Abstract
The goal for treatment in acute spinal cord injury (SCI) is to reduce the extent of secondary damage and facilitate neurologic regeneration and functional recovery. Although multiple studies have investigated potential new therapies for the treatment of acute SCI, outcomes and management protocols aimed at ameliorating neurologic injury in patients remain ineffective. More recent clinical and basic science research have shown surgical interventions to be a potentially valuable modality for treatment; however, the role and timing of surgical decompression, in addition to the optimal surgical intervention, remain one of the most controversial topics pertaining to surgical treatment of acute SCI. As an increasing number of potential treatment modalities emerge, animal models are pivotal for investigating its clinical application and translation into human trials. This review critically appraises the available literature for both clinical and basic science studies to highlight the extent of investigation that has occurred, specific therapies considered, and potential areas for future research.
Keywords: acute spinal cord injury, surgical decompression, durotomy, animal models
Acute spinal cord injury (SCI) is an important cause of morbidity and mortality with an annual incidence of 10000 to 12000 cases in the United States (Ackery et al., 2004). With life expectancy increasing for those with SCI, the prevalence worldwide is now approaching 2 million (Kirshblum et al., 2002; Ackery et al., 2004). The United States National Institute of Neurological Disorders and Stroke (NINDS) now estimates that over $4 billion are spent annually on medical treatment alone for acute SCI and management of chronically debilitated patients (Kirshblum et al., 2002; Ackery et al., 2004). The current standard of care for acute SCI is medical therapy with steroids; however, there have been multiple studies investigating the role of surgical intervention compared to conservative and medical treatments, as well as concerning the optimal therapeutic window for surgical intervention. Surgery has the potential advantage of obtaining greater neurological recovery and facilitating earlier rehabilitation through decompression of the spinal cord and nerve roots, in addition to preventing further neurological deterioration and secondary damage following SCI. Surgery has been widely used in patients with signs of progressive neurological deterioration, especially in which the injury is related to a herniated disk, intra-spinal hematoma, burst fracture, or other surgically correctable problems (Hawryluk et al., 2008). Despite recent advancements in understanding the pathophysiology of acute SCI, treatment outcomes and management protocols aimed at ameliorating neurologic damage in patients remain ineffective.
Study objectives and methodology
The continuing debate over whether the currently accepted standard of care, methylprednisolone, is truly efficacious or safe in the treatment of acute SCI has forced clinicians to look to alternatives in improving neurologic outcomes; however, adopting a novel treatment approach to acute SCI is not without its challenges. To overcome some of these obstacles, spinal cord injury clinical research must collaborate with neurobiological investigation in order to work toward the establishment of a successful translational model for patient care.
In this article, the authors will review the clinical and experimental evidence regarding the efficacy, therapeutic window, and optimal surgical interventions for the treatment of acute non-penetrating spinal cord injury in clinical studies as well as animal models, discuss experimental constraints, highlight the extent of investigation that has occurred, specific therapies considered, and potential areas for future research.
The authors conducted an evidence-based review of clinical studies as well as experimental research in animal models using a MEDLINE search of the literature from 1990 to 2013. The MEDLINE database was queried using the medical subject headings of “acute spinal cord injury,” “decompression,” and “surgical intervention.” For a summary of basic science research, the initial search yielded 130 articles, which were further limited to animal studies and the English language finally yielding 8 articles for appraisal. Histologic and behavioral outcomes in addition to surgical procedure and therapeutic time frames were compared and analyzed to meet inclusion criteria. For clinical studies over 100 articles were appraised and analyzed based on surgical intervention and therapeutic time window to meet inclusion and exclusion criteria. Each article underwent a detailed review by the investigators and the reference lists from select articles were further evaluated for relevance. Two tables are provided with the first table summarizing the clinical studies on the timing and type of surgical decompression after SCI (Table 1), and the second table summarizing the basic studies on decompression in animal models of SCI (Table 2).
Table 1.
