Abstract
Substantial population exposure to endocrine disrupting chemicals, combined with available biomarkers and public concern, has resulted in an explosion of human health effects research. At the same time, remarkable shifts in the regulations governing the composition of some consumer products that contain endocrine disruptors (EDs) has occurred. However, important questions remain as to the weight of evidence linking EDs to human health end points. In this review, we critically examine the literature linking ED exposures to child neurodevelopment, focusing in particular on two model exposures to demonstrate issues related to bioaccumulative [e.g., polychlorinated biphenyls (PCBs)] and rapidly metabolized (e.g., phthalates) compounds, respectively. Issues of study design, confounding, and exposure measurement are considered. Given widespread exposure to these compounds, the potential public health consequences of even small effects on human health are substantial. Therefore, advancing our understanding of any impact calls for careful attention to the principles of causal inference.
Keywords: endocrine disruptors, causation, neurodevelopment, behavior, phthalates, PCBs
INTRODUCTION
Environmental endocrine disruption of normal child development is a rapidly growing area of research. This increase in scientific attention is in part because exposure to many of these chemicals was only recently recognized (15, 14, 88). In addition, technological advances in exposure measurement have provided human biomonitoring data for chemicals of interest, documenting widespread exposure internationally and across all age ranges (11). The scientific basis for concern has been amplified by the public outcry about the perceived influences of endocrine disruptors (EDs) on human health and disease. As a result, remarkable shifts in regulations governing the composition of some consumer products have occurred both domestically and internationally, as has the voluntary removal of certain chemicals from other consumer products (10, 16, 23). Precautionary regulations may indeed be warranted, but carefully conducted observational studies may help guide future regulatory actions while defending against pressure to weaken the current protections.
By definition, the biological mechanism of EDs implies mediation by hormonal intermediates, not genotoxicity (Table 1), so association sizes are expected to be weak to moderate in observational studies. Therefore, large sample sizes and appropriate biological matrices for exposure measurement are a minimum study requirement. To the detriment of scientific inquiry, however, the single-minded pursuit of resources with these qualities has resulted in a comparative neglect of attention to principles of study design and inference. This trend has the potential either to result in the false dismissal of true associations or to direct inappropriate attention at associations that are not causal.
Table 1.
Endocrine disruptors and children's health in relation to causal inference considerations
| Endocrine disruptor exposure effects on child health and development | Environmental exposures considered by A.B. Hill in the mid-twentieth century (30) | |
|---|---|---|
| Mechanism | Action through hormonal or epigenetic intermediate | Genotoxic [tobacco smoke, benzo(a)pyrene], directly neurotoxic (lead), or infectious (cholera, meningococcus) |
| Susceptible window of exposure | In utero or early life | Assumed to be any time prior to the latent period |
| Health end point | Spectrum of severity or constellation of symptoms | Clear dichotomy (cancer, cardiovascular mortality, infection) |
| Presumed effect sizes | Weak to moderate | Strong |
APPLYING TOOLS OF CAUSAL INFERENCE
Research on EDs and children's health can benefit from careful consideration of causal inference guidelines tailored to fit diffuse exposures that likely act through biological intermediates on phenotypically diverse health end points. These circumstances may result in departures from guidelines for the classic infectious and genotoxic exposures considered by Hill (30) (Table 1). Endocrine disrupting chemicals have different biological properties, and health end points may involve a spectrum of severity or exist as a constellation of symptoms. In addition, disease end points may result from exposure during narrow windows of development. The wisdom of Hill still resonates despite these differences, but the nature of ED exposure calls for our attention to be focused on different aspects of study design and exposure measurement.
Guidelines and Rationale of A.B. Hill (1965) in His Order of Importance and Reconsiderations for Endocrine Disruptor Research
Strength of association
Hill suggested that stronger associations are more likely to have a causal basis, largely owing to his belief that unmeasured confounding may be less likely to completely erase or explain a strong association than a weak association. The problems of relying on association magnitude as a surrogate for causality have been described (62); many counterexamples of weak causal associations and strong noncausal associations exist. In addition, ED associations are expected to be relatively small because EDs act through hormonal intermediates. Many physiological processes can influence this mechanism and, likewise, can influence many outcomes.
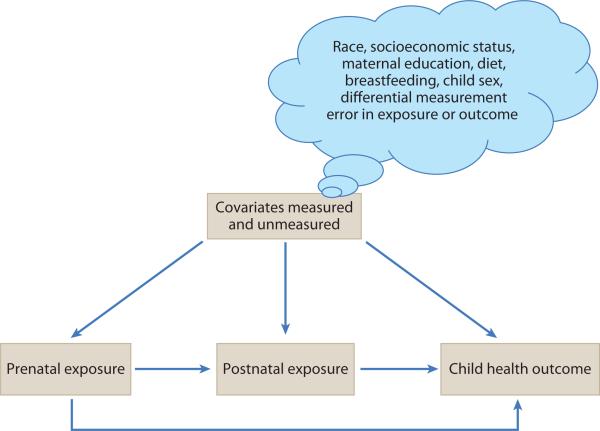
That being said, uncontrolled confounding is a major threat to validity in ED research, and the potential sources of it are diverse. A core assumption of causal inference in the context of observational studies is that of exchangeability: exposed and unexposed individuals are exchangeable on all factors apart from the exposure of interest (29). However, any factor that has the potential to be a common antecedent of ED exposure or the childhood health outcome of interest should be measured and evaluated as a confounder (Figure 1). This implicates a huge array of factors, including race, socioeconomic factors such as maternal education, home environment, diet, breastfeeding, and the sex of the child. Some problematic confounders include noncontaminant dietary constituents (e.g., omega-3 fatty acid intake, total calorie intake) that have independent causal associations, positive or negative, with important child health outcomes (e.g., neurodevelopment, childhood obesity). Yet, such factors also serve as general markers of exposure to certain ED compounds that are principally encountered through dietary intake. In this case, classical analytical approaches to dealing with confounders may be inadequate.
Figure 1.
Representative directed acyclic graph linking prenatal and postnatal exposure to a child health outcome. Direct paths connect prenatal exposure with postnatal exposure and with the child health outcome. Postnatal exposure has only one direct path to the child health outcome. Prenatal exposure, postnatal exposure, and the child health outcome all have common causes, which constitute the constellation of possible confounding factors and pathways. Included are race, socioeconomic status, maternal education, dietary components, breastfeeding (for postnatal exposure), sex of the child, and others. Notably, prenatal exposure will confound the independent association of postnatal exposure with any child health outcome if prenatal and postnatal exposure are correlated and prenatal exposure exerts an independent effect on the outcome of interest. Thus, the most informative measure of postnatal association will be conditioned on prenatal exposure. Prenatal exposure, however, is impacted by postnatal exposure only insofar as they are connected through common, and possibly unmeasured, causes—i.e., these exposures are potentially connected through a confounding pathway.
Replication and consistency in findings
Hill was ambivalent about how much importance to place on replication given that the lack of replication does not refute causality, nor does its presence definitively confirm it. The effects of some causal agents cannot occur unless all the necessary component causes are present (62). Indeed, in ED research, various unknown or unmeasured susceptibility factors may be differentially distributed across populations. These susceptibility factors have yet to be defined, but they almost certainly exist. In addition, a lack of replication may result from differences in how confounding was addressed across studies, or it may occur because a study simply lacked the power to observe any association. Carefully considering issues of power, residual confounding, and interaction may help clarify differences in observed associations across studies and point to future research needs. Still, a body of consistent evidence is essential to making causal inferences.
Specificity
Hill argues that an exposure linked to a specific site and type of disease that is not linked to any other mode of disease strongly supports a causal connection between those two factors. However, he also acknowledges that diseases may have more than one cause and that some exposures may indeed target multiple end points. ED action is theorized to work through hormonal intermediates, suggesting that multiple outcomes can be affected by disrupted hormonal pathways and therefore can also be associated with exposure. Thus, it is unlikely that associations will be restricted to a single disease end point. For example, thyroid antagonism is implicated in obesity, puberty and neurodevelopment. Additional considerations are raised in the Biological Plausibility section below.
