Abstract
Two conflicting phenomena, the bystander effect and the adaptive response, are important in determining biological responses at low doses of radiation and have the potential to have an impact on the shape of the dose–response relationship. Using the Columbia University charged-particle microbeam and the highly sensitive AL cell mutagenic assay, we reported previously that nonirradiated cells acquired mutagenesis through direct contact with cells whose nuclei had previously been traversed with either a single or 20 α particles each. Here we show that pretreatment of cells with a low dose of X rays 4 h before α-particle irradiation significantly decreased this bystander mutagenic response. Furthermore, bystander cells showed an increase in sensitivity after a subsequent challenging dose of X rays. Results from the present study address some of the pressing issues regarding both the actual target size and the radiation dose response and can improve on our current understanding of radiation risk assessment.
INTRODUCTION
There are many reports on the roles of the bystander effect and the adaptive response, two interesting and important phenomena, in the effects of low-dose radiation (reviewed in refs. 1–5). Bystander effects tend to exaggerate the effect of low doses by eliciting damage in nonirradiated cells, while the adaptive response, induced by a low initial priming dose, reduces damage from a subsequent challenging dose. Although these two conflicting phenomena have attracted much interest, there are very few data that directly address the interaction of the two effects (6–8).
Using the Columbia University charged-particle microbeam and the highly sensitive AL cell mutagenic assay, we reported previously that cells lethally irradiated with α particles could induce mutagenesis in neighboring cells not directly hit by the particles, and that reactive oxygen species were not directly involved (9). These observations were extended to cells traversed by a single α particle, and it was seen that gap junction-mediated cell–cell communication played an important role in mediating the process of bystander mutagenesis (10). In our present study, two related experiments were designed to explore the interactions between the bystander effect and the adaptive response. First, we addressed the question of whether low-dose radiation decreased bystander mutagenesis. Second, we examined the mutagenic response of the bystander cells to a subsequent high-dose irradiation. Our data show that in the presence of low-dose radiation stress, bystander mutagenesis is decreased by the adaptive response, whereas the bystander cells show an increase in sensitivity after a subsequent challenging dose of X rays. If these results were applicable in vivo, they might have significant consequences in terms of extrapolation of radiation risks from high to low doses, implying that the relevant target for radiation oncogenesis is larger than an individual cell, and that the risk of carcinogenesis would increase more slowly, if at all, at intermediate doses. Therefore, a simple linear extrapolation of radiation risk from intermediate doses (where they can be measured) to low doses (where they must be inferred) would be of questionable validity, at least at high LET.
MATERIALS AND METHODS
Cell Culture
Human–hamster hybrid AL cells, which contain a standard set of Chinese hamster ovary-K1 chromosomes and a single copy of human chromosome 11, were used in this study (11, 12). Cells were maintained in Ham’s F-12 medium supplemented with 8% heat-inactivated fetal bovine serum, 25 μg/ml gentamicin, and 2 × 10−4 M glycine at 37°C in a humidified 95% air/5% CO2 incubator and passaged as described previously (13–15).
Irradiation Procedure
Cells were irradiated with α particles using the Columbia University charged-particle microbeam as described (9, 10, 15, 16). Briefly, exponentially growing cells were plated on specially constructed microbeam dishes. Two days after plating, when the cultures were more than 80% confluent in the center of the growth surface, the nuclei of attached cells were stained with a 50 nM solution of Hoechst 33342 dye for 30 min. The image analysis system then located the centroid of each nucleus and irradiated them randomly one at a time with an exact number of α particles. For determining the adaptive response, cells were irradiated with a low dose of X rays (0.02–0.5 Gy) 4 h before the α-particle irradiation. To examine the response of the bystander cells to the subsequent challenging dose, 10% of the cells were randomly given a lethal dose of 20 α particles directed at the nuclear centroid. Four hours later, the cultures were irradiated with a subsequent challenging dose of 3 Gy X rays. After the second irradiation, cells were maintained in the dishes for 2 days before being removed by trypsinization and replated into culture flasks. After culture for 4–5 days, the cells were trypsinized and replated to measure the mutant fraction as described previously (13–15).
