Abstract
Protein separations in capillary zone electrophoresis (CZE) suffer from non-specific adsorption of analytes to the capillary surface. Semi-permanent phospholipid bilayers (PLBs) have been used to minimize adsorption, but must be regenerated regularly to ensure reproducibility. We investigated the formation, characterization, and use of hybrid phospholipid bilayers (HPBs) as more stable biosurfactant capillary coatings for CZE protein separations. HPBs are formed by covalently modifying a support with a hydrophobic monolayer onto which a self-assembled lipid monolayer is deposited. Monolayers prepared in capillaries using 3-cyanopropyldimethylchlorosilane (CPDCS) or n-octyldimethylchlorosilane (ODCS) yielded hydrophobic surfaces with lowered surface free energies of 6.0 ± 0.3 or 0.2 ± 0.1 mJ m−2, respectively, compared to 17 ± 1 mJ m−2 for bare silica capillaries. HPBs were formed by subsequently fusing vesicles comprised of 1,2-dilauroyl-sn-glycero-3-phosphocholine or 1,2-dioleoyl-sn-glycero-3-phosphocholine to CPDCS- or ODCS-modified capillaries. The resultant HPB coatings shielded the capillary surface and yielded reduced electroosmotic mobility (1.3 – 1.9 × 10−4 cm2 V−1s−1) compared to CPDCS- and ODCS-modified or bare capillaries (3.6 ± 0.2 × 10−4 cm2 V−1s−1, 4.8 ± 0.4 × 10−4 cm2 V−1s−1, and 6.0 ± 0.2 × 10−4 cm2 V−1s−1, respectively), with increased stability compared to PLB coatings. HPB-coated capillaries yielded reproducible protein migration times (RSD ≤ 3.6 %, n ≥ 6) with separation efficiencies as high as 200,000 plates m−1.
Keywords: Capillary coatings, Capillary zone electrophoresis, Hybrid bilayers, Phospholipids, Proteins
1. Introduction
Capillary zone electrophoresis (CZE) is frequently used to study biological analytes due to the small sample volume requirements and rapid, high efficiency separations. Yet, charged analytes, including proteins, readily adsorb to the capillary surface via electrostatic and hydrophobic interactions [1,2], thereby decreasing efficiency and detection sensitivity, as well as reducing the migration time repeatability by altering the zeta potential and electroosmotic mobility (μeof).
Non-specific adsorption can be minimized by altering the buffer composition [3–5] or coating the capillary surface [1,6]. Capillary coatings are separated into three classes: permanent coatings that are covalently bound to the surface, dynamic coatings that are in equilibrium with free molecules in solution, and semi-permanent coatings that are adsorbed to the surface, but removed from the BGE during separation [1,6,7]. Covalent coatings are expensive, sensitive to sample conditions and often exhibit limited lifetimes. Dynamic coatings are attractive since they are rapidly generated at low cost using small molecules, polymers, or surfactants [1,6,8]; however, dynamic coatings necessitate inclusion of the coating material in the running buffer, which may alter the separation via interactions between analytes and coating material [1,6,8,9]. Additionally, inclusion of surfactants in the running buffer complicates coupling of CZE to post-column analyses, such as mass spectrometry [8,10,11].
Phospholipid bilayers (PLBs) form semi-permanent coatings in capillaries and microchips in which excess lipid is removed prior to separation [1,8,10–12]. PLB coatings shield the charged capillary surface, leading to reduced μeof and high protein recoveries [9–11]. PLBs prepared using different phospholipids exhibit varying stabilities as semi-permanent coatings and typically require regeneration between separations, increasing the total analysis time and complexity of the separation process [10,11]. Bilayer stability can be increased via inclusion of cholesterol [13,14] or through polymerization of synthetic, polymerizable lipids [7,15–17] or monomers partitioned into the lamellar region of the bilayer [18,19]. Incorporating cholesterol at 30 mol % in PLB coatings increased stability when separations were performed above the phase transition temperature of the lipid, though the utility of these coatings for minimizing protein adsorption was not examined [14]. Polymerized PLB coatings were used for protein separations and shown to be stable after drying and rehydration, though the coating was formed from synthetic lipids that are not commercially available, minimizing widespread use [7,15].
Hybrid phospholipid bilayers (HPBs) provide an alternative to PLBs. In HPBs, moderately to very hydrophobic monolayers are formed on a substrate by adsorption or through covalent bonding of monomers [20,21]. A surfactant monolayer, formed from nonionic surfactants or phospholipids, self-assembles on this layer in a tails-down geometry due to hydrophobic interactions and van der Waals forces between the hydrophobic tails of the surfactant and the hydrophobic monolayer on the surface [22,23]. Detailed investigation of HPBs on planar substrates supports a model wherein the lipids are not intercalating, but rather a uniform lipid monolayer assembles on top of a low energy alkane or other modified surface [20,21,24–26]. HPBs formed with nonionic surfactants require the surfactant to be present in the aqueous medium to ensure the HPB structure is maintained [23]; however, HPBs prepared with phospholipids yield enhanced stability, even after exposure to air [27]. Additionally, the presence of zwitterionic phospholipids in HPBs reduces non-specific protein adsorption [28]. The reduction in non-specific adsorption, coupled with increased stability presents a potentially useful combination for utilizing HPBs as capillary coatings. Here, we explored the utility of HPBs for preparing stable, semi-permanent coatings for CZE separation of proteins. The coatings were characterized with respect to surface energy, stability, reproducibility, and separation performance.