Temporality
Exposure must clearly precede the onset of the disease for the association to be causal. Hill cautions in particular against reverse causality (exposure resulting from disease rather than causing it), which may be particularly difficult to discern in the context of diseases with long latent/silent periods or in cross-sectional studies. Attention to the correct temporal ordering of exposure and disease is critical for ED research. ED exposures may indeed result from the symptoms or treatment of the disease in question, leading to the threat of reverse causality. Incomplete knowledge of an exposure source may obscure opportunities to identify reverse causality.
Prospective designs are the best suited for establishing the link between ED exposure and childhood health outcomes, including studies that begin in utero and those that begin in postnatal life. However, in the absence of valid approaches for estimating exposure in the prenatal period, studies that begin in childhood may suffer from residual confounding by prenatal exposure if there is some correlation between pre- and postnatal exposure (Figure 1). For persistent exposures that pass from mother to child in utero or during breast-feeding, pre- and postnatal exposure biomarkers are almost certain to be highly correlated. In contrast, rapidly metabolized exposures arising from consumer product use may be less correlated over these periods. If the ED exposure levels are not highly correlated over the prenatal and postnatal exposure windows, postnatal exposure-outcome relations may suffer from less residual confounding due to unmeasured prenatal exposure, but other unmeasured common causes may still be problematic. In all cases, sensitivity analyses can be conducted to examine the implications of unmeasured confounding on the observed association (48).
Prospective studies that begin in utero have the advantage of temporal validity for assessing the causal association(s) of EDs with child health outcomes, but they are impractical for rare diseases or those with longer latent periods. Cross-sectional or case-control studies are often used to study the latter scenarios, but these designs have many limitations with respect to ED research. Cross-sectional studies cannot determine the temporal ordering of exposure and outcome, so there is an ever-present risk of reverse causality. In addition, a limitation of both study designs is that many outcomes of interest to child health researchers might in fact alter childhood exposure to ED compounds (e.g., obesity and/or metabolic syndrome, abnormal behavioral development). Moreover, many ED exposures are short-lived, in the range of hours to days, rendering it impossible for biomarker-based measures to represent temporally antecedent exposures in these study designs. Although National Health and Nutrition Examination Survey (NHANES) biomonitoring data have been an essential tool for researchers in establishing the prevalence and population correlates of exposure to many chemicals in the United States, it is uncertain whether analyses linking concurrent exposure to existing health complaints in such cross-sectional surveys are justified in light of their significant limitations.
Case-control studies, with the exception of those nested within prospective longitudinal cohorts, generally lack banked biological media with which to measure historical exposure during etiological windows of interest. Although biological measures obtained near the time of diagnosis are sometimes used as surrogates for exposures that persist in the body for longer periods [e.g., polychlorinated biphenyls (PCBs); Table 2], these measures may imperfectly estimate exposure during the etiological period of interest and may suffer from all the limitations of cross-sectional designs, including temporal ambiguity.
Table 2.
Approximate relative concentrations of exposure biomarkers in body compartments (whole basis, μg/liter or equivalent)
| Toxicant | Adiposea or bonec | Serum | Cord | Milk | Urine | Amniotic fluid | Half-life | Intracorrelations of biomarker levels over a window of ~1 year |
|---|---|---|---|---|---|---|---|---|
| PCBsa | 15 × 103 | 100 | 25 | 1,000 | – | – | 7–10 years | >0.8 |
| PFCsb | – | 100 | 50 | 1 | 0.1 | 1 | 4 years | >0.8 |
| Lead (Pb)c | 50 × 103 | 100 whole blood 0.5 plasma | 80 | 1 | 1 | <0.3 | 1 month | 0.7 |
| Phthalatesd | – | 2 | – | 7 | 100 | 7 | 12 hours | ~0.3-0.7 |
Biological gradient and dose response
Hill (30) gave greater weight to associations that were stronger at higher levels of exposure, specifically commenting on the persuasive evidence of a monotonic dose-response curve. He notes that causal evidence “would be weakened, though not necessarily destroyed, if it depended upon, say, a much heavier death rate in light smokers and a lower rate in heavier smokers” (p. 295). ED research has charted its own course with respect to the expected shape of the dose-response function. Low-dose and non-monotonic dose-response curves are supported by some biological evidence (80). Observational studies, however, should also recognize that biases may create irregular dose-response curves (62), such as apparent inverted U-shaped gradients, that result in the false suggestion of nonmonotonicity. Moreover, monotonicity does not necessarily mean the results are causal (if, for example, the confounding factor is also linearly associated with exposure). Therefore, a carefully considered a priori hypothesis about the expected shape of the dose-response curve, along with an examination of alternative, noncausal explanations for the reported dose-response function, is warranted.
Attention to dose response requires a deep understanding of exposure source and measurement. Although exposure to EDs in the United States is widespread, consumers are generally unaware of their exposure to these compounds. EDs that are incorporated into product packaging materials may migrate into the product. Other additives are exempted from public disclosure on product labels if they are considered trade secrets (e.g., proprietary fragrance formulations, patented delivery mechanisms for pharmaceuticals, or inactive ingredients) (39). This lack of transparency regarding exposure sources means that using questionnaire-based exposure ascertainment or exposure reconstruction to characterize an individual's total exposure profile is not currently feasible. As a result, biomarker-based exposure estimates are the current gold standard because they represent, in principle, an integrated metric of exposure from all sources, although their limitations are sometimes underappreciated. This generally limits research on EDs to those studies for which biospecimens are available. Moreover, many longitudinal cohort studies are not suited to the study of some EDs because they lack banked urine, which is the preferred analytic matrix for many rapidly metabolized ED contaminants. A principal concern is that poor exposure assessment leads to misclassification, which would influence a dose-response curve and, in extreme cases, invalidate associations. We present a discussion of different choices for exposure measurement and issues related to quantification, confounding, and cofactors (see sidebar, Criteria for Exposure Biomarker Measurement in Endocrine Disruptor Research).
Exposure biomarkers
Although individual-level biomarkers are invaluable and have become the sine qua non for many agents, their numerous pitfalls are often overlooked (see sidebar, Environmental Mixtures and Biomarkers). For example, twenty-first century EDs, such as bisphenol A (BPA) and phthalates, are rapidly metabolized, meaning that these exposure biomarkers reflect only relatively recent exposure (Table 2). Exposure to these compounds fluctuates over time, suggesting the need for repeated measures. Still, some urinary metabolites [e.g., monobutyl phthalate (MBP) and certain environmental phenols apart from BPA] appear to exhibit fairly good intraindividual reproducibility over 6-to-12 months, possibly owing to common patterns of product usage. Conversely, EDs with longer half-lives [e.g., PCBs, dioxins, polybrominated diphenyl ethers (PBDEs)] now exist at relatively low concentrations in the environment and are generally measured in blood samples. It is imperative to choose the correct biological matrix for sampling (Table 2; see sidebar, Unrecognized Misuse of Phthalate Biomarkers). Issues such as pharmacokinetics and possible biospecimen contamination are not adequately considered. There is also considerable interest in using blood products as a matrix for measuring biomarkers of rapidly metabolized compounds. However, the studies published to date reveal considerable opportunity for contamination by environmental sources of exposure and have produced exposure estimates that are orders of magnitude above any estimated ED chemical intake (Table 2; see sidebar, Unrecognized Misuse of Phthalate Biomarkers).
Most laboratories measure only selected agents (for example, NHANES data report one major sunscreen ingredient, whereas many products contain up to five). Although technology has achieved exceedingly low detection limits, in some circumstances, these advances may extend to a range in which it is unlikely that exposure would have biological effects.
