Significance
By suspending a lipid bilayer in an aperture or between water droplets, single-molecule transport through membrane channels can be electrically detected. To date, all suspended bilayers have been confined to fluid reservoirs. This study demonstrates that droplet interface bilayers can be created in an ambient environment using noncoalescing water droplets on an oil-infused surface. Air-stable droplet interface bilayers are easy to manipulate and electrically characterize, and could potentially allow for the biosensing of airborne molecules.
Keywords: nanofabrication, superhydrophobic, networks
Abstract
Droplet interface bilayers are versatile model membranes useful for synthetic biology and biosensing; however, to date they have always been confined to fluid reservoirs. Here, we demonstrate that when two or more water droplets collide on an oil-infused substrate, they exhibit noncoalescence due to the formation of a thin oil film that gets squeezed between the droplets from the bottom up. We show that when phospholipids are included in the water droplets, a stable droplet interface bilayer forms between the noncoalescing water droplets. As with traditional oil-submerged droplet interface bilayers, we were able to characterize ion channel transport by incorporating peptides into each droplet. Our findings reveal that droplet interface bilayers can function in ambient environments, which could potentially enable biosensing of airborne matter.
Inspired by the pitcher plant (1), it was recently found that nano/microstructured hydrophobic substrates can be impregnated with lubricating fluids to create slippery surfaces for droplets (2–5). In contrast to dry, superomniphobic surfaces (6), lubricant-infused surfaces demonstrate stable liquid repellency at extreme pressures and temperatures (5, 7), are self-healing to mechanical damage (5), and their wettability and optical properties can be tuned (7, 8). A wide variety of applications are being explored for lubricant-infused surfaces, such as enhancing condensation heat transfer (9, 10), self-cleaning (11), fog harvesting (12), and omniphobic textiles (13), or minimizing ice nucleation (14, 15), ice adhesion (16, 17), and biofouling (18). Though previous studies have characterized the dynamics and possible wetting states of isolated droplets on lubricant-infused surfaces (5, 19–22), the interactive behavior of multiple droplets has not been reported.
For the more traditional scenario of water droplets completely submerged in a reservoir of immiscible fluid, the physics of droplet–droplet interactions are well known. Water droplets submerged in crude oil can exhibit stable noncoalescence; this is because the crude oil contains surface-active components, such as resins and asphaltenes, which congregate at the droplet interfaces (23). When amphiphilic phospholipids are introduced into an oil reservoir containing water droplets, droplet interface bilayers (DIBs) can form between adjacent water droplets (24, 25). Recently, DIBs have emerged as an ideal model membrane system due to attractive features such as durability (26, 27), tunable size and curvature (28–30), deformability (31), facile electrical characterization of ion channels (32–35), the option to introduce asymmetry into the system (36), and droplet interchangeability (26, 32). In the absence of any stabilizing agents, water droplets colliding in an immiscible fluid will exhibit coalescence when their interaction time exceeds the time required to drain the film of fluid trapped between the droplets (37, 38). Droplet collision is typically controlled by applying a constant force (i.e., gravity) (39, 40), constant approach velocity (41, 42), or constant flow rate (43, 44). For experimental studies in pure oil baths, the time required for colliding water droplets to exhibit film rupture and coalesce typically ranges from 10−3 to 102 s, depending on parameters such as oil viscosity, droplet size, and the flow field (40, 42–44).
Here, we show that water droplets in an ambient environment exhibit noncoalescence when colliding on an oil-infused surface, even in the absence of any surfactants. This phenomenon is due to the oil meniscus that surrounds each water droplet; when the oil menisci of neighboring droplets overlap, the menisci spontaneously merge together to minimize their surface energies and an oil film is squeezed upward to form a barrier between the colliding droplets. Though droplet coalescence will eventually occur due to film drainage, the time required for film rupture is several hours for moderate-viscosity [∼100 centistokes (cSt)] oils and is 1–3 orders of magnitude longer compared with droplets submerged in an oil bath (40, 42–44). These findings should refine the understanding of using oil-infused substrates for processes involving droplet–droplet interactions, such as condensation (9, 10) and fog harvesting (12).
When incorporating amphiphilic phospholipids into the water droplets, we demonstrate that the thinning oil membrane between noncoalescing droplets gets replaced by a stable lipid bilayer, somewhat analogous to the formation of a black lipid membrane in an aperture painted with oil (45). To our knowledge, this is the first report of producing droplet interface bilayers in an ambient environment. We show that air-stable DIBs still allow for the robust electrical characterization of ion channels inserted in the lipid bilayer. Previously, it has been demonstrated that black lipid membranes or DIBs can be used for biosensing (46–50), light sensing (26), microscale biobatteries (26), electrical circuits (51, 52), and engineering tissue-like material (53). However, these suspended lipid bilayers have always been confined to fluid reservoirs (25, 45). We suggest that our air-stable DIBs will allow for an unprecedented degree of control regarding the fabrication, manipulation, transportation, and utilization of functional droplet networks.
Results and Discussion
Noncoalescence of Water Droplets on Oil-Infused Surfaces.
Droplet–droplet interactions were characterized on an oil-infused superhydrophobic surface. Listed in order of increasing viscosity, the five different oils used for experiments were hexadecane; Krytox 100; 50- and 350-cSt silicone oil (at 25 °C); and Fomblin 25/6 (SI Appendix, Table S1). These low surface-tension oils uniformly impregnate the surface roughness to prevent deposited water droplets from directly contacting the substrate: , where subscripts a, o, and w are the air, oil, and water phases, and s is the substrate (5, 21). The superhydrophobic surface was composed of silicon nanopillars, because it was previously found that nanotextured surfaces are best suited for locking in a film of oil that is long-lasting (54) and highly stable against external forces (55). When a water droplet was deposited onto an oil-infused surface, an oil meniscus rapidly formed around the droplet to balance its three-phase contact line: . It should be noted that because for all oils used here (except hexadecane; SI Appendix, Table S2), a thin film of oil is cloaked about the water–air interface, and is actually replaced by (21, 56). For brevity, the oil-cloaked droplet interface will henceforth be referred to simply as the water–air interface.
