Abstract
Background
Birch pollen allergies are frequently associated with adverse reactions to various fruits, nuts, or vegetables, described as pollen–food syndrome (PFS) and caused by cross-reactive IgE antibodies primarily directed against Bet v 1. Specific immunotherapy (SIT) represents an effective treatment for inhalant allergies; however, successful birch pollen SIT does not correlate well with the amelioration of concomitant food allergies.
Methods
As vaccine candidates, apple Mal d 1 as well as hazelnut Cor a 1 derivatives were designed by in silico backbone analyses of the respective allergens. The proteins were produced by site-directed mutagenesis as fold variants of their parental allergens. Because Mal d 1 and Cor a 1 form cysteine-mediated aggregates, nonaggregative cysteine to serine mutants were also generated. The proteins were characterized physicochemically, immunologically, and in in vivo models with or without adjuvant.
Results
The structurally modified proteins showed significantly decreased IgE binding capacity. Notably, both in vivo models revealed reduced immunogenicity of the hypoallergenic fold variants. When formulated with alum, the monomeric cysteine mutants induced a similar immune response as the aggregated parental allergens, which is in contrast with data published on Bet v 1.
Conclusion
These findings lead to the suggestion that the Bet v 1 structure has unique intrinsic properties, which could account for its high allergenicity. Obviously, these characteristics are not entirely shared with its food homologues from apple and hazelnut. Thus, it is important to tackle pollen-related food allergies from different angles for the generation of effective vaccine candidates to treat birch PFS.
Keywords: apple allergy, birch pollen-associated food allergies, hazelnut allergy, pollen–food syndrome, protein remodeling
To cite this article: Roulias A, Pichler U, Hauser M, Himly M, Hofer H, Lackner P, Ebner C, Briza P, Bohle B, Egger M, Wallner M, Ferreira F. Differences in the intrinsic immunogenicity and allergenicity of Bet v 1 and related food allergens revealed by site-directed mutagenesis. Allergy 2014; 69: 208–215.
Birch pollen allergies are frequently associated with adverse reactions to various fruits (i.e., pomaceous fruits, stone fruits), hazelnut, vegetables, and legumes and are generally described as pollen–food syndrome (PFS) 1. The symptoms of PFS are mediated by cross-reactive IgE antibodies primarily directed against the major birch pollen allergen Bet v 1 and usually manifest themselves either locally and mildly as oral itching and swelling 2 or, in rare occasions, systemically as urticaria or even anaphylaxis 3. In a recent study including 225 birch pollen-allergic patients from Austria, 73% of the patients reported Bet v 1-associated food allergies. 80% of the food-allergic patients showed hypersensitivity reactions to apple and 59.4% reacted with hazelnut; reactions to other Bet v 1-related food allergens were less frequently reported 4. Apple Mal d 1.0108 and hazelnut Cor a 1.0401 share 55% and 67% sequence identity with Bet v 1, respectively 5,6; however, the Bet v 1-fold seems very conserved within the whole allergen family 7. Successful allergen-specific immunotherapy (SIT) against birch pollinosis does not necessarily lead to a reduction of allergic reactions in concomitant food allergies 8–11, possibly due to the fact that antibody epitopes of Bet v 1 and associated food allergens only converge to a certain degree 12. Recently, it has been shown that continuous consumption of apple can reduce OAS symptoms in allergic individuals; however, this clinical improvement failed to create enduring immunologic effects 13. Of note, there are even reports on patients sensitized to the Bet v 1 allergen family who only experience food-associated allergic symptoms 14. Thus, such data highlight the need for specific treatment of birch pollen-related food allergies. For therapy of Bet v 1-mediated birch pollen allergies, a novel derivative termed BM4 has recently been developed 15. The molecule was generated by computer-aided fold analysis of the Bet v 1 backbone followed by site-directed mutagenesis. This rendered BM4 unable to adopt the Bet v 1-like fold, which abolished IgE binding, and moreover, the activation of antigen-presenting cells and T-cell polarization were changed 16. Using this model, we investigated the effect of structural modifications on the Bet v 1-associated food allergens Mal d 1 and Cor a 1, respectively.
Material and methods
Patients and sera
Patients with birch pollen allergy and concomitant adverse reactions toward apple and hazelnut were selected based on typical case history, positive skin prick test, and CAP class >3. Experiments with serum samples from allergic donors were approved by the Ethic Committee of the Medical University of Vienna (no. EK028/2006). Informed written consent was obtained from all subjects included in the study.