2. Materials and Methods
2.1 Materials
1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC, Tm = −1 °C) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Tm = −20 °C) were purchased from Avanti Polar Lipids (Alabaster, AL). 3-cyanopropyldimethylchlorosilane (CPDCS) was purchased from TCI America (Portland, OR) or Alfa Aesar (Ward Hill, MA). n-octyldimethylchlorosilane (ODCS) was purchased from Gelest (Morrisville, PA). MOPS, lysozyme (chicken egg white, Lyso), α-chymotrypsionogen A (bovine pancreas, α-Chymo), and myoglobin (equine heart, Myo) were purchased from Sigma (St. Louis, MO). All other chemicals were purchased from Fisher (Pittsburgh, PA) and used as received. Water was obtained from a Barnstead EasyPure UV/UF purification system.
2.2 Surface modification
CZE capillaries (50 μm i.d.; 360 μm o.d.; Polymicro Technologies., Phoenix, AZ) were 35 cm in length and detection windows were prepared 20 cm from the capillary inlet. Contact angle capillaries (100 μm i.d.; 360 μm o.d.) were 18 cm total length. Capillaries were washed by consecutive rinses of 1.0 M HNO3, H2O, and acetone before drying with He for 30 min. Dry capillaries were filled with 2 % silane (v/v) in dry solvent and sealed. The reaction proceeded at room temperature for 20 – 24 h. CPDCS or ODCS were dissolved in dry acetonitrile or toluene, respectively. After modification, capillaries were washed with consecutive rinses of acetonitrile (CPDCS) or toluene (ODCS), ethanol, and H2O for 5 min each. CZE capillaries were rinsed with background electrolyte (BGE) (20 mM MOPS, pH 7.4 or 10 mM phosphate, pH 7.4) prior to separations.
2.3 HPB formation
Chloroform was removed from phospholipids by evaporation under Ar followed by overnight vacuum drying. Dried lipids were suspended at 1 mg mL−1 in H2O and sonicated using either a cup horn (Model W-380, Heat Systems-Ultrasonics, Inc., Newtown, CT) or bath (Aquasonic Model 75T, VWR, Radnor, PA) sonicator to form small unilamellar vesicles (SUVs). Using the cup horn sonicator, lipids were sonicated at 25 °C for 0.5 – 1.0 h. With the bath sonicator, lipids were sonicated for 10 min followed by a 10 min period of rest at room temperature. This cycle was repeated until the solution was clear, approximately 3 – 4 cycles. To ensure SUV formation, lipids prepared with the bath sonicator were extruded 21 times (Avanti Polar Lipids miniextruder) at room temperature through membranes with 0.05 μm pores (Whatman, St. Louis, MO). Using a Malvern Zetasizer Nano-ZS (Worcetershire, United Kingdom) the mean SUV diameter was 43 ± 15 nm or 34 ± 8 nm (number distributions) when prepared with the cup horn sonicator or after extrusion, respectively. HPBs were prepared by vesicle fusion [25,26,29]. CPDCS- or ODCS-modified capillaries were washed with H2O for 5 min, the SUV solution for 1 h, and BGE for 10 min before usage. All washes were performed at 5 μL min−1 using a syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA).
Semi-permanent PLB coatings were prepared in bare capillaries by fusing SUVs of DLPC. Bare capillaries were washed with 0.1 M NaOH and H2O. The SUV solution was then flushed through the capillary for 45 min, after which, flow was stopped for 20 min to promote vesicle fusion. Capillaries were rinsed with BGE prior to separation.
2.4 Contact angle measurements and surface free energies
Water contact angles on planar substrates were measured immediately after surface modification using the sessile droplet method. H2O (2 μL) was manually deposited on each substrate using a micropipette. Images were acquired with a Pulnix TM-7CN video camera (JAI Inc., San Jose, CA) and contact angles were measured using a Kruss model DSA 10 Mk2 drop shape analysis system (Palo Alto, CA). For each surface modification, the mean contact angle was calculated from four measurements made at different locations on each of three substrates. Surface free energies (SFEs, γs) were calculated for planar substrates using the Fowkes method (Eq. (1)) [30,31], where Θ, Φ, and γl are the contact angle, a correction factor that can be approximated as unity [32], and the surface energy of water, 72.8 mJ m−2 at 21.5 °C [31], respectively.
