Abstract
Addictions are commonly presaged by problems in childhood and adolescence. For many individuals this starts with the early expression of impulsive risk-taking, social gregariousness and oppositional behaviors. We propose here that these early diverse manifestations reflect a heightened ability of emotionally salient stimuli to activate dopamine pathways that foster behavioral approach. If substance use is initiated, these at-risk youth can also develop heightened responses to drug-paired cues. Through conditioning and drug-induced sensitization, these effects strengthen and accumulate, leading to responses that exceed those elicited by other rewards. At the same time, cues not paired with drug become associated with comparatively lower dopamine release, accentuating further the difference between drug and non-drug rewards. Together, these enhancing and inhibiting processes steer a pre-existing vulnerability toward a disproportionate concern for drugs and drug-related stimuli. Implications for prevention and treatment are discussed.
Keywords: Drug Abuse, Alcohol Abuse, Reward, Conditioning, Sensitization, Incentive Salience, Externalizing, Allostasis
An integrative neurodevelopmental model of substance use disorders
Drug addiction is the most prevalent neuropsychiatric disorder affecting society today. The social, medical and economic costs are enormous, with drug use contributing to 12% of deaths worldwide [1] and costing the U.S. government alone an estimated $400 billion per year [2-3].
Because only a minority of people who try drugs of abuse develop a substance use disorder (SUD), attempts have been made to identify predisposing neurobiological features. One long considered hypothesis is that increased susceptibility reflects preexisting perturbations in the mesolimbic dopamine system [4]. Still debated though is whether this perturbation ultimately expresses itself as a decrease in dopamine activity, as in opponent-process and reward deficiency models [5-6], or heightened dopamine activity, as in incentive sensitization models [7-8]. The present neurodevelopmental model integrates each of these features. It recognizes a role for both hypo- and hyper-activity in mesolimbic dopamine systems, and outlines how each might become particularly pronounced in individuals at risk.
As summarized below, converging evidence from studies in human adolescents, young adults, and laboratory animals suggests that youth exhibiting heightened dopamine responses to emotionally intense stimuli are at increased susceptibility to engage in a wide range of impulsive, reward-seeking behaviors. Although these behaviors may initially target diverse non-drug stimuli, the initiation of drug use steers the heightened dopamine reactivity toward drug related cues, leading to drug conditioning and sensitization. These effects further enhance brain dopamine responses to the drugs and drug-paired cues, thereby augmenting the attentional focus of at-risk individuals on these stimuli and obtaining the drug. Because non-drug paired cues simultaneously become associated with comparatively lower dopamine responses, the overall result is a narrowed behavioral repertoire, setting the stage for progressively more frequent drug taking and a SUD.
This model represents a departure from single factor theories of drug abuse (Table 1). By incorporating both hypo- and hyper-dopamine activations, and combining this with identifiable predisposing factors, the present neurodevelopmental model provides a more comprehensive accounting of the addiction process. It is also, we propose, better positioned to inform the development of more effective therapeutic strategies.
Table 1. Comparison of Reward-Deficiency and Incentive Sensitization models of vulnerability to the Integrative model proposed here.
Two competing models of predisposing traits to addictions hypothesize increased vs. decreased reward system responsiveness. Here we propose an integrative neurodevelopmental model that incorporates both features. In brief, high-risk individuals with externalizing traits initially express elevated incentive motivational and striatal dopamine responses to diverse emotionally salient events. Once substance use begins, these responses become increasingly focused on the drugs and drug related cues. At the same time, cues paired with the absence of drug can come to inhibit dopamine release and associated motivational states. In this way, a pre-existing vulnerability is steered toward a disproportionate preference for drugs and drug-related stimuli, setting the stage for addictions. Positive reinforcement: Increased probability that a behavior will be repeated due to presentation of a positive event. Negative reinforcement: Increased probability that a behavior will be repeated due to the removal of an aversive event. Incentive salience: the property of a cue that renders it able to elicit approach and desire. Pre-existing susceptibility: Vulnerability traits that pre-exist substance use. Prevention: Interventions that can decrease the probability that vulnerable individuals will develop substance use problems.
| Feature | Opponent Process / Reward-Deficiency |
Incentive Sensitization | Integrative Neurodevelopmental Model |
|---|---|---|---|
| Positive reinforcement | No | Yes | Yes |
| Negative reinforcement | Yes | No | No |
| Hyperactive incentive salience | No | Yes | Yes |
| Hypoactive incentive salience | Yes | No | Yes |
| Pre-existing susceptibility | Yes | Yes | Yes |
| Intervention strategies Prevention Treat high dopamine responses Treat low dopamine responses Redirect attentional biases |
? No Yes No |
? Yes No Yes |
Yes Yes Yes Yes |
Increased impulsive reward-seeking and dopamine responsivity prior to drug use
A recent series of adoption, twin, and longitudinal follow-up studies have supported a strikingly consistent conclusion: many SUDs reflect the outcome of an ‘externalizing’ trajectory characterized by risky thrill-seeking, social gregariousness, and oppositional tendencies in childhood and adolescence [9-19]. The core processes underlying these predispositions are thought to include over- and under-sensitivity to reward and punishment related cues, respectively [20-22]. For example, adolescents with high externalizing traits make risky choices, preferring high frequency rewards even when the losses are higher [23-25].