Generation of Mal d 1 and Cor a 1 derivatives
Models of Mal d 1 WT and Cor a 1 WT were generated based on crystal structures of Pru av 1 (1E09) and Bet v 1 (1BV1), respectively, using the software Modeller (http://salilab.org/modeller/). In silico mutagenesis and Z-score calculations were performed as described 17. Mal d 1 and Cor a 1 variants were generated by PCR amplification of mutated fragments of Mal d 1.0108 (AJ417551) and Cor a 1.0401 (AF136945) using internal mismatch primers (Table S1) as described in the article's online Supporting Information. Mal d 1 variants were cloned into a pET28b vector (Merck KGaA, Darmstadt, Germany) using Nco I and Eco R I restriction sites, while Cor a 1 variants were cloned into a pHIS-Parallel2 vector using Nde I and Xho I restriction sites 18.
Recombinant production of Mal d 1, Cor a 1, and derivatives thereof
rMal d 1.0108 and rCor a 1.0401 termed Mal d 1 WT and Cor a 1 WT thereafter were produced as previously described 19,20. For the production of allergen derivatives, Escherichia coli BL21 Star™ (DE3) cells (Invitrogen Corp, Carlsbad, CA, USA) were transformed with the respective plasmids. Proteins were purified from inclusion bodies using a two-step chromatographic procedure as described in the article's online Supporting Information. Residual bacterial endotoxin was removed from recombinant allergens as described 21. Endotoxin content <3 EU/mg was verified by LAL assay (Associates of Cape Cod Inc., East Falmouth, MA, USA).
Physicochemical characterization of Mal d 1, Cor a 1, and variants thereof
Purity, identity, secondary structure, homogeneity, and aggregation of recombinant proteins were analyzed as previously described 22. A detailed methodology can be found in the article's online Supporting Information.
IgE binding, mediator release assays and ANS binding
ELISA as well as mediator release experiments were performed as previously described 15. A brief description of the assays is given in the article's online Supporting Information.
Endolysosomal degradation assay
Endolysosomes were isolated from JAWS II cells by differential centrifugation as previously described 23. 5 μg protein was digested with 7 μg of isolated microsomal proteins in a final volume of 20 μl containing 100 mM citrate buffer pH 4.8 and 2 mM dithiothreitol for 0, 2, 4, 6, 12, and 24 h at 37°C and stopped by heat denaturation. Qualitative analysis was performed by ESI-QTOF mass spectrometer fitted with a capillary reversed-phase HPLC (Waters Corp, Milford, MA, USA).
Mice and immunizations
Female BALB/c mice at 8–10 weeks of age (Charles River Laboratories, Wilmington, MA, USA) were immunized with either 5 μg antigen adsorbed to Alu-Gel-S (Serva, Heidelberg, Germany) or 10 μg of antigen in PBS pH 7.4 administered subcutaneously as two 50 μl injections in the back of the animals and boosted on days 14, 21, and 42 or 7, 14, and 21. Sera were collected on days 0, 21, and 49 or 0, 12, and 28, respectively. All animal experiments were conducted according to national guidelines approved by the Austrian Ministry of Science (BMWF-66.012/0011-II/10b/2010).
Serology and ELISPOT assays
IgG1 and IgE responses were determined by ELISA and β-hexosaminidase release assays, as described 24. IL-4, 5, 10, and 13 and IFN-γ were either assessed using mouse TH1/TH2/TH17/TH22 13plex FlowCytomix kit (eBioscience, San Diego, CA, USA) or by ELISPOT, according to kit's manual and as previously described 25.
Statistical analysis
Statistical differences were determined by paired samples t-test, Wilcoxon signed-rank test, and Mann–Whitney U-test, depending on source data. A value of P <0.05 was considered statistically significant.