Clinical research on timing and type of surgical decompression post-SCI
| Investigator | Sample size |
Location | Study type | Timing of decompression |
Intervention | Results |
|---|---|---|---|---|---|---|
| Levi et al. (1991) | 103 | Cervical | Retrospective | ± 24 hours | Anterior decompression and stabilization | Early decompression resulted in reduced hospitalization stay but no significant difference in functional grade improvement at discharge |
| Krengel et al. (1993) | 14 | Thoracic | Retrospective | ± 24 hours | Posterior and/or anterior decompression and fusion | Early surgical reduction, stabilization, and decompression is safe and improves neurologic recovery in comparison to historical controls treated by postural reduction or late surgical intervention |
| Duh et al. (1994) | 37 | Unknown | Retrospective | ± 24 hours | Anterior and posterior decompression with body and disk excision, internal fixation, and/or fusion | There is no statistically significant advantage to the timing of surgery. However, in these models, the degree of the initial injury is confirmed as the most powerful predictor of motor recovery. |
| Botel et al. (1997) | 178 | Unknown | Prospective | ± 24 hours | Anterior and posterior decompression and stabilization | Neurological recovery could only be found in those with incomplete lesions in more than 50% |
| Campagnolo et al. (1997) | 64 | Cervical, thoracic, lumbar, cauda equina | Retrospective | ± 24 hours | Spinal stabilization | Spinal stabilization performed < 24 hours after injury is associated with a significantly shorter length of stay in the hospital. |
| Tator, et al. (1999) | 88 | Cervical, thoracic, lumbar, cauda equina | Retrospective | ± 24 hours | Decompression and/or stabilization | There was a lack of consensus among centers concerning the optimum timing of surgical treatment, and only a minority of patients underwent surgery within 24 hours of trauma. Adequate long term follow-up was not obtained. |
| Guest et al. (2002) | 50 | Traumatic central cord | Retrospective | ± 24 hours | Anterior and/or posterior decompression and stabilization | Early surgery is safe and more cost effective than late surgery for the treatment of traumatic CCS, based on ICU stay and LOS and improved overall motor recovery, in patients whose CCS was related to acute disc herniation or fracture. In the setting of spinal stenosis or spondylosis, early surgery was safe but did not improve motor outcome compared with late surgery. |
| Pollard et al. (2003) | 412 | All | Retrospective | ± 24 hours | Anterior decompression | Improved neurologic outcomes were noted in younger patients and those with either a central cord or Brown-Sequard syndrome. |
| Ng et al. (1999) | 26 | Cervical | Prospective | ± 8 hours | Total laminectomy, flavectomy, and stabilization | Patients with surgical decompression within 8 hours showed significantly shorter overall hospital and intensive care unit stay and had lesser systemic complications and also exhibited better neurological improvement |
| Cengiz et al. (2008) | 27 | Thoracic lumbar | Prospective | ± 8 hours | Decompression and stabilization | Patients with surgical decompression within 8 hours showed significantly shorter overall hospital also exhibited better neurological outcomes |
| Fehlings et al. (2012) | 313 | Cervical | Prospective | ± 24 hours | Decompression and stabilization | Greater % patients with decompression within 24 hours had ≥2 ASIA scores at 6 month follow-up than those receiving delayed surgery (≥24 hours) |
| Wilson et al. (2012) | 84 | Cervical thoracic lumbar | Prospective | ± 24 hours | Decompression and stabilization | Patients with decompression surgery <24 hours post-injury had greater ASIA motor recovery than those with surgery ≥24 hours post-SCI. |
Table 2.
Basic research on decompression post-SCI
| Investigator | Species | Injury model | Timing of decompression |
Intervention | Results |
|---|---|---|---|---|---|
| Delamarter et al., (1995) | Dogs | Circumferential cable | 1 hour–1 week | Removal of the Pressure cable | Decompression is inversely proportional to duration of compression |
| Carlson et al., (1997) | Dogs | Piston | 5 min, 1, and 3 hours | Removal of pressure piston | Decompression at 5 min, 30 min, and 1 hour improves recovery of evoked potentials |
| Dimar et al., (1999) | Rats | Extradural impactor | 0, 2, 6, 24, and 72 hours | Removal of pressure spacer | Neurologic recovery is inversely related to extend and duration of compression |
| Carlson et al., (2003) | Dogs | Piston | 30 min and 3 hours | Removal of pressure piston | Recovery was obtained the 30 minute group but absent in the 3 hour group |
| Rabinowitz et al., (2008) | Dogs | Nylon tie | 6 hours | Surgery ± methyl prednisolone | Surgical decompression with or without methylprednisolone, improved recovery |
| Smith, (2010) | Rats | Infinite horizon impactor | 4 hours | Durotomy ± dural allograft | Durotomy alone showed increased scar and cavity formation while durotomy with dural allograft showed improved recovery compared to sham and durotomy alone |
| Jones et al., (2012a) | Pigs | Weight-drop impact, 8 hours weight compression | 8 hours | Removal of 100g weight | Decompression post-moderate SCI initially increased cord expansion that generally stabilized; Severe SCI caused sustained cord swelling and occlusion of subarachnoid space |
| Jones et al., (2012b) | Pigs | Weight-drop impact, 8 hours weight compression | 8 hours | Removal of 100g weight | Cranial CSFP elevated and caudal CSFP slightly decreased during spinal compression; Responses reversed immediately post-decompression, then resolution of pressure differential |
Mechanisms of secondary injury
Acute SCI involves a combination of primary mechanical and secondary cellular injury leading to neural tissue destruction (Tator and Fehlings, 1991; Rowland et al., 2008). Primary mechanisms refer to the initial rapid spinal cord compression and trauma induced by a fracture or shearing force (Tator and Fehlings, 1991; Rowland et al., 2008). Primary trauma to the cord is irreversible, and initiates a cascade of pathologic and molecular changes that contribute to secondary injury. Secondary injury mechanisms include hemorrhage, vasospasm, ischemia, edema, excitotoxicity, inflammation, and apoptosis (Tator and Fehlings, 1991; Tator and Koyanagi, 1997). Current therapy modalities focus on preventing and reducing damaging effects of secondary injury to improve neurological outcomes. Recent evidence suggests that neural tissue destruction is also enhanced by a persistent compression on the spinal cord, a reversible form of secondary injury following trauma (Vaccaro et al., 1997; Fehlings and Arvin 2009; Fehlings et al., 2012). Based on these data, surgical intervention has been explored as a potential treatment method. Surgery in acute SCI serves to decompress the spinal cord and restore spinal stability; thus reducing secondary injury and deterioration of function.
