Abstract
Life or death in hostile environments depends crucially on one's ability to detect and gate novel sounds to awareness, such as that of a twig cracking under the paw of a stalking predator in a noisy jungle. Two distinct auditory cortex processes have been thought to underlie this phenomenon: (i) attenuation of the so-called N1 response with repeated stimulation and (ii) elicitation of a mismatch negativity response (MMN) by changes in repetitive aspects of auditory stimulation. This division has been based on previous studies suggesting that, unlike for the N1, repetitive “standard” stimuli preceding a physically different “novel” stimulus constitute a prerequisite to MMN elicitation, and that the source loci of MMN and N1 are different. Contradicting these findings, our combined electromagnetic, hemodynamic, and psychophysical data indicate that the MMN is generated as a result of differential adaptation of anterior and posterior auditory cortex N1 sources by preceding auditory stimulation. Early (≈85 ms) neural activity within posterior auditory cortex is adapted as sound novelty decreases. This alters the center of gravity of electromagnetic N1 source activity, creating an illusory difference between N1 and MMN source loci when estimated by using equivalent current dipole fits. Further, our electroencephalography data show a robust MMN after a single standard event when the interval between two consecutive novel sounds is kept invariant. Our converging findings suggest that transient adaptation of feature-specific neurons within human posterior auditory cortex filters superfluous sounds from entering one's awareness.
Survival of higher organisms depends on their ability to automatically distinguish novel (“deviant”) sounds amongst background environmental noise. Because conscious attention can dwell on but few events at a time, it is clear that scanning the entire auditory scene bit-by-bit for novelty would be highly inefficient. Instead, novelty needs to be gated to awareness in a fast “bottom-up” manner. This requires preattentive “filtering out” of irrelevant stimuli, whilst letting the novel sounds swiftly enter our conscious attention (1). Although it is a widely held view that novel sounds are preattentively gated to awareness based on their degree of physical difference to preceding sensory input, the underlying neural mechanisms have remained elusive. Here, we investigated this question in healthy humans by using a combination of 3-T functional MRI (fMRI), magnetoencephalography (MEG), electroencephalography (EEG), and psychophysics.
Previous EEG and MEG studies, measuring scalp electric potentials and extracranial magnetic fields generated by neural responses to sensory stimuli, have suggested that two distinct auditory cortex processes underlie preattentive gating of novel sounds to awareness. First, attenuation of an early ≈100-ms “N1” sensory evoked electromagnetic response with repeated stimulation is thought to reflect short-lived adaptation of auditory cortex neurons (2, 3). Second, the so-called mismatch negativity response (MMN), elicited by stimulus change at ≈100-200 ms, is presumed to reflect relatively automatic (4) comparison of incoming sounds to putative auditory cortex sensory-memory representations that encode repetitive aspects of the auditory environment (5-7). This prevailing theory assumes that there are separate change-specific neurons in the human auditory cortex that give rise to the MMN (5). Alternatively, it has been suggested that the MMN could be elicited by a novel sound because preceding auditory stimuli transiently adapt feature-specific auditory cortex neurons (8). In this latter model (here termed the “adaptation hypothesis”) the N1 response to the superfluous (i.e., nonnovel) sounds is delayed and suppressed as a function of its similarity to the preceding auditory events, thus giving rise to the classic MMN.
To date, there has not been conclusive empirical data to decide between these two alternative interpretations. Although intracortical recordings in the macaque monkey (9), and recent single-neuron recordings in the cat (10), tentatively support the adaptation hypothesis, these studies were limited to the primary auditory cortex (A1) (9, 10). Thus, change-specific MMN neurons possibly located outside of this relatively small anatomical area were not measured (11). In contrast, empirical efforts in humans have suggested that separate change-specific neural populations, rather than stimulus-specific adaptation, would underlie the MMN (12). Specifically, previous MEG studies have consistently shown that the MMN originates ≈7-10 mm anterior to the N1 response in the auditory cortex (3, 12, 13). However, there are (at least) two auditory cortex sources that contribute to the N1 response; an early posterior N1 at ≈85 ms and a temporally lagging anterior N1 (≈150 ms) that closely matches the source locus of MMN (14-16). Changes in the relative amplitudes between these two sources could alter the center of gravity of the underlying source configuration that single equivalent current dipole (ECD) estimates approximate (17), possibly explaining the noted ≈7- to 10-mm differences between the N1 and MMN source loci. Another line of evidence that has been taken to support the hypothesis that MMN is generated by a mechanism separate from that of the N1 comes from reports showing that repetitive “standard” events constitute a prerequisite to elicitation of a robust MMN by the subsequent novel stimulus (5, 18, 19), whereas the N1 is elicited by any observable auditory stimulus. However, the novel-to-novel sound interval decreased as the number of intervening standard stimuli decreased in these studies (5, 18, 19). Thus, the failure to observe MMN after a single preceding/intervening standard stimulus may have resulted from attenuation of the novel-sound N1 response at short internovel intervals (2).
