Regorafenib is a novel oral agent with documented efficacy in advanced colorectal cancer. It has a characteristic adverse event profile that consists of hand-foot skin reaction, fatigue, diarrhea, hypertension, and other less common events. The authors detail practical management strategies of these adverse events to optimize patient care and maximize the clinical benefit patients can derive from regorafenib.
Keywords: Regorafenib, Adverse events, Dose optimization, Supportive management, Hand-foot skin reaction, Multikinase inhibitor
Abstract
Regorafenib is an oral multikinase inhibitor that inhibits several kinases relevant to tumor biology in several cancers, including colorectal carcinoma (CRC) and gastrointestinal stromal tumor (GIST). In phase III trials, regorafenib significantly improved overall survival versus placebo in patients with metastatic CRC progressing after all available standard therapies, and significantly prolonged progression-free survival in patients with advanced GIST in whom at least imatinib and sunitinib had failed. Thus, this agent holds promise as a new standard of care for CRC and GIST patients after disease progression following all other approved therapies. The clinical trials reported to date show that this new treatment has a consistent adverse event profile that is quite different from that of traditional cytotoxic chemotherapies. The most common adverse events of regorafenib include dermatologic and mucosal toxicities (especially hand-foot skin reaction, rash, and oral mucositis), constitutional symptoms (e.g., fatigue, nausea, and weight loss), vascular effects (especially hypertension), and gastrointestinal symptoms (e.g., diarrhea). To help health care professionals anticipate and manage the adverse events associated with regorafenib, we describe our experiences in clinical trials and show that such toxicities can be effectively managed with close observation of patients from initiation of dosing, along with prompt appropriate interventions, including dose modifications, if necessary.
Implications for Practice:
Regorafenib is a novel oral agent with documented efficacy in advanced colorectal cancer. It has a characteristic adverse event profile that consists of hand-foot skin reaction, fatigue, diarrhea, hypertension, and other less common events. This article details practical management strategies of these adverse events to optimize patient care and maximize the clinical benefit patients can derive from this novel agent.
Introduction
Regorafenib is an oral multikinase (serine-threonine and tyrosine-kinase) inhibitor that targets factors involved in angiogenesis (vascular endothelial growth factor [VEGFR] 1–3 and TIE2), oncogenesis (KIT, RET, RAF-1, and B-RAF), and regulation of the tumor microenvironment (platelet-derived growth factor receptor [PDGFR]-β and fibroblast growth factor receptors) [1]. In preclinical studies, regorafenib showed antitumor activity in a variety of tumor models, including models of colorectal carcinoma (CRC) and gastrointestinal stromal tumor (GIST) [1]. Regorafenib has been evaluated as a single agent in clinical trials in these two malignancies as well as other solid tumors (Table 1) [2–9].
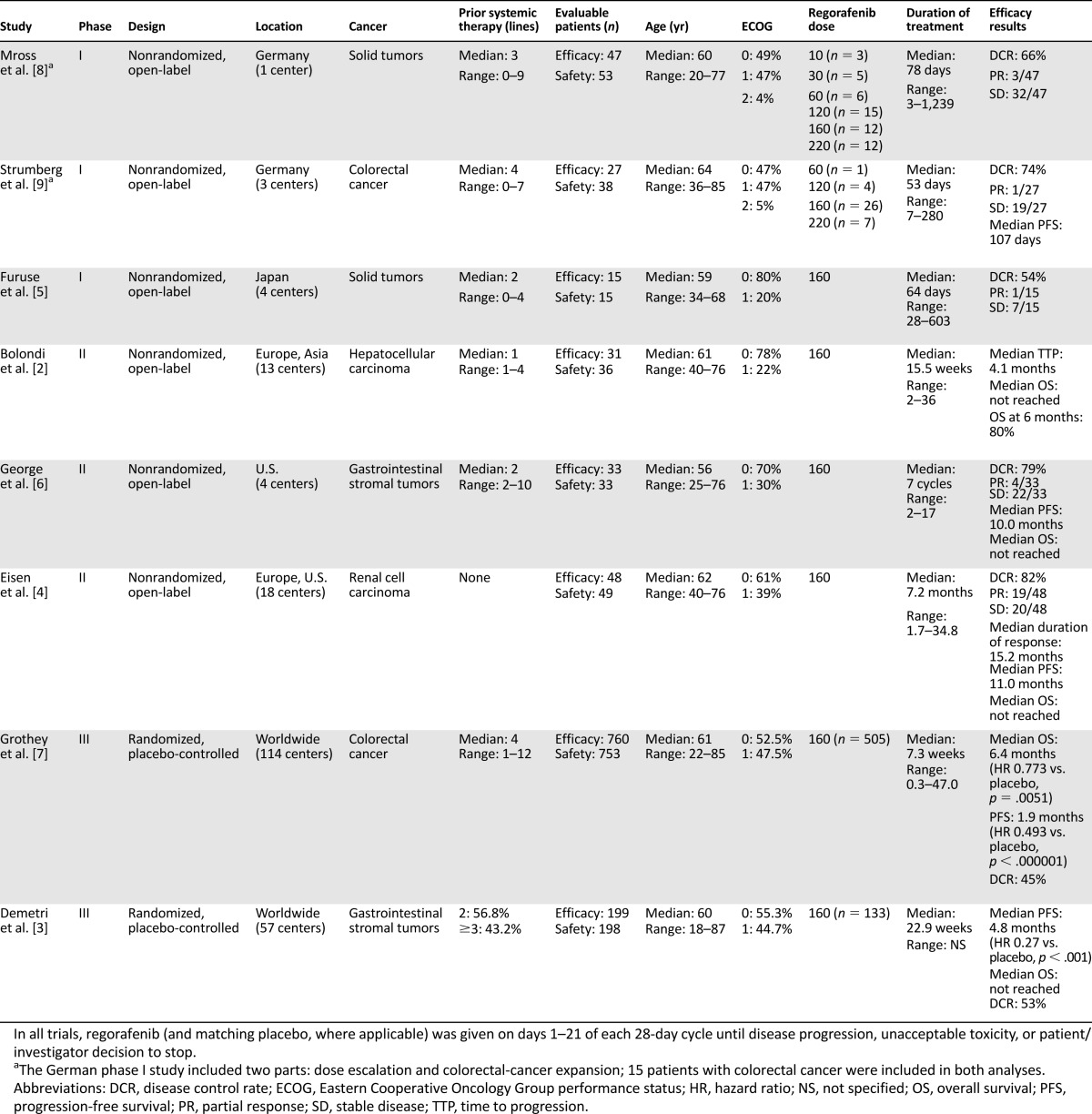
Table 1.