| (1) |
Contact angles were also calculated in 100 μm i.d. capillaries. Bare, CPDCS-, and ODCS-modified capillaries were dried and then positioned vertically. The lower ends of the capillaries were immersed in H2O, which was drawn into the capillary until it reached an equilibrium position. The distance the water traveled was measured and images of the water/air interface were recorded through a 10× stereoscope objective. Contact angles were measured from printed images using a protractor. SFEs (γ) for capillaries were calculated using the water rise height at its equilibrium position (h), contact angle of the surface (Θ), capillary radius (r), gravitational constant (g), and density of water (ρ) according to Eq. (2) [33].
| (2) |
2.5 Separations and capillary storage
The μeof of bare, silane-modified (CPDCS and ODCS), and HPB-coated capillaries was measured with a neutral marker in 20 mM MOPS, pH 7.4. Mesityl oxide was diluted to 1 mM in BGE and injected hydrodynamically at 5 cm for 5 s. Separation and detection were performed using a lab-built instrument with ultraviolet absorbance detection (Model 500 Detector, ChromTech, Apple Valley, MN) at 254 nm. Signal from the detector was collected using an A/D converter (NI USB-6221, National Instruments) and software written in LabVIEW (National Instruments, Austin, TX). Electric fields (571 V cm−1) were applied using a 30 kV power supply (CZE-1000, Spellman High Voltage Corporation, Hauppauge, NY). For all CZE separations, surface coatings were generated before positioning the capillary in the instrument. CZE was performed without regeneration of the coating between separations, though buffer was replaced in each vial after 30 min of cumulative applied high voltage.
Protein separations were performed in 10 mM phosphate buffer, pH 7.4 with an electric field of 514 V cm−1 and detection at 214 nm with 0.005 AUFS. Phosphate buffer was used as the BGE for protein separations since the MOPS buffer had impurities that were detected at 214 nm and interfered with detection and analysis of the protein peaks. Protein mixtures containing 100 μM Lyso, α-Chymo, and Myo were prepared daily in10 mM phosphate, pH 7.4.
Modified capillaries were stored in 20 mM acetate buffer, pH 4.0, to minimize degradation of the covalent modification [6]. All HPB-coated capillaries were washed with methanol to remove lipids before storage.
2.6 Data presentation and statistical evaluation
All data is presented as the mean ± standard deviation (graphically represented by error bars). In all cases, a minimum of 3 replicate measurements was obtained. Statistical comparison of data was performed using the Student’s t-test at the 95 % confidence interval.
3. Results and Discussion
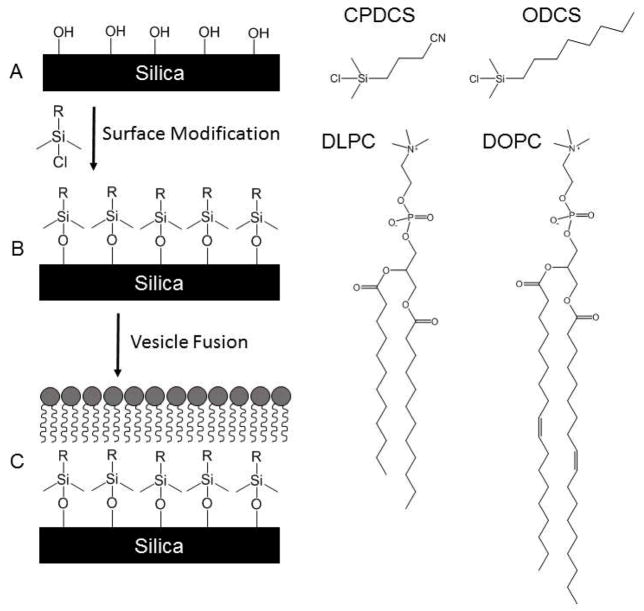
The general process for in situ preparation of HPBs within enclosed, fused silica capillaries is outlined in Figure 1. Briefly, fused silica capillaries were modified with CPDCS or ODCS to provide a nominally hydrophobic surface upon which lipid fusion could occur. Fusion of lipids to hydrophilic surfaces yields planar supported PLB coatings, whereas fusion to hydrophobic surfaces generates a lipid monolayer assembled in a tails-down orientation [29]. To better explore the formation and utility of HPB capillary coatings, HPBs were prepared using two surface modifiers and two different lipids.
Figure 1.
Scheme illustrating formation of HPB coatings. Bare silica (A) is reacted with CPDCS or ODCS to generate a covalently-bound, hydrophobic monolayer (B) onto which DLPC or DOPC can self-assemble, resulting in a HPB (C). Schematics not to scale.
3.1 HPB formation and characterization
Hydrophobicity of the modified silica surfaces was assessed using the SFE values determined by water contact angles on planar glass supports or within fused silica capillaries. SFEs for bare, CPDCS-, and ODCS-modified planar substrates were quantified to provide a baseline for in-capillary measurements. Images of droplets on planar surfaces and contact angles are shown in the Supporting Information (Figure SI-1 and Table SI-1). Calculated SFE values for bare, CPDCS- and ODCS-modified coverslips are presented in Table 1. The reduced SFE for CPDCS- and ODCS-modified planar substrates compared to bare planar substrates supports the increased hydrophobicity associated with the presence of organic modifiers on the surfaces. The reduced SFE for ODCS-modification compared to CPDCS-modification likely results from poorer wettability of the long hydrocarbon chain.
