WT and dre-1;daf-12 mutants grown on blmp-1 RNAi dtcs turn dorsally ahead of time. ***<0.001 (ANOVA). Mean±SD.
ns = not significant. nd = not determined. Scale bars = 10 μm.
Strikingly, depletion of blmp-1 suppressed the strong retarded phenotype of dre-1(dh99);daf-12(rh61rh411) mutants, restoring the dorsal turn in more than 80% of the animals (Figures 2B and C). In contrast, nhr-25 knockdown failed to suppress the gonadal migration defect (Figure 2C). blmp-1 depletion also restored gonadal reflection in dre-1;lin-29(n546) mutants, whereas gonadal migration defects in daf-12;lin-29 remained unaffected (Figure 2C). These observations reveal that blmp-1 specifically suppresses gonadal heterochronic phenotypes arising from dre-1, and not daf-12 or lin-29 mutation, suggesting that dre-1 and blmp-1 work in a unified pathway. Consistent with a role in gonadal developmental timing, blmp-1 mutants on their own showed precocious gonadal migration defects; dtcs navigated the first turn dorsally 2-3 hours ahead of schedule, but often failed the second turn and continued migration towards head and tail (Figures 2A, D and E). These phenotypes were rescued by the full-length blmp-1::gfp transgene (Figure 2E). Notably, the early gonadal migration defect prevailed in dre-1;daf-12 mutants grown on blmp-1 RNAi (Figures 2A and E), consistent with blmp-1 acting downstream of dre-1. Taken together these results show that dre-1/blmp-1 also function together to coordinate temporal patterning of gonadal outgrowth. Because blmp-1 and not nhr-25 suppressed dre-1 phenotypes in multiple tissues, we focused our further analyses on blmp-1.
blmp-1 Works Late in the Heterochronic Circuit
To further elucidate the role of blmp-1 in the seam cell heterochronic circuit, we first performed genetic epistasis experiments with precocious heterochronic loci that work at the larval-to-adult switch. Amongst them, hbl-1/Hunchback, lin-42/Period, lin-41/Trim71 and sop-2/PolycombG-like specify L4 programs by preventing lin-29/EGR expression and thus the adult fate (Abrahante et al., 2003; Cai et al., 2008; Rougvie and Ambros, 1995; Slack et al., 2000; Tennessen et al., 2006). Loss-of-function mutants typically show impenetrant precocious terminal differentiation, in which seam cells fuse and synthesize adult alae precociously in L3 larvae. Knockdown of blmp-1 variously suppressed precocious seam cell phenotypes of these mutants, suggesting it acts downstream or parallel to these components (Figures 3A-C and S1A). In particular, blmp-1 retarded phenotypes (incomplete alae formation) largely prevailed over hbl-1(ve18), lin-42(n1089) and lin-41i precocious phenotypes, possibly closely linking these genes in a pathway (Figures 3A, C and S1A). By comparison suppression of hypomorphic sop-2(bx91) phenotypes was relatively weak, suggesting blmp-1 might work upstream or in parallel to sop-2 (Figure 3A). We interpret these interactions with caution, since not all alleles used were null.
Figure 3. blmp-1 Interacts with Precocious and Retarded Heterochronic Loci.
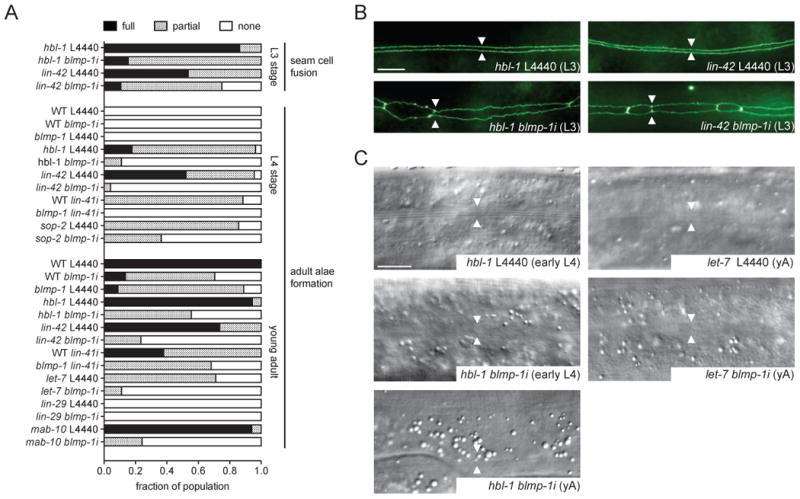
(A) Epistasis and synergy experiments of blmp-1(tm548)/blmp-1i with precocious (hbl-1(ve18), lin-41i, lin-42(n1089), sop-2(bx91)) and retarded (let-7(n2853ts), lin-29(n546), mab-10(e1248)) heterochronic loci (n>20). L4440 empty vector functions as control. Partial seam fusion refers to an incomplete seam syncytium with remaining unfused seam cells. Partial alae formation refers to poorly formed or incomplete adult alae. Temperature-sensitive alleles of sop-2 and let-7 were observed after shifting L1 larvae from 15°C to 23°C or 20°C, respectively. (B) Representative pictures of seam cell adherence junctions as visualized by ajm-1::gfp in hbl-1 and lin-42 L3 larvae grown on L4440 empty vector control or blmp-1 RNAi, respectively. Arrowheads indicate seam-seam boundaries. (C) Representative DIC images of hbl-1 and let-7 mutants showing adult alae formation under control (L4440) and blmp-1 depleted conditions at the early L4 or young adult (yA) stage. Arrowheads indicate positions of the seams.
Scale bars = 10 μm. See also Figure S1.
We next examined genetic interactions for synergy with various retarded heterochronic mutants working at the larval-to-adult transition. At this transition hbl-1 and lin-41 are downregulated post-transcriptionally by let-7/let-7-family microRNAs (Abbott et al., 2005; Abrahante et al., 2003; Slack et al., 2000). Consequentially lin-29/EGR is derepressed and triggers adult-specific cell fates. Thus let-7 and lin-29 loss-of-function cause retarded phenotypes: let-7 mutants display incomplete seam cell fusion and poor or no adult alae; in lin-29 mutants both events fail entirely. RNAi mediated knockdown of blmp-1 in temperature-sensitive let-7(n2853ts) mutants slightly enhanced the let-7 retarded adult alae formation and the bursting phenotype at a semi-permissive temperature (Figures 3A and C, data not shown). No enhancement was detected for lin-29(n546) as its retarded phenotypes are fully penetrant (Figures 3A and S1B). The transcriptional co-factor mab-10/NAB cooperates with lin-29/ZnF to specify the adult seam fate. Loss-of-function mutations of mab-10 do not affect seam cell fusion and adult alae formation, but subsets of seam cells undergo an extra nuclear division after they fuse into the seam syncytium (Harris and Horvitz, 2011). blmp-1 depletion enhanced the penetrance of the extra nuclear division phenotype of mab-10(e1248) (Figure S1C) and worms rarely formed adult alae (Figures 3A and S1B) suggesting that blmp-1 acts downstream or in parallel to mab-10. Altogether these analyses reveal a broad role for blmp-1 as a developmental timing gene promoting many aspects of seam cell terminal differentiation.