Design, baseline patient characteristics, and efficacy outcomes of regorafenib clinical trials
Two recent pivotal phase III trials with regorafenib demonstrated positive clinical results. In the “CORRECT” trial involving 760 patients with metastatic CRC in whom all available standard therapies had failed (or who were unable to tolerate available treatments), median overall survival was significantly prolonged with regorafenib compared with placebo (6.4 months vs. 5.0 months, respectively; hazard ratio 0.77, 95% confidence interval 0.64–0.94; one-sided p = .0052) [7]. In the “GRID” trial involving 199 patients with advanced GIST that had progressed despite treatment with at least imatinib and sunitinib, median progression-free survival was 4.8 months with regorafenib compared with 0.9 months with placebo (hazard ratio 0.27, 95% confidence interval 0.19–0.39; p < .0001) [3]. These data confirm the role of regorafenib as an effective treatment option for patients with metastatic CRC or GIST in whom all standard therapies have failed.
Similar to other oral therapies that target kinases relevant to cancer, regorafenib is associated with an adverse event profile that differs from that seen with traditional cytotoxic chemotherapy [10–12]. Health care professionals need to understand, recognize, and be able to manage these events so that patients have the best chance to benefit from this novel treatment. In this review, we highlight the adverse events that are most likely to occur with regorafenib and provide recommendations regarding their management.
Materials and Methods
To identify the most frequently reported adverse events associated with regorafenib treatment, we reviewed all of the presented and published safety and tolerability data on regorafenib reported in the clinical trials [2–9].
To identify relevant guidance on managing adverse events, we searched Medline (PubMed) using the search terms (kinase) AND (antagonist OR inhibitor) AND (safety OR toxicity OR tolerability OR adverse event) as well as abstracts from major oncology conferences (American Society of Clinical Oncology and the European Society for Medical Oncology annual meetings).
We have also drawn on our own databases and clinical experiences of using regorafenib in the clinical trials and after its regulatory approval in clinical practice to help inform the recommendations that we make in this article.
Regorafenib Adverse Event Profile
The study designs and patient characteristics for the regorafenib clinical trials are summarized in Table 1 [2–9]. Analysis of the most frequent drug-related adverse events reveals a consistent safety profile across all trials, regardless of patient population, ethnicity, previous treatment status, and tumor site or burden of disease (Table 2). The most common adverse events include dermatologic and mucosal toxicities (especially hand-foot skin reaction [HFSR], rash, and oral mucositis), constitutional symptoms (e.g., fatigue, nausea, and weight loss), systemic vascular effects (especially hypertension), and gastrointestinal symptoms (e.g., diarrhea).
Table 2.
Drug-related adverse events reported in regorafenib clinical trials
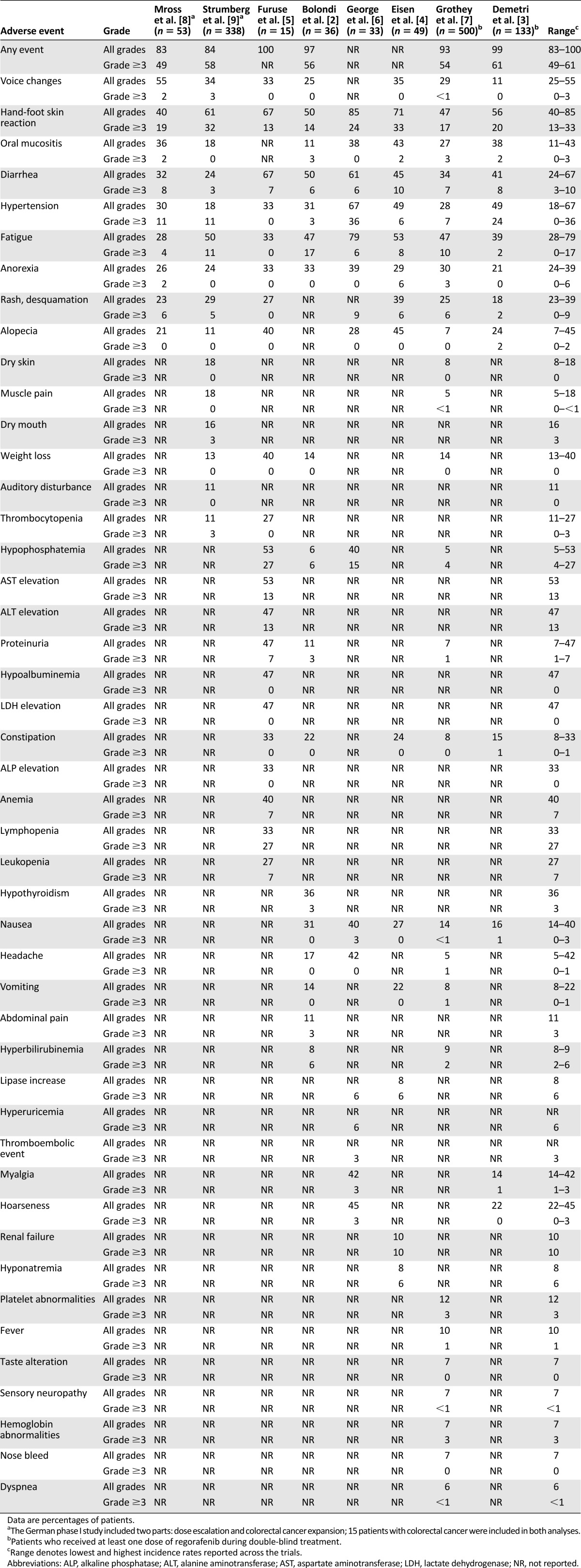
More than half of the patients in each trial (range 49%–61%) experienced grade 3 or greater toxicities (based on National Cancer Institute Common Terminology Criteria for Adverse Events [NCI-CTCAE] version 3.0 or 4.0) (Table 2) [2–9]. The most frequently reported grade 3 or higher events were HFSR (13%–33% of patients), hypertension (0%–36%), fatigue (0%–17%), diarrhea (3%–10%), and laboratory abnormalities, such as elevated aspartate or alanine aminotransferase (13% each, but reported in the Japanese trial only [5]), hypophosphatemia (4%–27%), or hyperbilirubinemia (2%–6%). The majority of grade 3 adverse events have appeared to be reversible with dose delays, dose reductions, and additional support [12]. It is important to note that side effects, in particular, HFSR, rash, and fatigue, occur early within the first cycle of therapy after a median of approximately 2 weeks [12]. To date, no clinical, biochemical, or molecular risk factors have been identified that predispose patients on regorafenib to the development of HFSR.