Quantification of Mutations at the CD59 Locus
Irradiated and control cultures were trypsinized 2 days after irradiation, plated in culture flasks, and incubated for 5 more days before the mutation assay was performed as described (13–15). Briefly, 5 × 104 cells were plated into each of six 60-mm dishes in 2 ml of growth medium. Cultures were incubated for 2 h to allow for cell attachment, after which 0.3% CD59 antiserum and 1.5% (v/v) freshly thawed complement were added to each dish (13–15). The cultures were further incubated for 7 days. At this time the cells were fixed and stained, and the number of CD59− mutant colonies was scored. The cultures derived from each treatment dose together with the appropriate controls were tested for mutant yield for 2 consecutive weeks to ensure full expression of the mutations.
Analysis of Mutant Spectrum by Multiplex PCR
Independently derived colonies were isolated by cloning and expanded in cultures. DNA was extracted using a salt-out method (17). To ensure their clonal origin, either a single colony or, periodically, two well-separated colonies per culture dish were isolated. Five marker genes located on both the long and short arms of human chromosome 11 (Wilm’s tumor, parathyroid hormone, catalase, RAS and apolipoprotein A-1) were selected for analysis using multiplex PCR as described previously (9, 16).
Statistical Analysis
All numerical data were calculated as means and standard deviations. Comparisons of induced mutation frequencies between treated groups and controls were made by Student’s t test. Differences in the mutant spectra between treated group and control were analyzed by χ2 analysis. A P value of 0.05 or less between groups was considered to be significant.
RESULTS
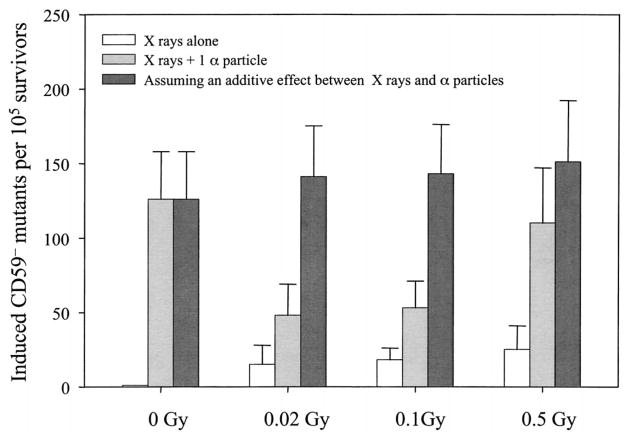
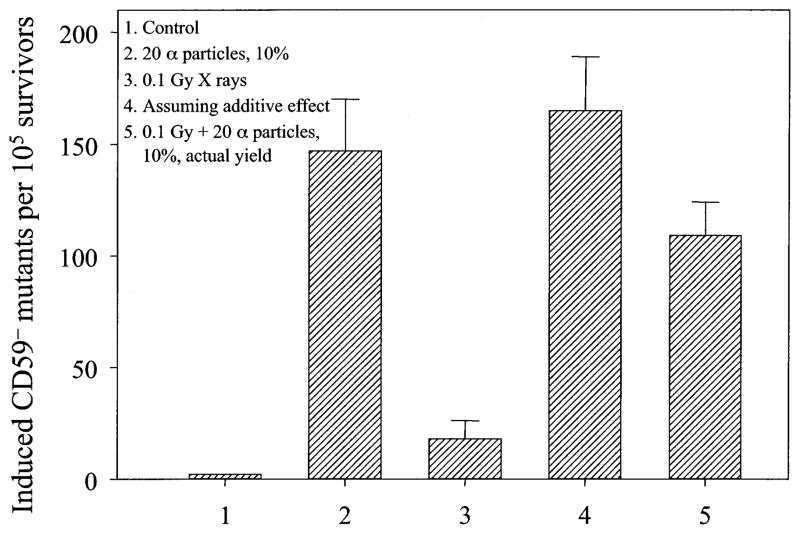
