Abstract
Cystic fibrosis (CF) patients are highly susceptible to chronic lung infections by the environmental bacterium Pseudomonas aeruginosa. The overproduction and accumulation of dehydrated viscous respiratory mucus and excessive inflammation represents a defining feature of CF and constitutes the major environment encountered by P. aeruginosa during chronic infections. We applied whole-genome microarray technology to investigate the ability of P. aeruginosa to respond to signals found in muco-purulent airway liquids collected from chronically infected CF patients. Particularly notable was the activation of the Rhl-dependent quorum-sensing (QS) network and repression of fliC, which encodes flagellin. Activation of the Rhl branch of the QS network supports the observation that QS molecules are produced in the chronically infected CF lung. The shut-off of flagellin synthesis in response to CF airway liquids was rapid and independent of QS and the known regulatory networks controlling the hierarchical expression of flagellar genes. As flagellin is highly immunogenic and subject to detection by host pattern recognition receptors, its repression may represent an adaptive response that allows P. aeruginosa to avoid detection by host defense mechanisms and phagocytosis during the chronic phase of CF lung infections.
A number of environmental microorganisms possess the ability to cause serious human infections. These life-threatening situations result from a combination of factors, including host predisposition and the ability of these opportunistic pathogens to regulate expression of specific virulence factors in response to the changing host environment. For example, cystic fibrosis (CF) patients are highly susceptible to chronic lung infections by the environmental bacterium Pseudomonas aeruginosa (1). The predisposition of these individuals is a consequence of the pleiotropic effects of mutations in the host gene encoding the CF transmembrane conductance regulator (CFTR), but the pathogenesis of CF lung infections is poorly understood.
Several hypotheses have been proposed to explain this hypersusceptibility, including deficient antimicrobial peptide activity, reduced production of nitric oxide, increased adherence of bacteria to epithelial cells, and decreased epithelial cell ingestion (2). However, consistent in vivo evidence for these hypotheses has not yet emerged. It is likely, however, that defective mucus clearance contributes to the infection process. Mucus clearance is a key mechanism for defending the respiratory tract from bacterial challenge (3). Protection may in part be mediated by the ability of airway surface liquid components to specifically bind to pathogenic bacterial species and facilitate their removal. This clearance mechanism becomes defective because of specific genetic or acquired disorders. Recent studies indicate that in CF airways altered ion transport and accelerated airway surface liquid absorption results in decreased volume of the periciliary liquid layer, leading to mucus stagnation and the accumulation of adherent mucus plugs (4, 5). A number of studies have shown that during chronic CF lung infections P. aeruginosa resides within the viscous mucus layer and appears to form macrocolonies or biofilms embedded in mucus obstructions (6, 7). Despite these findings, it remains unclear why P. aeruginosa infections predominate in the CF lung given the myriad of infectious organisms that are likely encountered. We propose that adaptive microbial behavior may play a key role in enhancing the preferential survival and persistence of P. aeruginosa in this environment.
Although most P. aeruginosa strains in the environment rarely encounter a suitable human host, they possess a set of highly conserved genes encoding virulence determinants whose products have specific host targets (8). The pathogenesis of P. aeruginosa lung infections in CF likely requires the coordinately regulated expression of distinct sets of virulence determinants at different disease stages. As such, the genes necessary for initiation of infection from an environmental reservoir likely differ from those required for persistence and chronic infection. To recognize the adaptive responses that occur during the chronic phase of CF infections, we identified all of the genes differentially expressed by P. aeruginosa during growth in muco-purulent respiratory liquid (defined here as muco-purulent material, MPM) derived from chronically infected adult CF patients.
We show that exposure of P. aeruginosa to MPM from CF patients results in the activation of the Rhl-dependent quorum-sensing (QS) network and repression of a number of genes encoded proteins involved in or predicted to be involved in flagellar-mediated chemotactic motility including fliC, which encodes flagellin. Activation of the Rhl branch of the QS network indicates that bacterial cell-to-cell signaling molecules are present in CF respiratory liquid at biologically active concentrations and further supports the observation that QS molecules are produced during chronic CF lung disease (9). By comparing the transcriptional response of wild-type P. aeruginosa and QS signaling mutants, we identified a number of QS-dependent genes that appear to be specific to the CF airway environment.