dre-1/blmp-1 Regulate C. elegans Life History
The interactions described above reveal that dre-1 and blmp-1 act in opposition for developmental timing events. We wondered whether this relationship also applied to other processes. Interestingly, we found that blmp-1 and dre-1 both function to regulate entry into the long-lived dauer stage, an alternate third larval stage triggered under harsh environmental conditions and food scarcity. In particular blmp-1 mutants were defective in dauer formation (Daf-d) while dre-1 mutants constitutively formed dauer larvae (Daf-c) albeit at low levels, suggesting the two genes normally promote and prevent dauer formation, respectively (Figure 4A). Consistent with our previous data, the blmp-1 mutant phenotype (Daf-d) prevailed in the double mutant (Figure 4A). blmp-1 depletion also suppressed canonical Daf-c loci, daf-2/InsR, daf-7/TGFβ, and daf-9/CYP27A1 (Figures 4B and C), placing blmp-1 downstream of insulin/IGF, TGFβ, and steroidal signaling at a late step in the dauer signaling pathways. In addition blmp-1 and dre-1 affected organismal life span. Knockdown of blmp-1 from L4 onwards shortened wild type (WT) lifespan, similar to previous reports (Samuelson et al., 2007), whereas dre-1 depletion resulted in a modest, but reproducible lifespan increase (Figure 4D). Therefore the two genes work in opposite ways for the life history traits of dauer formation and longevity.
Figure 4. dre-1 and blmp-1 Show Opposite Phenotypes in Dauer Formation and Lifespan.
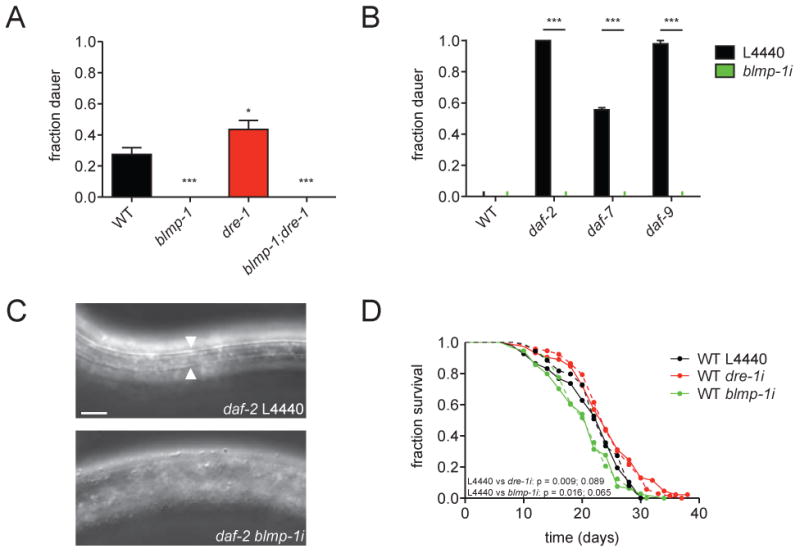
(A) Dauer formation of WT, dre-1(dh99), blmp-1(tm548) and dre-1(dh99);blmp-1(tm548) animals scored on plates lacking cholesterol at 27°C. *p<0.05, ***p<0.001 (ANOVA). Mean+SEM (n≥8, >100 worms each). (B) Dauer formation of WT, daf-2(e1368), daf-7(e1372) and daf-9(dh6) animals scored on regular NGM plates at 25°C under control (L4440) or blmp-1 depleted conditions . In the latter genotypes, blmp-1 depletion led to L3/L4 arrest without dauer alae and SDS sensitivity. ***p<0.001 (ANOVA). Mean+SEM (n=3, 15 worms each). (C) Representative DIC images of daf-2(e1368) mutants grown on L4440 empty vector control or blmp-1 RNAi at 25°C for 48 h. Arrowheads indicate dauer alae. Scale bar = 10 μm. (D) Lifespan analysis: WT animals were grown on RNAi from the L4 stage as indicated. Dashed lines represent an independent repeat of the experiment. First given p value (Mantel-Cox Log Rank method) corresponds to the experiment displayed with solid lines.
Just as blmp-1 loss suppressed dre-1 phenotypes, blmp-1 overexpression conversely further enhanced dre-1 phenotypes: First, the blmp-1 overexpressor evoked strong retarded gonadal migration defects when grown on dre-1 RNAi (Figure S2A). Second, it enhanced molting defects of dre-1 depleted worms (Figures S2B and C). Third, it was synthetic lethal in a dre-1(dh99) background, similar to dre-1 null mutants. In sum, our genetic data reveal intimate genetic interaction between dre-1 and blmp-1 for multiple processes.
The SCFDRE-1 Complex Degrades BLMP-1 via the Proteasome
The genetic epistasis experiments described above place blmp-1 downstream of dre-1, supporting the idea that BLMP-1 could be a substrate of the SCFDRE-1 E3-ubiquitin ligase. To directly test this hypothesis, we examined the effect of dre-1 depletion on BLMP-1 protein levels. BLMP-1::GFP showed overlapping expression with DRE-1::GFP in seam and hypodermal cells as well as the dtcs (Figure S3A, data not shown). Consistent with a role in BLMP-1 proteolysis, we found that knockdown of dre-1 resulted in a striking 7-8-fold increase in BLMP-1::GFP levels in seam and hypodermal cells (Figures 5A and B). Knockdown of other SCF complex components — the scaffold cul-1 and the skr-1 adaptor — also led to a substantial accumulation of BLMP-1::GFP, whereas knockdown of related molecules (cul-4, skr-2 and -3) had little or no effect (Figure 5B and S3B). In contrast NHR-25::GFP expression showed no obvious dre-1 dependence during L2 to L4 larval stages (Figure S3C). To test whether the UPS mediates BLMP-1 proteolysis, we inhibited the proteasome using the synthetic inhibitor bortezomib or rpn-8 RNAi, which depletes a subunit of the proteasomal 19S cap. Both treatments significantly increased BLMP-1::GFP levels in seam and hypodermis (Figures 5C, D and S3B).
Figure 5. DRE-1 Targets BLMP-1 for Proteasomal Degradation.