Table 1.
Surface characterization of planar supports and fused silica capillaries
| Planar Supports | 100 μm i.d. capillaries | 50 μm i.d. capillaries | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Surface | Surface energy (mJ m−2) | Surface energy (mJ m−2) | μeof (10−4 cm2 V−1 s−1) | RSD (%) | nb | μeof Reduction (%) |
|
| ||||||
| Bare | 73a | 17 ± 1 | 6.0 ± 0.2 | 3.5 | 40 | |
|
| ||||||
| CPDCS- modified | 31 ± 3 | 6.0 ± 0.3 | 3.6 ± 0.2 | 6.3 | 140 | 40 |
| CPDCS/DOPC | 1.3 ± 0.2 | 12 | 48 | 78 | ||
| CPDCS/DLPC | 1.9 ± 0.2 | 11 | 44 | 68 | ||
|
| ||||||
| ODCS-modified | 14 ± 2 | 0.2 ± 0.1 | 4.8 ± 0.4 | 8.4 | 100 | 20 |
| ODCS/DOPC | 1.4 ± 0.2 | 15 | 38 | 77 | ||
| ODCS/DLPC | 1.7 ± 0.2 | 9.2 | 41 | 71 | ||
The Kruss analysis system cannot accurately quantify water contact angles less than 10 °. Therefore, the contact angle of bare coverslips was reported as less than 10 ° (Table SI-1) and the SFE was approximated and presented without a standard deviation.
The total n value represents a minimum of 3 separations from a minimum of 4 different capillaries.
While planar substrates provide a good first approximation, the surface of coverslips may present some differences compared to curved fused silica capillaries. Thus, SFEs for silane-modified capillaries were measured and compared to the planar supports (Table 1, Figure SI-1, and Table SI-1). Though the absolute values of these measurements differ from the planar supports, the underlying trend is the same, demonstrating increased hydrophobicity upon modification with CPDCS and ODCS. Unfortunately, it was not possible to obtain similar measurements directly on HPBs and PLBs. Lipid membranes are held together by relatively weak non-covalent interactions. Removal of water disrupts these interactions leading to catastrophic breakdown of the membrane in both vesicle and supported lipid membrane geometries. Thus, alternate methods were required to assess the resultant coatings.
μeof can be used to indirectly monitor chemical changes on the capillary surface. Table 1 lists the average μeof for bare, modified, and HPB-coated capillaries (n ≥ 4 capillaries for each coating). μeof in CPDCS- and ODCS- modified capillaries is reduced by 20 – 40 % compared to μeof in bare capillaries. During capillary modification, CPDCS and ODCS react with surface silanols, decreasing the number of free silanols and thus, the negative surface charge and μeof. Both modifications result in μeof reductions, indicating that the surfaces have been functionalized. Interestingly, though ODCS-modification yields more hydrophobic surfaces than CPDCS-modification (Table 1), higher μeof was observed in ODCS-modified capillaries. At low surface densities, including the initial time during surface modification, alkyl chains are predicted to be bent and reside parallel to silica surfaces [34]. Longer alkyl chains (such as the n-octyl-chain) can cover more surface silanols than shorter chains (the propyl chain of CPDCS), preventing additional reactions of silanes at neighboring sites and resulting in lower surface coverage and an associated higher μeof as observed in these measurements.
Though the μeof for CPDCS- and ODCS-modified capillaries suggest that the surfaces have been chemically altered, the true test is whether these surfaces can be used to form HPBs. HPBs were prepared by fusing DLPC or DOPC vesicles with CPDCS- or ODCS-modified capillaries. The μeof values for CPDCS/DOPC, CPDCS/DLPC, ODCS/DOPC, and ODCS/DLPC capillaries are shown in Table 1. In all HPB-coated capillaries, μeof was reduced compared to CPDCS- or ODCS-modified capillaries, indicating that a lipid layer had formed, which shielded the capillary surface. Fluorescence microscopy of HPB coated glass substrates verified that the lipid structure was a monolayer formed on the hydrophobic surface (Figure SI-2 and Table SI-2). Additionally, HPB coatings represent μeof reductions of 78 % (CPDCS/DOPC), 68 % (CPDCS/DLPC), 77 % (ODCS/DOPC), and 71 % (ODCS/DLPC) compared to bare capillaries. Direct measurement of zeta-potentials for silica nanoparticles demonstrated similar trends with respect to surface coatings (Table SI-3).
Additionally, the fluorescence intensity of bare, CPDCS-modified, and CPDCS/DOPC capillaries was measured and normalized after staining with a membrane indicator, FM1-43 (Figure SI-3). CPDCS/DOPC-coated capillaries yielded the highest fluorescence intensity, indicating a high lipid density on the capillary surface. Bare capillaries had the lowest fluorescence intensity due to the absence of lipids. CPDCS-modified capillaries exhibited approximately 20 % of the fluorescence intensity of the CPDCS/DOPC capillaries, suggesting that FM1-43 may interact with the moderately hydrophobic cyanopropyl-monolayer, though the intensity was significantly lower than that of CPDCS/DOPC capillaries. Prior studies with FM1-43 have shown high levels of fluorescence when PLB-coated capillaries were prepared [7].