Mild asthenia and noticeable voice changes (most commonly hoarseness) are also frequent early features of regorafenib therapy and may occasionally occur with low-grade fever. Skin toxicities such as HFSR have also been observed with other oral therapies that target kinases including VEGFRs. In clinical trials, HFSR symptoms of any grade were reported in 34% of patients on sorafenib and 19% of patients on sunitinib; grade 3 or higher events affected 9% and 6% of patients, respectively [13, 14]. The mechanism of action of kinase inhibition responsible for HFSR remains to be elucidated, but it is believed to be a result of inhibition of multiple molecular pathways targeted by these agents, including VEGFRs, PDGFRs, and c-KIT [15, 16]. Of note, HFSR associated with kinase inhibitor therapy differs in important ways from the classic hand-foot syndrome seen with traditional cytotoxic agents such as 5-fluorouracil, capecitabine, doxorubicin, or liposomal doxorubicin (Table 3). Initial symptoms of HFSR mediated by kinase inhibitor therapies may manifest with early signs of tingling or subtle discomfort, even after only 5–7 days on therapy. These symptoms may progress in some patients to worsening pain, tenderness, callus formation, redness, and edema (occasionally associated with a burning sensation) in the palms of the hands or soles of the feet and especially in the folds between joints or pressure points of the feet. Other areas that may be involved include the tips of the fingers and toes, heels, and areas of flexure or overlying skin (Fig. 1). These pressure areas are where most severe symptoms are typically seen, with formation of blisters that can severely impair the ability to walk. These blisters can burst and discharge serous fluid, although, commonly, thick callus formation may occur. Signs and symptoms may appear concomitantly or sequentially, and can affect both hands and both feet [17, 18].
Table 3.
Clinical characteristics of hand-foot syndrome and hand-foot skin reaction
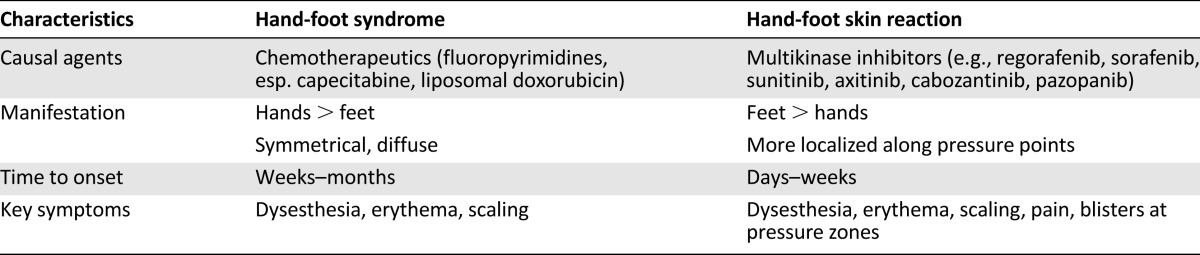
Figure 1.
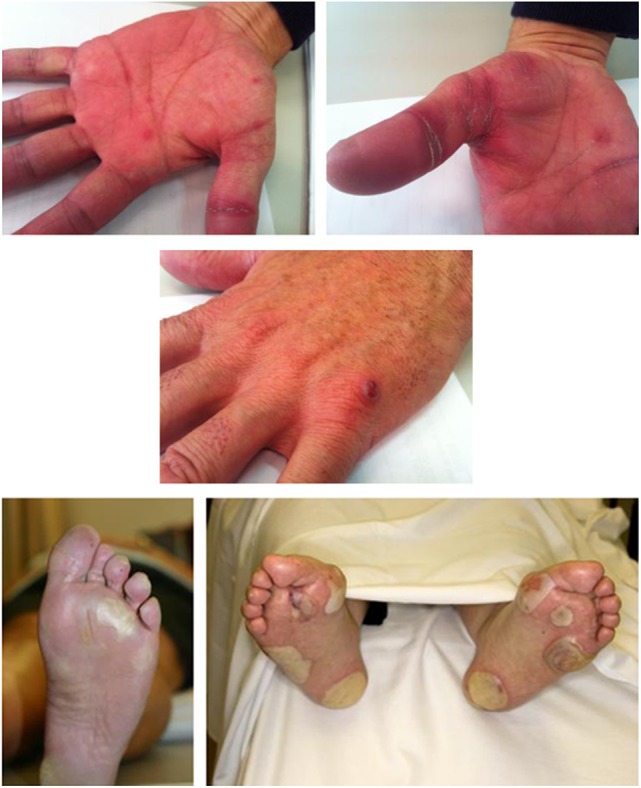
Clinical manifestations of hand-foot skin reaction.