Here we hypothesized that a robust MMN is elicited by a novel stimulus even after a single preceding standard stimulus when the internovel interval is kept fixed while varying the number of preceding standard stimuli. We also hypothesized that the posterior N1 source is more robustly adapted than the anterior N1 source by preceding standard stimuli, and that this explains the previously reported differences in the center of gravity of N1 and MMN source loci. Further, by explicitly combining fMRI and MEG data, we aimed at pinpointing the anatomical structures involved in preattentive gating of novel sounds to awareness.
Methods
Subjects. A total of 17 healthy volunteers (ages 21-42 years, three females) with normal hearing participated in four separate experiments after voluntary consent was obtained. Human subjects' approval was obtained from the local institution in accordance with the Helsinki declaration.
Data Recording, Tasks, and Stimuli. Experiment 1. Subjects (n = 7) underwent two separate sessions, wherein 50-ms “novel” tones (5-ms rise/fall times, in separate blocks of either 1,020, 1,127, or 1,320 Hz) were presented once every 3.5 s to the left ear at 60 dB over hearing threshold during simultaneous recording of 122-channel MEG and nose-referenced 64-channel EEG (Neuromag, Helsinki) (0.03- to 100-Hz bandpass, 397-Hz digitization rate). The frequency and number of standard stimuli that preceded each novel stimulus was manipulated as follows: in one of the sessions, three “test” blocks were presented wherein a single 1,000-Hz standard tone occurred 0.35 s before each novel tone. In three control blocks, this preceding tone was of the same frequency as the subsequent novel tone. In the other session, multiple (two to four) stimuli, as opposed to a single tone, preceded each novel tone. Order of the sessions and blocks were counterbalanced across subjects. For an illustration of the paradigm used, see Fig. 1. The subjects were watching a silent movie of their own preference, ignoring the tones presented. At least 300 artifact-free (peak-to-peak amplitude <300 μV and 3,000 fT/cm in electrooculography/EEG and MEG, respectively) novel-tone EEG epochs were collected and averaged per block. The averaged EEG epochs were filtered off-line at 1.0-15 Hz, and a 50-ms prestimulus baseline was used.
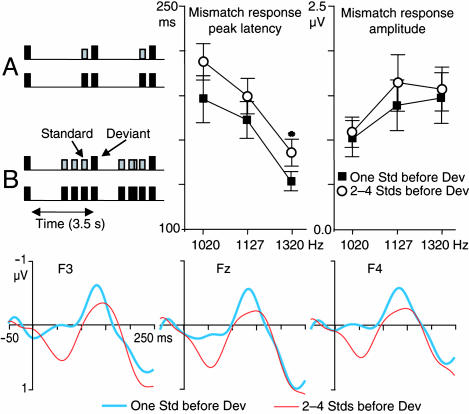
Fig. 1.
MMN to a novel stimulus presented after a single standard stimulus. (Upper Left) The experimental paradigm. (Upper Right) The mean (±SEM) MMN peak latencies and amplitudes in the different stimulus conditions (n = 7). Peak latencies were shorter, and amplitudes larger, with larger standard-novel sound difference, thus replicating previous observations (5). (Lower) Grand-average subtraction waveforms showing the MMN to 1,320-Hz novel tones at frontal EEG leads. Tentatively, the slight between-condition differences in the responses suggest that repeated stimulus presentation may enhance stimulus-specific adaptation, as reflected in longer latencies and diminished response amplitudes. [Note that to correct for possible baseline shifts, the MMN amplitude was, conservatively, quantified (at Fz) as the difference between the negative-going peak and the average of the preceding and subsequent positive-going peaks. Without this highly conservative correction, somewhat larger MMN amplitudes were observed with a single vs. multiple standard stimuli preceding the novel stimuli.]
Experiment 2. Eighty-millisecond pink-noise bursts (1/5-octave in bandwidth, 10-ms rise/fall times) were presented to the right ear of seven subjects at 60 dB over hearing threshold during simultaneous recording of 306-channel MEG and nose-referenced 60-channel EEG (Neuromag) (600-Hz sampling rate, 0.03- to 172-Hz bandpass). Fig. 2 illustrates the stimulus paradigm. In three novel sounds with standards stimulus blocks, repetitive homogeneous standard stimuli (241 Hz in sound center frequency, 2-Hz presentation rate) were presented in between the novel stimuli (P = 0.15) that were, in different blocks, either one, two, or four octaves higher in sound center frequency than the standard stimuli. In three separate novel sounds without standard stimulus blocks, the novel stimuli were presented without intervening standard stimuli. Order of the stimulus blocks was counterbalanced across subjects. The subjects were instructed to ignore the stimuli presented. At least 150 artifact-free epochs were averaged per stimulus type per subject (artifact rejection criteria as in experiment 1).