Results
Generation of the Mal d 1 and Cor a 1 structural variants
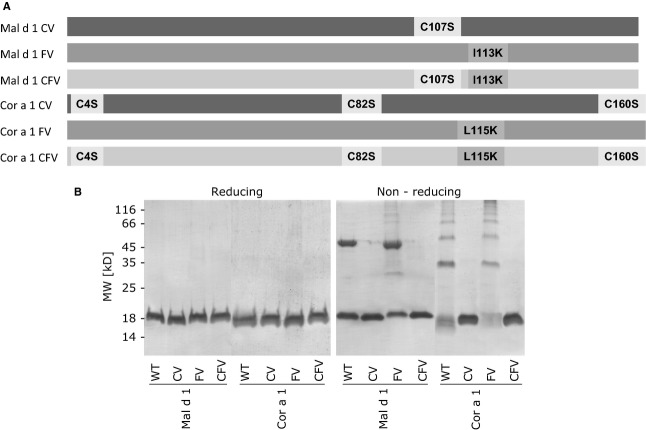
Because no structural data of neither Mal d 1 nor Cor a 1 were available, models were calculated based on protein structures of highly homologous allergens. Using in silico mutagenesis, we searched for mutation sites on each of the WT allergens, which would lead to a Z-score increase – an indicator for the structural stability of proteins. Thus, mutations giving high Z-score values show maximal effect on structural stability. The most destabilizing single-point mutation was selected for each protein, resulting in the generation of Mal d 1 I113K and Cor a 1 L115K, which were designated Mal d 1 FV and Cor a 1 FV, respectively (Fig.1A).
Figure 1.
Graphic representation of the mutated residues and their positions on the generated structural variants (A). Coomassie Brilliant Blue-stained reducing and nonreducing SDS-PAGE of Mal d 1, Cor a 1, and mutants thereof (B).
Similarly to Bet v 1.0102 24, Mal d 1 WT and Cor a 1 WT appeared to form oligomers under nonreducing conditions in SDS-PAGE (Fig.1B). Both allergens contain free cysteine residues; thus, the data suggest disulfide-mediated aggregation. To investigate the effects of oligomerization on the molecules' structural and immunologic behavior, we mutated the cysteins of the WT proteins and their FV mutants to serines. This lead to the production of the cysteine variants (CVs) Mal d 1 C107S and Cor a 1 C4,82,160S as well as the cysteine fold variants (CFVs) Mal d 1 C107S, I113K and Cor a 1 C4,82,160S, L115K (Fig.1A). The recombinant variants were cloned, expressed in E. coli, and purified to homogeneity (Fig.1B).
Structural characteristics of the generated variants
Protein secondary structure was determined by circular dichroism spectroscopy. Far-UV spectra were recorded to compare the structures of allergen variants with their WT counterparts (Fig.2A). CD spectra from all Mal d 1 and Cor a 1 variants were highly similar to their respective WT proteins and resembled the spectra typical for Bet v 1-like proteins. Allergens of the Bet v 1 family, including Mal d 1 and Cor a 1, have highly homologous structures with a characteristic hydrophobic cavity traversing the proteins 26. A wide variety of ligands, one of which is 8-anilino-1-naphthalenesulfonic acid (ANS), were shown to bind to this cavity 27,28. Fluorescence spectroscopy was used to indicate structural alterations in the variants via changes in their ability to bind ANS (Fig.2B). A profound signal decrease was observed for the FVs and CFVs of both Mal d 1 and Cor a 1. The decrease for the CVs was far less significant, albeit stronger for Cor a 1 CV. Altogether, these data indicated an overall preservation of the original protein fold for the WTs and CVs, although a significant structural alteration was observed for FVs and CFVs.
Figure 2.
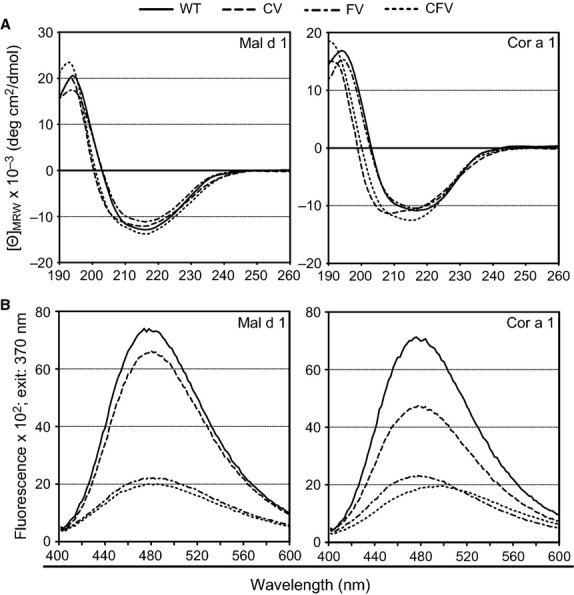
Circular dichroism spectra of the structural variants compared with the respective WT proteins at 20°C. Data are baseline-subtracted, normalized, and presented as mean residue molar ellipticity [Θ]MRW (A). Fluorescence emission spectra of the structural variants compared with the respective WT proteins. Data are baseline-subtracted and presented in fluorescence units (B).