Combined, these data support the formation of HPB coatings in fused silica separation capillaries that have been modified with either CPDCS or ODCS followed by lipid fusion and that the resultant HPBs reduce EOF similarly to PLBs. Unmodified capillaries coated with DOPC or DLPC to form PLBs yielded lower μeof, 1.0 × 10−4 cm2 V−1s−1 [11] or 0.22 × 10−4 cm2 V−1s−1 [10], respectively, representing μeof reductions of approximately 80 % (DOPC) and 95 % (DLPC) compared to bare capillaries (4.6 × 10−4 cm2 V−1s−1) [11]. These data support that HPB and PLB coatings formed with DOPC result in similar reductions of μeof; however, HPB coatings prepared with DLPC exhibit less μeof suppression compared to PLBs formed with the same lipid. Since DLPC has fully saturated carbon tails, the difference in μeof reduction may be due to different packing densities of DLPC lipids in PLBs and HPBs, resulting in differential shielding of the charged capillary surface.
3.2 Coating stability and reproducibility
A primary drawback of semi-permanent PLB coatings is the limited and varying stability. Capillaries coated with DLPC bilayers were stable for 105 min of applied voltage [10]; however, DOPC bilayers started to degrade immediately upon application of an electrical field as evidenced by the increasing μeof [11]. Since, HPBs formed on planar substrates exhibit enhanced stability relative to PLBs [27], we tested the stability of HPB capillary coatings by monitoring μeof over 100 min of applied high voltage without coating regeneration within the same capillary. RSDs of mesityl oxide tm were 1.6 % (n = 55) and 2.6 % (n = 20) for CPDCS-modified and CPDCS/DOPC-coated capillaries, respectively (Table SI-4). Within 100 min of run time, the μeof reduction in CPDCS/DOPC-coated capillaries decreased by 4 %, whereas in DOPC PLB-coated capillaries the μeof reduction decreased by 15 % [11], illustrating enhanced stability of HPBs compared to PLB coatings.
To validate that the reduced μeof and increased stability resulted from the HPB, the reproducibility of each capillary modification was evaluated as a function of μeof. In Figure 2, mean μeof is plotted for the first 10 runs in capillaries prepared with the indicated surface modification (n ≥ 3 capillaries for each coating condition). Similar to Table 1, bare capillaries (diamonds) had the highest μeof, while CPDCS- (Figure 2A) and ODCS-modified (Figure 2B) capillaries (squares) showed μeof reductions. Finally, HPB coatings prepared using either of two common lipids, DLPC (triangle) or DOPC (circles), yielded the lowest μeof and thus the highest shielding of capillary surface charges. The stable μeof values demonstrate the increased stability of the HPB coatings for at least 10 runs (approximately 1 hour of applied high voltage for HPB capillaries) without regeneration.
Figure 2.

Stability and reproducibility of HPB coatings. Coating reproducibility for CPDCS-(A) and ODCS-modified (B) capillaries in the presence and absence of the HPB. Each plot shows the mean μeof of 10 consecutive runs in bare (diamonds), CPDCS- or ODCS-modified (squares), DLPC HPB (triangles), and DOPC HPB (circles) capillaries (n ≥ 3 capillaries). Coatings were not regenerated between separations.
3.3 Protein separations
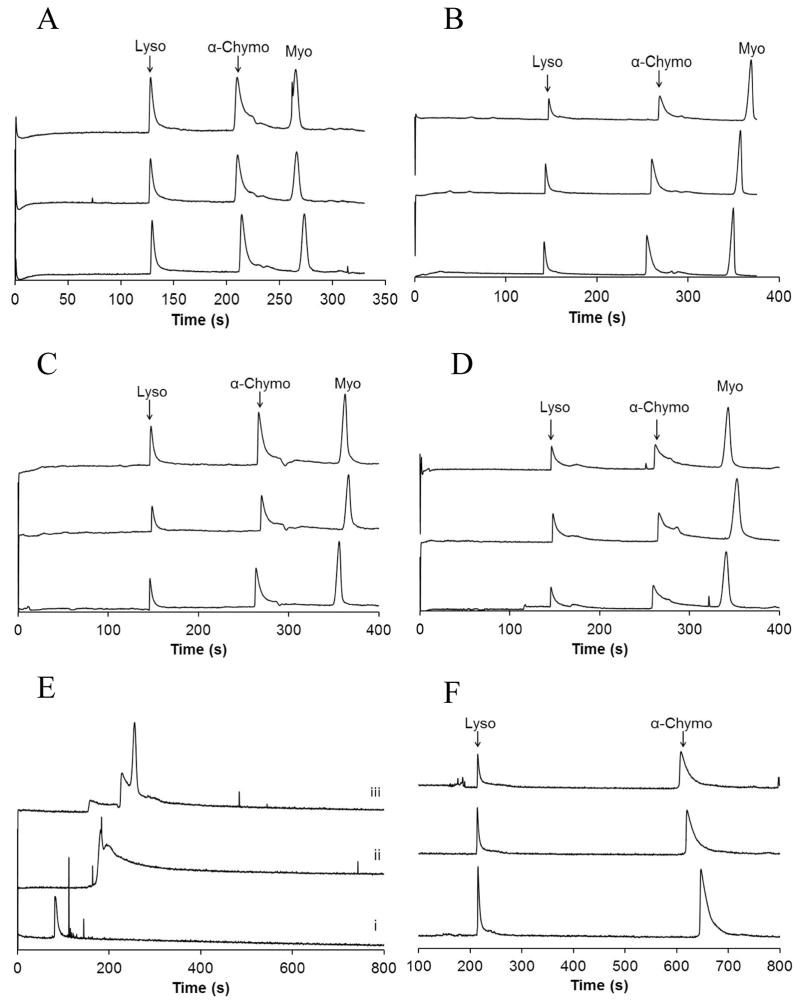