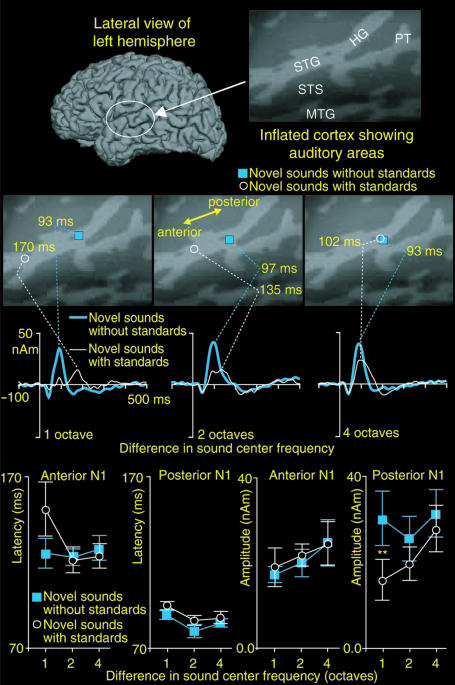
Fig. 2.
EEG responses to novel sounds with and without intervening standard stimuli. (Upper Left) A schematic illustration of the stimulus paradigm. (Upper Right) Mean (±SEM) novel sound response latencies and amplitudes in the “novel sounds with standards” and “novel sounds without standards” conditions. (Lower) Grand-averaged (n = 7) novel sound responses in the novel sounds with standards and novel sounds without standards conditions are shown at a frontal (Fz) electrode position. The convergence of response waveforms with the large (four octave) physical difference between the novel sounds and the intervening standard stimuli suggests that the MMN arises because of selective adaptation of the N1 response by preceding standard stimuli, rather than being generated by distinct neural populations.
Experiment 3. The same stimuli were used as in experiment 2. However, in these separate psychophysical distraction experiments we measured both increases in reaction time and reductions in hit rate that were induced by the three (unattended) novel sounds on simultaneously presented visual forced-choice trials (20), in both novel sounds with standards and novel sounds without standards conditions. The visual trials were even (i.e., 2, 4, 6, and 8) and odd (1, 3, 5, and 7) digits presented on a computer screen. The subjects (n = 7) had 1.3 s to press either of two response keys during each trial in response to odd vs. even digits and were instructed to ignore the auditory stimuli. During half of the blocks, a varying number of repetitive standard stimuli preceded the visual digits, and either an additional standard or one of the novel sounds cooccurred with the presentation of the visual digit. The rest of the blocks were otherwise identical but lacked the standard stimuli.
Experiment 4. Subjects (n = 7) were scanned by using a 3-T Siemens Allegra fMRI scanner, using identical stimulus/task paradigm as in experiment 2. A “silent” fMRI blood oxygenation level-dependent (BOLD) sequence was used (21) (time to echo = 30 ms, flip angle = 90°, time to repetition = 1,051 ms). Sixteen axial slices with 3 × 3 × 3-mm voxel size were aligned along the superior aspect of the left temporal lobe that contains the auditory cortex contralateral to the stimulated right ear. To circumvent scanner noise contamination of the hemodynamic responses (21), the echo planar imaging (EPI) volumes were obtained once every 10 s during alternating 30-s silent baseline, standard sounds alone, novel sounds alone, and novel sounds with standard sounds conditions (three novel sounds were presented, at 4.5, 3, and 1.5 s, before each EPI volume acquisition). A 10-cm-diameter transmit-receive surface coil, placed over the left temporal bone, was used to enhance the signal-to-noise ratio by a factor of ≈3 in the regions of interest. Sixty EPI volumes were obtained per condition to reach sufficient experimental power (1 - β > 0.8) to detect fMRI activations due to putative change-specific auditory cortex neurons. The scanner's coolant pump was switched off during the functional scans to avoid contamination by its low-frequency acoustic noise.
Data Analysis. EEG responses. EEG responses collected in experiments 1 and 2 were analyzed as follows. The amplitude and peak latency of MMN and N1 responses were quantified from the Fz electrode position, where the signals are largest (5). Repeated-measures ANOVA was used in testing for statistically significant differences across experimental conditions. In experiment 1, to make the results directly comparable to previous reports showing lack of MMN after a single standard stimulus, the MMN was obtained by subtracting the novel-standard difference waveforms of the control blocks from the novel-standard difference waveforms of the respective test blocks (the two to four standard responses before the novel sound were averaged for the subtraction procedure). In experiment 2 the response peak latencies and amplitudes were quantified from the novel-sound response (as the most negative-going peak between 100-200 ms), rather than novel minus standard difference waveforms, as recently suggested (8, 22). The characteristic reversal of N1 and MMN amplitude polarity at mastoid recording sites was used in dissociating the MMN from overlap of the subsequent attention-dependent N2b component in subjects eliciting the N2b. Baseline was set at -50-0 ms, and the responses were filtered at 1-30 Hz.