Cysteine substitutions abrogate the aggregation tendency of Mal d 1 WT, Cor a 1 WT, and the FVs thereof
For detailed aggregation behavior analysis of the variants compared to WT allergens, we performed high-performance size-exclusion chromatography (HPSEC). As shown in Table1, both Mal d 1 and Cor a 1 WTs are prone to form aggregates with monomeric fractions of 56% and 45%, respectively. This aggregation tendency aggravates to an indeterminable level for the FVs. However, for the CVs and CFVs, the Cys to Ser mutations almost lead to an elimination of aggregation tendency, confirmed by dynamic light scattering (DLS) experiments (see Fig. S1 of the article's online Supporting Information).
Table 1.
Summary of the HPSEC results
| Proteins | Monomers | Dimers | Tri-/Tetramers | High MW aggregates | Recovery (%) |
|---|---|---|---|---|---|
| Mal d 1 | 56% | 34% | 8% | 2% | >99 |
| Mal d 1 CV | >98% | <1% | nd | <0.5% | >99 |
| Mal d 1 FV | ws | ws | ws | ws | <15 |
| Mal d 1 CFV | >99% | nd | nd | nd | >99 |
| Cor a 1 WT | 45% | 28% | 18% | 9% | >99 |
| Cor a 1 CV | 93% | 7% | nd | nd | >99 |
| Cor a 1 FV | ws | ws | ws | ws | <16 |
| Cor a 1 CFV | >99% | nd | nd | nd | >99 |
Recovery, amount of protein eluting from the column relative to the amount expected to be recovered. Low values indicate that high MW aggregates might have bound to column material or parts of HPLC system; nd, not detected; ws, weak signal. Amount of protein reaching the detector was too low to create reading strong enough to allow required calculations.
FV and CFV mutants display significantly reduced human IgE binding and cross-linking capacity
The structural variants were tested in ELISA for their ability to bind human IgE antibodies from 50 patients (see Table S2 of the article's online Supporting Information) with birch pollinosis and concomitant oral allergy syndrome (OAS) to apples and/or hazelnuts (Fig.3A). Both FVs and CFVs showed significantly reduced IgE binding capacity compared to the respective WT allergens, the reduction in CFVs being slightly stronger than the one of FVs. Allergenic activity was analyzed by RBL mediator release assays using sera from seven birch pollen-allergic patients (Fig.3B). Although the reaction of each patient to the antigens varied (see Fig. S2 of the article's online Supporting Information), both FVs and CFVs needed >100 times increased antigen concentrations to trigger a 50% mediator release compared with the respective WT molecules. In both assays, also the CVs showed reduced allergenicity, but not as profound as the one of the FVs and CFVs.
Figure 3.
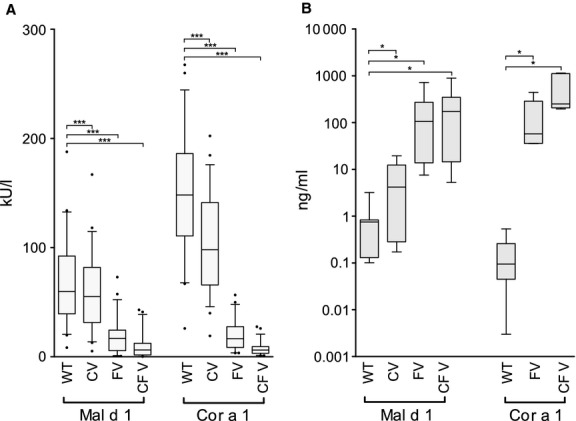
Human IgE ELISA using sera from 50 patients with birch pollen allergy and concomitant OAS. IgE binding of the structural variants was compared with the parental proteins. Data are expressed as IgE concentration (kU/L) ± SEM. P-values were calculated with paired samples t-test (***P <0.001) (A). RBL assays using patients' sera (n = 7). Data are expressed as antigen concentration (ng/ml) needed to trigger 50% mediator release ± SEM. P-values were calculated with Wilcoxon signed-rank test (*P <0.05) (B).