AL cells irradiated with doses of X rays ranging from 0.02 to 0.5 Gy resulted in a low but significant induction of mutations at the CD59 locus as shown in Fig. 1. The background CD59− mutant fraction among the population of AL cells used in these experiments averaged 61 ± 19 per 105 survivors, and it was subtracted from both control and experimental values in the data shown in Figs. 1–3. Consistent with our previously published data, irradiation of 10% of a confluent cell population with a lethal dose of 20 α particles each through the nuclei resulted in a mutant yield that was approximately three times higher than the background among the nonirradiated neighboring cells (9). Likewise, irradiation of 10% of cells with a single α particle each resulted in a mutant yield similar to that observed when all of the cells in the population were hit by a single α particle (10). Pretreatment of cells with a low (0.02 or 0.1 Gy) dose of X rays significantly reduced this bystander mutagenesis (P < 0.05, Fig. 1) by 62% and 58%, respectively. An increase in the priming dose decreased the inhibitory effect such that pretreatment with 0.5 Gy of X rays reduced the bystander mutant yield by only 12%, and the difference was no longer statistically significant. A similar mutagenic response was found if 10% of the cells were given a near lethal dose of 20 α particles each delivered to the nuclei. As shown in Fig. 2, if the cells were pretreated with a dose of 0.1 Gy X rays, the mutant yield from the population in which 10% of randomly selected cells were irradiated with 20 α particles decreased significantly (P < 0.05). These results imply that in the presence of low-dose radiation stress, bystander mutagenesis is suppressed by the adaptive response, though the mechanism(s) is unclear.
Fig. 1.
Effect of the pretreatment with X rays on bystander mutagenesis in AL cells. Cells were pretreated with graded doses of X rays 4 h before targeted nuclear irradiation of 10% of randomly selected cells with a single α particle. Data are pooled from four independent experiments. Bars represent ±SD.
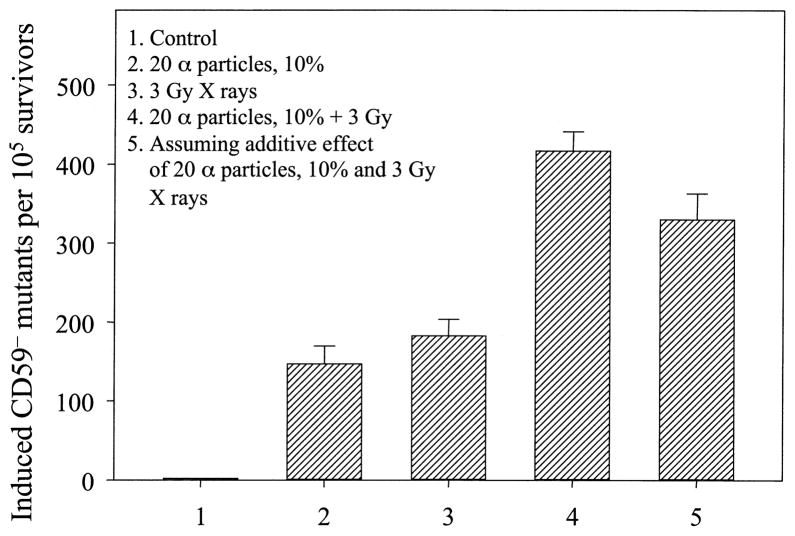
Fig. 3.
Response of bystander cells to a subsequent challenging high-dose irradiation. Ten percent of randomly selected cells were irradiated with a lethal dose of 20 α particles each. Four hours later, cultures were irradiated with 3 Gy of X rays. Data were pooled from three independent experiments. Error bars represent ±SD.