MEG responses. The MEG responses collected in experiments 1 and 2 were off-line filtered at 1-40 Hz and baseline-corrected at -50-0 ms. A fixed subset of 34 MEG channels over the left temporal lobe were used in fitting the ECD models of the recorded data in a least squares sense. The loci, amplitudes, and latencies of the anterior and posterior N1 responses were estimated using ECD fits as described (14-16). Specifically, the posterior N1 was fitted at the ascending phase (≈85 ms) and the anterior N1 was fitted at the descending phase, or during a second peak, of the N1 response. The resulting ECDs were then entered to a time-varying multi-ECD model to validate the goodness of fit of the model with the recorded signals. Statistically significant differences across experimental conditions were tested using repeated-measures ANOVA.
fMRI data analysis. The fMRI data collected in experiment 4 were analyzed as follows. Each voxel exhibiting significant (>3 Z scores) BOLD signal increases in any of the stimulation conditions vs. the silent baseline within the left temporal lobe was included to the region of interest (ROI). Each voxel within the ROI exhibiting larger (>2 Z scores) signal intensity changes during either novel sounds with standards or novel sounds without standards conditions vs. the standard sounds alone condition was further coded as being sensitive to novel sounds. For each of these it was then tested, using repeated-measures ANOVA, whether the BOLD signal increases induced by novel sounds with standards stimulation significantly exceeded the linear sum of the signal increases induced by standard and novel stimuli when presented alone. This test (described in ref. 23) was adopted to specifically pinpoint auditory cortex fMRI activations due to activation of any putative change-specific neurons generating the MMN response.
Combined fMRI/MEG estimates. Combined fMRI/MEG estimates of cortical source configurations were obtained by constraining the well defined minimum norm estimate (based on all 306 MEG channels) to cortical gray matter in each of the four subjects who participated both in experiment 2 and 4. The solutions were 90% weighted by significant fMRI signal changes observed in the novel sounds with standards condition vs. the silent baseline (for a detailed description of the method, see refs. 24 and 25). Noise-normalized estimates were used to obtain time-varying statistical parametric maps (SPM) (26). Whole-head fMRI scans were taken to extend the coverage of the functional activations to the whole brain for calculation of the fMRI-constrained MEG continuous source estimates. Separately obtained T1-weighted 3D anatomical MRIs were used to construct individual realistically shaped boundary element models for MEG forward solutions (26, 27).
Results
EEG Responses. Experiment 1. When the interval between two consecutive novel stimuli was held constant at 3.5 s, and each of the novel tones was preceded either by a single or two to four standard stimuli, the MMN tended to be more robust after a single than after two to four preceding standard stimuli (Fig. 1). Further, the peak latency of the MMN was significantly longer than that of the N1 responses (P < 0.01 for each condition). Experiment 2. The response amplitudes to novel stimuli, separated by one and two octaves from the standard stimuli, were significantly suppressed in the novel sounds with standards conditions, as compared with the novel sounds without standards conditions (P < 0.001 and 0.05, respectively). Significantly delayed peak latencies were also noted for the one-octave difference in the novel sounds with standards condition (P < 0.05). In contrast, the responses to novel sounds separated by four octaves from the standards were identical, irrespective of whether the novel stimuli were preceded by standard stimuli (Fig. 2).
MEG Responses: Experiment 2. We observed more anterior ECD loci in the novel sounds with standards than in the novel sounds without standards conditions when the source loci were estimated at response peak latencies. However, this effect vanished with the large (four-octave) frequency separation between the novel and standard stimuli (Fig. 3). At the group level (mean ± SEM), 6.2 ± 3.1, 1.1 ± 2.8, and -0.6 ± 2.9 mm more anterior ECD loci were observed in the novel sounds with standards than in the novel sounds without standards conditions with the one-, two-, and four-octave standard-novel differences, respectively (P < 0.07). When we separately analyzed the relative amplitudes of anterior and posterior N1 responses, we observed that the amplitude of the posterior N1 response was rapidly suppressed with decreasing sound novelty (P < 0.01). In contrast, the amplitude of the anterior N1 response was little affected (Fig. 3). Statistically significant 55% reductions also in the amplitude of the anterior N1 response amplitude were, however, noted when the standard-novel difference was 1/3 octave (P < 0.02) in experiment 1. This finding suggests that the neurons generating the anterior N1 source are more narrowly tuned on sound frequency than those underlying the posterior N1 response. Further, the anterior N1 response was significantly increased in latency as a function of decreasing sound novelty (Fig. 3). The estimated ECD loci of neither the anterior nor the posterior N1 responses differed across experimental conditions.