From our experiences in the phase III CRC and GIST trials, as well as earlier phase trials of regorafenib and in its use in clinical practice, we have noted that adverse events are likely to occur early—even during the first 3 days of regorafenib dosing—and the incidence of many adverse events is highest during the first cycle of treatment [12]. This time profile is similar to the timing of adverse events seen with other kinase inhibitors, such as sunitinib or sorafenib [19, 20].
Experience in the clinical trials indicates that most adverse events can be effectively managed with treatment breaks, dose adjustment, and appropriate intervention. By managing these events appropriately and proactively, particularly within the initial one or two cycles of therapy, most patients do not need to stop treatment permanently because of intolerable toxicities.
Experience in the clinical trials indicates that most adverse events can be effectively managed with treatment breaks, dose adjustment, and appropriate intervention. By managing these events appropriately and proactively, particularly within the initial one or two cycles of therapy, most patients do not need to stop treatment permanently because of intolerable toxicities.
It is important to note that to date no correlation has been established between the severity of specific adverse events such as hand-foot skin reaction and efficacy of regorafenib (data on file). This is in contrast to a report from a nonrandomized population study in which the presence of skin toxicity was associated with outcome parameters in patients with renal cell cancer treated with sunitinib [21].
Management of Regorafenib-Related Adverse Events
Our literature search identified 6,656 articles addressing the toxicity profile of kinase inhibitors and other targeted agents, 64 of which were focused on adverse event management (supplemental online Table 1). Understandably, given that experience with regorafenib to date has been mainly limited to clinical trials, no articles have yet been published that focus specifically on the practical management of regorafenib-related adverse events. The following information is therefore based on data and experience gleaned from the phase III clinical trials and the use of regorafenib in clinical practice, as well as our own and other groups’ experience with other kinase inhibitors (in particular, multikinase inhibitors that inhibit VEGFRs, such as sunitinib and sorafenib).
Dose and Schedule Adjustments
As mentioned previously, the pertinent toxicities associated with regorafenib, in particular HFSR, may appear very early after initiation of therapy. Prompt management of these early events is critical to ensure tolerable treatment continuation. We advise evaluation of patients within 1 week of starting treatment and then at least every 2 weeks during the first 2 months (which corresponds to the first two treatment cycles) to be able to address adverse events using dose delays or dose reductions. This evaluation would preferably be a face-to-face clinical assessment, but it could also be done by a telephone call conducted by a health care provider trained in regorafenib management. In the phase III clinical trials of regorafenib, only 8% of patients in the CRC study and 2% of patients in the GIST study permanently stopped regorafenib treatment because of adverse events unrelated to disease progression [8, 9]. Treatment modifications (dose reduction or delayed start of the next cycle) were used to manage adverse events in 56% of patients in the CRC study and 72% of patients in the GIST study [3, 7]. In some cases, patients were eventually able to return to the full dose of regorafenib once the toxicity had resolved to baseline levels without encountering recurrence of any severe adverse events. Table 4 shows the prespecified dose adjustments defined in the protocols of the phase III CRC and GIST trials.
Table 4.
Recommended regorafenib dose modifications to manage adverse events (based on protocol-specified dose modifications in the phase III trials) [8, 9]