The hypoallergenic variants show reduced immunogenicity in an alum-based in vivo model
To compare the immune responses induced by the WT proteins and their structural variants, we analyzed IgG1 and IgE antibody responses (Fig.4A,B) of BALB/c mice immunized with the respective antigen 1 week after the last booster injection. Both the FVs and CFVs induced significantly lower levels of IgG1 antibodies than the respective WT allergens. Of note, immunization with the FVs and CFVs also resulted in lower induction of antigen-specific IgE antibodies. A reduction in the antibody levels induced by the CVs was also observed, although minimal and not as profound as the one of the FV and CFV mutants.
Figure 4.
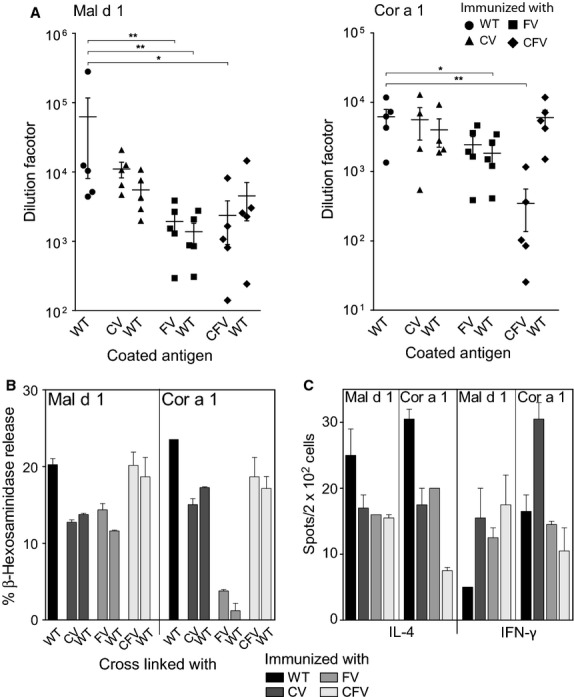
IgG1 antibody responses at day 49 analyzed by ELISA. IgG1 levels of each variant against itself and the WT, compared with the IgG1 response of the respective WT protein. The y-axis shows Δ preserum values of the serum dilution at which 50% of the maximum absorption (405 nm) was measured. Each symbol represents sera from one of the five mice immunized with each protein (A). IgE antibody responses by mouse RBL assays. 1 : 40 dilutions of sera pools from mice immunized with each of the antigens were used to passively sensitize mouse RBL cells. Recognition and cross-linking of IgE antibodies was evaluated for the homologous molecules and the WT proteins. Data are expressed as means ± SEM of% mediator release (B). ELISPOT analysis of splenocytes from immunized mice expressed as the mean of cytokine-secreting cells per 2 × 105 cells ± SEM (C). P-values were calculated using the Mann–Whitney U-test (*P <0.05; **P <0.01).
Using ELISPOT assays, we evaluated the T-cell reactivity for all the mutants and found that immunization with either of them induced IL-4 as well as IFN-γ-secreting T cells (Fig.4C). The IL-4 immune response against all variants was reduced compared to the WT proteins. The levels of IFN-γ-secreting T cells were increased after immunization with the Mal d 1 variants, while in the case of Cor a 1 the IFN-γ response to all variants was comparable to WT. Only Cor a 1 CV induced almost double the amount of IFN-γ-producing T cells. Furthermore, we could observe some difference in the number of IL-5-secreting T cells with an increase for Mal d 1 mutants and the opposite for the Cor a 1 mutants; this trend is not supported by the data obtained with IL-4. Of note, IL-10 levels were all the same for the Mal d 1 proteins, but the two Cor a 1 derivatives FV and CFV completely failed to induce IL-10-producing cells (see Fig. S4 of the article's online Supporting Information). Although no statistical analysis could be performed for RBL as well as ELISPOT assays due to the small number of repetitions, the data showed clear differences between wild-type and mutated allergens.
In an adjuvant-free mouse model, we observed similar immunologic potential with the exception of the FVs, which induced high levels of IgE and TH2 cytokines. Detailed analysis of the results is found in the article's online Supporting Information (see Fig. S5).
Structural variants show the same endolysosomal degradation patterns but different kinetics
Because an antigen's ability to prime T cells and to induce an immune response has recently been linked to its resistance to proteolysis 23, we performed in vitro endolysosomal degradation analyses of Mal d 1, Cor a 1, and the structural variants thereof. Analysis of the degradation-derived peptides via tandem mass spectrometry (see Fig. S3 of the article's online Supporting Information) revealed highly similar degradation patterns between the mutants and the respective WT proteins. However, at all time points, degradations of FVs and CFVs resulted in less peptides, while the peptide patterns of CVs reflected the ones of the WT allergens.