Pharmacological treatments for SCI
The National Acute Spinal Cord Injury Studies (NASCIS trials II and III) have shown that patients treated with the steroid methylprednisolone within 8 h of acute SCI exhibit improved neurologic outcomes at 1 year compared to placebo (Bracken et al., 1990; Bracken et al., 1997). Although methylprednisolone has been established as the only standard of care for acute SCI, recent evidence suggests that there is no benefit in both short-term and long-term results and that the risks of high doses of methylprednisolone outweigh the benefits (Hurlbert, 2000). Numerous other therapies for acute SCI have appeared promising in preclinical trials. A recent phase III randomized controlled trial evaluating GM-1 ganglioside and its neuroprotective properties showed potential at 3 months post-injury, but ultimately failed to provide significant benefit in outcomes at 6 months follow-up (Baptiste and Fehlings, 2008). Multiple other pharmacologic compounds have been investigated as potential adjuncts for reducing injury and improving neurologic recovery, however, such therapeutics from animal studies are not easily, or ever, translated clinically (Amar and Levy, 1999; Baptiste and Fehlings, 2008). Continued research on the pathophysiology of acute SCI is needed to develop pharmacologic agents that reduce secondary injury and stimulate neurologic regeneration.
Potential benefits of surgical intervention
To date, surgical decompression via laminectomy remains a valid practice option for the treatment of acute SCI. However, there is no conclusive Class I clinical data that suggest an enhanced benefit over conservative treatment approaches (Vaccaro et al., 1997; Fehlings and Arvin, 2009; Fehlings et al., 2012). Class II evidence suggests that early surgical intervention is safe and effective, though no standardized guidelines or algorithms exist regarding the timing and optimal surgical intervention for extra-dural and intra-dural decompression in acute SCI (Fehlings and Arvin 2009; Fehlings et al., 2012). Few prospective randomized trials investigating surgical management of SCI has been published (Ng et al., 1999; Cengiz et al., 2008; Fehlings et al., 2012; Wilson et al., 2012), and among these the results are conflicting; timing appears to be one of the most crucial factors in improving neurological recovery following surgical intervention.
The results of a study by Cengiz et al., study showed no significant neurologic benefit of cervical spinal cord decompression performed less than 72 h with a mean of 43 h compared to time points beyond 5 days post-injury (Cengiz et al., 2008). Alternatively, the results of the highly anticipated randomized multicenter STASCIS (Surgical Treatment of Acute Spinal Cord Injury Study) trial supports acute decompressive surgery for improving functional recovery following cervical SCI (Fehlings et al., 2012). This result was supported by a smaller prospective study also performed under direction by Michael Fehlings (Wilson et al., 2012). Many class II and III clinical studies have shown surgical approaches to be promising intervention options for acute SCI; however, the timing of surgical decompression in addition to determination of the optimal specific surgical intervention remain among the most controversial topics pertaining to surgical treatment of acute SCI (Ng et al., 1999; Cengiz et al., 2008; Rabinowitz et al., 2008).
Accurate appraisals of a surgical therapeutic window for neurologic improvement in acute SCI are fundamental to the establishment of optimal treatment modalities and algorithms. Unfortunately, this topic remains understudied. Prevention of secondary injury in animal models is suggested to be dependent on the timing of surgical decompression. Therefore, it is hypothesized that early surgical interventions will improve neurologic outcome. Unfortunately, the timing of “early” surgical decompression has been difficult to establish because it is defined at different times by different authors and there is a lack of evidence supporting the definition. One systematic review on class II evidence concluded that early surgery (< 24 h) results in better neurological outcome, reduced complications, and reduced length of ICU and overall hospital stay when compared to delayed surgery (> 24 h) (Cengiz et al., 2008; Fehlings and Arvin 2009; Fehlings et al., 2012).
The results of the STASCIS trial investigating the outcomes of early (< 24 h) vs. late (~48 h) extradural surgical decompression of SCI, has shed light on the current debate (Fehlings and Arvin, 2009; Fehlings et al., 2012). In this study involving 313 patients diagnosed with acute cervical SCI, approximately 20% of those who underwent extradural surgical decompression within 24 h of injury experienced a 2-grade or greater improvement on the American Spinal Injury Association (ASIA) scale compared with approximately 9% in those patients with delayed decompressive surgery. It was also observed that systemic complications, particularly involving the cardiopulmonary and urinary systems, were reduced in patients who underwent early extradural surgical decompression at 24.2% compared to those with delayed decompression at 30.5%. Based on the aforementioned literature, the authors of this study focused on the appraisal of clinical and experimental studies in which surgical intervention was performed within 24 h in patients presenting with acute SCI. Nevertheless, further animal studies are necessary to establish a potential treatment algorithm as well as determine the optimal therapeutic window and surgical technique.
Discussion
Surgical decompression may be beneficial for recovery
Secondary injury is an active process that requires both energy and cellular mediators making it a feasible target for therapeutic intervention. Investigation of pharmacologic compounds that facilitate the regenerative processes while inhibiting detrimental aspects of inflammation has been at the forefront of SCI research; however, to date, these agents have failed to show significance clinical translation (Hall and Braughler, 1982; Nagata and Golstein, 1995; Juurlink and Paterson, 1998; Park et al., 2004). Despite these shortcomings, surgical interventions to prevent edema, restore vascular perfusion, and reduce the mechanisms that perpetuate secondary injury are important future directions for research.
Currently no standards of care regarding the timing and indications of surgical decompression in acute SCI exist, although the literature suggests that early extradural surgical decompression may play a significant role in functional recovery (Fehlings and Arvin, 2009; Fehlings et al., 2012; Wilson et al., 2012). Surgical decompression has the potential to reduce intra-dural pressure and thus increasing blood flow to the spinal cord, reducing ischemia, and preventing secondary injury mechanisms. The optimal combination of decompression (laminectomy, durotomy, piotomy or myelotomy) along with adjunctive therapies, such as the use of methylprednisolone, has not yet been established despite studies investigating the role of a myriad of agents and interventions for mediating neuroprotection and reducing secondary injury. We believe that the acute SCI treatments should focus on developing or improving combinational therapeutic regimens, instead of single therapies alone, to ameliorate the extent of secondary injury and improve neurological outcomes. Investigating optimal surgical interventions for the treatment of acute non-penetrating SCI in clinical studies, particularly focusing on therapeutic window and surgical procedures, may provide potential ideas for future treatment algorithms, modalities, and research.