Fig. 2.
Induced mutant fraction of AL cells in which 10% had been irradiated with 20 α particles through the nucleus with or without pretreatment with 0.1 Gy X rays. Data are pooled from three independent experiments. Bar represents ±SD.
To determine the genotoxic response of the bystander cells to a subsequent high-dose irradiation, 10% of randomly selected cells were irradiated with a lethal dose of 20 α particles each. Four hours later, cultures were irradiated with a dose of 3 Gy X rays. We found that bystander cells that were not directly hit by α particles exhibited a significantly higher mutant yield than control cells when exposed to X rays under similar conditions (Fig. 3, bar 4 compared to bar 3, P < 0.05). Furthermore, the mutant yield among the bystander cells exposed to a dose of 3 Gy X rays was significantly higher than a simple additive effect of the bystander mutation and X-ray-induced mutagenesis (Fig. 3, bar 4 compared to bar 5, P < 0.05). These data indicate that bystander cells show an increase in sensitivity after a subsequent challenging dose of X rays.
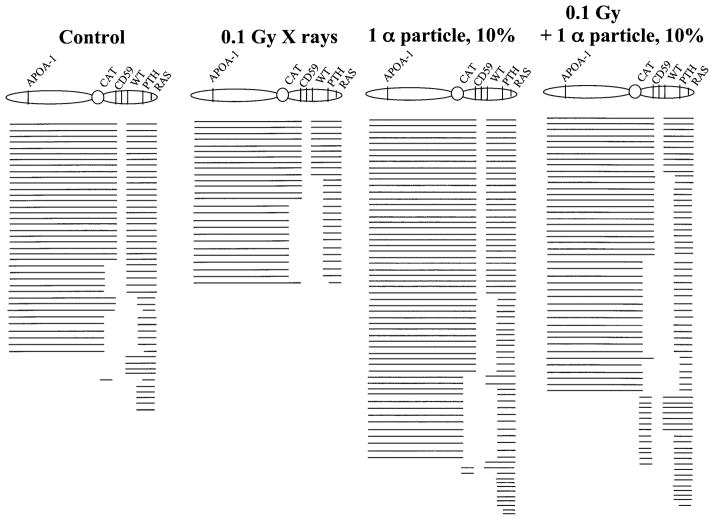
To further probe the possible mechanism of the interaction of bystander effects and adaptive response, we examined the types of mutation associated with the CD59− phenotype in AL cells using multiplex PCR techniques. A total of 212 CD59− mutants of either spontaneous or radiation-induced origin were analyzed to ascertain the presence or absence of five chromosome 11 markers located on either side of the CD59 gene. As shown in Fig. 4, about half of the spontaneous CD59− mutants showed no detectable changes in any of the marker genes examined. Compared with those of spontaneous origin, 36% and 43% of the mutants from cells irradiated with 0.1 Gy X rays or from the population in which 10% of randomly selected cells were traversed by a single α particle, respectively, showed the presence of all five marker genes examined. The difference was not significant. In contrast, 85% of the mutants from the population in which 10% of randomly selected cells were pretreated with 0.1 Gy of X rays and irradiated with a single α particle had lost at least one additional marker. Pretreatment with a dose of 0.1 Gy X rays followed by exposure of 10% of the cells to a single α particle through the nucleus increased the incidence of complex mutations (complex mutants have lost markers in a discontinuous fashion) from 3% to 20%. The difference in mutant spectrum between the two groups with or without X-ray pretreatment was statistically significant (χ2 = 12.98, P < 0.001).
Fig. 4.
Mutation spectra of CD59− mutants isolated either of spontaneous origin or induced by radiation. Each line depicts a single mutant. Blank spaces depict missing markers on human chromosome 11 as determined by multiplex PCR.