Exposure reconstruction
Classic exposure reconstruction has been a cornerstone of environmental epidemiology, but much of it was based on unique high-level exposures that could be historically characterized, particularly in the workplace. Accurate reconstruction is difficult for exposures from multiple and unknown sources. However, if such approaches could be developed and validated, reconstruction could be more economical and could provide more accurate information across a window of susceptibility. For example, both 2,3,7,8-tetrachlorodibenzodioxin (TCDD) and PCB exposure biomarkers have been used to validate job history or fish consumption as markers of exposure, which has allowed cost-effective retrospective research on large populations. Preliminary research attempting to quantify exposures to phthalates using questionnaires or job exposure matrices has not yet succeeded in supplanting biomarker-based ED exposure estimates, although in theory, the sources and patterns of exposure should permit such a partial exposure reconstruction strategy.
Environmental monitoring
Although environmental monitoring is more expensive than reconstruction, it can be more affordable than biomonitoring and has some advantages. Air or dust measurements can provide a basis for exposure ranking, but, like biomonitoring, these techniques may be costly if they require special training and equipment. Although ambient measurements would have the limitation of assuming single exposure sources, as discussed above, investigators have used this approach successfully to estimate individual-level exposures to pesticides, polycyclic aromatic hydro-carbons (PAHs), and phthalates.
Biological plausibility
Hill notes that finding a biological basis that can explain the association under study is helpful. However, he also notes that such information is inherently limited to what is currently known, which is incomplete. A biological basis is inherent in (and indeed eponymous with) ED research. By definition, EDs act primarily through a neuroendocrine pathway upon an end point that is intermediate to classic health outcomes or disease. Therefore, the mechanistic basis of ED research is a primary and essential element of causal inference in this field.
In studies of the impact of EDs on child development, biological plausibility, and its corollary, temporality, are best embodied in longitudinal cohort study designs that begin in the prenatal period. Brain development proceeds on a trajectory from early pregnancy to the postpartum period and into early childhood (Table 3). Both experimental and epidemiologic evidence support the embryonic and fetal periods as windows of vulnerability for insults to brain development. However, both in utero and childhood exposure to toxicants such as lead and PCBs have been shown to have a strong influence on child development, although the relative contribution may vary depending on relative toxicity and window of action. That being said, prenatal and postnatal exposures are often correlated, and exposure in each period may independently influence the outcome of interest. Therefore, the most informative postnatal association likely requires conditioning on a prenatal exposure level, i.e., prenatal exposure is clearly a confounder of postnatal exposure. However, the reverse is only possible insofar as postnatal exposure is on a confounding pathway through both known and potentially unmeasured common causes (Figure 1). Very few studies in the ED literature have conducted this type of analysis.
Table 3.
Trajectory of neurological development
| Developmental process | Timing |
|---|---|
| Neurulation | 3–4 prenatal weeks |
| Neuronal migration | 6–24 prenatal weeks |
| Synaptogenesis | Third trimester to adolescence |
| Postnatal neurogenesis | Birth to adulthood |
| Myelination | Third trimester to adulthood |
| Gyrification (brain folds) | Third trimester to adulthood |
| Structural development of prefrontal cortex | Birth to adulthood |
| Neurotransmitter development of prefrontal cortex | In utero to adolescence |
Adapted from Nelson (55).
Coherence and analogy
In Hill's paper, coherence and analogy are interrelated concepts. Ideally, new mechanisms explaining the association between an exposure and a disease would cohere with what is currently known and shed light on related exposures. In the case of EDs, these factors become closely related to biological plausibility. For example, it might be suspected that chemical homologs would act similarly. Carefully considering the impact(s) of important effect measure modifiers related to a biological pathway may also help establish coherence with the existing literature.
Experimental evidence
Hill described scenarios that might provide experimental or quasi-experimental evidence of a causal relation. For example, a scenario in which the frequency of disease changes if some preventive measure is implemented and the exposure is removed would constitute powerful evidence. As Hill (30) points out, “our object is usually to take action” (p. 300). Exposure intervention or reduction may occur by design (e.g., policy-level interventions) or by nature. However, population-level interventions for ED exposures may be almost impossible. Because there are multiple related ED exposures that may impact children's health, health improvement following the elimination or reduction of a single agent may not be realistic.
CASE STUDIES OF ENDOCRINE DISRUPTOR RESEARCH IN LIGHT OF CAUSAL INFERENCE GUIDELINES
To illustrate how these causal guidelines can be used to evaluate the evidence linking ED chemical exposure and child health end points, we selected two quite different model exposures, PCBs and phthalates, on the basis of approximately 200 reviews on EDs and child health that have appeared in the twenty-first century. PCBs, which are persistent and bioaccumulative, are represented in a large toxicology and epidemiologic literature. Phthalates are rapidly metabolized compounds of emerging interest with respect to reproductive function and neurodevelopment. Great diversity exists in the potential child health outcomes associated with both chemicals, including birth size, reproductive toxicity, growth, obesity, asthma, metabolic syndrome, and pubertal maturation, each of which has a unique constellation of predictors. Thus, we focus the remainder of our review on aspects of neurodevelopment. For each model exposure, we evaluate the evidence using biological plausibility, temporality, dose response (including exposure source and measurement), consistency and replication.
PCBs AND NEURODEVELOPMENT
Biological Plausibility of PCB Research
PCBs and related organohalogens were twentieth-century toxins; they are still studied because of ongoing environmental exposures, albeit at low and declining levels. Regulatory action in the mid-1970s banned PCB production internationally, which resulted in a progressive decline in exposure levels.
However, structurally analogous chemicals such as PBDEs have emerged in recent years with toxicology and exposure patterns similar to PCBs, thereby extending this area of investigation (see sidebar, Environmental Mixtures and Biomarkers). Health concerns were raised in the 1970s because of the long half-life and widespread dissemination of PCB residues worldwide.
The structural homology of PCBs and related halogenated aromatic compounds with thyroxine and the estrogenic activity of some PCBs (50) are the basis for their endocrine activity. Extensive molecular research—more than 13,000 references in PubMed—during the next three decades supported the neurotoxic potential of PCBs. The results of studies of similarly persistent chlorinated hydrocarbons with thyroid hormone regulation capabilities (12), including 1,1-dichloro-2,2 -bis(4-chlorophenyl)ethylene (DDE) and 1,1,1-trichloro-2,2 -bis(4-chlorophenyl)ethane (DDT), have been mixed. However, other agents, such as PBDE, perfluorinated compounds (PFCs), and polybrominated biphenyls, demonstrate similar associations with thyroid hormone levels. In 1968, the Yusho (rice oil) incident, in which ingestion of PCB-contaminated rice oil resulted in dramatic harmful effects on multiple child health outcomes, occurred in Japan. This disaster, along with early PCB research in rodents and primates, provides the necessary biological link to neurological impairment.
Temporality in PCB Research
In the 1970s, two birth cohorts were established specifically to examine the neurotoxicity of population exposures in North Carolina and Michigan, and additional studies followed (see Table 4). Birth cohorts provide a longitudinal design encompassing a vulnerable window for neurodevelopment consistent with both biological findings and the temporal rela tionship of exposure to neurodevelopment. Neurobehavior in relation to prenatal exposure has been examined in these kinds of studies from birth to late adolescence. Sometimes, childhood exposure was also measured by analyzing PCBs in breast milk or in the children's blood. Many other studies followed, resulting in a robust literature including a total of 16 birth cohorts (most of which had assessments at several ages) (59).
Table 4.