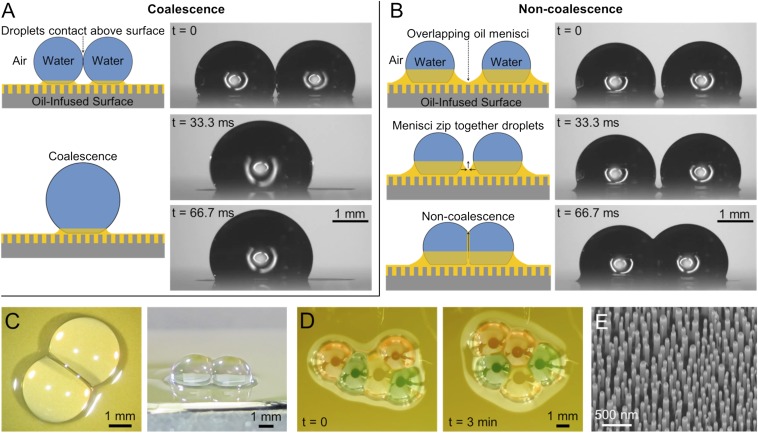
When two water droplets came into contact on an oil-infused surface, they exhibited either coalescence or noncoalescence depending on their initial point of contact (Fig. 1). Droplets colliding at the water-air interfaces experienced immediate coalescence, revealing that the thin cloak of oil surrounding the droplets is not sufficient to prevent coalescence (Fig. 1A). This scenario will not be considered any further, because the dynamics of conventional droplet coalescence have been reported elsewhere (57). When the initial contact occurred at the droplets’ surrounding oil menisci, however, the droplets exhibited noncoalescence for minutes or even hours despite becoming firmly attached to each other (Fig. 1 B–D).
Fig. 1.
Interactive behavior of water droplets on an oil-infused surface. (A) Droplets colliding at the liquid–air interfaces exhibited coalescence. (B) When the oil menisci of two droplets overlapped, an oil film formed between the droplets to enable noncoalescence. (C) Top-down and isometric photographs of noncoalescing droplets. (D) Multiple droplets could be connected into a network; due to the negligible hysteresis, these networks spontaneously rearranged over time to minimize their surface energy. (E) SEM of nanopillared substrate. The oils used were (A and B) Krytox 100 and (C and D) Fomblin 25/6; food coloring was used in D. See SI Appendix, Movies S1–S3.
The mechanism for noncoalescence is a thin film of oil that gets squeezed upward between the droplets as the menisci merge. Surface-tension measurements of the oil–water interfaces confirmed that all of the oils (except for Fomblin) did not contain any appreciable surface-active materials (SI Appendix, Fig. S1), indicating that noncoalescence occurs independently of any stabilizing agents. It has previously been shown that water condensing in an oil bath exhibits an ordered hexagonal structure due to delayed coalescence (58); however, without a supporting substrate, these condensate drops were primarily submerged beneath the oil surface, whereas the noncoalescing droplets observed here are suspended almost entirely above the oil interface.
By varying the amount of excess oil on the surface, the contact angle of a deposited droplet and the shape of its oil meniscus could be tuned to control the occurrence of coalescence vs. noncoalescence (SI Appendix, Fig. S2). In short, noncoalescence will occur if the oil menisci extend beyond the droplet profiles, which is the case if either of the following conditions are met:
| [1] |
where is the contact angle of the water droplet on the oil-infused surface (with respect to the horizontal), R is the droplet’s radius of curvature, L is the droplet’s contact radius with the oil meniscus, and is the decay length of the oil meniscus (SI Appendix, Fig. S3). Over hundreds of trials, Eq. 1 was confirmed for a variety of droplet sizes (2.5–10 μL) and oil viscosities (∼1–1,000 mPa⋅s). The cutoff point is illustrated in Fig. 1, where droplets exhibited coalescence for an oil film spin-coated at 1,000 rpm for 45 s (Fig. 1A) and noncoalescence for a thicker film spin-coated at 500 rpm for 45 s (Fig. 1B). For the remainder of the experiments reported here, an excess oil thickness of ∼50 ± 10 μm was deposited over the infused nanostructure; this was chosen because 50 μm of excess oil is thick enough to ensure that the criteria for noncoalescence will consistently be met but is also thin enough to elevate the majority of the water droplet(s) above the oil–air interface.
When the oil menisci of multiple droplets overlap, the droplets spontaneously pull together to minimize the surface energies of the oil menisci. The collision velocity of the droplets is proportional to the viscous-capillary velocity (57) of the oil,
| [2] |
where v is the maximum cumulative velocity exhibited by the droplets before deceleration and and are the surface tension and viscosity of the oil menisci pulling the droplets together (SI Appendix, Fig. S4). When Eq. 1 is satisfied, the water droplets exhibit noncoalescence upon collision due to the formation of an intermediate oil film. After collision, the immobilized droplets remain stuck together and exhibit a near-constant profile over time (the surface was cooled to the dew point to prevent droplet evaporation).
Thin Film Drainage Between Water Droplets.