Furthermore we discovered that in chronically infected CF airway liquid P. aeruginosa flagellin expression is repressed. The shut-off of flagellin is rapid, resulting in an 80% reduction of fliC mRNA within 2 h of MPMexposure. After prolonged exposure, fliC mRNA, flagellin, and flagellar filaments could not be detected. The repression of fliC expression was independent of QS and the known regulatory networks controlling the hierarchical expression of flagellar genes.
We propose that the cessation of fliC expression, as well as other transcriptional events including activation of the QS network, represent adaptive responses that contribute to the selective persistence of P. aeruginosa during the chronic phase of CF lung infections. The findings presented here provide insight into the transcriptional adaptations that occur during the pathogenesis of P. aeruginosa lung infections in CF.
Materials and Methods
Bacterial Strains, Media, and Transcriptional Profiling. For transcriptional profiling, wild-type P. aeruginosa strain PAK and an isogenic QS mutant lacking the acyl-homoserine lactone (HSL) responsive transcriptional regulators RhlR and LasR were used. The QS mutant was constructed by sequentially deleting the coding sequences of rhlR and lasR by a previously described technique (10). Bacteria were grown at 37°C in 12.5 ml of M63 medium [15 mM (NH4)2SO4/22 mM KH2PO4/40 mM K2HPO4/40 mM glucose/1 mM MgSO4/25 μM FeCl2] or in M63 supplemented with 10% (vol/vol) MPM collected from CF patients. MPM was obtained at the University of Florida Medical Center after chest physiotherapy to clear airway obstructions in chronically infected adult CF patients. MPM was sterilized by UV-irradiation and stored at -80°C. The addition of 10% MPM to M63 medium did not significantly influence bacterial growth rate (data not shown). Material collected from two different CF patients (samples 1 and 2) was used to assess the effect of MPM on global gene expression in wild-type P. aeruginosa. A single sample (sample 1) was used for transcriptional profiling of the rhlR, lasR double mutant. Duplicate experiments were performed for each condition. For each experiment bacteria were cultured to mid-exponential growth phase (OD600 ≈ 0.5) and total RNA was extracted at identical culture densities (confirmed by plating serial dilutions), converted to cDNA targets, and hybridized to GeneChip P. aeruginosa Genome Arrays (Affymetrix, Santa Clara, CA) as described (10).
Microarray Analysis. Hybridization data for all microarray experiments was analyzed by using GENESPRING 4.2.1 software (Silicon Genetics, Redwood City, CA) as described (10). See Table 1, which is published as supporting information on the PNAS web site, for a complete summary of microarray results. To identify a common set of MPM-responsive genes, MPM samples from two different patients were analyzed by microarray as described above. Those genes that showed a statistically significant (P < 0.1) change in expression and a >5-fold change in magnitude when grown in the presence of 10% MPM relative to M63 medium alone were regarded as significant. In total, 181 genes met this condition, with 81 genes responding to sample 1 and 160 genes showing a response to sample 2. Sixty-one of the 181 genes responded to both MPM samples. Of the remaining 120 genes 100 showed a similar trend; however, the response to one of the two samples did not pass the statistical criteria described above (Table 1). The remaining 20 genes represent a limited set of patient-specific responses (Table 1).
Electron Microscopy. P. aeruginosa cultures were grown overnight as described above in M63 alone or supplemented with 10% MPM. A drop of culture was placed on a carbon-coated grid for 10 sec, excess liquid was drained, and the grid was rinsed in a drop of saline and adherent cells stained with 1% aqueous uranyl acetate for 30 sec. Samples were examined with a Hitachi H-7000 transmission electron microscope.
