Abstract
Argonaute proteins are central players in small RNA-mediated silencing mechanisms such as RNA interference (RNAi), microRNA repression and piRNA-mediated transposon silencing. In eukaryotes, Argonautes bind small RNAs that guide them to RNA targets in order to regulate gene expression and repress invasive genomic elements. Although Argonaute proteins are conserved in all life forms from bacteria to eukaryotes, until now studies have focused on the biological functions of eukaryotic Argonautes. Here we highlight two recent studies that discover the functions of prokaryotic Argonautes in defence against exogenous DNA.
Argonaute family proteins (Ago) are found in all domains of life. However, their functions have mainly been studied in eukaryotic models, where Ago proteins constitute the core of several silencing mechanisms. In these pathways Argonautes use tightly bound small RNA guides to identify target sequences via sequence complementarity [1]. Upon recognition of a target such as viral RNA, the Ago protein induces target cleavage by its endonuclease Piwi domain (the so-called ‘slicing’ activity). However, the endonuclease activity of Ago is not required for all small RNA-dependent mechanisms. For example, in the micro RNA (miRNA) pathway partial base-complementarity of the miRNA and the target is typically sufficient to induce molecular events that lead to silencing without the need for endonucleolytic target cleavage [2].
Although many Argonautes repress their targets on a post-transcriptional level, certain members in fungi, plants and Metazoa use small RNA guides to repress transcription at target genomic loci [3]. In the pathways studied so far, Ago proteins seem to recognize nascent RNA transcripts of target loci and recruit factors required for chromatin silencing [4]. In addition, even more exotic pathways were described in the macronucleus of ciliates where Ago proteins utilize small RNAs to mark certain genomic regions for DNA elimination [3]. Even in this case, however, no direct association of Ago protein with DNA has been observed and it was proposed that Ago recognizes nascent RNA targets prior to affecting chromatin modifications.
Argonaute proteins are also present in many bacterial and archaeal species: bioinformatic analysis of the phylogenetic distribution of Argonautes among bacteria and archaea reveals that about 20% of sequenced strains contain at least one Ago gene [5]. Several structural studies of bacterial and archaeal Ago helped to elucidate the mechanism of small RNA silencing in eukaryotes [6, 7]. Indeed, the insight that Argonaute provides enzymatic endonuclease activity to RNA induced silencing complexes (RISC) was inferred by structures of Agos from the archaeon Pyrococcus furiosus and the bacterium Aquifex aeolicus [6, 7]. Interestingly, these studies revealed that several prokaryotic Agos, unlike their eukaryotic homologs, have the remarkable ability to bind single-stranded DNA guides in vitro and are capable of utilizing them for cleavage of RNA targets. In addition, the presence of an Ago gene correlated with other known genome protection systems, suggesting that prokaryotic Agos, like some of their eukaryotic homologs, might protect cells from invasive nucleic acids [5]. However, until recently neither the in vivo function nor the nucleic acid partners of bacterial or archaeal Ago proteins were known.
Recently, two studies addressed the biological functions of Argonautes in the gram-negative bacteria Rhodobacter sphaeroides (RsAgo) [8] and Thermus thermophilus (TtAgo) [9]. Both studies revealed that Agos protect the genome against foreign and possibly invasive genomic elements, such as plasmids. In striking contrast to eukaryotic Agos, the Agos from both bacterial species directly target DNA molecules. Indeed, it was shown that in the host species, TtAgo decreases transformation efficiency and plasmid yield, indicative of its function in suppressing foreign DNA. However, there are important differences between their repressive roles in R. sphaeroides and T. thermophilus: whereas RsAgo uses ~18 nt small RNAs that are likely derived from cellular transcripts as guide molecules, TtAgo associates with ~15 nt small DNAs. In both species, guide molecules show a strong nucleotide bias at the 5’ position: uridine for RsAgo-associated small RNA and deoxycytidine for TtAgo-associated small DNA. This finding suggests that the Ago 5’ binding pocket discriminates its guide by the nature of the first nucleotide.
The Argonautes from the two species are also different in their abilities to cleave DNA targets. TtAgo has endonuclease activity that it uses to cleave DNA targets in the middle of the region targeted by the guide DNA [9]. By contrast, the residues that are critical for endonuclease activity are not conserved in RsAgo [8], similar to the majority of other prokaryotic Ago proteins [5]. Accordingly, it was found that target recognition by RsAgo results in cleavage immediately outside of the region targeted by the guide sequence, which implies that cleavage activity is likely mediated by other nucleases and not Ago itself. Despite this difference in endonucleoytic activities, the Agos in both species are (directly or indirectly) able to degrade target plasmid DNA. Furthermore, it was proposed that the binding of small RNA-loaded RsAgo to DNA targets might induce transcriptional inhibition without DNA cleavage. Indeed, in its host species RsAgo represses gene expression from exogenous plasmids without apparent degradation of the DNA target.
Although the pathways that involve prokaryotic Argonautes seem to be simpler than eukaryotic analogues, the principles that allow them to recognize foreign DNAs are still not clear. In both studies discussed here, Ago proteins recognize properties inherent to invading DNAs and silence them without using small RNA or DNA guides encoded in separate genomic loci. This property is strikingly different from CRISPR-Cas, which is another prokaryotic genomic ‘immune system’ where effector proteins associate with host-encoded RNA guides to determine DNA cleavage loci [10]. Eukaryotic Argonautes have surprised researchers for many years and, through the elucidation of their biological functions and nucleic acid partners, prokaryotic Agos might surprise researchers in the years to come.