Fig. 3.
ECD analyses of anterior and posterior auditory cortex N1 responses. (Top) Lateral view of single-subject reconstructed left hemisphere and a patch of inflated cortex [i.e., cortical curvature maps (30)] disclosing auditory areas hidden within the Sylvian fissure. (Middle) Single-subject ECD fits at the novel-response peak latencies show more anterior source loci when the novel sounds are preceded by standard stimuli that are similar in sound frequency. With large (four octave) novel-standard difference this effect disappears. Correspondingly, the amplitude waveforms of the ECDs fitted at response peak latencies converge. (Bottom) Mean (±SEM) amplitudes and latencies of the anterior and posterior N1 responses are shown (n = 7). Notably, the differential adaptation of anterior and posterior N1 responses by preceding standard stimuli may explain the previously observed differences in the single-ECD estimated N1 and MMN source loci (3, 12, 13), by way of altering the center of gravity of the underlying source configuration.
Psychophysical Measures: Experiment 3. Novel sounds separated by 1 octave from the preceding standard stimuli induced significantly less distraction in the novel sounds with standards than in the novel sounds without standards condition (P < 0.05; combined reaction time/hit rate index scores 1.7 ± 1.2 and 3.3 ± 1.1, respectively). In contrast, novel stimuli separated from the standard stimuli by four octaves caused similar distraction in the novel sounds with standards and novel sounds without standards conditions (index scores 2.2 ± 1.0 and 2.5 ± 1.3).
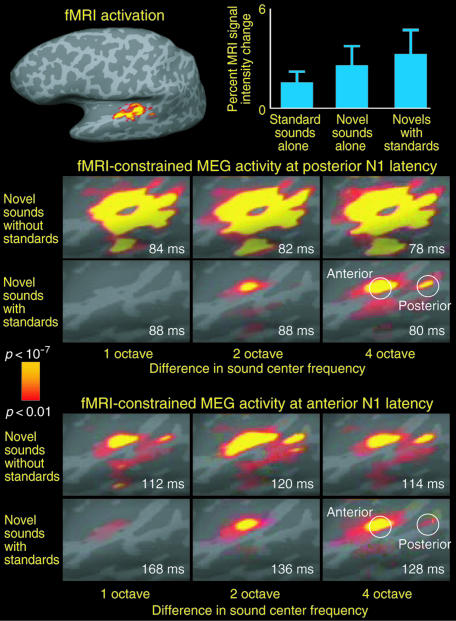
fMRI Measures: Experiment 4. Robust auditory cortex activations were observed for each of the conditions when contrasted against the silent baseline. Within voxels exhibiting sensitivity to sound novelty, the BOLD signal increases in the novel sounds with standards condition did not significantly exceed the sum of activations caused by the standard and novel stimuli when presented in isolation (uncorrected α = 0.05) in any of the subjects [for group mean (±SEM) signal intensity changes, see Fig. 4]. This finding suggests that MMN is not generated by separate change-specific auditory cortex neurons, as such would had given rise to additional BOLD signal increases (23).
Fig. 4.
The fMRI activations and fMRI-constrained MEG activity (24-26) at anterior and posterior N1 response latencies in the novel sounds with standards and novel sounds without standards conditions. (Top Left) fMRI data used in the fMRI-constrained MEG estimates. (Top Right) Group mean (±SEM) MRI signal intensity changes in the standard sounds alone, novel sounds alone, and novel sounds with standards sounds conditions. (Middle) fMRI-constrained MEG activity, at the latency of the posterior auditory cortex N1 response extends from HG onto PT, STS, MTG, and posterior STG. Note that the activity in areas posterior to the primary auditory cortex is suppressed as a function of decreasing standard-novel sound frequency separation. (Bottom) The estimated auditory cortex activity at the latency of the temporally lagging anterior N1 response, encompassing areas anterior to the primary auditory cortex (i.e., medial two-thirds of the HG), was relatively little affected as the standard-novel difference decreased.
Combined fMRI and MEG Estimates. Fig. 4 shows estimated neural activity underlying anterior and posterior N1 responses within the left auditory cortex. A combination of fMRI (experiment 3) and MEG (experiment 2) data were used to calculate these estimates (24-26). Neural activity at the latency of the posterior N1 response was estimated to encompass Heschl's gyrus (HG), planum temporale (PT), superior temporal sulcus (STS), middle temporal gyrus (MTG), and the posterior aspects of superior temporal gyrus (STG). Consistent with the adaptation of the posterior N1 response with decreasing sound novelty, activity within posterior STG and PT was robustly suppressed as sound novelty decreased. Moreover, activity originating from areas anterior to the primary auditory cortex (which appeared to dominate at the latency of the anterior N1 response) was relatively less influenced by the preceding standard sounds. Patterns of fMRI-constrained MEG activity in the three other subjects who participated in both experiments 2 and 4 corroborated these results (Fig. 6, which is published as supporting information on the PNAS web site).