Reverse Transcription Polymerase Chain Reactions. Wild-type P. aeruginosa was grown overnight in M63 and subcultured in either M63 alone or supplemented with 10% MPM. Total RNA was isolated at 0, 2, and 4 h after subculture. Reverse transcription was performed by using 200 ng of each RNA sample, random hexamer primer mix, and Superscript II reverse transcriptase according to the manufacturers recommendations (Invitrogen). After RNaseH digestion, 2 μl of each cDNA sample were amplified by PCR with primers specific for either fliC or the constitutively expressed control gene clpX.
β-Galactosidase Assays. A chromosomal fliC transcriptional reporter was engineered by cloning a 521-bp DNA fragment carrying the fliC promoter and 107 base pairs of coding sequence upstream of the lacZ gene encoding β-galactosidase as described (10). The resulting construct was moved onto the chromosome of wild-type strain PAK and the isogenic mutants fliA, fliC, fliD, and flgM (11-13). Before assays strains were grown overnight in the presence and absence of 10% MPM, diluted into the same medium, and grown for 3 h. β-galactosidase activity was measured in triplicate and normalized based on colony-forming units.
Results and Discussion
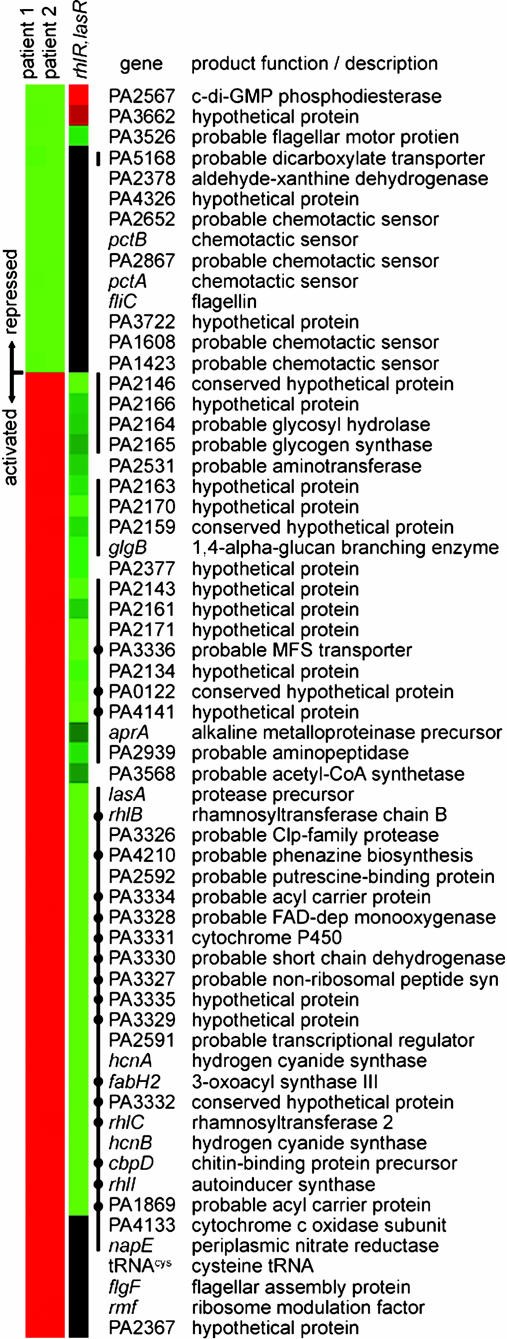
Using P. aeruginosa whole-genome DNA microarrays, we identified 61 genes that exhibited a statistically significant change of >5-fold after growth in M63 medium containing 10% MPM from two different CF patients relative to M63 medium alone (Fig. 1). This group includes 14 repressed and 47 activated genes. Eight of the repressed genes encoded proteins involved in or predicted to be involved in flagellar-mediated chemotactic motility, including six putative methyl-accepting chemotactic sensor proteins, flagellin, and a protein with homology to the flagellar motor protein MotY.
Fig. 1.