Non-specific adsorption to bare capillaries presents a number of challenges that are often overcome using PLB coatings, though at the expense of coating stability. To validate the overall functional utility of HPB coatings, protein mixtures were analyzed. Figure 3 shows three electropherograms for each HPB coating without regeneration between separations. All HPB coatings yielded peaks corresponding to Lyso, α-Chymo, and Myo, compared to bare capillaries, where only a single peak was observed (Figure 3E,i). Lyso and α-Chymo were also separated in a DLPC PLB capillary (Figure 3F). PLBs of DLPC were prepared since literature showed that these coatings were more stable than DOPC PLBs [10,11]. The EOF in the DLPC PLB-coated capillary was more reduced than the EOF of the HPB capillaries; thus, Myo was not included in the protein mixture since long run times would be required to detect the tm of the anionic protein (Myo pI = 7.2). The protein peaks for all HPB- and DLPC PLB-coated capillaries exhibited varying degrees of peak asymmetry, perhaps due to interactions between the analytes and possible domains of poor coating, e.g. near the ends of the capillary where lipid coatings may be destroyed upon exposure to air or drying. However, in spite of this, all proteins were resolved and readily detected using all four HPB coatings.
Figure 3.
Protein separations in HPB-coated capillaries. Electropherograms showing multiple separations of Lyso, α-Chymo, and Myo in capillaries with HPBs formed from CPDCS/DOPC (A), CPDCS/DLPC (B), ODCS/DOPC (C), and ODCS/DLPC (D). (E) Protein separations in bare silica (i), CPDCS-modified (ii), and ODCS-modified (iii) capillaries show broad peaks that are not resolved. (F) Separations of Lyso and α-Chymo in capillaries coated with a DLPC PLB are presented for comparison of protein peak symmetry and efficiency. Coatings were not regenerated between any protein separations. Electropherograms offset for clarity.
For comparison, Figure 3E shows a single electropherogram for the protein mixture in a bare (i), CPDCS-modified (ii), and ODCS-modified (iii) capillary. In each electropherogram, broad, unresolved peaks are detected. The μeof for CPDCS- and ODCS-modified capillaries suggest that a fraction of the silanols are unreacted after surface modification (Table 1); therefore, protein adsorption is still possible in bare and silane-modified capillaries. This conclusion is supported by fluorescence imaging studies with six-histidine-tagged enhanced green fluorescent protein (6×His-EGFP), a highly fluorescent protein (Figure SI-4). 6×His-EGPF was incubated in bare, CPDCS-modified, DOPC PLB-coated, and CPDCS/DOPC-coated capillaries. After rinsing the capillaries, intense fluorescence was observed in bare and CPDCS-modified capillaries, indicating non-specific adsorption of the protein to the capillary surfaces. However, the fluorescence intensity was diminished by more than 90 % with a DOPC bilayer or CPDCS/DOPC coating, demonstrating that DOPC bilayers and CPDCS/DOPC-coated capillaries decrease non-specific adsorption to a similar extent in regions of good coating uniformity. Hydrated phosphorylcholine headgroups are inherently resistant to non-specific protein adsorption [7,35], a property that is retained in both PLB and HPB capillary coatings, likely due to similar arrangements of lipid headgroups within the coating.
The tm repeatability for all three proteins are presented in Table 2 (n ≥ 6) where RSD values ranging from 0.7 to 3.6 % were obtained in each HPB, without coating regeneration. The CPDCS/DLPC capillary had one run in which the migration times varied more than the other runs, though statistically the migration times appeared to be part of the data set. However, if the protein migration times were removed for that single run, the RSD values for protein tm ranged from 0.7 – 2.0 % for HPB-coated capillaries. For the DLPC PLB capillary, the tm repeatability for Lyso and α-Chymo were 1.6 % and 5.3 % RSD, respectively. The RSD for α-Chymo in PLB capillaries was larger than the RSD calculated in any of the HPB capillaries, likely because the semi-permanent PLB was not regenerated between runs, indicating the lower stability of PLBs compared to HPBs.
Table 2.