Marked individual differences in substance use are also seen in laboratory animals, and not all readily develop drug self-administration behaviors [26]. One of the best-described predictors of susceptibility to acquire drug self-administration is a greater tendency to explore novel environments [26-29]. Among those animals that acquire drug self-administration, only a subset will transition to compulsive use, as defined by willingness to work more for the drug, endure aversive events to obtain it, and persist in drug seeking for much longer than average [30-31]. These “compulsive” drug-using rats are distinguished by high novelty preference and forms of impulsivity, such as premature responding to cues [32].
The behavioral traits that predict drug use behaviors co-vary with the tendency to engage with other rewarding stimuli and individual differences in dopamine cell responsiveness. In rats, high dopamine cell firing at baseline and release in response to diverse challenges predict greater novelty exploration [29,33], greater sugar feeding [29,34], more incentive learning [35], and the more rapid acquisition of drug self-administration [4,29,36-38]. The evidence is more than just correlational. Dopamine agonists increase premature responses during tests of impulsivity and a wide range of situation dependent reward-seeking behaviors including drug seeking (Box 1).
Box 1. Dopamine and reward.
Animal studies indicate that risky, reward-seeking behaviors are potently influenced by dopamine. Different components of these behaviors can be anatomically dissected. The best studied is the willingness to approach and sustain effort to obtain a reward, behaviors that are closely influenced by dopamine transmission in the ventral striatum, amygdala, and anterior cingulate [7-8,39-44]. Dopamine also affects the tendency to prematurely respond to reward cues [45], reflecting effects in the striatum [46], the willingness to tolerate delay for a larger reward, reflecting effects in the amygdala and orbitofrontal cortex [42-43,47], and executive control engagement with the task, reflecting effects in the orbitofrontal cortex [47]. The weight of evidence suggests that dopamine is not closely related to pleasure [7,48].
In humans too individual differences in externalizing behaviors may be related to differences in dopamine responsiveness. In young healthy adults, greater striatal dopamine responsiveness co-varies with novelty seeking [49-50] and other impulsivity related traits [50-52]. In fMRI studies, similar results are seen. The greater the striatal responses to monetary reward, the greater the tendency to risky behavior [53-55]. The greater the striatal response to monetary reward anticipation, the higher the positive affective response scores [56]. The greater the striatal response to cues paired with erotic images, the more likely these cues will be chosen two months later [57]. And the greater the striatal responses to images of food and sex, the greater the weight gain and sexual activity at follow-up six months later [58].
The above associations in humans are thought to reflect causal effects since manipulating dopamine transmission alters many of the same processes [59-61]. Lowered dopamine transmission disrupts corticostriatal functional connectivity [62], top-down regulation by the cortex and the ability of reward related cues to activate the striatum [63-64]. These neurophysiological effects are associated with a decreased behavioral tendency to preferentially respond to rewards [65-67], and a decreased willingness to sustain effort to obtain rewards, including alcohol [68], tobacco [69] and money [70]. Elevated dopamine function, in comparison, increases the ability of reward related cues to guide behavioral choices [65], diminishes the ability to differentiate between high and low value rewards [71], and induces steeper temporal discounting, a form of impulsivity defined by preference for immediately available small rewards over larger, more distal ones [72]. In clinical populations, patients with schizophrenia – considered a hyper-dopamine disease – have very high rates of substance use problems [73] while those with Parkinson’s disease exhibit, if anything, decreased rates of substance abuse [60]. Indeed, administering Parkinson’s patients dopamine agonist medications can induce a dysregulation syndrome characterized by various impulse-control problems, including pathological gambling, hyper-sexuality, and substance abuse [60].
Hyper- and hypo-dopamine activity following the initiation of drug use
Once drug use begins, some of the effects can become sensitized; i.e., previously ineffective low doses can now produce a response and previously effective doses elicit larger responses. In laboratory animals, repeated drug administration regimens can lead to progressive increases in drug-induced behavioral activation, greater willingness to sustain effort to obtain drug reward, and greater drug-induced dopamine release [7-8].
The conditions most likely to produce sensitization resemble early drug use patterns in humans: multiple exposures to moderate to high doses taken days apart in the presence of the same environmental stimuli. When these conditions have been simulated in human research, drug-induced sensitization has been demonstrated including greater drug-induced dopamine release and greater energizing effects [74-76]. This noted, even under these conditions, not all subjects exhibit the augmented responses. In rats, sensitization is more likely to develop in those that exhibit high reactivity to novel environments [27,33]. In humans, dopamine sensitization was greater in those with high novelty seeking scores [74].
Repeated drug administration can also lead to conditioned effects; i.e., environmental stimuli paired with the drug can come to elicit many of the same effects as the drug itself, including behavioral activation, dopamine release and reward-seeking [77-81]. The optimal conditions for producing these conditioned effects are the same as those for eliciting sensitization. Moreover, individual differences are also apparent [82]. Finally, high novelty exploring rats engage more actively with cocaine cues, and are more susceptible to the cue-induced reinstatement of drug-seeking following an extinction procedure [83].
In humans too, cues paired with drug use can come to elicit many of the same effects as the drugs, including increased reward-seeking [84], conditioned place preferences [85-86], greater drug-induced drug craving [87], and dopamine pathway activation [88-89]. Individual differences in cue-induced dopamine [88] and craving responses are seen [21], and some evidence suggests that this could reflect a trait [21].