Cluster analysis (14) of P. aeruginosa genes showing altered expression after growth in the presence of MPM collected from the airways of chronically infected CF patients. Indicated in the first and second columns are genes showing a >5-fold change in expression in M63 medium containing 10% MPM collected from two different CF patients relative to M63 alone (samples 1 and 2, respectively). Genes represented in green are repressed in response to signal(s) present in the MPM samples. Genes in red are activated. The role of the Rhl and Las QS systems in controlling the expression of these MPM-responsive genes was determined by comparing gene expression in a rhlR, lasR double mutant relative to wild type, both in the presence of 10% MPM (sample 1). In the third column, genes represented in green require the QS system for activation in MPM, genes in red require QS for repression in MPM and genes in black are QS-independent (<2-fold change). Black lines indicate genes previously shown to be regulated by the QS system (15, 16). Black dots denote genes specifically regulated by the Rhl QS system as inferred from Schuster and coworker (15).
Evidence indicates that flagella play a critical role in initial colonization and acute infection and that they are important in facilitating the formation of resilient bacterial communities or biofilms (17-19). Despite the apparent importance of flagella in the early stages of infection it has been shown that flagellin, the structural subunit of the flagellar fiber, is recognized by the host toll-like receptor 5 and leads to the mobilization of various innate defense mechanisms via the NF-κB signaling pathway (20, 21). In addition, it is known that flagellin-specific antibodies are produced during CF lung infections and that flagellin can serve as an efficient ligand for opsonic and nonopsonic phagocytosis (22-25).
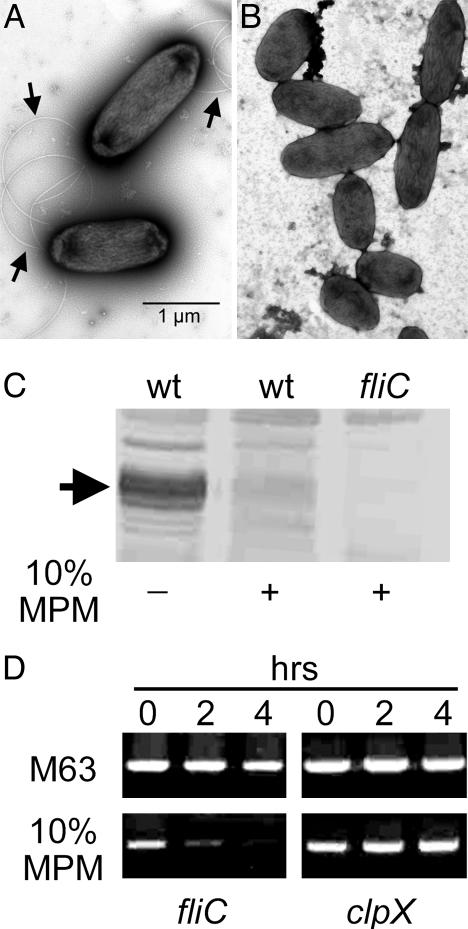
Given the dual role of P. aeruginosa flagella as an important virulence factor and a highly potent activator of host innate and acquired immunity, we carried out a series of studies to corroborate the findings of the DNA microarrays. Electron microscopic examination demonstrated that flagella were absent from the surface of 10% MPM grown P. aeruginosa, whereas these structures were readily visible on the same bacteria grown in medium lacking CF airway liquid (Fig. 2 A and B). Immunoblot analysis of whole-cell protein extracts using flagellin specific polyclonal antiserum showed that P. aeruginosa did not synthesize flagellin in the presence of MPM (Fig. 2C). In addition, the kinetics of fliC repression was examined by reverse transcription PCR (Fig. 2D). An 80% reduction in the amount of flagellin-specific mRNA was detected after a 2-h exposure to 10% MPM. After a 4-h exposure to 10% MPM, flagellin mRNA was almost completely absent.
Fig. 2.