Birth cohorts that have reported neurodevelopmental outcomes in relation to PCBs or phthalates
| Location and year of recruitment | Estimated median exposure, as ng/g lipid PCB 153 unless indicated otherwisea | N b | Reference for at least one citation |
|---|---|---|---|
| Denmark/Faroe Islands 1994–1995 | 450 | 182 | 46, 72 |
| United States/11 cities 1959–1965 | 140 | 1,207 | 17, 46 |
| Germany/Düsseldorf 1993–1995 | 140 | 171 | 46, 83 |
| Germany/Duisburg 2000–2002 | 1/3–1/2 lower than 1993 cohort above | 86 | |
| United States/California 1964–1967 | 130 | 399 | 36, 46 |
| United States/Michigan 1980–1981 | 120 | 212 | 33, 35, 46 |
| Netherlands/2 cities 1990–1992 | 100 | 395 | 46, 57 |
| Netherlands 2001–2002 | 63 | 62 | 63 |
| Canada/Northern Quebec 1995–1998 (Nunavik, Inuiti) | 100 | 175 | 46, 53 |
| United States/North Carolina 1978–1982 | 80 | 912 | 46, 61 |
| United States/New York 1991–1994 | 40 | 216 | 18, 46 |
| United States/New York | Placenta 1.5 μg/g wet wt (~ 0.3 μg/g wt PCB 153) | 156 | 74 |
| United States/Massachusetts 1993–1998 | 30 | 542 | 46, 64, 65 |
| Japan/ Hokkaido 2002–2004 | 23 | 134 | 19, 54 |
| Spain 1997–1999 | 1.9 μg/L | 62 | 58 |
| United States/New York City 1998–2001 | 0.8 μg/L sum 4 PCBs | 151 | 20 |
| United States/California 1998–2001 | 59.8 ng/gL sum (~12 ng/g PCB 153) | 285 | 12 |
| Maternal urinary phthalate metabolites (median or other, μg/L) | N | ||
|---|---|---|---|
| United States/New York 1998–2001 | 130 high-MW 430 low-MW | 295 | 21, 22, 52 |
| United States/New York City 1999–2006 | ~88 DEHP and ~47 DBP metabolite sums | 319 | 85 |
| United States/Ohio 2003–2006 | DEHP sum: 86 at 16 weeks’ gestation, 68 at 26 weeks’ gestation DBP sum: 29 at 16 weeks’ gestation, 25 at 26 weeks gestation |
330 | 90 |
| Korea 2006–2009 | ~18 sum of 2 DEHP metabolite; MBP 17 | 460 | 42 |
| United States/3 cities 1999–2005 | 23 DEHP and 15 DBP metabolite sums | 145 | 77 |
Abbreviations: DBP, dibutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; MBP, monobutyl phthalate; MW, molecular weight; PCB, polychlorinated biphenyl.
From Longnecker et al. (46), estimated prepregnancy maternal serum concentration, from two reviews (6, 59) and listed sources.
N varies in multiple reports.
Exposure Source, Measurement, and Dose Response in PCB Research
Diet is the chief source of PCB exposure, mainly due to seafood or, in the Faroes, whale blubber consumption. Importantly, other substances from those sources, such as mercury, other organochlorines including DDT, lead, and fatty acids, are coexposures that have also been measured in some of the birth cohort studies. Exposure reconstruction methods have been used in neurobehavioral research in which fish intake was validated as a surrogate for maternal PCB exposure. Recent recognition of indoor air contamination of volatilized PCBs from construction materials in schools adds another exposure source for consideration (47).
Exposure biomarkers for PCBs are equivalent in three matrices and have a relatively long half-life in the body compared with the in utero window (Table 2), so there is some flexibility regarding when and how to measure prenatal exposure. Although serum PCB concentrations vary somewhat during pregnancy owing to the progressive mobilization of maternal fat stores and plasma expansion, a single assay is broadly representative of the in utero window if it is properly normalized for lipids (45). In addition to maternal blood, other matrices, including cord blood, breast milk, and placenta, have been useful for PCB biomarker measures. Childhood PCB levels have been assessed in a few studies to determine whether they exacerbate existing conditions or are incremental to prenatal exposure. However, child PCB levels cannot be used as a surrogate for perinatal exposure because body size changes dramatically during early life, and similarly dramatic and unpredictable changes in child PCB levels occur due to breastfeeding, weight gain, and continued dietary exposure (40).
Meaningful dose-response analyses require attention to laboratory issues regarding exposure assessment. Both inter- and intra-laboratory variability in PCB analysis have been investigated (see sidebar, Environmental Mixtures and Biomarkers). To provide a basis for comparing PCB-neurodevelopment asso ciations across studies, Longnecker et al. (46) used a number of assumptions and laboratory data to compute the median exposures among 10 cohorts. The total PCB concentrations determined in the individual studies were approximately five times the PCB 153 levels. In this and later studies, the median perinatal PCB 153 levels were 13–450 μg/kg lipid (nine cohorts). Within each of these cohorts, the fifth through ninety-fifth percentile ranges were 5- to 10-fold around the median (e.g., for Canada: median 100 μg/kg lipid, range 30–400). Comparable exposure measures in Yusho victims (1968) and in victims of a similar accident in Taiwan (Yucheng, in 1979) were more than 10 times as high.
Among the 16 birth cohorts that have been studied, neurodevelopment has been evaluated from infancy to childhood using a range of age-appropriate assessment tools, including cognitive (visual-spatial, IQ, and executive functioning), psychomotor, and neurobehavioral measures (59). Most studies made assessments at several ages. Outcomes were measured on a continuous scale, but they could also be categorized at levels that identify children at risk of clinically significant impairment. Recent reports have included outcomes related to attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Overall, outcomes are rather nonspecific in relation to the theoretical trajectory of development (Table 3). There are several consistent aspects to the observed dose-response relations among diverse international studies. The relations between PCBs and adverse neurodevelopment in later childhood were strongest for motor, cognitive, visual recognition, and executive function (including ADHD and ASD-like behaviors). Overall, studies of earlier cohorts with higher exposure levels found associations with prenatal but not postnatal exposure to PCBs. Cognitive and motor deficits in the range of 1 to 5 standard units per unit of prenatal exposure are nicely illustrated in the Ribas-Fito et al. (59) review of the birth cohorts, whereas children who had very high prenatal exposures (Yusho and Yucheng) exhibited deficits of 4 to 10 and 4 to 20 units (cognitive and motor, respectively). However, recent studies indicate that childhood exposure may also contribute to impaired neurodevelopment. Perhaps childhood and prenatal exposures are more similar and lower now than in the earlier cohorts. Simulated exposures based on pharmacokinetics indicated that prenatal and postnatal exposure may act on different axes (infant attention and activity, respectively) (81) (Table 3). High exposure has also been related to clinically relevant test results (60). Jacobson & Jacobson (34) argued that “functionally significant impairment” was twice as likely to occur in the most highly exposed Michigan children; this kind of impairment was not seen in less exposed populations. Jacobson & Jacobson (34) also noted that in the earliest two US birth cohorts, associations with PCBs were seen in the highest 3–5% of the exposure range in NC and the highest 11% in Michigan. Stewart et al. (73) reported an association with higher-chlorinated PCBs, which are most persistent, but not the lower chlorinated.
Consistency and Replication in PCB Research
Dose-response models are rarely independent of confounding, which varies in complexity (Figure 1). Coexposures to xenobiotics are often not measured and may also be linearly associated with an outcome of interest. For example, PCBs and mercury have similar dietary origins (29), specifically fish consumption, and some research suggests coexposure to mercury may explain any PCB associations (72, 76). However, exposure through fish consumption also results in exposure to nutrients that have beneficial effects on neurodevelopment, such as omega-3 fatty acids. Although few studies adjust for such coexposures (and in some circumstances, doing so may not be possible), they can be presumed to be present with widely varying distributions across populations. Considering the possible implications of cofactors, particularly unmeasured ones, may be helpful when contrasting association sizes across studies.