Discussion
A plethora of previous electrophysiological studies have documented well the association between N1 and MMN sensory evoked responses and the degree that auditory stimuli enter one's awareness (5). However, the neurophysiological mechanisms underlying the MMN and the sensory memory representations have remained vague, largely because of limitations in the noninvasive research methods. Recent computational modeling work (8) suggested that MMN is an N1 response suppressed and delayed by stimulus specific adaptation (i.e., that the responses would be generated by common cortical sources). Although recent animal work (9, 10) support this hypothesis, it is inconsistent with previous human observations suggesting that repetitive standard sounds constitute a prerequisite to MMN elicitation (5, 18, 19), and that MMN and N1 source loci are different (3, 12, 13). In the present study, we conducted a series of studies to critically examine these seeming discrepancies.
Supporting our stimulus specific adaptation hypothesis, Fig. 1 shows robust MMN to novel stimuli when they were preceded by a single standard stimulus. This finding is in sharp contrast with previous studies that failed to observe MMN to novel stimuli when preceded by a single standard stimulus (5, 18, 19). However, the novel-to-novel interval in these previous studies decreased as the number of intervening standard stimuli was decreased. In contrast, in the present study the interval between two consecutive novel stimuli was constant at 3.5 s, and each of the novel tones was preceded either by a single or two to four standard stimuli. Thus, our results suggest that the lack of MMN in the previous studies resulted from suppression of novel-tone N1 responses at short internovel intervals (2). Our results are consistent with the hypothesis that preceding auditory events (cumulatively) adapt the feature-specific neurons in the auditory cortex, resulting in suppressed and delayed N1 responses to novel stimuli presented shortly afterward (8, 9). However, although suggestive, these results per se do not establish whether the auditory cortex sources of the MMN and N1 responses are the same or different.
Fig. 2 shows additional EEG data suggesting that the MMN arises through adaptation of N1 activity (8), rather than being generated by independent auditory cortex sources (5, 12). Specifically, responses to novel sounds separated by four octaves from the standards were identical, irrespective of whether the novel stimuli were preceded by standard stimuli. Any independent sources generating the MMN (5, 12) should have superimposed on the N1 response in the novel sounds with standards condition, thus increasing the amplitude and altering the morphology of the novel-sound response. The convergence of the waveforms, therefore, suggests that MMN arises through stimulus-specific adaptation of N1 activity (8), rather than being generated by distinct auditory cortex sources (5, 12). Consistent with these EEG results, our fMRI data failed to reveal any additional change-specific activations.
However, these results do not explain why previous MEG studies have consistently shown that MMN originates ≈7-10 mm anterior to N1 response in the auditory cortex (3, 12, 13). Previous MEG studies have shown that the N1 is generated by two auditory cortex sources that partly overlap in time: an early posterior and a late anterior source dominating the ascending and descending aspects of the response, respectively (14-16). Here, we investigated whether differential effects of stimulus-specific adaptation on these anterior and posterior N1 sources could explain the observed differences in MMN and N1 source loci by altering the center of gravity of activity that an ECD fitted at response peak latency approximates (17). Indeed, Fig. 3 shows that this may be the case. The amplitude of the posterior N1 source was rapidly suppressed with decreasing sound novelty, whereas that of the anterior N1 source was less affected. This finding suggests that neurons generating the anterior N1 source are more narrowly tuned on sound frequency than the neurons underlying the posterior N1 source. The anterior N1 response was also robustly increased in latency as a function of decreasing sound novelty, thus suggesting that it might in fact correspond to the “classic” MMN response customarily recorded to very small changes in sound frequency (5, 7). Previously noted similarities in ECD-estimated source loci of the anterior N1 and MMN support this interpretation (15).
Our results can also be interpreted within the context of recent monkey data suggesting the existence of segregated pathways within the auditory cortex for processing of auditory object/content and location features (28), the so-called “what” and “where” processing streams. These have been reported to reside in areas anterior and posterior to the A1 (28), thus matching closely the anatomical structures giving rise to the anterior and posterior N1 responses in our experiments (Fig. 4). Tentatively, the functional properties of these processing streams might explain the differential adaptation of anterior and posterior N1 responses by the preceding standard stimuli. Our psychophysical data show that the degree to which unattended novel sounds distracted visual forced-choice task performance coincided well with the extent that the posterior N1 response was uninhibited with increasing sound novelty. This finding suggests that adaptation of the posterior N1 response serves as the preattentive gating mechanism that determines the extent to which unattended novel sounds enter the subjects' awareness, agreeing well with the notion that fast analysis of sound location is fundamentally important for attentional orienting. On the other hand, the anterior N1 response might be more involved in subsequent attentional analysis of fine object features (7).