Exposure to CF derived muco-purulent respiratory material results in transcriptional repression of fliC, encoding flagellin. Electron microscopic observation indicates that polar flagellar filaments, composed of flagellin (arrows) are present (A) on the surface of P. aeruginosa grown in M63 medium and absent (B) from the surface of bacteria grown in the same medium containing 10% MPM. (C) Immunoblot analysis of whole-cell protein samples with flagellin-specific antiserum confirms that P. aeruginosa grown in the presence of 10% MPM produce significantly reduced amounts of flagellin (arrow) compared to equivalent samples derived from bacteria grown in medium lacking MPM. No flagellin is detectable in protein samples from bacteria lacking the flagellin gene fliC. (D) Reverse transcription polymerase chain reactions with fliC-specific oligonucleotide primers and total mRNA extracted from wild-type P. aeruginosa at 0, 2, and 4 h after subculture in M63 medium with or without 10% MPM. Samples were equalized based on RT-PCR products for the constitutively expressed clpX gene.
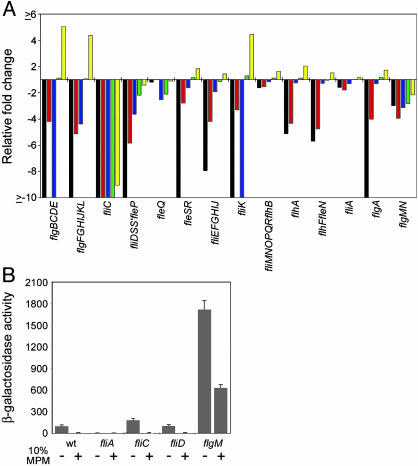
The flagellum of P. aeruginosa, like that of other bacteria, is assembled in a hierarchical fashion in a process driven by the sequential expression of flagellar genes in the order of their assembly (11, 26). To determine whether the MPM response correlated with a specific defect in the flagellar biosynthesis pathway, we examined transcriptomes previously derived for a set of flagellar regulatory mutants (11). The effect of MPM on flagellar gene expression was most similar to that seen in a mutant lacking the alternative sigma factor FliA (11, 27) (Fig. 3A). However, repression of fliA transcription does not account for the results seen here, as fliA mRNA levels were unchanged in MPM-containing medium relative to M63 alone (Table 1). In light of this finding, we examined the effect of a mutation in flgM, which encodes a FliA-specific anti-sigma factor (13), on fliC transcription in the presence and absence of 10% MPM (Fig. 3B). The mutation in flgM resulted in an ≈17-fold increase in the expression of a fliC::lacZ transcriptional reporter compared to wild-type in M63 medium. However, in the absence of FlgM, transcription from the fliC promoter was still repressed by medium containing CF respiratory liquid (Fig. 3B). To examine the possibility that flagellar binding of a mucus component directly initiated a cessation of fliC expression we assayed the effect of MPM exposure on transcription of the fliC::lacZ reporter in mutants lacking flagellin or the mucin-specific adhesin FliD (12) (Fig. 3B). In both cases, expression from the fliC promoter was repressed by 10% MPM. These findings suggest that the repressive signal likely acts through a previously uncharacterized transcriptional mechanism that alters FliA function. Moreover, we have demonstrated that mutants that did not assemble flagellar filaments or bind mucin are still subject to the same repressive signal.
Fig. 3.
The mechanism of fliC repression in response to MPM likely acts through the alternative sigma factor FliA. (A) Presented is a comparison of the transcriptional response of flagellar biogenesis genes in mutants lacking known flagellar regulators relative to wild type and the expression change in wild type grown in 10% MPM relative to medium lacking MPM. The transcriptional response is given as the average fold change for each of the 14 flagellar biogenesis gene operons (11). Expression data for the flagellar regulatory mutants was derived from previously published transcriptomes (11). Black bars, rpoN mutant vs. wild type; red bars, fleQ mutant vs. wild type; blue bars, fleR mutant vs. wild type; green bars, fliA mutant vs. wild type; yellow bars, wild type grown in M63 containing 10% MPM vs. M63 alone. (B) Response of a fliC::lacZ transcriptional reporter in wild type and flagellar mutants in the absence and presence of 10% MPM.