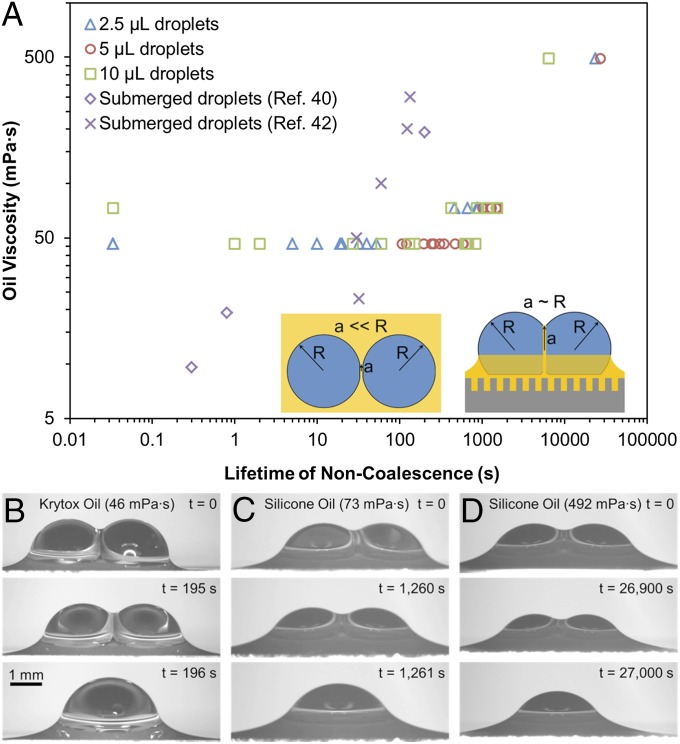
After a period that ranges from seconds to hours depending on the experimental conditions, the intermediate oil film collapses, causing the droplets to finally coalesce together. The drainage time required for film rupture depended primarily upon the viscosity of the oil, with tc ∼10–1,000 s for μo ∼10 mPa⋅s and tc ∼1,000–10,000 s for μo ∼100 mPa⋅s (Fig. 2). These lifetimes of noncoalescence are 1–3 orders of magnitude longer compared with submerged water droplets (∼1 mm) colliding in an oil bath, where tc ∼0.1–10 s for μo ∼ 10 mPa⋅s and tc ∼10–100 s for μo ∼100 mPa⋅s (40, 42, 44). When an oil containing surfactants is used (Fomblin), the noncoalescing droplets were stable for at least 24 h in the absence of evaporation (SI Appendix, Fig. S5).
Fig. 2.
(A) Time required to drain an oil film between water droplets on surfaces infused with Krytox or silicone oils. The substrate was held at 8 °C to prevent droplet evaporation. The drainage time was 1–3 orders of magnitude longer compared with droplets submerged in oil baths (whose data are shown here from refs. 40 and 42), most likely due to the larger film radius (Inset). (B–D) Side-view imaging of the lifetime of noncoalescing 5-μL droplets on surfaces infused with (B) Krytox, (C) 50 cSt silicone oil, and (D) 350 cSt silicone oil. See SI Appendix, Movies S4 and S5.
In addition to oil viscosity, the volume of the colliding water droplets also affected the lifetime of noncoalescence, particularly for the low-viscosity Krytox oil. The tested droplet volumes were 2.5, 5, and 10 μL. The 2.5-μL droplets consistently exhibited shorter droplet lifetimes compared with the 5-μL droplets; this is because droplets with a smaller radius of curvature exhibit a larger Laplace pressure. It would therefore be expected that the 10-μL droplets would last even longer than the 5-μL droplets; however, this was not always the case. The 10-μL droplets exhibited the widest range of noncoalescence lifetimes, with some coalescing almost immediately while others did indeed last longer than the 5-μL droplets. One possible explanation is that larger droplets collide at larger Weber numbers , which could reduce the initial film thickness. For example, droplet collisions on Krytox oil exhibited We ∼0.001 for 2.5-μL droplets compared with We ∼0.01 for 10-μL droplets. The importance of inertial effects is attested by the observation that droplets colliding with externally imposed velocities exhibited higher rates of early film rupture compared with static droplets joined together solely by the oil menisci’s surface tension.
The drainage and eventual collapse of the oil film is primarily due to the Laplace pressure and long-range intermolecular forces acting on the film (37),
| [3] |
where is the pressure difference between the droplets and oil film, R is the radius of curvature of two same-sized droplets, is the Hamaker constant, and is the thickness of the oil film. The droplets used here were smaller than the capillary length, (4), so gravity is not expected to play a significant role. Because the intermolecular forces scale with , the oil film rapidly collapses as intermolecular forces become dominant at a critical thickness (37, 38),
| [4] |
which is approximately for (56), , and .
For an oil film of thickness and constant radius a, it can be assumed that . Therefore, the lubrication approximation can be invoked, where only the radial velocity of the draining oil film, , is considered. Neglecting inertial terms and assuming axisymmetry and an immobile interface (i.e., no plug flow), the Navier–Stokes velocity profile for a given film thickness is given by
| [5] |
where z is the perpendicular distance from the center line of the oil film. Though it is likely that the film interfaces exhibit some degree of curvature and/or deformation (38, 42), an analytical approximation of the drainage rate may be obtained by assuming rigid, parallel interfaces, such that (full derivation in SI Appendix):
| [6] |
Eq. 6 reveals that, all other parameters being equal, the drainage rate is inversely proportional to , which is important because for conventional film drainage between two submerged same-sized water droplets of radius R, the point contact made between colliding droplets results in film drainage where (42); this is in sharp contrast to our large-area oil film that forms between two colliding droplets on an oil-infused surface, where (Fig. 2A, Inset). The large increase in the size of a is one possible explanation for the dramatically long lifetime of noncoalescence observed here compared with lifetimes reported for submerged droplets. Another possible reason is that droplets on an oil-infused surface require no external forces to collide together, whereas submerged droplets require gravity or an applied flow field which can accelerate the drainage rate.