Discussion
Recently, it has been demonstrated that structural alterations of Bet v 1 can not only affect IgE binding but can also improve the immunologic behavior of the protein dramatically 15. Therefore, we investigated whether it is possible to transfer this knowledge to Bet v 1-related food homologues in a generic and unison approach to generate vaccine candidates for the birch pollen-related food allergies. Structural variants of Mal d 1 and Cor a 1, two Bet v 1-food homologues that are commonly associated with birch PFS, were created and extensively characterized.
Three variants of both Mal d 1 and Cor a 1 were generated based on in silico mutagenesis of models of each WT allergen using an algorithm, which has been successfully applied to modify Bet v 1.0101. Unlike Bet v 1.0101, both WT Mal d 1 and WT Cor a 1 have free cysteine residues, which form disulfide bridges under physiologic conditions leading to oligomerization of the native allergens. Thus, the cysteins were replaced by serines to mimic the behavior of monomeric Bet v 1. Cysteine and cysteine fold variants were generated, which did not form aggregates any longer. Of note, circular dichroism spectroscopy of the candidate molecules revealed that the serine mutations did not change secondary structure elements. Thus, this approach offers an effective possibility of overcoming disulfide-mediated aggregation of Bet v 1-like allergens.
Next, the immunologic properties of the candidate proteins were investigated. As predicted, the structural alterations of the FVs as well as CFVs significantly decreased their IgE binding capacity as determined by ELISA and mediator release assays. Thereafter, a BALB/c mouse model with alum as adjuvant was established. However, the in vivo experiments revealed that the hypoallergenic FVs and CFVs induced a decreased humoral immune response reflected by reduced IgG as well as IgE antibody levels when compared to parental allergens. Recently, an in vitro model to predict immunogenicity has been published 23. By digesting proteins with endolysosomal proteases from dendritic cells, conclusions on the immunologic behavior of the antigens in vivo can be drawn. Using this method, we found that the fold variants showed a higher susceptibility toward proteolysis, a fact that could explain their low immunogenic potential. This was not expected, given that the Bet v 1 fold variant BM4 was recently shown to display increased resistance toward proteolytic digestion 15. Of note, both CVs appeared stable.
The immune response triggered by the monomeric CVs was comparable to their WT allergens, which formed aggregates. The observation is almost opposite of what has recently been presented by Zaborsky et al. 24 where aggregation of Bet v 1.0102 (formerly Bet v 1.0401) was linked to significantly increased immunogenicity compared to the monomeric Bet v 1 isoform 0101. Moreover, in the absence of alum, the FVs raised a strong TH2 response with high IgE titers and significant increase in IL-13 and IL-5 cytokines. In fact, any modifications we made on Mal d 1 and Cor a 1 would always lead to either similar or weaker immune responses showing a drift toward TH2. On the contrary, manipulations of Bet v 1a constantly result in stronger responses with a clear TH1 bias 15,16,25.
These findings allow us to speculate that Bet v 1 has some unique intrinsic characteristics that are not shared by its food homologues from apple and hazelnut. Thus, manipulation of the Bet v 1-fold does not automatically lead to similar results for any Bet v 1-like allergen. Our results clearly point out that even though the Bet v 1 molecule and birch pollen allergy are intensively studied, it is important to tackle related food allergies from different angles.
Acknowledgments
This work was supported by the Austrian Science Fund (FWF), projects L688 and W1213, and the Christian Doppler Research Association. We thank Dr. Rob Aalberse for the helpful discussions and Markus Steiner, MSc, for technical support for HPSEC and DLS experiments.
Author contributions
AR performed cloning, protein purification, immunologic experiments and contributed in writing the manuscript. UP and HH contributed in the immunologic experiments. MHa contributed with the mouse models. MHi and PB performed physicochemical analyses. PL carried out the in silico calculations. CE and BB collected and provided patients' sera. ME contributed to study design. MW and FF performed the study design, contributed in writing and editing of the manuscript.
Conflicts of interest
Fatima Ferreira has received research support from the Austrian Science Fund (FWF), the Christian Doppler Research Association, and Biomay AG and provides legal consultation or expert witness testimony for the AllergenOnline Database, Indoor Biotechnologies, and HAL Allergy. The rest of the authors declare no competing financial interest.