To access whether the host signal responsible for the shut-off of flagellin is restricted to MPM from CF patients, we examined the transcriptional response of fliC to respiratory fluid collected from individuals with non-CF bronchiectasis. Although the pathogenesis of this disease is poorly understood, it is characterized by the chronic production of muco-purulent sputum, neutrophil-dominated airway inflammation, and hypersusceptibility to chronic and recurrent bacterial infections including P. aeruginosa (28, 29). Using P. aeruginosa carrying the fliC::lacZ transcriptional reporter, we demonstrated that M63 medium containing non-CF bronchiectasis respiratory fluid was also capable of repressing the transcription of fliC (data not shown).
The repression of flagellin in response to signals in chronically infected host airway liquid may allow P. aeruginosa to avoid clearance by phagocytosis. In fact, a number of studies have shown that P. aeruginosa lacking flagella are resistant to phagocytosis by macrophages and polymorphonuclear leukocytes (25, 30). This observation is particularly relevant as the chronic phase of CF lung infections is characterized by considerable neutrophil dominated inflammation. Interestingly, most environmental P. aeruginosa strains and those isolated early in CF infections are highly motile, whereas those isolated from older chronically infected CF patients show a high frequency of mutations leading to the loss of flagellar formation (30). As such, it appears that sustained genetic repression of flagellin expression in chronic infections may be replaced by mutations that result in a permanent adaptation.
Among the genes significantly activated in the presence of CF MPM was rhlI encoding the N-butyryl-L-HSL (C4-HSL) autoinducer synthesis protein and a number of genes indicative of a QS response (Fig. 1). The detection of a quorum response under the low culture density conditions used in this study indicated the presence of biologically significant levels of HSLs in the airway fluids of chronically infected CF patients. This result was consistent with previous studies which have shown that sputum samples from CF patients colonized with P. aeruginosa contain detectable levels of C4-HSL and N-3-oxododecanoyl-L-HSL (3OC12-HSL) the autoinducers for the Rhl and Las QS systems, respectively (9). Activation of the P. aeruginosa QS regulatory network during chronic infections correlates with the formation of polysaccharide encapsulated biofilm-like structures within airway mucus plugs (7, 9, 31). These bacterial communities appear to be highly resistant to innate and adaptive host defense mechanisms (32, 33). In addition, the P. aeruginosa QS system has been shown to control the expression of 6-10% of P. aeruginosa genes, a number of which encode known extracellular virulence factors (15, 16).
Given the significant role that QS plays in gene regulation, we engineered a strain of P. aeruginosa lacking both of the quorum responsive transcriptional regulators RhlR and LasR and analyzed gene expression in this strain after growth in 10% MPM-containing medium. A substantial fraction of the MPM-regulated genes were under the control of RhlR and/or LasR (Fig. 1). However, the expression of the MPM-repressed flagellar genes including fliC and the chemotactic sensors did not depend on RhlR or LasR, indicating that repression was independent of QS (Fig. 1). This result was confirmed by assaying the activity of the fliC::lacZ transcriptional fusion in the rhlR, lasR double mutant grown in the presence and absence of 10% MPM (data not shown).
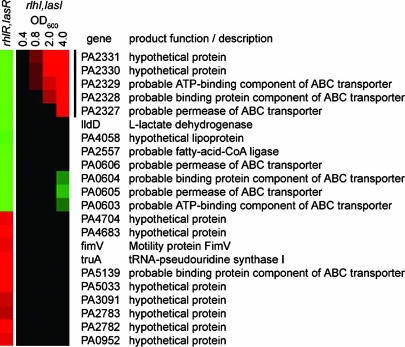
Examination of the list of MPM-responsive genes that were specifically dependent on the RhlR and LasR regulators revealed that many had been previously identified during recent exhaustive attempts to catalog QS responsive genes under various laboratory conditions (15, 16) (Fig. 1). Many of the most highly MPM-activated genes are specifically regulated by the Rhl-dependent QS system implicated in biofilm formation (9, 15, 31) (Fig. 1). In addition, our approach showed that a number of genes, not identified as part of the QS regulon under laboratory conditions, were significantly activated or repressed in the context of M63 medium containing CF respiratory liquid (15) (Fig. 4). Of note is a putative operon of five genes encoding a complete ABC transport system (PA2327-PA2331), which was previously identified as activated by QS (15). Here we found that in the presence of CF respiratory liquid, this cluster of genes is repressed by the QS system (Fig. 4). These results highlight the fact that QS signaling in the CF lung environment extends beyond the network observed in laboratory media.