Due to the substantial changes in body volume during pregnancy (see Exposure Source, Measurement, and Dose Response in PCB Research, above), caution is required in constructing statistical models because of the strong intercorrelations among maternal weight gain, lipid levels, age, race/ethnicity, socioeconomic status, and PCB levels, even across the relatively restricted pregnancy time frame, that may result in residual confounding (89). An interesting recent investigation uncovered an inverse association of PCBs with IQ after adjusting a null or slightly positive association for socioeconomic negative suppressors (75). Thus, confounding may cloud associations with temporally declining levels of PCBs, in part due to an incomplete appreciation of pharmacokinetics or coexposures.
Prenatal and postnatal PCB exposure levels are correlated and practically collinear if a child is breastfed, so it may be difficult to separate the effects of prenatal and postnatal exposure. In early investigations of their independent associations, some studies observed a stronger association of prenatal PCB exposure than breast milk PCB exposure with neurodevelopment. Explanations include a specific, sensitive prenatal window of susceptibility. A more likely explanation, however, is that breastfeeding carries benefits that can offset xenobiotic toxicity, including nurturing and favorable physiological components. Various approaches have been undertaken to isolate prenatal and postnatal exposure windows in breastfed infants, such as including the duration of nursing to calculate a total exposure through breastfeeding in the analysis, examining bottle-feeding versus breastfeeding, and examining important multivariable statistical models that account for many important confounders (including exposure in a prior period) (6, 27, 32, 57, 83). Some recent studies with lower prenatal PCB exposures do find associations with child exposure.
Summary of Evidence
Taken together, the structural homology of PCBs and related halogenated aromatic compounds with thyroxine, the Yusho disaster involving the ingestion of PCB-contaminated rice oil, which had dramatic harmful effects on multiple child health outcomes; and early PCB research in rodents and primates provide sufficient biological plausibility for any association of PCBs with neurodevelopmental impairment. Birth cohorts with both prenatal and postnatal/breast milk measured PCB exposure were established to evaluate the association between exposure during vulnerable windows and neurodevelopment. Most studies of PCB exposure and neurodevelopment have observed associations with one or more domains, particularly earlier studies with higher exposure levels. Because different studies employed different neurodevelopmental assessment tools, the outcomes are not always aligned, which may be due more to study design than to biology. In the current setting of low and declining levels of PCB exposure, important coexposures to both other pollutants and beneficial nutrients are important to consider.
PHTHALATES AND NEURODEVELOPMENT
Biological Plausibility of Phthalate Research
Early environmental health research on phtha-late diesters focused on potential carcinogenicity. Later, phthalates were classified as EDs based on extensive research that documented phthalate antiandrogenicity in rodents (15). Parallels in the antiandrogenic action of phthalates were observed in rodents and humans in the compromised development of male genitalia. Although male androgen insufficiency was emphasized, female reproductive toxicity was also detected. The possible biological link between phthalates and brain development relies on the disruption of intermediate hormonal markers, including the thyroid hormone signaling pathway and/or sex steroid hormones. Concern about widespread exposure and evidence of reproductive toxicity in animals resulted in regulatory action in the United States and abroad that aimed to limit exposure to some phthalates in specific product lines (10, 16, 23). To date, six cohort studies (five with biomarkers) and one cross-sectional study have investigated phthalate exposure in relation to child behavior, intelligence, and/or neuropsychiatric conditions (13, 21, 41, 42, 44, 77, 85, 90; Table 4).
Temporality in Phthalate Research
Five of the six cohorts we have listed utilized at least one prenatal urine sample to estimate exposure to phthalates during pregnancy (21, 42, 77, 85, 90), which effectively established exposure as a temporal antecedent to the measured neurobehavioral outcome. In contrast, cross-sectional surveys (13, 41) are unable to establish the temporal ordering of exposure and outcome, and they fail to address one of the key areas of concern with phthalate exposure and neuropsychiatric conditions: reverse causality (e.g., urinary phthalate levels could be elevated due to medical treatment for ADHD or other neurodevelopmental problems).
Exposure Source, Measurement, and Dose Response in Phthalate Research
Sources of exposure to phthalates are incredibly diverse, the scope of which has only recently been appreciated. Exposure occurs through the diet, personal care products (such as cosmetics, perfumes, lotions, and shampoos), adhesives, detergents, PVC products, and some pharmaceuticals.
Phthalate exposure measurement generally relies on urinary biomarkers. Many urinary metabolites are measurable and derived from at least ten diesters (dimethyl-, diethyl-, dipropyl-, dibutyl-, diethylhexyl-, dioctyl-, dinonyl-, didecyl-, and others). A very wide range of exposure exists, which varies by phthalate type and differs across studies; measured exposures may range from <1 to > 1,000 μg/liter for some urinary metabolites. Given such a wide variety of potential exposure sources, it is necessary to measure many metabolites (which are polar) in an appropriate aqueous matrix (see sidebar, Unrecognized Misuse of Phthalate Biomarkers; Table 2). Utilization of the oxidative metabolites of phthalates with side chains of four carbons or more (dibutyl-, diethylhexyl-, dioctyl-, dinonyl-phthalates) when measuring exposure reduces the likelihood of exposure misclassification due to contamination in the exposure metric. The relative levels of different metabolites reflect their common use and the rapidity of their metabolism. There is also some evidence that multiple biologically active metabolites may have additive effects (15). Bound (e.g., glucuronidated) metabolites are an underutilized group of exposure biomarkers; current technology requires a second assay to measure the glucuronide, and the additional expense has generally been warranted only to verify that high analyte levels are not purely a result of contamination by free monoesters.
Although the extrapolation of exposure back to a preceding period is sometimes possible with persistent contaminants, it is not possible with rapidly metabolized compounds, such as phthalates, or with other similar agents such as environmental phenols and phytoestrogens. A one-time biomarker can represent only a relatively recent period with accuracy. However, some phthalate metabolites appear to demonstrate reasonable temporal stability over periods of weeks to months (1, 2, 28, 51, 79) (Table 2), which likely reflects common patterns of product usage over this same time period. This justifies the use of a single urinary assay if only one is available when analyzing exposure over a relatively limited time frame (see sidebar, Unrecognized Misuse of Phthalate Biomarkers).
The wide range of phthalate exposure within populations allows the investigation of dose-response functions. However, the weak biological activity of these agents requires some attention to potential nonlinearities. Some studies have examined these possibilities by using graphical techniques or by categorizing the exposure into micromolar-based quantiles (21, 42, 52, 90). The sizes of the associations between phthalates and neurodevelopment are similar in magnitude to those observed for PCBs, organophosphates, and low levels of lead.
Confounding is a major concern in studies of phthalate exposure and neurodevelopment. Phthalate exposure biomarkers are known to vary according to age, sex, body size, and race (69), and these variations likely reflect the typical usage of common consumer products such as cosmetics and hair treatments. Reproductive-aged women have higher exposures than men, and non-Hispanic blacks have higher exposures than Hispanics and whites. These kinds of demographic features may also correlate with known or suspected risk factors for neurodevelopmental impairment in children, either through poverty or through other features of a disrupted home environment that correlate with socioeconomic position. In addition, coexposures, including exposures to multiple phthalate metabolites, similar agents such as phenols, or environmental contaminants with similar population distributions such as PAHs or tobacco smoke, should be considered. Two studies adjusted for some of these coexposures in their analyses (85, 90). Dietary sources of exposure present all the same complications mentioned previously. In addition, the occult presence of phthalates in medications and dietary supplements poses a unique challenge when estimating phthalate exposure (39). This can be particularly problematic for cross-sectional studies; however, prospective studies should also consider the implications of this in their analysis.
Consistency and Replication in Phthalate Research
Neurodevelopment outcomes for which results have been reported thus far include infant neurobehavioral organization (22, 90), early measures of cognitive and psychomotor development (42, 85), behavioral development and social responsiveness (21, 52, 77, 85), neuropsychiatric conditions (ASD and ADHD) (41, 44), and IQ (13). Unlike PCBs, no study has addressed both prenatal and childhood phthalate exposure. Because of the possibility that boys and girls will be differentially affected by exposure, several studies have examined boys and girls separately or have focused on male-like behaviors for similar reasons. As a cautionary note, although the incidence of some conditions is higher in boys than in girls (e.g., ADHD and ASD), it has not been established that the mechanism for this difference is hormonal.