By inserting electrodes into each of the noncoalescing droplets and applying a triangular waveform voltage, the average thickness of an oil film can be estimated:
| [7] |
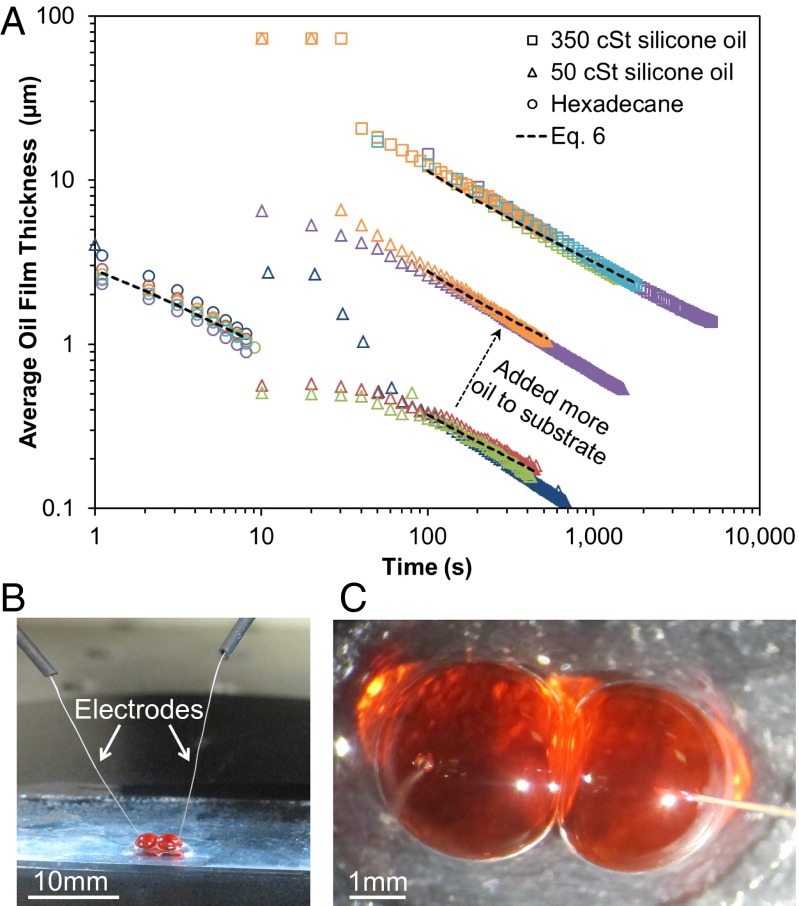
where is the relative permittivity of the oil (2.14 for hexadecane and 2.75 for silicone oils), is the vacuum permittivity, is the approximate surface area of the oil film between 5-μL droplets , and is the capacitance calculated from current measurements (SI Appendix, Figs. S6 and S7). The electrodes were inserted into two separate 5-μL water droplets, and then the droplets were joined together using a micromanipulator to measure for the complete lifetime of noncoalescence (Fig. 3). For the remainder of the experiments shown here, a nonconductive superhydrophobic surface composed of silica nanoparticles on a glass slide (SI Appendix, Fig. S8) was used to prevent current from passing into the substrate.
Fig. 3.
(A) Electrical measurements of the average oil film thickness between noncoalescing water droplets for substrates infused with hexadecane (○) at room temperature (22 °C), 50 cSt silicone oil (Δ) cooled to the dew point (17 °C), or 350 cSt silicone oil (□) cooled to the dew point (now 7 °C due to change in humidity). Multiple trials for each oil are denoted by different colors, and the final data point for each trial indicates film collapse and coalescence. The drainage rates were in good agreement with the model (Eq. 6) as denoted by the dotted trend lines; see SI Appendix, Table S3 for the values used in Eq. 6. The initial film thickness increased with viscosity and could also be increased by adding more excess oil to the substrate, as demonstrated here with 50 cSt silicone oil. (B and C) Side-view and top-down photographs of noncoalescing water droplets with inserted electrodes.
For each trial, the oil film collapsed and coalescence occurred at a critical film thickness of , ∼1 order of magnitude larger than predicted by Eq. 4. It is therefore likely that the oil film exhibits a slight curvature in its profile, such that is only obtained at a localized area with larger thicknesses elsewhere. Nevertheless, the parallel disk drainage model given by Eq. 6 reasonably approximated the experimental drainage rates (Fig. 3). Values of a used to fit Eq. 6 with the data ranged from to (depending on oil viscosity and excess oil thickness; SI Appendix, Table S3), which further supports the hypothesis that for air-stable interdroplet films. Though the insertion of electrodes into the droplets did reduce the lifetime of noncoalescence compared with free droplets (Fig. 2A), it was confirmed that the applied voltage did not have any appreciable effect on the capacitance measurements (SI Appendix, Fig. S9).
Air-Stable Droplet Interface Bilayers.
Thus far, we have characterized the formation and drainage of a thin oil film between noncoalescing water droplets on an oil-infused surface. The functionality of these noncoalescing droplets would be greatly enhanced if the intermediate oil film could be replaced by a lipid bilayer, because a lipid membrane would provide a stable droplet–droplet interface of known thickness that can facilitate molecular transport between droplets. In this final section, we mixed 2.4 mM of diphytanoyl phosphocholine (DPhPC) phospholipids in the water phase before droplet deposition on the oil-infused surface. Colliding droplets still exhibit noncoalescence in the same manner as before, but now lipids assemble at the interfaces of the intermediate oil film. Instead of droplet coalescence occurring once the oil film drains and collapses, the droplet–droplet interface is stabilized by the formation of a lipid bilayer that is only 5–7 nm thick (analogous to the formation of a black lipid membrane). Hexadecane oil was chosen for these experiments due to its compatibility with phospholipids and because its low viscosity ensures that the initial oil film will rapidly drain and become replaced by a droplet interface bilayer.