Fig. 4.
Environment-specific QS gene response. Cluster analysis (14) of genes that show CF respiratory liquid-specific patterns of QS-dependent expression not previously identified under laboratory growth conditions. Indicated in the first column are genes showing a >5-fold change in expression in wild-type P. aeruginosa relative to a rhlR, lasR double mutant, both grown in M63 medium containing 10% MPM. Green and red depict QS-dependent genes that are repressed or activated, respectively, in 10% MPM. The second through fifth columns represent the expression pattern of these genes at the indicated culture densities (OD600) in laboratory medium supplemented with synthetic autoinducers (rhlI, lasI mutant with added autoinducers versus no autoinducer). Genes in green and red are repressed and activated, respectively, by the addition of exogenous autoinducers. Genes in black are QS-independent (<2-fold change). Data for growth phase-dependent gene expression in the presence of P. aeruginosa QS autoinducers has been published (15).
In summary, we have identified two major bacterial adaptive responses that occur during growth in the presence of MPM collected from the airways of chronically infected CF patients. These responses included the repression of a subset of genes involved in flagellar biogenesis and function and activation of a set of HSL- or quorum-responsive genes. The findings presented here indicate that during the course of CF respiratory infections P. aeruginosa utilizes different strategies to evade host defenses by systematically activating transcriptional programs in response to the changing lung environment. As such, any antimicrobial strategies aimed at preventing early infection in CF have to be different from those used to treat chronically infected older patients.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01-AI45014 (to R.R.) and R01-AI21451 (to S.L.). M.C.W. was supported by a Cystic Fibrosis Foundation Postdoctoral Research Fellowship. A.L.G. is a Howard Hughes Medical Institute Predoctoral Fellow. Cystic Fibrosis Foundation Therapeutics, Inc., subsidized the purchase of GeneChip P. aeruginosa genome arrays.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CF, cystic fibrosis; HSL, homoserine lactone; MPM, muco-purulent material; QS, quorum-sensing.
References
- 1.Lyczak, J. B., Cannon, C. L. & Pier, G. B. (2002) Clin. Microbiol. Rev. 15, 194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pier, G. B. (2002) Curr. Opin. Microbiol. 5, 81-86. [DOI] [PubMed] [Google Scholar]
- 3.Knowles, M. R. & Boucher, R. C. (2002) J. Clin. Invest. 109, 571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui, H., Grubb, B. R., Tarran, R., Randell, S. H., Gatzy, J. T., Davis, C. W. & Boucher, R. C. (1998) Cell 95, 1005-1015. [DOI] [PubMed] [Google Scholar]
- 5.Tarran, R., Grubb, B. R., Parsons, D., Picher, M., Hirsh, A. J., Davis, C. W. & Boucher, R. C. (2001) Mol. Cell 8, 149-158. [DOI] [PubMed] [Google Scholar]
- 6.Worlitzsch, D., Tarran, R., Ulrich, M., Schwab, U., Cekici, A., Meyer, K. C., Birrer, P., Bellon, G., Berger, J., Weiss, T., et al. (2002) J. Clin. Invest. 109, 317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam, J., Chan, R., Lam, K. & Costerton, J. W. (1980) Infect. Immun. 28, 546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfgang, M. C., Kulasekara, B. R., Liang, X., Boyd, D., Wu, K., Yang, Q., Miyada, C. G. & Lory, S. (2003) Proc. Natl. Acad. Sci. USA 100, 8484-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh, P. K., Schaefer, A. L., Parsek, M. R., Moninger, T. O., Welsh, M. J. & Greenberg, E. P. (2000) Nature 407, 762-764. [DOI] [PubMed] [Google Scholar]