Two studies report poorer psychomotor development with increased prenatal exposure to MBP, although at widely different ages (42, 85). Some consistency is emerging with respect to childhood behavioral domains. Associations between prenatal exposure to low-molecular-weight phthalates [e.g., monoethyl phthalate (MEP), MBP] and maladaptive child behaviors have been reported in three analyses from two cohorts using standardized instruments, although the ages at administration were different (4–9 years, 7–9 years, and 3 years, respectively). Two studies report associations with depressive and/or internalizing symptoms as well as with domains that strongly correlate with anxiety and aggression (21, 85). In these latter domains, the associations were stronger among boys than among girls in both studies. Although not all the most notable associations were consistent between these studies, differences in their findings may be attributable to differences in a parent's awareness of his/her child's internal emotional state, which tends to vary according to the age of the child.
Summary of Evidence
Like PCBs, phthalates have been shown to interfere with thyroid hormone regulation, which forms a biological basis for concern with respect to child neurobehavioral and cognitive development. Because phthalates are rapidly metabolized, prospective cohort studies with exposure biomarker measures in the prenatal period are essential for establishing temporally valid associations between exposure and neurodevelopment, and most studies have utilized this design. The tremendous diversity in sources of phthalate exposure presents serious concerns about residual/uncontrolled confounding; key areas of concern include exposure to copollutants such as tobacco smoke, PAHs, or other environmental contaminants with similar population distributions. Although some agreement among the early studies of phthalates and neurodevelopment is emerging, there have been very few studies overall. Moreover, two of the key studies are of US-based, urban cohorts (in New York City) that predominantly represent a low socioeconomic status, minority population with a high risk of behavioral problems. More studies in other populations are required to advance our understanding of this evolving area of research.
Emerging Toxicants
Two examples of emerging toxicants complement the issues raised by the exposures we have already discussed in more detail. PFCs are moderately persistent in the body (Table 2), and some evidence of their hormonal activity has been found. Their possible associations with multiple domains (e.g., subfecundity, preeclampsia, thyroid hormone regulation, and neurodevelopment) point to the dilemma of studying EDs with diffuse and sometimes overlapping outcomes (24, 56, 71, 84). The exposures are well measured, but the matrix dilution has not been resolved, although it is known that PFCs attach to albumin in blood. There are very few human data on any health outcome, including neurobehavioral development, although populations with high exposure due to industrial contamination are being followed (26, 71). Future research may be able to sharpen both the exposure measurement and the windows of susceptibility.
BPA is very rapidly metabolized, but it has exquisitely powerful estrogenic activity, which secures its biological plausibility as an ED. Yet few compelling epidemiology data link exposure to human health effects, and the most widely publicized findings have arisen from cross-sectional studies. BPA biomarker levels are uniformly low (e.g., population medians of 1 to 3 μg/liter in urine), which may be because spot urine samples fail to capture episodes of high exposure and are thus unable to accurately rank participants according to their usual, total, or peak exposure level (7, 8). Some reports reveal large proportions of unbound urinary metabolites that are likely to have arisen from specimen contamination, reminiscent of failures in phthalate biomarker measures (see sidebar, Unrecognized Misuse of Phthalate Biomarkers). More generally, the impact of matrix dilution for analytes at low urinary concentration is problematic because the limited range of biomarker concentrations may lead to instability in the exposure estimate, particularly at the extremes of the urine osmolality distribution. Dubious efforts to measure BPA in blood samples are likely to overestimate true exposures due to uncontrolled sources of sample contamination, in a similar manner to phthalates. Recent studies confirm that BPA has a similar physiological distribution to that of phthalates, i.e., blood concentrations of BPA ~50 times lower than those in urine have been reported (25, 78) (Table 2). Thus, aside from acute exposure conditions, blood biomarker levels greater than 1 μg/liter are suspect (25); for example, according to NHANES data, the 95th percentile of BPA urinary concentration is 16 μg/liter, which corresponds to a serum level of less than 0.5 μg/liter. This urinary BPA level (16 μg/liter) is roughly equivalent to the peak urinary level following a high-exposure event, such as receiving BPA-containing dental sealants (median urinary level approximately 1 h post treatment of 12 μg/liter) (37, 49). Therefore, although BPA may very well present a hazard to human health, it is not clear that the current approaches to exposure estimation on the basis of a single, or even a few, spot urine sample(s) are accurate enough to isolate signal from noise.
SUMMARY
Sufficient data exist to make a strong case for continued research aimed at investigating the role of EDs in child health and development. However, future epidemiologic research should base study designs on a cogent hypothesis with attention to the principles of causal inference. Cross-sectional studies of ED exposure and prevalent health complaints often do little to advance our understanding of any causal associations and create a body of literature that has the potential to distract both researchers and the public from pressing public health concerns. Prudent attention to the principles of causal inference with attention to issues of exposure assessment will assist in focusing scientific attention toward associations of public health importance.
ED: endocrine disruptor
NHANES: National Health and Nutrition Examination Survey
PCBs:
polychlorinated biphenyls
BPA: bisphenol A
PBDEs:
polybrominated diphenyl ethers
CRITERIA FOR EXPOSURE BIOMARKER MEASUREMENT IN ENDOCRINE DISRUPTOR RESEARCH.
To be informative in epidemiologic research, environmental exposure biomarkers should fit the scientific hypothesis. Choice of biomarkers should address basic criteria encompassing questions of toxicology, pharmacokinetics, quality assurance, and confounding. Convenient or technologically feasible biomarkers may not always be causally informative.
Relevance: A clear mechanism linking hormonal disruption with the outcome of interest is required.
Prevalence: A sufficient range of concentrations and detectability of the exposure measure is required.
Reliability: Low intraindividual variability in the exposure metric over a window of interest (high reproducibility over time) or multiple measures are required. The baseline measure with regard to the prospective outcome must be taken during the proper window of action; a concurrent biomarker can be used to represent retrospective exposure only if it is not confounded by extraneous exposure or pharmacokinetic factors that can be traced to the putative action point.
Measurement: To avoid misclassification, care must be taken with respect to quality control during sample collection and laboratory analysis, contamination control, and measurement technology.
Proper statistics: Data analysis will also consider factors affecting exposure biomarkers (i.e., confounders, including pharmacokinetics, dilution, and susceptibility factors, and analytic data, e.g., laboratory batch).
ENVIRONMENTAL MIXTURES AND BIOMARKERS.
PCBs are complex mixtures with more than 60 congeners detected in humans. Cohort studies have used different summary measures. Most studies report some estimate of total PCBs (e.g., a sum of 3–50 congeners or a 4-congener sum). In dose-response models, some studies have examined individual congeners plus a total. Noise may be introduced when congeners that have different concentration ranges are compared if a lower-concentration compound is less precise. The Longnecker standardization attempted to avoid this (46) by using PCB 153, which has a very long half-life and is the most commonly detected, highest concentration congener in nonoccupational exposures. A disadvantage of using a single PCB proxy is that the resulting associations may suffer from lack of specificity because PCB 153 is not a surrogate for all types of PCBs (e.g., coplanar). PCB subgroups with different biological activities also differ in exposure level. Thus PCB 153 and other noncoplanar PCBs generally have much higher concentrations than the dioxin-like coplanar PCBs and short-lived hormonally active congeners. This can lead to erroneous dose-response trends because of the greater error associated with low-concentration PCBs. By definition, the precision near the detection limit is three times the standard deviation of a measurement. For example, if the detection limit is 0.09 μg/liter (SD 0.03), then a serum PCB level of 0.1 μg/liter (20 μg/kg lipid) has an analytic precision of 30%. Acceptable intralaboratory precision at higher exposure levels is 5–10%.