DISCUSSION
Based principally on the cancer incidence found in survivors of the atomic bombs in Japan, the International Commission on Radiation Protection (ICRP) and the U.S. National Council on Radiation Protection and Measurements (NCRP) have recommended that estimates of cancer risk for low-dose exposure be extrapolated from higher doses where data are available using a linear, no-threshold model (18, 19). This recommendation is based on the dogma that the DNA of the nucleus is the main target for radiation-induced genotoxicity and, since fewer cells are directly damaged, the deleterious effects of radiation decline proportionally. However, two conflicting phenomena, the bystander effect and the adaptive response, may be important in determining biological responses to low doses of radiation and might have the potential to have an impact on the shape of the dose–response relationship. A better understanding of the mechanisms of radiobiological effects at low doses would shed light on the validity of the model used currently and provide a rationale for the best estimates of risk.
Considerable evidence is now emerging that targeted nuclei may not always be required in mediating the genotoxic effects of radiation. Nonirradiated bystander cells have been shown to have similar cytotoxic and genotoxic responses to those detected in directly irradiated cells (20–34). Early investigations of radiation-induced bystander effects measured the frequency of sister chromatid exchanges (SCEs) in populations of CHO cells exposed to low fluences of α particles. It was found that SCE levels were significantly higher than expected from calculations of the number of cells likely to have been hit by an α particle (20, 22). Furthermore, such biological effects as induction of micronuclei (25, 33), gene mutation (9, 10, 26), expression of stress-related genes (21, 26), and malignant transformation of mammalian cells in vitro (30) can occur in a significantly higher proportion of cells than in those traversed by an α particle. There is evidence that gap junction-mediated cell–cell communication plays a critical role in the bystander response (9, 10, 26, 29, 32), while secretion of cytokines or other growth-promoting factors by irradiated cells has been suggested to modulate the bystander response (23, 24, 28). However, the precise mechanism of the bystander effect is not clear. It is likely that different signaling pathways are required in either confluent or sparsely populated cultures. Since CHO cells have been shown to exhibit a bystander response and these cells contain mutant TP53, it is likely that a TP53-dependent signaling pathway may not be critical in the process. The observation that bystander micronucleus induction in human fibroblasts can be attributed to a redox-sensitive signaling pathway that is linked to gap junction communication (33) suggests that a cascade of events may be necessary for the signaling process.
The adaptive response is characterized by a reduction of radiobiological response in cells pretreated with a low dose of radiation followed by exposure to a higher challenging dose. Since the original experiments reported in 1984 (35), numerous studies have shown the existence of such a response with a variety of end points in various cell types (reviewed in ref. 4). Although the mechanism(s) of the adaptive response has not been elucidated, there is some evidence that the protein kinase C-mediated signaling pathway is a key step for the transduction of the low-dose-induced signal (4). Although the bystander effect and adaptive response have attracted considerable attention, there are only limited data available comparing the bystander effect and the adaptive response (6–8). Sawant et al. (6) reported that an adaptive dose of 2 cGy of γ rays, delivered 6 h beforehand, canceled out about half of the cytotoxic bystander effect produced by the subsequent α-particle irradiation. Using transfer of supernatants of normal human lung fibroblasts (HFL-1) irradiated with 1 cGy of α particles or γ rays to unirradiated HFL-1 cells as a bystander model, Iyer et al. (7, 8) found that clonogenic survival after subsequent exposure to α particles or γ rays was significantly increased, and increases in AP endonuclease were found in the bystander cells but not in directly irradiated cells.