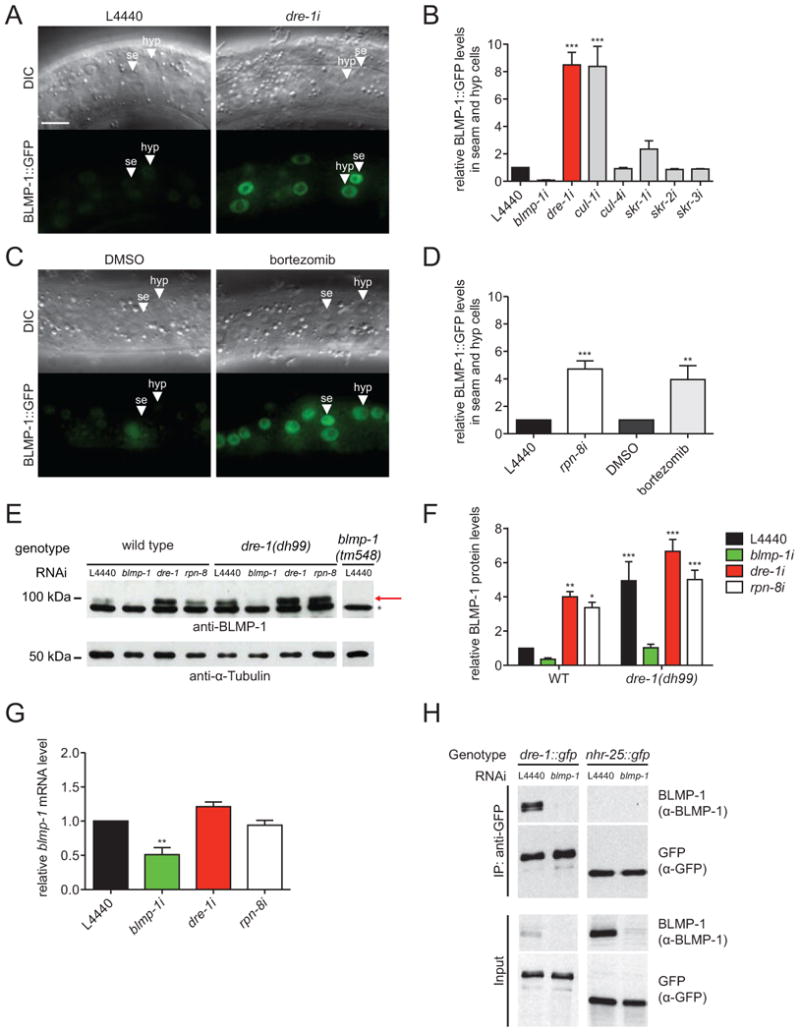
(A) Representative DIC and fluorescence images of BLMP-1::GFP expressing L4 larvae grown on L4440 empty vector control or dre-1 RNAi. (B) BLMP-1::GFP levels in seam and hypodermal cells are significantly increased upon dre-1 and cul-1 depletion. ***p<0.001 (ANOVA). Mean+SEM (n≥3, ≥10 cells of 5 worms each). (C) Representative DIC and fluorescence images of BLMP-1::GFP expressing L4 larvae treated with 100 μM bortezomib or DMSO control. (D) Proteasome inhibition (bortezomib and rpn-8 RNAi) increases BLMP-1::GFP levels in seam and hypodermal cells. **p<0.01, ***p<0.001 (ANOVA). Mean+SEM (n≥3, ≥10 cells of 5 worms each). (E) Western blot analysis of endogenous BLMP-1 protein levels in WT, dre-1 and blmp-1 L4 larvae after various RNAi treatments from egg stage onwards. The asterisk marks a nonspecific band. The arrow points to the BLMP-1 band. (F) Quantification of Western blot analyses. *P<0.05, **P<0.01, ***P<0.001 (ANOVA). Mean +SD (n=5). (G) qPCR analysis of WT L4 larvae treated with the indicated RNAis starting at the egg stage. **p<0.01 (ANOVA). Mean+SEM (n=3). (H) L3 larvae expressing integrated DRE-1::GFP or NHR-25::GFP as control were grown from egg stage onwards on L4440 empty vector control or blmp-1 RNAi expressing bacteria, respectively and utilized for anti-GFP immunoprecipitation (IP). Lysates and IP were analyzed by immunoblotting. Detected sizes: BLMP-1: ≈ 98 kDa, DRE-1::GFP: ≈ 145 kDa and NHR-25::GFP: ≈ 120 kDa
se = seam cell. hyp = hypodermal cell. Scale bars = 10 μm. See also Figures S2 and S3.
We next analyzed endogenous BLMP-1 levels by Western blotting using antibodies to visualize the native protein. In accord with our blmp-1::gfp transgene results, dre-1 loss-of-function and proteasome inhibition by RNAi both resulted in a significant increase in endogenous BLMP-1 protein (Figures 5E and F). Whereas BLMP-1 levels were strongly elevated in a dre-1(dh99) mutant background, blmp-1 mRNA levels were unchanged upon dre-1 depletion or proteasome inhibition (Figures 5E-G). These results further support the notion that the SCFDRE-1 complex post-transcriptionally regulates BLMP-1 through proteasome-mediated degradation.
If BLMP-1 were a direct substrate of DRE-1, then the two proteins would be predicted to physically interact. Therefore we performed co-immunoprecipitation experiments in worms expressing DRE-1::GFP under its native promoter. We found that immunoprecipitation of DRE-1::GFP specifically co-precipitated a small fraction of endogenous BLMP-1 (< 1% of input), whereas a NHR-25::GFP control did not (Figure 5H). When expressed in HEK293T cells under proteasome inhibition FLAG-HA-tagged DRE-1 specifically co-precipitated with 6xHis-tagged C. elegans BLMP-1, but not the closely related Zn-finger protein LIN-29 (Figure S3D). However, we could not detect ubiquitylation of C. elegans BLMP-1 in our assays (data not shown), but such species might be of low abundance or unstable in vivo, and difficult to ubiquitylate in a heterologous system in vitro.
The DRE-1/BLMP-1 Interaction is Conserved to Mammals
Importantly, the association of BLMP-1 with DRE-1 is evolutionarily conserved. First, like the worm proteins, human BLIMP-1 specifically co-precipitated with human FBXO11 when co-expressed in cultured HEK293T cells treated with proteasome inhibitor (Figures 6A and S4A). BLIMP-1 also weakly interacted with the closely related FBXO10, but not other controls (Figures 6A). Interestingly, the ability to bind BLIMP-1 was substantially reduced when the Q491L amino acid substitution identified in the Jeff mouse mutant (Hardisty-Hughes et al., 2006) was introduced into human Fbxo11, suggesting that the conserved region containing the point mutation may facilitate interaction (Figure 6A). Second, we found that immunopurified FBXO11 promoted BLIMP-1 ubiquitylation in vitro, dependent upon a functional F-box domain, which tethers FBXO11 to the SCF complex: F-box domain mutation (F-boxMUT/V98A, C99A, F102A) abolished binding to the SKP1 adaptor protein and, consistently, suppressed ubiquitylated BLIMP-1 higher molecular weight species (Figure 6B). Third, cycloheximide (CHX) treated cells showed progressive proteolysis of worm or human BLMP-1/BLIMP-1 upon co-transfection of DRE-1/FBXO11, which strongly depended on a functional proteasome (Figures 6C and S4B), Rates of human BLIMP-1 proteolysis were significantly dampened by deletion of the FBXO11 F-box domain (ΔF-box) or by introducing the amino acid substitution FBXO11/Q491L (Figure 6C). Furthermore, inclusion of a deletion mutant, dominant negative form of the SCF scaffold CUL1, but not other dominant negative cullins, prevented FBXO11-dependent BLIMP-1 degradation (Figures S4C and D). In accord with a low BLIMP-1 binding affinity, FBXO10 overexpression slightly destabilized BLIMP-1 after CHX treatment (Figure 6C), indicating that FBXO10 might also harbor a minor capability to target BLIMP-1. Finally, in agreement with accelerated BLIMP-1 degradation we found that short hairpin RNA (shRNA)-mediated depletion of FBXO11 in ARP1 multiple myeloma cells using two different shRNAs, led to stabilization of endogenous BLIMP-1 in the presence of CHX (Figure 6D). Altogether these experiments demonstrate that SCFFBXO11 mediates ubiquitin-mediated degradation of BLIMP-1, providing strong evidence for conserved proteolytic regulation across taxa.