UNRECOGNIZED MISUSE OF PHTHALATE BIOMARKERS.
Untransformed di(2-ethylhexyl) phthalate (DEHP) and some phthalate monoesters, including mono(2-ethylhexyl) phthalate (MEHP), are not appropriate biomarkers for phthalates because they can easily originate from contamination during sample collection or analysis. The first step of phthalate diester metabolism involves lipase (an enzyme commonly found in blood, saliva, or skin), which makes the conversion of DEHP to MEHP [or dibutyl phthalate (DBP) to MBP] possible following contact with contaminated materials. For DEHP and other high-molecular-weight phthalate diesters, the oxidative metabolites are resistant to contamination because they require hepatic conversion. For lower-molecular-weight phthalates, urine clearance of the monoesters is quite rapid (15). A few carefully controlled studies are helpful for understanding contamination issues. In these studies, blood levels of MEHP in the range of 100 to 1,000 μg/liter accompanied urine levels ~ 50 fold higher, far beyond what has been reported in humans. In one report of unusually elevated exposures (15, table 2.3), the highest median MEHP level in urine was 400 μg/liter, which corresponds to a predicted serum level of 8 μg/liter. If a huge exposure had happened to occur within the past 5 hours (as seen in some experiments), the serum level could approach the urine level. However, the serum level of MEHP would never exceed the urine level by orders of magnitude because clearance is so rapid. Questionable reports found median serum levels of DEHP and MEHP that would be equivalent to urinary levels at least six- to sevenfold higher than the NHANES ninety-fifth percentile for children. These implausible levels likely arose from contamination during sample collection, processing, and analysis. Thus, any associations between serum phthalate biomarkers and outcomes (such as puberty, birth weight, endometriosis, and gynecomastia) likely reflect differences in handling during sample collection.
PFCs: perfluorinated compounds
ACKNOWLEDGMENTS
The authors gratefully acknowledge Drs. Lawrence Engel and David Savitz for their thoughtful reviews of this manuscript. M.S.W. acknowledges support from grant U01ES019454 and the Mount Sinai Children's Environmental Health Center.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ. Health Perspect. 2008;116(4):467–73. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird DD, Saldana TM, Nepomnaschy PA, Hoppin JA, Longnecker MP, et al. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J. Expo. Sci. Environ. Epidemiol. 2010;20(2):169–75. doi: 10.1038/jes.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbosa F, Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ. Health Perspect. 2005;113(12):1669–74. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergdahl IA, Schutz A, Gerhardsson L, Jensen A, Skerfving S. Lead concentrations in human plasma, urine and whole blood. Scand. J. Work Environ. Health. 1997;23(5):359–63. doi: 10.5271/sjweh.232. [DOI] [PubMed] [Google Scholar]
- 5.Bergdahl IA, Skerfving S. Biomonitoring of lead exposure-alternatives to blood. J. Toxicol. Environ. Health A. 2008;71(18):1235–43. doi: 10.1080/15287390802209525. [DOI] [PubMed] [Google Scholar]
- 6.Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ. Health Perspect. 2009;117(1):7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ. Health Perspect. 2011;119(1):131–37. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, et al. Variability of urinary phtha-late metabolite and bisphenol A concentrations before and during pregnancy. Environ. Health Perspect. 2012;120(5):739–45. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brede E, Wilhelm M, Goen T, Muller J, Rauchfuss K, et al. Two-year follow-up biomonitoring pilot study of residents’ and controls’ PFC plasma levels after PFOA reduction in public water system in Arnsberg, Germany. Int. J. Hyg. Environ. Health. 2010;213(3):217–23. doi: 10.1016/j.ijheh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Calif. Safe Cosmetics Act of 2005. 2005 Senate Bill No. 484. Chapter 729. http://www.leginfo.ca.gov/pub/05-06/bill/sen/sb_0451-0500/sb_484_bill_20051007_chaptered.pdf.
- 11.Cent. Dis. Control Prev. (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. CDC; Atlanta, GA: 2009. [Google Scholar]
- 12.Chevrier J, Eskenazi B, Bradman A, Fenster L, Barr DB. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ. Health Perspect. 2007;115(10):1490–96. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, et al. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ. Health Perspect. 2010;118(7):1027–32. doi: 10.1289/ehp.0901376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993;101(5):378–84. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comm. Health Risks Phthalates . Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Natl. Acad. Press.; Washington, DC: 2008. http://dels.nas.edu/resources/static-assets/materials-based-on-reports/reports-in-brief/phthalates_final.pdf. [PubMed] [Google Scholar]
- 16.Consum. Prod. Saf. Improv. Act of 2008. 2008;122:3016–77. Public Law 110-314. U.S. Statutes at Large. [Google Scholar]
- 17.Daniels JL, Longnecker MP, Klebanoff MA, Gray KA, Brock JW, et al. Prenatal exposure to low-level polychlorinated biphenyls in relation to mental and motor development at 8 months. Am. J. Epidemiol. 2003;157(6):485–92. doi: 10.1093/aje/kwg010. [DOI] [PubMed] [Google Scholar]
- 18.Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to PCBs and infant performance on the Fagan Test of Infant Intelligence. Neurotoxicology. 2000;21(6):1029–38. [PubMed] [Google Scholar]
- 19.Dorman PM. A hospital-based day camp for children with diabetes. Diabetes Educ. 1989;15(6):514–17. doi: 10.1177/014572178901500608. [DOI] [PubMed] [Google Scholar]
- 20.Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007;165(12):1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- 21.Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010;118(4):565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–28. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eur. Parliam. Counc. Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending Council Directive 76/769/EEC on the approximation of the laws of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles). Off. J. Eur. Union L344. 2005:40–43. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:344:0040:0043:EN:PDF.