Therapeutic window and clinical considerations
Based on a review of literature and in accordance with the STASCIS trial, the authors of this review have defined early surgical decompression as such intervention performed within 24 h of acute SCI, although this definition has varied from 8 h to 4 days in various clinical studies. Delayed surgical intervention has also been more commonly classified in the literature as between 24 h to 5 days; however, less consistently.
We identified 7 retrospective and 5 prospective clinical studies investigating the efficacy, complications, and/or optimal therapeutic window of surgical intervention for acute SCI. Table 1 shows 7 out of 12 studies concluding that patients who undergo early surgical intervention have shorter hospitalization or shorter length of stay in intensive care compared to those undergoing medical management. Additionally, 7 out of those 12 studies also concluded that better neurologic outcomes were observed in those patients undergoing early surgical intervention. One main argument for delaying surgery is the concern for a higher incidence of medical complications. One study showed no difference and even less systemic complications in those patients who undergo early surgical intervention, consistent with preliminary data presented in the STASCIS trial. Improved neurologic outcomes were noted in patients who were younger, those who presented with incomplete injury such as Brown-Sequard syndrome, or in Central Cord Syndrome related to acute disc herniation or fractures. The literature suggests that in the setting of spondylosis or stenosis, there was no difference between early and later surgical stabilization, though, early surgery was deemed safe in those patients presenting acutely.
The National Acute Spinal Cord Injury Studies (NASCIS trials II and III) have shown that patients treated with methylprednisolone within 8 h of acute SCI improve neurologic outcomes at 1 year compared to placebo (Bracken et al., 1990; Bracken et al., 1997). This suggests a potential therapeutic window during this timeframe for preventing the cascade of secondary injury. Ng et al. (1999) and Cengiz et al. (2008) represent two prospective studies in which patients underwent surgical decompression of the cervical, thoracic, and lumbar segments of the spine within 8 h of acute traumatic SCI. Both of these studies observed shorter intensive care and hospital stay, with fewer secondary complications and better neurologic outcomes in those patients who underwent early surgical decompression less than 8 h after acute SCI compared to other patients operated on at a later time. Despite these optimistic results, one study showed no difference in mortality when comparing early versus late surgical decompression (8 h). Additionally, it could be argued that further neurological recovery could potentially show no difference although it is equally possible that an even greater difference in neurologic improvement could be observed with later follow-up.
Decisions about the timing of surgery could also have been made (especially in retrospective studies), based on factors that were not measured in the study, for example, worsening neurological or clinical status indicating the need for emergent surgical intervention. Patients who do not fit in this category and who participate in standardized prospective studies may potentially benefit to a greater degree. Major limitations in many of the aforementioned studies are the lack of a randomized, double-blind, multicenter clinical trial designed to determine the efficacy and optimal therapeutic window for various surgical interventions in acute SCI. The outcome of the STASCIS and follow-up trials provide more reliable insight for clarifying these important clinical considerations.
Clinical evidence from Table 1 suggesting that early surgical decompression with or without spinal fusion within 24 h, and especially within 8 h of acute SCI is safe, more cost effective, and results in improved overall neurologic recover in patients with incomplete spinal trauma. In spite of these findings, the application of surgical decompression is to reduce secondary injury mechanisms. Early surgical interventions that serve to reduce mechanisms such as ischemia, free radical formation, lipid peroxidation, and calcium channel mediated cytotoxicity, should be explored.
While the most traditional form of surgical decompression of the spinal canal is through performance of a total laminectomy with flavectomy with or without spinal stabilization, which decompresses extra-dural elements, Perkins and Deane (1988) reported six cases of patients with acute SCI who underwent surgical decompression of the dura. At the time of surgery (five of six within 24 h), the dural sac failed to show normal pulsations. Presence of congested epidural veins was also noted. During the surgery, the dura was exposed and incised longitudinally. At the time of incision cerebrospinal fluid escaped under considerable pressure with a mean of 15 mmHg followed by hemorrhage from surrounding epidural veins. The dura was subsequently closed following an expansion duraplasty in a water-tight fashion with a continuous locking suture and spinal stabilization was obtained through instrument assisted fusion.
Perkins and Deane (1988) followed their patients for an average of 4–5 years after emergency intra-dural decompression. The authors reported full neurological recovery in three of the six previously impaired patients and partial recovery in the remaining three. Perkins and colleagues hypothesized that edema in acute SCI restricts the normal flow of cerebrospinal fluid exacerbating intra-dural pressure, ultimately causing a compartment-like syndrome within the neural structures. This phenomenon is hypothesized to restrict normal arterial perfusion, supporting surgical durotomy as an effective measure of preventing secondary injury.
Although intra-dural decompression via durotomy has been implicated to reduce secondary injury and improve neurologic outcomes in acute SCI, such surgery is not without complications. Spinal pseudomeningoceles and cerebrospinal fluid fistulas are rare extradural complications that result following a failure to obtain a water-tight closure of the dura. The incidence of pseudomeningoceles following durotomy has been estimated to be less than 0.1% (Schumacher et al., 1988); however, clinically significant cases are even more infrequent. More common complications of intra-dural surgery include low-pressure headache with meningitis, while transient quadriplegia is a more severe, but rare, complication (Desai et al., 2011).