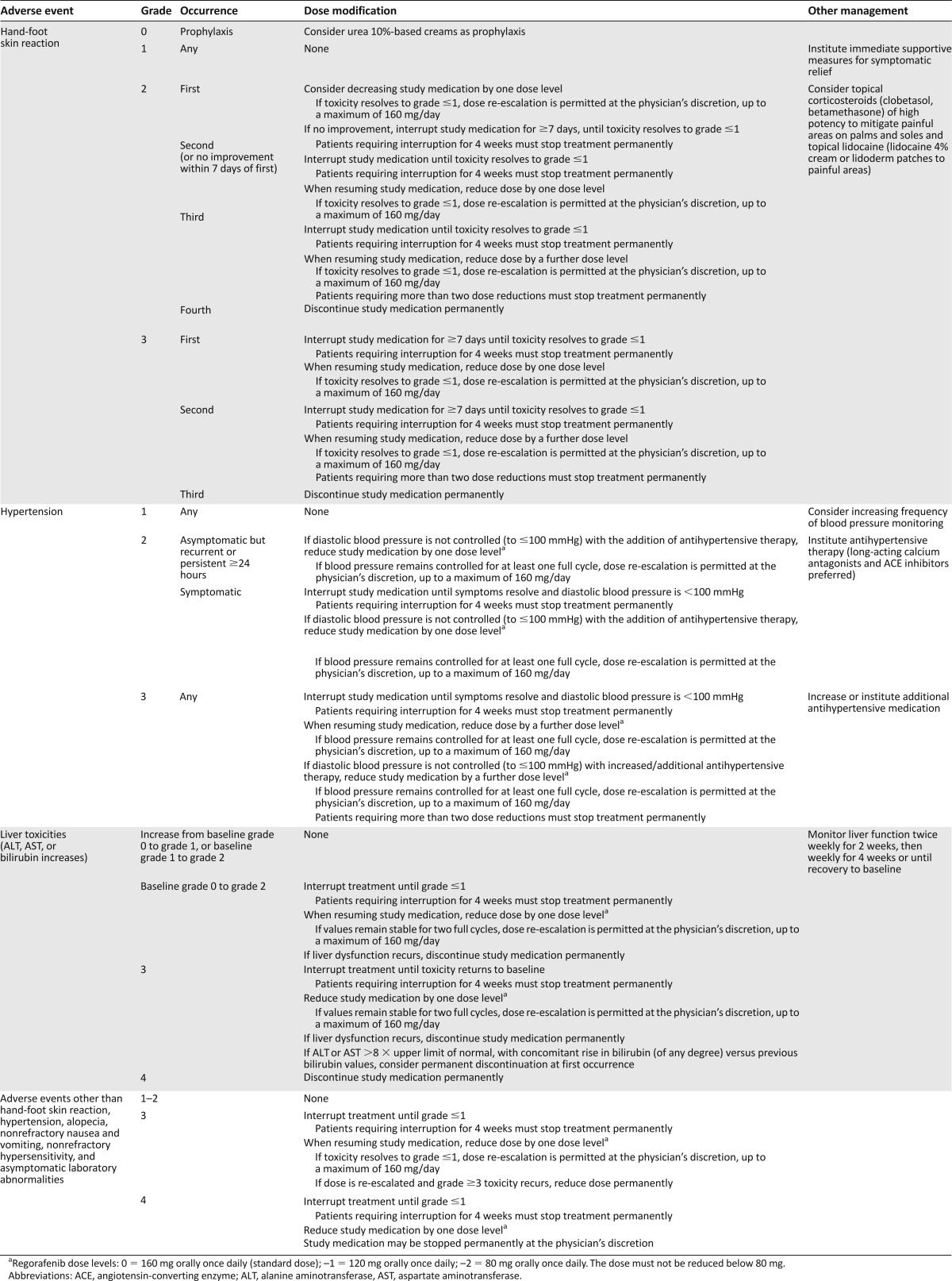
For most patients, physicians were able to titrate regorafenib to an appropriately tolerable dose using the dose adjustment guidelines provided within the pivotal phase III studies [3, 7]. After initial dose delays and dose adjustments, patients tolerated long-term treatment with regorafenib with tolerable adverse events, with some patients now on regorafenib for more than 3 years, even if they had experienced severe (grade 3) toxicities within the first one or two cycles. Of note, the minimal daily dose of regorafenib allowed per protocol was 80 mg. This minimal daily dose was selected in view of the fact that no data on antitumor activity had been generated with lower doses in prior studies [9]. Experience with other kinase inhibitors also shows that dose and schedule modifications are important to minimize risk and reduce the frequency or severity of adverse events, thereby enabling patients to remain on treatment over time [18, 22, 23].
Supportive Care
There is little prospective research reported on appropriate supportive care interventions to manage adverse events associated with molecular targeted therapy. Much of the published advice exists in the form of expert opinion, consensus, or anecdotes (supplemental online Table 1). The advice provided in Table 4 and in the following text is similarly based on expert recommendations for other kinase inhibitors and our own experience managing patients during the course of regorafenib in clinical trials and clinical practice.
Dermatologic and Mucosal Toxicities
Although skin and mucosal toxicities are virtually never life-threatening, such symptoms can be painful, distressing, or even disabling. In our experience, HFSR, mucositis, and to a lesser extent rash, have a substantial impact on patients’ quality of life [24]. Health care professionals therefore need to take proactive measures to prevent or minimize the impact of these symptoms.
Prevention
Patients should be educated about the potential risks of skin and mucosal toxicities, especially HFSR, and should have a full-body examination before the start of treatment to identify areas of pre-existing skin damage or hyperkeratosis/calluses, which should ideally be removed (e.g., by manicure or pedicure) before initiating regorafenib [15, 16, 25]. Pressure points should continue to be exfoliated with emollient or kerotolytic agent creams (e.g., salicylic acid 3%-, ammonium lactate/lactic acid 12%-, or urea 10%-based creams). Patients should be advised to wear cotton socks, avoid constrictive footwear, and prevent excessive friction or trauma to the hands and feet. In addition, patients should be advised to apply moisturizers and sunscreen, and avoid contact with hot water or chemicals (including household cleaners). Gloves should be worn if potentially skin-irritating chemicals are to be handled. Health care staff should frequently examine patients and encourage them to discuss any skin concerns to ensure early diagnosis of skin toxicities, particularly within the first 2 months of therapy.
Management
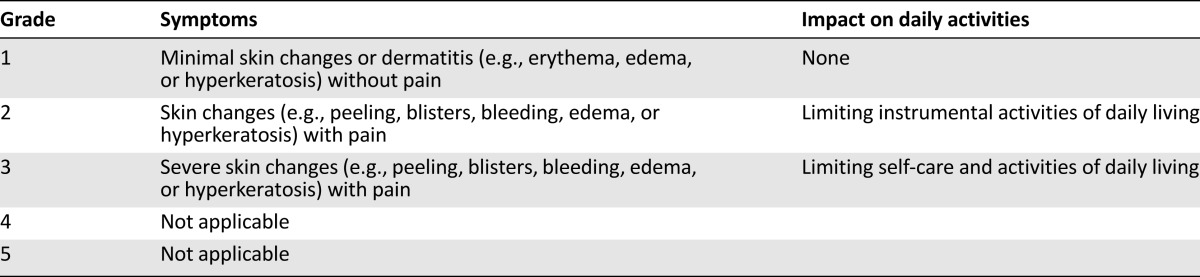
Table 5 shows the standardized NCI-CTCAE version 4.0 grading for dermatologic toxicities, which may be helpful in guiding treatment decisions [11, 17, 18, 25]. However, these criteria were not developed specifically for skin symptoms associated with targeted therapies. The Multinational Association of Supportive Care in Cancer (MASCC) Skin Toxicity Study Group has therefore proposed a modified grading scale tailored for epidermal growth factor receptor inhibitor therapy, which may also be relevant for VEGFR inhibitors [26]. Although the MASCC scale needs to be validated prospectively, this nonetheless may better enable physicians to detect and report skin toxicities with greater sensitivity, specificity, and range than is possible with previous scales or in conventional clinical practice.
Table 5.