The cue-induced effects appear to be particularly marked in subjects at risk for addictions. In heavy drinkers at risk for alcohol use disorders, alcohol related cues induce a heightened electroencephalogram (EEG) P300 signal, an index of motivational salience [90]. In fMRI studies, high externalizing adolescents show greater responses to monetary reward notification than control subjects in the ventral striatum [54]. Similarly, compared to healthy controls, subjects with a family history of alcohol use disorders exhibit larger responses to alcohol associated cues in the nucleus accumbens and other aspects of the mesocorticolimbic circuit [91-93]. Indeed, in a large study of heavy drinkers (n=326), the greater the severity of alcohol use problems, the greater the alcohol cue-induced striatal activation [94-95]. Finally, intriguing preliminary evidence suggests that a subpharmacological taste of beer leads to significant striatal dopamine responses in participants with a family history of alcohol use disorders, but not in low risk drinkers [96].
The presence vs. absence of drug related cues and contexts can modify the readiness to respond to other events [76,97-99]. If a natural reward is presented in a place previously paired with drug, the animal will exhibit invigorated engagement with this natural reward [82,100]. If, more typically, drug cues are presented in association with the possibility of receiving drug, drug-seeking behaviors are fostered [77,81,101]; if the drug is administered, the expression of dopamine [101] and behavioral sensitization is enabled [102-103]. Conversely, cues explicitly paired with the absence of drug reward can have potent inhibitory effects, actively decreasing dopamine release [104], behavioral activation [97,102-103,105-106] as well as drug-taking and reinstatement [107-108].
The effects of stimuli explicitly paired with the absence of drug reward are less well studied in humans. However, recent evidence suggests that inhibitory processes can be engaged. For example, when non-dependent smokers were presented with cigarette cues, craving scores increased significantly above baseline; presentation of cues explicitly paired with the absence of cigarettes, in comparison, significantly decreased craving below baseline [109]. Evidence of these diminished effects can be seen in brain also. High-risk subjects who have begun substance use exhibit smaller EEG P300 responses to positive non-substance related cues such as erotica than drug related cues [90]. fMRI studies support the same conclusion: compared to healthy controls, at-risk subjects exhibit smaller striatal-limbic responses to various low non-drug cues, perhaps particularly those with low immediate salience [110-112; cf, 55].
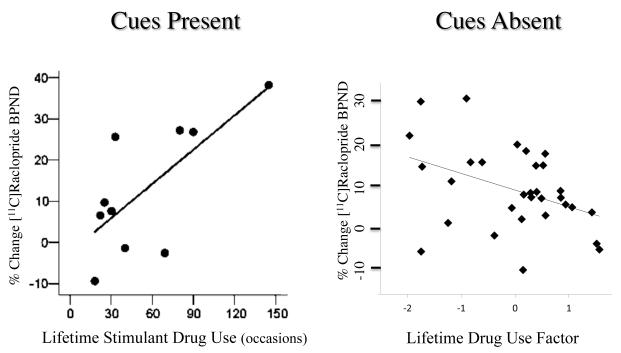
The presence vs. absence of drug related cues might also affect the readiness of dopamine cells to respond in humans. For example, when non-dependent stimulant drug users ingested cocaine in the presence of drug related cues (immersed in the familiar microenvironment of preparing and inhaling cocaine powder) [113], the greater the lifetime history of stimulant drug use, the greater the drug-induced striatal dopamine response. In comparison, in non-dependent stimulant users tested in the absence of drug-related stimuli, greater lifetime histories of substance use were associated with smaller drug-induced striatal dopamine responses [114] (Figure 1). One interpretation of these results is that the absence of drug related cues dampens dopamine cell reactivity (Figure 2).
Figure 1. The presence or absence of drug cues differentially regulates drug-induced dopamine release as a function of lifetime history of drug use.
In the study depicted at left [113], 10 non-dependent stimulant drug users self-administered intra-nasal cocaine powder (1 mg/kg, i.n.) in their usual fashion, immersed in the drug cue rich microenvironment. The greater the lifetime history of stimulant drug use, the greater the dopamine response (r = 0.715, p = 0.02). In the study at right [114], 31 non-dependent stimulant drug users were administered a d-amphetamine tablet (0.3 mg/kg, p.o.) in the absence of drug-related cues. The greater the lifetime history of all drug use (derived omnibus factor) [114], the smaller the dopamine response (r = 0.407, p = 0.023).
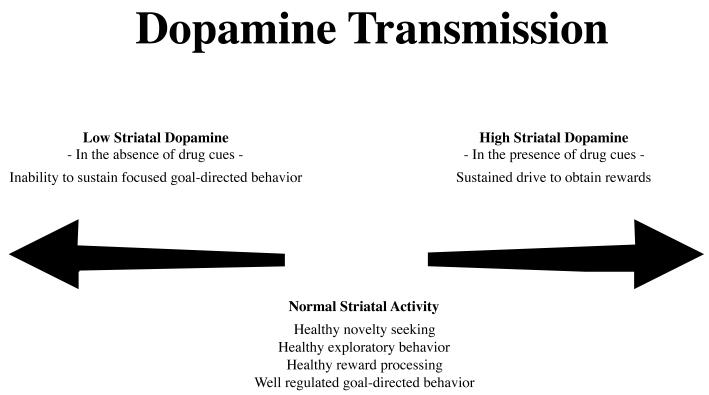
Figure 2. Model of dopamine activations and behavioral effects in addictions.
Patients with SUDs may experience periods of striatal dopamine hyper- and hypo-activations related to the presence vs. absence of drug related cues. With sustained drug use, both effects become exaggerated leading to a progressively increased preoccupation with drug taking and a progressively decreased interest in normal daily non-drug activities. Adapted from [59,76].