Supporting Information
Methods and results.
Aggregation behaviour analysis of Mal d 1, Cor a 1 and their mutants via HPSEC (big graphs) and DLS (small graphs).
Three characteristic RBL titration curves for each of the Mal d 1 (up) and Cor a 1 (down) protein group.
Chronology of peptide cluster formation during in vitro endolysosomal degradation.
ELISPOT analysis of splenocytes from immunized mice expressed as the mean of cytokine-secreting cells per 2 × 105 cells ± SEM.
IgG1 antibody responses at day 28 analysed by ELISA.
List of primers used for the construction of the Mal d 1 and Cor a 1 structural variants.
Patients' sera used within this study.
References
- Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006;61:461–476. doi: 10.1111/j.1398-9995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- Mari A, Ballmer-Weber BK, Vieths S. The oral allergy syndrome: improved diagnostic and treatment methods. Curr Opin Allergy Clin Immunol. 2005;5:267–273. doi: 10.1097/01.all.0000168793.27948.b0. [DOI] [PubMed] [Google Scholar]
- Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003;112:784–788. doi: 10.1016/s0091-6749(03)02008-6. [DOI] [PubMed] [Google Scholar]
- Geroldinger-Simic M, Zelniker T, Aberer W, Ebner C, Egger C, Greiderer A, et al. Birch pollen-related food allergy: clinical aspects and the role of allergen-specific IgE and IgG4 antibodies. J Allergy Clin Immunol. 2011;127:616–622. doi: 10.1016/j.jaci.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Vanekkrebitz M, Hoffmannsommergruber K, Machado MLD, Susani M, Ebner C, Kraft D, et al. Cloning and sequencing of Mal d 1, the major allergen from apple (Malus domestica), and its immunological relationship to Bet v 1, the major birch pollen allergen. Biochem Biophys Res Commun. 1995;214:538–551. doi: 10.1006/bbrc.1995.2320. [DOI] [PubMed] [Google Scholar]
- Luttkopf D, Muller U, Skov PS, Ballmer-Weber BK, Wuthrich B, Skamstrup Hansen K, et al. Comparison of four variants of a major allergen in hazelnut (Corylus avellana) Cor a 1.04 with the major hazel pollen allergen Cor a 1.01. Mol Immunol. 2002;38:515–525. doi: 10.1016/s0161-5890(01)00087-6. [DOI] [PubMed] [Google Scholar]
- Schirmer T, Hoffimann-Sommergrube K, Susani M, Breiteneder H, Markovic-Housley Z. Crystal structure of the major celery allergen Api g 1: molecular analysis of cross-reactivity. J Mol Biol. 2005;351:1101–1109. doi: 10.1016/j.jmb.2005.06.054. [DOI] [PubMed] [Google Scholar]
- Bolhaar ST, Tiemessen MM, Zuidmeer L, van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CA, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. 2004;34:761–769. doi: 10.1111/j.1365-2222.2004.1939.x. [DOI] [PubMed] [Google Scholar]
- Bucher X, Pichler WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. 2004;59:1272–1276. doi: 10.1111/j.1398-9995.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004;48:441–448. doi: 10.1002/mnfr.200400037. [DOI] [PubMed] [Google Scholar]
- Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwolfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–943. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Hecker J, Diethers A, Schulz D, Sabri A, Plum M, Michel Y, et al. An IgE epitope of Bet v 1 and fagales PR10 proteins as defined by a human monoclonal IgE. Allergy. 2012;67:1530–1537. doi: 10.1111/all.12045. [DOI] [PubMed] [Google Scholar]
- Kopac P, Rudin M, Gentinetta T, Gerber R, Pichler C, Hausmann O, et al. Continuous apple consumption induces oral tolerance in birch-pollen-associated apple allergy. Allergy. 2012;67:280–285. doi: 10.1111/j.1398-9995.2011.02744.x. [DOI] [PubMed] [Google Scholar]
- Rashid RS, Smith KA, Nambiar KZ, Frew AJ, Tarzi MD. Pollen-food syndrome is related to Bet v 1/PR-10 protein sensitisation, but not all patients have spring rhinitis. Allergy. 2011;66:1391–1392. doi: 10.1111/j.1398-9995.2011.02618.x. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hauser M, Himly M, Zaborsky N, Mutschlechner S, Harrer A, et al. Reshaping the Bet v 1 fold modulates T(H) polarization. J Allergy Clin Immunol. 2011;127:1571–1578. doi: 10.1016/j.jaci.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmuller C, Wallner M, Deifl S, Mutschlechner S, Walterskirchen C, Zlabinger GJ, et al. A hypoallergenic variant of the major birch pollen allergen shows distinct characteristics in antigen processing and T-cell activation. Allergy. 2012;67:1375–1382. doi: 10.1111/all.12016. [DOI] [PubMed] [Google Scholar]