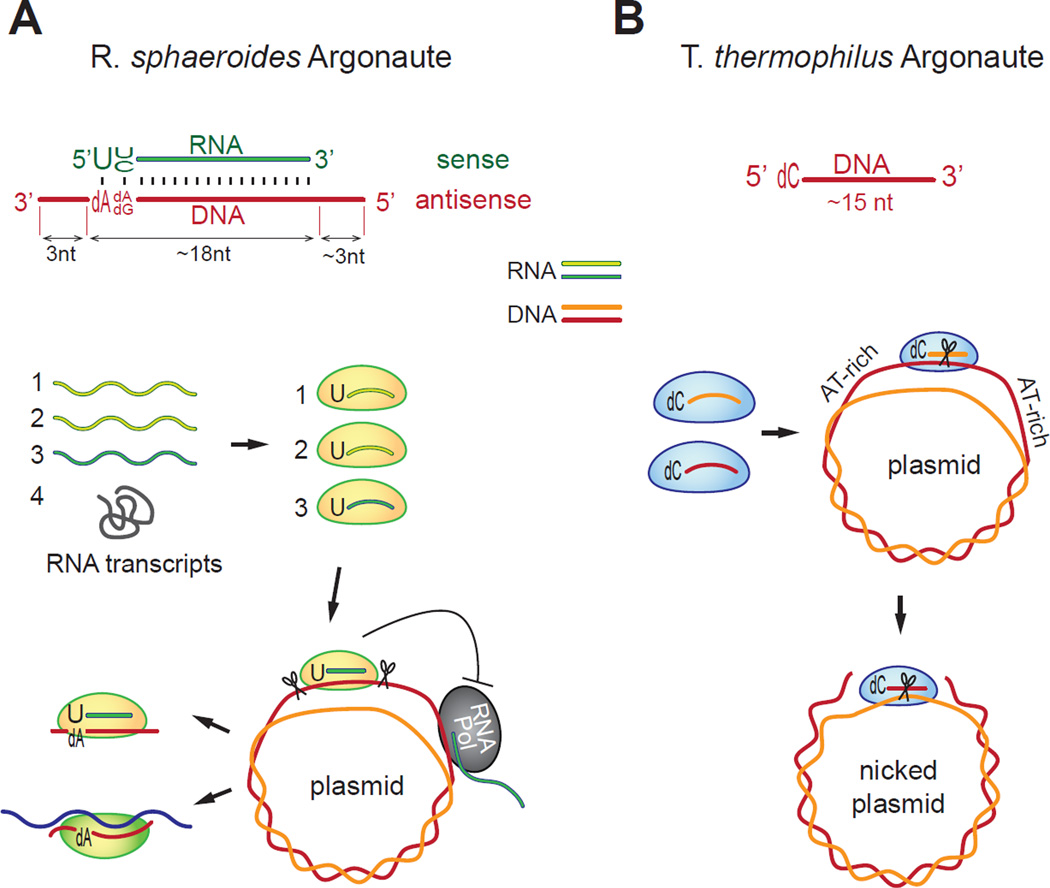
Figure 1. Models of RsAgo and TtAgo functions.
(A) RsAgo associates with ~18 nt small RNAs with a strong bias towards uridine at the first position, and ~24 nt small DNAs that are complementary to the small RNAs but contain ~3 nt overhangs on both sides. Small RNAs are derived from mRNAs (1-3) or their degraded products, however structured RNAs (4) are inefficient in small RNA production. RsAgo associated with cognate small RNA recognizes foreign DNA targets such as plasmids and induces excision of ~24 nt small DNA. Binding of RsAgo to DNA plasmids might also repress transcription by RNA polymerase. Following excision, small DNAs form duplexes with small RNAs associated with RsAgo. (B) TtAgo associates with ~15 nt small DNA with a strong bias towards deoxycytidine at the first position. TtAgo associated with small DNA induces cleavage of plasmid DNA. Flanking AT-rich regions and negative supercoiling of plasmid DNA facilitate TtAgo cutting of the first DNA strand. This results in a nicked plasmid that exposes the other DNA strand to another TtAgo-DNA complex that cuts and linearizes the plasmid. Nicking also removes the supercoiled topology of the plasmid, resulting in repression of transcription by RNA polymerase.
Acknowledgements
We thank members of the Aravin lab for discussion and Alexandre Webster for critical reading of the manuscript. Research in Aravin lab is supported by grants from the National Institutes of Health (HD057233, GM097363, and OD007371A) and by the Searle Scholar and the Packard Fellowship Awards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malone CD, Hannon GJ. Small RNAs as guardians of the genome. Cell. 2009;136:656–668. doi: 10.1016/j.cell.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuwe E, et al. Small but sturdy: small RNAs in cellular memory and epigenetics. Genes & development. 2014;28:423–431. doi: 10.1101/gad.236414.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarova KS, et al. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biology direct. 2009;4:29. doi: 10.1186/1745-6150-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song JJ, et al. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 7.Yuan YR, et al. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell. 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olovnikov I, et al. Bacterial argonaute samples the transcriptome to identify foreign DNA. Molecular cell. 2013;51:594–605. doi: 10.1016/j.molcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swarts DC, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. 2014;507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiedenheft B, et al. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]