Figure 6. FBXO11 Directly Interacts with BLIMP-1 and Triggers its Degradation.
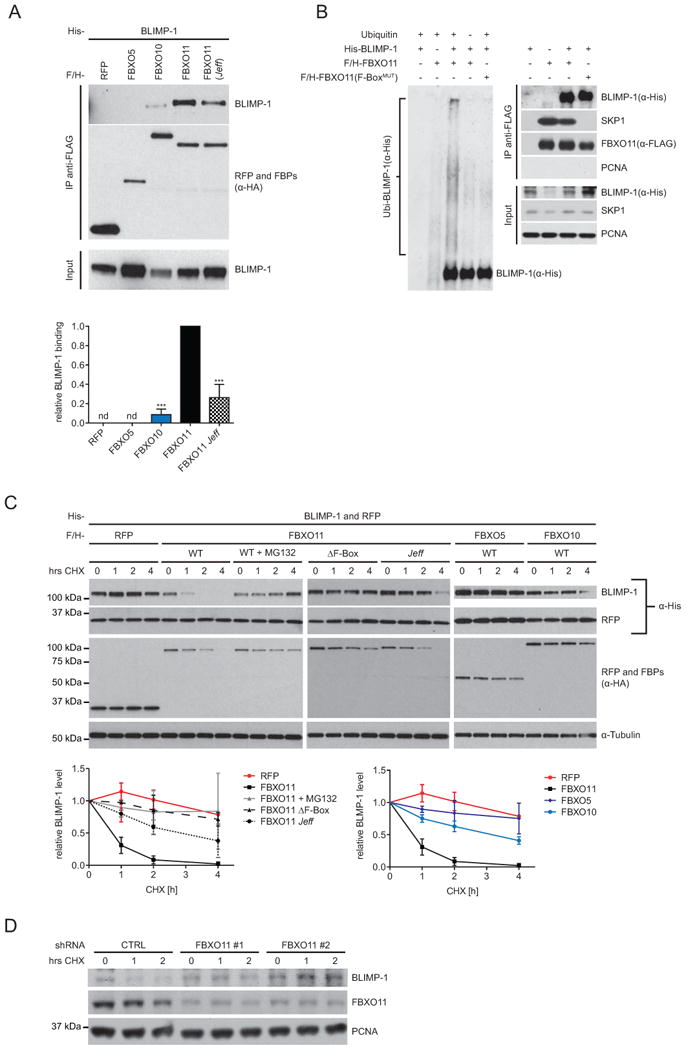
(A) anti-FLAG IP of human FLAG-HA-tagged (F/H) RFP, -FBXO5, -FBXO10, -FBXO11 and -FBXO1 1(Q491L/Jeff) from HEK293T cells treated with 15 μM MG132. Human His-BLIMP-1 was co-expressed. Detected protein sizes: see panel C. Quantification of RFP/F-box-protein binding intensity to BLIMP-1 was normalized to BLIMP-1 input and FBXO1 1-BLIMP-1 association. ***p<0.001 (ANOVA). Mean+SD (n=5). nd = not detectable. (B) HEK-293T cells were transfected with His-tagged BLIMP-1, FLAG-tagged FBXO11 or -FBXO11(F-boxMUT), and an empty vector as indicated. After immunopurification with anti-FLAG resin, in vitro ubiquitylation of BLIMP-1 was performed in the presence of E1 and E2s. Where indicated, ubiquitin was added. Samples were analyzed by immunoblotting (left panel). Immunoblots of whole-cell extracts and immunoprecipitations are shown in the right panel. Detected size SKP1 ≈ 19kDA, other proteins as indicated in panels C and D. (C) Human BLIMP-1 stability was assessed in HEK293T cells transfected with His-BLIMP-1 and -RFP in combination with either F/H-tagged RFP, -FBXO11, -FBXO11 (Δ F-box), -FBXO1 1(Q491L/Jeff), -FBXO5 or –FBXO10. Cells were treated with cycloheximide (CHX) or CHX ± 15μM MG132 for 0, 1, 2 or 4 hrs prior to harvesting and analysis by immunoblotting. Human BLIMP-1 stability was quantified from ≥3 independent experiments and normalized to co-expressed His-RFP. Mean+SD (n≥3). (D) Stability of endogenous BLIMP-1 was analyzed in ARP1 multiple myeloma cells infected with either viruses expressing two different FBXO11 shRNAs or an empty virus (CTRL), and selected for 72 hours. Cells were then treated with CHX for the indicated times and protein extracts were analyzed by immunoblotting. Detected sizes: BLIMP-1 ≈ 90 kDA, FBXO11 ≈ 125 kDA.
See also Figure S4.
BLMP-1 is Dynamically Regulated in a Tissue-Specific Manner
Having established BLMP-1 as a DRE-1 substrate for proteolysis, we sought to better understand the dynamics of regulation in vivo. The genetic studies in C. elegans described above reveal that blmp-1 acts at an intermediate step in dtc migration, but promotes terminal differentiation in the hypodermis. To clarify the tissue-specific regulation of developmental timing by DRE-1/BLMP-1, we followed BLMP-1::GFP levels in these tissues throughout larval development.
In the dtcs BLMP-1::GFP was expressed from mid-L2, when gonadal outgrowth initiates towards head and tail. By mid-L3 (28-30 h developmental age (DA)), just prior to the dorsal gonadal turn BLMP-1::GFP levels in the dtcs dropped dramatically (Figures 7A and B). Correlatively DRE-1::GFP levels in the dtcs increased steadily from L2 to L4 (Figure S5A). In contrast, when worms were grown on dre-1 RNAi, BLMP-1::GFP levels persisted to later ages and the dtcs frequently failed to turn dorsally (Figures 7A, B and S2A). Our data therefore strongly suggest that timely DRE-1-mediated BLMP-1 degradation permits the gonadal turn, while high BLMP-1 levels in gonadal dtcs impede this event (Figure 7D).
Figure 7. BLMP-1::GFP Levels are Dynamically Regulated by DRE-1.