Together, the above studies suggest that low dopamine transmission in the absence of drug-related cues can result from two processes. The first is a passive process in which dopamine transmission is low as compared to responses seen when drug cues are present. The second is an active process, reflecting conditioned inhibition (Box 2). Moreover, not only can these non-drug cues usher in a period of low dopamine activity and motivation, their lack of attractiveness cannot compete with the pull of drug-paired cues. These effects may also have implications for behavior during withdrawal, and, indeed, the heightened susceptibility to seek and use drugs when in drug withdrawal may well reflect the same processes. Just as deprivation states can enhance the incentive value of natural reward cues, such as food [116], compelling evidence suggests that drug seeking observed during drug withdrawal may also reflect the heightened incentive salience of drug cues rather than avoidance of withdrawal [117-119]. Thus, drug use during withdrawal may reflect elements of positive rather than negative reinforcement processes. In these ways, cues unpaired with drug may be critical for the development of two overarching features of SUD: the progressive narrowing of interests toward drug related cues and drug taking and a diminished interest in pursuing the non-drug related goals necessary to thrive.
Box 2. Environmental cues and reward.
Imagine you are walking up a steep hill. If past experience has taught you that an enticing reward is at the top, your motivation to continue will be high, and cues indicating that the reward is forthcoming will augment and sustain your drive. These motivational states are closely related to changes in dopamine transmission; i.e., reward-paired contexts increase the readiness of dopamine cells to burst fire in response to discrete reward-paired cues [44,98,115]. In comparison, environments explicitly paired with the absence of reward can acquire the properties of a conditioned inhibitor [99] and the ability to actively inhibit dopamine readiness and the ability to respond to rewards and reward-related cues [76,104]. Together, this combination of effects produces strong preferences for drug-paired environments and cues, steering individuals away from non-drug related activities and events.
Two very recent studies suggest that subjects at high risk for SUDs might be particularly susceptible to these effects (Figure 3). First, a distinctively high dopamine response was seen in impulsive substance users at elevated risk for addictions, as compared to low risk users, when they were tested with drug cues present (alcohol ingested with the beverage’s sight, smell, taste and touch) [120]. Second, and in striking contrast, exceptionally low dopamine release was observed in impulsive substance users at elevated risk for addictions when they were tested without drug cues present (d-amphetamine tablets hidden in nondescript gelcaps) [114]. In both of these studies, the group differences persisted after controlling for lifetime substance use. Indeed, in these high-risk users, the dopamine responses in the absence of drug-related cues were significantly lower than those seen in low risk subjects matched for personal drug use histories [114]. Such observations raise the possibility that, in these high-risk populations, conditioned control over the response to rewards is developing faster or more extensively. Together, the findings reviewed here suggest that the combination of drug-induced sensitization, conditioning, and individual differences in susceptibility to these effects could come to steer at-risk youth toward progressively more frequent drug use, setting the stage for a SUD.
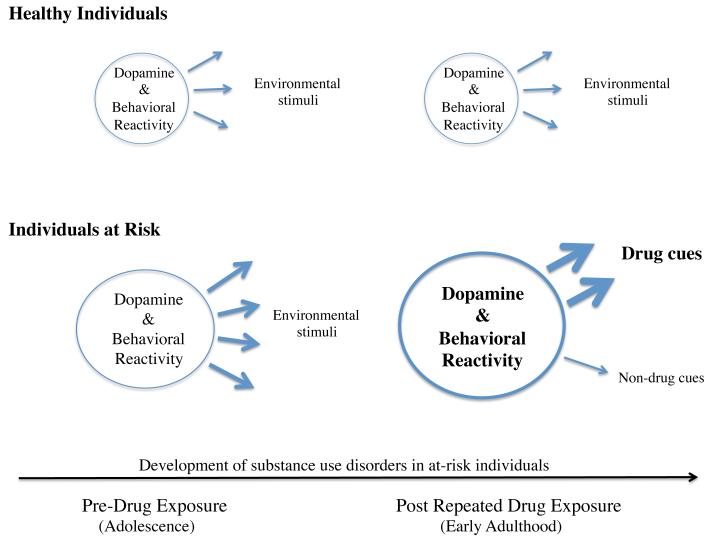
Figure 3. Dopamine and the development of substance use disorders in high externalizing individuals.
Mesolimbic dopamine is critical for attribution of salience to, approach toward, and interaction with environmental stimuli. In healthy individuals, this manifests as relatively stable dopamine and behavioral reactivity (circles) directed at (arrows) diverse stimuli in the environment. In the present model, prior to drug use, high externalizing at-risk individuals exhibit augmented limbic dopamine and behavioral responses to a diverse range of emotionally salient environmental stimuli. With the initiation of substance use, these responses progressively increase in magnitude and become increasingly focused on drug paired stimuli, reflecting drug-induced sensitization and conditioning. Concurrently, dopamine and behavioral responses to non-drug cues diminish in magnitude and frequency, reflecting the decreasing ability of these stimuli to compete with sensitized drug effects and drug-paired cues as well as the potential for conditioned inhibition of dopamine pathways by cues specifically paired with the absence of drug. The net result is a progressive narrowing of interests toward drug related cues and drug taking with a diminished interest in pursuing non-drug related goals.
Implications for prevention and treatment
Unlike single factor views of addiction that focus on either hyper- or hypo-mesolimbic dopamine activations, the integrative model proposed here combines both features, thus providing a novel neurobiological starting point for intervention strategies, including prevention (Box 3). Recent work gives reason for optimism. For example, externalizing adolescents given impulse-control training exhibit fewer substance use problems at two-year follow-up [129].