Quantitative characterization of protein separations in HPB capillaries
| Coating | Parameters | Lyso | a-Chymo | Myo |
|---|---|---|---|---|
| CPDCS/DOPC | Time (s), (%RSD) N (plates m−1) |
128.0 (0.85) 39,000 ± 8,000 |
210.2 (1.3) 44,000 ± 10,000 |
266.5 (1.8) 74,000 ± 14,000 |
| CPDCS/DLPC | Time (s), (%RSD) N (plates m−1) |
143.2 (2.0) 91,000 ± 5,000 |
258.2 (3.2) 66,000 ± 9,000 |
354.1 (3.6) 200,000 ± 35,000 |
| ODCS/DOPC | Time (s), (%RSD) N (plates m−1) |
147.5 (0.82) 56,000 ± 15,000 |
266.8 (1.1) 66,000 ± 5,000 |
361.3 (1.4) 150,000 ± 11,000 |
| ODCS/DLPC | Time (s), (%RSD) N (plates m−1) |
146.8 (0.74) 28,000 ± 4,000 |
262.9 (0.78) 17,000 ± 6,000 |
346.3 (1.2) 83,000 ± 7,000 |
| DLPC bilayer | Time (s), (%RSD) N (plates m−1) |
215.5 (1.6) 110,000 ± 8,000 |
634.3 (5.3) 60,000 ± 10,000 |
HPB coating performance was further evaluated as a function of separation efficiency (Table 2). The efficiency of Myo was 74,000 – 200,000 plates m−1 in HPB capillaries. Of the separated proteins, Myo was closest to neutrally charged, yielded the highest separation efficiencies, and showed little peak asymmetry in the electropherograms, indicating the protein had little interaction with the HPB-coated capillary surfaces. Lyso yielded efficiencies between 28,000 and 91,000 plates m−1, while α-Chymo had efficiencies between 17,000 and 66,000 plates m−1. The lowest efficiencies for Lyso and α-Chymo were observed in ODCS/DLPC capillaries, whereas the highest efficiencies were measured in the CPDCS/DLPC capillaries. When proteins were separated using a DLPC PLB capillary, efficiencies of 110,000 plates m−1 (Lyso) and 60,000 plates m−1 (α-Chymo) were measured. Thus, proteins separated in HPB-coated capillaries had similar efficiencies to PLB-coated capillaries when using the same conditions, high migration time repeatability, and the HPB-coated capillaries can be used without regular coating regeneration.
4 Concluding Remarks
HPBs formed with either CPDCS- or ODCS-modified surfaces and either of two lipids resulted in stable coatings for CZE separations. Covalent modification of the capillary surface decreased the SFE, allowing lipids to self-assemble to form a coating that exhibited similar reduction to non-specific adsorption compared to PLB coatings, yet with higher stability. Protein separation in HPB-coated capillaries facilitated reproducible migration and detection of Lyso, α-Chymo, and Myo, which were not resolved in bare capillaries.
Supplementary Material
Acknowledgments
ESG was supported by a graduate training fellowship through the Biological Chemistry Program (NIH T32 GM008804). This work was funded in part by the National Institutes of Health (GM095763). Certain commercial equipment, instruments, or materials are identified in this document. Such identification does not imply recommendations or endorsement by the National Institute of Standards and Technology, nor does it imply that the products identified are necessarily the best available for the purpose.
Abbreviations
- α-Chymo
α-chymotrypsinogen A
- CPDCS
3-cyanopropyldimethylchlorosilane
- DLPC
1,2-dilauroyl-sn-glycero-3-phosphocholine
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- HPB
Hybrid phospholipid bilayer
- Lyso
Lysozyme
- Myo
Myoglobin
- ODCS
n-octyldimethylchlorosilane
- PBS
Phosphate buffered saline
- PLB
Phospholipid bilayer
- Rh-DPPE
1,2-dipalmitoyl-sn-glycerol-3-phosphoethanolamine-N-lissamine rhodamine B sulfonyl
- SFE
Surface free energy
- SUV
Small unilamellar vesicle
Footnotes
Contribution of NIST, an agency of the US government; not subject to copyright.
Conflict of Interest
The authors claim no financial or commercial conflicts of interest associated with this work.