- 10.Wolfgang, M. C., Lee, V. T., Gilmore, M. E. & Lory, S. (2003) Dev. Cell 4, 253-263. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta, N., Wolfgang, M. C., Goodman, A. L., Arora, S. K., Jyot, J., Lory, S. & Ramphal, R. (2003) Mol. Microbiol. 50, 809-824. [DOI] [PubMed] [Google Scholar]
- 12.Arora, S. K., Ritchings, B. W., Almira, E. C., Lory, S. & Ramphal, R. (1998) Infect. Immun. 66, 1000-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisk, A., Jyot, J., Arora, S. K. & Ramphal, R. (2002) J. Bacteriol. 184, 1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisen, M. B., Spellman, P. T., Brown, P. O. & Botstein, D. (1998) Proc. Natl. Acad. Sci. USA 95, 14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster, M., Lostroh, C. P., Ogi, T. & Greenberg, E. P. (2003) J. Bacteriol. 185, 2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner, V. E., Bushnell, D., Passador, L., Brooks, A. I. & Iglewski, B. H. (2003) J. Bacteriol. 185, 2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman, M., Bryan, R., Rajan, S., Scheffler, L., Brunnert, S., Tang, H. & Prince, A. (1998) Infect. Immun. 66, 43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klausen, M., Heydorn, A., Ragas, P., Lambertsen, L., Aaes-Jorgensen, A., Molin, S. & Tolker-Nielsen, T. (2003) Mol. Microbiol. 48, 1511-1524. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole, G. A. & Kolter, R. (1998) Mol. Microbiol. 30, 295-304. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi, F., Smith, K. D., Ozinsky, A., Hawn, T. R., Yi, E. C., Goodlett, D. R., Eng, J. K., Akira, S., Underhill, D. M. & Aderem, A. (2001) Nature 410, 1099-1103. [DOI] [PubMed] [Google Scholar]
- 21.Ciacci-Woolwine, F., McDermott, P. F. & Mizel, S. B. (1999) Infect. Immun. 67, 5176-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson, T. R. & Montie, T. C. (1987) Infect. Immun. 55, 3204-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagace, J., Peloquin, L., Kermani, P. & Montie, T. C. (1995) J. Med. Microbiol. 43, 270-276. [DOI] [PubMed] [Google Scholar]
- 24.Anderson, T. R., Montie, T. C., Murphy, M. D. & McCarthy, V. P. (1989) J. Clin. Microbiol. 27, 2789-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahenthiralingam, E. & Speert, D. P. (1995) Infect. Immun. 63, 4519-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chilcott, G. S. & Hughes, K. T. (2000) Microbiol. Mol. Biol. Rev. 64, 694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starnbach, M. N. & Lory, S. (1992) Mol. Microbiol. 6, 459-469. [DOI] [PubMed] [Google Scholar]
- 28.Angrill, J., Agusti, C. & Torres, A. (2001) Curr. Opin. Infect. Dis. 14, 193-197. [DOI] [PubMed] [Google Scholar]
- 29.Barker, A. F. (2002) N. Engl. J. Med. 346, 1383-1393. [DOI] [PubMed] [Google Scholar]
- 30.Mahenthiralingam, E., Campbell, M. E. & Speert, D. P. (1994) Infect. Immun. 62, 596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies, D. G., Parsek, M. R., Pearson, J. P., Iglewski, B. H., Costerton, J. W. & Greenberg, E. P. (1998) Science 280, 295-298. [DOI] [PubMed] [Google Scholar]
- 32.Meluleni, G. J., Grout, M., Evans, D. J. & Pier, G. B. (1995) J. Immunol. 155, 2029-2038. [PubMed] [Google Scholar]
- 33.Jesaitis, A. J., Franklin, M. J., Berglund, D., Sasaki, M., Lord, C. I., Bleazard, J. B., Duffy, J. E., Beyenal, H. & Lewandowski, Z. (2003) J. Immunol. 171, 4329-4339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.