Box 3. Dopamine and impulsive behavior.
The relation between impulsive behaviors, heightened dopamine release, and greater susceptibility to substance abuse can propagate across generations. In addition to propagation via heritable traits, impulsive rodents exhibit less maternal care [121], leading to greater impulsivity, reward cue sensitivity, dopamine release, and drug self-administration in their offspring [122-124]. In a natural environment, these animals may also be more likely to come in contact with adverse events. These stressors also induce dopamine release, and can lead to long lasting behavioral and dopaminergic cross-sensitization to drugs of abuse [125-127], aggravating further the pre-existing tendencies. The same effects may be occurring in humans too. Indeed, children growing up in families characterized by externalizing behaviors are at elevated risk for stress, trauma and neglect, putting them at even higher risk for SUDs [128].
It remains speculative whether the processes described above (externalizing traits, alternating hyper- and hypo-dopamine function) are relevant once a severe addiction has developed. On the one hand, drug related cues consistently induce striatal activations in people with current addictions, these activations are larger than those seen in healthy controls, and individual differences in the magnitude of drug cue-induced dopamine responses correlate with craving [76]. Based on these observations, we propose that it is premature to reject elevated dopamine transmission as a target for treatment.
At the same time, individuals with current SUDs are also consistently reported to have decreased striatal dopamine release, compared to healthy controls, when challenged with amphetamine [61]. Two points are of interest here. First, in all but one of these studies [130], amphetamine was administered without drug related cues being present (Box 4). Second, not all individuals with current SUDs exhibit diminished amphetamine-induced dopamine release when tested in the absence of drug-paired cues. This differential response appears to have clinical significance: the roughly 50% of subjects who exhibit a normal dopamine response under these conditions are also better responders to monetary reinforcement-based behavioral therapies, raising the intriguing possibility that patients who can express a dopamine response in the absence of drug related cues are also better able to learn new reward related behaviors [138-139]. It remains unclear whether the low dopamine release seen in the other substance dependent patients reflects the absence of drug-related cues, differential vulnerability to neurotoxic effects of extensive substance abuse, a pre-existing trait, dopamine D2 pre- and post-synaptic receptor super-sensitivity, or some combination of these factors. Irrespective, Martinez and colleagues [138] intriguingly noted that these individuals might display a biomarker indicating that they would benefit better from behavioral therapies if they were pre-treated with agents that increase presynaptic dopamine function, such as L-DOPA [140].
Box 4. Dopamine and “behavioral addictions”.
Evidence of augmented dopamine responses in the presence of addiction related cues has been seen consistently in people with ‘behavioral addictions’. Compared to healthy controls, people with non-substance related ‘behavioral addictions’ (Pathological Gambling, Binge Eating Disorder) exhibit evidence of exaggerated striatal dopamine responses to food, monetary rewards and undisguised amphetamine tablets [131-134; cf, 135]. The greater the elicited dopamine release, the more severe the clinical problems [132,134,136-137]. Low dopamine release has not been reported in these populations. However, the fMRI pathological gambling literature reports both increases and decreases in striatal activations, and these differential responses appear to reflect in substantial part the presence vs. absence of explicit gambling related cues [76].
Other dopamine based treatment strategies are also under development. Dopamine D1 and D2 receptor ligands have shown little efficacy but D3 receptor antagonists have tentatively shown potential [141]. Other receptor subtypes (D4, D5) have yet to be examined. Finally, since addicts appear to experience dopamine spikes in response to drug cues and dips when the cues are absent, dopamine modulators may provide a novel treatment consistent with the present model. The proposition is that these compounds will diminish the increases in dopamine that reinstate drug seeking without negating all dopamine transmission and producing a pervasive loss of interest [142].
Concluding remarks
The present model combines a neurodevelopmental perspective with evidence that the presence vs. absence of drug-related cues can come to regulate dopamine reactivity, directing motivational processes and setting the stage for progressively more frequent drug use and a SUD. This integrated perspective shows promise for guiding early intervention preventative strategies, and suggests that a fruitful direction for novel pharmacotherapeutic approaches could be to develop compounds that foster the ability to sustain interest in non-drug related activities. Strengthening the appeal of these goals may help those with SUDs steer away from drug related cues and attend better to ones necessary for healthy living.
Highlights.
Addictions are commonly presaged by problem behaviors in childhood
Susceptibility might reflect increased dopamine responses to salient events
Drugs hijack dopamine responses, directing behavior preferentially toward drugs
Non-drug events become less salient, and less able to activate dopamine
Narrowed interests develop, setting the stage for frequent drug use and addictions
Acknowledgements
This review was made possible by grants from the Canadian Institutes for Health Research (MOP-36429 and MOP-64426, ML) and the National Institutes of Health (DA09397, PV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO Management of Susbtance abuse: the global burden. 2013 http://www.who.int/substance_abuse/facts/global_burden/en/
- 2.Harwood H. Updating estimates of the economic costs of alcohol abuse in the United States: Estimates, update, and data. U.S. Department of Health and Human Services, U.S. Public Health Service, National Institutes of Health, National Institute of Alcohol Abuse and Alcoholism; Rockville, MD: 2000. [Google Scholar]; Office of National Drug Control Policy . The economic costs of drug abuse in the United States, 1992-1998. Executive Office of the President; Washington, DC: 2001. [Google Scholar]
- 3.United States Department of Justice The Economic Impact of Illicit Drug Use on American Society. 2011 Retrieved from http://www.justice.gov/ndic.