In our present studies, we found that pretreatment of cells with a low dose of X rays (≤0.1 Gy) 4 h before α-particle irradiation (1 or 20 α particles) significantly decreased the bystander mutagenic response. These results are consistent with the previous findings of Sawant et al. (6). Although the nature of the molecular mechanism is not clear, it is possible that an inducible protein is triggered by low-dose radiation that leads to protection of cells against the deleterious effects of a subsequent irradiation. In this regard, our data are consistent with those of Ueno et al. (36), who demonstrated a reduction in CD59− mutants among AL cells pretreated with a dose of 0.04 Gy γ rays before they were exposed to a subsequent challenging dose of 4 Gy. Furthermore, the increase in the incidence of complex mutations among the bystander mutants pretreated with a dose of 0.1 Gy X rays is also consistent with the findings of Ueno et al. (36). There is an indication that many of these complex mutations are unstable in time and may be a marker of transmissible genomic instability (37). Additionally, we found that the bystander cells showed an increase in sensitivity after a subsequent challenging dose of 3 Gy of X rays. Radiobiological responses at low doses are likely to be a complex interplay among direct effects, the adaptive response, and the bystander effect. The use of a linear, no-threshold extrapolation model for low-dose risk assessment has become even more controversial in light of the recently reported studies of the bystander phenomenon. The important question is, which is more important, the bystander effect or the adaptive response? This question remains unanswered, and more studies are needed to elucidate the mechanism(s) involved.
Acknowledgments
We gratefully acknowledge Mr. Stephen Marino and Mr. Mutian Zhang for performing the irradiations. We also thank Dr. Katherine Mitchell for her critical reading of the manuscript. This work was supported in part by NIH grants CA 49062, CA 75384, Environmental Center grant ES 10349, and funding from the U.S. Department of Energy DEFG-ER 63441. The Columbia Microbeam Facility is funded by NIH Research Resource Center grant RR 11623.
References
- 1.Iyer R, Lehnert BE. Effects of ionizing radiation in targeted and non-targeted cells. Arch Biochem Biophys. 2000;376:14–25. doi: 10.1006/abbi.1999.1684. [DOI] [PubMed] [Google Scholar]
- 2.Mothersill C, Seymour C. Radiation-induced bystander effects: Past history and future directions. Radiat Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Ballarini F, Biaggi M, Ottolenghi A, Sapora O. Cellular communication and bystander effects: A critical review for modeling low-dose radiation action. Mutat Res. 2002;501:1–12. doi: 10.1016/s0027-5107(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Rigaud O, Moustacchi E. Radioadaptation for gene mutation and the possible molecular mechanisms of the adaptive response. Mutat Res. 1996;358:127–134. doi: 10.1016/s0027-5107(96)00113-3. [DOI] [PubMed] [Google Scholar]
- 5.Upton AC. Radiation hormesis: Data and interpretations. Crit Rev Toxicol. 2001;31:681–695. doi: 10.1080/20014091111956. [DOI] [PubMed] [Google Scholar]
- 6.Sawant SG, Randers-Pehrson G, Metting NF, Hall EJ. Adaptive response and the bystander effect induced by radiation in C3H 10T1/2 cells in culture. Radiat Res. 2001;156:177–180. doi: 10.1667/0033-7587(2001)156[0177:aratbe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Iyer R, Lehnert BE. Low dose, low-LET ionizing radiation-induced radioadaptation and associated early responses in unirradiated cells. Mutat Res. 2002;503:1–9. doi: 10.1016/s0027-5107(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 8.Iyer R, Lehnert BE. Alpha-particle-induced increases in the radioresistance of normal human bystander cells. Radiat Res. 2002;157:3–7. doi: 10.1667/0033-7587(2002)157[0003:apiiit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 2000;97:2099– 2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Waldren CA, Cheng G, Trosko JE, Hei TK. Radiation risk to low fluences of α particles may be greater than we thought. Proc Natl Acad Sci USA. 2001;98:14410–14415. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waldren CA, Jones C, Puck TT. Measurement of mutagenesis in mammalian cells. Proc Natl Acad Sci USA. 1979;76:1358–1362. doi: 10.1073/pnas.76.3.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldren CA, Correll L, Sognier MA, Puck TT. Measurement of low levels of X-ray mutagenesis in relation to human disease. Proc Natl Acad Sci USA. 1986;83:4839–4843. doi: 10.1073/pnas.83.13.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hei TK, Waldren CA, Hall EJ. Mutation induction and relative biological effectiveness of neutrons in mammalian cells. Radiat Res. 1988;115:281–291. [PubMed] [Google Scholar]