References
- 1.Lucy CA, MacDonald AM, Gulcev MD. J Chromatogr A. 2008;1184:81–105. doi: 10.1016/j.chroma.2007.10.114. [DOI] [PubMed] [Google Scholar]
- 2.Yeung KKC, Lucy CA. Anal Chem. 1997;69:3435–3441. doi: 10.1021/ac961231k. [DOI] [PubMed] [Google Scholar]
- 3.Lauer HH, McManigill D. Anal Chem. 1986;58:166–170. [Google Scholar]
- 4.McCormick RM. Anal Chem. 1988;60:2322–2328. doi: 10.1021/ac00172a003. [DOI] [PubMed] [Google Scholar]
- 5.Green JS, Jorgenson JW. J Chromatogr. 1989;478:63–70. [Google Scholar]
- 6.Horvath J, Dolnik V. Electrophoresis. 2001;22:644–655. doi: 10.1002/1522-2683(200102)22:4<644::AID-ELPS644>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Mansfield E, Ross EE, Aspinwall CA. Anal Chem. 2007;79:3135–3141. doi: 10.1021/ac0618829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baryla NE, Lucy CA. J Chromatogr A. 2002;956:271–277. doi: 10.1016/s0021-9673(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 9.Baryla NE, Melanson JE, McDermott MT, Lucy CA. Anal Chem. 2001;73:4558–4565. doi: 10.1021/ac0105134. [DOI] [PubMed] [Google Scholar]
- 10.Cunliffe JM, Baryla NE, Lucy CA. Anal Chem. 2002;74:776–783. doi: 10.1021/ac015627u. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Lucy CA. Anal Chem. 2005;77:2015–2021. doi: 10.1021/ac0489622. [DOI] [PubMed] [Google Scholar]
- 12.Phillips KS, Kottegoda S, Kang KM, Sims CE, Allbritton NL. Anal Chem. 2008;80:9756–9762. doi: 10.1021/ac801850z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hautala JT, Linden MV, Wiedmer SK, Ryhanen SJ, Saily MJ, Kinnunen PKJ, Riekkola ML. J Chromatogr A. 2003;1004:81–90. doi: 10.1016/s0021-9673(03)00570-3. [DOI] [PubMed] [Google Scholar]
- 14.Linden MV, Holopainen JM, Laukkanen A, Riekkola ML, Wiedmer SK. Electrophoresis. 2006;27:3988–3998. doi: 10.1002/elps.200600002. [DOI] [PubMed] [Google Scholar]
- 15.Adem SM, Mansfield E, Keogh JP, Hall HK, Jr, Aspinwall CA. Anal Chim Acta. 2013;772:93–98. doi: 10.1016/j.aca.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross EE, Bondurant B, Spratt T, Conboy JC, O’Brien DF, Saavedra SS. Langmuir. 2001;17:2305–2307. [Google Scholar]
- 17.Ross EE, Rozanski LJ, Spratt T, Liu S, O’Brien DF, Saavedra SS. Langmuir. 2003;19:1752–1765. [Google Scholar]
- 18.Hotz J, Meier W. Langmuir. 1998;14:1031–1036. [Google Scholar]
- 19.Graff A, Winterhalter M, Meier W. Langmuir. 2001;17:919–923. [Google Scholar]
- 20.Silin VI, Wieder H, Woodward JT, Valincius G, Offenhausser A, Plant AL. J Am Chem Soc. 2002;124:14676–14683. doi: 10.1021/ja026585+. [DOI] [PubMed] [Google Scholar]
- 21.Plant AL. Langmuir. 1993;9:2764–2767. [Google Scholar]
- 22.Plant AL. Langmuir. 1999;15:5128–5135. [Google Scholar]
- 23.Towns JK, Regnier FE. Anal Chem. 1991;63:1126–1132. doi: 10.1021/ac00011a013. [DOI] [PubMed] [Google Scholar]
- 24.Plant AL, Gueguetchkeri M, Yap W. Biophys J. 1994;67:1126–1133. doi: 10.1016/S0006-3495(94)80579-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard JB, Silin V, Plant AL. Biophys Chem. 1998;75:163–176. doi: 10.1016/s0301-4622(98)00199-9. [DOI] [PubMed] [Google Scholar]
- 26.Ratnayaka SN, Wysocki RJ, Jr, Saavedra SS. J Colloid Interface Sci. 2008;327:63–74. doi: 10.1016/j.jcis.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meuse CW, Krueger S, Majkrzak CF, Dura JA, Fu J, Connor JT, Plant AL. Biophys J. 1998;74:1388–1398. doi: 10.1016/S0006-3495(98)77851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plant AL, Brigham-Burke M, Petrella EC, O’Shannessy DJ. Anal Biochem. 1995;226:342–348. doi: 10.1006/abio.1995.1234. [DOI] [PubMed] [Google Scholar]
- 29.White RJ, Ervin EN, Yang T, Chen X, Daniel S, Cremer PS, White HS. J Am Chem Soc. 2007;129:11766–11775. doi: 10.1021/ja073174q. [DOI] [PubMed] [Google Scholar]
- 30.Zenkiewicz M. Journal of Achievements in Materials and Manufacturing Engineering. 2007;24:137–145. [Google Scholar]
- 31.Nakajima A. NPG Asia Materials. 2011;3:49–56. [Google Scholar]
- 32.Girifalco LA, Good RJ. J Phys Chem. 1957;61:904–909. [Google Scholar]
- 33.Adamson AW. Physical Chemistry of Surfaces. John Wiley and Sons, Inc; New York: 1990. [Google Scholar]
- 34.Yarovsky I, Aguilar MI, Hearn MTW. Anal Chem. 1995;67:2145–2153. [Google Scholar]
- 35.Glasmastar K, Larsson C, Hook F, Kasemo B. J Colloid Interface Sci. 2002;246:40–47. doi: 10.1006/jcis.2001.8060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.