Zhu et al. (2008) reported a total of 30 patients with “complete” acute SCI who underwent internal fixation of the vertebral column, bilateral laminectomy for epidural decompression, separation of arachnoid adhesions, and intramedullary decompression through debridement of the necrotic lesion epicenter at 4–14 days after injury. Although all 30 patients presented initially with an American Spinal Injury Association (ASIA)-A score, after three months of rehabilitation, all patients recovered some ability to walk. Within this group, 40% of the patients were able to walk with wheeled weight support and 43% with crutches or without support.
The authors hypothesized that the volume of necrosis in the spinal cord from secondary injury is much greater than the extent of primary injury and early debridement may serve as a method to stop further expansion of secondary injury by removing activators of the secondary injury cascade (Tator, 1991; Lu et al., 2000; Profyris et al., 2004). The authors propose intramedullary decompression may serve as a potential intervention to increase cerebral perfusion pressure in spared tissue thus reducing further ischemia and secondary injury mechanisms. Intramedullary decompression is another potential novel therapeutic neurosurgical intervention that should be investigated for clinical translation. Given the low incidence of adverse effects following intra-dural surgery pooled with a high potential for neurological benefit following acute SCI, durotomy is a viable treatment option. Intramedullary decompression has also shown considerable promise; however, due to the high risk of adverse events, further investigation using animal models is required.
Surgical decompression in animal models
There is convincing evidence from laboratory studies in rat and dog animal models that show persistent compression of the spinal cord to be a reversible form of secondary injury. Table 2 summarizes those studies using animal models published within the past 20 years. The investigators have concluded with consistent evidence, that not only is neurological recovery inversely related to the extent and duration of spinal cord compression after primary acute SCI, but early decompression of the spinal cord, especially within the first hour, improved neurological recovery. Acute SCI without surgical decompression within the first three hours of injury has also shown to produce irreversible neurologic damage. Additionally, Rabinowitz et al. (2008) investigated new treatment modalities such as combining surgical decompression and methylprednisolone to reduce the extent of secondary injury. They concluded that surgical decompression, with or without methylprednisolone, were superior in recovery compared to methylprednisolone alone. Although this experiment highlights the potential role of surgical decompression in acute SCI, it also emphasizes the importance considering adjunctive therapies in preventing progression of secondary injury.
Investigating decompression in animal models could shed light on potential benefits of such an approach for acute SCI. Dimar et al. (1999) previously showed that both histological damage and neurological dysfunction increased with incrementally prolonged intraspinal cord compression in a rat model. Smith et al. (2010) devised an experiment focusing on intra-dural decompression as a result of the study conducted by Perkins and Deane (1988). Smith et al. (2010) hypothesized that performance of a durotomy following acute SCI may abate potential deleterious secondary injury that results from intact dural compression secondary to primary contusive trauma.
The authors concluded that durotomy alone showed increased scar and cavity formation while durotomy with dural allograft showed improved recovery compared to sham and durotomy alone at 4 h after injury (Iannotti et al., 2006). Despite the evidence, there are limitations to their study design. First, the stated SCI protocol does not specify whether the same investigator performed the durotomy on each of the animals. Variations in surgical skill play a large role in observed inconsistency within the results. In addition, the details of the transplanted dural allograft and its potential neuroprotective and anti-inflammatory effects on the spinal cord are unclear and require further investigation. Furthermore, experiments are required to determine if the improved neurological outcomes extend beyond their study period of four weeks. It seems intuitive that the effect of surgical decompression of the dura alone would also produce a beneficial outcome. Although the results of the study by Smith et al. (2010) showed that durotomy alone may not play a role in acute SCI, further studies must be conducted in order to establish a standardized treatment algorithm and to better understand the progression of disease. Without such knowledge, the potential realization of clinical application of durotomy as a clinically applicable treatment modality is greatly diminished.
Jones et al. described two separate studies in 2012 utilizing a porcine model of weight drop and compression SCI followed by decompression surgery 4 h after primary injury (Jones et al., 2012a, 2012b). In one study, the authors investigated the morphological alterations to the dura and spinal cord following persistent compression and surgical decompression in Yucatan pigs. Their results show that decompression following such SCI results in varying degrees of cord swelling, occlusion of subarachnoid space, and blockage of CSF flow (Jones et al., 2012a). Jones and colleagues posit that intradural swelling may induce secondary pathology due to interruption of normal flow of CSF and constraints of swelling by the surrounding meninges, leading to the inconclusive results of decompression and patient neurological outcome following SCI. In the other study, the CSF blockade was documented to induce elevated intracranial pressure, which could induce separate but additional neurological deficits (Jones et al., 2012b). Figure 1A &B illustrates the hemorrhage, edema and swelling of the rat cervical spinal cord following hemi-contusion SCI three days post-insult. As suggested in human SCI, removal of pressure from bone fragmentation or dislocation following trauma may benefit from additional decompression by dural or pial opening to alleviate intramedullary pressure on the cord. The timing of such interventions and restoration of normal CSF circulation will require further research in animal models as well as in human SCI to obtain an optimal surgical approach to decompressing the traumatized spinal cord. Figure 2 illustrates an axial view of the normal spinal cord, canal and vertebral column, and the pathology that follows traumatic injury to the spine. A series of illustrations highlight combination decompression approaches targeting laminectomy for reducing external pressure from the cord, and meningeal opening to minimize subsequent intraspinal pressure caused by cord swelling and constraints of the pia and dura matter and blockade of CSF flow.