- 14.Hei TK, Piao CQ, He ZY, Vannais D, Waldren CA. Chrysotile fiber is a strong mutagen in mammalian cells. Cancer Res. 1992;52:6305–6309. [PubMed] [Google Scholar]
- 15.Hei TK, Wu LJ, Liu SX, Vannais D, Waldren CA. Mutagenic effects of a single and an exact number of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 1997;94:3765–3770. doi: 10.1073/pnas.94.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z, Hei TK. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci USA. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ICRP. Report 60. International Commission on Radiological Protection, Pergamon Press; New York: 1991. 1990 Recommendation of the International Commission on Radiological Protection. [Google Scholar]
- 19.NCRP. Report 116. National Council on Radiation Protection and Measurements; Bethesda, MD: 1993. Limitation of Exposure to Ionizing Radiation. [Google Scholar]
- 20.Nagasawa H, Little J. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 21.Hickman AW, Jaramillo RJ, Lechner JF, Johnson NF. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994;54:5797–5800. [PubMed] [Google Scholar]
- 22.Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: Evidence for an extranuclear target. Radiat Res. 1996;145:260–267. [PubMed] [Google Scholar]
- 23.Narayanan PK, Goodwin EH, Lehnert BE. α Particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 24.Mothersill C, Seymour CB. Cell–cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: Evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256– 262. [PubMed] [Google Scholar]
- 25.Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol. 1998;74:793–798. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- 26.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 27.Nagasawa H, Little JB. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: Evidence for a bystander effect. Radiat Res. 1999;152:552–557. [PubMed] [Google Scholar]
- 28.Iyer R, Lehnert BE. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- 29.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawant SG, Randers-Pehrson G, Geard CR, Brenner DJ, Hall EJ. The bystander effect in radiation oncogenesis: I. Transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbors of irradiated cells. Radiat Res. 2001;155:397–401. doi: 10.1667/0033-7587(2001)155[0397:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Huo L, Nagasawa H, Little JB. HPRT mutants induced in bystander cells by very low fluences of alpha particles result primarily from point mutations. Radiat Res. 2001;156:521–525. doi: 10.1667/0033-7587(2001)156[0521:hmiibc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Randers-Pehrson G, Suzuki M, Waldren CA, Hei TK. Genotoxic damage in non-irradiated cells: Contribution from the bystander effect. Radiat Prot Dosim. 2002;99:227–232. doi: 10.1093/oxfordjournals.rpd.a006769. [DOI] [PubMed] [Google Scholar]
- 33.Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62:5436–5442. [PubMed] [Google Scholar]
- 34.Little JB, Nagasawa H, Li GC, Chen DJ. Involvement of the nonhomologous end joining DNA repair pathway in the bystander effect for chromosomal aberrations. Radiat Res. 2003;159:262–267. doi: 10.1667/0033-7587(2003)159[0262:iotnej]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 36.Ueno AM, Vannais DB, Gustafson DL, Wong JC, Waldren CA. A low, adaptive dose of gamma-rays reduced the number and altered the spectrum of S1– mutants in human-hamster hybrid AL cells. Mutat Res. 1996;358:161–169. doi: 10.1016/s0027-5107(96)00117-0. [DOI] [PubMed] [Google Scholar]
- 37.Ueno A, Vannais DB, Lenarczyk M, Waldren CA. Ascorbate, added after irradiation, reduces the mutant yield and alters the spectrum of CD59− mutations in AL cells irradiated with high LET carbon ions. J Radiat Res. 2002;43:S245–S249. doi: 10.1269/jrr.43.s245. [DOI] [PubMed] [Google Scholar]