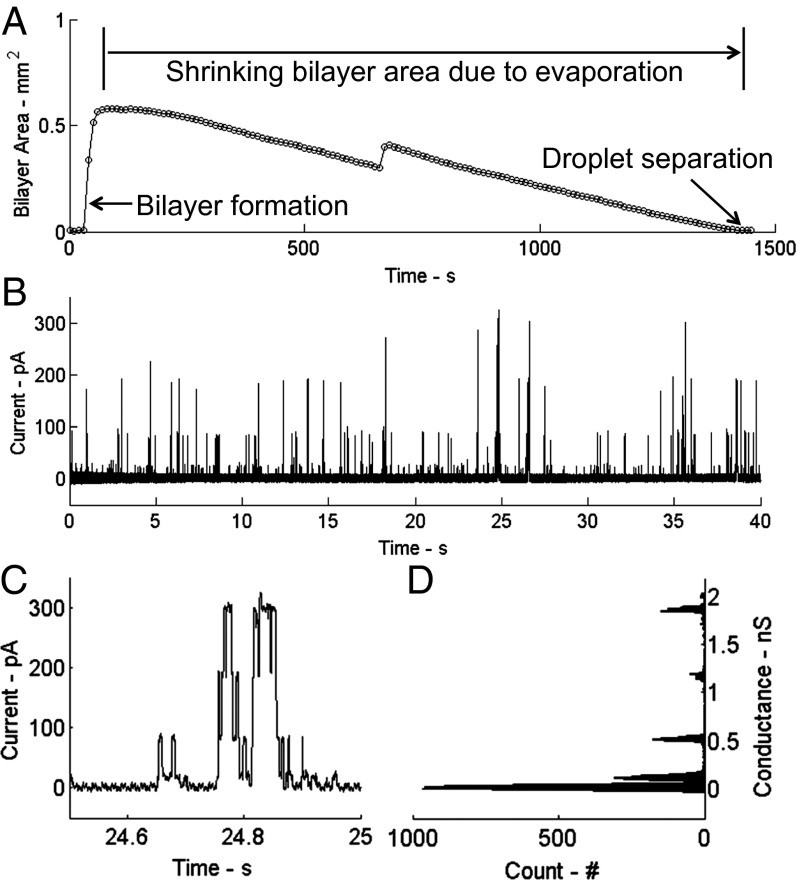
Using the same electrical characterization technique described in the previous section, capacitance measurements reveal that the oil film is completely replaced by a lipid bilayer within 50 s (Fig. 4A). Due to the high freezing temperature of hexadecane (18 °C), the surface could not be cooled down to the dew point, so over time the area of the bilayer steadily decreased to zero due to droplet evaporation. It is remarkable that the droplets never coalesced throughout the shrinkage of the droplet interface bilayer. In the future, a different choice of oil and/or humidity control could allow for the droplets to be held at the dew point; it should also be possible to use condensation in addition to evaporation to enable bilayers with reversibly tunable geometries.
Fig. 4.
(A) Bilayer area vs. time for an air-stable droplet interface bilayer. The area is determined from a capacitance measurement of the interface (SI Appendix). The maximum area (0.58 mm2) corresponds to the maximum expansion of the thinned interface between droplets soon after initial bilayer formation, and the area reduces to zero over the next 23 min due to evaporation of the electrode-pinned droplets that causes them to pull apart as they shrink. The slight kink at was caused by one droplet suddenly shifting its position on the slippery surface. (B) Measurement of single-channel currents produced by alamethicin peptides at a voltage of +160 mV. (C) A detailed view of one gating event shows the characteristic, multilevel current response seen for alamethicin oligomers. (D) A corresponding histogram of the conductance levels.
Finally, we incorporated transmembrane peptides into the droplets to alter the ionic conductance of the interface as a way to verify that the lipid-stabilized interface between droplets is a bilayer membrane. Specifically, alamethicin peptides from the fungus Trichoderma viride were incorporated into the droplets at 10 nM to encourage the formation of voltage-dependent ion channels through the bilayer. Fig. 4B shows measured current for a holding potential of +160 mV, where the transient increases correspond to the insertion and assembly of channel-forming alamethicin oligomers that span the thickness of the membrane. An example of the discrete changes in current that occur during these rapid pore-forming events is shown in more detail in Fig. 4C. A histogram of the channel conductance (i.e., current/voltage) during these gating transitions confirms the measured current levels to be those corresponding to the first four conductance levels (110 pS, 520 pS, 1.20 nS, and 1.85 nS) for alamethicin (59, 60). These results verify that the droplet–droplet interface is a bilayer membrane whose properties can be controlled and characterized using transmembrane biomolecules.
To our knowledge, this is the first report of fabricating a suspended lipid bilayer in an ambient environment. Black lipid membranes have always been confined to aqueous baths (45), and droplet interface bilayers have always been constructed in oil reservoirs (25) or in oil-in-water emulsions (61). Recent reports have obtained air-stable supported lipid membranes formed on a solid substrate (62, 63); however, the fluidity of such membranes is highly dependent on humidity, and transmembrane studies cannot be achieved with solid supported bilayers. Our air-stable droplet interface bilayers mitigate both of these limitations: the adjacent droplets maintain bilayer fluidity and suspend the bilayer to allow for molecular transport. The use of an oil-infused surface in place of an oil reservoir will enable the robust fabrication and modulation of functional droplet networks via techniques such as micropositioning, condensation, evaporation, vibration (64), or magnetic fields (65); such techniques are typically more practical in an ambient environment.
Conclusion
Using oil-infused superhydrophobic surfaces, we have demonstrated that colliding water droplets exhibit noncoalescence due to the spontaneous formation of a microscopic oil film between the droplets. In the absence of surfactants the Laplace pressure and intermolecular forces eventually cause the intermediate film to collapse, but the lifetime of noncoalescence is 1–3 orders of magnitude longer compared with droplets colliding in a submerged fluid bath. When phospholipids are mixed into the noncoalescing water droplets, an air-stable droplet interface bilayer forms that was used to detect single-channel gating events. To our knowledge, this is the first time that suspended lipid bilayers have been produced and characterized in an ambient environment instead of in a fluid reservoir. We envision that our air-stable suspended bilayers will enable the robust construction and manipulation of functional droplet networks and potentially allow for the stochastic biosensing of airborne molecules.
Materials and Methods
Superhydrophobic Silicon Nanopillars.