Figure 1.
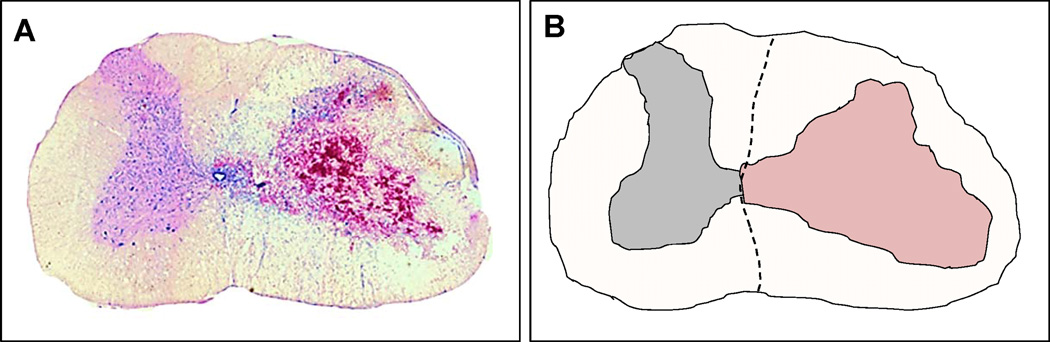
Spinal cord swelling following traumatic C5 hemicontusion injury in rats. (A) Photomicrograph depicting hemorrhage and swelling due to trauma on the side ipsilateral to injury (right), and no swelling or morphological change on the contralateral uninjured side (left) of the cord. (B) Camera lucida drawing of the photomicrograph in (A) highlighting the morphological differences between the ipsilateral and contralateral sides of injury in the rat cervical spinal cord. The lesion is indicated by the region colored in light-red. Scale bar = 1 mm.
Figure 2.
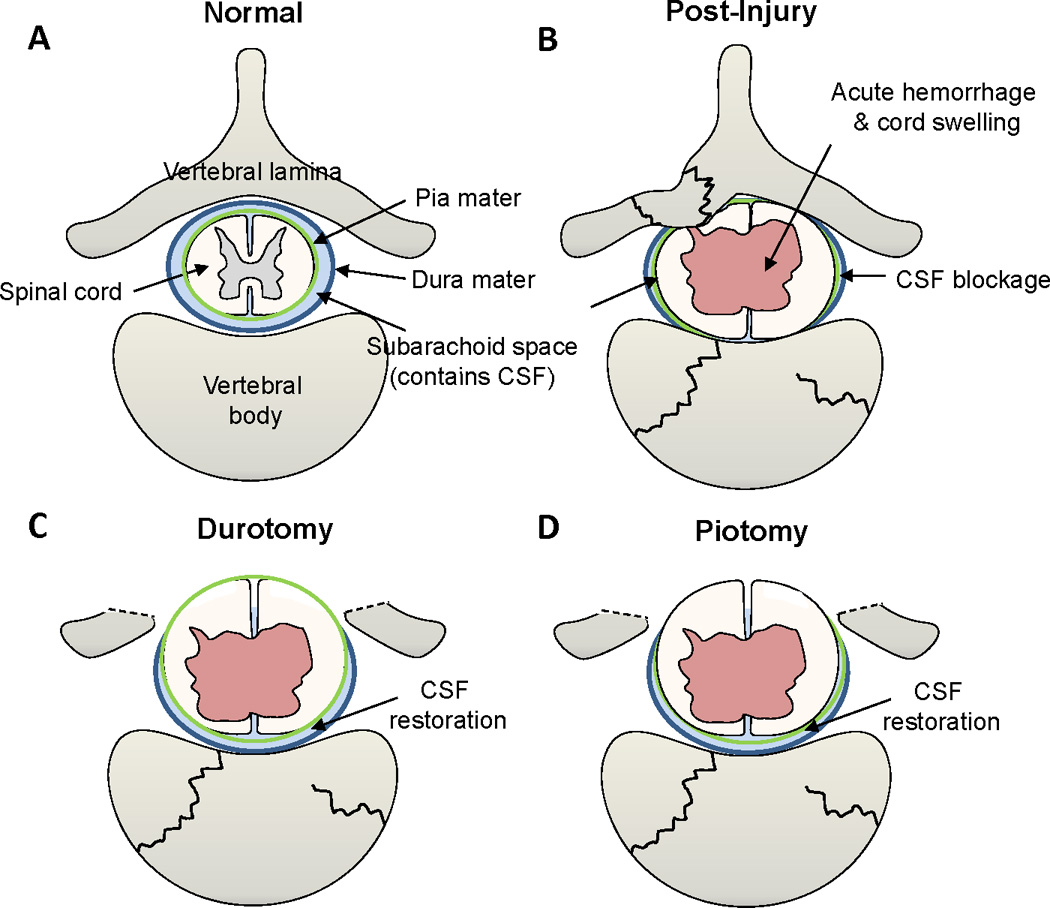
Illustrations of the normal spine, injured vertebra and spinal cord, and surgical decompression methods to alleviate pressure from cord swelling. (A) Transverse illustration of a normal vertebra, dura mater (dark blue), subarachnoid space containing the cerebrospinal fluid (CSF, light blue), pia mater (green), and the spinal cord. (B) Traumatic spine fracture inflicts compressive spinal cord damage, leading to vascular rupture, hemorrhage and swelling of the cord resulting in the occlusion of the subarachnoid space and blockade of the CSF flow. (C) Durotomy, i.e., longitudinal incision of the dura mater, may release the cord pressure caused by tissue swelling and, therefore, reduce secondary tissue damage. (D) Piotomy, i.e., longitudinal incision of the pia mater, may afford further cord expansion to reduce further tissue damage of the injured spinal cord.