- 4.Piazza PV, et al. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 6.Blum K, et al. “Liking” and “wanting” linked to reward deficiency syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr. Pharmaceut. Des. 2012;18:113–118. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev, 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Conrod PJ, et al. Validation of a system of classifying female substance abusers on the basis of personality and motivational risk factors for substance abuse. Psychol. Addict. Behav. 2000;14:243–256. doi: 10.1037//0893-164x.14.3.243. [DOI] [PubMed] [Google Scholar]
- 10.Tarter RE, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, et al. Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch. Gen. Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendler KS, et al. Dimensions of Parental Alcohol Use/Problems and Offspring Temperament, Externalizing Behaviors, and Alcohol Use/Problems. Alcohol: Clin. Exp. Res. 2013 doi: 10.1111/acer.12196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. PNAS USA. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks BM, et al. Genetic and Environmental Influences on the Familial Transmission of Externalizing Disorders in Adoptive and Twin Offspring. JAMA Psychiatry. 2013a;70:1076–1083. doi: 10.1001/jamapsychiatry.2013.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks BM, et al. Identifying childhood characteristics that underlie premorbid risk for substance use disorders: socialization and boldness. Dev. Psychopathology. 2013b;26:1–17. doi: 10.1017/S0954579413000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pingault JB, et al. Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol. Psychiatry. 2013;18:806–812. doi: 10.1038/mp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick DM, et al. Adolescent alcohol use is predicted by childhood temperament factors before age 5, with mediation through personality and peers. Alc: Clin. Exp. Res. 2013;37:2108–2117. doi: 10.1111/acer.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyton M. Are addictions diseases or choices? J. Psychiatry Neurosci. 2013;38:219–221. doi: 10.1503/jpn.130097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter M. Developmental psychopathology: a paradigm shift or just a relabeling? Dev Psychopathology. 2013;25:1201–1213. doi: 10.1017/S0954579413000564. [DOI] [PubMed] [Google Scholar]
- 20.Newman JP, Lorenz AR. Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology. In: Davidson RJ, Scherer K, Goldsmith HH, editors. Handbook of affective sciences. Oxford University Press; Oxford: 2002. pp. 1043–1067. [Google Scholar]
- 21.Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohbot VD, et al. Caudate nucleus-dependent navigational strategies are associated with increased use of addictive drugs. Hippocampus. 2013;23:973–984. doi: 10.1002/hipo.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane SD, Cherek DR. Risk taking by adolescents with maladaptive behavior histories. Exp. Clin. Psychopharmacol. 2001;9:74–82. doi: 10.1037/1064-1297.9.1.74. [DOI] [PubMed] [Google Scholar]
- 24.Séguin JR, et al. Response perseveration in adolescent boys with stable and unstable histories of physical aggression: the role of underlying processes. J. Child. Psychol. Psychiatry. 2002;43:481–494. doi: 10.1111/1469-7610.00039. [DOI] [PubMed] [Google Scholar]
- 25.Fairchild G. The developmental psychopathology of motivation in adolescence. Dev. Cog. Neurosci. 2011;1:414–429. doi: 10.1016/j.dcn.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piazza PV, et al. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 27.Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- 28.Suto N, et al. Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology. 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- 29.Marinelli M. The many facets of the locomotor response to a novel environment test: theoretical comment on Mitchell, Cunningham, and Mark (2005) Behav. Neurosci. 2005;1194:1144–1151. doi: 10.1037/0735-7044.119.4.1144. [DOI] [PubMed] [Google Scholar]
- 30.Deroche-Gamonet V, et al. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 31.Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 32.Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb. Perspect. Med. 2012;2:a011940. doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooks MS, et al. Sensitization and individual differences in IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Ann. NY Acad. Sci. 1992;654:444–447. doi: 10.1111/j.1749-6632.1992.tb25993.x. [DOI] [PubMed] [Google Scholar]
- 34.Sills TL, Crawley JN. Individual differences in sugar consumption predict amphetamine-induced dopamine overflow in nucleus accumbens. Eur. J. Pharmacol. 1996;303:177–181. doi: 10.1016/0014-2999(96)00161-6. [DOI] [PubMed] [Google Scholar]
- 35.Flagel SB, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–59. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sills TL, Vaccarino FJ. Individual differences in sugar intake predict the locomotor response to acute and repeated amphetamine administration. Psychopharmacology. 1994;116:1–8. doi: 10.1007/BF02244864. [DOI] [PubMed] [Google Scholar]
- 37.Zocchi A, et al. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82:521–528. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]
- 38.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J. Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor JR, Horger BA. Enhanced responding for conditioned reward by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- 40.Schweimer J, et al. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behavioral Neuroscience. 2005;119:1687–1692. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- 41.Salamone JD, et al. Dopamine, behavioral economics, and effort. Frontiers in Behavioral Neuroscience. 2009;3:1–12. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winstanley CA, et al. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neuroscience. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb. Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- 44.Howe MW, et al. Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 2013;500:575–579. doi: 10.1038/nature12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gaalen MM, et al. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 46.Pattij T, et al. Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl) 2007;191:587–598. doi: 10.1007/s00213-006-0533-x. [DOI] [PubMed] [Google Scholar]
- 47.Winstanley CA, et al. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav. Brain Res. 2010;210:263–272. doi: 10.1016/j.bbr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 48.Leyton M. The neurobiology of desire: dopamine and the regulation of mood and motivational states in humans. In M. L. Kringelbach & K. C. Berridge (Eds.), Pleasures of the Brain. New York: Oxford University Press, Ch. 2009;13 [Google Scholar]