The silicon nanopillars were fabricated using a lithography-free technique adapted from a previous report (66). First, 100 nm of silicon dioxide (SiO2) was thermally grown onto a 〈100〉 Si substrate and 5 nm of platinum was then deposited onto the SiO2 using an electron beam evaporator. The sample was heated in a rapid thermal processor furnace (Easy Tube 3000; First Nano) at full power for 8 s in a H2 and Ar ambient to dewet the platinum film into an etch mask. The maximum temperature of the chamber at full power was ∼850 °C. The SiO2 was etched using 2 standard cubic centimeters per minute (sccm) O2 and 45 sccm C4F8 for 55 s at 15 °C, 7 mTorr, and 200 W rf (Oxford Plasmalab 100). The Si was subsequently etched using 5 sccm Ar, 25 sccm SF6, and 58 sccm C4F8 for 6 min at 20 °C, 10 mTorr, and 30 W RF. Scanning electron microscopy revealed an etch depth of 500 ± 100 nm and pillar diameters ranging from 10 to 100 nm (Fig. 1E). The nanostructure was rendered superhydrophobic by immersion in a solution of 0.1% (vol/vol) of (tridecafluoro-1,1,2,2-tetrahydrooctyl)triethoxysilane (Gelest Inc.) in hexane for 12 h. Droplets exhibited contact angles of and (66).
Superhydrophobic Glass.
SiO2 powder (SI Appendix, Fig. S8A) was annealed at 70 °C for 24 h. The mean size of the particle agglomerates was 610 ± 140 nm (SI Appendix, Fig. S8B). The powder was suspended in a solution of hexane and (tridecafluoro-1,1,2,2-tetrahydrooctyl)trichlorosilane (Gelest Inc.). The concentrations of the SiO2 particles and silane in the hexane are 6 and 0.6 wt%, respectively. After stirring the suspension for 8 h at room temperature, the powder was washed with hexane to remove nonreacted silane groups and then dried at 60 °C for 24 h. The resulting functionalized particles (SI Appendix, Fig. S8C) were suspended in a solution of polyurethane resin and acetone in a weight ratio . Superhydrophobic substrates with water contact angle ∼170° were obtained by spin coating the suspension with a Speedline Technologies G3-8 at 1,000 rpm for 30 s on glass slides (SI Appendix, Fig. S8D).
Electrical Measurements.
Electrical measurements of oil- and bilayer-stabilized interfaces were performed by measuring the current induced by a triangle waveform voltage applied to two wire-type silver–silver chloride electrodes (125 μm) inserted into the droplets. The triangle voltage waveform was the output of a function generator (Agilent 33210A), and the resulting current was recorded using an Axopatch 200B patch-clamp amplifier and Digidata 1440A data acquisition device (Molecular Devices). Measurements of current were sampled at 20 kHz, and all electrical measurements are performed on glass substrates.
Droplets Solutions for Bilayer Tests.
Hexadecane [99% (vol/vol); Sigma] was used as the infusing oil on the glass substrates for bilayer formation between droplets. Liposome solutions consisted of 2.4 mM DPhPC (Avanti Polar Lipids) suspended as 100-nM liposomes in a 200-mM NaCl (Sigma), 10-mM Mops (Sigma) buffer. Alamethicin peptides (A.G. Scientific) were stored in ethanol at 10 mg/mL, and then diluted to 10 nM (19.64 ng/mL) in liposome solution.
Supplementary Material
Acknowledgments
We thank J. Fowlkes, P. Rack, and S. Retterer for helpful discussions. This research was conducted at the Center for Nanophase Materials Sciences, which is sponsored at Oak Ridge National Laboratory by the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy. Funding was provided by Air Force Office of Scientific Research Basic Research Initiative Grant FA9550-12-1-0464 (to S.A.S.) and the SunShot Program of the Office of Energy Efficiency and Renewable Energy (G.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400381111/-/DCSupplemental.
References
- 1.Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc Natl Acad Sci USA. 2004;101(39):14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verheijen HJJ, Prins MWJ. Reversible electrowetting and trapping of charge: model and experiments. Langmuir. 1999;15(20):6616–6620. [Google Scholar]
- 3.Krupenkin T, Yang S, Mach P. Tunable liquid microlens. Appl Phys Lett. 2003;82:316–318. [Google Scholar]
- 4.Quere D. Non-sticking drops. Rep Prog Phys. 2005;68(11):2495–2532. [Google Scholar]
- 5.Wong TS, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature. 2011;477(7365):443–447. doi: 10.1038/nature10447. [DOI] [PubMed] [Google Scholar]
- 6.Tuteja A, et al. Designing superoleophobic surfaces. Science. 2007;318(5856):1618–1622. doi: 10.1126/science.1148326. [DOI] [PubMed] [Google Scholar]
- 7.Daniel D, Mankin MN, Belisle RA, Wong TS, Aizenberg J. Lubricant-infused micro/nano-structured surfaces with tunable dynamic omniphobicity at high temperatures. Appl Phys Lett. 2013;102:231603. [Google Scholar]
- 8.Yao X, et al. Adaptive fluid-infused porous films with tunable transparency and wettability. Nat Mater. 2013;12(6):529–534. doi: 10.1038/nmat3598. [DOI] [PubMed] [Google Scholar]
- 9.Anand S, Paxson AT, Dhiman R, Smith JD, Varanasi KK. Enhanced condensation on lubricant-impregnated nanotextured surfaces. ACS Nano. 2012;6(11):10122–10129. doi: 10.1021/nn303867y. [DOI] [PubMed] [Google Scholar]