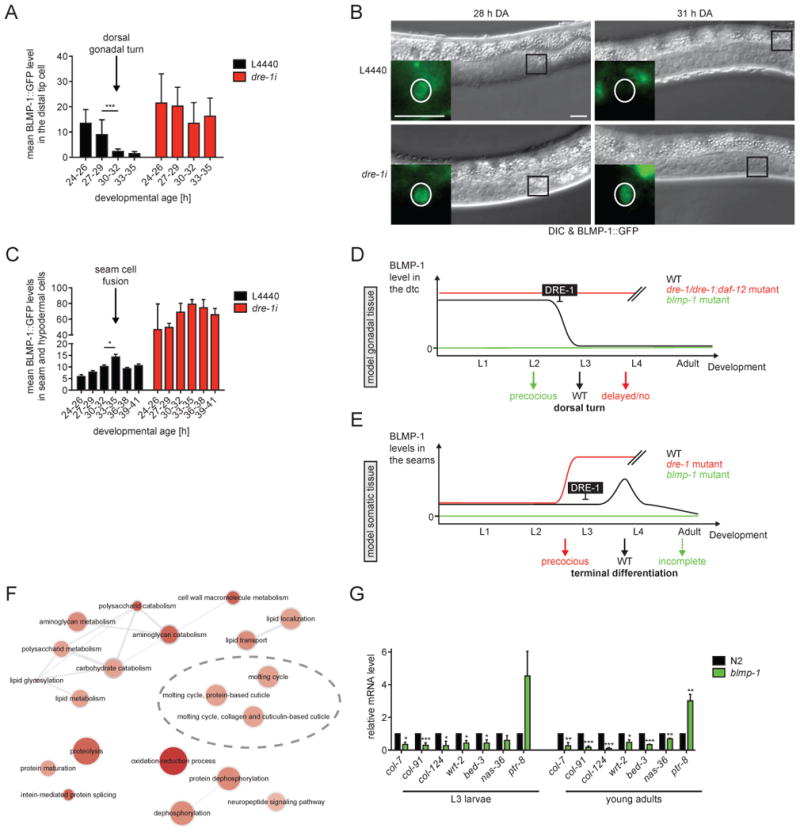
(A) BLMP-1::GFP levels in the dtcs were assessed in ≥5 worms per grouped DA (developmental age). ***p<0.001 (ANOVA). Mean+SD. (B) Representative DIC images of age-matched L3 larvae with zoom in on BLMP-1::GFP expression in the dtcs. Black box indicates the area enlarged in the fluorescence image. White circle roughly marks the dtc nucleus. Scale bars = 10 μm. (C) BLMP-1::GFP levels in seam and hypodermal cells were assessed in ≥5 worms per grouped DA. *p<0.05 (ANOVA). Mean+SEM. (D and E) Model for dre-1/blmp-1 action in (D) gonadal tissue: BLMP-1 is downregulated in a dre-1-dependent manner in mid-L3 to allow the dorsal gonadal turn. In dre-1 or dre-1/daf-12 mutants BLMP-1 levels persist to later ages, leading to retarded migration defects, whereas the dorsal turn is preceded in the absence of BLMP-1. (E) somatic tissue: Elevated BLMP-1 levels contribute to seam cell terminal differentiation, leading to precocious terminal differentiation in dre-1 mutants and consequentially impaired terminal differentiation in the absence of BLMP-1. Double-slashes indicate the end of data assessment. (F) Summary of gene-ontology/biological processes derived from DAVID analysis (Huang et al., 2009) of differentially regulated genes (q<0.05, fold change >±1.5) in WT vs blmp-1 mutant animals from RNA-Seq datasets (n=3). ReviGO was used for visualization of the resulting GO term classes and its semantic similarity (Supek et al., 2011). Bubble color, size, and line widths respectively indicate significance of enrichment, frequency of GO term in underlying data sets, and degree of similarity. More intense colors indicate a higher significance of enrichment. (G) qPCR analysis of blmp-1-regulated molting and cuticle-related genes in L3 larvae and young adult worms. *P<0.05, **P<0.01, ***P<0.001 (t-test). Mean+SEM (n≥3).
See also Figure S5.
Surprisingly, in seam and hypodermal cells BLMP-1::GFP levels were largely constant throughout larval development except for a transient peak early in L4, which coincides with the time of seam cell fusion (Figure 7C), suggesting this BLMP-1 pulse could trigger the fusion event. Consistent with this, dre-1 knockdown provoked a strong increase of seam and hypodermal BLMP-1::GFP expression earlier in development (Figure 7C), resulting in seam fusion as early as 28 h of DA during the L3 stage. These observations are consistent with a model in which increased BLMP-1 levels initiate terminal differentiation events in the seams (Figure 7E). Unexpectedly, DRE-1::GFP levels in seam and hypodermis did not obviously change during this time (Figure S5B), implying that other instructive components or co-factors (e.g. a kinase or phosphatase) may contribute towards BLMP-1 regulation. Altogether our data suggest that DRE-1 maintains a low steady state level of BLMP-1 to prevent precocious terminal differentiation in the epidermis (Figure 7E).
blmp-1 Expression Profiles
To identify downstream effectors regulated by the transcription factor blmp-1 we performed RNA-seq analysis of blmp-1 mutant and WT animals harvested at the mid-L3 stage. We identified 325 significantly up- and 303 downregulated genes (≥1.5-fold difference from WT, q-value<0.05) (Table S2). Differentially regulated genes included those enriched in the biological processes of molting and cuticle development, sugar and aminosugar metabolism, and protein modification (Figure 7F). The molting and cuticle-related regulated genes we included ptr-8, wrt-2, bed-3, nas-36 and collagens (e.g. col-7, col-91 and col-124). blmp-1-dependent regulation of these genes was further confirmed by qPCR in L3 larvae and young adult animals (Figure 7G). These data support the idea that blmp-1 is a key regulator of C. elegans epidermal terminal differentiation.
Discussion
In this study we discovered that the highly conserved SCFDRE-1/FBXO11 E3-ubiquitin ligase complex targets the Zn-finger transcriptional repressor BLMP-1 as a substrate. DRE-1 and BLMP-1 work as a functional module to regulate not only C. elegans developmental timing, but also the processes of dauer formation, molting and longevity, revealing a global role in life history regulation. Remarkably, we find the DRE-1/BLMP-1 molecular interaction to be conserved in human cells suggesting that FBXO11 and BLIMP-1 could act together to regulate related processes in mammals.
Multiple lines of evidence argue that BLMP-1/BLIMP-1 is an endogenous substrate degraded by the SCFDRE-1/FBXO11 complex. First, BLMP-1 and DRE-1 co-immunoprecipitate in vivo from C. elegans and when expressed in cell culture, as do the human counterparts. Second, mammalian FBXO11 ubiquitylates BLIMP-1 in vitro. Third, BLMP-1 protein levels strikingly depend on the SCFDRE-1 complex and the proteasome. Loss of DRE-1 and other components of the SCF complex, as well as proteasome inhibition result in elevated endogenous BLMP-1 or BLMP-1::GFP levels in C. elegans. Conversely DRE-1 overexpression in cell culture enhances BLMP-1 degradation. This molecular function is conserved since mammalian FBXO11 degrades BLIMP-1 when expressed in cultured cells and shRNA mediated depletion of FBOX11 in the ARP1 multiple myeloma cell line leads to stabilization of endogenous BLIMP-1. Finally, blmp-1 depletion strongly suppresses dre-1 heterochronic phenotypes in seam and gonadal tissue. This strict epistasis indicates that dre-1 acts through blmp-1 in a regulatory pathway, and suggest that dre-1 phenotypes arise largely from elevated BLMP-1 activity. In accord with this, blmp-1 overexpression exacerbates dre-1 phenotypes. Taken together our results demonstrate that BLMP-1 is a substrate of the SCFDRE-1 complex, and that this molecular activity is evolutionarily conserved. This study therefore provides critical evidence for BLMP-1/BLIMP-1 being specifically targeted for proteasomal degradation.