Taken together, our findings suggest that transient adaptation (8-10) of feature-specific neurons (3, 28, 29) within the anterior and posterior parts of the human auditory cortex can explain both the sensory memory representations underlying the MMN and preattentive gating of novel sounds to awareness (Fig. 5). Our results suggest that neurons within posterior auditory cortex are more broadly tuned on sound frequency than those within the anterior auditory cortex. Thus, their responses to subsequent stimuli that are close in sound frequency (and thus low in novelty) are robustly suppressed. Further, processing of sounds with low novelty value is significantly delayed within the anterior auditory cortex. In contrast, a highly novel sound enters consciousness via stimulating unadapted feature-specific neurons within the posterior auditory cortex. In this way, the human auditory cortex might accomplish the fast and coarse stimulus novelty analysis required for swift orientation of conscious attention to the features of the relevant stimulus, which is decisive for proper “flight-or-fight” responding in the face of impending danger.
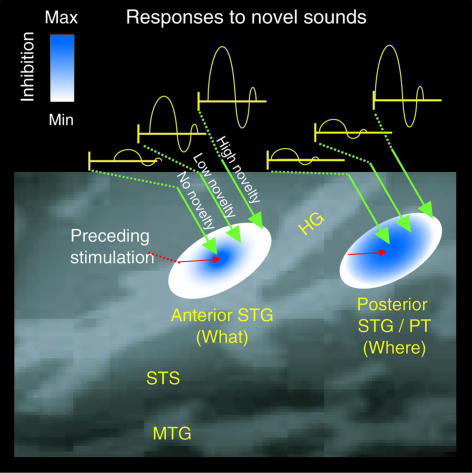
Fig. 5.
A schematic model illustrating how the human posterior auditory cortex gates novel sounds to awareness. The approximate locations of the anterior and posterior auditory cortex areas are shown. In this model, neurons within the anterior auditory cortex are narrowly tuned, whereas those within the posterior auditory cortex are more broadly tuned, on sound frequency (28). Thus, adaptation caused by preceding auditory stimulation (8-10) encompasses a greater extent of cortex in the posterior than anterior auditory areas, as indicated by gradients of blue. Thus, responses to subsequently presented sounds with relatively low novelty are robustly suppressed within the posterior auditory cortex. In contrast, on presentation of a highly novel sound, unadapted feature-specific neurons are activated within posterior auditory cortex, thus accelerating ensuing stimulus feature processing within the anterior auditory cortex and allowing the novel sound to enter one's awareness. Note that the present studies were limited to investigating MMN responses elicited by changes in sound frequency. The fact that feature-specific neurons tuned on sound duration, intensity, and periodicity have been documented in animal studies suggests that the governing principles of our model could be generalized to explain MMN responses to changes in other stimulus features; however, this remains to be determined in future investigations.
Supplementary Material
Acknowledgments
We thank Drs. Risto Näätänen and Tommi Raij for comments, and Dr. Matti Hämäläinen for technical help. This work was supported by National Institutes of Health Grants R01 HD040712, R01 NS037462, and P41 RR14075, the Mental Illness and Neuroscience Discovery Institute, the American Heart Association, the Academy of Finland, the Ella and Georg Ehrnrooth Foundation, and the Finnish Cultural Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BOLD, blood oxygenation level-dependent; ECD, equivalent current dipole; EEG, electroencephalography; fMRI, functional MRI; MEG, magnetoencephalography; MMN, mismatch negativity response.
References
- 1.James, W. (1890) Principles of Psychology (Holt, New York).
- 2.Hari, R., Kaila, K., Katila, T., Tuomisto, T. & Varpula, T. (1982) Electroencephalogr. Clin. Neurophysiol. 54, 561-569. [DOI] [PubMed] [Google Scholar]
- 3.Tiitinen, H., Alho, K., Huotilainen, M., Ilmoniemi, R. J., Simola, J. & Näätänen, R. (1993) Psychophysiology 30, 537-540. [DOI] [PubMed] [Google Scholar]
- 4.Woldorff, M. G., Hillyard, S. A., Gallen, C. C., Hampson, S. R. & Bloom, F. E. (1998) Psychophysiology 35, 283-292. [DOI] [PubMed] [Google Scholar]
- 5.Näätänen, R. (1992) Attention and Brain Function (Erlbaum, Mahwah, NJ).