- 10.Xiao R, Miljkovic N, Enright R, Wang EN. Immersion condensation on oil-infused heterogeneous surfaces for enhanced heat transfer. Sci Rep. 2013;3:1988. doi: 10.1038/srep01988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Zhang P, Liu M, Wang S, Jiang L. Organogel-based thin films for self-cleaning on various surfaces. Adv Mater. 2013;25(32):4477–4481. doi: 10.1002/adma.201301289. [DOI] [PubMed] [Google Scholar]
- 12.Lalia BS, Anand S, Varanasi KK, Hashaikeh R. Fog-harvesting potential of lubricant-impregnated electrospun nanomats. Langmuir. 2013;29(42):13081–13088. doi: 10.1021/la403021q. [DOI] [PubMed] [Google Scholar]
- 13.Shillingford C, MacCallum N, Wong TS, Kim P, Aizenberg J. Fabrics coated with lubricated nanostructures display robust omniphobicity. Nanotechnology. 2014;25(1):014019. doi: 10.1088/0957-4484/25/1/014019. [DOI] [PubMed] [Google Scholar]
- 14.Kim P, et al. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano. 2012;6(8):6569–6577. doi: 10.1021/nn302310q. [DOI] [PubMed] [Google Scholar]
- 15.Wilson PW, et al. Inhibition of ice nucleation by slippery liquid-infused porous surfaces (SLIPS) Phys Chem Chem Phys. 2013;15(2):581–585. doi: 10.1039/c2cp43586a. [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, et al. Ice-phobic coatings based on silicon-oil-infused polydimethylsiloxane. ACS Appl Mater Interfaces. 2013;5(10):4053–4062. doi: 10.1021/am400704z. [DOI] [PubMed] [Google Scholar]
- 17.Subramanyam SB, Rykaczewski K, Varanasi KK. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir. 2013;29(44):13414–13418. doi: 10.1021/la402456c. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AK, Wong TS, Belisle RA, Boggs EM, Aizenberg J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc Natl Acad Sci USA. 2012;109(33):13182–13187. doi: 10.1073/pnas.1201973109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafuma A, Quere D. Slippery pre-suffused surfaces. Europhys Lett. 2011;96:56001. [Google Scholar]
- 20.Hejazi V, Nosonovsky M. Wetting transitions in two-, three-, and four-phase systems. Langmuir. 2012;28(4):2173–2180. doi: 10.1021/la2038284. [DOI] [PubMed] [Google Scholar]
- 21.Smith JD, et al. Droplet mobility on lubricant-impregnated surfaces. Soft Matter. 2013;9:1772–1780. [Google Scholar]
- 22.Jenner E, D’Urso B. Wetting states on structured immiscible liquid coated surfaces. Appl Phys Lett. 2013;103:251606. [Google Scholar]
- 23.McLean JD, Kilpatrick PK. Effects of asphaltene solvency on stability of water-in-crude-oil emulsions. J Colloid Interface Sci. 1997;189:242–253. doi: 10.1006/jcis.1997.5177. [DOI] [PubMed] [Google Scholar]
- 24.Funakoshi K, Suzuki H, Takeuchi S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem. 2006;78(24):8169–8174. doi: 10.1021/ac0613479. [DOI] [PubMed] [Google Scholar]
- 25.Leptihn S, et al. Constructing droplet interface bilayers from the contact of aqueous droplets in oil. Nat Protoc. 2013;8(6):1048–1057. doi: 10.1038/nprot.2013.061. [DOI] [PubMed] [Google Scholar]
- 26.Holden MA, Needham D, Bayley H. Functional bionetworks from nanoliter water droplets. J Am Chem Soc. 2007;129(27):8650–8655. doi: 10.1021/ja072292a. [DOI] [PubMed] [Google Scholar]
- 27.Sarles SA, Leo DJ. Physical encapsulation of droplet interface bilayers for durable, portable biomolecular networks. Lab Chip. 2010;10(6):710–717. doi: 10.1039/b916736f. [DOI] [PubMed] [Google Scholar]
- 28.Sarles SA, Leo DJ. Regulated attachment method for reconstituting lipid bilayers of prescribed size within flexible substrates. Anal Chem. 2010;82(3):959–966. doi: 10.1021/ac902555z. [DOI] [PubMed] [Google Scholar]
- 29.Punnamaraju S, Steckl AJ. Voltage control of droplet interface bilayer lipid membrane dimensions. Langmuir. 2011;27(2):618–626. doi: 10.1021/la1036508. [DOI] [PubMed] [Google Scholar]
- 30.Dixit SS, Pincus A, Guo B, Faris GW. Droplet shape analysis and permeability studies in droplet lipid bilayers. Langmuir. 2012;28(19):7442–7451. doi: 10.1021/la3005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boreyko JB, Mruetusatorn P, Sarles SA, Retterer ST, Collier CP. Evaporation-induced buckling and fission of microscale droplet interface bilayers. J Am Chem Soc. 2013;135(15):5545–5548. doi: 10.1021/ja4019435. [DOI] [PubMed] [Google Scholar]
- 32.Hwang WL, Holden MA, White S, Bayley H. Electrical behavior of droplet interface bilayer networks: Experimental analysis and modeling. J Am Chem Soc. 2007;129(38):11854–11864. doi: 10.1021/ja074071a. [DOI] [PubMed] [Google Scholar]
- 33.Aghdaei S, Sandison ME, Zagnoni M, Green NG, Morgan H. Formation of artificial lipid bilayers using droplet dielectrophoresis. Lab Chip. 2008;8(10):1617–1620. doi: 10.1039/b807374k. [DOI] [PubMed] [Google Scholar]
- 34.Zagnoni M, Sandison ME, Marius P, Morgan H. Bilayer lipid membranes from falling droplets. Anal Bioanal Chem. 2009;393(6-7):1601–1605. doi: 10.1007/s00216-008-2588-5. [DOI] [PubMed] [Google Scholar]
- 35.Sarles SA. The use of virtual ground to control transmembrane voltages and measure bilayer currents in serial arrays of droplet interface bilayers. Smart Mater Struct. 2013;22(9):094023. [Google Scholar]
- 36.Hwang WL, Chen M, Cronin B, Holden MA, Bayley H. Asymmetric droplet interface bilayers. J Am Chem Soc. 2008;130(18):5878–5879. doi: 10.1021/ja802089s. [DOI] [PubMed] [Google Scholar]
- 37.Marrucci G. A theory of coalescence. Chem Eng Sci. 1969;24:975–985. [Google Scholar]
- 38.Chesters AK. The modelling of coalescence processes in fluid-liquid dispersions: A review of current understanding. Chem Eng Res Des. 1991;69:259–270. [Google Scholar]