- Thalhamer T, Dobias H, Stepanoska T, Proll M, Stutz H, Dissertori O, et al. Designing hypoallergenic derivatives for allergy treatment by means of in silico mutation and screening. J Allergy Clin Immunol. 2010;125:926–934. doi: 10.1016/j.jaci.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Sheffield P, Garrard S, Derewenda Z. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr Purif. 1999;15:34–39. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- Ma Y, Gadermaier G, Bohle B, Bolhaar S, Knulst A, Markovic-Housley Z, et al. Mutational analysis of amino acid positions crucial for IgE-binding epitopes of the major apple (Malus domestica) allergen, Mal d 1. Int Arch Allergy Immunol. 2006;139:53–62. doi: 10.1159/000089756. [DOI] [PubMed] [Google Scholar]
- Lauer I, Alessandri S, Pokoj S, Reuter A, Conti A, Vieths S, et al. Expression and characterization of three important panallergens from hazelnut. Mol Nutr Food Res. 2008;52(S2):S262–S271. doi: 10.1002/mnfr.200700426. [DOI] [PubMed] [Google Scholar]
- Jensen LB, Torp AM, Andersen SB, Skov PS, Poulsen LK, Knol EF, et al. The biological activity of a recombinantly expressed (His)6-tagged peanut allergen (rAra h 1) is unaffected by endotoxin removal. J Immunol Methods. 2008;335:116–120. doi: 10.1016/j.jim.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Himly M, Nony E, Chabre H, Van Overtvelt L, Neubauer A, Van Ree R, et al. Standardization of allergen products: 1. Detailed characterization of GMP-produced recombinant Bet v 1.0101 as biological reference preparation. Allergy. 2009;64:1038–1045. doi: 10.1111/j.1398-9995.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Jurets A, Wallner M, Briza P, Ruzek S, Hainzl S, et al. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS One. 2011;6:e17278. doi: 10.1371/journal.pone.0017278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborsky N, Brunner M, Wallner M, Himly M, Karl T, Schwarzenbacher R, et al. Antigen aggregation decides the fate of the allergic immune response. J Immunol. 2010;184:725–735. doi: 10.4049/jimmunol.0902080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Scheiblhofer S, Kern K, Gruber C, Stepanoska T, Thalhamer T, et al. Generation of hypoallergenic DNA vaccines by forced ubiquitination: preventive and therapeutic effects in a mouse model of allergy. J Allergy Clin Immunol. 2006;118:269–276. doi: 10.1016/j.jaci.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Sommergruber K, Mills EN. Food allergen protein families and their structural characteristics and application in component-resolved diagnosis: new data from the EuroPrevall project. Anal Bioanal Chem. 2009;395:25–35. doi: 10.1007/s00216-009-2953-z. [DOI] [PubMed] [Google Scholar]
- Mogensen JE, Wimmer R, Larsen JN, Spangfort MD, Otzen DE. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J Biol Chem. 2002;277:23684–23692. doi: 10.1074/jbc.M202065200. [DOI] [PubMed] [Google Scholar]
- Kofler S, Asam C, Eckhard U, Wallner M, Ferreira F, Brandstetter H. Crystallographically mapped ligand binding differs in high and low IgE binding isoforms of birch pollen allergen Bet v 1. J Mol Biol. 2012;422:109–123. doi: 10.1016/j.jmb.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods and results.
Aggregation behaviour analysis of Mal d 1, Cor a 1 and their mutants via HPSEC (big graphs) and DLS (small graphs).
Three characteristic RBL titration curves for each of the Mal d 1 (up) and Cor a 1 (down) protein group.
Chronology of peptide cluster formation during in vitro endolysosomal degradation.
ELISPOT analysis of splenocytes from immunized mice expressed as the mean of cytokine-secreting cells per 2 × 105 cells ± SEM.
IgG1 antibody responses at day 28 analysed by ELISA.
List of primers used for the construction of the Mal d 1 and Cor a 1 structural variants.
Patients' sera used within this study.