Grading of hand-foot skin reaction symptoms, based on National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 for palmar-plantar erythrodysesthesia syndrome [11]
At the first signs of redness, patients should be advised to moisturize the area with topical creams (e.g., zinc oxide and magnesium silicate lenitive cream without petrolatum), and preventive measures should be discussed again with the patient and caregivers. If blisters form, pressure should be avoided, and good supportive care to skin integrity should be pursued. Sometimes blisters have to be opened and drained under sterile conditions. Antibiotics should be prescribed only if there is evidence of infection. To prevent the risk of superinfection if fissures form, patients may be advised to soak their hands for 10 minutes every morning and evening in a solution of vinegar and water (equal measures). To reduce pain associated with walking, proper shoe fit is important, and podiatrist evaluation and support may be worth considering. A recent study has indicated that urea-based creams may provide prophylaxis for HFSR [27], but these creams should not be used if the patient develops broken skin, as such topical application may further irritate the skin.
If severe symptoms develop, patients should be offered pain relief and appropriate management to reduce the risk of infection. The regorafenib dose should be held until symptoms resolve and subsequently should be decreased (Table 4) to manage the toxicity and reduce the risk of treatment discontinuation.
Changes in Liver Enzymes and Bilirubin
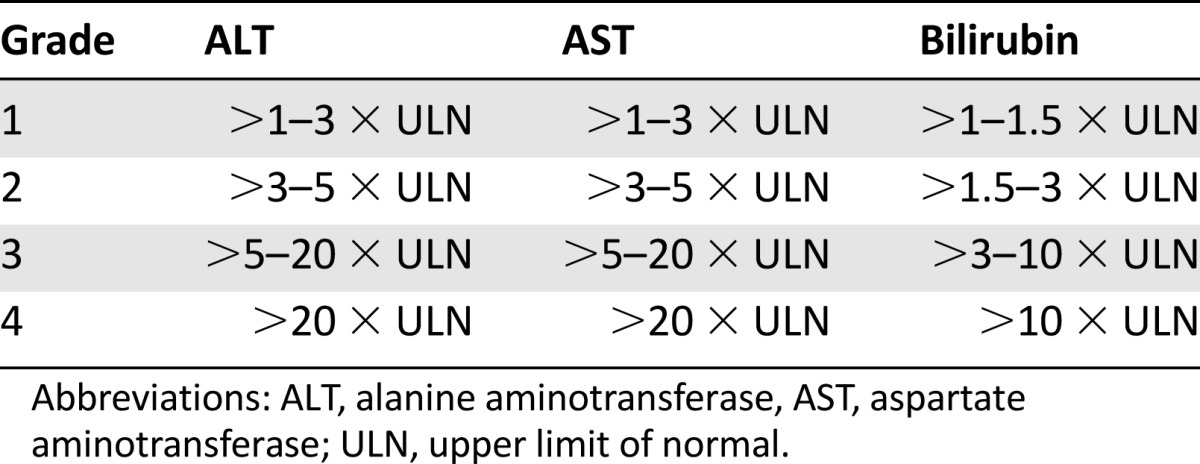
Asymptomatic laboratory abnormalities (e.g., circulating levels of liver enzymes or bilirubin) have been relatively common in clinical trials of regorafenib. However, few patients experienced clinically significant abnormalities, although one death in each of the phase III trials was attributed to liver dysfunction, usually in the setting of concurrent metastatic disease burden affecting the liver [3, 7]. Because patients with advanced metastatic CRC and GIST commonly have liver metastases, the distinction between abnormalities in liver function due to tumor progression and regorafenib can be unclear. Until further evidence is available on the mechanism of regorafenib-induced liver dysfunction and the likely risk to patients in the general population, careful monitoring of liver function before and during treatment is recommended in the U.S. Federal Drug Administration-approved dosing information for regorafenib. Dose modifications or treatment discontinuation (Table 4) are mandated in the case of elevated liver function test results or hepatocellular necrosis, depending on severity and persistence (Table 6 shows the standardized NCI-CTCAE version 4.0 grading for liver function tests).
Table 6.
Grading of liver toxicities, based on National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 for alanine aminotransferase, aspartate aminotransferase, and bilirubin [11]
Systemic Vascular Toxicities and Potential Cardiovascular Risks
Hypertension was reported in 28% of patients in the phase III CRC trial and in 49% of patients in the phase III GIST trial at any grade, with 7% and 24%, respectively, experiencing grade 3 or higher hypertension [3, 7]. The higher rates of hypertension in the GIST trial could reflect the longer treatment duration compared with the CRC study (mean duration of treatment 20.2 weeks vs. 12.1 weeks, respectively), although this hypothesis has not been formally assessed. Abnormally elevated blood pressure may have an important impact on patients, who may experience clinical symptoms (e.g., headache) in the short term, or may have significant detrimental cardiovascular effects with longer term or severely uncontrolled hypertension. Therefore, the need for close and careful blood pressure monitoring and adjustment of medications should not be underestimated. Fortunately, with the introduction of VEGF inhibitors in the treatment algorithms of various cancers, blood pressure monitoring and management have become part of standard oncologic practice. Although hypertension is not a contraindication for regorafenib treatment, patients should have good control of any pre-existing hypertension, with normal baseline blood pressure. Patients should also be counseled and educated on antihypertensive treatment. Blood pressure should be monitored regularly while patients are taking regorafenib. In the GIST trial protocol, it was recommended that blood pressure should be monitored at least weekly for the first 6 weeks of treatment. In addition, we suggest further monitoring at least during the first week of subsequent cycles. Table 4 suggests that appropriate antihypertensive management and regorafenib dose adjustment be considered in patients with hypertension. The health care team needs to ensure that the patient has access to a blood pressure monitor at home, records blood pressure regularly in a log or diary, and is given instructions to contact the health care team for specified elevations in blood pressure. For example, we recommend that patients be instructed to contact the doctor’s office if any single blood pressure reading is above 160/90 mmHg, and to review home blood pressures monthly, with a goal of keeping blood pressure below 140/90 mmHg. Patients should also be educated on the potential symptoms of hypertensive crisis and the appropriate actions to take.