- 6.Näätänen, R., Gaillard, A. W. K. & Mantysalo, S. (1978) Acta Psychologica 42, 313-329. [DOI] [PubMed] [Google Scholar]
- 7.Tiitinen, H., May, P., Reinikainen, K. & Näätänen, R. (1994) Nature 372, 90-92. [DOI] [PubMed] [Google Scholar]
- 8.May, P., Tiitinen, H., Ilmoniemi, R. J., Nyman, G., Taylor, J. G. & Näätänen, R. (1999) J. Comput. Neurosci. 6, 99-120. [DOI] [PubMed] [Google Scholar]
- 9.Javitt, D. C., Steinschneider, M., Schroeder, C. E. & Azerro, J. C. (1996) Proc. Natl. Acad. Sci. USA 93, 11962-11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulanovsky, N., Las, L. & Nelken, I. (2003) Nat. Neurosci. 6, 391-398. [DOI] [PubMed] [Google Scholar]
- 11.Halgren, E., Baudena, P., Clarke, J. M., Heit, G., Liegeois-Chauvel, S., Chauvel, P. & Musolino, A. (1995) EEG Clin. Neurophysiol. 94, 191-220. [DOI] [PubMed] [Google Scholar]
- 12.Korzyukov, O., Alho, K., Kujala, A., Gumenyuk, V., Ilmoniemi, R. J., Virtanen, J., Kropotov, J. & Näätänen, R. (1999) Neurosci. Lett. 276, 169-172. [DOI] [PubMed] [Google Scholar]
- 13.Hari, R., Rif, J., Tiihonen, J. & Sams, M. (1992) Electroencephalogr. Clin. Neurophysiol. 82, 152-154. [DOI] [PubMed] [Google Scholar]
- 14.Sams, M., Hari, R., Rif, J. & Knuutila, J. (1993) J. Cognit. Neurosci. 5, 363-370. [DOI] [PubMed] [Google Scholar]
- 15.Loveless, N., Levänen, S., Jousmaki, V., Sams, M. & Hari, R. (1996) Electroencephalogr. Clin. Neurophysiol. 100, 220-228. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy, L., Levänen, S. & Loveless, N. (1997) Psychophysiology 34, 308-316. [DOI] [PubMed] [Google Scholar]
- 17.Hamalainen, M., Hari, R., Ilmoniemi, R. J., Knuutila, J. & Lounasmaa, O. V. (1993) Rev. Mod. Phys. 65, 413-497. [Google Scholar]
- 18.Sams, M., Alho, K. & Näätänen, R. (1984) Psychophysiology 21, 434-441. [DOI] [PubMed] [Google Scholar]
- 19.Sams, M., Alho, K. & Näätänen, R. (1983) Biol. Psychol. 17, 41-58. [DOI] [PubMed] [Google Scholar]
- 20.Jääskeläinen, I. P., Alho, K., Escera, C., Winkler, I., Sillanaukee, P. & Näätänen, R. (1996) Alcohol 13, 153-156. [DOI] [PubMed] [Google Scholar]
- 21.Eden, G. F., Joseph, J. E., Brown, H. E., Brown, C. P. & Zeffiro, T. A. (1999) Magn. Reson. Med. 41, 13-20. [DOI] [PubMed] [Google Scholar]
- 22.Walker, L. J., Carpenter, M., Downs, C. R., Cranford, J. L., Stuart, A. & Pravica, D. (2001) J. Am. Acad. Audiol. 12, 348-356. [PubMed] [Google Scholar]
- 23.Calvert, G. A., Hansen, P. C., Iversen, S. D. & Brammer, M. J. (2001) NeuroImage 14, 427-438. [DOI] [PubMed] [Google Scholar]
- 24.Liu, A. K., Belliveau, J. W. & Dale, A. M. (1998) Proc. Natl. Acad. Sci. USA 95, 8945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, A. K., Dale, A. M. & Belliveau, J. W. (2002) Hum. Brain Mapp. 16, 47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale, A. M., Liu, A. K., Fischl, B. R., Buckner, R. L., Belliveau, J. W., Lewine, J. D. & Halgren, E. (2000) Neuron 26, 55-67. [DOI] [PubMed] [Google Scholar]
- 27.Oostendorp, T. F. & van Oosterom, A. (1992) in Biomagnetic Localization and 3D Modeling, Report TKK-F-A689, eds. Nenonen, J., Rajala, H. M. & Katila, T. (Helsinki Univ of Technol., Helsinki).
- 28.Rauschecker, J. P. & Tian, B. (2000) Proc. Natl. Acad. Sci. USA 97, 11800-11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talavage, T. M., Ledden, P. J., Benson, R. R., Rosen, B. R. & Melcher, J. R. (2000) Hear. Res. 150, 225-244. [DOI] [PubMed] [Google Scholar]
- 30.Fischl, B., Sereno, M. I. & Dale, A. M. (1999) NeuroImage 9, 195-207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.