DRE-1 and BLMP-1 work as a module to regulate several aspects of C. elegans life history. In particular, our studies establish dre-1/blmp-1 as key regulators of developmental timing within the heterochronic circuit during late larval development. Our previous work demonstrated that dre-1 loss-of-function induces precocious seam cell fusion and synthetic retarded gonadal migration defects. Here we discovered blmp-1 depletion to efficiently suppress both phenotypes. On its own, blmp-1 mutation leads to phenotypes opposite to dre-1, namely retarded terminal differentiation of the seam cells (e.g. poor alae formation) on the one hand and precocious migration of gonadal dtcs on the other hand. These findings imply that blmp-1 like its mammalian counterpart mediates terminal differentiation in some tissues, but also controls intermediate stages of differentiation in others, presumably by regulating distinct targets. Importantly blmp-1 genetically interacts with multiple heterochronic loci that govern the larval-to-adult transition. blmp-1 loss-of-function enhances retarded (let-7, mab-10/NAB) and suppresses precocious (lin-41/Trim71, lin-42/Period, hbl-1/Hunchback, sop-2/PolycombG-like) heterochronic mutants for phenotypes in the epidermal seam cells. These interactions firmly place blmp-1 within the heterochronic circuit controlling late larval development. Understanding how these genetic interactions translate into molecular regulatory events should be interesting to dissect in the future.
Many of the C. elegans heterochronic genes governing seam cell development have counterparts in mammals regulating skin stem cell homeostasis including blmp-1/BLIMP-1 itself, daf-12/FXR/VDR, lin-4/mir-125, let-7, lin-42/Period, and others (Cianferotti et al., 2007; Horsley et al., 2006; Janich et al., 2011; Magnúsdóttir et al., 2007; Zhang et al., 2011). In particular BLIMP-1 is required for terminal differentiation of the skin specifying the transition from granule to cornified layers in keratinocytes and stem cell lineages in the sebaceous gland (Horsley et al., 2006; Magnúsdóttir et al., 2007). Therein BLIMP-1 and the vitamin D receptor were shown to be downregulated by mir-125 to prevent skin stem cell differentiation (Zhang et al., 2011). Our data show that C. elegans blmp-1 specifies terminal differentiation in the epidermis and indicate that this regulation could stem from blmp-1-dependent transcriptional changes in molting and cuticle synthesis genes. Moreover dauer epistasis analyses place blmp-1 at a late step in dauer morphogenesis pathways proximal to daf-12/FXR/VDR, bearing remarkable similarity to the mammalian situation in the skin. Interestingly FBXO11 too is expressed in the skin (Hardisty-Hughes et al., 2006), suggesting a possible role for FBXO11 in BLIMP-1-dependent regulation of skin maturation.
Our studies also reveal that DRE-1/BLMP-1 affect organism-wide fates including the entry into the dauer diapause, molting and adult longevity. Moreover, dre-1 was shown to function in male gonadal linker cell engulfment and blmp-1 in male tail retraction (Chiorazzi et al., 2013; Nelson et al., 2011). Although much is known about vertebrate BLIMP-1, the rich signaling circuits presiding over C. elegans developmental timing, dauer signaling, molting, and life span might further inform mammalian stem cell progression and quiescence. In this view, it could be argued that the FBXO11/BLIMP-1 module not only acts as fate switch regulating cellular differentiation, but perhaps more broadly functions as part of a developmental timer across tissues and species.
Indeed the notion that DRE-1/BLMP-1 comprise a conserved functional module may have several implications in mammals. Among other things, BLIMP-1 is a key regulator in primordial germ cell specification (Ohinata et al., 2005; 2009; Vincent et al., 2005), forelimb development, pharyngeal and heart morphogenesis (Vincent et al., 2005), T cell differentiation (Martins and Calame, 2008), pluripotency and reprogramming (Nagamatsu et al., 2011). By inference, FBXO11 could work in tandem with BLIMP-1 in at least some of these contexts by controlling its turnover.
Despite its high conservation, relatively little is known about the biological function of mammalian FBXO11. Its loss-of-function is associated with chronic otitis media, facial clefting and other developmental abnormalities (Hardisty et al., 2003; Hardisty-Hughes et al., 2006; Segade et al., 2006). Our studies suggest these phenotypes could in some measure result from aberrantly elevated BLIMP-1 levels, since FBXO11-dependent BLIMP-1 degradation is significantly impaired by introducing the FBXO11 Jeff mutation, which affects a highly conserved residue. FBXO11 is also known to play a critical role in tumorigenesis in the immune system. Duan et al. recently showed FBXO11 to target BCL6 for proteasomal degradation and FBXO11 inactivation results in aberrantly elevated BCL6 levels in diffuse large B-cell lymphomas (Duan et al., 2012). Zn-finger proteins BLIMP-1 and BCL6 are reciprocally antagonistic transcription factors that serve as a self-reinforcing switch to determine B- and T- cell fates (Crotty et al., 2010). Specifically, BLIMP-1 promotes terminal differentiation of B-plasma cells, while BCL6 functions in the maintenance of germinal center B-cells. Blimp-1 inactivation is also associated with the onset of B-Cell-like diffuse large B-cell lymphomas (Mandelbaum et al., 2010). Intriguingly, FBXO11 thus targets both BLIMP-1 (this work) and BCL6 for proteolytic degradation, but how their regulation is coordinated within normal immune lineages and in tumorigenesis is not yet understood. The closely related FBXO10 has also been implicated in degrading BCL2 (Chiorazzi et al., 2013) suggesting a general role for FBXO10/11 complexes in immune function. It is noteworthy that immune development, too, employs many homologs of developmental timing genes including lin-4, LIN28 and let-7 modules (O'Connell et al., 2010; Yuan et al., 2012).
Taken together, we discovered BLMP-1 as a substrate of the SCFDRE-1 complex, modulating developmental timing and other life history traits in C. elegans. Given the remarkable degree of structural and functional conservation of these two proteins, we hypothesize that the FBXO11/BLIMP-1 module could regulate metazoan cellular fate decisions across tissues.
Experimental Procedures
C. elegans Strains, RNAi and Treatments
C. elegans strains were grown under standard conditions at 20°C unless otherwise noted (Brenner, 1974). Strains used are listed in the Extended Experimental Procedures. RNAi treatment was performed as described (Kamath and Ahringer, 2003). In general, L4 larvae were moved to corresponding RNAi plates. RNAi clones were available from the Ahringer collection, except for dre-1 RNAi, which we described previously (Fielenbach et al., 2007). Proteasome inhibition was achieved by transferring L3 larvae to a plate containing 100 μM bortezomib (in DMSO) on top of the bacteria lawn, for 6 h.