Other cardiac and vascular toxicities have been reported with therapies that inhibit the VEGF signaling pathways, such as the monoclonal antibody bevacizumab [28, 29], and other targeted therapies have also been implicated in cardiac dysfunction [30]. Bleeding disorders or thromboembolic events have only rarely been reported in the regorafenib clinical trials and have not been of major relevance in clinical practice. Because of the potential impact of VEGF inhibition on cardiac and cardiovascular function, the clinical trials of regorafenib excluded patients with known pre-existing cardiovascular comorbidities, including arterial or venous thrombotic or embolic events such as stroke or transient ischemic attacks, deep vein thrombosis, or pulmonary embolism within the 6 months before the start of treatment, cardiac arrhythmias requiring antiarrhythmic therapy, or uncontrolled hypertension despite optimal medical management. Therefore, in the absence of data on the effect of regorafenib in these patients, we strongly advise caution when considering regorafenib treatment in such patients. Patients who were on stable anticoagulation treatment were allowed to participate in the trials, and excess bleeding was not seen. However, close monitoring of patients on Coumadin (warfarin) is recommended, or consideration should be given to using an alternative anticoagulant, such as a low-molecular-weight heparin.
Other Adverse Effects
Other frequent adverse events that may have a substantial impact on patients include fatigue, diarrhea, muscle aches, fever, hair thinning, and voice changes. Patients should be educated on these potential adverse events and the need to report any adverse events to the oncology team to allow appropriate and prompt intervention.
Fatigue can be significant and debilitating in the first few cycles, and symptoms, which are most prominent toward the end of dosing in each cycle, can begin early (e.g., hoarseness within the first 3–4 days) [12]. As many multikinase inhibitors have been noted to induce hypothyroidism, particularly after several months of therapy [31], we recommend checking thyroid-stimulating hormone (TSH) levels in patients with any level of fatigue (abnormally high TSH levels on regorafenib therapy are often clinically unapparent and not accompanied by changes in free triiodothyronine and thyroxine). If there is no laboratory evidence of hypothyroidism, the patient may be encouraged to exercise, which may help to mitigate mild fatigue. For grade 2 or 3 fatigue, regorafenib dose interruption or reduction should be considered. Indeed, decreased doses to 120 or even 80 mg/day may be necessary. There are no clinical data to support dosing regorafenib lower than 80 mg/day.
Diarrhea is generally a highly manageable adverse event with the use of standard antidiarrheal agents. Patients should be encouraged to keep a dietary log, which may identify the need for diet modification, including probiotics, lactose avoidance, and adequate hydration. If the patient experiences persistent grade 3 diarrhea despite antidiarrheal drugs and dietary modification, regorafenib dose interruption or reduction should be considered.
For other significant adverse events, regorafenib dose modifications (interruption or reduction) should be considered.
Discussion and Recommendations for Practical Management
Our experience in the phase III clinical trials, together with evidence from earlier phase trials and postapproval practice, indicates that regorafenib has a generally consistent and predictable profile of adverse events that can be managed with appropriate patient education, dose interruptions, dose modification, and supportive care. Adverse events are usually of a low grade, and events of grade 3 or higher are typically of short duration when identified promptly and managed optimally [12]. Due to their reversibility, adverse events can normally be managed without the need for permanent discontinuation of regorafenib treatment, and most patients are able to continue treatment until disease progression.
Close communication between the patient and the health care team allows prompt identification and management of toxicities to achieve adherence to regorafenib therapy. It is important to see or at least contact patients very soon after starting treatment (i.e., within the first week), and then see patients every 2 weeks during the first two cycles of regorafenib treatment, to facilitate early recognition of incipient adverse effects and initiate optimal strategies for prevention and management (with appropriate regorafenib dose holding or dose reduction, if needed).
It may be that, in patients who are frailer than those included in the clinical trials, a starting dose lower than 160 mg (e.g., 120 or 80 mg per day) might be appropriate. The dose can then be escalated within the first one or two cycles to reach the target dose of 160 mg; however, there are no data yet available to support this approach. Therefore, we currently recommend starting at the approved dose and stress the importance of assessment after 3–7 days to allow the dosing to be modified immediately as shown in Table 4 to address any evidence of toxicities. Once the patient is on a stable dose and tolerating regorafenib well, the follow-up interval can be expanded to less frequent visits (e.g., monthly or even longer after many months of benefit with good tolerance).
With the rapid growth in the number of targeted therapies being introduced into clinical practice in oncology, there is a need for high-quality evidence on the appropriate management of adverse events, supported by a greater understanding of the mechanism of action of these events. Side effects associated with targeted therapies that include VEGF inhibition appear to be relatively similar between therapeutic drugs, indicating that these adverse effects may represent a class effect resulting from inhibition of shared molecular targets. Further linking the mechanistic etiologies of these adverse events to the pathways inhibited by biologic agents represents an area of active research [15, 16].
Side effects associated with targeted therapies that include VEGF inhibition appear to be relatively similar between therapeutic drugs, indicating that these adverse effects may represent a class effect resulting from inhibition of shared molecular targets. Further linking the mechanistic etiologies of these adverse events to the pathways inhibited by biologic agents represents an area of active research.
For regorafenib, data continue to be collected for long-term follow-up analyses to assess whether delivered dose intensity correlates with therapeutic outcomes, as well as to assess interindividual differences in pharmacologic levels of regorafenib and its metabolites to guide optimal dose adjustment. Further research is also needed into the profiles of regorafenib-associated adverse effects over time; the potential impact of patients’ age, ethnicity, or pharmacologic biomarkers; and the effect of sequential or combination therapy with other targeted therapies (evidence from the phase III trials indicates that previous treatment with bevacizumab, imatinib, or sunitinib does not appear to lead to any “holdover” impact or worsening of adverse events on regorafenib).