- 24.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ. Health Perspect. 2011;119(4):573–78. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher JW, Twaddle NC, Vanlandingham M, Doerge DR. Pharmacokinetic modeling: prediction and evaluation of route dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol. Appl. Pharmacol. 2011;257(1):122–36. doi: 10.1016/j.taap.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, et al. The C8 health project: design, methods, and participants. Environ. Health Perspect. 2009;117(12):1873–82. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladen BC, Rogan WJ, Hardy P, Thullen J, Tingelstad J, Tully M. Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. J. Pediatr. 1988;113(6):991–95. doi: 10.1016/s0022-3476(88)80569-9. [DOI] [PubMed] [Google Scholar]
- 28.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ. Health Perspect. 2004;112(17):1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernan MA. Beyond exchangeability: the other conditions for causal inference in medical research. Stat. Methods Med. Res. 2012;21(1):3–5. doi: 10.1177/0962280211398037. [DOI] [PubMed] [Google Scholar]
- 30.Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and serum of lactating North Carolina women. Environ. Health Perspect. 2009;117(1):86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson JL, Jacobson SW. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): the Michigan and North Carolina cohort studies. Toxicol. Ind. Health. 1996;12(3–4):435–45. doi: 10.1177/074823379601200315. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N. Engl. J. Med. 1996;335(11):783–89. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson JL, Jacobson SW. Evidence for PCBs as neurodevelopmental toxicants in humans. Neurotoxicology. 1997;18(2):415–24. [PubMed] [Google Scholar]
- 35.Jacobson JL, Jacobson SW. Association of prenatal exposure to an environmental contaminant with intellectual function in childhood. J. Toxicol. Clin. Toxicol. 2002;40(4):467–75. doi: 10.1081/clt-120006749. [DOI] [PubMed] [Google Scholar]
- 36.James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963–1967. Environ. Health Perspect. 2002;110(7):617–24. doi: 10.1289/ehp.02110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J. Am. Dent. Assoc. 2006;137(3):353–62. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 38.Karrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, et al. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007;115(2):226–30. doi: 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2012;120(3):379–84. doi: 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerger BD, Leung HW, Scott PK, Paustenbach DJ. Refinements on the age-dependent half-life model for estimating child body burdens of polychlorodibenzodioxins and dibenzofurans. Chemosphere. 2007;67(9):S272–78. doi: 10.1016/j.chemosphere.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 41.Kim BN, Cho SC, Kim Y, Shin MS, Yoo HJ, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol. Psychiatry. 2009;66(10):958–63. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Ha EH, Kim EJ, Park H, Ha M, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children's Environmental Health (MOCEH) study. Environ. Health Perspect. 2011;119(10):1495–500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koyashiki GA, Paoliello MM, Tchounwou PB. Lead levels in human milk and children's health risk: a systematic review. Rev. Environ. Health. 2010;25(3):243–53. doi: 10.1515/reveh.2010.25.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30(5):822–31. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. Serial levels of serum organochlorines during pregnancy and postpartum. Arch. Environ. Health. 1999;54(2):110–14. doi: 10.1080/00039899909602244. [DOI] [PubMed] [Google Scholar]
- 46.Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health Perspect. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macintosh DL, Minegishi T, Fragala MA, Allen JG, Coghlan KM, et al. Mitigation of building-related polychlorinated biphenyls in indoor air of a school. Environ. Health. 2012;11(1):24. doi: 10.1186/1476-069X-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacLehose RF, Kaufman S, Kaufman JS, Poole C. Bounding causal effects under uncontrolled confounding using counterfactuals. Epidemiology. 2005;16(4):548–55. doi: 10.1097/01.ede.0000166500.23446.53. [DOI] [PubMed] [Google Scholar]
- 49.Martin MD. Exposure to Bisphenol A (BPA) from dental sealants is detectable in saliva and urine, and varies significantly between sealant formulations. J. Evid. Based Dent. Pract. 2007;7(2):79–80. doi: 10.1016/j.jebdp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 50.McKinney JD, Waller CL. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Health Perspect. 1994;102(3):290–97. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J. Expo. Sci. Environ. Epidemiol. 2012;22(4):376–85. doi: 10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–67. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muckle G, Ayotte P, Dewailly EE, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Quebec Inuit infants to environmental contaminants. Environ. Health Perspect. 2001;109(12):1291–99. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakajima S, Saijo Y, Kato S, Sasaki S, Uno A, et al. Effects of prenatal exposure to polychlorinated biphenyls and dioxins on mental and motor development in Japanese children at 6 months of age. Environ. Health Perspect. 2006;114(5):773–78. doi: 10.1289/ehp.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson CA. Brain development during puberty and adolescence: comments on part II. Ann. N. Y. Acad. Sci. 2004;1021:105–9. doi: 10.1196/annals.1308.011. [DOI] [PubMed] [Google Scholar]
- 56.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009;27(3–4):212–30. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Patandin S, Lanting CI, Mulder PG, Boersma ER, Sauer PJ, Weisglas-Kuperus N. Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. J. Pediatr. 1999;134(1):33–41. doi: 10.1016/s0022-3476(99)70369-0. [DOI] [PubMed] [Google Scholar]
- 58.Ribas-Fito N, Cardo E, Sala M, Eulalia de Muga M, Mazon C, et al. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111(5 Pt 1):e580–85. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- 59.Ribas-Fito N, Sala M, Kogevinas M, Sunyer J. Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J. Epidemiol. Community Health. 2001;55(8):537–46. doi: 10.1136/jech.55.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogan WJ, Gladen BC, Hung KL, Koong SL, Shih LY, et al. Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science. 1988;241(4863):334–36. doi: 10.1126/science.3133768. [DOI] [PubMed] [Google Scholar]
- 61.Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, et al. Neonatal effects of transplacental exposure to PCBs and DDE. J. Pediatr. 1986;109(2):335–41. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- 62.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Lippincott Williams & Wilkins.; Philadelphia: 2008. [Google Scholar]
- 63.Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009;117(12):1953–58. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ. Health Perspect. 2012;120(6):904–9. doi: 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010;171(5):593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seals R, Bartell SM, Steenland K. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ. Health Perspect. 2011;119(1):119–24. doi: 10.1289/ehp.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seegal RF, Fitzgerald EF, Hills EA, Wolff MS, Haase RF, et al. Estimating the half-lives of PCB congeners in former capacitor workers measured over a 28-year interval. J. Expo. Sci. Environ. Epidemiol. 2011;21(3):234–46. doi: 10.1038/jes.2010.3. [DOI] [PubMed] [Google Scholar]
- 68.Sexton K, Adgate JL, Fredrickson AL, Ryan AD, Needham LL, Ashley DL. Using biologic markers in blood to assess exposure to multiple environmental chemicals for inner-city children 3–6 years of age. Environ. Health Perspect. 2006;114(3):453–59. doi: 10.1289/ehp.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004;112(3):331–38. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 2010;118(8):1100–8. doi: 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ. Health Perspect. 2011;119(10):1466–71. doi: 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steuerwald U, Weihe P, Jorgensen PJ, Bjerve K, Brock J, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J. Pediatr. 2000;136(5):599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 73.Stewart P, Reihman J, Lonky E, Darvill T, Pagano J. Prenatal PCB exposure and neonatal behavioral assessment scale (NBAS) performance. Neurotoxicol. Teratol. 2000;22(1):21–29. doi: 10.1016/s0892-0362(99)00056-2. [DOI] [PubMed] [Google Scholar]
- 74.Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ. Health Perspect. 2008;116(10):1416–22. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart PW, Reihman J, Lonky E, Pagano J. Issues in the interpretation of associations of PCBs and IQ. Neurotoxicol. Teratol. 2012;34(1):96–107. doi: 10.1016/j.ntt.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol. 2003;25(1):11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 77.Swan SH, Liu F, Hines M, Kruse RL, Wang C, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int. J. Androl. 2010;33(2):259–69. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, et al. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol. Sci. 2011;123(1):48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- 79.Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008;106(2):257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verner MA, Plusquellec P, Muckle G, Ayotte P, Dewailly E, et al. Alteration of infant attention and activity by polychlorinated biphenyls: unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology. 2010;31(5):424–31. doi: 10.1016/j.neuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 82.von Ehrenstein OS, Fenton SE, Kato K, Kuklenyik Z, Calafat AM, Hines EP. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 2009;27(3–4):239–45. doi: 10.1016/j.reprotox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 83.Walkowiak J, Wiener JA, Fastabend A, Heinzow B, Kramer U, et al. Environmental exposure to polychlorinated biphenyls and quality of the home environment: effects on psychodevelopment in early childhood. Lancet. 2001;358(9293):1602–7. doi: 10.1016/S0140-6736(01)06654-5. [DOI] [PubMed] [Google Scholar]
- 84.Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012;23(2):257–63. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect. 2012;120(2):290–95. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilhelm M, Ranft U, Kramer U, Wittsiepe J, Lemm F, et al. Lack of neurodevelopmental adversity by prenatal exposure of infants to current lowered PCB levels: comparison of two German birth cohort studies. J. Toxicol. Environ. Health A. 2008;71(11–12):700–2. doi: 10.1080/15287390801984904. [DOI] [PubMed] [Google Scholar]
- 87.Wolff MS. Occupationally derived chemicals in breast milk. Am. J. Ind. Med. 1983;4(1–2):259–81. [PubMed] [Google Scholar]
- 88.Wolff MS. Endocrine disruptors: challenges for environmental research in the 21st century. Ann. N. Y. Acad. Sci. 2006;1076:228–38. doi: 10.1196/annals.1371.009. [DOI] [PubMed] [Google Scholar]
- 89.Wolff MS, Anderson HA, Britton JA, Rothman N. Pharmacokinetic variability and modern epidemiology—the example of dichlorodiphenyltrichloroethane, body mass index, and birth cohort. Cancer Epidemiol. Biomarkers Prev. 2007;16(10):1925–30. doi: 10.1158/1055-9965.EPI-07-0394. [DOI] [PubMed] [Google Scholar]
- 90.Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol. Teratol. 2011;33(5):558–66. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]