- 39.Yiantsios SG, Davis RH. Close approach and deformation of two viscous drops due to gravity and van der Waals forces. J Colloid Interface Sci. 1991;144:412–433. [Google Scholar]
- 40.Steinhaus B, Spicer PT, Shen AQ. Droplet size effects on film drainage between droplet and substrate. Langmuir. 2006;22(12):5308–5313. doi: 10.1021/la0531300. [DOI] [PubMed] [Google Scholar]
- 41.Abid S, Chesters AK. The drainage and rupture of partially-mobile films between colliding drops at constant approach velocity. Int J Multiph Flow. 1994;20:613–629. [Google Scholar]
- 42.Klaseboer E, Chevaillier JP, Gourdon C, Masbernat O. Film drainage between colliding drops at constant approach velocity: Experiments and modeling. J Colloid Interface Sci. 2000;229:274–285. doi: 10.1006/jcis.2000.6987. [DOI] [PubMed] [Google Scholar]
- 43.Borrell M, Leal LG. Viscous coalescence of expanding low-viscosity drops; the dueling drops experiment. J Colloid Interface Sci. 2008;319:263–269. doi: 10.1016/j.jcis.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Gong J, Ngan KH, Angeli P. Effect of glycerol on the binary coalescence of water drops in stagnant oil phase. Chem Eng Res Des. 2009;87:1640–1648. [Google Scholar]
- 45.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci USA. 1972;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci USA. 1996;93(24):13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howorka S, Cheley S, Bayley H. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat Biotechnol. 2001;19(7):636–639. doi: 10.1038/90236. [DOI] [PubMed] [Google Scholar]
- 48.Bayley H, Cremer PS. Stochastic sensors inspired by biology. Nature. 2001;413(6852):226–230. doi: 10.1038/35093038. [DOI] [PubMed] [Google Scholar]
- 49.Guan X, Gu LQ, Cheley S, Braha O, Bayley H. Stochastic sensing of TNT with a genetically engineered pore. ChemBioChem. 2005;6(10):1875–1881. doi: 10.1002/cbic.200500064. [DOI] [PubMed] [Google Scholar]
- 50.Poulos JL, et al. Ion channel and toxin measurement using a high throughput lipid membrane platform. Biosens Bioelectron. 2009;24(6):1806–1810. doi: 10.1016/j.bios.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 51.Sapra KT, Bayley H. Lipid-coated hydrogel shapes as components of electrical circuits and mechanical devices. Sci Rep. 2012;2:848. doi: 10.1038/srep00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maglia G, et al. Droplet networks with incorporated protein diodes show collective properties. Nat Nanotechnol. 2009;4(7):437–440. doi: 10.1038/nnano.2009.121. [DOI] [PubMed] [Google Scholar]
- 53.Villar G, Graham AD, Bayley H. A tissue-like printed material. Science. 2013;340(6128):48–52. doi: 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vogel N, Belisle RA, Hatton B, Wong TS, Aizenberg J. Transparency and damage tolerance of patternable omniphobic lubricated surfaces based on inverse colloidal monolayers. Nat Commun. 2013;4:3167. doi: 10.1038/ncomms3176. [DOI] [PubMed] [Google Scholar]
- 55.Kim P, Kreder MJ, Alvarenga J, Aizenberg J. Hierarchical or not? Effect of the length scale and hierarchy of the surface roughness on omniphobicity of lubricant-infused substrates. Nano Lett. 2013;13(4):1793–1799. doi: 10.1021/nl4003969. [DOI] [PubMed] [Google Scholar]
- 56.Carlson A, Kim P, Amberg G, Stone HA. Short and long time drop dynamics on lubricated substrates. Europhys Lett. 2013;104:34008. [Google Scholar]
- 57.Eggers J, Lister JR, Stone HA. Coalescence of liquid drops. J Fluid Mech. 2013;401:293–310. [Google Scholar]
- 58.Steyer A, Guenoun P, Beysens D, Knobler CM. Two-dimensional ordering during droplet growth on a liquid surface. Phys Rev B Condens Matter. 1990;42(1):1086–1089. doi: 10.1103/physrevb.42.1086. [DOI] [PubMed] [Google Scholar]
- 59.Sansom MSP. The biophysics of peptide models of ion channels. Prog Biophys Mol Biol. 1991;55(3):139–235. doi: 10.1016/0079-6107(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 60.Sarles SA, Stiltner LJ, Williams CB, Leo DJ. Bilayer formation between lipid-encased hydrogels contained in solid substrates. ACS Appl Mater Interfaces. 2010;2(12):3654–3663. doi: 10.1021/am100826s. [DOI] [PubMed] [Google Scholar]
- 61.Villar G, Heron AJ, Bayley H. Formation of droplet networks that function in aqueous environments. Nat Nanotechnol. 2011;6(12):803–808. doi: 10.1038/nnano.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holden MA, Jung SY, Yang T, Castellana ET, Cremer PS. Creating fluid and air-stable solid supported lipid bilayers. J Am Chem Soc. 2004;126(21):6512–6513. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 63.Albertorio F, et al. Fluid and air-stable lipopolymer membranes for biosensor applications. Langmuir. 2005;21(16):7476–7482. doi: 10.1021/la050871s. [DOI] [PubMed] [Google Scholar]
- 64.Noblin X, Kofman R, Celestini F. Ratchetlike motion of a shaken drop. Phys Rev Lett. 2009;102(19):194504. doi: 10.1103/PhysRevLett.102.194504. [DOI] [PubMed] [Google Scholar]
- 65.Timonen JVI, Latikka M, Leibler L, Ras RHA, Ikkala O. Switchable static and dynamic self-assembly of magnetic droplets on superhydrophobic surfaces. Science. 2013;341(6143):253–257. doi: 10.1126/science.1233775. [DOI] [PubMed] [Google Scholar]
- 66.Boreyko JB, et al. Dynamic defrosting on nanostructured superhydrophobic surfaces. Langmuir. 2013;29(30):9516–9524. doi: 10.1021/la401282c. [DOI] [PubMed] [Google Scholar]