Future experimental direction
The authors of this paper hypothesize that breaching the relatively non-compliant dura early in the clinical course will reduce compression of the injured spinal cord, promoting adequate vascular perfusion, and reducing the spread of secondary events. Other surgical interventions such as intramedullary decompression, which has the potential to ameliorate damaged vasculature and prevent further cell death, should be on the forefront of future investigation. Future studies utilizing animal models should determine the role of surgery for the treatment of acute spinal cord injury, establish a therapeutic window, and evaluate the effects of durotomy and intramedullary decompression on inflammation, scar formation, functional, histological, and neurological outcomes.
Acknowledgements
This work was supported by National Institutes of Health (NIH/NINDS R01 NS059622, R01 NS050243, R01 NS073636 to XMX, and F31NS 071863 to CLW), the Indiana Clinical and Translational Sciences Institute (CTSI) Collaboration in Biomedical/Translational Research (CBR/CTR) Pilot Program Grants (Grant #RR025761) from the NIH, the Indiana Spinal Cord and Brain Injury Research Funds, the Mari Hulman George Endowment Funds. We also appreciate the use of the Core facility of the Spinal Cord and Brain Injury Research Group/Stark Neurosciences Research Institute at Indiana University School of Medicine, and collaboration with Norton Healthcare, Louisville, KY.
Footnotes
Compliance with ethics guidelines
Yiping Li, Chandler L. Walker, Yi Ping Zhang, Christopher B. Shields, and Xiao-Ming Xu declare that they have no conflict of interest.
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. doi: 10.1089/neu.2004.21.1355. PMID:15672627. [DOI] [PubMed] [Google Scholar]
- Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44(5):1027–1039. doi: 10.1097/00006123-199905000-00052. discussion 1039-1040. PMID:10232536. [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Emerging drugs for spinal cord injury. Expert Opin Emerg Drugs. 2008;13(1):63–80. doi: 10.1517/14728214.13.1.63. PMID:18321149. [DOI] [PubMed] [Google Scholar]
- Bötel U, Gläser E, Niedeggen A. The surgical treatment of acute spinal paralysed patients. Spinal Cord. 1997;35(7):420–428. doi: 10.1038/sj.sc.3100407. PMID:9232746. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, Marshall LF, Perot PL, Jr, Piepmeier J, Sonntag VKH, Wagner FC, Wilberger JE, Winn HR. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. PMID:2278545. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597–1604. PMID:9168289. [PubMed] [Google Scholar]
- Campagnolo DI, Esquieres RE, Kopacz KJ. Effect of timing of stabilization on length of stay and medical complications following spinal cord injury. J Spinal Cord Med. 1997;20(3):331–334. doi: 10.1080/10790268.1997.11719484. PMID:9261779. [DOI] [PubMed] [Google Scholar]
- Carlson GD, Gorden CD, Oliff HS, Pillai JJ, LaManna JC. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am. 2003;85-A(1):86–94. PMID:12533577. [PubMed] [Google Scholar]
- Carlson GD, Minato Y, Okada A, Gorden CD, Warden KE, Barbeau JM, Biro CL, Bahnuik E, Bohlman HH, Lamanna JC. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J Neurotrauma. 1997;14(12):951–962. doi: 10.1089/neu.1997.14.951. PMID:9475376. [DOI] [PubMed] [Google Scholar]
- Cengiz SL, Kalkan E, Bayir A, Ilik K, Basefer A. Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course. A real prospective (rct) randomized controlled study. Arch Orthop Trauma Surg. 2008;128(9):959–966. doi: 10.1007/s00402-007-0518-1. PMID:18040702. [DOI] [PubMed] [Google Scholar]
- Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77(7):1042–1049. doi: 10.2106/00004623-199507000-00010. PMID:7608226. [DOI] [PubMed] [Google Scholar]
- Desai A, Ball PA, Bekelis K, Lurie J, Mirza SK, Tosteson TD, Weinstein JN. SPORT: does incidental durotomy affect long-term outcomes in cases of spinal stenosis? Neurosurgery. 2011;69(1):38–44. doi: 10.1227/NEU.0b013e3182134171. discussion 44 PMID:21358354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimar JR, 2nd, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24(16):1623–1633. doi: 10.1097/00007632-199908150-00002. PMID:10472095. [DOI] [PubMed] [Google Scholar]
- Duh MS, Shepard MJ, Wilberger JE, Bracken MB. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35(2):240–248. doi: 10.1227/00006123-199408000-00009. discussion 248–249 PMID:7969831. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10(1):1–2. doi: 10.3171/2008.10.SPI0822. PMID:19119924. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS ONE. 2012;7(2):e32037. doi: 10.1371/journal.pone.0032037. PMID:22384132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J, Eleraky MA, Apostolides PJ, Dickman CA, Sonntag VK. Traumatic central cord syndrome: results of surgical management. J Neurosurg. 2002;97(1 Suppl):25–32. doi: 10.3171/spi.2002.97.1.0025. PMID:12120648. [DOI] [PubMed] [Google Scholar]
- Hall ED, Braughler JM. Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol. 1982;18(5):320–327. doi: 10.1016/0090-3019(82)90140-9. PMID:7179094. [DOI] [PubMed] [Google Scholar]
- Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25(5):E14. doi: 10.3171/FOC.2008.25.11.E14. PMID:18980474. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(1 Suppl):1–7. doi: 10.3171/spi.2000.93.1.0001. PMID:10879751. [DOI] [PubMed] [Google Scholar]
- Iannotti C, Zhang YP, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J Neurotrauma. 2006;23(6):853–865. doi: 10.1089/neu.2006.23.853. PMID:16774471. [DOI] [PubMed] [Google Scholar]
- Jones CF, Cripton PA, Kwon BK. Gross morphological changes of the spinal cord immediately after surgical decompression in a large animal model of traumatic spinal cord injury. Spine. 2012a;37(15):E890–E899. doi: 10.1097/BRS.0b013e3182553d1d. PMID:22433504. [DOI] [PubMed] [Google Scholar]
- Jones CF, Newell RS, Lee JH, Cripton PA, Kwon BK. The pressure distribution of cerebrospinal fluid responds to residual compression and decompression in an animal model of acute spinal cord injury. Spine. 2012b;37(23):E1422–E1431. doi: 10.1097/BRS.0b013e31826ba7cd. PMID:22869059. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J Spinal Cord Med. 1998;21(4):309–334. doi: 10.1080/10790268.1998.11719540. PMID:10096045. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Campagnolo DI, DeLisa JA. Spinal cord medicine. x. Philadelphia: Lippincott Williams & Wilkins; 2002. p. 655. [Google Scholar]
- Krengel WF, 3rd, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine. 1993;18(14 Supplement):2080–2087. doi: 10.1097/00007632-199310001-00027. PMID:8272964. [DOI] [PubMed] [Google Scholar]
- Levi L, Wolf A, Rigamonti D, Ragheb J, Mirvis S, Robinson WL. Anterior decompression in cervical spine trauma: does the timing of surgery affect the outcome? Neurosurgery. 1991;29(2):216–222. PMID:1886659. [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25(14):1859–1866. doi: 10.1097/00007632-200007150-00022. PMID:10888960. [DOI] [PubMed] [Google Scholar]
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–1456. doi: 10.1126/science.7533326. PMID:7533326. [DOI] [PubMed] [Google Scholar]
- Ng WP, Fehlings MG, Cuddy B, Dickman C, Fazl M, Green B, Hitchon P, Northrup B, Sonntag V, Wagner F, Tator CH. Surgical treatment for acute spinal cord injury study pilot study #2: evaluation of protocol for decompressive surgery within 8 hours of injury. Neurosurg Focus. 1999;6(1):e3. doi: 10.3171/foc.1999.6.1.4. PMID:17031916. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–774. doi: 10.1089/0897715041269641. PMID:15253803. [DOI] [PubMed] [Google Scholar]
- Perkins PG, Deane RH. Long-term follow-up of six patients with acute spinal injury following dural decompression. Injury. 1988;19(6):397–401. doi: 10.1016/0020-1383(88)90132-5. PMID:3267644. [DOI] [PubMed] [Google Scholar]
- Pollard ME, Apple DF. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine. 2003;28(1):33–39. doi: 10.1097/00007632-200301010-00009. PMID:12544952. [DOI] [PubMed] [Google Scholar]
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–436. doi: 10.1016/j.nbd.2003.11.015. PMID:15056450. [DOI] [PubMed] [Google Scholar]
- Rabinowitz RS, Eck JC, Harper CM, Jr, Larson DR, Jimenez MA, Parisi JE, Friedman JA, Yaszemski MJ, Currier BL. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine. 2008;33(21):2260–2268. doi: 10.1097/BRS.0b013e31818786db. PMID:18827690. [DOI] [PubMed] [Google Scholar]
- Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. doi: 10.3171/FOC.2008.25.11.E2. PMID:18980476. [DOI] [PubMed] [Google Scholar]
- Schumacher HW, Wassmann H, Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29(1):77–78. doi: 10.1016/0090-3019(88)90127-9. PMID:3336842. [DOI] [PubMed] [Google Scholar]
- Smith JS, Anderson R, Pham T, Bhatia N, Steward O, Gupta R. Role of early surgical decompression of the intradural space after cervical spinal cord injury in an animal model. J Bone Joint Surg Am. 2010;92(5):1206–1214. doi: 10.2106/JBJS.I.00740. PMID:20439667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH. Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie. 1991;37(5):291–302. PMID:1758561. [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15–26. doi: 10.3171/jns.1991.75.1.0015. PMID:2045903. [DOI] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG, Thorpe K, Taylor W. Current use and timing of spinal surgery for management of acute spinal surgery for management of acute spinal cord injury in North America: results of a retrospective multicenter study. J Neurosurg. 1999;91(1 Suppl):12–18. doi: 10.3171/spi.1999.91.1.0012. PMID:10419357. [DOI] [PubMed] [Google Scholar]
- Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86(3):483–492. doi: 10.3171/jns.1997.86.3.0483. PMID:9046306. [DOI] [PubMed] [Google Scholar]
- Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, Herbison GJ, Northrup BE. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22(22):2609–2613. doi: 10.1097/00007632-199711150-00006. PMID:9399445. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Singh A, Craven C, Verrier MC, Drew B, Ahn H, Ford M, Fehlings MG. Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord. 2012;50(11):840–843. doi: 10.1038/sc.2012.59. PMID:22565550. [DOI] [PubMed] [Google Scholar]
- Zhu H, Feng YP, Young W, You SW, Shen XF, Liu YS, Ju G. Early neurosurgical intervention of spinal cord contusion: an analysis of 30 cases. Chin Med J (Engl) 2008;121(24):2473–2478. PMID:19187581. [PubMed] [Google Scholar]