- 49.Leyton M, et al. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- 50.Buckholtz JW, et al. Dopaminergic network differences in human impulsivity. Science. 2010a;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckholtz JW, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010b;13:419–421. doi: 10.1038/nn.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cherkasova MV, et al. Amphetamine-induced dopamine release in treatment-naïve adults with ADHD: a PET/[11C]raclopride study. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.349. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvan A, et al. Risk-taking and the adolescent brain: who is at risk? Dev. Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- 54.Bjork JM, et al. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychology Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bjork JM, et al. Psychosocial problems and recruitment of incentive neurocircuitry: exploring individual differences in health adolescents. Dev. Cog. Neurosci. 2011;1:570–577. doi: 10.1016/j.dcn.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu CC, et al. Affective traits link to relaible markers of incentive anticipation. NeuroImage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chumbley JR, et al. Fatal attraction: ventral striatum predicts costly choice errors in humans. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.11.039. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Demos KE, et al. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 60.Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 61.Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.06.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagano-Saito A, et al. Dopamine facilitates fronto-striatal functional connectivity during a set-shifting task. J. Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagano-Saito A, et al. From anticipation to action, the role of dopamine in perceptual decision-making: an fMRI-tyrosine depletion study. J. Neurophysiol. 2012;108:501–512. doi: 10.1152/jn.00592.2011. [DOI] [PubMed] [Google Scholar]
- 64.Bjork JM, et al. Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.232. doi: 10.1038/npp.2013.232. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frank MJ, et al. By carrot or by stick: cognitive reinforcement learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 66.Leyton M, et al. Cocaine craving, euphoria, and self-administration: A preliminary study of the effect of catecholamine precursor depletion. Behav. Neuroscience. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- 67.Leyton M, et al. Mood-elevating effects of d-amphetamine and incentive salience: The effect of acute dopamine precursor depletion. J. Psychiatry Neurosci. 2007;32:129–136. [PMC free article] [PubMed] [Google Scholar]
- 68.Barrett SP, et al. The role of dopamine in alcohol self-administration in humans: Individual differences. Eur. Neuropsychopharmacology. 2008;18:439–447. doi: 10.1016/j.euroneuro.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Venugopalan VV, et al. Acute phenylalanine/tyrosine depletion reduces motivation to smoke cigarettes across stages of addiction. Neuropsychopharmacology. 2011;36:2469–2476. doi: 10.1038/npp.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cawley EI, et al. Dopamine and light: Dissecting effects on mood and motivational states in women with sub-syndromal seasonal affective disorder. J. Psychiatry & Neuroscience. 2013;38:388–397. doi: 10.1503/jpn.120181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simioni AC, et al. Dissecting the effects of disease and treatment on impulsivity in Parkinson’s disease. J. Int. Neuropsychol. Soc. 2012;18:942–951. doi: 10.1017/S135561771200094X. [DOI] [PubMed] [Google Scholar]
- 72.Pine A, et al. Dopamine, time, and impulsivity in humans. J. Neurosci. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Regier DA, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 74.Boileau I, et al. Modeling sensitization to stimulants in humans: A [11C]raclopride/PET study in healthy volunteers. Arch. Gen. Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 75.O’Daly OG, et al. Investigation of the amphetamine sensitization model of schizophrenia in healthy male volunteers. Arch. Gen. Psychiatry. 2011;68:545–554. doi: 10.1001/archgenpsychiatry.2011.3. [DOI] [PubMed] [Google Scholar]
- 76.Leyton M, Vezina P. Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neurosci. Biobehav. Rev. 2013;37:1999–2014. doi: 10.1016/j.neubiorev.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stewart J, Eikelboom R. Conditioned drug effects. In: Iversen LL, Iversen SD, Snyder SH, editors. Handbook of Psychopharmacology. Plenum Press; New York: 1987. pp. 1–57. [Google Scholar]
- 78.Aragona BJ, et al. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur. J. Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Ciano P, et al. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur. J. Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- 80.Ito R, et al. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J. Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss F, et al. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc. Natl. Acad. Sci. USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robinson TE, et al. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flagel SB, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panlilio LV, et al. Human cocaine-seeking behavior and its control by drug-associated stimuli in the laboratory. Neuropsychopharmacology. 2005;30:433–443. doi: 10.1038/sj.npp.1300599. [DOI] [PubMed] [Google Scholar]
- 85.Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol. Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mayo LM, et al. Conditioned preference to a methamphetamine-associated contextual cue in humans. Neuropsychopharmacology. 2013;38:921–929. doi: 10.1038/npp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Childs E, de Wit H. Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans. Addiction Biology. 2013;18:985–992. doi: 10.1111/j.1369-1600.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boileau I, et al. Conditioned dopamine release in humans: A PET [11C]raclopride study with amphetamine. J. Neuroscience. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang DW, et al. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology Behavior. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Bartholow BD, et al. Specificity of P3 event-related potential reactivity to alcohol cues in individuals low in alcohol sensitivity. Psych. Addictive Behaviors. 2010;24:220–228. doi: 10.1037/a0017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kareken DA, et al. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol: Clin. Exp. Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- 92.Kareken DA, et al. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. NeuroImage. 2010;50:267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dager AD, et al. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcohol: Clin. Exp. Res. 2013;37(Suppl 1):E161–171. doi: 10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Claus ED, et al. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Filbey FM, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oberlin BG, et al. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holland PC. Occasion setting in Pavlovian conditioning. In: Medin DL, editor. The psychology of learning and motivation. Academic press; San Diego, CA: 1992. pp. 69–125. [Google Scholar]