Microscopy and Phenotype Analysis
Heterochronic phenotypes were analyzed at specific larval stages as assessed by gonad morphology and vulva formation. Nomarski DIC microscopy was used to score seam cell number (scm-1::gfp marker), seam cell fusion (ajm-1::gfp marker) and adult alae formation, each on one side of individual animals. Worms were anesthetized with 1 mM levamisole. Fluorescence microscopy was performed on a Carl Zeiss Axio Imager Z1. GFP intensity in seam/hypodermal cells was leveled from 5-10 cells per animal, whereas for measurements in the dtc, one dtc per animal was analyzed. Fluorescence intensity was determined using the ImageJ software (http://rsbweb.nih.gov/ij/).
Dauer and Lifespan Analysis
For dauer assays worms were synchronized by egg-lay on OP50 or HT115 bacteria and scored after 48 h (27°C) or 60 h (25°C). Lifespan assays were performed at 20°C. Worms were synchronized by egg-lay and transferred to RNAi plates at the L4 stage. Worms undergoing internal hatching, bursting vulva or crawling off the plates were censored.
Antibodies
The following antibodies were used in this study: BLMP-1 (rb, Novus Bio), GFP (ms, Clontech), α-Tubulin (ms, Sigma), HA (rat, Roche), His (ms, Qiagen and rb, Santa Cruz), FLAG (ms and rb, SIGMA), PCNA (ms, Invitrogen), BLIMP-1 (rb, ab59369, Abcam and ms, 3H2-E8, Novus Bio), FBXO11 (rb, NB100-59826, Novus Bio), and SKP1 (Pagano lab).
C. elegans Biochemistry
For Western blot analysis synchronized L4 larvae were lysed in SDS sample buffer and equal volumes were applied to SDS-PAGE and immunblotting. Band intensity was quantified with Adobe Photoshop.
For Immunoprecipitation (IP) assays eggs were isolated by bleach treatment. After a 3 h hatching-period L1 larvae were washed off with M9 buffer and transferred to RNAi plates. L3 larvae were lysed in worm IP buffer (50 mM Tris/HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, protease and phosphatase inhibitor cocktail (Roche)) using a dounce homogenizer. Worm lysates were incubated with 40 μl GFP-Trap agarose beads (Chromotek) for 1 h at 4°C and washed 5x with worm washing buffer (as worm IP buffer, but 300 mM NaCl and 0.75% NP-40). Precipitants were eluted with SDS sample buffer and analyzed by SDS-PAGE and immunoblotting.
Cell Culture and IP Experiments
HEK293T cells were maintained in DMEM supplemented with 10% fetal bovine serum (Gibco). Transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instruction. Plasmid construction is described in the Extended Experimental Procedures. For IP experiments (not prior to ubiquitylation assay), cells were treated with 15 μM MG132 or DMSO control for 4 h prior to harvesting. Cells were lysed 20 h post-transfection in lysis buffer (50 mM Tris/HCl pH 7.4, 120 mM NaCl, 0.5% NP-40, protease and phosphatase inhibitor cocktail (Roche)) and subsequently incubated with anti-FLAG-M2 resin (Sigma). Precipitated proteins were eluted by competition with 3xFLAG peptide (Sigma) and analyzed by immunoblotting. To study BLIMP-1 turnover, transfected cells were treated with 100 μg/ml cycloheximide (AppliChem) for the indicated time intervals 20 h post-transfection. Cells were lysed in lysis buffer and analyzed by SDS-PAGE and immunoblotting. Whole cell lysates and CHX treatment in ARP1 cells was performed as described (Duan et al., 2012).
Gene silencing by shRNAs
Sequences of FBXO11 shRNAs were as follows:. #1: GAGTTTACATCTTTGGTGA, #2: CAATTGTTCGGCATAACAA. shRNA lentiviruses were produced as described (Phan et al., 2007). ARP1 multiple myeloma cells were infected by re-suspension in virus-containing supernatants and centrifugation. 24 h after infection, cells were re-suspended in fresh medium and selected with puromycin.
In vitro ubiquitylation
HEK293T cells were co-transfected with His-BLIMP-1 and FLAG-FBXO11 or -FBXO11(F-boxMUT) and lysed using isotonic NP40 buffer with Turbonuclease (Accelagen). Lysates were precleared with 0.40 μm filters and FLAG-tagged complexes were isolated using anti-FLAG-M2 resin (Sigma). Complexes were eluted by competition with 3xFLAG peptide (Sigma). In vitro ubiquitylation assays were performed in a volume of 30 μl containing 0.1 μM UBE1 (Boston Biochem), 10 ng/ml Ubch3, 10 ng/ml Ubch5c, 1 μM ubiquitin aldehyde, ±2.5 μg/μl ubiquitin (Sigma), and either SCF FBXO11 or SCFFBXO11Fbox-MUT complex in a ubiquitylation buffer (50 mM Tris [pH 7.6], 2 mM ATP, 5 mM MgCl2, 0.6 mM DTT, okadaic acid 0.1 mM) for 45 min at 37°C.
Statistical Analysis
Statistical analyses were performed as indicated using GraphPad Prism (GraphPad software).