Conclusion
While acknowledging that management of adverse events associated with targeted therapies is currently largely based on clinical experience rather than prospectively tested, randomized strategies, we believe that our experience has generated clinical guidance that will be helpful to clinicians treating patients with regorafenib. The adverse event profile associated with regorafenib appears to be quite predictable across tumor types and patient populations, and symptoms can be effectively managed as long as health care professionals know what to expect, assess patients early and regularly (starting within 3–4 days of treatment initiation), take prompt action, and educate, advise, and manage patients appropriately.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The academic authors take full responsibility for the content of this review. Sara Black, of Succinct Healthcare Communication, provided editorial assistance, which was funded by Bayer HealthCare Pharmaceuticals. Bayer HealthCare Pharmaceuticals was allowed to review the manuscript for factual correctness, but did not influence the content or decision to publish. Axel Grothey and Suzanne George contributed equally to this article.
Author Contributions
Conception/design: Axel Grothey, Suzanne George, George Demetri
Data analysis and interpretation: Axel Grothey, Suzanne George, Eric van Cutsem, Jean-Yves Blay, Alberto Sobrero, George Demetri
Manuscript writing: Axel Grothey, Suzanne George, George Demetri
Final approval of manuscript: Axel Grothey, Suzanne George, Eric van Cutsem, Jean-Yves Blay, Alberto Sobrero, George Demetri
Disclosures
Axel Grothey: Bayer (C/A, RF); Alberto Sobrero: Roche, Amgen, Sanofi, Bayer (C/A, H); Suzanne George: Novartis, Bayer, Pfizer, Ariad (C/A, RF); Eric van Cutsem: Bayer (RF); Jean-Yves Blay: Bayer, Novartis, Roche, Pharmamar, GlaxoSmithKline (C/A, RF); George Demetri: Novartis (IP), Bayer, Pfizer, Novartis (C/A), Bayer (ET).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 2.Bolondi L, Tak WY, Gasbarrini A, et al. 6576 POSTER phase II safety study of the oral multikinase inhibitor regorafenib (BAY 73-4506) as second-line therapy in patients with hepatocellular carcinoma. Eur J Cancer. 2011;47(suppl 1):S464. [Google Scholar]
- 3.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen T, Joensuu H, Nathan PD, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: A single-group phase 2 trial. Lancet Oncol. 2012;13:1055–1062. doi: 10.1016/S1470-2045(12)70364-9. [DOI] [PubMed] [Google Scholar]
- 5.Furuse J, Sasaki Y, Okusaka T, et al. 1236 POSTER phase I study to assess the safety, tolerability and pharmacokinetics of the multikinase inhibitor regorafenib (BAY 73-4506) in Japanese patients with advanced solid tumours. Eur J Cancer. 2011;47(suppl 1):S155. [Google Scholar]
- 6.George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: A multicenter phase II trial. J Clin Oncol. 2012;30:2401–2407. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grothey A, van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 8.Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–2667. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 9.Strumberg D, Scheulen ME, Schultheis B, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: A phase I study. Br J Cancer. 2012;106:1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: A meta-analysis. Invest New Drugs. 2013;31:1078–1086. doi: 10.1007/s10637-013-9977-0. [DOI] [PubMed] [Google Scholar]
- 11.Gomez P, Lacouture ME. Clinical presentation and management of hand-foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: Experience in breast cancer. The Oncologist. 2011;16:1508–1519. doi: 10.1634/theoncologist.2011-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grothey A, Sobrero AF, Siena S et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol 2013;31:3637a. [Google Scholar]
- 13.Chu D, Lacouture ME, Fillos T, et al. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 14.Chu D, Lacouture ME, Weiner E, et al. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: A meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 15.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1955–1961. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 16.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. The Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 17.Bracarda S, Ruggeri EM, Monti M, et al. Early detection, prevention and management of cutaneous adverse events due to sorafenib: Recommendations from the Sorafenib Working Group. Crit Rev Oncol Hematol. 2012;82:378–386. doi: 10.1016/j.critrevonc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Manchen E, Robert C, Porta C. Management of tyrosine kinase inhibitor-induced hand-foot skin reaction: Viewpoints from the medical oncologist, dermatologist, and oncology nurse. J Support Oncol. 2011;9:13–23. doi: 10.1016/j.suponc.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Iijima M, Fukino K, Adachi M, et al. Sorafenib-associated hand-foot syndrome in Japanese patients. J Dermatol. 2011;38:261–266. doi: 10.1111/j.1346-8138.2010.01059.x. [DOI] [PubMed] [Google Scholar]
- 20.La Vine DB, Coleman TA, Davis CH, et al. Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma. Am J Clin Oncol. 2010;33:217–220. doi: 10.1097/COC.0b013e3181a650a6. [DOI] [PubMed] [Google Scholar]
- 21.Poprach A, Pavlik T, Melichar B, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: A national registry-based study. Ann Oncol. 2012;23:3137–3143. doi: 10.1093/annonc/mds145. [DOI] [PubMed] [Google Scholar]
- 22.O’Dwyer M, Atallah E. Practical considerations for the management of patients in the tyrosine kinase inhibitor era. Semin Hematol. 2009;46(suppl 3):S16–S21. doi: 10.1053/j.seminhematol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 24.Nardone B, Hensley JR, Kulik L, et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol. 2012;11:e61–e65. [PubMed] [Google Scholar]
- 25.Anderson R, Jatoi A, Robert C, et al. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs) The Oncologist. 2009;14:291–302. doi: 10.1634/theoncologist.2008-0237. [DOI] [PubMed] [Google Scholar]
- 26.Lacouture ME, Maitland ML, Segaert S, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–522. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 27.Ren Z, Zhu K, Kang H et al. A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol 2012;30:4008a. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Mayer EL, Je Y, et al. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J Clin Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 29.Schutz FA, Je Y, Azzi GR, et al. Bevacizumab increases the risk of arterial ischemia: A large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 30.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106:21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- 31.Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660–664. doi: 10.7326/0003-4819-145-9-200611070-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