- 98.Grace AA, et al. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. TiNS. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56(Suppl 1):160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat in the presence of stimuli previously paired with systemic injections of morphine. PBB. 1990;35:367–372. doi: 10.1016/0091-3057(90)90171-d. [DOI] [PubMed] [Google Scholar]
- 101.Duvauchelle CL, et al. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav. Neurosci. 2000;114:1156–1166. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- 102.Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the nervous system. Telford press; Caldwell, New Jersey: 1988. pp. 207–224. [Google Scholar]
- 103.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav. Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 104.Guillory AM, et al. Effects of conditioned inhibition on neurotransmitter overflow in the nucleus accumbens. Soc. Neurosci. 2006 Abstr. 32, 483.3. [Google Scholar]
- 105.Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav. Pharmacology. 1991;2:65–71. [PubMed] [Google Scholar]
- 106.Anagnostaras SG, et al. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- 107.Cortright JJ, et al. Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology. 2012;37:2277–2284. doi: 10.1038/npp.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neugebauer NM, et al. Exposure to nicotine enhances its subsequent self-administration: contribution of nicotine-associated contextual stimuli. Behav. Brain Res. 2014;260:155–161. doi: 10.1016/j.bbr.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wray JM, et al. The magnitude and reliability of cue-specific craving in nondependent smokers. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.10.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 110.Andrews MM, et al. Individuals family history positive for alcoholism show functional resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schneider S, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am. J. Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 112.Yau W-YW, et al. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neuroscience. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cox SM, et al. Striatal dopamine responses to intranasal cocaine self-administration in humans. Biol. Psychiatry. 2009;15:846–850. doi: 10.1016/j.biopsych.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 114.Casey KF, et al. Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol. Psychiatry. 2013 Oct 16; doi: 10.1016/j.biopsych.2013.08.033. 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 115.Schultz W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toates F. Motivational Systems. Cambridge University Press; Cambridge, UK: 1986. [Google Scholar]
- 117.Stewart J, Wise RA. Reinstatement of heroin self-administration habits: morphine prompts and naltrexone discourages renewed responding after extinction. Psychopharmacology. 1992;108:79–84. doi: 10.1007/BF02245289. [DOI] [PubMed] [Google Scholar]
- 118.Hutcheson DM, et al. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat. Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- 119.Minhas M, Leri F. The effect of heroin dependence on resumption of heroin self-administration in rats. Drug Alc. Depend. 2014 doi: 10.1016/j.drugalcdep.2014.01.007. http://dx.doi.org/10.1016/j.drugalcdep.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 120.Setiawan E, et al. Differential striatal dopamine responses following oral alcohol in individuals at varying risk for dependence. Alcohol: Clin. Exp. Res. 2013 doi: 10.1111/acer.12218. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Lovic V, et al. Impulsive rats are less maternal. Dev. Psychobiol. 2011;53:13–22. doi: 10.1002/dev.20481. [DOI] [PubMed] [Google Scholar]
- 122.Kosten TA, et al. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Research. 2000;875:44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- 123.Meaney MJ, et al. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- 124.Lomanowska AM, et al. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav. Brain Res. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 125.Antelman SM, et al. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 126.Leyton M, Stewart J. Pre-exposure to repeated footshock sensitizes the locomotor activity from systemic morphine and intra-nucleus accumbens amphetamine. PBB. 1990;37:303–310. doi: 10.1016/0091-3057(90)90339-j. [DOI] [PubMed] [Google Scholar]
- 127.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 128.Nelson EC, et al. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: A twin study. Psychol. Med. 2006;36:1473–1483. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- 129.Conrod PJ, et al. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry. 2013;70:334–342. doi: 10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]
- 130.Volkow ND, et al. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. NeuroImage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steeves TDL, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C]raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Joutsa J, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. NeuroImage. 2012;60:1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Wang GJ, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boileau I, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [11C]-(+)-PHNO. Mol. Psychiatry. 2013 doi: 10.1038/mp.2013.163. doi:10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- 135.Broft A, et al. Striatal dopamine in bulimia nervosa: a PET imaging study. Int. J. Eat. Disord. 2012;45:648–656. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Linnet J, et al. Dopamine release in ventral striatum of pathological gamblers losing money. Acta Psychiatrica Scandinavica. 2010;122:326–333. doi: 10.1111/j.1600-0447.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- 137.Linnet J, et al. Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand. J. Psychology. 2011;52:28–34. doi: 10.1111/j.1467-9450.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 138.Martinez D, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang GJ, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol. Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmitz JM, et al. Contingency management and levodopa-carbidopa for cocaine treatment: a comparison of three behavioral targets. Exp. Clin. Psychopharmacology. 2010;18:238–244. doi: 10.1037/a0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Le Foll B, Boileau I. Repurposing buspirone for drug addiction treatment. Int. J Neuropsychopharmacol. 2013;16:251–253. doi: 10.1017/S1461145712000995. [DOI] [PubMed] [Google Scholar]
- 142.Steensland P, et al. The monoamine stabilizer (-)-OSU6162 attenuates voluntary ethanol intake and ethanol-induced dopamine output in nucleus accumbens. Biol. Psychiatry. 2012;72:823–831. doi: 10.1016/j.biopsych.2012.06.018. [DOI] [PubMed] [Google Scholar]