Supplementary Material
Acknowledgments
We would like to thank members of the Antebi lab for scientific discussion and review of the manuscript and the C. elegans Genetic Center (CGC) at the University of Minnesota as well as the Japanese National Bioresource Project Knockout Consortium for strains. This work was supported by the DFG (SFB635) (CG, AA), the Max Planck Society (AA), CECAD/DFG (MH), and the National Institutes of Health (R01-GM057587, R37-CA076584, R21-CA161108, and R03 TW009040) (MP). MP is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas T, Mueller AC, Shibata E, Keaton M, Rossi M, Dutta A. CRL1-FBXO11 promotes Cdt2 ubiquitylation and degradation and regulates Pr-Set7/Set8-mediated cellular migration. Mol Cell. 2013;49:1147–1158. doi: 10.1016/j.molcel.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, Ambros V. The let-7 MicroRNA Family Members mir-48, mir-84, and mir-241 Function Together to Regulate Developmental Timing in Caenorhabditis elegans. Dev Cell. 2005;9:403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahante JE, Daul AL, Li M, Volk ML, Tennessen JM, Miller EA, Rougvie AE. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev Cell. 2003;4:625–637. doi: 10.1016/s1534-5807(03)00127-8. [DOI] [PubMed] [Google Scholar]
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science. 1984;226:409–416. doi: 10.1126/science.6494891. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Sun Y, Huang X, Guo C, Zhang Y, Zhu Z, Zhang H. The Caenorhabditis elegans PcG-like gene sop-2 regulates the temporal and sexual specificities of cell fates. Genetics. 2008;178:1445–1456. doi: 10.1534/genetics.108.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi M, Rui L, Yang Y, Ceribelli M, Tishbi N, Maurer CW, Ranuncolo SM, Zhao H, Xu W, Chan WCC, et al. Related F-box proteins control cell death in Caenorhabditis elegans and human lymphoma. Proc Natl Acad Sci USA. 2013;110:3943–3948. doi: 10.1073/pnas.1217271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan SS, Cermak LL, Pagan JKJ, Rossi MM, Martinengo CC, di Celle PFP, Chapuy BB, Shipp MM, Chiarle RR, Pagano MM. FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature. 2012;481:90–93. doi: 10.1038/nature10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N, Guardavaccaro D, Neubert K, Chan T, Li D, Feng Q, Hutter H, Pagano M, Antebi A. DRE-1: An Evolutionarily Conserved F Box Protein that Regulates C. elegans Developmental Age. Dev Cell. 2007;12:443–455. doi: 10.1016/j.devcel.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Gissendanner CR, Sluder AE. nhr-25, the Caenorhabditis elegans ortholog of ftz-f1, is required for epidermal and somatic gonad development. Dev Biol. 2000;221:259–272. doi: 10.1006/dbio.2000.9679. [DOI] [PubMed] [Google Scholar]
- Hada K, Asahina M, Hasegawa H, Kanaho Y, Slack FJ, Niwa R. The nuclear receptor gene nhr-25 plays multiple roles in the Caenorhabditis elegans heterochronic gene network to control the larva-to-adult transition. Dev Biol. 2010;344:1100–1109. doi: 10.1016/j.ydbio.2010.05.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardisty RE, Erven A, Logan K, Morse S, Guionaud S, Sancho-Oliver S, Hunter AG, Brown SDM, Steel KP. The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J Assoc Res Otolaryngol. 2003;4:130–138. doi: 10.1007/s10162-002-3015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardisty-Hughes RE, Tateossian H, Morse SA, Romero MR, Middleton A, Tymowska-Lalanne Z, Hunter AJ, Cheeseman M, Brown SDM. A mutation in the F-box gene, Fbxo11 causes otitis media in the Jeff mouse. Hum Mol Genet. 2006;15:3273–3279. doi: 10.1093/hmg/ddl403. [DOI] [PubMed] [Google Scholar]
- Harris DT, Horvitz HR. MAB-10/NAB acts with LIN-29/EGR to regulate terminal differentiation and the transition from larva to adult in C. elegans. Development. 2011;138:4051–4062. doi: 10.1242/dev.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Janich P, Pascual G, Merlos-Suárez A, Batlle E, Ripperger J, Albrecht U, Cheng HYM, Obrietan K, Di Croce L, Benitah SA. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480:209–214. doi: 10.1038/nature10649. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Magnúsdóttir E, Kalachikov S, Mizukoshi K, Savitsky D, Ishida-Yamamoto A, Panteleyev AA, Calame K. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc Natl Acad Sci USA. 2007;104:14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum J, Bhagat G, Tang H, Mo T, Brahmachary M, Shen Q, Chadburn A, Rajewsky K, Tarakhovsky A, Pasqualucci L, et al. BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer Cell. 2010;18:568–579. doi: 10.1016/j.ccr.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Nagamatsu G, Kosaka T, Kawasumi M, Kinoshita T, Takubo K, Akiyama H, Sudo T, Kobayashi T, Oya M, Suda T. A germ cell-specific gene, Prmt5, works in somatic cell reprogramming. J Biol Chem. 2011;286:10641–10648. doi: 10.1074/jbc.M110.216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Zhou E, Kiontke K, Fradin H, Maldonado G, Martin D, Shah K, Fitch DHA. A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002010. doi: 10.1371/journal.pgen.1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T, Saitou M. A signaling principle for the specification of the germ cell lineage in mice. Cell. 2009;137:571–584. doi: 10.1016/j.cell.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee ST, Sohn YB, Cho SY, Kim SH, Kim SJ, Kim CH, Ko AR, Paik KH, Kim JW, et al. LIN28B polymorphisms are associated with central precocious puberty and early puberty in girls. Korean J Pediatr. 2012;55:388–392. doi: 10.3345/kjp.2012.55.10.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan RT, Saito M, Kitagawa Y, Means AR, Dalla-Favera R. Genotoxic stress regulates expression of the proto-oncogene Bcl6 in germinal center B cells. Nat Immunol. 2007;8:1132–1139. doi: 10.1038/ni1508. [DOI] [PubMed] [Google Scholar]
- Resnick TD, McCulloch KA, Rougvie AE. miRNAs give worms the time of their lives: small RNAs and temporal control in Caenorhabditis elegans. Dev Dyn. 2010;239:1477–1489. doi: 10.1002/dvdy.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Duan S, Jeong YT, Horn M, Saraf A, Florens L, Washburn MP, Antebi A, Pagano M. Regulation of the CRL4(Cdt2) ubiquitin ligase and cell-cycle exit by the SCF(Fbxo11) ubiquitin ligase. Mol Cell. 2013;49:1159–1166. doi: 10.1016/j.molcel.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougvie AE, Ambros V. The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development. 1995;121:2491–2500. doi: 10.1242/dev.121.8.2491. [DOI] [PubMed] [Google Scholar]
- Rybak A, Fuchs H, Hadian K, Smirnova L, Wulczyn EA, Michel G, Nitsch R, Krappmann D, Wulczyn FG. The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nat Cell Biol. 2009;11:1411–1420. doi: 10.1038/ncb1987. [DOI] [PubMed] [Google Scholar]
- Samuelson AV, Carr CE, Ruvkun G. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev. 2007;21:2976–2994. doi: 10.1101/gad.1588907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segade F, Daly KA, Allred D, Hicks PJ, Cox M, Brown M, Hardisty-Hughes RE, Brown SDM, Rich SS, Bowden DW. Association of the FBXO11 gene with chronic otitis media with effusion and recurrent otitis media: the Minnesota COME/ROM Family Study. Arch Otolaryngol Head Neck Surg. 2006;132:729–733. doi: 10.1001/archotol.132.7.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhánková M, Jindra M, Asahina M. Nuclear receptor NHR-25 is required for cell-shape dynamics during epidermal differentiation in Caenorhabditis elegans. J Cell Sci. 2005;118:223–232. doi: 10.1242/jcs.01609. [DOI] [PubMed] [Google Scholar]
- Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013;14:369–381. doi: 10.1038/nrm3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell. 2000;5:659–669. doi: 10.1016/s1097-2765(00)80245-2. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:e21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennessen JM, Gardner HF, Volk ML, Rougvie AE. Novel heterochronic functions of the Caenorhabditis elegans period-related protein LIN-42. Dev Biol. 2006;289:30–43. doi: 10.1016/j.ydbio.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Tunyaplin C, Shapiro MA, Calame KL. Characterization of the B lymphocyte-induced maturation protein-1 (Blimp-1) gene, mRNA isoforms and basal promoter. Nucleic Acids Res. 2000;28:4846–4855. doi: 10.1093/nar/28.24.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Mack DH, Davis MM. Blimp-1, a zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Sciammas R, Shapiro-Shalef M, Davis MM, Calame K, Bikoff EK, Robertson EJ. The zinc finger transcriptional repressor Blimp1/Prdm1 is dispensable for early axis formation but is required for specification of primordial germ cells in the mouse. Development. 2005;132:1315–1325. doi: 10.1242/dev.01711. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Stokes N, Polak L, Fuchs E. Specific MicroRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell. 2011;8:294–308. doi: 10.1016/j.stem.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


